Abstract
Aging causes changes in the hippocampus that may lead to cognitive decline in older adults. In young animals, exercise increases hippocampal neurogenesis and improves learning. We investigated whether voluntary wheel running would benefit mice that were sedentary until 19 months of age. Specifically, young and aged mice were housed with or without a running wheel and injected with bromodeoxyuridine or retrovirus to label newborn cells. After 1 month, learning was tested in the Morris water maze. Aged runners showed faster acquisition and better retention of the maze than age-matched controls. The decline in neurogenesis in aged mice was reversed to 50% of young control levels by running. Moreover, fine morphology of new neurons did not differ between young and aged runners, indicating that the initial maturation of newborn neurons was not affected by aging. Thus, voluntary exercise ameliorates some of the deleterious morphological and behavioral consequences of aging.
Keywords: exercise, neurogenesis, learning, aging, angiogenesis, spines
Introduction
Aging leads to functional changes in the hippocampus, a brain structure that is important for learning (Jarrard, 1995). The ability to learn new tasks decreases with age (Gage et al., 1984; Smith et al., 2000). On the cellular level, synaptic contacts, synaptic strength, and plasticity are reduced (Geinisman et al., 1992; Barnes, 1994). In addition, hippocampal neurogenesis (Altman and Das, 1965; Eriksson et al., 1998) is diminished with aging (Kuhn et al., 1996; Heine et al., 2004). In elderly humans, imaging studies have shown hippocampal atrophy (West, 1993; Small et al., 2002). These deleterious consequences of aging may be prevented or reversed by exercise. Indeed, older adults who exercised throughout life had less brain tissue loss than sedentary individuals (Colcombe et al., 2003). Moreover, physically fit aged individuals performed better on cognitive tests than their sedentary counterparts (Kramer et al., 1999; Yaffe et al., 2001).
The functional benefits of exercise have been well studied in young adult animals. Wheel running and treadmill training improve spatial learning in rodents (Fordyce and Farrar, 1991; van Praag et al., 1999a; Anderson et al., 2000; Vaynman et al., 2004). In addition, exercise enhances hippocampal neurogenesis (van Praag et al., 1999a,b; Fabel et al., 2003; Farmer et al., 2004), a process that may contribute to cognition (Lemaire et al., 2000; Shors et al., 2001). Moreover, synaptic plasticity (van Praag et al., 1999a; Farmer et al., 2004), neurotransmission, and growth factor gene expression (Cotman and Berchtold, 2002) are increased in the hippocampus of physically active rats and mice. Similar changes in the brain and behavior of older animals may occur with exercise.
Reduced neurogenesis in the aged hippocampus has been associated with cognitive deficits (Drapeau et al., 2003; Merrill et al., 2003; Bizon et al., 2004). In the dentate gyrus, new cells are clustered close to blood vessels (Palmer et al., 2000) and proliferate in response to vascular growth factors (Jin et al., 2002). Thus, limited angiogenesis and decreased cerebral blood flow in the aged brain may contribute to the decline in cell genesis (Black et al., 1989; Sonntag et al., 1997). In young adult animals, exercise increases endothelial cell proliferation, vascular growth factor levels (Fabel et al., 2003; Lopez-Lopez et al., 2004), and angiogenesis (Swain et al., 2003) throughout the brain. In the dentate gyrus, however, the effects of exercise on the vasculature may also be important for enhancing neurogenesis.
Here, we show that exercise improves learning and neurogenesis in aged mice. In addition, using retroviral labeling, we demonstrate that newborn neurons have a similar morphology in the young and aged hippocampus. However, angiogenesis is not increased in old runners. Overall, aging-associated deficits in learning and neurogenesis are ameliorated by voluntary exercise.
Materials and Methods
Subjects. Fifteen young (3 months of age) and 18 old (19 months of age) male C57BL/6 (Harlan Sprague Dawley, Indianapolis, IN) mice were housed individually and divided into sedentary [young control (Y), n = 8; old control (O), n = 8] or running [young runner (YR), n = 7; old runner (OR), n = 10 (8 of 10 were tested behaviorally; all 10 were used for histology)] groups. The median life span for C57BL/6 mice is ∼26 months (Rowlatt et al., 1976; Kempermann et al., 1998). Runners had unlimited access to a running wheel in their cage for 45 d. Running distance (in kilometers) was monitored electronically (Lafayette Instrument, Lafayette, IN). Animals were given a daily intraperitoneal bromodeoxyuridine (BrdU) injection (dissolved in 0.9% NaCl, 50 μg/g body weight, 10 mg/ml; Sigma, St. Louis, MO) during the first week. Learning was tested between days 35 and 39. On day 45, animals were anesthetized with ketamine/xylazine (3:1) and perfused transcardially with 4% paraformaldehyde. Brains were postfixed for 24 h and equilibrated in 30% sucrose. Sequential 40 μm coronal sections were taken and stored at –20°C.
Spatial learning. Mice were trained on the Morris water maze (Morris et al., 1982) with four trials per day over 5 d. The platform was hidden 1 cm below the surface of water made opaque with white nontoxic paint. Starting points were changed every day. Each trial lasted either until the mouse found the platform or for 40 s. Mice rested on the platform for 10 s after each trial. Four hours after the last training session on day 5, the platform was removed for a 40 s probe trial. Swim path length and speed were recorded (Ethovision; Noldus Information Technology, Wageningen, The Netherlands).
Quantity and phenotype of newly born cells. Immunohistochemistry for BrdU and immunofluorescent triple labeling for BrdU, neuronal nuclei (NeuN), and S100β were performed as described previously (Kuhn et al., 1996). The antibodies used were rat anti-BrdU ascites (1:100; Accurate; Harlan Sera-Lab, Loughborough, UK), rabbit anti-S100β (1:2500; Swant, Bellinzona, Switzerland), and mouse anti-NeuN (1:20; R. J. Mullen, University of Utah, Salt Lake City, UT). Staining for BrdU with the peroxidase method was used (Vector Laboratories, Burlingame, CA) for cell counts. The fluorescent secondary antibodies used were antimouse FITC, anti-rat Texas Red, and anti-rabbit cyanine 5 (6 μl/ml; Jackson ImmunoResearch, West Grove, PA).
BrdU-positive cells were counted in a one-in-six series of sections as described previously (van Praag et al., 1999a). For phenotype analysis, 30 BrdU-positive cells per animal (except for aged sedentary mice, in which a minimum of 10 cells was used) were analyzed for the coexpression of BrdU and NeuN (neurons) and S100β (astrocytes). Ratios of colabeling were determined.
Fine morphology of new neurons. Mice were injected with green fluorescent protein (GFP)-expressing retrovirus and perfused 4 weeks later as described previously (van Praag et al., 2002). The pCAG-GFP retrovirus containing plasmid with enhanced GFP and replication-deficient Moloney murine leukemia virus retrovirus (Chunmei Zhao, The Salk Institute, La Jolla, CA) was used. Immunocytochemistry for GFP was performed using rabbit anti-GFP (Chemicon, Temecula, CA) as a primary antibody and donkey anti-rabbit Alexa 488 (Molecular Probes, Eugene, OR) as a secondary antibody followed by 4′6′-diamidino-2-phenylindole (DAPI) in 40 μm tissue sections. To assess dendritic length and branching, cells were imaged through a 40× objective, and Z-series of 1 μm optical sections were merged for measurements. For the spine density counts, dendrites were imaged through a 40× objective with a digital zoom of 3, and Z-series of 0.5 μm optical sections were merged for quantification.
Quantitation and analysis of blood vessels. Lectin staining (Lycopersicon esculentum; 1:200; Vector Laboratories) followed by streptavidin cyanine 3 was used. The optical fractionator method (Stereo Investigator; MicroBrightField, Williston, VT) was used to estimate blood vessel number. The counting frame was 100 × 100 μm, and the grid overlay was 200 × 200 μm. Analysis was done on six equidistant sections (240 μm apart).
Vessel cross-sectional surface area and perimeter were measured using Neuroleucida (MicroBrightField). Two vessels for two coronal midbrain sections were analyzed per animal. Vessels originated within the medial boundaries of the dentate and were a minimum length of 300 μm.
Statistical analysis. A two-way ANOVA (age × exercise) with Fisher's PLSD for post hoc tests was used (GraphPad Prism 4; SAS version 8.02; SAS Institute, Cary, NC).
Results
Running distance
There was a nonsignificant trend in the old mice to run less (4.9 ± 0.2 km/d and 3.9 ± 0.1 km/d for young and old mice, respectively; F(1,15) = 3.65; p > 0.07).
Spatial learning
Mice were tested in the water maze between days 35 and 39. There was a significant interaction between exercise and age for latency (F(1,27) = 4.89; p < 0.035) (Fig. 1a) and swim path length (F(1,27) = 8.62; p < 0.007) (Fig. 1b), but not swim speed (F(1,27) = 0.08; p > 0.77) (Fig. 1c), suggesting that exercise had a differential effect on acquisition of the water maze task in young versus aged mice. Post hoc comparisons showed that O mice had the longest latency and swim path to the platform (p < 0.01), indicating they did not learn the task as well as the others. With regard to swim speed, it should be noted that there was a main effect of age (F(1,27) = 8.16; p < 0.008) (Fig. 1c). Post hoc comparisons for each day of testing indicated that the O mice swam more slowly than all of the other groups only on day 5 of training (p < 0.03). Floating was observed in O mice (three of eight).
Figure 1.
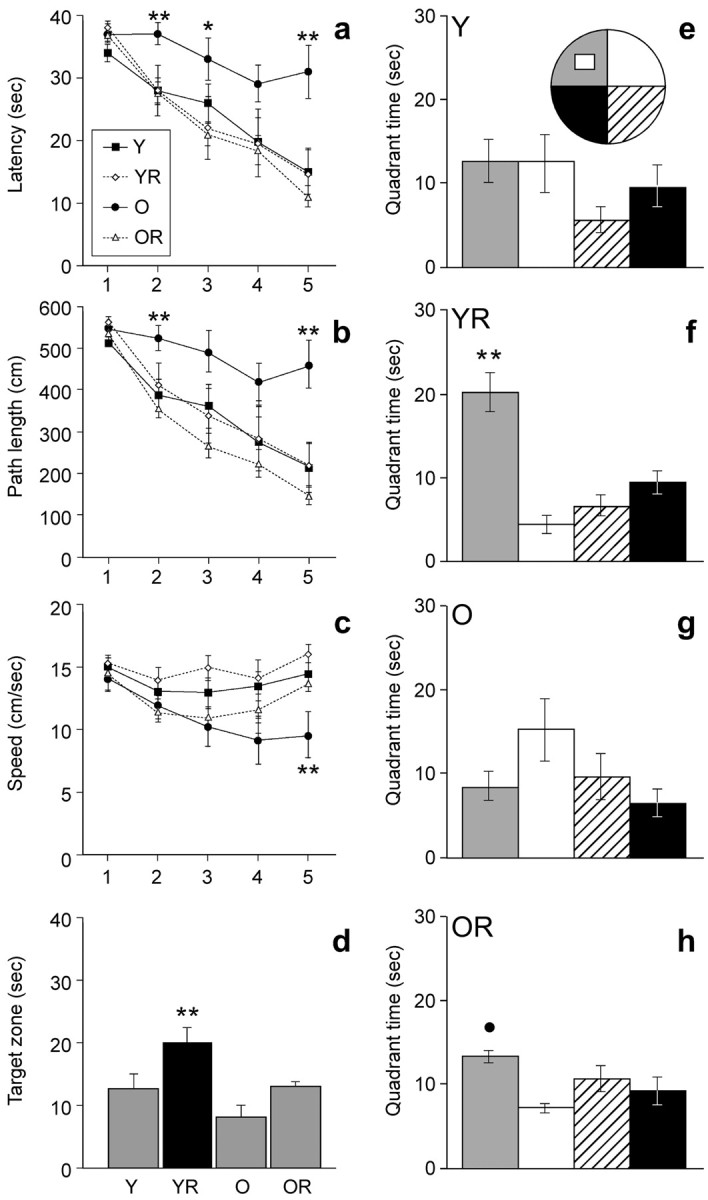
Water maze learning in young and aged mice housed with or without running wheel access. Aged controls (O) had significantly longer latency (a) and swim path (b) to the platform than all other groups (p < 0.01). Swim speed (c) was slower in old controls than in all other groups on day 5 of training (p < 0.03). The probe test 4 h after the last trial on day 5 showed that YR mice (d, f) and OR mice (h) had a significant preference for the platform quadrant but not the Y mice (e) and O mice (g). Asterisks indicate a significant difference from YR and OR mice (*) and a significant difference from all other groups/quadrants (**); the filled circle indicates a significant difference from adjacent quadrants (p < 0.05).
On day 5, the platform was removed for a 40 s probe test 4 h after the last trial to test recall. Analysis of time spent in the target zone revealed significant main effects for exercise (F(1,27) = 8.9; p < 0.006) and age (F(1,27) = 7.1; p < 0.01). YR mice spent more time in the platform quadrant than other groups (p < 0.02) (Fig. 1d). However, both YR mice (F(3,24) = 18.6; p < 0.0001) and OR mice (F(3,24) = 4.2; p < 0.017) showed a significant bias for the target zone, whereas Y mice (F(3,24) = 1.6; p > 0.22) and O mice (F(3,24) = 2.7; p > 0.07) did not (Fig. 1e–h).
Cell counts
BrdU-positive cells in the dentate gyrus were counted. A main effect of age confirmed that cell genesis declined with aging (F(1,29) = 51.9; p < 0.0005) (Kuhn et al., 1996). O mice had fewer new cells than Y mice (p < 0.03). Wheel running increased the number of BrdU-labeled cells in both age groups (F(1,29) = 56.12; p < 0.0001). Indeed, YR and OR mice had more new cells than age-matched sedentary controls (p < 0.0001 and p < 0.01, respectively). Furthermore, the effect of running on cell number was more pronounced in young mice than in aged mice, as indicated by a significant interaction between exercise and age (F(1,29) = 15.6; p < 0.0005). Specific comparisons showed that YR mice had more new cells than the other groups (p < 0.0001). Remarkably, OR mice did not differ from Y mice in new cell number, suggesting that exercise restores cell genesis in aging (Table 1).
Table 1.
Number and phenotype of BrdU-positive cells
|
|
Y |
YR |
O |
OR |
|---|---|---|---|---|
| Cell number | 613 (59)a | 2355 (343)c | 117 (50)a | 656 (43) |
| Neuron (%) | 49.9 (4.5)a | 81.3 (3.3)c | 9.5 (4.3)a | 25.6 (7.5) |
| Astrocyte (%) | 7.9 (2.5) | 3.4 (1.3) | 20.7 (5.5)b | 19.1 (2.3)b |
| Other (%) | 41.5 (3.7)a | 14.7 (3.3)c | 69.8 (6.9) | 55.3 (5.8) |
| Volume (mm3) |
0.39 (0.025) |
0.39 (0.03) |
0.42 (0.025) |
0.40 (0.025) |
Young and aged C57BL/6 mice were housed with or without a running wheel. All mice received BrdU injections (50 mg/kg) daily for the first 7 d. Survival and phenotype of BrdU-labeled cells as well as dentate gyrus volume were assessed 1 month after the last BrdU injection. The percentages of BrdU cells double labeled for NeuN (neurons), S100β (astrocytes), or neither marker are presented. Data are presented as means with SEM in parentheses.
Significantly different from age-matched animals (p<0.05).
Significantly different from Y, YR.
Significantly different from all other groups.
Phenotype analysis
Cells were analyzed for coexpression of BrdU and NeuN for neuronal phenotype and S100β for glial phenotype. Significant main effects of exercise (F(1,29) = 30.4; p < 0.0001) and age (F(1,29) = 116.5; p < 0.0001) indicated that running enhanced neurogenesis in both young and aged groups. Young runners had the most neurogenesis (∼81.3%), as evidenced by a significant interaction between age and exercise (F(1,29) = 5.3; p < 0.05) and post hoc comparisons (p < 0.001). In the O group, very few BrdU-positive cells became neurons (∼9.5%). There was significantly more neurogenesis in the OR mice (∼25.6%) than in the O mice (p < 0.01). However, the increase in percentage of BrdU/NeuN-positive cells in OR mice was still less than that in Y mice (∼49.9%; p < 0.04). The groups also differed with regard to the astrocytic fate of the newborn cells. The greatest percentage of gliogenesis was observed in the aged mice (∼20%) compared with young mice (<8%). Analysis showed a main effect of age (F(1,29) = 19.2; p < 0.0001) but not of exercise or an interaction (p > 0.05). Overall, the young groups had relatively less gliogenesis than the aged mice (p < 0.02) (Fig. 2a–d; Table 1).
Figure 2.
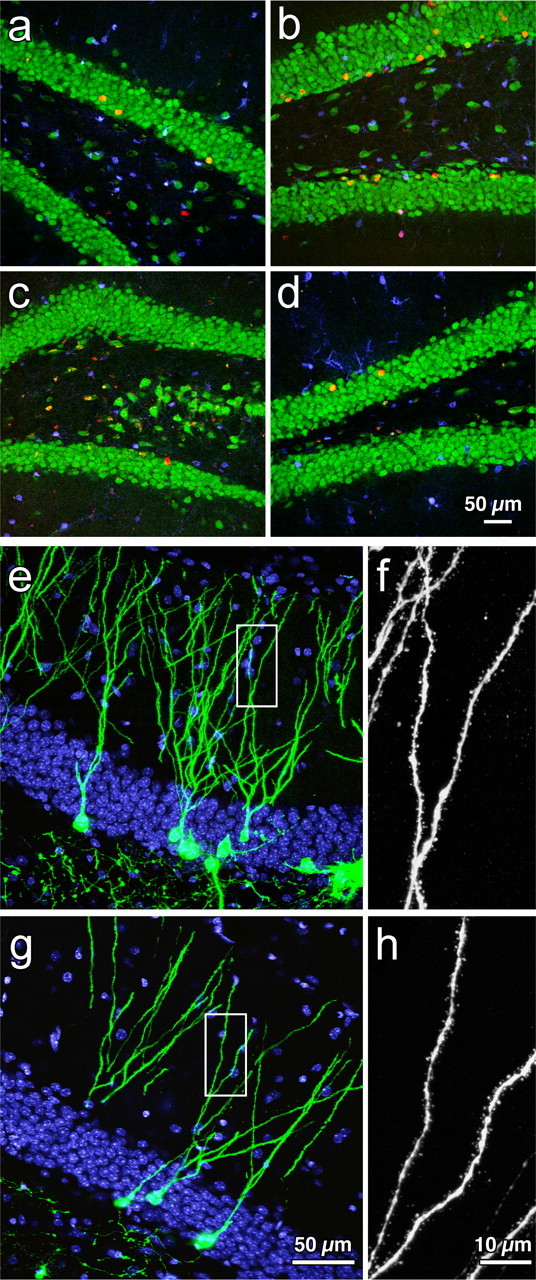
Neurogenesis in the young and aged dentate gyrus. Confocal images of immunofluorescent triple-labeled sections for BrdU (red), NeuN (green), and S100β (blue) [BrdU-labeled neurons are orange(redplusgreen)]inyoungcontrols(a), old controls (c), young runners (b), and old runners (d) are shown, as well as photomicrographs of GFP+ new neurons in young (e) and aged (g) running mice at 4 weeks after virus injection. The boxed areas in e and f correspond to the enlarged images of spines in young (f) and aged (h) mice (DAPI, blue).
Fine morphology
To determine whether aging affects dendritic length, dendritic branching, and spine density in new cells, a retrovirus-expressing GFP selective for dividing cells was used. No GFP+ neurons were found in aged controls, most likely because retroviral labeling is less efficient than BrdU (Y, 314 ± 46 GFP+ cells; YR, 550 ± 90 GFP+ cells; OR, 15 ± 7 GFP+ cells). Dendritic length (Y, 532.5 ± 87 μm; YR, 572.1 ± 73 μm; OR, 600 ± 37 μm) and number of branch points (Y, 5.9 ± 0.85; YR, 6.6 ± 0.82; OR, 6.3 ± 0.53) did not differ between cells from young and aged animals (n = 8 cells per group). Spines were counted in a total dendritic length of 483.8 μm in cells from young mice (n = 4) and 631.3 μm in cells from aged mice (n = 5). Spine density did not differ between the groups (YR, 1.61 ± 0.06 spines/μm; OR, 1.77 ± 0.13 spines; p > 0.29). Thus, new neurons in aged animals appear to be similar to those produced in young mice (Fig. 2e–h).
Blood vessels in the dentate gyrus
To investigate whether exercise enhances angiogenesis, dentate gyrus blood vessels were counted. There was no difference in vessel number between the groups [Y, 227 (±9); YR, 235 (±9); O, 229 (±10); OR, 257 (±10); p > 0.14]. However, analysis of individual vessels revealed qualitative changes in surface area and perimeter. Specifically, wheel running increased both parameters in young mice, as evidenced by a significant main effect for exercise (surface area, F(1,29) = 4.45, p < 0.043; perimeter, F(1,29) = 4.58, p < 0.04), a significant interaction between age and exercise for vessel surface area (F(1,29) = 4.18; p < 0.05), and an almost significant interaction for perimeter (F(1,29) = 4.05; p < 0.054). Indeed, specific comparisons showed that the vessel surface in YR mice was increased compared with all other groups (p < 0.05). Blood vessel perimeter was greater in YR mice than in Y mice (p < 0.04) but not in O or OR mice (p > 0.21). Thus, exercise may improve dentate gyrus perfusion in young runners (Fig. 3).
Figure 3.
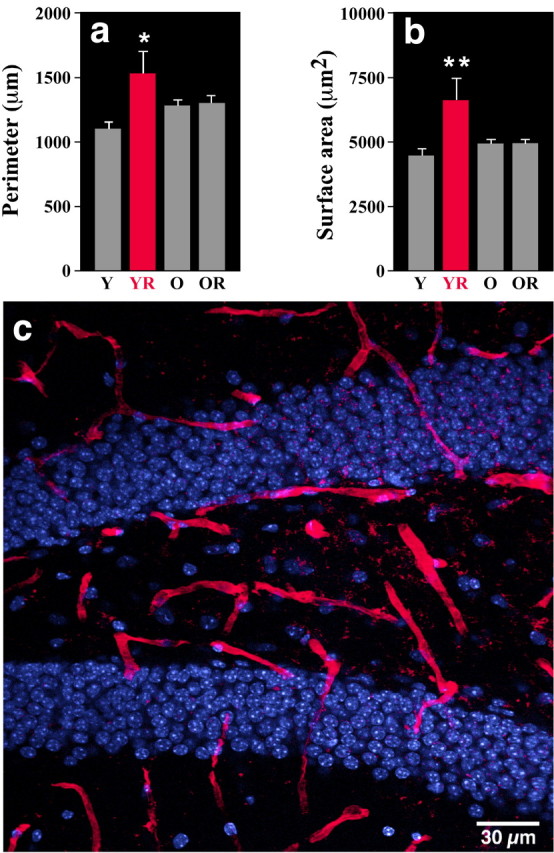
Blood vessel size in the dentate gyrus of young and aged mice housed with or without a running wheel. a, The perimeter of the vessels is larger in YR mice compared with age-matchedcontrols(Y;p<0.04) but did not differ from the aged controls (O) or aged runners (OR) (p > 0.21). b, Vessel surface area was greater in YR mice than in all of the other groups (p < 0.05). c, Lectin-stained vessels (red) and DAPI (blue) in the dentate gyrus.
Discussion
The present study was designed to determine whether cognition, neurogenesis, and angiogenesis in aged mice could be increased by exercise. We found that both learning and hippocampal neurogenesis were enhanced in aged running mice. There was no detectable decline in blood vessel surface area or perimeter in the aged groups. These parameters were only increased in the young runners. Interestingly, although the aged mice only started running at 19 months of age, the average running distance did not differ significantly from that of 3-month-old runners. However, our equipment could not measure whether the old mice ran more slowly or spent more time in the wheel than the young mice.
Exercise enhanced acquisition of the hidden platform task in the water maze in old mice compared with age-matched sedentary controls. This finding is consistent with research by some investigators who found that passive-avoidance learning is improved in physically active aged mice (Samorajski et al., 1985). However, others have reported that there was no effect of exercise on spatial learning in aged rats (Barnes et al., 1991). In the latter study, forced treadmill training was used rather than voluntary wheel running. In addition, mice were tested on the circular platform maze instead of the more physically demanding water maze (Barnes et al., 1991). However, enhanced fitness in runners is probably not the main reason for improved learning. Performance on the probe trial, which is more indicative of recall ability than fitness of the subject, was better in both young and old runners than in sedentary controls. It should be noted, however, that the aged mice had been sedentary until they were 19 months of age. It is possible that earlier onset of running would have maintained cognitive function to an even greater extent.
Previous studies have suggested that cognitive decline in aging may be attributable to decreased dentate gyrus neurogenesis (Kempermann et al., 1998; Drapeau et al., 2003; Merrill et al., 2003; Bizon et al., 2004). Several researchers have shown that cell genesis can be restored in the aged brain. Administration of insulin-derived growth factor-1 (IGF-1) (Lichtenwalner et al., 2001) and epidermal and fibroblast growth factor (Jin et al., 2003), reduction of corticosterone levels by adrenalectomy (Cameron and McKay, 1999), and environmental enrichment (Kempermann et al., 1998, 2002) increase new cell numbers in aged animals. However, learning was only tested and found to be improved in the enrichment studies (Kempermann et al., 1998, 2002). Exercise in young and aged animals enhances cell proliferation (van Praag et al., 1999a; Kim et al., 2004). In aged runners, cell survival returned to the level of young controls. However, the functional contribution may depend on the percentage of cells that become neurons.
Consistent with previous work, we found that relatively more cells became neurons in runners compared with age-matched controls (van Praag et al., 1999a,b). The greatest percentage of new neurons was found in the young runners (∼81.3%). In addition, although the total number of BrdU+ cells was similar in young controls and aged runners, there was more neurogenesis in young sedentary mice (49.9%) than in active aged mice (25.6%). Interestingly, the probe trial indicated that old runners may learn better than young controls. Thus, there appears to be no simple relationship between the number of new neurons and learning. For example, changes in the physiological properties of new cells may occur with exercise. Furthermore, other factors associated with running, such as increased neurotrophin and neurotransmitter levels, may contribute to improved learning (Cotman and Berchtold, 2002), although it is not known whether these changes occur in aged runners. The percentage of cells that become neurons in aged sedentary mice (9.5%) was consistent with previous work (Kempermann et al., 1998; Heine et al., 2004), although some researchers report up to 50% BrdU/NeuN cells in aged animals (Drapeau et al., 2003; Bizon et al., 2004). The increase in neurogenesis observed with exercise is comparable with enrichment data in aged mice (Kempermann et al., 1998). Neither exercise nor enrichment affected the percentage of cells that became glia in either age group.
To investigate how the aged hippocampal environment influences new neurons, mice were injected with a retrovirus selective for dividing cells (van Praag et al., 2002). Four weeks after injection, dendritic length, dendritic branching, and spine density of the new cells in the aged brain were similar to those in the young brain, suggesting that the function of the new cells in the aged brain was intact. However, more detailed physiological studies and a time course of the further development of these cells are needed to draw a more definite conclusion. Full maturation of new neurons takes several more weeks (van Praag et al., 2002) and may be delayed in the aged brain (Rao et al., 2005). In addition, new cells in the old brain may lose functional synapses much faster than in the young brain as a result of diminished trophic support (Sonntag et al., 1997). Furthermore, because of the limitations of the retroviral labeling technique, spine number was compared only between young and aged runners and was found not to differ between the groups. We do not know whether spine quantity increases as a function of running in aged animals. In young mice, however, spine density does not differ between controls and runners (C. Zhao, E. M. Teng, R. G. Summers, and F. H. Gage, personal communication).
In the present study, we found no decline in dentate gyrus blood vessel size or number with aging. Exercise did enhance the perimeter and surface area of blood vessels in young but not in aged mice. The findings for the young mice are consistent with other studies. For example, activity matched to a motor skill-learning task enhanced capillary density in the cerebellum (Black et al., 1990). More recently, voluntary wheel running has been found to increase angiogenesis in the motor cortex, cerebellum, and hippocampus (Swain et al., 2003; Lopez-Lopez et al., 2004). The lack of vascular plasticity with exercise in the older animals may be a result of reduced IGF-1 (Sonntag et al., 1997) and vascular endothelial growth factor levels (Shetty et al., 2005). In addition, decreased mitochondria content in capillary endothelial cells has been reported previously (Burns et al., 1981). However, lack of exercise-induced angiogenesis is not a rate-limiting factor for neurogenesis, given the significant increase in new neurons in aged runners.
In summary, exercise restores spatial learning and neurogenesis in aged mice. Interestingly, the properties of new neurons do not appear to change with aging, suggesting that the local hippocampal environment of the aged dentate gyrus can sustain neurogenesis.
Footnotes
This work was supported by grants from the Pritzker Neurogenesis Research Consortium, the Defense Advanced Research Projects Agency, the National Institutes of Health–National Institute on Aging (5 R01 AG020938), and the Lookout Fund. We thank Eve Taylor for the aged mice; Jae Ik Lee, Matthew Enhung Teng, Melanie J. Lucero, Steve Forbes, and Bobbi Miller for technical assistance; Jamie Simon, Linda R. Kitabayashi, and Robert G. Summers for help with figure preparation; and Mary Lynn Gage for comments on this manuscript. We also thank A. Smith, K. Suter, and their colleagues from the Animal Research Department at The Salk Institute for their support of these experiments.
Correspondence should be addressed to Fred H. Gage, Laboratory of Genetics, The Salk Institute for Biological Studies, 10010 North Torrey Pines Road, La Jolla, CA 92037. E-mail: gage@salk.edu.
DOI:10.1523/JNEUROSCI.1731-05.2005
Copyright © 2005 Society for Neuroscience 0270-6474/05/258680-06$15.00/0
References
- Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319–335. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Rapp DN, Baek DH, McCloskey DP, Coburn-Litvak PS, Robinson JK (2000) Exercise influences spatial learning in the radial arm maze. Physiol Behav 70: 425–429. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Forster MJ, Fleshner M, Ahanotu EN, Laudenslager ML, Mazzeo RS, Maier SF, Lal H (1991) Exercise does not modify spatial memory, brain autoimmunity, or antibody response in aged F-344 rats. Neurobiol Aging 12: 47–53. [DOI] [PubMed] [Google Scholar]
- Barnes CA (1994) Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci 17: 13–18. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Lee HJ, Gallagher M (2004) Neurogenesis in a rat model of age-related cognitive decline. Aging Cell 3: 227–234. [DOI] [PubMed] [Google Scholar]
- Black JE, Polinsky M, Greenough WT (1989) Progressive failure of cerebral angiogenesis supporting neural plasticity in aging rats. Neurobiol Aging 10: 353–358. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT (1990) Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA 87: 5568–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns EM, Kruckeberg TW, Gaetano PK (1981) Changes with age in cerebral capillary morphology. Neurobiol Aging 2: 283–291. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD (1999) Restoring production of hippocampal neurons in old age. Nat Neurosci 10: 894–897. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF (2003) Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci 58: M176–M180. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC (2002) Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 25: 295–301. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN (2003) Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA 100: 14385–14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4: 1313–1317. [DOI] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD (2003) VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci 18: 2803–2812. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR (2004) Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 124: 71–79. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Farrar RP (1991) Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning. Behav Brain Res 46: 123–133. [DOI] [PubMed] [Google Scholar]
- Gage FH, Kelly PA, Bjorklund A (1984) Regional changes in brain glucose metabolism reflect cognitive impairments in aged rats. J Neurosci 4: 2856–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F, Persina IS, Rossi M (1992) Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus 2: 437–444. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Joels M, Lucassen PJ (2004) Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging 25: 361–375. [DOI] [PubMed] [Google Scholar]
- Jarrard LE (1995) What does the hippocampus really do? Behav Brain Res 71: 1–10. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA (2002) Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA 99: 11946–11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA (2003) Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell 2: 175–183. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH (1998) Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci 18: 3206–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH (2002) Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol 52: 135–143. [DOI] [PubMed] [Google Scholar]
- Kim YP, Kim H, Shin MS, Chang HK, Jang MH, Shin MC, Lee SJ, Lee HH, Yoon JH, Jeong IG, Kim CJ (2004) Age-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Neurosci Lett 355: 152–154. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A (1999) Ageing, fitness and neurocognitive function. Nature 400: 418–419. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH (1996) Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 16: 2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN (2000) Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA 97: 11032–11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenwalner R, Forbes M, Bennett S, Lynch C, Sonntag W, Riddle D (2001) Intracerebroventricular infusion of insulin-like growth factor-1 ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience 107: 606–613. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez C, LeRoith D, Torres-Aleman I (2004) Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci USA 101: 9833–9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill DA, Karim R, Darraq M, Chiba AA, Tuszynski MH (2003) Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. J Comp Neurol 459: 201–207. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297: 681–683. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite A, Gage FH (2000) Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425: 479–494. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK (2005) Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci 21: 464–476. [DOI] [PubMed] [Google Scholar]
- Rowlatt C, Chesterman FC, Sheriff MU (1976) Lifespan, age changes and tumour incidence in an ageing C57BL mouse colony. Lab Anim 10: 419–442. [DOI] [PubMed] [Google Scholar]
- Samorajski T, Delaney C, Durham L, Ordy JM, Johnson JA, Dunlap WP (1985) Effect of exercise on longevity, body weight, locomotor performance, and passive-avoidance memory of C57BL/6J mice. Neurobiol Aging 6: 17–24. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA (2005) Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia 51: 173–186. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001) Neurogenesis in the adult is involved in the formation of trace memories. Nature 410: 372–376. [DOI] [PubMed] [Google Scholar]
- Small SA, Tsai WY, DeLaPaz R, Mayeux R, Stern Y (2002) Imaging hippocampal function across the human life span: is memory decline normal or not? Ann Neurol 51: 290–295. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR (2000) Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci 20: 6587–6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cooney PT, Hutchins PM (1997) Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology 138: 3515–3520. [DOI] [PubMed] [Google Scholar]
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT (2003) (2003) Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience 117: 1037–1046. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999a) Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 96: 13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH (1999b) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2: 266–270. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH (2002) Functional neurogenesis in the adult hippocampus. Nature 415: 1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F (2004) Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J Neurosci 20: 2580–2590. [DOI] [PubMed] [Google Scholar]
- West MJ (1993) Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging 14: 287–293. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K (2001) A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med 161: 1703–1708. [DOI] [PubMed] [Google Scholar]


