Abstract
Patients with corticospinal tract dysfunction have slow voluntary movements with brisk stretch reflexes and spasticity. Previous studies reported reduced firing rates of motor units during voluntary contraction. To assess whether this firing behavior occurs because motor neurons do not respond normally to excitatory inputs, we studied motor units in patients with primary lateral sclerosis, a degenerative syndrome of progressive spasticity. Firing rates were measured from motor units in the wrist extensor muscles at varying levels of voluntary contraction ≤10% maximal force. At each force level, the firing rate was measured with and without added muscle vibration, a maneuver that repetitively activates muscle spindles. In motor units from age-matched control subjects, the firing rate increased with successively stronger contractions as well as with the addition of vibration at each force level. In patients with primary lateral sclerosis, motor-unit firing rates remained stable, or in some cases declined, with progressively stronger contractions or with muscle vibration. We conclude that excitatory inputs produce a blunted response in motor neurons in patients with primary lateral sclerosis compared with age-matched controls. The potential explanations include abnormal activation of voltage-activated channels that produce stable membrane plateaus at low voltages, abnormal recruitment of the motor pool, or tonic inhibition of motor neurons.
INTRODUCTION
During voluntary movement, the nervous system normally regulates the strength of muscle contraction by modulating the number of active motor neurons and their firing rates. Stronger contractions are normally produced both by increasing the firing rate of active motor neurons and by recruiting inactive motor neurons (reviewed in Doherty et al. 2002). After injury to the motor cortex or the corticospinal tract in humans, voluntary movements become slow and effortful, and recruitment and firing rates of motor units are reduced (Frascarelli et al. 1998; Gemperline et al. 1995; Grimby et al. 1974; Rosenfalck and Andreassen 1980; Wiegner et al. 1993). This reduced ability to activate motor neurons is generally attributed to the loss of descending excitatory input. However, chronic loss of corticospinal input may also produce adaptations in spinal neurons or afferents. For example, the hyperactive stretch reflexes that develop after injury to the corticospinal tract are associated with reduced presynaptic inhibition and reduced postactivation depression of Ia spindle afferents (Aymard et al. 2000; Calancie et al. 1993; Faist et al. 1994; Nielsen et al. 1995).
Only a few studies have addressed whether spinal motor neurons undergo changes in excitability after loss of corticospinal inputs. In acute spinal injury, the flaccid paralysis and reduced stretch reflexes of spinal shock have been attributed, in part, to transient hypo-excitability of motor neurons (Hiersemenzel et al. 2000; Leis et al. 1996a,b). However, the time course of recovery from spinal shock and development of spasticity corresponds to the increasing excitability of presynaptic Ia afferents (Calancie et al. 1993; Schindler-Ivens and Shields 2000). Nevertheless, enlarged F-waves seen late in spasticity (Fierro et al. 1990) provide indirect evidence for enhanced firing by motor neurons. Recent studies in patients with chronic spinal injury also report that motor neurons can exhibit persistent firing after activation and propose sustained firing as a possible basis for spasms (Gorassini et al. 2004; Nickolls et al. 2004; Thomas and Ross 1997). These studies could indicate that motor neurons themselves become hyper-excitable in spasticity, i.e., with greater than normal firing in response to excitatory input.
To test whether motor neurons have an altered response to excitatory inputs in chronic spasticity, we examined the firing behavior of motor units in a group of patients with primary lateral sclerosis (PLS). PLS is a rare, degenerative condition that consists of slowly progressive limb and bulbar spasticity with relative sparing of spinal motor neurons (Erb 1875). Although the etiology of PLS is unknown and there is considerable debate whether it constitutes a distinct disorder (Rowland 1999; Swash et al. 1999), patients with PLS can be defined by clinical criteria (Pringle et al. 1992). In a cohort of patients defined by these clinical criteria, we previously demonstrated corticospinal impairment using transcranial magnetic stimulation and magnetic resonance spectroscopy imaging (Zhai et al. 2003). In contrast to spasticity from causes such as spinal injury, PLS patients have no physical disruption of spinal tracts, and physiologic testing showed normal ascending sensory potentials as well as intact reflexes mediated by reticulospinal pathways (Zhai et al. 2003). Clinically, PLS patients have marked spasticity, diffusely brisk stretch reflexes, clonus, and slow voluntary movements but relatively preserved strength.
In this study, motor neuron responsiveness was tested by measuring firing rate changes in wrist extensor motor units with sustained excitatory inputs. To obtain at least two levels of input, we looked at the additive effect of a second excitatory input on firing produced by the first. The first source of excitation was voluntary effort to produce a steady level of force. Increments of voluntary effort normally produce stepwise increments in motor unit firing even in acutely paralyzed muscles (Gandevia et al. 1993). Patients with PLS can produce increments of steady force, but because the pathways used to produce voluntary contraction may differ from those normally used, graded contraction alone may not provide a reliable source of graded input to motor neurons. For this reason, voluntary effort was used to provide a tonic level of excitation to motor neurons, and a second excitatory input was added by vibrating the muscle during the sustained contraction. Vibration of muscle repetitively activates muscle Ia spindle afferents (Burke et al. 1976) and excites motor neurons (Jack and Roberts 1978). Because previous studies of patients with spasticity showed that Ia terminals are subject to less pre-synaptic inhibition and postactivation depression than in healthy subjects (Aymard et al. 2000; Calancie et al. 1993; Faist et al. 1994; Nielsen et al. 1995), we reasoned that several seconds of muscle vibration would provide an effective source of excitatory input to motor neurons in PLS patients. In healthy persons, vibration produces postactivation depression that reduces the effectiveness of Ia stimulation, but vibration-induced depression of Ia afferents is markedly attenuated in patients with spasticity (Desmedt and Godaux 1978; Ongerboer de Visser et al. 1989). Thus we predicted that muscle vibration would provide an effective source of excitatory input to motor neurons in PLS patients that would add to excitation from voluntary drive.
METHODS
Nine patients with PLS [5 men and 4 women, aged 55 ± 10 (SD) yr] and 7 healthy volunteers (5 men and 2 women, aged 51.1 ± 9.9 yr) participated in the study. All subjects gave written, informed consent in accord with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board. The PLS patients fulfilled the clinical criteria of Pringle (Pringle et al. 1992) and have been described in detail in our previous report (patients 1–3, 5–8, 10, and 11 in Zhai et al. 2003). All PLS patients had longstanding disease (range 5–11 yr) that began with spasticity in the legs and slowly ascended over the years, producing four-limb and bulbar spasticity. At the time of study, all had arm spasticity and impaired finger tapping movements for ≥1 yr. None had evidence for muscle denervation with diagnostic needle electromyography (EMG). Table 1 describes the motor findings of the PLS patients. Finger tapping speed on a keyboard was measured for three 15-s epochs, using a custom software program (LabView, National Instruments). The physiology experiments were performed with the subject seated in a chair, with the hand and arm strapped to a horizontal platform that incorporated a force transducer to measure isometric wrist extension. The elbow was bent at ~90° and rested on the platform, with straps applied to the proximal forearm to prevent movement at the elbow or shoulder from being transmitted to the transducer. Subjects viewed a computer screen that displayed the output of the force transducer in one color and target levels of force to be produced in another color. A vibrating hammer (Bruel and Kjaer 4810, Denmark) rested on the wrist extensor muscles distal to the recording sites. Vibration consisted of 3-ms taps of 1-mm undamped displacement at a frequency of 50 Hz.
TABLE 1.
Clinical features of PLS patients
| Subject | Motor Units (n) | Age | Disease Duration, yr | Motor Score* | Finger Taps/s |
|---|---|---|---|---|---|
| 1 | 4 | 43 | 5 | 34 | 2.5 |
| 2 | 1 | 54 | 5 | 35 | 2.5 |
| 3 | 4 | 45 | 6 | 35 | 3.4 |
| 4 | 3 | 47 | 6 | 35 | 2.7 |
| 5 | 2 | 53 | 7 | 34.5 | 3.0 |
| 6 | 4 | 65 | 7 | 35 | 2.3 |
| 7 | 3 | 65 | 9 | 34.5 | 1.7 |
| 8 | 1 | 58 | 10 | 35 | 3.9 |
| 9 | 3 | 72 | 11 | 34.5 | 2.3 |
PLS, primary lateral sclerosis.
Strength of 7 muscles, each graded 0–5 using the Medical Research Council scale (a score of 35 indicates full strength).
Motor units were recorded from two to four intramuscular wires (Nicolet Biomedical, Madison, WI) inserted into the extensor carpi radialis muscle. A counterpoint electromyograph (Dantec, Denmark) was used, with filter settings of 500 Hz to 20 kHz to reduce pick-up from distant units. Surface EMG was recorded from wrist extensor and flexor muscles using disposable silver/silver chloride electrodes (Teca) in a bipolar configuration with filter settings 30 Hz to 2 kHz to monitor the contraction of muscles of interest. EMG signals and the output from the force transducers were digitized using a CED 1401 and analyzed with spike2 software (Cambridge Electronic Devices).
The peak maximal force was first measured from three maximal voluntary wrist extensions, and thereafter, forces were expressed as a percentage of each subject’s maximum voluntary contraction (MVC). To obtain the initial target force level, subjects made a small, sustained contraction of the wrist extensors that produced steady firing of one or two motor units. This corresponded to either 1 or 2% of maximal force in most subjects. A target line showing this level of force was then displayed on the screen, and subjects were instructed to reach and hold the wrist extension for 10 s on the target line. After 5–10 s of rest, contraction at the target level was repeated with muscle vibration. Each target level was tested three times with and without vibration. The target force level was then doubled and the series of contractions was repeated with and without vibration. In most subjects (all but 2), a third or fourth additional target force level was recorded, ≤10% maximal force. Spike discrimination was too difficult to accurately identify units at higher force levels. Figure 1A shows the sequence of force levels and addition of vibration as the experiment was carried out in patient 4.
Fig. 1.
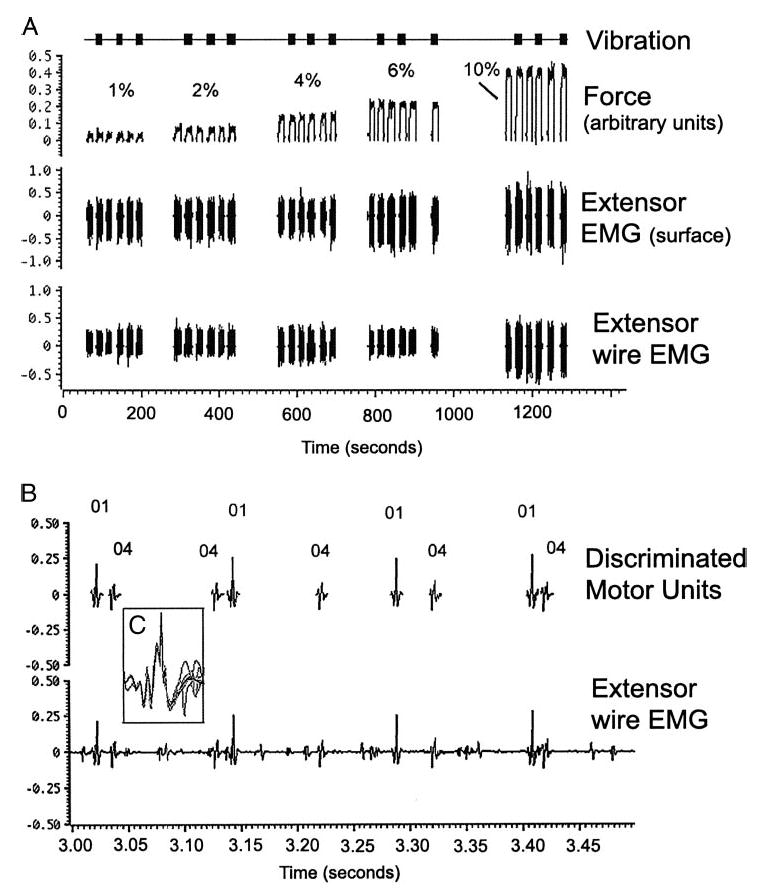
Example of the experimental procedure (patient 4). A: wrist extension is performed at a steady level for 10-s epochs with several seconds rest between contractions. In this example, there are 6 10-s epochs at each of 5 force levels: 1, 2, 4, 6, and 10% of maximal voluntary contraction. Top: timing of the muscle vibration. Middle and bottom: extensor muscle surface electromyography (EMG) and recordings from intramuscular wires. B: expanded scale (sweep = 500 ms) shows motor unit recordings and spike discrimination of 2 units during a contraction epoch at 10% maximal force. Both units were initially recruited at 1% force with firing frequencies of 9.7 and 11.3 Hz, similar to that seen at 10% force. Note that not all motor units visible in the recording were discriminated. C: inset showing overlay of 10 spikes discriminated by their morphology.
Discrimination of individual motor units by spike morphology was carried out off-line using the template matching routine within spike2, with interactive editing by the investigators to ensure correct classification of motor units in the event of superimposition (Fig. 1, B and C). In some cases, the same motor unit was identified on two separate channels to ensure correct discrimination. As shown in Fig. 1B, particular units were discriminated, but not every unit visible on the recording was identified. Those units that had regular firing and distinctive morphologic features that allowed them to be easily identified were studied. The mean firing rate was determined from a 5-s epoch in the middle of each contraction when force was held most steady, and data from the three epochs at each force level were averaged. The slope of the firing rate-force relationship was calculated by linear regression for all force levels for each unit with and without vibration. A repeated-measures ANOVA was used to assess changes in patient’s motor unit firing rates with increasing force and vibration. Paired t-test were used to assess differences between each unit’s slope with and without vibration, and unpaired t-test were used to compare PLS patients to healthy controls. Changes in the direction of slopes were compared with the Mann-Whitney U test. Data are expressed as mean ± SD.
RESULTS
Subjects
All patients had slow voluntary hand movements. Finger tapping speeds of PLS patients were about half as fast as those of age-matched control subjects (PLS: 2.7 ± 0.6 taps/s; controls: 6.2 ± 0.9 taps/s; P ≤ 0.01). Wrist extensor strength, however, was preserved in PLS patients (Table 1) with no significant difference in maximal voluntary wrist extension torque measures (PLS: 5.4 ± 2.2 N · m; controls: 6.6 ± 0.16 N · m; P = 0.16). All patients and control subjects were able to grade the force of wrist extension and to maintain a steady contraction for 5- to 10-s epochs at multiple levels of force <10% MVC, as shown in Fig. 1 and at higher levels as shown in Fig. 6.
Fig. 6.
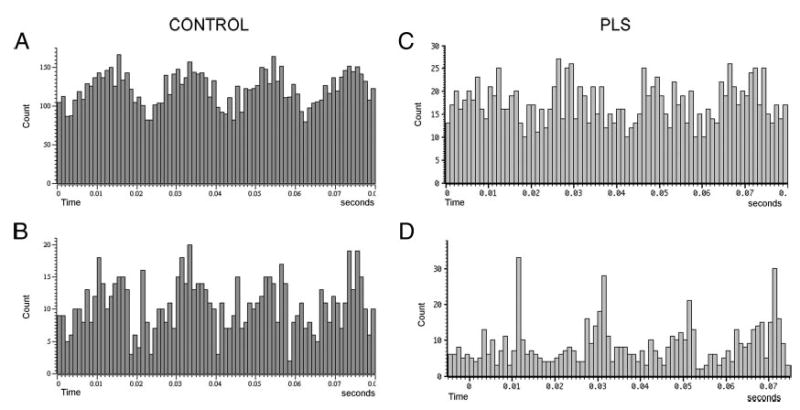
Histograms of motor unit spikes triggered from the timing pulse driving the vibrating hammer. Slight variations in firing intervals at the frequency of vibration produce periodicities in the histograms. Four cycles are shown in each 80-ms sweep with a 20-ms interval between peaks. A and B: examples from 2 motor units of control subjects. C and D: examples from 2 motor units in PLS patients.
Motor-unit firing
Twenty-five motor units were recorded from PLS patients and 10 motor units from the control subjects. The initial firing rates of motor units at the lowest target force level were similar for patients and controls (PLS: 13.1 ± 2.9 Hz; controls: 14.1 ± 2.1 Hz; P = 0.19). With more forceful contractions, motor units in the control subjects typically increased their firing rates, by an average of 1.7 Hz at each successive force level. In contrast, motor units from the PLS patients did not significantly change their firing rates with increasing force (mean change for successive force level: 0.4 Hz), as shown in Fig. 2. In 7 of the 25 motor units recorded from PLS patients, the firing rate declined at higher forces.
Fig. 2.
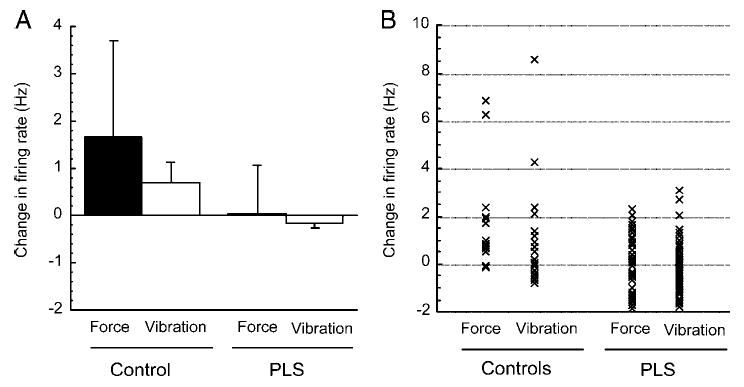
A: change in motor-unit firing rate between successive force levels (▪) or with addition of vibration at each force level (□) for control subjects (left) and primary lateral sclerosis (PLS) patients (right). Mean ± SD. B: data points for each force level of each motor unit represented in group data of A.
The addition of muscle vibration to the contraction modestly increased the motor unit firing rates in control subjects (0.72 ± 0.6 Hz; P < 0.05) but did not change the firing rate in motor units of PLS patients (−0.14 ± 0.19 Hz; P = 0.14). In some motor units, vibration produced a decline in firing rates. This occasionally happened in some motor units of control subjects at the lowest force levels but not at higher force levels (Fig. 3A). In motor units of PLS patients, vibration and force led to decreased firing rates across all force levels in many motor units (Fig. 3B).
Fig. 3.
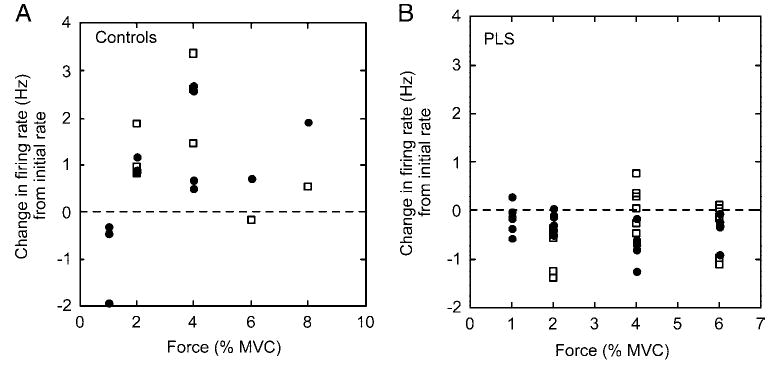
Examples of units in which firing rates declined with vibration (•) or at successive force levels (□). A: 4 motor units from control subjects. Declines in firing rate from vibration were limited to the lowest force levels. B: 6 motor units from PLS patients. Declines in firing rate occurred across all force levels.
Slope of firing rate/force
During epochs of contraction alone, the firing rate increased as contraction force increased in all motor units from control subjects, producing a positive slope of the regression line for firing rate force (Fig. 4A, slope values >0; Fig. 5, A–C). With addition of vibration, the slopes of the firing rate-force regression line remained positive, and often increased compared with contraction alone (Fig. 4A). In patient motor units, the slopes of the firing rate-force regression line were significantly flatter than those of controls (P < 0.01) during epochs of contraction alone and negative slopes were observed in motor neurons in which the firing rate declined at higher forces (Fig. 4B, slope values <0). With addition of vibration, the slope was unchanged for most motor units from PLS patients, and in some cases declined (Figs. 4B and 5, D and E). The effect of vibration on the direction of the slope was significantly different in PLS patients compared with controls (P < 0.01 Mann Whitney U test).
Fig. 4.
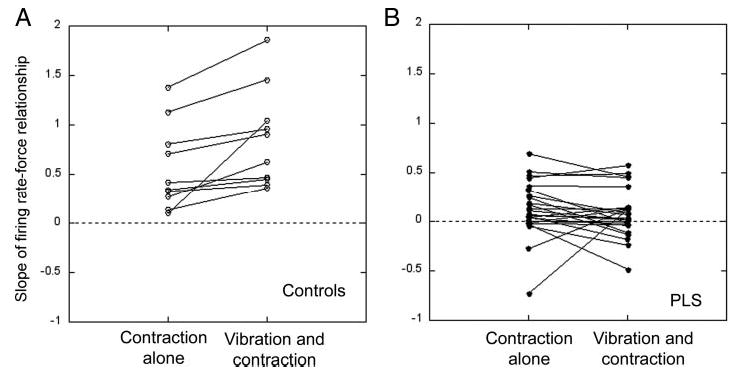
Slopes of the firing rate-force relationship of individual motor units (Hz/N · m), calculated using linear regression. A: most motor units in healthy volunteers have a positive slope during contraction alone, indicating that firing rates increase with stronger contractions. The slope remains positive and often increases further when vibration of the muscle is added during contraction. B: the slopes of most motor units in PLS patients are lower than controls with contractions of increasing force and some are even negative. When the muscle is vibrated during contraction, most motor-unit slopes fail to increase and some decline.
Fig. 5.
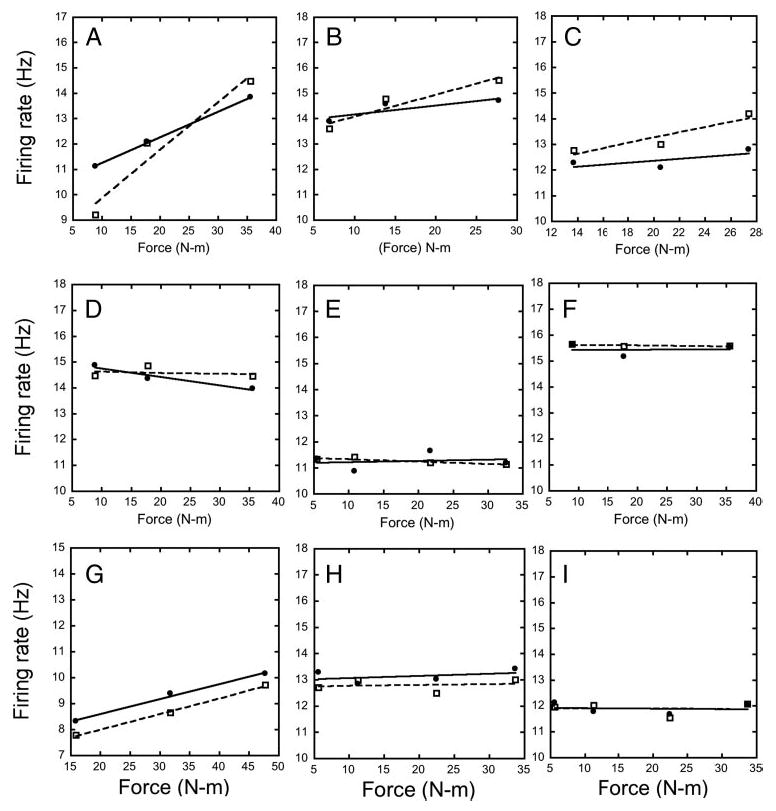
Examples of firing rate changes of individual motor units at successive force levels during contraction alone (—) and with vibration (- - -) from control subjects (A–C) and PLS patients (D–I).
Variability
Variability of motor unit firing rate was similar in PLS patients and control subjects during epochs of contraction alone (coefficient of variation PLS: 0.15; controls 0.16). During vibration, the variability of motor-unit firing increased in PLS patients (coefficient of variation = 0.47) but was unchanged in controls (coefficient of variation = 0.17). In many motor units, the vibration introduced a 50-Hz periodicity in firing, the frequency of the vibrating hammer. Figure 6 shows examples of this periodicity from several motor units in which a firing histogram was constructed by triggering from the vibration timing pulse. Periodicities were seen in motor units of patients and controls, so are unlikely to have introduced the differences in variability of firing rate. Nevertheless, these periodicities demonstrate that the vibration stimulus produced excitation that reached the motor neurons in patients and controls, although it did not alter the main firing frequency in patients.
Surface EMG
Surface EMG from the extensor muscles was rectified and averaged at each force level. In many patients, surface EMG recordings were also available for force levels >10% MVC, although individual units could not be discriminated at these force levels. In controls and patients, the mean surface EMG increased with increasing force (Fig. 7, A and B, —), indicating that extensor muscles were increasingly activated to produce the measured force. Surface EMG recordings of the flexor muscles (Fig. 7, A and B, —) showed a relatively small amount of co-contraction in most of the patients, primarily at higher force levels.
Fig. 7.
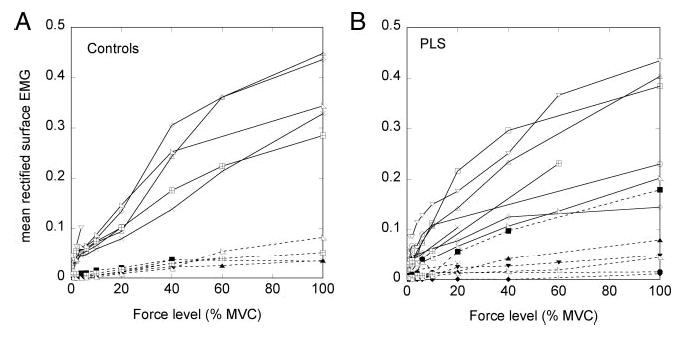
Mean rectified surface EMG from wrist extensor muscles (—) and flexor muscles (- - -) at progressive force levels up to maximal voluntary contraction. A: control subjects. B: PLS patients
Recruitment
The finding that motor-unit firing rates remained low with increasing force suggests that additional motor units were recruited to produce the higher forces in PLS patients. To quantify the number of motor units, we employed a “turns” analysis (Nandedkar et al. 1986; Willison 1968) of the same epochs of EMG that were used for assessing motor-unit firing rates. This analysis gives an indication of the total number of motor units recorded by the intramuscular wires, including motor units that were not discriminated and classified. (The number of additional, unclassified motor units was considerable at the higher force levels.) The number of voltage turns per second was calculated at each force level, with and without vibration, using a threshold of 100 μV to define a voltage change or “turn.” The number of turns/s is a combined measure of recruitment and firing rate: turns/s can increase by recruiting additional motor units or by increasing firing rates. The change in turns/s were expressed as a percentage of the lowest force level with contraction alone for each subject. (Differences in inter-electrode distance between the wire pairs in different subjects do not allow inter-subject comparison). In both patients and controls, turns per second increased with increasing force, and to a lesser extent, with vibration (Fig. 8).
Fig. 8.
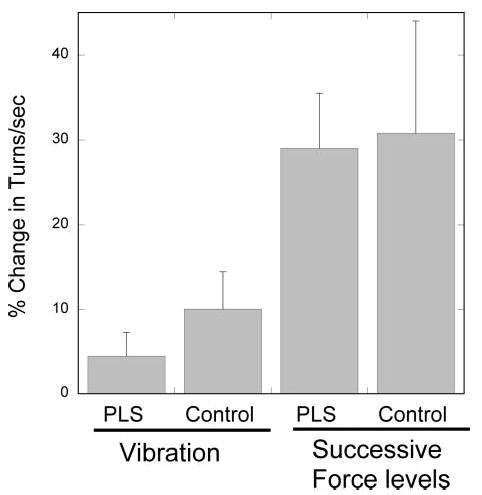
Turns analysis of wire EMG recordings. Turns were defined as a change in voltage direction with a threshold of 100 μV. Change in mean turns/s with addition of vibration or between successive force levels in controls and PLS patients. Means ± SD.
DISCUSSION
The goal of this study was to assess changes in motor neuron responsiveness in patients with PLS who had long-standing spasticity. In accord with other studies of spasticity (Gemperline et al. 1995; Rosenfalck and Andreassen 1980), we found that the firing rate of individual motor units during progressively stronger contractions of the wrist extensors did not increase as occurred with motor units from age-matched healthy controls. To produce the additional force, patients recruited additional motor units, as demonstrated by an increase in the surface EMG signal and analysis of the turns in the intramuscular EMG signal. Motor units are usually recruited in order of their size, and later-recruited units produce stronger forces but are more easily fatigued (Burke et al. 1973; Henneman 1957; Zajac 1990). Because PLS patients rely on recruiting additional units to generate increased force, rather than optimizing the firing rate of already active units, it is likely that they recruit units higher up the recruitment sequence than control subjects do. Thus PLS patients would generate stronger forces during voluntary contraction by activating units more susceptible to fatigue. The recruitment of higher threshold motor units with voluntary contraction could theoretically be attributed to changes in descending control because PLS patients, having lost corticospinal input, may have developed alternative pathways that distribute excitation uniformly across the motor pool (Edgley et al. 2004; Marchand-Pauvert et al. 2000). However, in this study, we also tested the response of motor neurons to peripheral inputs and found a similar alteration: using vibration of the muscle to stimulate spindle afferents (Burke et al. 1976; Jack and Roberts 1978), motor units of aged-matched controls increased their firing rates, whereas motor units from patients with PLS did not.
Because voluntary effort and peripheral inputs both failed to increase motor neuron firing rate, it is likely that the underlying mechanism limiting the firing rate in patients is within the motor neuron or is acting directly on the motor neuron. An alternative possibility, that vibration did not produce effective activation of Ia afferents in patients, seems unlikely because vibration affected motor units enough to produce a periodic variation in inter-spike intervals. Previous studies in patients with spasticity showed less presynaptic inhibition of Ia afferents (Aymard et al. 2000; Calancie et al. 1993; Faist et al. 1994; Nielsen et al. 1995), and because the PLS patients in this study exhibited spasticity with brisk reflexes, we had anticipated that vibration would be a more effective excitatory stimulus than in controls. Vibration has been shown to augment contraction force and increase motor-unit firing rates during fatigue in normal subjects (Griffin et al. 2001). Vibration has similarly been shown to increase force output during contraction of weak muscles of some, but not all, patients with spinal cord injury (Ribot-Ciscar et al. 2003). Our finding that vibration failed to increase motor-unit firing in motor units of PLS patients was unexpected.
There are several possible mechanisms that could produce the blunted responsiveness of the motor neurons. One possibility is that patient motor neurons receive tonic inhibitory synaptic inputs that effectively clamp them at a hyperpolarized level. Potential sources of inhibition would include spinal interneurons such as the Ia inhibitory interneurons that are activated during activity in antagonist wrist flexor motor neurons. Against this possibility is the finding of previous studies of reciprocal inhibition after cortical stroke that found less reciprocal inhibition between wrist flexors and extensors (Artieda et al. 1991; Panizza et al. 1995). Also, as shown (Fig. 6), most PLS patients exhibited relatively little co-contraction during voluntary wrist extension, suggesting that there is no change in reciprocal inhibition. Further studies would be needed to assess whether there is increased activity of the Ia inhibitory pathway at rest or of other inhibitory interneurons whose effects could become unmasked in chronic spasticity (Calancie et al. 2002; Crone et al. 2003; Mailis and Ashby 1990).
A more likely possibility would be a change in the intrinsic properties of the motor neuron that govern its excitability and firing behavior. Reduced motor neuron membrane resistance, for example, would lead to reduced effectiveness of excitatory and inhibitory inputs. Another candidate mechanism would be abnormal activation of voltage-activated conductances. Studies in animals have found that dendritic channels that produce persistent inward currents are activated when the motor neuron is depolarized in the presence of neuromodulators such as monoamines (reviewed in Heckman et al. 2003, 2004). Activation of these channels can amplify excitatory inputs or produce stable membrane plateaus that resist changes in response to small inputs because of hysteresis in the voltage levels at which persistent inward currents activate and inactivate (Lee and Heckman 1998, 2000). Vibration can trigger plateau potentials. In animals, the effective synaptic current induced by vibration displays voltage sensitivity compatible with the action of persistent inward currents (Lee and Heckman 2000). In normal human subjects, brief muscle vibration induced sustained firing of active motor units in leg muscles that was attributed to turning on plateau potentials (Gorassini et al. 2002). The stable firing rates of motor neurons of PLS patients would be compatible with activation of a plateau potential, albeit at an abnormally low membrane potential. Low-voltage plateau potentials could occur if channels were activated at an extremely low voltage, even below threshold for firing, as could theoretically occur under conditions of high monoaminergic tone (Hounsgaard and Kiehn 1989; Lee and Heckman 1999) or if the numbers of channels were reduced. In animal models of chronic spinal injury, the voltage threshold for persistent inward currents was found to be less than the firing threshold for the motor neuron (Li and Bennett 2003; Li et al. 2004). Persistent inward currents are also modulated by other metabotropic neurotransmitter pathways of supraspinal and spinal origin (Svirskis and Hounsgaard 1998) that could contribute to such properties.
Our finding that motor neurons maintain low firing rates in response to sustained excitatory inputs may seem paradoxical because PLS patients have significant spasticity. Spasticity is a clinical syndrome with manifestations of motor hyperexcitability—increased muscle tone at rest, hyperactive stretch reflexes, stimulus-induced and spontaneous spasms—as well as motor weakness and slow, effortful voluntary movements. The symptoms of spasticity may be caused by several different spinal mechanisms. Activation of plateau potentials at low voltages could contribute to tonic motor unit firing, producing increased muscle tone, and to limiting the firing rate of motor units during voluntary movements. Hyperactive stretch reflexes and spasms have been associated with changes in excitability of primary afferent terminals (Aymard et al. 2000; Calancie et al. 1993; Faist et al. 1994; Nielsen et al. 1995). Increased excitability of high-threshold sensory circuits may trigger spasms, possibly due to plateau potentials in interneurons (Bennett et al. 2004; Calancie et al. 2002; Hornby et al. 2003; Roby-Brami and Bussel 1987). In chronic spinal cord injury, persistent inward currents have been proposed to underlie hyperexcitability phenomena such as spasms (Gorassini et al. 2004; Nickolls et al. 2004). It will be important to investigate spasticity of various causes to better understand whether differences in surviving descending inputs lead to changes in the activation of motor neuron and interneuron intrinsic properties, the state of neuromodulation, or in new expression of channels producing voltage-sensitive currents (Li and Bennett 2003; Li et al. 2004).
In PLS patients, spasticity and slow movements are more clinically evident than weakness (Zhai et al. 2003). The finding that motor units are less responsive to muscle vibration indicates that adding exogenous excitatory input to motor neurons may not be helpful for improving movement in these patients, unlike the effect of vibration to increase contraction strength in patients with spinal injury (Ribot-Ciscar et al. 2003). Further investigation into the mechanisms causing the altered responsiveness of motor neurons may point to candidate targets for therapeutic intervention.
Acknowledgments
We thank L. Danielian for superb assistance with instrumentation and programming.
References
- Artieda J, Quesada P, Obeso JA. Reciprocal inhibition between forearm muscles in spastic hemiplegia. Neurology. 1991;41:286–289. doi: 10.1212/wnl.41.2_part_1.286. [DOI] [PubMed] [Google Scholar]
- Aymard C, Katz R, Lafitte C, Lo E, Penicaud A, Pradat-Diehl P, Raoul S. Presynaptic inhibition and homosynaptic depression: a comparison between lower and upper limbs in normal human subjects and patients with hemiplegia. Brain. 2000;123:1688–1702. doi: 10.1093/brain/123.8.1688. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol. 2004;91:2247–2258. doi: 10.1152/jn.00946.2003. [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth K-E, Lofstedt L, Wallin BG. The response of human muscle spindle endings to vibration of non-contracting muscles. J Physiol. 1976;261:673–693. doi: 10.1113/jphysiol.1976.sp011580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calancie B, Broton JG, Klose KJ, Traad M, Difini J, Ayyar DR. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal injury in man. Electrophysiol Clin neurophysiol. 1993;89:177–186. doi: 10.1016/0168-5597(93)90131-8. [DOI] [PubMed] [Google Scholar]
- Calancie B, Molano MR, Broton JG. Interlimb reflexes and synaptic plasticity become evident months after human spinal cord injury. Brain. 2002;125:1150–1161. doi: 10.1093/brain/awf114. [DOI] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain. 2003;126:495–507. doi: 10.1093/brain/awg036. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Godaux E. Mechanism of the vibration paradox: excitatory and inhibitory effects of tendon vibration on single soleus muscle motor units in man. J Physiol. 1978;285:197–207. doi: 10.1113/jphysiol.1978.sp012567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TJ, Chan KM, and Brown WF. Motor neurons, motor units, and motor unit recruitment. In: Neuromuscular Function and Disease, edited by Brown WF, Bolton CF, and Aminoff MJ. Philadelphia: W. B. Saunders, 2002, p. 247–273.
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci. 2004;24:7804–7813. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb WH. Ueber einen wenig bekannten spinalen Symptomencomplex. Berliner Klin Wochenschrift. 1875;26:357–359. [Google Scholar]
- Faist M, Mazavet D, Dietz V, Pierrot-Deseilligny E. A quantitative assessment of presynaptic inhibition of Ia afferents in spastics. Differences in hemiplegics and paraplegics. Brain. 1994;117:1449–1455. doi: 10.1093/brain/117.6.1449. [DOI] [PubMed] [Google Scholar]
- Fierro B, Raimondo D, Modica A. Analysis of F response in upper motoneurone lesions. Acta Neurol Scand. 1990;82:329–334. doi: 10.1111/j.1600-0404.1990.tb03311.x. [DOI] [PubMed] [Google Scholar]
- Frascarelli M, Mastrogregori L, Conforti L. Initial motor unit recruitment in patients with spastic hemiplegia. Electromyogr Clin Neurophysiol. 1998;38:267–271. [PubMed] [Google Scholar]
- Gandevia SC, Macefield VG, Bigland-Ritchie B, Gorman RB, Burke D. Motoneuronal output and gradation of effort in attempts to contract acutely paralysed leg muscles in man. J Physiol. 1993;471:411–427. doi: 10.1113/jphysiol.1993.sp019907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve. 1995;18:1101–1114. doi: 10.1002/mus.880181006. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol. 2002;87:1850–1858. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127:2247–2258. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Griffin L, Garland SJ, Ivanova T, Gossen ER. Muscle vibration sustains motor unit firing rate during submaximal isometric fatigue in humans. J Physiol. 2001;535:929–936. doi: 10.1111/j.1469-7793.2001.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimby L, Hannerz J, Ranlund T. Disturbances in the voluntary recruitment order of anterior tibial motor units in spastic paraparesis upon fatigue. J Neurol Neurosurg Psychiatry. 1974;37:40–46. doi: 10.1136/jnnp.37.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, and Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 2004. [DOI] [PubMed]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1346. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Hiersemenzel LP, Curt A, Dietz V. From spinal shock to spasticity: neuronal adaptations to a spinal cord injury. Neurology. 2000;54:1574–1582. doi: 10.1212/wnl.54.8.1574. [DOI] [PubMed] [Google Scholar]
- Hornby TG, Rymer WZ, Benz EN, Schmit BD. Windup of flexion reflexes in chronic human spinal cord injury: a marker for neuronal plateau potentials? J Neurophysiol. 2003;89:416–426. doi: 10.1152/jn.00979.2001. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack JJ, Roberts RC. The role of muscle spindle afferents in stretch and vibration reflexes of the soleus muscle of the decerebrate cat. Brain Res. 1978;146:366–372. doi: 10.1016/0006-8993(78)90981-2. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol. 1998;80:572–582. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha1 agonist methoxamine. J Neurophysiol. 1999;81:2164–2174. doi: 10.1152/jn.1999.81.5.2164. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci. 2000;20:6734–6740. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis AA, Kronenberg MF, Stetkarova I, Paske WC, Stokic DS. Spinal motoneuron excitability after acute spinal cord injury in humans. Neurology. 1996a;47:231–237. doi: 10.1212/wnl.47.1.231. [DOI] [PubMed] [Google Scholar]
- Leis AA, Zhou HH, Mehta M, Harkey HL, 3rd, Paske WC. Behavior of the H-reflex in humans following mechanical perturbation or injury to rostral spinal cord. Muscle Nerve. 1996b;19:1373–1382. doi: 10.1002/(SICI)1097-4598(199611)19:11<1373::AID-MUS1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol. 2004;91:767–783. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Mailis A, Ashby P. Alterations in group Ia projections to motoneurons following spinal lesions in humans. J Neurophysiol. 1990;64:637–647. doi: 10.1152/jn.1990.64.2.637. [DOI] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Mazevet D, Nielsen J, Petersen N, Pierrot-Deseilligny E. Distribution of non-monosynaptic excitation to early and late recruited units in human forearm muscles. Exp Brain Res. 2000;134:274–278. doi: 10.1007/s002210000498. [DOI] [PubMed] [Google Scholar]
- Nandedkar SD, Sanders DB, Stalberg EV. Automatic analysis of the electromyographic interference pattern. I. Development of quantitative features. Muscle Nerve. 1986;9:431–439. doi: 10.1002/mus.880090508. [DOI] [PubMed] [Google Scholar]
- Nickolls P, Collins DF, Gorman RB, Burke D, Gandevia SC. Forces consistent with plateau-like behavior of spinal neurons evoked in patients with spinal cord injuries. Brain. 2004;127:660–670. doi: 10.1093/brain/awh073. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Crone C. Changes in transmission across synapses of Ia afferents in spastic patients. Brain. 1995;118:995–1004. doi: 10.1093/brain/118.4.995. [DOI] [PubMed] [Google Scholar]
- Ongerboer de Visser BW, Bour LJ, Koelman JH, Speelman JD. Cumulative vibratory indices and the H/M ratio of the soleus H-reflex: a quantitative study in control and spastic subjects. Electroencephalogr Clin Neurophysiol. 1989;73:162–166. doi: 10.1016/0013-4694(89)90196-x. [DOI] [PubMed] [Google Scholar]
- Panizza M, Balbi P, Russo G, Nilsson J. H-reflex recovery curve and reciprocal inhibition of H-reflex of the upper limbs in patients with spasticity secondary to stroke. Am J Phys Med Rehabil. 1995;74:357–363. [PubMed] [Google Scholar]
- Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC. Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain. 1992;115:495–520. doi: 10.1093/brain/115.2.495. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Butler JE, Thomas CK. Facilitation of triceps brachii muscle contraction by tendon vibration after chronic cervical spinal cord injury. J Appl Physiol. 2003;94:2358–2367. doi: 10.1152/japplphysiol.00894.2002. [DOI] [PubMed] [Google Scholar]
- Roby-Brami A, Bussel B. Long-latency spinal reflex in man after flexor reflex afferent stimulation. Brain. 1987;110:707–725. doi: 10.1093/brain/110.3.707. [DOI] [PubMed] [Google Scholar]
- Rosenfalck A, Andreassen S. Impaired regulation of force and firing pattern of single motor units in patients with spasticity. J Neurol Neurosurg Psychiatry. 1980;43:907–916. doi: 10.1136/jnnp.43.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LP. Primary lateral sclerosis: disease, syndrome, both or neither? J Neurol Sci. 1999;170:1–4. doi: 10.1016/s0022-510x(99)00183-5. [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens S, Shields RK. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp Brain Res. 2000;133:233–241. doi: 10.1007/s002210000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svirskis G, Hounsgaard J. Transmitter regulation of plateau properties in turtle motoneurons. J Neurophysiol. 1998;79:45–50. doi: 10.1152/jn.1998.79.1.45. [DOI] [PubMed] [Google Scholar]
- Swash M, Desai J, Misra VP. What is primary lateral sclerosis? J Neurol Sci. 1999;170:5–10. doi: 10.1016/s0022-510x(99)00184-7. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Ross BH. Distinct patterns of motor unit behavior during muscle spasms in spinal cord injured subjects. J Neurophysiol. 1997;77:2847–2850. doi: 10.1152/jn.1997.77.5.2847. [DOI] [PubMed] [Google Scholar]
- Wiegner AW, Wierzbicka MM, Davies L, Young RR. Discharge properties of single motor units in patients with spinal cord injuries. Muscle Nerve. 1993;16:661–671. doi: 10.1002/mus.880160613. [DOI] [PubMed] [Google Scholar]
- Willison RG. Quantitative analysis of the EMG. Electroencephalogr Clin Neurophysiol. 1968;25:401. [PubMed] [Google Scholar]
- Zajac FE III. Coupling of recruitment order to the force produced by motor units: the “size principle hypothesis” revisited. In: The Segmental Motor System, edited by Binder MD and Mendell LM. New York: Oxford, 1990, p. 96–111.
- Zhai P, Pagan F, Statland J, Butman JA, Floeter MK. Primary lateral sclerosis: a heterogeneous disorder composed of different subtypes? Neurology. 2003;60:1258–1265. doi: 10.1212/01.wnl.0000058900.02672.d2. [DOI] [PubMed] [Google Scholar]


