Abstract
Background
The melanocortin (MC) system is composed of peptides that are cleaved from the polypeptide precursor proopiomelanocortin. A growing body of literature suggests that the MC system modulates neurobiological responses to drugs of abuse. Because ethanol has direct effects on central proopiomelanocortin activity, it is possible that MC neuropeptides participate in the control of voluntary ethanol consumption. Here we assessed the possibility that MC receptor (MCR) agonists modulate ethanol intake via the MC3 receptor (MC3R) and/or the MC4 receptor (MC4R) and whether the MCR antagonist AgRP-(83-132) controls ethanol consumption.
Methods
Mc3r-deficient (Mc3r−/−) and wild-type (Mc3r+/+) littermate mice were given intraperitoneal (10 mg/kg) and intracerebroventricular (1.0 μg ICV) doses of melanotan II (MTII), a nonselective MCR agonist. To assess the role of MC4R, C57BL/6J mice were given an ICV infusion of the highly selective MC4R agonist cyclo(NH-CH2-CH2-CO-His-d-Phe-Arg-Trp-Glu)-NH2 (1.0 or 3.0 μg). Finally, naïve C57BL/6J mice were given an ICV infusion of AgRP-(83-132) (0.05 and 1.0 μg).
Results
MTII was similarly effective at reducing ethanol drinking in Mc3r-deficient (Mc3r−/−) and wild-type (Mc3r+/+) littermate mice. Furthermore, ICV infusion of the MC4R agonist significantly reduced ethanol drinking, whereas ICV infusion of AgRP-(83-132) significantly increased ethanol drinking in C57BL/6J mice. Neither MTII nor AgRP-(83-132) altered blood ethanol levels at doses that modulated ethanol drinking.
Conclusions
The present results suggest that MC4R, and not MC3R, modulates MCR agonist–induced reduction of ethanol consumption and that ethanol intake is increased by the antagonistic actions of AgRP-(83-132). These findings strengthen the argument that MCR signaling controls ethanol consumption and that compounds directed at MCR may represent promising targets for treating alcohol abuse disorders in addition to obesity.
Keywords: AgRP-(83-132), Ethanol consumption, MC3 receptor, Melanocortin, MTII, MC4 receptor
THE MELANOCORTIN (MC) system is composed of peptides that are cleaved from the polypeptide precursor proopiomelanocortin (POMC). Central MC peptides are produced by neurons within the hypothalamic arcuate nucleus and the medulla (Dores et al., 1986; Jacobowitz and O’Donohue, 1978; O’Donohue and Dorsa, 1982). These peptides include adrenocorticotropic hormone, α-melanocyte–stimulating hormone (α-MSH), β-MSH, and γ-MSH (Hadley and Haskell-Luevano, 1999). Genetic and pharmacological evidence reveals that MC receptor (MCR) signaling is involved in grooming behavior (Gispen et al., 1975), antipyretic (Murphy et al., 1983) and anti-inflammatory (Macaluso et al., 1994) responses, learning (Zhao et al., 1995), reproductive function (Schioth and Watanobe, 2002), and regulation of appetite and energy homeostasis (Chen et al., 2000; Fan et al., 1997; Huszar et al., 1997; Marsh et al., 1999; Schwartz and Wisse, 2000).
There are several observations that suggest that the MC system is a prime candidate for regulating drug-seeking behavior. MCRs are expressed in brain regions thought to mediate drug self-administration, including the nucleus accumbens (NAcc), the hypothalamus, and the ventral tegmental area (VTA) (Alvaro et al., 1996; Griffon et al., 1994; Mountjoy et al., 1994; Roselli-Rehfuss et al., 1993). Interestingly, α-MSH administered into the VTA increases dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC) levels in the NAcc (Lindblom et al., 2001), and chronic intracerebroventricular (ICV) infusion of the nonselective MCR agonist melanotan II (MTII) to rats increases dopamine D1 receptor binding in the NAcc and dopamine D2 receptor binding in the VTA, suggesting that MTII alters dopamine signaling in these regions (Lindblom et al., 2002a). Chronic treatment of a high dose of morphine decreases MC4 receptor (MC4R) mRNA expression in the NAcc, the periaqueductal gray, and neostriatum (Alvaro et al., 1996), brain regions that modulate drug reward, opiate tolerance, and psychomotor stimulation, respectively (Kalivas and Stewart, 1991; Koob and Bloom, 1988; Wise and Bozarth, 1987). On the other hand, chronic treatment with low doses of morphine or cocaine increases MC4R mRNA in the striatum and hippocampus (Alvaro et al., 2003). Consistent with a role in drug self-administration, central infusion of an MCR agonist decreases the acquisition of heroin self-administration in rats (van Ree et al., 1981).
Ethanol has direct effects on central POMC and α-MSH activity (Angelogianni and Gianoulakis, 1993; Rainero et al., 1990). It is therefore possible that MC neuropeptides modulate neurobiological responses to ethanol and participate in the control of voluntary ethanol consumption. Several observations are consistent with this hypothesis. First, α-MSH is expressed in brain regions involved with neurobiological responses to ethanol, including the striatum, NAcc, VTA, amygdala, hippocampus, and hypothalamus (Bloch et al., 1979; Dube et al., 1978; Jacobowitz and O’Donohue, 1978; O’Donohue and Jacobowitz, 1980; O’Donohue et al., 1979; Yamazoe et al., 1984). Second, rats selectively bred for high ethanol drinking (AA [Alko, alcohol]) have low levels of MC3R in the shell of the NAcc but have high levels of MC3R and MC4R in various regions of the hypothalamus, when compared with low–ethanol-drinking rats (Lindblom et al., 2002b). Third, central infusion of MTII significantly reduces voluntary ethanol drinking in AA rats with an established history of ethanol intake (Ploj et al., 2002). Recently, MTII-induced reduction of ethanol consumption was shown to be receptor mediated and not associated with alterations of ethanol metabolism in C57BL/6 mice (Navarro et al., 2003).
One objective of the present report was to study the role of selected MCRs in the modulation of MCR agonist–induced reduction of ethanol intake. In rodents, MC peptides act through five receptors (MC1R–MC5R) (Hadley and Haskell-Luevano, 1999). MC3R and MC4R are expressed at high levels in the brain (Alvaro et al., 1997), whereas MC1R and MC5R are detected at low levels and in only limited brain regions (Adan and Gispen, 1997; Barrett et al., 1994; Xia et al., 1995). MTII binds, with varying affinity, to all centrally expressed MCRs (Haskell-Luevano et al., 1997; Schioth et al., 1997). Thus, it is unclear which MCRs are important for modulating MTII-induced reductions of ethanol consumption (Navarro et al., 2003; Ploj et al., 2002). To assess the contribution of MC3R, we examined MTII-induced alteration of ethanol drinking in Mc3r–knock-out (Mc3r−/−) and wild-type (Mc3r+/+) mice. To assess the role of MC4R, we studied ethanol intake by C57BL/6J mice after central infusion of the highly selective MC4R agonist cyclo(NH-CH2-CH2-CO-His-d-Phe-Arg-Trp-Glu)-NH2. A second objective was to determine whether central administration of AgRP-(83-132) would increase ethanol consumption by mice. In vivo, AgRP-(83-132) is a potent and nonselective MCR antagonist (Quillan et al., 1998). Such results would strengthen the argument that MCR signaling plays an important role in the modulation of ethanol self-administration. Because MCR agonists reduce and MCR antagonists increase feeding (Hagan et al., 2000; Hohmann et al., 2000; Hollopeter et al., 1998; Marsh et al., 1999; Thiele et al., 1998), food intake was concurrently assessed in most studies described in the present report.
METHODS
Animals
The generation of Mc3r−/− mice has been described (Chen et al., 2000). Mc3r−/− mice are born at the expected frequency and are viable and fertile. Furthermore, gross anatomical and histological assessment of these mice has revealed no abnormalities of brain or other organs (Chen et al., 2000). For the first 6 months of development, Mc3r−/− mice show normal body weight, food intake, and activity. Beginning at approximately 25 weeks of age, Mc3r−/− mice maintained on normal chow begin to show increased body weight associated with increased fat mass and reduced lean mass. Increased fat mass in Mc3r−/− mice is associated with increased blood levels of leptin and insulin. Despite increased fat mass, Mc3r−/− mice show hypophagia (Chen et al., 2000). Therefore, to avoid possible confounding associated with late-onset developmental changes, the studies in this report used 8- to 12-week-old mice at study onset to allow sufficient time for testing before changes occurred. Mc3r−/− mice were originally maintained on a C57BL/6J × 129/SvJ genetic background (Chen et al., 2000) but have now been backcrossed to a C57BL/6J genetic background seven times. Nonlittermate heterozygous (Mc3r±) mice were bred, resulting in N7 Mc3r−/− and Mc3r+/+ littermate mice. The studies described included male and female Mc3r−/− and littermate Mc3r+/+mice. Because sex was not found to interact with the experimental manipulations in these studies, data for male and female mice were collapsed for statistical analyses. Some studies used male C57BL/6J mice purchased from Jackson Laboratory (Bar Harbor, ME). Mice were individually housed in polypropylene cages with wood-chip bedding and had ad libitum access to standard rodent chow (Teklad, Madison, WI) and water throughout the experiments, except where noted. The colony room was maintained at approximately 22°C with a 12-hr/12-hr light/dark cycle. All procedures used in the present research were in compliance with National Institutes of Health guidelines, and the protocols were approved by the University of North Carolina Animal Care and Use Committee.
Cannulation Surgery and Infusion Procedures
For studies involving ICV infusions, mice were anesthetized with a cocktail of ketamine (117 mg/kg) and xylazine (7.92 mg/kg) and surgically implanted with a 26-gauge cannula (Plastic One, Roanoak, VA) aimed at the left lateral ventricle, with the following stereotaxic coordinates: 0.2 mm posterior to the bregma, 1.0 mm lateral to the midline, and 2.3 mm ventral to the skull surface. Mice were allowed to recover for approximately 2 weeks before experimental procedures were initiated. After experimental procedures, cannula placement was verified histologically. The ICV infusions were given in a 1.0 μl volume over a 1-min period with a 5.0 μl Hamilton syringe, infused manually or with a Harvard Apparatus PHD 2000 microinfusion pump.
Ethanol Consumption and Food Intake After Peripheral Injection of MTII to Mc3r−/− and Mc3r+/+ Mice
Mc3r−/− and Mc3r+/+ mice were given 24-hr access to two bottles, one containing plain water and the other containing ethanol in water. To habituate mice to drinking ethanol, the concentration (v/v) was increased every 8 days; mice received 3, 6, 10, and finally 20% ethanol. The positions of the bottles were changed every 2 days to control for position preferences. These data were analyzed with a two-way, 2 × 4 (genotype × concentration) repeated-measures ANOVA. Mice were then distributed to two groups per genotype based on average voluntary ethanol consumption (g/kg/day) of 20% ethanol. Two hours before the beginning of the dark phase of the light cycle, mice were weighed, and ethanol, water, and food were removed from the cages. One hour later, mice were given an intraperitoneal (ip) injection of either 10 mg/kg MTII (2.0 mg/ml in isotonic saline; Bachem, Torrance, CA) (Mc3r−/− mice, n = 11; Mc3r+/+ mice, n = 12) or an equal volume of isotonic saline (Mc3r−/− mice, n = 11; Mc3r+/+ mice, n = 13). When administered peripherally, this dose of MTII was found to decrease food intake by mice without producing aversive side effects (Chen et al., 2000). Mice were immediately returned to their cages. Just before the dark cycle, ethanol, water, and food were returned to the cages. Intake measures were recorded every 2 hr for up to 8 hr into the dark cycle and again at 24 hr after treatment. Two-way, 2 × 2 (genotype × drug) ANOVAs were used to assess consumption data. All data in this report are presented as mean ± SEM, and ethanol and food intake data are presented to the longest time point that the drug [MTII, MC4RA, or AgRP-(83-132)] significantly altered consumption. In all cases, significance was accepted at p < 0.05 (two tailed).
Ethanol Consumption and Food Intake After Central Infusion of MTII to Mc3r−/− and Mc3r+/+ Mice
After recovery from surgery, mice were habituated to drinking 20% ethanol similar to the procedure already described and were distributed to groups based on average ethanol consumption (g/kg/day).
Mice were weighed, and ethanol, water, and food were removed from their cages 2 hr before the beginning of the dark cycle. One hour before the beginning of the dark cycle, mice were given an ICV infusion of either 1.0 μg MTII (Mc3r−/− mice, n = 9; Mc3r+/+ mice, n = 8) dissolved in artificial cerebrospinal fluid (aCSF; Harvard Apparatus, Holliston, MA) or an equal volume of aCSF (Mc3r−/− mice, n = 9; Mc3r+/+ mice, n = 9) and were returned to their cages. The 1.0 μg dose of MTII was chosen because a similar dose (1.0 nmol, where 1.0 nmol = 1.02 μg) was found to reduce ethanol drinking by AA rats (Ploj et al., 2002) and C57BL/6 mice (Navarro et al., 2003). The 20% ethanol solution, water, and food were returned immediately before the dark cycle. Two-way, 2 × 2 (genotype × drug) ANOVAs were used to assess consumption data. To determine whether the MTII-induced reduction of ethanol drinking depended on the presence of food, an additional study was performed in which C57BL/6J mice were given an ICV infusion of aCSF (n = 10) or 1.0 μg MTII (n = 9) in a similar procedure as describe previously, except that food was not returned to the cage with ethanol and water immediately before the dark cycle (food was returned 10 hr later). Data from this study were analyzed with a one-way (drug) ANOVA.
Ethanol Consumption and Food Intake After Central Infusion of a Selective MC4R Agonist
C57BL/6J mice were implanted with an ICV cannula and acclimated to the two-bottle consumption procedure as described before. On the test day, mice were weighed, and ethanol, water, and food were removed from their cages 2 hr before the beginning of the dark cycle. One hour before the beginning of the dark cycle, mice were given ICV infusions of either 1.0 (n = 7) or 3.0 (n = 8) μg of the MC4R-selective agonist cyclo(NH-CH2-CH2-CO-His-d-Phe-Arg-Trp-Glu)-NH2 (Phoenix Pharmaceuticals, Inc., Belmont, CA) dissolved in aCSF or an equal volume of aCSF (n = 10). This compound is a potent agonist at the human MC4R with 90-fold selectivity over human MC3R and approximately 3400-fold selectivity over MC5R, as assessed by adenosine 3’:5’-cyclic monophosphate induction (Bednarek et al., 2001). We included a larger dose of the selective MC4R agonist than MTII (3.0 vs. 1.0 μg) because this compound has weaker binding potency (~16%) and adenosine 3’:5’-cyclic monophosphate induction (~80%) at MC4R relative to MTII (Bednarek et al., 2001). The 20% ethanol solution, water, and food were returned immediately before the dark cycle. One-way (drug) ANOVAs were used to assess consumption measures after ICV infusion of the MC4R agonist or aCSF.
Ethanol Consumption and Food Intake After Central Infusion of AgRP-(83-132)
C57BL/6J mice were implanted with an ICV cannula and acclimated to the two-bottle consumption procedures as described before. Mice were weighed, and ethanol, water, and food were removed from their cages 2 hr before the beginning of the dark cycle. One hour before the beginning of the dark cycle, mice were given ICV infusions of 0.05 (n = 6) or 0.1 (n = 11) μg of AgRP-(83-132) (Phoenix Pharmaceuticals, Inc., Belmont, CA) or an equal volume of aCSF (n = 9). The 20% ethanol solution, water, and food were returned immediately before the dark cycle. One-way (drug) ANOVAs were used to assess consumption data.
Blood Ethanol Level Assessment
To determine whether peripherally administered MTII influenced blood ethanol levels, C57BL/6J mice were divided into two groups based on body weight and given an ip injection of either 10 mg/kg MTII (n = 19) or an equal volume of saline (n = 18). Ten minutes later, all mice were given ip injections of ethanol (3.0 g/kg; 20% [w/v] in isotonic saline) and immediately returned to their homecages. Two hours after injection, half the mice from each group were rapidly decapitated for blood collection. The remaining mice were decapitated 4 hr after ethanol injection. Blood ethanol levels were determined by spectrophotometric methods (Sigma Diagnostics, Enzymatic Determination of Ethanol Test, St. Louis, MO) and calculated as mg/dl. A two-way 2 × 2 (drug × time interval) ANOVA was used to analyze the data.
To determine whether ICV infusion of AgRP-(83-132) influenced blood ethanol levels, naïve C57BL/6J mice were divided into two groups based on body weight and given ICV infusions of either 0.05 μg AgRP-(83-132) (n = 8) or an equal volume of aCSF (n = 7) according to the procedures described before. Approximately 10 min later, all mice were given an ip injection of ethanol (4.0 g/kg; 20% [w/v] mixed in isotonic saline) and immediately returned to their homecages. Two and 4 hr after injection, mice tails were cleaned, and sterile single-blade razors were used to make small tail-tip nicks for blood collection. Blood ethanol samples were analyzed with gas chromatographic methods described elsewhere (Knapp et al., 1993; Navarro et al., 2003). A two-way, 2 × 2 (genotype × time interval) repeated-measures ANOVA was used to assess blood ethanol levels using gas chromatography.
RESULTS
Peripheral Injection of MTII Reduces Ethanol Drinking and Food Intake in Mc3r−/− and Mc3r+/+ Mice
Relative to wild-type mice, Mc3r−/− mice did not show altered ethanol preference during habituation to ethanol consumption (Fig. 1A). ANOVA performed on the ethanol-preference ratio data showed a significant effect of ethanol concentration [F(3,138) = 131.49; p < 0.001]; as the concentration of ethanol increased from 10 to 20%, the ethanol-preference ratio decreased below 0.5, a finding indicating that the mice preferred water to the ethanol solution. Ethanol-preference ratios ranging from approximately 0.2 to 0.8 have been reported with mice given access to a 20% ethanol solution (Thiele et al., 2000; Wang et al., 2003). Intraperitoneal injection of MTII (10.0 mg/kg) significantly reduced ethanol intake (Fig. 1B) and the ethanol-preference ratio (Fig. 1C) for up to 24 hr, regardless of genotype. ANOVA performed on consumption data (g/kg) showed a significant effect of the drug [F(1,43) = 5.08; p = 0.029]. Neither the genotype nor interaction effects reached significance. Similarly, ANOVA performed on preference ratio data showed a significant drug effect [F(1,43) = 7.19; p = 0.01], but the genotype and interaction effects were not significant. While causing 24-hr reductions of ethanol drinking, ip injection of MTII did not alter 24-hr water drinking by Mc3r−/− (204.5 ± 16.38 ml/kg) or Mc3r+/+ (215.31 ± 15.69 ml/kg) mice relative to Mc3r−/− (169.41 ± 16.3 ml/kg) and Mc3r+/+ (200.12 ± 15.07 ml/kg) mice injected with saline. Finally, ip administration of MTII caused significant reductions of food intake only up to the 4-hr measure in both Mc3r−/− (44.29 ± 4.02 g/kg) and Mc3r+/+ (41.02 ± 3.85 g/kg) mice relative to Mc3r−/− (67.13 ± 4.03 g/kg) and Mc3r+/+ (71.12 ± 3.70 g/kg) mice injected with saline. ANOVA performed on 4-hr food intake data showed a significant effect of drug [F(1,43) = 45.88; p < 0.001], but once again, the genotype and interaction effects were not significant.
Fig. 1.
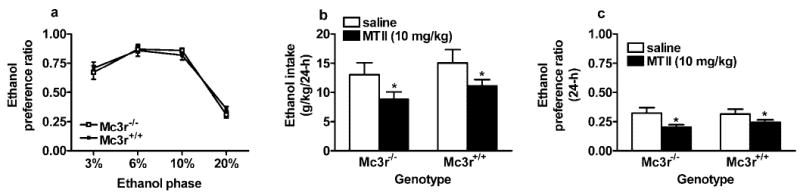
Consumption of solutions containing ethanol by mice lacking Mc3r (Mc3r−/−) and by littermate wild-type (Mc3r+/+) mice maintained on an inbred C57BL/6J genetic background. (A) Ethanol-preference ratios (volume of ethanol consumed/total volume of fluid consumed) as a measure of relative ethanol preference. (B) Consumption (g/kg/24 hr) of 20% ethanol solution after ip injection of MTII (10 mg/kg) or an equal volume of saline. (C) 20% ethanol–preference ratio (24 hr) after ip injection of MTII or saline. All values are means ± SEM. ANOVAs indicated no significant differences between Mc3r−/− and Mc3r+/+ mice in ethanol preference (A). MTII caused significant and similar reductions of ethanol drinking in both Mc3r−/− and Mc3r+/+ mice (B and C).
*p < 0.05 relative to saline injection.
Peripheral Injection of MTII Does Not Alter Blood Ethanol Levels
Relative to saline, ip injection of MTII (10.0 mg/kg) did not significantly alter blood ethanol levels 2 hr after a 3.0 g ethanol/kg dose (MTII, 232.09 ± 7.44 mg/dl; saline, 233.60 ± 7.43 mg/dl). Four hours after ethanol injection, plasma ethanol levels were lower, but again, there was no significant effect of MTII treatment (MTII, 89.62 ± 7.83 mg/dl; saline, 74.25 ± 8.31 mg/dl). ANOVA performed on plasma ethanol data showed a significant effect of time [F(1,33) = 377.85; p < 0.001], which reflected the reduced levels of ethanol in plasma from 2 to 4 hr after injection. However, neither the effect of MTII injection nor the interaction effect was significant.
Central Infusion of MTII Reduces Ethanol Drinking and Food Intake in Mc3r−/− and Mc3r+/+ Mice
Relative to mice given ICV infusions of aCSF, central infusion of a 1.0 μg dose of MTII significantly reduced ethanol intake (Fig. 2A) and ethanol-preference ratio (Fig. 2B) for up to 24 hr after infusion in both Mc3r−/− and Mc3r+/+ mice. ANOVA performed on consumption data (g/kg) revealed a significant effect of drug [F(1,31) = 9.98; p = 0.004]; however, neither the genotype nor the interaction effects reached levels of significance. ANOVA performed on 24-hr preference ratio data also showed a significant drug effect [F(1,31) = 19.86; p < 0.001] and no significant genotype or interaction effects. Interestingly, ethanol consumption levels were higher in the ICV study (Fig. 2A) than in the study involving ip injection of MTII (Fig. 1B). Ethanol consumption levels by C57BL/6 mice are reported to be approximately 20 g/kg/day in young mice (6–10 weeks) and decrease to approximately 10 to 15 g/kg/day as mice age (26–28 weeks) (Wang et al., 2003). Because mice from the ip injection study were older (~17 weeks) than mice from the ICV study (~10 weeks), difference in ethanol consumption between studies may be age dependent.
Fig. 2.
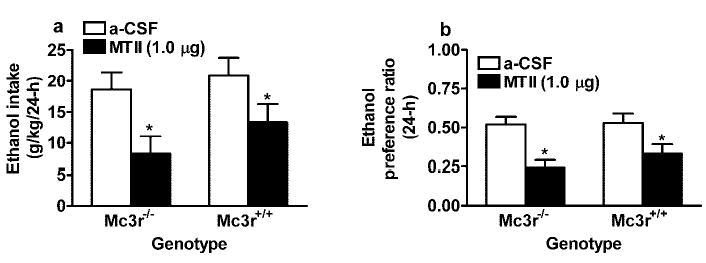
Consumption of 20% ethanol expressed as g/kg/24 hr (A) and ethanol-preference ratio (B) by Mc3r−/− and Mc3r+/+mice after ICV infusion of MTII (1.0 μg) or aCSF. All values are means ± SEM. ANO-VAs indicated that MTII significantly reduced ethanol drinking in both Mc3r−/− and Mc3r+/+ mice.
*p < 0.05 relative to aCSF infusion.
Infusion (ICV) of MTII was not associated with altered drinking from the water bottle at the 24-hr measure by Mc3r−/− (158.64 ± 16.29 ml/kg) and Mc3r+/+ (159.59 ± 22.68 ml/kg) mice relative to Mc3r−/− (115.32 ± 10.96 ml/kg) and Mc3r+/+ (126.25 ± 26.27 ml/kg) treated with aCSF, although there was a trend for increased water drinking by MTII-treated mice (p = 0.06). As with the ip injection study, ICV infusion of MTII caused significant reductions of food intake only up to 4 hr in both Mc3r−/− (20.21 ± 4.29 g/kg) and Mc3r+/+ (36.64 ± 7.28 g/kg) mice relative to Mc3r−/− (58.80 ± 4.04 g/kg) and Mc3r+/+ (52.12 ± 5.87 g/kg) mice infused with aCSF. ANOVA performed on 4-hr food intake data revealed a significant drug effect [F(1,31) = 25.02; p < 0.001]. Finally, ICV infusion of a 1.0 μg dose of MTII significantly reduced ethanol intake (1.19 ± 0.31 g/kg/10 hr) relative to treatment with aCSF (5.77 ± 1.09 g/kg/10 hr) in C57BL/6J mice presented with ethanol and water but no food [F(1,17) = 14.71; p = 0.001]. Thus, the presence of food is not necessary for MTII-induced reduction of ethanol drinking.
Central Infusion of a Selective MC4R Agonist Reduces Ethanol Drinking and Food Intake
Infusion (ICV) of the MC4R agonist significantly and dose-dependently reduced ethanol consumption (Fig. 3A) and food intake (Fig. 3B) for up to 2 hr after infusion when compared with aCSF-treated mice. ANOVAs performed on ethanol consumption data [F(1,22) = 4.75; p = 0.001] and food intake data [F(1,22) = 10.31; p = 0.001] were both significant, and post hoc tests confirmed that the 1.0 and 3.0 μg doses of the MC4R agonist reduced ethanol and food ingestion. Relative to aCSF infusion (24.88 ± 5.51 ml/kg), neither the 1.0 (11.13 ± 5.55 ml/kg) nor the 3.0 (8.45 ± 4.40 ml/kg) μg dose of the MC4R agonist significantly reduced 2-hr water drinking, although there was a trend (p = 0.07). Although reductions of 2-hr water drinking may suggest possible aversive side effects, it is typical to find that MCR agonists concurrently reduce short-term feeding and water drinking, whereas MCR antagonists increase short-term food intake and water consumption (Fan et al., 1997; Grill et al., 1998). This pattern of results suggests the possibility that alterations of water drinking caused by MCR compounds may be the direct result of changes in food intake, rather than a marker of aversion.
Fig. 3.
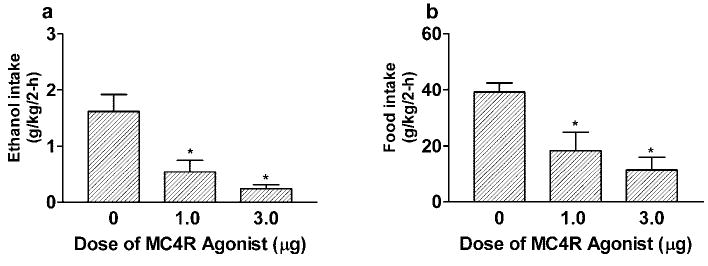
Consumption of 20% ethanol (B) and food intake (B) expressed as g/kg/2 hr by C57BL/6J mice after ICV infusion of MC4R agonist (1.0 or 3.0 μg) or aCSF. All values are means ± SEM. ANOVAs indicated that the MC4R agonist significantly reduced ethanol drinking and food intake by mice.
*p < 0.05 relative to aCSF infusion.
Central Infusion of AgRP-(83-132) Increases Ethanol Drinking
As shown in Fig. 4A, ICV infusion of the 0.05 μg dose of AgRP-(83-132) significantly increased ethanol consumption for up to 8 hr when compared with mice treated with aCSF [F(2,23) = 4.63; p = 0.02]. Post hoc tests confirmed this conclusion. However, neither the 0.05 nor the 0.1 μg dose of AgRP-(83-132) altered food intake at the 8- (Fig. 4B) or 24-hr measure. A higher, 5.0 μg dose of AgRP-(83-132) was also found not to alter 8-hr food intake or ethanol drinking by C57BL/6J mice (Navarro et al., 2003). Further assessment of these data revealed that the 5.0 μg dose of AgRP-(83-132) caused a significant increase of food intake at the 24-hr measure (165.24 ± 20.36 g/kg) relative to aCSF (105.84 ± 13.56 g/kg) [F(1,26) = 5.89; p = 0.02] without influencing ethanol consumption.
Fig. 4.
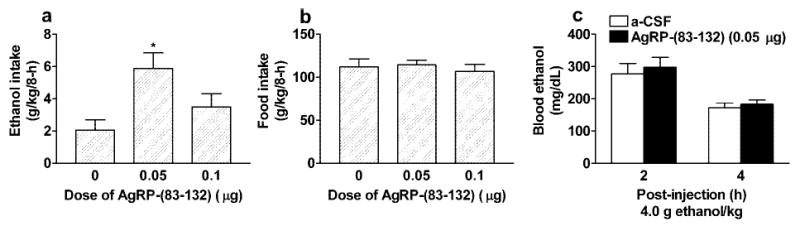
Consumption of 20% ethanol (A) and food intake (B) expressed as g/kg/8 hr by C57BL/6J mice after ICV infusion of the MCR antagonist AgRP-(83-132) (0.05 or 0.1 μg) or aCSF. Plasma ethanol levels 2 and 4 hr after ip injection of 4.0 g ethanol/kg (C). Ten minutes before ethanol injection, mice were given ICV infusion of AgRP-(83-132) (0.05 μg) or aCSF. All values are means ± SEM. ANOVAs indicated that the 0.05-μg dose of AgRP-(83-132) significantly increased ethanol drinking but did not alter food intake. Furthermore, pretreatment with AgRP-(83-132) did not influence plasma ethanol levels
*p < 0.05 relative to aCSF infusion.
Central Infusion AgRP-(83-132) Does Not Alter Blood Ethanol Levels
Data from blood ethanol levels after ICV infusion of AgRP-(83-132) are presented in Fig. 4C. Blood ethanol levels 2 hr after ip injection of a 4.0 g ethanol/kg dose did not differ significantly between mice pretreated with 0.05 μg AgRP-(83-132) versus aCSF. Four hours after ethanol injection, blood ethanol levels were lower, but again, there was no significant effect of ICV treatment. A two-way, 2 × 2 (ICV treatment × hr) repeated-measures ANOVA performed on these data revealed a significant effect of hr [F(1,13) = 28.10; p < 0.001], which reflected the reduced levels of blood ethanol from 2 to 4 hr after injection. However, neither the effect of ICV treatment nor the interaction effect was significant.
DISCUSSION
The present studies reveal four important points regarding the role of MCR signaling in ethanol consumption. First, a highly selective MC4R agonist reduces ethanol consumption by C57Bl/6J mice, suggesting that MC4R is a likely receptor for mediating MCR agonist–induced reduction of ethanol consumption. Second, Mc3r−/− mice respond normally to MTII, suggesting that MC3R is not a likely receptor for mediating MCR agonist–induced reduction of ethanol drinking. Third, ethanol intake is increased by the antagonistic actions of AgRP-(83-132) in C57BL/6J mice. Fourth, compounds that target MCR modulate ethanol intake without altering ethanol metabolism.
The MC4R agonist cyclo(NH-CH2-CH2-CO-His-d-Phe-Arg-Trp-Glu)-NH2 significantly reduces ethanol drinking. This compound is a highly selective agonist for MC4R, with 90-fold selectivity over MC3R and a > 3400-fold selectivity over MC5R (Bednarek et al., 2001). Thus, we conclude that activation of MC4R modulates ethanol consumption in mice. However, the present results do not support a role for MC3R. When compared with littermate control mice, Mc3r−/− mice exhibit a normal preference of solutions containing various concentrations of ethanol. However, the Mc3r−/− mice were maintained on a high–ethanol-drinking C57Bl/6J genetic background (Belknap et al., 1993), which may have masked genotype differences in ethanol consumption. Additional studies with Mc3r−/− mice maintained on more moderate ethanol-consuming genetic backgrounds will be necessary to more firmly establish the role of MC3R in voluntary ethanol consumption. Consistent with previous research (Navarro et al., 2003; Ploj et al., 2002), MTII administered via ICV infusion (1.0 μg) or by ip injection (10 mg/kg) causes reductions of voluntary ethanol drinking for up to 24 hr, and these routes of administration are similarly effective in Mc3r−/− and Mc3r+/+ mice. These findings indicate that MTII’s effect on ethanol drinking does not require MC3R signaling. Because the MC4R agonist reduces ethanol drinking by mice and MTII-induced reduction of ethanol drinking is receptor mediated (Navarro et al., 2003) and given the limited expression of MC1R and MC5R in the brain (Adan and Gispen, 1997; Barrett et al., 1994; Xia et al., 1995), MTII likely reduces ethanol drinking by acting at MC4R.
Reductions of ethanol drinking caused by MTII and the MC4R agonist cannot be explained by general decreases in fluid consumption. Neither administration of MTII (ip or ICV) nor ICV infusion of the MC4R agonist significantly altered water drinking. Furthermore, MTII-induced reduction of ethanol drinking is not likely related to ethanol metabolism, as we have previously shown that ICV infusion of a 1.0-μg dose of MTII does not alter blood ethanol levels in C57BL/6 mice (Navarro et al., 2003), and we show here that ip injection of MTII does not alter plasma ethanol levels. Central and peripheral routes of administration of MTII promote conditioned taste aversion to novel flavors in rats (Benoit et al., 2003; Thiele et al., 1998); thus, MTII-induced reduction of feeding and ethanol drinking could be mediated, at least in part, by aversive consequences of MTII. Although we cannot completely rule out aversive effects here, as noted earlier, MTII and the MC4R agonist do not reduce water drinking by mice. We would predict that mice should show general decreases of consummatory behavior (including water drinking) if they experience aversive effects such as visceral illness or malaise. Furthermore, increased ethanol drinking caused by AgRP-(83-132) administration is unlikely to be related to any possible aversive consequences.
AgRP-(83-132) has been shown to increase food intake after central infusion (Hagan et al., 2000; Small et al., 2001). Here we show that ICV infusion of a 0.05-μg dose of AgRP-(83-132) increases ethanol drinking by C57BL/6J mice without modifying food intake. Because a 0.05-μg dose of AgRP-(83-132) does not alter blood ethanol levels, AgRP-(83-132)–induced increases of ethanol drinking are not caused by increased ethanol metabolism. Interestingly, a recent report showed that an MC4R-selective antagonist (HS014) has no effect on ethanol drinking by rats selectively bred for ethanol preference (Ploj et al., 2002). However, only one dose of HS014 was tested in rats (Ploj et al., 2002), and other doses may be found to increase ethanol drinking. It is also possible that AgRP-(83-132) modulates ethanol drinking by acting at receptors other than the MC4R.
We propose two possible mechanisms by which MCR signaling modulates ethanol intake. The MC system has been shown to modulate ingestive behavior (Fan et al., 1997; Hohmann et al., 2000; Hollopeter et al., 1998; Marsh et al., 1999; Pierroz et al., 2002), and we show here that MCR agonists and antagonist modulate food intake. Thus, MCR agonists and antagonists may regulate feeding and ethanol consumption via the same or similar pathways. In fact, we believe that this possibility should not come as a surprise in light of recent electrophysiological evidence demonstrating that both drugs of abuse and “natural” reinforcers (food and water) produce similar cell firing in the NAcc (Carelli et al., 2000; Hollander et al., 2002; Roop et al., 2002). Furthermore, there are examples of overlapping control of feeding and ethanol intake by other neurochemical systems (Thiele et al., 2003; Thiele et al., 2004). It has been known for some time that the peptide cholecystokinin reduces food intake and ethanol consumption in a dose-dependent fashion (Avery and Livosky, 1986; DiBattista et al., 2003; Kulkosky and Chavez, 1984; Kulkosky et al., 1991; Kulkosky and Glazner, 1988; Le Sauter et al., 1988), and recent evidence shows that the orexigenic neuropeptide galanin (Kyrkouli et al., 1990; Tempel et al., 1988) controls ethanol consumption (Leibowitz et al., 2003; Lewis et al., 2004). A growing body of literature also shows that the central cannabinoid system modulates ethanol self-administration and feeding (Colombo et al., 1998; Freedland et al., 2003; Poncelet et al., 2003; Wang et al., 2003). Interestingly, it has been proposed that cannabinoid-1 receptor antagonists may be useful therapeutic agents for treating obesity (Cota et al., 2003) and alcoholism (Racz et al., 2003). Similarly, MCR agonists may be useful for treating alcoholism, in addition to obesity. This being said, ICV infusion of a 0.05-μg dose of AgRP-(83-132) increases ethanol drinking by C57BL/6J mice without modifying food intake. On the other hand, the 5.0-μg dose of AgRP-(83-132) increases food, but not ethanol, consumption. Furthermore, MTII reduced feeding for 4 hr but reduced ethanol drinking for up to 24 hr. Thus, MCR signaling may modulate ethanol drinking and food intake through different central pathways. Regardless, an important issue requiring resolution is whether MCR signaling controls ethanol drinking through a mechanism involving the regulation of calories or by a mechanism that modulates the pharmacodynamic effects of ethanol (e.g., the reinforcing properties of ethanol as discussed next).
A second possibility is that compounds targeting MCRs modulate ethanol consumption by acting on pathways involved with drug reward. MCR signaling has been implicated in opioid tolerance and dependence (Contreras and Takemori, 1984; Szekely et al., 1979) and amphetamine reward (Cabeza de Vaca et al., 2002), and MCRs are expressed in brain regions that modulate the reinforcing properties of drugs of abuse and ‘natural’ reinforcers (e.g., food and water), including the NAcc, the VTA, and the hypothalamus (Hadley and Haskell-Luevano, 1999; Lindblom et al., 2002b). It is tempting to speculate that MCR agonists reduce and AgRP-(83-132) increases ethanol consumption by acting on MCRs within the VTA–NAcc circuit, perhaps by modulating the reinforcing properties associated with ethanol. Ploj et al. (2002) proposed a mechanism that involves an interaction between endogenous opioids and melanocortins. In addition to reducing ethanol drinking, central infusion of MTII prevents ethanol-induced changes in opioid peptide levels in the VTA and the substantia nigra of AA rats (Ploj et al., 2002). Thus, MCR signaling may regulate ethanol drinking by modulating endogenous opioid activity within mesolimbic dopamine pathways (Ploj et al., 2002).
In conclusion, we extend recent findings (Lindblom et al., 2002b; Navarro et al., 2003; Ploj et al., 2002) by showing that a highly selective MC4R agonist reduces ethanol drinking and feeding, that MTII-induced reductions of ethanol drinking and food intake do not involve MC3R, and that ethanol consumption is increased by antagonizing MCR signaling. These findings strengthen the argument that modulation of MCR signaling controls ethanol consumption. Important future research will determine whether MCR signaling controls ethanol drinking through a mechanism involving the regulation of calories or by a mechanism that modulates the pharmacodynamic effects of ethanol and whether ethanol administration modulates central MC peptide, MCR, and/or agonti-related protein (AgRP) activity and expression.
Acknowledgments
The authors thank Tanya MacNeil for helpful discussions regarding the selective MC4R agonist.
Footnotes
This work was supported by National Institutes of Health grants AA13573 (TET) and AA11605 (TET, DJK, GRB) and grant FPD/I from the Spanish Government (MN, IC).
References
- Adan RA, Gispen WH. Brain melanocortin receptors: from cloning to function. Peptides. 1997;18:1279–1287. doi: 10.1016/s0196-9781(97)00078-8. [DOI] [PubMed] [Google Scholar]
- Alvaro JD, Tatro JB, Duman RS. Melanocortins and opiate addiction. Life Sci. 1997;61:1–9. doi: 10.1016/s0024-3205(97)00029-5. [DOI] [PubMed] [Google Scholar]
- Alvaro JD, Tatro JB, Quillan JM, Fogliano M, Eisenhard M, Lerner MR, Nestler EJ, Duman RS. Morphine down-regulates melanocortin-4 receptor expression in brain regions that mediate opiate addiction. Mol Pharmacol. 1996;50:583–591. [PubMed] [Google Scholar]
- Alvaro JD, Taylor JR, Duman RS. Molecular and behavioral interactions between central melanocortins and cocaine. J Pharmacol Exp Ther. 2003;304:391–399. doi: 10.1124/jpet.102.040311. [DOI] [PubMed] [Google Scholar]
- Angelogianni P, Gianoulakis C. Chronic ethanol increases proopio-melanocortin gene expression in the rat hypothalamus. Neuroendocrinology. 1993;57:106–114. doi: 10.1159/000126348. [DOI] [PubMed] [Google Scholar]
- Avery DD, Livosky M. Peripheral injections of bombesin and cholecystokinin affect dietary self-selection in rats. Pharmacol Biochem Behav. 1986;25:7–11. doi: 10.1016/0091-3057(86)90221-2. [DOI] [PubMed] [Google Scholar]
- Barrett P, MacDonald A, Helliwell R, Davidson G, Morgan P. Cloning and expression of a new member of the melanocyte-stimulating hormone receptor family. J Mol Endocrinol. 1994;12:203–213. doi: 10.1677/jme.0.0120203. [DOI] [PubMed] [Google Scholar]
- Bednarek MA, MacNeil T, Tang R, Kalyani RN, Van der Ploeg LH, Weinberg DH. Potent and selective peptide agonists of alpha-melanotropin action at human melanocortin receptor 4: their synthesis and biological evaluation in vitro. Biochem Biophys Res Commun. 2001;286:641–645. doi: 10.1006/bbrc.2001.5444. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Sheldon RJ, Air EL, Messerschmidt P, Wilmer KA, Hodge KMB, Jones MB, Eckstein DMM, McOsker CC, Woods SC, Seeley RJ. Assessment of the aversive consequences of acute and chronic administration of melanocortin agonist, MTII. Int J Obes. 2003;27:550–556. doi: 10.1038/sj.ijo.0802280. [DOI] [PubMed] [Google Scholar]
- Bloch B, Bugnon C, Fellmann D, Lenys D, Gouget A. Neurons of the rat hypothalamus reactive with antisera against endorphins, ACTH, MSH and beta-LPH. Cell Tissue Res. 1979;204:1–15. doi: 10.1007/BF00235160. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Kim GY, Carr KD. The melanocortin receptor agonist MTII augments the rewarding effect of amphetamine in adlibitum-fed and food-restricted rats. Psychopharmacology. 2002;161:77–85. doi: 10.1007/s00213-002-0998-1. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus ‘natural’ (water and food) reward. J Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho E, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LHT. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Fa M, Guano L, Lobina C, Loche A, Reali R, Gessa GL. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol. 1998;33:126–130. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- Contreras PC, Takemori AE. Antagonism of morphine-induced analgesia, tolerance and dependence by alpha-melanocyte-stimulating hormone. J Pharmacol Exp Ther. 1984;229:21–26. [PubMed] [Google Scholar]
- Cota D, Marsicano G, Lutz B, Vicennati V, Stalla GK, Pasquali R, Pagotto U. Endogenous cannabinoid system as a modulator of food intake. Int J Obes Relat Metab Disord. 2003;27:289–301. doi: 10.1038/sj.ijo.0802250. [DOI] [PubMed] [Google Scholar]
- DiBattista D, McKenzie TLB, Hollis-Walker L. Cholecystokinin reduces ethanol consumption in golden hamsters. Alcohol. 2003;29:173–181. doi: 10.1016/s0741-8329(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Dores RM, Jain M, Akil H. Characterization of the forms of beta-endorphin and alpha-MSH in the caudal medulla of the rat and guinea pig. Brain Res. 1986;377:251–260. doi: 10.1016/0006-8993(86)90866-8. [DOI] [PubMed] [Google Scholar]
- Dube D, Lissitzky JC, Leclerc R, Pelletier G. Localization of alpha-melanocyte-stimulating hormone in rat brain and pituitary. Endocrinology. 1978;102:1283–1291. doi: 10.1210/endo-102-4-1283. [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Whitlow CT, Smith HR, Porrino LJ. Functional consequences of the acute administration of the cannabinoid receptor antagonist, SR141716A, in cannabinoidnaive and -tolerant animals: a quantitative 2-[14C]deoxyglucose study. Brain Res. 2003;962:169–179. doi: 10.1016/s0006-8993(02)03999-9. [DOI] [PubMed] [Google Scholar]
- Gispen WH, Wiegant VM, Greven HM, de Wied D. The induction of excessive grooming in the rat by intraventricular application of peptides derived from ACTH: structure-activity studies. Life Sci. 1975;17:645–652. doi: 10.1016/0024-3205(75)90103-4. [DOI] [PubMed] [Google Scholar]
- Griffon N, Mignon V, Facchinetti P, Diaz J, Schwartz JC, Sokoloff P. Molecular cloning and characterization of the rat fifth melanocortin receptor. Biochem Biophys Res Commun. 1994;200:1007–1014. doi: 10.1006/bbrc.1994.1550. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci. 1998;18:10128–10135. doi: 10.1523/JNEUROSCI.18-23-10128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley ME, Haskell-Luevano C. The proopiomelanocortin system. Ann NY Acad Sci. 1999;885:1–21. doi: 10.1111/j.1749-6632.1999.tb08662.x. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van Der Ploeg LH, Woods SC, Seeley RJ. Long-term orexigenic effects of AgRP-(83-132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol. 2000;279:R47–R52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C, Nikiforovich G, Sharma SD, Yang YK, Dickinson C, Hruby VJ, Gantz I. Biological and conformational examination of stereochemical modifications using the template melanotropin peptide, Ac-Nle-c[Asp-His-Phe-Arg-Trp-Ala-Lys]-NH2, on human melanocortin receptors. J Med Chem. 1997;40:1738–1748. doi: 10.1021/jm960845e. [DOI] [PubMed] [Google Scholar]
- Hohmann JG, Teal TH, Clifton DK, Davis J, Hruby VJ, Han G, Steiner RA. Differential role of melanocortins in mediating leptin’s central effects on feeding and reproduction. Am J Physiol. 2000;278:R50–R59. doi: 10.1152/ajpregu.2000.278.1.R50. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Ijames SG, Roop RG, Carelli RM. An examination of nucleus accumbens cell firing during extinction and reinstatement of water reinforcement behavior in rats. Brain Res. 2002;929:226–235. doi: 10.1016/s0006-8993(01)03396-0. [DOI] [PubMed] [Google Scholar]
- Hollopeter G, Erickson JC, Seeley RJ, Marsh DJ, Palmiter RD. Response of neuropeptide Y-deficient mice to feeding effectors. Reg Peptides. 1998;75–76:383–389. doi: 10.1016/s0167-0115(98)00092-5. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Jacobowitz DM, O’Donohue TL. alpha-Melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. Proc Natl Acad Sci USA. 1978;75:6300–6304. doi: 10.1073/pnas.75.12.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Saiers JA, Pohorecky LA (1993) Observations of novel behaviors as indices of ethanol withdrawal-induced anxiety, in Advances in Biomedical Alcohol Research (Taberner PV, Badaway AA, eds), pp 489–493, Pergamon, New York. [PubMed]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Kulkosky PJ, Chavez MR. Sulphated cholecystokinin octapeptide inhibits ethanol consumption in the rat. Alcohol. 1984;1:409–412. doi: 10.1016/0741-8329(84)90012-0. [DOI] [PubMed] [Google Scholar]
- Kulkosky PJ, Foderaro MA, Sandoval SL, Cesar SS, Marrinan DA. Cholecystokinin-induced satiation with ethanol: effects of lighting cycle and limited access procedures. Alcohol. 1991;8:223–227. doi: 10.1016/0741-8329(91)90886-2. [DOI] [PubMed] [Google Scholar]
- Kulkosky PJ, Glazner GW. Dose-additive inhibition of intake of ethanol by cholecystokinin and bombesin. Alcohol Clin Exp Res. 1988;12:277–281. doi: 10.1111/j.1530-0277.1988.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Kyrkouli SE, Stanley BG, Seirafi RD, Leibowitz SF. Stimulation of feeding by galanin: anatomical localization and behavioral specificity of this peptide’s effects in the brain. Peptides. 1990;11:995–1001. doi: 10.1016/0196-9781(90)90023-x. [DOI] [PubMed] [Google Scholar]
- Le Sauter J, Goldberg B, Geary N. CCK inhibits real and sham feeding in gastric vagotomized rats. Physiol Behav. 1988;44:527–534. doi: 10.1016/0031-9384(88)90314-9. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Avena NM, Chang G, Karatayev O, Chau DT, Hoebel BG. Ethanol intake increases galanin mRNA in the hypothalamus and withdrawal decreases it. Physiol Behav. 2003;79:103–111. doi: 10.1016/s0031-9384(03)00110-0. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Johnson DF, Waldman D, Leibowitz SF, Hoebel BG. Galanin microinjection in the third ventricle increases voluntary ethanol intake. Alcohol Clin Exp Res. 2004;28:1822–1828. doi: 10.1097/01.alc.0000148099.12344.c8. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Kask A, Hagg E, Harmark L, Bergstrom L, Wikberg J. Chronic infusion of a melanocortin receptor agonist modulates dopamine receptor binding in the rat brain. Pharmacol Res. 2002a;45:119–124. doi: 10.1006/phrs.2001.0913. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Opmane B, Mutulis F, Mutule I, Petrovska R, Klusa V, Bergstrom L, Wikberg JE. The MC4 receptor mediates alpha-MSH induced release of nucleus accumbens dopamine. NeuroReport. 2001;12:2155–2158. doi: 10.1097/00001756-200107200-00022. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Wikberg JES, Bergstrom L. Alcohol-preferring AA rats show a derangement in their central melanocortin signalling system. Pharm Biochem Behav. 2002b;72:491–496. doi: 10.1016/s0091-3057(02)00719-0. [DOI] [PubMed] [Google Scholar]
- Macaluso A, McCoy D, Ceriani G, Watanabe T, Biltz J, Catania A, Lipton JM. Antiinflammatory influences of alpha-MSH molecules: central neurogenic and peripheral actions. J Neurosci. 1994;14:2377–2382. doi: 10.1523/JNEUROSCI.14-04-02377.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet. 1999;21:119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- Murphy MT, Richards DB, Lipton JM. Antipyretic potency of centrally administered alpha-melanocyte stimulating hormone. Science. 1983;221:192–193. doi: 10.1126/science.6602381. [DOI] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Knapp DJ, Thiele TE. MTII-induced reduction of voluntary ethanol drinking is blocked by pretreatment with AgRP-(83-132) Neuropeptides. 2003;37:338–344. doi: 10.1016/j.npep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- O’Donohue TL, Dorsa DM. The opiomelanotropinergic neuronal and endocrine systems. Peptides. 1982;3:353–395. doi: 10.1016/0196-9781(82)90098-5. [DOI] [PubMed] [Google Scholar]
- O’Donohue TL, Jacobowitz DM. Studies of alpha-MSH-containing nerves in the brain. Prog Biochem Pharmacol. 1980;16:69–83. [PubMed] [Google Scholar]
- O’Donohue TL, Miller RL, Jacobowitz DM. Identification, characterization and stereotaxic mapping of intraneuronal alpha-melanocyte stimulating hormone-like immunoreactive peptides in discrete regions of the rat brain. Brain Res. 1979;176:101–123. doi: 10.1016/0006-8993(79)90873-4. [DOI] [PubMed] [Google Scholar]
- Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS, Mantzoros CS. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes. 2002;51:1337–1345. doi: 10.2337/diabetes.51.5.1337. [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Kask A, Hyytia P, Schioth HB, Wikberg J, Nylander I. Effects of melanocortin receptor ligands on ethanol intake and opioid levels in alcohol-preferring AA rats. Brain Res Bull. 2002;59:97–104. doi: 10.1016/s0361-9230(02)00844-4. [DOI] [PubMed] [Google Scholar]
- Poncelet M, Maruani J, Calassi R, Soubrie P. Overeating, alcohol and sucrose consumption decrease in CB1 receptor deleted mice. Neurosci Lett. 2003;343:216–218. doi: 10.1016/s0304-3940(03)00397-5. [DOI] [PubMed] [Google Scholar]
- Quillan JM, Sadee W, Wei ET, Jimenez C, Ji L, Chang JK. A synthetic human Agouti-related protein-(83-132)-NH2 fragment is a potent inhibitor of melanocortin receptor function. FEBS Lett. 1998;428:59–62. doi: 10.1016/s0014-5793(98)00487-6. [DOI] [PubMed] [Google Scholar]
- Racz I, Bilkei-Gorzo A, Toth ZE, Michel K, Palkovits M, Zimmer A. A critical role for the cannabinoid CB1 receptors in alcohol dependence and stress-stimulated ethanol drinking. J Neurosci. 2003;23:2453–2458. doi: 10.1523/JNEUROSCI.23-06-02453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainero I, De Gennaro T, Visentin G, Brunetti E, Cerrato P, Torre E, Portaleone P, Pinessi L. Effects of chronic ethanol treatment on alpha-MSH concentrations in rat brain and pituitary. Neuropeptides. 1990;15:139–141. doi: 10.1016/0143-4179(90)90145-o. [DOI] [PubMed] [Google Scholar]
- Roop RG, Hollander JA, Carelli RM. Accumbens activity during a multiple schedule for water and sucrose reinforcement in rats. Synapse. 2002;43:223–226. doi: 10.1002/syn.10041. [DOI] [PubMed] [Google Scholar]
- Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA. 1993;90:8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schioth HB, Muceniece R, Mutulis F, Prusis P, Lindeberg G, Sharma SD, Hruby VJ, Wikberg JE. Selectivity of cyclic [D-Nal7] and [D-Phe7] substituted MSH analogues for the melanocortin receptor subtypes. Peptides. 1997;18:1009–1013. doi: 10.1016/s0196-9781(97)00079-x. [DOI] [PubMed] [Google Scholar]
- Schioth HB, Watanobe H. Melanocortins and reproduction. Brain Res Brain Res Rev. 2002;38:340–350. doi: 10.1016/s0165-0173(01)00159-x. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Wisse BE. Role of melanocortins in control of obesity. Nat Genet. 2000;26:8–9. [Google Scholar]
- Small CJ, Kim MS, Stanley SA, Mitchell JR, Murphy K, Morgan DG, Ghatei MA, Bloom SR. Effects of chronic central nervous system administration of agouti-related protein in pair-fed animals. Diabetes. 2001;50:248–254. doi: 10.2337/diabetes.50.2.248. [DOI] [PubMed] [Google Scholar]
- Szekely JI, Miglecz E, Dunai-Kovacs Z, Tarnawa I, Ronai AZ, Graf L, Bajusz S. Attenuation of morphine tolerance and dependence by alpha-melanocyte stimulating hormone(alpha-MSH) Life Sci. 1979;24:1931–1938. doi: 10.1016/0024-3205(79)90302-3. [DOI] [PubMed] [Google Scholar]
- Tempel DL, Leibowitz KJ, Leibowitz SF. Effects of PVN galanin on macronutrient selection. Peptides. 1988;9:309–314. doi: 10.1016/0196-9781(88)90265-3. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Navarro M, Sparta DR, Fee JR, Knapp DJ, Cubero I. Alcoholism and obesity: overlapping neuropeptide pathways? Neuropeptides. 2003;37:321–337. doi: 10.1016/j.npep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Stewart RB, Badia-Elder NE, Geary N, Massi M, Leibowitz SF, Hoebel BG, Egli M. Overlapping peptide control of alcohol self-administration and feeding. Alcohol Clin Exp Res. 2004;28:288–294. doi: 10.1097/01.alc.0000113777.87190.9c. [DOI] [PubMed] [Google Scholar]
- Thiele TE, van Dijk G, Yagaloff KA, Fisher SL, Schwartz M, Burn P, Seeley RJ. Central infusion of melanocortin agonist MTII in rats: assessment of c-Fos expression and taste aversion. Am J Physiol. 1998;274:R248–R254. doi: 10.1152/ajpregu.1998.274.1.R248. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS (2000) High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci (online) 20:RC75. [DOI] [PMC free article] [PubMed]
- van Ree JM, Bohus B, Csontos KM, Gispen WH, Greven HM, Nijkamp FP, Opmeer FA, de Rotte GA, van Wimersma Greidanus TB, Witter A, de Wied D. Behavioral profile of gamma-MSH: relationship with ACTH and beta-endorphin action. Life Sci. 1981;28:2875–2978. doi: 10.1016/0024-3205(81)90104-1. [DOI] [PubMed] [Google Scholar]
- Wang L, Lui J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci USA. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Xia Y, Wikberg JE, Chhajlani V. Expression of melanocortin 1 receptor in periaqueductal gray matter. NeuroReport. 1995;6:2193–2196. doi: 10.1097/00001756-199511000-00022. [DOI] [PubMed] [Google Scholar]
- Yamazoe M, Shiosaka S, Yagura A, Kawai Y, Shibasaki T, Ling N, Tohyama M. The distribution of alpha-melanocyte stimulating hormone (alpha-MSH) in the central nervous system of the rat: an immunohistochemical study: II–lower brain stem. Peptides. 1984;5:721–727. doi: 10.1016/0196-9781(84)90013-5. [DOI] [PubMed] [Google Scholar]
- Zhao W, Sedman G, Gibbs M, Ng KT. Phosphorylation changes following weakly reinforced learning and ACTH-induced memory consolidation for a weak learning experience. Brain Res Bull. 1995;36:161–168. doi: 10.1016/0361-9230(94)00184-3. [DOI] [PubMed] [Google Scholar]


