Abstract
Background
Recent genetic and pharmacological evidence indicates that low neuropeptide Y (NPY) levels in brain regions involved with neurobiological responses to ethanol promote increased ethanol consumption. Because of their opposing actions, it has been suggested that NPY and corticotropin releasing factor (CRF) exert a reciprocal regulation on drug self-administration. It has been widely reported that inbred C57BL/6 mice consume significantly higher amounts of ethanol than do DBA/2 mice. Therefore, we used immunohistochemical techniques to determine if basal NPY and/or CRF levels differed in predicted directions between C57BL/6J and DBA/2J mice.
Methods
Ethanol-naive C57BL/6J and DBA/2J mice were deeply anesthetized with sodium pentobarbital (100 mg/kg) and perfused transcardially with 0.1 mM of phosphate-buffered saline followed by 4% paraformaldehyde in buffered saline. Brains were collected and postfixed for 4 hr at 4°C and then were cut into 35-μm sections. Tissues containing the nucleus accumbens (NAc), hypothalamus, and amygdala were processed for NPY or CRF immunoreactivity using immunofluorescent or DAB techniques. Immunoreactivity was quantified from digital images using Image J software.
Results
The C57BL/6J mice showed reduced NPY expression in the NAc shell, the basolateral amygdala, and the central nucleus of the amygdala when compared with DBA/2J mice. However, these strains did not differ in CRF expression in any of the brain regions analyzed.
Conclusions
These data suggest that low NPY levels in the amygdala and/or the shell of the NAc, which are not compensated for by similar changes in CRF levels, may contribute to the high ethanol consumption characteristic of C57BL/6J mice.
Keywords: Neuropeptide Y, Corticotropin Releasing Factor, Alcohol, Immunohistochemistry, C57BL/6J, DBA/2J
NEUROPEPTIDE Y (NPY) is a 36 amino acid neuro-modulator belonging to the PP-fold family of peptides (Berglund et al., 2003; Colmer and Wahlestedt, 1993; Dumont et al., 1992). NPY immunoreactivity (IR) has been noted in brain regions such as the amygdala, cerebral cortex, caudate putamen, hippocampus, hypothalamus, and nucleus of the solitary tract (Gray and Morley, 1986) and has been shown to be involved in the regulation of a wide-ranging group of biological functions. It has been implicated in the control of food intake (Clark et al., 1984; Levine and Morley, 1984), cardiovascular homeostasis (Pedrazzini et al., 1998), integration of emotional behavior (Heilig et al., 1993; Heilig and Widerlov, 1995), neuronal development (Hansel et al., 2001a,b), seizure activity (Woldbye et al., 1996, 1997), pain modulation (Shi et al., 1999, 2001), thermogenesis (Lopez-Valpuesta et al., 1996), circadian rhythms (Biello et al., 1997; Golombek et al., 1996; Gribkoff et al., 1998; Harrington and Schak, 2000), and reproduction (Kalra et al., 1998; Kasuya et al., 1998).
In recent years, evidence has emerged showing that NPY is also involved with neurobiological responses to ethanol (Pandey et al., 2003a; Thiele and Badia-Elder, 2003; Thiele et al., 2003, 2004b). Specifically, administration of ethanol and ethanol withdrawal alter central NPY expression in rodents (Bison and Crews, 2003; Clark et al., 1998; Ehlers et al., 1998; Kinoshita et al., 2000; Roy and Pandey, 2002; Thiele et al., 2000a). Further, NPY has been implicated in the modulation of ethanol consumption in rats selectively bred for ethanol preference. A genetic linkage analysis conducted in F2 intercross progenies of selectively bred alcohol-preferring (P) and -nonpreferring (NP) rat lines identified a chromosomal region that includes the gene for the NPY precursor (Bice et al., 1998; Carr et al., 1998; Foroud et al., 2000). P rats and the high alcohol drinking (HAD) rats have low levels of NPY in the amygdala when compared with controls (Ehlers et al., 1998; Hwang et al., 1999), and ventricular administration of NPY reduces ethanol drinking in P and HAD rats but not in NP rats, low alcohol drinking (LAD) rats, or outbred Wistar rats (Badia-Elder et al., 2003; Badia-Elder et al., 2001; Gilpin et al., 2003; Katner et al., 2002a,b; Slawecki et al., 2000). Interestingly, in genetically modified mice, voluntary ethanol consumption and resistance to the intoxicating effects of ethanol are inversely related to NPY levels (Thiele et al., 1998, 2000b, 2002, 2004a).
Corticotropin releasing factor (CRF) is a 41 amino acid polypeptide that is expressed in many of the same brain regions as NPY, including the hypothalamus and amygdala (Swanson et al., 1983). Interestingly, NPY and CRF often produce opposing neurobiological actions. Thus, after ethanol withdrawal, there are low levels of NPY (Pandey et al., 2003b; Roy and Pandey, 2002) and increased levels of CRF (Merlo Pich et al., 1995) in the amygdala. Furthermore, central infusion of NPY (Pandey et al., 2003b) or CRF receptor antagonists (Breese et al., 2004; Knapp et al., 2004; Overstreet et al., 2004; Rassnick et al., 1993) reverses the anxiogenic effect of ethanol withdrawal. Of critical interest, while NPY decreases ethanol consumption in high ethanol drinking models (Badia-Elder et al., 2003; Badia-Elder et al., 2001; Gilpin et al., 2003), central infusion of the CRF receptor antagonist D-Phe-CRF(1,2-14) eliminates excessive ethanol drinking by rats made dependent with chronic exposure to ethanol vapor (Valdez et al., 2002). Because of their opposing actions, it has been suggested that CRF and NPY exert a reciprocal regulation of drug self-administration through allostatic interactions within the extended amygdala (Koob, 2003; Koob and Le Moal, 2001).
The goal of the present study was twofold. First, we determined if ethanol consumption was inversely related to NPY levels in two inbred strains of mice that differ dramatically in their voluntary consumption of ethanol (Belknap et al., 1993; Le et al., 1994; Meliska et al., 1995; Mittleman et al., 2003; Risinger et al., 1998). C57BL/6J mice voluntary consume 10–12 g/kg/day of ethanol when offered a 10% (v/v) solution, while DBA/2J mice consume approximately 1.0 g/kg/day (Belknap et al., 1993). Second, because of the proposed relationship between NPY and CRF, we determined if C57BL/6J and DBA/2J mice also differed in central CRF levels. Immunohistochemistry procedures were used to determine NPY and CRF levels in critical brain regions within the hypothalamus and the extended amygdala. On the basis of observations utilizing genetically altered mice and selectively bred rats, we predicted that C57BL/6J mice would have low NPY levels in specific brain regions when compared with DBA/2J mice. Furthermore, because excessive ethanol drinking may be modulated by enhanced CRF receptor signaling (Valdez et al., 2002), we predicted that the C57BL/6J mice would also differ from the DBA/2J mice in brain CRF levels.
MATERIALS AND METHODS
Subjects
Consistent with prior experiments that assessed NPY levels in P/NP and HAD/LAD rats (Ehlers et al., 1998; Hwang et al., 1999), male mice were used in the present study. Subjects were 13 DBA/2J mice and 12 C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) obtained at 7 weeks of age and weighing between 20 and 26 g at the beginning of the experiment. Mice were individually housed for 2 weeks with ad libitum access to standard rodent chow (Teklad, Madison, WI) and water and maintained at 22°C on a 12 hr:12 hr light/dark cycle. All experiments were conducted in compliance with the National Institutes of Health guidelines, and the University of North Carolina Animal Care and Use Committee approved the protocols.
Tissue Preparation
Animals were weighed approximately 90 min before the beginning of the perfusions. Mice were injected intraperitoneally with pentobarbital (100 mg/kg) in their home cages in pairs, one C57BL/6J and one DBA/2J, and were then transported and perfused transcardially with 0.1 mM of phosphate-buffered saline (PBS; pH 7.4) followed by 4% paraformaldehyde in buffered saline. Because of rapid onset of hypnosis, perfusions were begun within 5 min of injection. Because mice were injected in their home cages and because perfusions were conducted within 5 min of drug injection, stress- or drug-induced alteration of either CRF or NPY appears unlikely. In fact, heteronuclear transcripts of CRF do not appear to be increased until 20–40 min after ethanol injection (Rivier and Lee, 1996). The brains were collected and postfixed in paraformaldehyde for 4 hr at 4°C, at which point they were transferred to PBS. The brains were then packaged and sent to NeuroScience Associates (Knoxville, TN) for multi-brain processing in which the 25 brains were embedded together in a matrix and sliced as a unit into 35-μm sections. Brains were returned in a cryopreservation solution (1:1 0.2 M phosphate buffer: ethylene glycol with 1% w/v polyvinylpyrrolidone 40) and stored at 4°C until immunohistochemical analyses.
NPY Immunohistochemical Analysis and Quantification
Tissue slices containing the brain regions of interest were washed in PBS and then blocked in a solution containing 10% goat serum and 0.1% Triton X-100 for 1 hr. The slices were then incubated with rabbit anti-NPY antibody (1:1000; Peninsula Laboratories, San Carlos, CA) and 3% goat serum for approximately 72 hr at 4°C. After multiple washes with PBS, slices were incubated with Alexa Flour 594 antirabbit secondary antibody (1:1000; Molecular Probes, Eugene, OR) and 3% goat serum for 1 hr at room temperature in the dark. As a control to assess potential nonspecific staining, some sections were run through the assay without primary or without secondary antibodies, and no staining occurred. Slices were then mounted onto 75 × 50-mm Corning microslides (Corning, Corning, NY) using fluorescence mounting media. The tissue was visualized using an Olympus M081 microscope with a 10× objective (Olympus, Melville, NY). Photographs were obtained with a 35-mm Olympus OM-4Ti camera using Fujifilm Provia Professional film (Fujifilm, Edison, NJ). Photographs were converted to 35-mm slides and then to digital images using a Polaroid Sprint Scan 4000 with PolarInsight software (Polaroid, Charlotte, NC). Immunofluorescence was quantified using Image J software (Image J, National Institutes of Health, Bethesda, MD) by calculating the percent of the total area examined that showed immunofluorescence reactivity relative to a subthreshold background. For analyses, great care was taken to match sections through the same region of brain and at the same level using anatomical landmarks. Anatomically matched pictures of the left and right sides of the brain were used to produce an average IR score for each brain region in each animal.
CRF Immunohistochemical Analysis and Quantification
CRF IR utilized the DAB method of staining rather than immunofluorescence because a constant procedure has been established in our laboratory. However, a pilot comparison of CRF IR using DAB and immunofluorescent procedures yielded similar levels of expression (data not shown). Tissue slices were washed in PBS and then blocked in a solution containing 0.1% Triton X-100 and 10% goat serum for 1 hr. The slices were then incubated with rabbit anti-CRF antibody (1:1000; Peninsula Laboratories) and 3% goat serum for approximately 72 hr at 4°C. After multiple PBS washes, slices were incubated with biotinylated secondary antibody from the VectaStain rabbit ABC kit (Vector Laboratories, Burlingame, CA) and 3% goat serum for 1 hr at room temperature. After several PBS washes, tissue slices were transferred to the VectaStain ABC reagent mixture and left for 1 hr; the slices were then stained using the DAB method (Vector Laboratories) with 0.0063% H2O2, 0.05% DAB, 0.0075% NiAmSO4, and 0.005% CaCl2. As a control to assess potential nonspecific staining, some sections were run through the assay without primary or without secondary antibody, and no staining occurred. Slices were mounted onto microslides using ShurMount mounting media (Durham, NC). Flat-field corrected digital pictures (8-bit gray scale) were taken on a SPOT camera (model 1.1.0, SPOT, Melville, NY) connected to an Olympus BX60 scope (10–20× objectives) with accompanying software (SPOT version 3.5.8). Image J software was used to process background (noncellular regions or corpus callosum) and signal (cell body or processes) intensities that were standardized with densitometric standards (Kodak T14 Control Scale, Kodak, Rochester, NY). As above, anatomically matched pictures of the left and right sides of the brain were used to produce an average density for each brain region in each animal. Data were presented as background-corrected standardized image densities for each brain region.
Data Analyses
For each brain region examined, differences between C57BL/6J and DBA/2J mice were analyzed using one-way analysis of variance (ANOVA). In all cases, p < 0.05 (two-tailed) was used to indicate statistical significance.
RESULTS
NPY Immunoreactivity
In general, differences in NPY IR between the strains were observed within the extended amygdala but not within hypothalamic nuclei. Representative photomicrographs and data of NPY IR in the nucleus accumbens (NAc) are depicted in Fig. 1A and 1B. Relative to the low alcohol drinking DBA/2J mice, the high alcohol drinking C57BL/6J mice showed lower NPY IR in the shell of the NAc (Fig. 1C), an observation confirmed by statistically significant results of analysis of variance [F(1,16) = 7.04; p = 0.02]. Importantly, strain differences in NPY IR were specific to the NAc shell, because C57BL/6J and DBA/2J mice showed similar NPY IR in the NAc core (Fig. 1D) [F(1,16) = 7.04; p = 0.33]. Representative photomicrographs and data of NPY IR in the amygdala are depicted in Fig. 2A and 2B. Analyses revealed that the C57BL/6J mice showed significantly lower NPY IR in the central nucleus of the amygdala [F(1,16) = 4.36; p = 0.05] and the basolateral amygdala [F(1,16) = 6.23; p = 0.02] (Fig. 2C and 2D). Photomicrographs of NPY IR in the paraventricular nuclei and arcuate nuclei of the hypothalamus are shown in Fig. 3A-3D. NPY IR did not significantly differ between the strains in the arcuate nucleus [F(1,18) = 0.90; p = 0.36] or the paraventricular nucleus [F(1,19) = 0.20; p = 0.66] (Fig. 4).
Fig. 1.
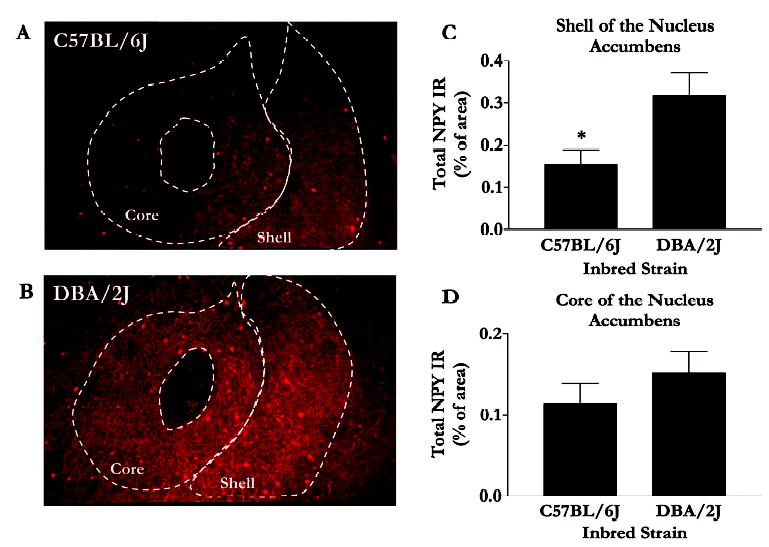
NPY immunofluorescence in the NAc. Representative photomicrographs are coronal brain slices (at approximately 1.0 mm anterior to bregma) taken from C57BL/6J (A) and DBA/2J (B) mice. Quantification of immunofluorescence in the shell (C) and core (D) regions of the NAc were performed using Image J software, and values are represented as mean area ± SEM. *p < 0.05.
Fig. 2.
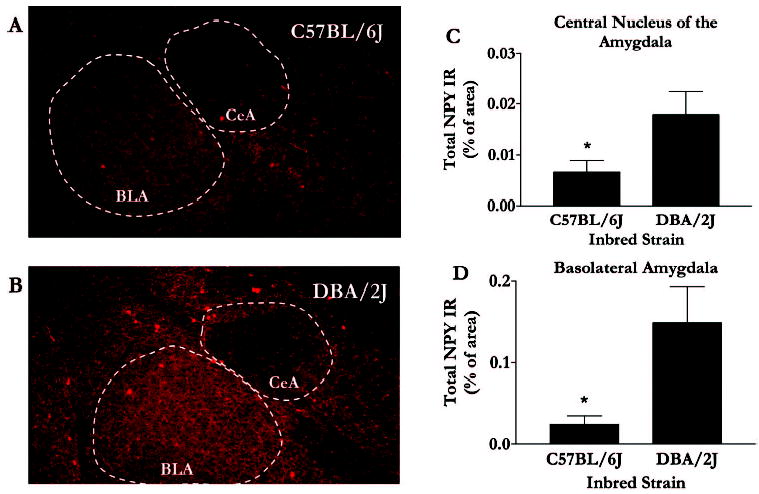
NPY immunofluorescence in the amygdala. Representative photomicrographs are coronal brain slices (at approximately 1.5 mm posterior to bregma) taken from C57BL/6J (A) and DBA/2J (B) mice. Quantification of immunofluorescence in the central nucleus (CEA) of the amygdala (C) and the basolateral (BLA) amygdala (D) were performed using Image J software, and values are represented as mean area ± SEM. *p < 0.05.
Fig. 3.
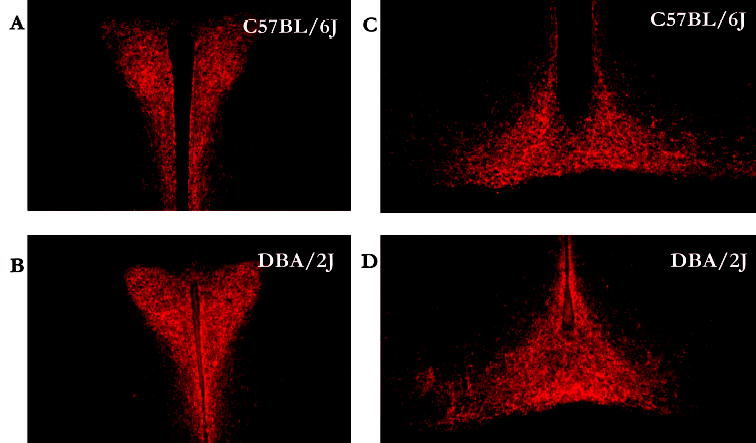
NPY immunofluorescence in the hypothalamus. Representative photomicrographs are coronal brain slices taken from C57BL/6J (A and C) and DBA/2J (B and D) mice in the paraventricular nucleus of the hypothalamus (A and B) and the arcuate nucleus of the hypothalamus (C and D).
Fig. 4.
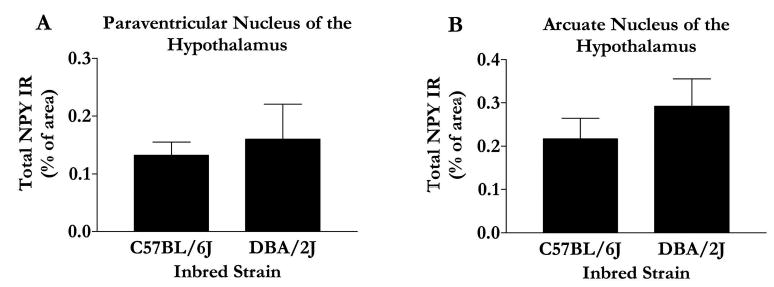
Quantification of immunofluorescence in the paraventricular nucleus (A) and arcuate nucleus (B) of the hypothalamus was performed using Image J software, and values are represented as mean area ± SEM. *p < 0.05.
CRF Immunoreactivity
Representative photomicrographs of CRF IR in the NAc and amygdala of C57BL/6J and DBA/2J mice are shown in Fig. 5A-5D, and a graphical representation of all quantified CRF IR is presented in Fig. 6. There were no observed statistical differences between strains in CRF IR within the NAc shell [F(1,22) = 3.63; p = 0.07] or core [F(1,22) = 2.85; p = 0.12], the basolateral amygdala [F(1,23) = 1.27; p = 0.27] or central nucleus of the amygdala [F(1,20) = 3.96; p = 0.06], or the arcuate nucleus of the hypothalamus [F(1,20) = 0.31; p = 0.58]. Due to lack of tissue, quantification of CRF IR in the paraventricular nucleus of the hypothalamus was not possible. As an alternative region, we quantified CRF IR in the suprachiasmatic nucleus of the hypothalamus because the suprachiasmatic nucleus is a major source of CRF that innervates the paraventricular nucleus (Vrang et al., 1995). Consistent with other regions, there were no strain differences in CRF IR in the supra-chiasmatic nucleus [F(1,18) = 0.46; p = 0.51].
Fig. 5.
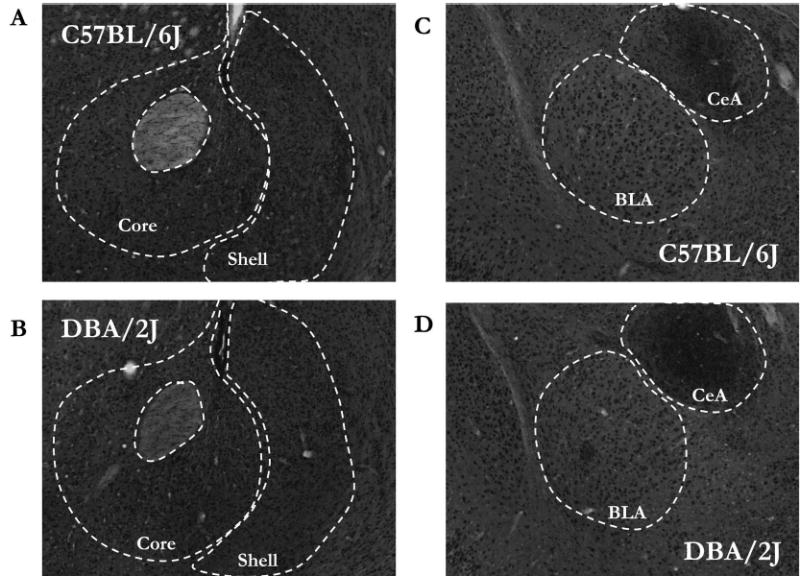
CRF IR in the NAc (A and B) and the amygdala (C and D). Representative photomicrographs are coronal brain slices taken from C57BL/6J (A and C) and DBA/2J (B and D) mice.
Fig. 6.
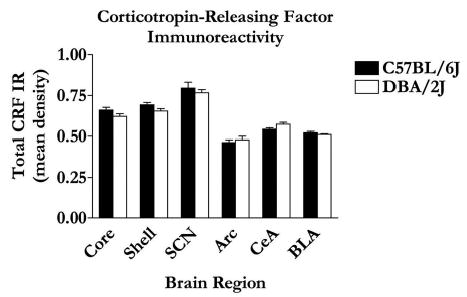
Mean area of CRF IR quantification was performed using Image J software. Values are expressed as mean area ± SEM.
DISCUSSION
We show that relative to the low ethanol drinking DBA/2J mice, the high ethanol consuming C57BL/6J mice have significantly lower baseline levels of NPY in several regions of the extended amygdala, namely, the shell of the NAc (Fig. 1C) and the central nuclei (Fig. 2C) and baso-lateral amygdala (Fig. 2D). These findings are consistent with and extend a recent report that also found low levels of NPY in the shell of the NAc of C57BL/6J mice relative to DBA/2J mice (Misra and Pandey, 2003). On the other hand, there are no strain differences in NPY levels in the core of the NAc or various nuclei examined within the hypothalamus. Although the present results do not provide a causal link between NPY and ethanol consumption, these findings offer an initial comparison of basal NPY levels between inbred strains of mice that show extreme differences in ethanol preference (Belknap et al., 1993; Le et al., 1994; Meliska et al., 1995; Mittleman et al., 2003; Risinger et al., 1998). However, utilizing genetically altered mice, it has been shown that mice lacking NPY consume significantly higher amounts of ethanol than do wild-type strains, while mice that overexpress the NPY gene exhibit a reduced preference for ethanol (Thiele et al., 1998). Therefore, taken together with this previous research, these data suggest the possibility that low NPY levels, in part, may contribute to the high ethanol consumption characteristic of C57BL/6J mice. Despite strain differences in NPY levels and contrary to expectations, the C57BL/6J and DBA/2J mice do not differ in baseline CRF levels in any of the regions examined.
It must be cautioned that differential expression of NPY between C57BL/6J and DBA/2J mice may modulate any of a number of phenotypic parameters that differ between these strains, and in fact, differences in ethanol consumption between strains could be completely independent of NPY signaling. However, the observation that low basal NPY levels are associated with high ethanol intake in inbred mice is consistent with several other reports in the literature. The selectively bred P and HAD rat lines show low NPY levels in the amygdala (Ehlers et al., 1998; Hwang et al., 1999), and voluntary ethanol consumption is inversely related to NPY signaling in knockout and trans-genic mice (Thiele et al., 1998, 2002, 2000b, 2004a). In addition, amygdalar infusion of a PKA inhibitor causes local reductions of NPY levels that are associated with increased ethanol intake in Sprague Dawley rats, and furthermore, elevated levels of ethanol drinking are rescued by amygdalar coadministration of NPY (Pandey et al., 2003b). Thus, the present results are consistent with previous research and further suggest that low NPY levels, specifically within the extended amygdala, predispose high ethanol consumption. Hypothalamic infusion of NPY increases ethanol intake by Long-Evans rats (Kelley et al., 2001). However, because the C57BL/6J and DBA/2J mice do not differ in hypothalamic NPY levels, it is unlikely that hypothalamic NPY levels modulate ethanol intake by these inbred strains.
It is of interest to consider the possible mechanisms by which NPY signaling modulates ethanol consumption. Because of their opposing actions, it has been suggested that CRF and NPY exert a reciprocal regulation of drug self-administration through allostatic interactions within the extended amygdala (Koob, 2003; Koob and Le Moal, 2001). Theoretically, repeated abstinence and relapse weaken the NPY system and augment CRF signaling, ultimately creating an imbalance that results in reduced sensitivity to drug reward and a negative affective state and that increases the likelihood of relapse and uncontrolled ethanol drinking. We suggest that our observations are in fact consistent with the allostasis model of uncontrolled ethanol drinking (Koob, 2003; Koob and Le Moal, 2001). Relative to DBA/2J mice, C57BL/6J mice have low baseline NPY levels but similar CRF expression. Viewed this way, naive C57BL/6J mice possess an inherent imbalance between NPY and CRF that theoretically resembles a dependent animal. This inherent imbalance, in turn, could motivate the high ethanol drinking that is characteristic of C57BL/6 mice with little or no prior ethanol experience.
It has been suggested that factors that may contribute to the initiation of ethanol consumption and/or continued use of this drug include high basal levels of anxiety and increased anxiety caused by ethanol withdrawal (Bibb and Chambless, 1986; Cappell and Herman, 1972; Cornelius et al., 2003; Koob, 2003; Schuckit and Hesselbrock, 1994). NPY possesses anxiolytic properties when infused into the amygdala (Heilig et al., 1993, 1989). Thus, organisms with innately low levels of NPY, or with low levels of NPY caused by withdrawal, may drink increased amounts of ethanol in an attempt to reduce anxiety (i.e., increased ethanol drinking represents self-medication) (Pandey, 2003; Pandey et al., 2003a; Thiele et al., 2004b). Consistent with this view, P rats, which have low amygdalar NPY levels (Ehlers et al., 1998; Hwang et al., 1999), show high basal levels of anxiety (Stewart et al., 1993) that may predispose them to drink large quantities of ethanol. Central infusion of NPY may rescue the high ethanol drinking in P rats by reducing their high levels of anxiety (Badia-Elder et al., 2000; Stewart et al., 1993). However, C57BL/6J mice show low basal levels of anxiety when compared with DBA/2J mice (Griebel et al., 2000; Lamberty, 1998; Misra and Pandey, 2003; Ohl et al., 2001), thus making altered anxiety an unlikely explanation of increased ethanol drinking by the C57BL/6J strain.
It has also been suggested that NPY may influence ethanol consumption by modulating the sedative effects of this drug. There is increasing evidence from both human and animal research indicating that resistance to the intoxicating effects of ethanol is often associated with high levels of ethanol drinking (Schuckit, 1986, 1988, 1994). Low NPY signaling is associated with increased ethanol consumption and resistance to the sedative effects of ethanol, while NPY overexpression promotes increased sensitivity to ethanol-induced sedation and inhibits ethanol drinking (Thiele et al., 1998, 2002). Interestingly, there is evidence that C57BL/6J mice are less sensitive to ethanol-induced sedation than DBA/2J mice (Camjanovich and MacInnes, 1973; Tabakoff and Ritzmann, 1979), which could be the result of low NPY signaling. However, differences in sensitivity to the sedative effects of ethanol between C57BL/6J and DBA/2J mice have not always been observed (Alkana et al., 1988; Browman et al., 2000).
Finally, it has been suggested that central NPY signaling modulates the rewarding properties associated with ethanol (Thiele et al., 2004b). The NAc is a brain region with one of the highest levels of NPY IR (Hendry, 1993). Furthermore, the NAc is thought to be a key brain region for the modulation of ethanol reward (Koob, 2003). NPY infused directly into the NAc supports conditioned place preference in rats; thus, NPY signaling in the NAc is apparently rewarding (Brown et al., 2000; Josselyn and Beninger, 1993). In addition, pretreatment with a dopamine receptor antagonist prevents NPY from producing a conditioned place preference, a finding that suggests that NPY-mediated reward involves an interaction with the dopamine system (Josselyn and Beninger, 1993). Thus, low NPY function in the NAc may, in part, drive the high ethanol drinking in C57BL/6 mice (Misra and Pandey, 2003; Thiele et al., 2004b).
In summary, we provide an initial comparison of basal NPY and CRF levels between C57BL/6J and DBA/2J mice, strains that differ dramatically in their level of voluntary ethanol consumption. Relative to DBA/2J mice, the C57BL/6J strain showed significantly lower NPY levels within the amygdala and the shell region of the NAc. However, there were no corresponding differences in CRF levels between the strains. These findings suggest that low NPY levels, and possibly an imbalance between NPY and CRF, contribute to the high ethanol consumption characteristic of C57BL/6J mice. However, to firmly establish the possible role of endogenous NPY signaling in ethanol intake by inbred mice, future research will involve a systematic assessment of mechanisms affecting NPY function, at baseline and in response to an ethanol challenge, over a range of inbred strains that differ in ethanol intake. It will also be necessary to determine and control for strain differences in synthesis, storage, and release of NPY, as well as possible stain differences in NPY receptor subtype populations. Future research should also include female animals to address the possibility that these differences may be attributable to gender effects.
Acknowledgments
The authors thank Dr. Thomas McCown for providing expert assistance with immunofluorescent procedures.
Footnotes
Supported by Grants AA13573, AA011605, and AA014949 from the National Institutes of Health.
References
- Alkana RL, Finn DA, Bejanian M, Crabbe JC. Genetically determined differences in ethanol sensitivity influenced by body temperature during intoxication. Life Sci. 1988;43:1973–1982. doi: 10.1016/0024-3205(88)90570-x. [DOI] [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Murphy JM, Li TK. Effects of neuropeptide Y on sucrose and ethanol intake and on anxiety-like behavior in high alcohol drinking (HAD) and low alcohol drinking (LAD) rats. Alcohol Clin Exp Res. 2003;27:894–899. doi: 10.1097/01.ALC.0000071929.17974.DA. [DOI] [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, Li TK. Effect of neuropeptide Y (NPY) on ethanol intake in Wistar, alcohol-preferring (P), and -nonpreferring (NP) rats. Alcohol Clin Exp Res. 2000;24:82A. [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, Li TK. Effect of neuropeptide Y (NPY) on oral ethanol intake in Wistar, alcohol-preferring (P), and -nonpreferring (NP) rats. Alcohol Clin Exp Res. 2001;25:386–390. [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Berglund MM, Hipskind PA, Gehlert DR. Recent developments in our understanding of the physiological role of PP-fold peptide receptor subtypes. Exp Biol Med. 2003;228:217–244. doi: 10.1177/153537020322800301. [DOI] [PubMed] [Google Scholar]
- Bibb JL, Chambless DL. Alcohol use and abuse among diagnosed agoraphobics. Behav Res Ther. 1986;24:49–58. doi: 10.1016/0005-7967(86)90149-x. [DOI] [PubMed] [Google Scholar]
- Bice P, Foroud T, Bo R, Castelluccio P, Lumeng L, Li TK, Carr LG. Genomic screen for QTLs underlying alcohol consumption in the P and NP rat lines. Mamm Genome. 1998;9:949–955. doi: 10.1007/s003359900905. [DOI] [PubMed] [Google Scholar]
- Biello SM, Golombek DA, Harrington ME. Neuropeptide Y and glutamate block each other’s phase shifts in the suprachiasmatic nucleus in vitro. Neuroscience. 1997;77:1049–1057. doi: 10.1016/s0306-4522(96)00547-7. [DOI] [PubMed] [Google Scholar]
- Bison S, Crews FT. Alcohol withdrawal increases neuropeptide Y immunoreactivity in rat brain. Alcohol Clin Exp Res. 2003;27:1173–1183. doi: 10.1097/01.ALC.0000075827.74538.FE. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: Inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browman KE, Rustay NR, Nikolaidis N, Crawshaw L, Crabbe JC. Sensitivity and tolerance to ethanol in mouse lines selected for ethanol-induced hypothermia. Pharmacol Biochem Behav. 2000;67:821–829. doi: 10.1016/s0091-3057(00)00427-5. [DOI] [PubMed] [Google Scholar]
- Brown CM, Coscina DV, Fletcher PJ. The rewarding properties of neuropeptide Y in perifornical hypothalamus vs. nucleus accumbens. Peptides. 2000;21:1279–1287. doi: 10.1016/s0196-9781(00)00270-9. [DOI] [PubMed] [Google Scholar]
- Camjanovich RP, MacInnes JW. Factors involved in ethanol narcosis: Analysis in mice of three inbred strains. Life Sci. 1973;13:55–65. doi: 10.1016/0024-3205(73)90277-4. [DOI] [PubMed] [Google Scholar]
- Cappell H, Herman CP. Alcohol and tension reduction. A review. Q J Stud Alcohol. 1972;33:33–64. [PubMed] [Google Scholar]
- Carr LG, Foroud T, Bice P, Gobbett T, Ivashina J, Edenberg H, Lumeng L, Li T-K. A quantitative trait locus for alcohol consumption in selectively bred rat lines. Alcohol Clin Exp Res. 1998;22:884–887. [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Clark JT, Keaton AK, Sahu A, Kalra SP, Mahajan SC, Gudger JN. Neuropeptide Y (NPY) levels in alcoholic and food restricted male rats: Implications for site selective function. Regul Pept. 1998;75–76:335–345. doi: 10.1016/s0167-0115(98)00086-x. [DOI] [PubMed] [Google Scholar]
- Colmer WF, Wahlestedt C (1993) The biology of neuropeptide Y and related peptides. Humana Press, Totowa.
- Cornelius JR, Bukstein O, Salloum I, Clark D. Alcohol and psychiatric comorbidity. Recent Dev Alcohol. 2003;16:361–374. doi: 10.1007/0-306-47939-7_24. [DOI] [PubMed] [Google Scholar]
- Dumont Y, Martel JC, Fournier A, St-Pierre S, Quirion R. Neuropeptide Y and neuropeptide Y receptor subtypes in brain and peripheral tissues. Prog Neurobiol. 1992;38:125–167. doi: 10.1016/0301-0082(92)90038-g. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Li TK, Lumeng L, Hwang BH, Somes C, Jimenez P, Mathe AA. Neuropeptide Y levels in ethanol-naive alcohol-preferring and -nonpreferring rats and in Wistar rats after ethanol exposure. Alcohol Clin Exp Res. 1998;22:1778–1782. [PubMed] [Google Scholar]
- Foroud T, Bice P, Castelluccio P, Bo R, Miller L, Ritchotte A, Lumeng L, Li TK, Carr LG. Identification of quantitative trait loci influencing alcohol consumption in the high alcohol drinking and low alcohol drinking rat lines. Behav Genet. 2000;30:131–140. doi: 10.1023/a:1001955205117. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Li TK, Badia-Elder NE. Neuropeptide Y reduces oral ethanol intake in alcohol-preferring (P) rats following a period of imposed ethanol abstinence. Alcohol Clin Exp Res. 2003;27:787–794. doi: 10.1097/01.ALC.0000065723.93234.1D. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Biello SM, Rendon RA, Harrington ME. Neuropeptide Y phase shifts the circadian clock in vitro via a Y2 receptor. Neuroreport. 1996;7:1315–1319. doi: 10.1097/00001756-199605170-00020. [DOI] [PubMed] [Google Scholar]
- Gray TS, Morley JE. Neuropeptide Y: Anatomical distribution and possible function in mammalian nervous system. Life Sci. 1986;38:389–401. doi: 10.1016/0024-3205(86)90061-5. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Wisialowski TA, van den Pol AN, Yocca FD. Phase shifting of circadian rhythms and depression of neuronal activity in the rat suprachiasmatic nucleus by neuropeptide Y: Mediation by different receptor subtypes. J Neurosci. 1998;18:3014–3022. doi: 10.1523/JNEUROSCI.18-08-03014.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature (Lond) 2001a;410:940–944. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Regulation of olfactory neurogenesis by amidated neuropeptides. J Neurosci Res. 2001b;66:1–7. doi: 10.1002/jnr.1191. [DOI] [PubMed] [Google Scholar]
- Harrington ME, Schak KM. Neuropeptide Y phase advances the in vitro hamster circadian clock during the subjective day with no effect on phase during the subjective night. Can J Physiol Pharmacol. 2000;78:87–92. [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: Mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heilig M, Soderpalm B, Engel JA, Widerlov E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl) 1989;98:524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- Heilig M, Widerlov E. Neurobiology and clinical aspects of neuropeptide Y. Crit Rev Neurobiol. 1995;9:115–136. [PubMed] [Google Scholar]
- Hendry SH (1993) Organization of neuropeptide Y neurons in the mammalian central nervous system, in The Biology of Neuropeptide Y and Related Peptides (Wahlestedt C, Colmers WF eds), pp 65–156. Humana Press, Totowa.
- Hwang BH, Zhang JK, Ehlers CL, Lumeng L, Li TK. Innate differences of neuropeptide Y (NPY) in hypothalamic nuclei and central nucleus of the amygdala between selectively bred rats with high and low alcohol preference. Alcohol Clin Exp Res. 1999;23:1023–1030. [PubMed] [Google Scholar]
- Josselyn SA, Beninger RJ. Neuropeptide Y: Intraaccumbens injections produce a place preference that is blocked by cis-flupenthixol. Pharmacol Biochem Behav. 1993;46:543–552. doi: 10.1016/0091-3057(93)90542-2. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Xu B, Dube MG, Moldawer LL, Martin D, Kalra PS. Leptin and ciliary neurotropic factor (CNTF) inhibit fasting-induced suppression of luteinizing hormone release in rats: Role of neuropeptide Y. Neurosci Lett. 1998;240:45–49. doi: 10.1016/s0304-3940(97)00896-3. [DOI] [PubMed] [Google Scholar]
- Kasuya E, Mizuno M, Watanabe G, Terasawa E. Effects of an antisense oligodeoxynucleotide for neuropeptide Y mRNA on in vivo luteinizing hormone-releasing hormone release in ovariectomized female rhesus monkeys. Regul Pept. 1998;75-76:319–325. doi: 10.1016/s0167-0115(98)00084-6. [DOI] [PubMed] [Google Scholar]
- Katner SN, Slawecki CJ, Ehlers CL. Neuropeptide Y administration into the amygdala does not affect ethanol consumption. Alcohol. 2002a;28:29–38. doi: 10.1016/s0741-8329(02)00235-5. [DOI] [PubMed] [Google Scholar]
- Katner SN, Slawecki CJ, Ehlers CL. Neuropeptide Y administration into the third ventricle does not increase sucrose or ethanol self-administration but does affect the cortical EEG and increases food intake. Psychopharmacology (Berl) 2002b;160:146–154. doi: 10.1007/s00213-001-0950-9. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Nannini MA, Bratt AM, Hodge CW. Neuropeptide-Y in the paraventricular nucleus increases ethanol self-administration. Peptides. 2001;22:515–522. doi: 10.1016/s0196-9781(01)00361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita H, Jessop DS, Finn DP, Coventry TL, Roberts DJ, Ameno K, Ijiri I, Harbuz MS. Acute ethanol decreases NPY mRNA but not POMC mRNA in the arcuate nucleus. Neuroreport. 2000;11:3517–3519. doi: 10.1097/00001756-200011090-00023. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: Allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Lamberty Y. The mirror chamber test for testing anxiolytics: Is there a mirror-induced stimulation? Physiol Behav. 1998;64:703–705. doi: 10.1016/s0031-9384(98)00124-3. [DOI] [PubMed] [Google Scholar]
- Le AD, Ko J, Chow S, Quan B. Alcohol consumption by C57BL/6, BALB/c, and DBA/2 mice in a limited access paradigm. Pharmacol Biochem Behav. 1994;47:375–378. doi: 10.1016/0091-3057(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Levine AS, Morley JE. Neuropeptide Y: A potent inducer of consummatory behavior in rats. Peptides. 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- Lopez-Valpuesta FJ, Nyce JW, Griffin-Biggs TA, Ice JC, Myers RD. Antisense to NPY-Y1 demonstrates that Y1 receptors in the hypothalamus underlie NPY hypothermia and feeding in rats. Proc R Soc Lond B Biol Sci. 1996;263:881–886. doi: 10.1098/rspb.1996.0130. [DOI] [PubMed] [Google Scholar]
- Meliska CJ, Bartke A, Vandergriff JL, Jensen RA. Ethanol and nicotine consumption and preference in transgenic mice overexpressing the bovine growth hormone gene. Pharmacol Biochem Behav. 1995;50:563–570. doi: 10.1016/0091-3057(94)00345-9. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra K, Pandey SC. Differences in basal levels of CREB and NPY in nucleus accumbens regions between C57BL/6 and DBA/2 mice differing in inborn alcohol drinking behavior. J Neurosci Res. 2003;74:967–975. doi: 10.1002/jnr.10831. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Van Brunt CL, Matthews DB. Schedule-induced ethanol self-administration in DBA/2J and C57BL/6J mice. Alcohol Clin Exp Res. 2003;27:918–925. doi: 10.1097/01.ALC.0000071930.48632.AE. [DOI] [PubMed] [Google Scholar]
- Ohl F, Sillaber I, Binder E, Keck ME, Holsboer F. Differential analysis of behavior and diazepam-induced alterations in C57BL/6N and BALB/c mice using the modified hole board test. J Psychiatr Res. 2001;35:147–154. doi: 10.1016/s0022-3956(01)00017-6. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC. Anxiety and alcohol abuse disorders: A common role for CREB and its target, the neuropeptide Y gene. Trends Pharmacol Sci. 2003;24:456–460. doi: 10.1016/S0165-6147(03)00226-8. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Carr LG, Heilig M, Ilveskoski E, Thiele TE. Neuropeptide Y and alcoholism: Genetic, molecular, and pharmacological evidence. Alcohol Clin Exp Res. 2003a;27:149–154. doi: 10.1097/01.ALC.0000052706.21367.0E. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H. The decreased phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats. Alcohol Clin Exp Res. 2003b;27:396–409. doi: 10.1097/01.ALC.0000056616.81971.49. [DOI] [PubMed] [Google Scholar]
- Pedrazzini T, Seydoux J, Kunstner P, Aubert JF, Grouzmann E, Beermann F, Brunner HR. Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nat Med. 1998;4:722–726. doi: 10.1038/nm0698-722. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Brown MM, Doan AM, Oakes RA. Mouse strain differences in oral operant ethanol reinforcement under continuous access conditions. Alcohol Clin Exp Res. 1998;22:677–684. doi: 10.1111/j.1530-0277.1998.tb04311.x. [DOI] [PubMed] [Google Scholar]
- Rivier C, Lee S. Acute alcohol administration stimulates the activity of hypothalamic neurons that express corticotropin-releasing factor and vasopressin. Brain Res. 1996;726:1–10. [PubMed] [Google Scholar]
- Roy A, Pandey SC. The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcohol Clin Exp Res. 2002;26:796–803. [PubMed] [Google Scholar]
- Schuckit MA. Biological markers in alcoholism. Prog Neuropsycho-pharmacol Biol Psychiatry. 1986;10:191–199. doi: 10.1016/0278-5846(86)90073-4. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Reactions to alcohol in sons of alcoholics and controls. Alcohol Clin Exp Res. 1988;12:465–470. doi: 10.1111/j.1530-0277.1988.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Hesselbrock V. Alcohol dependence and anxiety disorders: What is the relationship? Am J Psychiatry. 1994;151:1723–1734. doi: 10.1176/ajp.151.12.1723. [DOI] [PubMed] [Google Scholar]
- Shi TJ, Cui JG, Meyerson BA, Linderoth B, Hokfelt T. Regulation of galanin and neuropeptide Y in dorsal root ganglia and dorsal horn in rat mononeuropathic models: Possible relation to tactile hypersensitivity. Neuroscience. 1999;93:741–757. doi: 10.1016/s0306-4522(99)00105-0. [DOI] [PubMed] [Google Scholar]
- Shi TJ, Tandrup T, Bergman E, Xu ZQ, Ulfhake B, Hokfelt T. Effect of peripheral nerve injury on dorsal root ganglion neurons in the C57 BL/6J mouse: Marked changes both in cell numbers and neuropeptide expression. Neuroscience. 2001;105:249–263. doi: 10.1016/s0306-4522(01)00148-8. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Walpole T, Ehlers CL. Increases in sucrose consumption, but not ethanol consumption, following ICV NPY administration. Pharmacol Biochem Behav. 2000;66:591–594. doi: 10.1016/s0091-3057(00)00215-x. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, Li T-K, Murphy JM. Comparison of alcohol-preferring (P) and -nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of alcohol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: An immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Ritzmann RF. Acute tolerance in inbred and selected lines of mice. Drug Alcohol Depend. 1979;4:87–90. doi: 10.1016/0376-8716(79)90043-7. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Badia-Elder NE. A role for neuropeptide Y in alcohol intake control: Evidence from human and animal research. Physiol Behav. 2003;79:95–101. doi: 10.1016/s0031-9384(03)00109-4. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Cubero I, van Dijk G, Mediavilla C, Bernstein IL. Ethanol-induced c-Fos expression in catecholamine- and neuropeptide Y-producing neurons in rat brainstem. Alcohol Clin Exp Res. 2000a;24:802–809. [PubMed] [Google Scholar]
- Thiele TE, Koh MT, Pedrazzini T. Voluntary alcohol consumption is controlled via the neuropeptide Y Y1 receptor. J Neurosci. 2002;22:RC208. doi: 10.1523/JNEUROSCI.22-03-j0006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature (Lond) 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Miura GI, Marsh DJ, Bernstein IL, Palmiter RD. Neurobiological responses to ethanol in mutant mice lacking neuropeptide Y or the Y5 receptor. Pharmacol Biochem Behav. 2000b;67:683–691. doi: 10.1016/s0091-3057(00)00413-5. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Navarro M, Sparta DR, Fee JR, Knapp DJ, Cubero I. Alcoholism and obesity: Overlapping neuropeptide pathways? Neuropeptides. 2003;37:321–337. doi: 10.1016/j.npep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Naveilhan P, Ernfors P. Assessment of ethanol consumption and water drinking by NPY Y2 receptor knockout mice. Peptides. 2004a;25:975–983. doi: 10.1016/j.peptides.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Sparta DR, Hayes DM, Fee JR. A role for neuropeptide Y in neurobiological responses to ethanol and drugs of abuse. Neuropeptides. 2004b;38:235–243. doi: 10.1016/j.npep.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: Regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Mikkelsen JD. Direct projection from the suprachiasmatic nucleus to hypophysiotrophic corticotropin-releasing factor immunoreactive cells in the paraventricular nucleus of the hypothalamus demonstrated by means of Phaseolus vulgaris-leucoagglutinin tract tracing. Brain Res. 1995;684:61–69. doi: 10.1016/0006-8993(95)00425-p. [DOI] [PubMed] [Google Scholar]
- Woldbye DP, Larsen PJ, Mikkelsen JD, Klemp K, Madsen TM, Bolwig TG. Powerful inhibition of kainic acid seizures by neuropeptide Y via Y5-like receptors. Nat Med. 1997;3:761–764. doi: 10.1038/nm0797-761. [DOI] [PubMed] [Google Scholar]
- Woldbye DP, Madsen TM, Larsen PJ, Mikkelsen JD, Bolwig TG. Neuropeptide Y inhibits hippocampal seizures and wet dog shakes. Brain Res. 1996;737:162–168. doi: 10.1016/0006-8993(96)00730-5. [DOI] [PubMed] [Google Scholar]


