Abstract
Background
Genetic and pharmacological evidence suggests that the cyclic adenosine monophosphate–dependent protein kinase A pathway modulates neurobiological responses to ethanol. Mutant mice lacking the RIIβ subunit of protein kinase A (RIIβ−/−) are resistant to ethanol-induced sedation and drink significantly more ethanol than littermate wild-type mice (RIIβ+/+). We determined whether high ethanol intake by the RIIβ −/− mice on alternate genetic backgrounds is reliably predicted by high basal levels of anxiety or resistance to the sedative effects of ethanol.
Methods
Two-bottle choice procedures and a battery of behavioral tests (elevated plus maze, open-field activity, and zero maze) were used to assess voluntary ethanol consumption and basal levels of anxiety in RIIβ−/− and RIIβ+/+ mice on either a C57BL/6J or a 129/SvEv × C57BL/6J genetic background. Additionally, ethanol-induced sedation and blood ethanol levels were determined in RIIβ−/− and RIIβ+/+ mice after intraperitoneal injection of ethanol (3.8 g/kg).
Results
RIIβ−/− mice on both genetic backgrounds consumed more ethanol and had a greater preference for ethanol relative to RIIβ+/+ mice. However, RIIβ−/− mice showed reduced basal levels of anxiety when maintained on the C57BL/6J background but showed increased anxiety when maintained on the 129/SvEv × C57BL/6J background. Consistent with prior research, RIIβ−/− mice were resistant to the sedative effects of ethanol, regardless of the genetic background. Finally, RIIβ−/− and RIIβ+/+ mice showed similar blood ethanol levels.
Conclusions
These results indicate that high ethanol consumption is associated with resistance to the sedative effects of ethanol but that basal levels of anxiety, as well as ethanol metabolism, do not reliably predict high ethanol drinking by RIIβ−/− mice.
Keywords: Alcohol, Anxiety, Protein Kinase A, Sedation, Voluntary Consumption
MANY NEUROTRANSMITTERS, NEUROMODULATORS, and hormones transduce their signal into cells by activating G protein–coupled receptors. After G protein–coupled receptor binding, cyclic adenosine monophosphate (cAMP) levels are either enhanced or inhibited via changes in adenylyl cyclase activation. This action leads to concomitant increases or decreases, respectively, in cAMP-dependent protein kinase A (PKA) activity. PKA is a holoenzyme that consists of a regulatory (R) subunit homodimer and two catalytic (C) subunits (Brandon et al., 1997). In the mouse, PKA includes four regulatory subunits (RIα, RIβ, RIIα, and RIIβ) and two catalytic subunits (Cα and Cβ), which are expressed in tissue-specific patterns (McKnight, 1991).
A growing body of evidence from both human and animal studies suggests that variation of the cAMP/PKA system alters ethanol-seeking behavior and acute alcohol-induced physiological responses. Human alcoholics have lower platelet adenylyl cyclase activity when compared with controls (Ikeda et al., 1998; Parsian et al., 1996), and cAMP levels in lymphocytes collected from alcoholics are higher than normal under basal conditions but are lower after chronic exposure to ethanol (Nagy et al., 1988). In vitro studies indicate that NG108-15 cells exposed to ethanol show translocation of the Cα subunit from the Golgi area to the nucleus as long as ethanol is present (Dohrman et al., 1996). Furthermore, long-term (12-hr) exposure to ethanol causes translocation of both the Cα and RIIβ subunits to the nucleus (Dohrman et al., 2002). This translocation and the phosphorylation of cAMP-response element binding protein (CREB) are blocked by pretreatment with a type II PKA antagonist (Constantinescu et al., 2002). In vivo studies show that mutant drosophila lacking production of the RII subunit of PKA are resistant to the intoxicating effects of ethanol (Park et al., 2000). In agreement, we have reported that genetically altered mice lacking the RIIβ subunit of PKA (RIIβ−/−) are resistant to ethanol-induced sedation and drink significantly more ethanol than litter-mate wild-type (RIIβ+/+) mice (Thiele et al., 2000b).
The goal of this research was 2-fold. First, we determined whether RIIβ−/− mice drink increased amounts of ethanol when maintained on alternate genetic backgrounds. A growing body of literature is emerging indicating that phenotypes, including neurobiological responses to ethanol, can depend on the genetic background of the knock-out model (Bowers et al., 1999; Howe et al., 2002; Kelly et al., 1998; Palmer et al., 2003; Phillips et al., 1999; Simpson et al., 1997; Thiele et al., 2000a). We have previously studied RIIβ−/− mice maintained on a mixed 129/SvJ × C57BL/6J genetic background (Thiele et al., 2000b). To extend these findings in this study, we assessed ethanol consumption by RIIβ−/− mice backcrossed over eight generations to a pure C57BL/6J inbred background and by RIIβ−/− mice maintained on a mixed 129/SvEv × C57BL/6J background. In addition to background strain (Belknap et al., 1993), sex is a factor that can influence ethanol consumption (Middaugh et al., 1999). Thus, we also determined whether the high ethanol drinking by RIIβ−/− mice is sex dependent. If both male and female RIIβ−/− mice showed increased ethanol intake on multiple genetic backgrounds, this would strengthen the argument that the RIIβ subunit of PKA is a key mediator of voluntary ethanol consumption.
The second goal was to identify possible predictors of the high ethanol consumption that is characteristic of RIIβ−/− mice. One factor that has been proposed to contribute to the initiation of ethanol consumption or to continued use of this drug is high basal levels of anxiety (Bibb and Chambless, 1986; Cappell and Herman, 1972; Cornelius et al., 2003; Koob, 2003; Schuckit and Hesselbrock, 1994). Low PKA, phosphorylated CREB (p-CREB), and CREB-targeted gene activity in the central and medial amygdala—brain regions that modulate fear and anxiety (Davis, 1997)—are associated with increased anxiety-like behavior in rodents (Pandey et al., 2003a,b; Roy and Pandey, 2002). Because RIIβ−/− mice have blunted cAMP-induced PKA activity in the amygdala (Thiele et al., 2000b), we used a battery of behavioral tests to determine whether the increased ethanol drinking by RIIβ−/− mice is associated with increased basal levels of anxiety. Sex and strain are factors that influence anxiety in mice (Contet et al., 2001; Voikar et al., 2001); therefore, we studied anxiety-like behavior in male and female RIIβ−/− mice maintained on multiple genetic backgrounds. A second factor that may predispose to high ethanol consumption is an inherent resistance to the intoxicating effects of ethanol (Hodge et al., 1999; Schuckit, 1994; Thiele et al., 1998, 2000b, 2002; Wand et al., 2001; Weinshenker et al., 2000). Recent evidence indicates that low cAMP/PKA signaling promotes resistance to the intoxicating properties of ethanol (Park et al., 2000; Thiele et al., 2000b; but see Wand et al., 2001); therefore, we further characterized male and female RIIβ−/− mice to determine whether they consistently show low sensitivity to ethanol-induced sedation when maintained on alternate genetic backgrounds.
MATERIALS AND METHODS
Animals
RIIβ−/− mice were created through disruption of the RIIβ gene by homologous recombination in embryonic stem cells from 129/SvJ mice (Brandon et al., 1998). Chimeras were bred with C57BL/6J mice to obtain heterozygotes (50% 129/SvJ × 50% C57BL/6J). These heterozygotes were backcrossed with C57BL/6J mice for eight generations to yield RIIβ+/− mice on an approximately 100% C57BL/6J genetic background. For some experiments described here, nonlittermate RIIβ+/− mice on the 100% C57BL/6J background were bred to provide RIIβ+/− and RIIβ+/+ F(2) littermate mice. Additional experiments involved RIIβ−/− and RIIβ+/+ F(2) littermate mice on a 50% 129/SvEv × 50% C57BL/6J background that were created by crossing the RIIβ−/− mice with wild-type 129/SvEv mice. The genetic status of all mice was determined by using polymerase chain reaction procedures as described elsewhere (Thiele et al., 2000b). Animals weighed approximately 20 g, were 3 to 6 months old at the beginning of experiments, and were individually housed in polypropylene cages with corncob bedding. Mice had ad libitum access to water and standard rodent chow (Tekland, Madison, WI) except where noted. The colony room was maintained at approximately 22°C with a 12-hr/12-hr light/dark cycle with lights off at 3:00 PM. All procedures used in this study were in compliance with the NIH guidelines, and the protocols were approved by the University of North Carolina Animal Care and Use Committee.
Voluntary Ethanol Consumption
RIIβ−/− (male, n = 9; female, n = 10) and RIIβ+/+ (male, n = 10; female, n = 10) mice maintained on a pure C57BL/6J background were tested with a two-bottle continuous-access paradigm (ethanol solution in one bottle and water in the other bottle). A 3% (v/v) solution was presented for the first 4 days. The concentrations were then increased to 5, 8, 10, 13, 15, 18, and 20% every 4 days. Each drinking bottle was weighed every 2 days, and body weights were recorded every 8 days. The position of the bottle containing the ethanol solution was reversed every 2 days to prevent the development of a position preference. To obtain a measure that corrected for individual differences in body weight, grams of ethanol consumed per kilogram of body weight were calculated. As a measure of relative ethanol preference, ethanol preference ratios were calculated at each ethanol concentration by dividing the total ethanol solution consumed by the total fluid (ethanol plus water) consumed. RIIβ−/− (male, n = 10; female, n = 10) and RIIβ+/+ (male, n = 10; female, n = 10) mice maintained on the mixed 129/SvEv × C57BL/6J background were also tested by using the previously described two-bottle procedure. Three-way 2 × 2 × 8 (genotype × sex × concentration) repeated-measures ANOVAs were performed to analyze consumption data for both genetic backgrounds.
Elevated Plus Maze Testing
The elevated plus maze is a pharmacologically validated model for the assessment of anxiety in rodents (Pellow et al., 1985). Drug-naive RIIβ−/− (male, n = 10; female, n = 5) and RIIβ+/+ (male, n = 10; female, n = 11) mice on a pure C57BL/6J background, as well as RIIβ−/− (male, n = 12; female, n = 12) and RIIβ+/+ (male, n = 11; female, n = 12) mice on a mixed 129/SvEv × C57BL/6J background, were tested during the last 3 to 6 hr of their light phase. Animals were transported to the room immediately adjacent to the testing room and were allowed to habituate for approximately 30 min before procedures began. A small fan was used to provide masking noise. The plus maze (MED Associates, Inc., St. Albans, VT) was positioned in the center of the room directly below a ceiling-mounted lamp fitted with a single 25-W red lightbulb that provided the only light for the room. Each mouse was individually removed from its home cage and immediately placed onto the center square of the plus maze with its nose pointing toward one of the open arms. The 5-min test session was video-recorded with a tripod-mounted camcorder to eliminate the need for an investigator’s presence in the testing room. Sessions were scored by genotype-blind investigators for the time spent in open or closed arms and the proportion of total time spent in the open arm, defined as open-arm time divided by total time spent in both arms. An animal was considered to have entered an arm of the plus maze if all four paws had left the center square. Open- and closed-arm time was considered terminated once a single paw was placed back into the center square. These data were analyzed using two-way 2 × 2 (genotype × sex) ANOVAs.
Open-Field Testing
The open-field test is commonly used to assess anxiety in rodents. This test has been validated in part by demonstration that benzodiazepines increase the amount of time a mouse will spend in the center of the activity chamber (e.g., Choleris et al., 2001). Drug-naive RIIβ−/− (male, n = 10; female, n = 10) and RIIβ+/+ (male, n = 10; female, n = 10) mice on a pure C57BL/6J background, as well as RIIβ−/− (male, n = 10; female, n = 10) and RIIβ+/+ (male, n = 10; female, n = 10) mice on a mixed 129/SvEv × C57BL/6J background, were tested during their light phase. All animals were transported to the testing room and allowed to habituate for at least 30 min before testing. A fan provided masking noise during the study, and testing was conducted under ambient lighting conditions. Mice were placed into the center of an open-field arena that automatically recorded activity via photo beam breaks (Harvard Apparatus, Inc., Holliston, MA). The open-field arena measured 40.64 × 40.64 × 30.48 cm and was made of clear Plexiglas (Rohm & Haas Co., Philadelphia, PA). Testing sessions were 30 min long, and the chambers were cleaned with isopropyl ethanol wipes after each session. Time spent and distance traveled in the center section of the chamber were recorded (center time and center distance). Similar measures were recorded for time spent and distance traveled in the portions of the chamber closest to the walls (margin time and margin distance). For analyses, data were converted to the proportion of total time spent in the center of the chamber, calculated as center time/(center time + margin time), and the proportion of total locomotor activity in the center of the chamber, calculated as center distance/(center distance + margin distance). Data for each genetic background were analyzed with a two-way 2 × 2 (genotype × sex) ANOVA.
Zero Maze Testing
The zero maze has been validated as a test of anxiety from observations that anxiolytic drugs increase open-arm time in the maze, whereas anxiogenic drugs reduce open-arm time (Shepherd et al., 1994). Approximately 3 weeks after the open-field test, the mice used in the open-field test described previously were transported to a room immediately adjacent to the testing room and allowed to habituate for at least 30 min before testing began. A fan was used to provide masking noise. The zero maze (Hamilton-Kinder, Poway, CA) was positioned in the center of a room below a ceiling-mounted lamp fitted with a single 25-W red lightbulb that provided the only light for the room. Each mouse was individually removed from its home cage and immediately placed just inside a closed arm of the zero maze with its nose pointing into the closed-arm section. The 5-min test session was video-recorded with a tripod-mounted camcorder to eliminate the need for an investigator’s presence in the testing room. Sessions were scored by genotype-blind investigators for the proportion of time spent in open or closed arms and for the number of open- and closed-arm entries. An animal was considered to have entered the open arm if all four paws had left the closed arm. Open-arm time was considered terminated once a single paw was placed back into the closed arm. Data for each genetic background were analyzed with a two-way (genotype × sex) ANOVA.
Ethanol-Induced Sedation Testing
RIIβ−/− (male, n = 10; female, n = 10) and RIIβ+/+ (male, n = 10; female, n = 10) mice on a pure C57BL/6J background and RIIβ−/− (male, n = 10; female, n = 10) and RIIβ+/+ (male, n = 10; female, n = 10) mice on a 129/SvEv × C57BL/6J background were tested for ethanol-induced sedation. Mice on the C57BL/6J background were drug naïve, and mice on the 129/SvEv × C57BL/6J background received an intraperitoneal injection of sodium pentobarbital (50 or 60 mg/kg, distributed evenly between genotypes) 3 weeks before testing with ethanol. Mice were transported to the testing room 45 min before procedures for a period of habituation. Body weights were recorded, and mice were returned to their home cages. Mice received an intraperitoneal injection of ethanol (3.8 g/kg; 19% w/v, mixed in isotonic saline) and were returned to their home cages for observation. Upon onset of loss of the righting reflex, mice were removed from their home cages and placed on their backs in a plastic U-shaped trough. Mice were monitored, and the righting reflex was considered regained when mice could right themselves onto all four paws three times within 30 sec. Duration of loss of righting reflex data were analyzed with two-way 2 × 2 (genotype × sex) ANOVAs.
Blood Ethanol Concentrations
Drug-naive RIIβ−/− (male, n = 3; female, n = 3) and RIIβ+/+ (male, n = 3; female, n = 3) mice on a pure C57BL/6J background received an intraperitoneal injection of ethanol (3.8 g/kg; 19% w/v, mixed in isotonic saline) and were returned to their home cages. As adapted from breath-sampling procedures for rats (Pohorecky and Brick, 1982), breath ethanol samples were taken from mice at 5, 17, 29, 41, 53, 130, 207, 284, 361, and 438 min after injection and immediately processed with gas chromatograph procedures described previously (Knapp et al., 1993; Navarro et al., 2003). To collect breath, a mouse was placed into a 50-ml polypropylene centrifuge tube (Corning Inc., Corning, NY) with its nose positioned at the 10-ml mark. Animals were allowed to breathe for 20 sec (approximately 60 breaths at three breaths per second) while enclosed in the tube before a 1.5-ml sample was removed through a needle placed through a small hole drilled in the normally closed conical end of the tube. This needle extended into the tube to approximately the 5-ml mark and was angled dorsally relative to the mouse’s nose.
To extrapolate blood ethanol levels from breath, 6-μl tail nick blood samples from each animal were also collected into capillary tubes at 41 and 207 min and were dispensed immediately into 12 × 75-mm borosilicate glass tubes containing 375 μl of water and 0.5 g of NaCl. These liquid samples were capped and frozen until processing in the gas chromatograph. Liquid ethanol standards (also 6 μl; 0–400 mg/100 ml) and samples were similarly prepared and heated in a water bath at 55°C for 10 min. Subsequently, a 1.5-ml sample of headspace gas was removed from the glass tubes with a plastic 3.0-ml syringe and injected directly into an SRI 8610C gas chromatograph (SRI, Torrance, CA) equipped with an external syringe adapter and a 1.0-ml external loading loop. Samples were run at 140°C through a Hayesep D column (Hayes Separations, Bandera, TX) and detected with a flame ionization detector at approximately 2 min after injection. Hydrogen gas, carrier gas (also hydrogen), and internal air generator flow rates were 13.3, 25, and 250 ml/min, respectively. Areas under the curve for both breath and blood samples were analyzed with SRI PeakSimple software for Windows running on a laptop computer and were converted to milligrams per 100 ml in blood on the basis of the curve generated for the standards and the relationship between blood and breath samples taken from the same mouse. Blood ethanol data were analyzed with a three-way 2 × 2 × 10 (genotype × sex × postinjection time) repeated-measures ANOVA.
Data Analyses
All data in this report are presented as mean ± SEM. We used ANOVA to analyze all data, and t tests were used for planned comparisons (Winer et al., 1991). Significance was accepted at p < 0.05 (two tailed).
Voluntary Ethanol Consumption
Voluntary ethanol consumption data are presented in Fig. 1. RIIβ−/− mice on the C57BL/6J background consumed significantly more ethanol than RIIβ+/+ mice (Fig. 1A). ANOVA performed on these data revealed that the genotype [F(1,35) = 11.20], sex [F(1,35) = 10.29], and ethanol concentration [F(7,245) = 27.52] main effects were all significant. These significant main effects reflected the increased consumption of ethanol by RIIβ−/− mice, greater consumption of ethanol by female mice (data collapsed across males and females for simplicity of presentation), and increased consumption of ethanol as the concentration of the ethanol solution was increased, respectively. Similar amounts of ethanol consumption have been reported with mice of a C57BL/6J (Wang et al., 2003) and 129/SvJ × C57BL/6J (Thiele et al., 2000b) background, and high consumption of ethanol by female C57BL/6 mice relative to males has been reported previously (Middaugh et al., 1999). The genotype × concentration [F(7,245) = 2.19] and sex × concentration [F(7,245) = 4.64] interaction effects were also significant. Planned comparisons confirmed that male and female RIIβ−/− mice drank significantly more ethanol than RIIβ+/+ mice at each of the concentrations tested. Mice maintained on the C57BL/6J background consistently showed preference for ethanol solution, as evidenced by preference ratios of greater than 50%. ANOVA run on ethanol preference ratio data from mice on the C57BL/6J background (Fig. 1B) revealed a significant main effect of genotype [F(1,35) = 7.25], and planned comparisons revealed that RIIβ−/− mice had significantly higher ethanol preference ratios during access to the 3, 5, 8, 15, and 20% ethanol concentrations. There were no genotype differences in water consumption at any point during testing (data not shown).
Fig. 1.
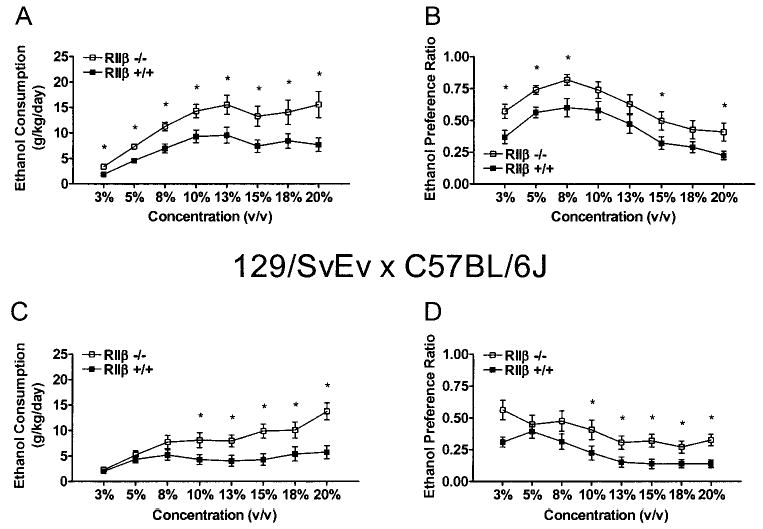
Consumption of ethanol solutions (A and C) and ethanol preference ratios (B and D) by mutant mice lacking the RIIβ subunit of PKA (RIIβ−−) and littermate wild-type (RIIβ+/+) mice. Mice were maintained on either a C57BL/6J (A and B) or 129/SvEv × C57BL/6J (C and D) genetic background. Consumption data are expressed as grams of ethanol consumed per kilogram of body weight per day, and each data point represents a 4-day average. Ethanol preference ratios are expressed as volume of ethanol solution consumed/total fluid consumption. All values reported are mean ± SEM (p < 0.05 relative to RIIβ+/+ mice).
In general, the RIIβ−/− mice on the 129/SvEv × C57BL/6J background consumed significantly more ethanol than RIIβ+/+ mice (Fig. 1C). An ANOVA performed on the data revealed significant genotype [F(1,36) = 9.70], sex [F(1,36) = 9.46], and concentration [F(7,252) = 18.25] main effects. The genotype × concentration [F(7,252) = 5.91] and sex × concentration [F(7,252) = 4.76] interaction effects were also significant. Planned comparisons revealed that male and female RIIβ−/− mice drank significantly more ethanol than RIIβ+/+ mice during access to the 10–20% ethanol concentrations. RIIβ−/− mice on the 129/SvEv × C57BL/6J background showed significantly greater preference of ethanol than RIIβ+/+ mice [F(1,36) = 4.98], and planned comparisons revealed that genotypes differed during access to the 10–20% ethanol concentrations (Fig. 1D). The 129/SvEv × C57BL/6J mice rarely showed a true ethanol preference (<50% preference ratio) during the course of the experiment, as was observed in mice maintained on the pure C57BL/6J genetic background. C57BL/6J mice consume more ethanol than other inbred strain, including 129 strains (Belknap et al., 1993). Thus, the absolute consumption and preference ratio would be predicted to be less in mice on a mixed 129/SvEv × C57BL/6J background relative to pure C57BL/6J mice. There were no significant differences in water consumption between genotypes at any point during two-bottle testing (data not shown).
Elevated Plus Maze Testing
Elevated plus maze data are presented in Fig. 2. RIIβ−/− mice on the C57BL/6J background showed significantly longer open-arm time [F(1,34) = 7.01], a significantly greater proportion of total time spent in the open arm [F(1,34) = 6.89], and significantly less closed-arm time [F(1,34) = 6.61] relative to RIIβ+/+ mice (Fig. 2A–C). In general, RIIβ−/− mice on the 129/SvEv × C57BL/6J background did not differ from RIIβ+/+ mice during elevated plus maze testing (Fig. 2D–F). However, there was a significant sex × genotype interaction with respect to the proportion of time spent in the open arm [F(1,43) = 4.59]. Planned comparisons revealed that male RIIβ−/− mice showed a significantly lower proportion of time spent in the open arm relative to RIIβ+/+ mice (0.13 ± 0.03 vs. 0.26 ± 0.04, respectively). However, there were no significant differences between female RIIβ−/− and RIIβ+/+ mice (0.31 ± 0.08 vs. 0.20 ± 0.06, respectively). Similar sex differences in plus maze behavior have been observed in mice of a 129 × C57BL/6 mixed genetic background (Voikar et al., 2001).
Fig. 2.
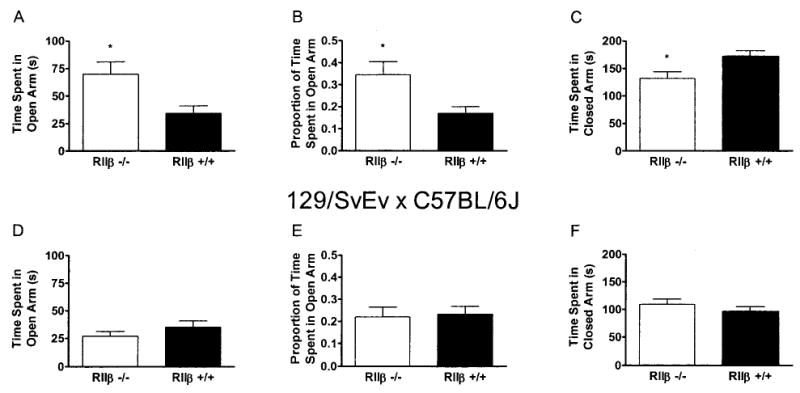
Elevated plus maze performance by RIIβ−/− and RIIβ+/+ mice maintained on either a C57BL/6J (A–C) or a 129/SvEv × C57BL/6J (D–F) genetic background. Data from 5-min test sessions are expressed as time spent in the open arm (A and D), the proportion of total time that was spent in the open arm (B and E), and the time spent in the closed arm (C and F). All values reported are mean ± SEM (p < 0.05 relative to RIIβ+/+ mice).
Open-Field Testing
Open-field activity data are presented in Fig. 3. RIIβ−/− mice on the C57BL/6J background failed to show genotype differences (Fig. 3A–C). However, RIIβ−/− mice on the 129/SvEv × C57BL/6J background spent significantly less of their total time [F(1,36) = 4.18] and total locomotor activity [F(1,36) = 7.99] in the center of the chamber relative to RIIβ+/+ mice (Fig. 3D and 3E). There was no significant difference between genotypes with respect to total locomotor activity (Fig. 3F).
Fig. 3.
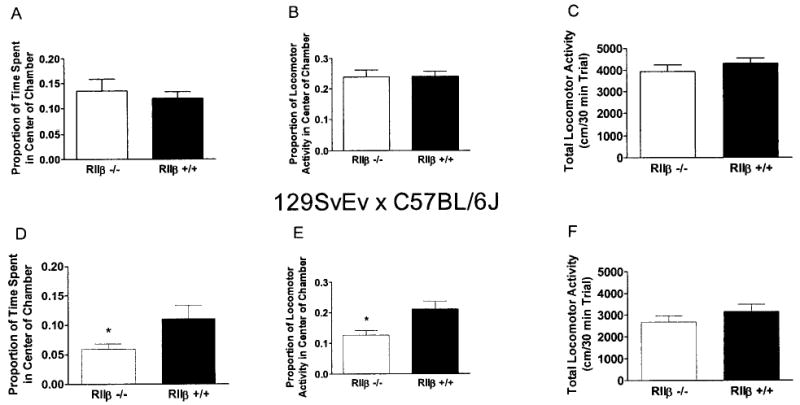
Open-field locomotor activity by RIIβ−/− and RIIβ+/+ mice maintained on either a C57BL/6J (A–C) or a 129/SvEv × C57BL/6J (D–F) genetic background. Data from 30-min test sessions are expressed as the proportion of total time spent in the center of the chamber (A and D), the proportion of total activity that occurred in the center chamber (B and E), and total locomotor activity expressed as centimeters traveled during the 30-min test (C and F). All values reported are mean ± SEM (p < 0.05 relative to RIIβ+/+ mice).
Zero Maze Testing
Zero maze data are presented in Fig. 4. There were no significant differences between RIIβ−/− mice and RIIβ+/+ mice in the proportion of time spent in the open arm or open-arm entries, regardless of the genetic background.
Fig. 4.
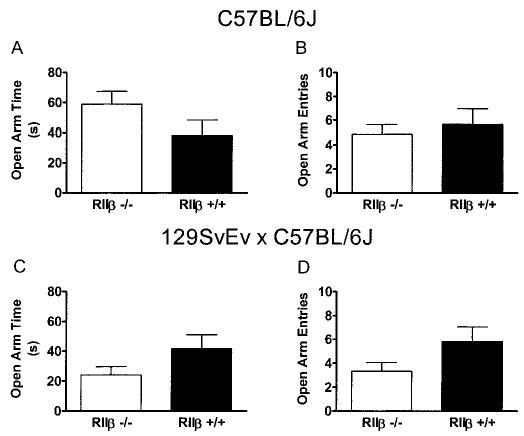
Elevated zero maze performance by RIIβ−/− and RIIβ+/+ mice maintained on either a C57BL/6J (A and B) or a 129/SvEv × C57BL/6J (C and D) genetic background. Data from the 5-min test session are expressed as the proportion of time spent in the open arm (A and C) and the number of open-arm entries (B and D). All values reported are mean ± SEM. There were no significant genotype differences.
Ethanol-Induced Sedation Testing
Results from the ethanol-induced sedation test are presented in Fig. 5. In C57BL/6J mice, there was a significant main effect of genotype [F(1,39) = 7.85]: the RIIβ−/− mice regained their righting reflex in less time than RIIβ+/+ mice (44.10 ± 7.29 min versus 73.60 ± 7.56 min, respectively). A similar main effect of genotype [F(1,39) = 6.52] was observed in mice maintained on the 129/SvEv × C57BL/6J background. RIIβ−/− mice regained their righting reflex in less time than RIIβ+/+ mice (42.64 ± 7.11 min versus 69.43 ± 8.51 min, respectively). As mentioned previously, mice on the mixed genetic background had previous experience with pentobarbital injection. The RIIβ−/− mice were found to be more sensitive to pentobarbital-induced sedation [F(1,39) = 4.70] compared with the RIIβ+/+ mice (68.78 ± 8.29 min versus 44.04 ± 7.89 min sleep time, respectively). For this reason, the possibility of cross-tolerance seems unlikely because of the opposite direction of the results. In addition, sleep times were quite similar to those observed previously in RIIβ−/− mice maintained on a similar genetic background (Thiele et al., 2000b).
Fig. 5.
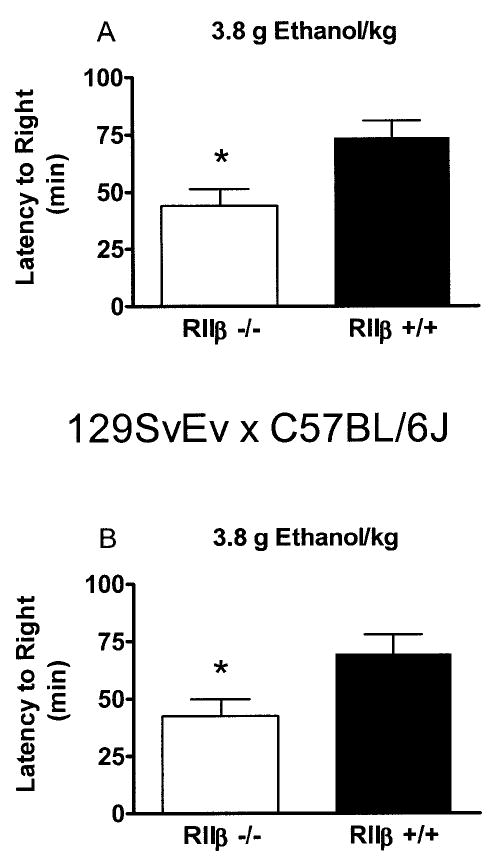
Time to regain the righting reflex after the injection of ethanol (3.8 g/kg intraperitoneally) by RIIβ−/− and RIIβ+/+ mice maintained on a C57BL/6J genetic background. Values are reported as mean ± SEM (p < 0.05 relative to RIIβ+/+ mice).
Blood Ethanol Assessments
Figure 6 depicts blood ethanol levels over the 7-hr monitoring period. As extrapolated from breath samples, blood ethanol levels seemed to increase rapidly for all animals within minutes and peaked within the first 15 to 20 min. Levels then fell to nearly zero within 6 to 7 hr. ANOVA revealed significant effects of postinjection time, as expected because of normal absorption and metabolism [F(9,72) = 238.76]. There was a significant sex × postinjection time interaction that seemed primarily due to the more rapid decline of blood ethanol levels in female mice [F(9,72) = 2.63]. However, there were no significant main effects of genotype status or sex and no other significant interactions.
Fig. 6.
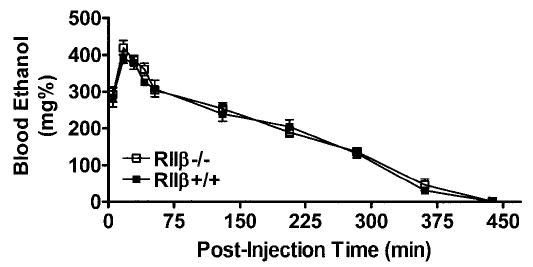
Blood ethanol levels from RIIβ−/− and RIIβ+/+ mice maintained on a C57BL/6J background after injection of ethanol (3.8 g/kg intraperitoneally). Breath samples were taken at various time points after ethanol administration until ethanol was no longer detected. All values reported are mean ± SEM. There were no significant genotype differences.
DISCUSSION
Consistent with prior research (Thiele et al., 2000b), RIIβ−/− mice drank significantly more ethanol and had greater ethanol preference relative to RIIβ+/+ mice. A growing body of literature is emerging indicating that phenotypes, including neurobiological responses to ethanol, can depend on the genetic background of the knock-out model (Bowers et al., 1999; Howe et al., 2002; Kelly et al., 1998; Palmer et al., 2003; Phillips et al., 1999; Simpson et al., 1997; Thiele et al., 2000a). It is important to note that because male and female RIIβ−/− mice maintained on the C57BL/6J, 129/SvEv × C57BL/6J, and 129/SvJ × C57BL/6J (Thiele et al., 2000b) genetic backgrounds show increased consumption and preference for ethanol, the contribution of the RIIβ subunit of PKA to ethanol intake does not seem to be genetic background or sex dependent. Furthermore, this study shows that male and female RIIβ−/− mice on each genetic background tested are resistant to ethanol-induced sedation relative to RIIβ+/+ mice. We have previously shown that RIIβ−/− mice on a mixed 129/SvJ × C57BL/6J background are resistant to ethanol-induced sedation (Thiele et al., 2000b); therefore, reduced sensitivity to the intoxicating effects of ethanol is an additional phenotype observed on multiple genetic backgrounds in both male and female RIIβ−/− mice. Taken together, these findings strengthen the argument that the RIIβ subunit of PKA is a key mediator of neurobiological responses to ethanol.
Increased consumption of ethanol by the RIIβ−/− mice does not seem to be related to the taste or caloric properties of ethanol, because these mice show normal consumption of solutions containing either sucrose or quinine and have normal food intake (Thiele et al., 2000b). Furthermore, increased consumption of ethanol and reduced sensitivity to ethanol-induced sedation are not related to alterations of ethanol metabolism, because RIIβ−/− mice on the C57BL/6J background in this study and on the mixed 129/SvJ × C57BL/6J background (Thiele et al., 2000b) show normal blood ethanol levels relative to RIIβ+/+ mice. Thus, we conclude that the increased consumption of ethanol and resistance to ethanol-induced sedation reflect altered sensitivity to the pharmacological effects of ethanol in RIIβ−/− mice.
One of the goals of this report was to identify factors that predict the high ethanol consumption characteristic of the RIIβ−/− mice. High basal levels of anxiety and increased anxiety associated with ethanol withdrawal are factors that have been suggested to drive increased ethanol intake (Bibb and Chambless, 1986; Cappell and Herman, 1972; Cornelius et al., 2003; Koob, 2003; Schuckit and Hesselbrock, 1994). Low PKA, p-CREB, and CREB-targeted gene (i.e., neuropeptide Y) activity in the central and medial amygdala are associated with increased anxiety-like behavior (Pandey et al., 2003a,b; Roy and Pandey, 2002). Because RIIβ−/− mice have blunted cAMP-induced PKA activity in the amygdala (Thiele et al., 2000b), we hypothesized that the increased ethanol drinking by RIIβ−/− mice would be associated with increased basal levels of anxiety. However, although RIIβ−/− mice on both the C57BL/6J and 129/SvEv × 129/SvEv genetic backgrounds drink more ethanol than RIIβ+/+ mice, differences in basal levels of anxiety are not consistently observed between the genotypes, as assessed by a battery of tests commonly used to assess anxiety in rodents (Choleris et al., 2001; Pellow et al., 1985; Shepherd et al., 1994). Relative to RIIβ+/+ mice, RIIβ−/− mice on the C57BL/6J background spend significantly more time and a greater proportion of their total time in the open arm of the plus maze, an outcome suggesting low levels of basal anxiety. However, male RIIβ−/− mice on the C57BL/6J × 129/SvEv background spend a smaller proportion of their total time in the open arm of the plus maze, and male and female mice on this background spend less time in the center portion of the open-field chamber—outcomes indicating that the RIIβ−/− mice on the mixed genetic background are more anxious relative to RIIβ+/+ mice. There were no genotype differences on the zero maze test regardless of the genetic background. These observations suggest that altered basal levels of anxiety (either high or low) do not consistently predict high ethanol consumption by RIIβ−/− mice. Protein kinase C (PKC) isozyme knock-outs have also been used to evaluate the role of anxiety in ethanol consumption. PKCɛ knockout mice show reduced basal anxiety-like behavior and reduced consumption and operant self-administration of ethanol relative to wild-type mice (Hodge et al., 1999, 2002; Olive et al., 2000). However, PKCγ knock-out mice show reduced basal anxiety but self-administer more ethanol than wild-type controls (Bowers and Wehner, 2001; Bowers et al., 2000). Basal anxiety is not a consistent predictor of ethanol consumption in the knock-out mice discussed previously.
It is important to point out that quantitative trait locus analyses suggest that there are multiple candidate genes for the modulation of anxiety-like behavior, and each are revealed with different testing procedures (e.g., elevated plus maze and open-field activity; Henderson et al., 2004; Turri et al., 2001). We suggest that anxiety, as measured by the battery of tests used in this study, is not predictive of ethanol consumption. It is possible, however, that different anxiety-testing procedures could produce alternative results. Additionally, mice tested with the zero maze had experience with the open-field procedure, and it cannot be ruled out that the use of strictly naive animals could have produced different results in the zero maze. Along these lines, alternate environmental factors such as lighting conditions (ambient versus low light) and time of day (light cycle versus dark cycle) could have also influenced our results. Finally, we cannot eliminate the possibility that genotype differences in anxiety could emerge after long-term ethanol consumption. Because anxiety stemming from withdrawal after chronic ethanol exposure is modulated by PKA signaling and p-CREB activity (Pandey, 2003; Pandey et al., 2001, 2003a, Pandey et al., b; Roy and Pandey, 2002), it will be important to determine whether RIIβ−/− and RIIβ+/+ mice differ in anxiety levels after ethanol withdrawal.
There is increasing evidence that high levels of voluntary ethanol consumption are associated with resistance to the intoxicating effects produced by this drug, and vice versa (Choi et al., 2002; Harris et al., 1995; Hodge et al., 1999; Thiele et al., 1998, 2002; Wand et al., 2001; Weinshenker et al., 2000). Furthermore, this relationship has also been reported in the human research literature (Schuckit, 1994). Thus, we determined whether male and female RIIβ−/− mice on the C57BL/6J and 129/SvEv × C57BL/6J backgrounds are resistant to the sedative effects of ethanol, as is observed in RIIβ−/− mice maintained on the C57BL/6J × 129/SvJ mixed background (Thiele et al., 2000b). Consistent with previous observations, RIIβ−/− mice regain their righting reflex in less time than RIIβ+/+ mice after intra-peritoneal injection of a 3.8 g/kg dose of ethanol, regardless of sex or genetic background. Thus, because RIIβ−/− mice drink high amounts of ethanol and regain their righting reflex significantly faster than RIIβ+/+ mice in all cases, resistance to the sedative effects of ethanol seems to be a good predictor of high ethanol drinking. It will be important to study the RIIβ−/− mice with a range of behavioral tests to more fully characterize the extent to which these mice are resistant to the intoxicating effects of ethanol (Crabbe et al., 2003; Rustay et al., 2003).
Since ethanol-associated phenotypes were first described in RIIβ−/− mice (Thiele et al., 2000b), other mutant models have been characterized and support a role for endogenous cAMP/PKA signaling in neurobiological responses to ethanol (Moore et al., 1998; Park et al., 2000; Rodan et al., 2002; Wand et al., 2001; Yang et al., 2003). These results reinforce the argument that the RIIβ subunit of PKA is a key mediator of voluntary ethanol consumption; however, the mechanism involved remains unclear. It has been suggested that constitutive PKA activation promotes increased ethanol consumption and resistance to the acute effects of ethanol in RIIβ−/− mice (Thiele et al., 2000b). PKA activation occurs when cAMP binds to the R subunit of the PKA complex, a process that liberates catalytically active C subunits (Brandon et al., 1997). Increases in expression of RIα and RIβ do not compensate fully for the loss of RIIβ (Amieux et al., 1997; Brandon et al., 1998); therefore, an increased proportion of active C subunits are chronically unregulated in RIIβ−/− mice to produce a state of constitutive PKA activation. Reduced cAMP-induced PKA activity in striatum, amygdala, and hippocampus (Thiele et al., 2000b) occurs because R subunits also protect C subunits from proteolysis (Hemmings, 1986). Unbound (i.e., active) C subunits are more rapidly degraded in RIIβ−/− mice, and this leads to dramatic decreases in steady-state levels of both Cα and Cβ (Brandon et al., 1998). In addition to possible constitutive PKA activity, it could be that activity downstream of the PKA mutation is blunted in RIIβ−/− mice. In fact, RIIβ−/− mice demonstrate low expression of several CREB-targeted genes in the striatum, including low basal levels of dynorphin messenger RNA (Brandon et al., 1998), an absence of haloperidol- and amphetamine-induced c-fos messenger RNA (Adams et al., 1997; Brandon et al., 1998), and a lack of haloperidol-induced neurotensin messenger RNA (Adams et al., 1997). Dynorphin (Lindholm et al., 2001; Sandi et al., 1988), neurotensin (Ehlers et al., 1999; Erwin et al., 1994, 2001), and c-fos (Bachtell et al., 1999; Ryabinin et al., 2003) have all been implicated in the modulation of neurobiological responses to ethanol; thus, high ethanol consumption and resistance to the intoxicating effects of ethanol by RIIβ−/− mice may reflect a perturbation to these or other (e.g., neuropeptide Y; Pandey et al., 2003b) CREB-targeted systems.
In summary, we extend previous results by showing that male and female RIIβ−/− mice on multiple genetic backgrounds consume more ethanol and are resistant to the sedative effects of ethanol relative to RIIβ+/+ mice. Basal anxiety, as determined by a battery of behavioral tests, is not a reliable predictor of high ethanol drinking by RIIβ−/− mice. However, resistance to the sedative effects of ethanol seems to be a reliable predictor of high ethanol consumption. Future experiments will study the RIIβ−/− mice with a range of behavioral tests to more fully characterize the extent to which these mice are resistant to the intoxicating effects of ethanol (Crabbe et al., 2003; Rustay et al., 2003).
Acknowledgments
We thank G. Stanley McKnight for providing the RIIβ−/− mice.
Footnotes
Supported by NIH Grants AA13573, DA10277, and AA011605.
References
- Adams MR, Brandon EP, Chartoff EH, Idzerda RL, Dorsa DM, McK-night GS. Loss of haloperidol induced gene expression and catalepsy in protein kinase A-deficient mice. Proc Natl Acad Sci USA. 1997;94:12157–12161. doi: 10.1073/pnas.94.22.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amieux PS, Cummings DE, Motamed K, Brandon EP, Wailes LA, Le K, Idzerda RL, McKnight GS. Compensatory regulation of RIalpha protein levels in protein kinase A mutant mice. J Biol Chem. 1997;272:3993–3998. doi: 10.1074/jbc.272.7.3993. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Wang YM, Freeman P, Risinger FO, Ryabinin AE. Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res. 1999;847:157–165. doi: 10.1016/s0006-8993(99)02019-3. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bibb JL, Chambless DL. Alcohol use and abuse among diagnosed agoraphobics. Behav Res Ther. 1986;24:49–58. doi: 10.1016/0005-7967(86)90149-x. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, Collins AC, Tritto T, Wehner JM. Mice lacking PKC gamma exhibit decreased anxiety. Behav Genet. 2000;30:111–121. doi: 10.1023/a:1001951104208. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, Owen EH, Collins AC, Abeliovich A, Tonegawa S, Wehner JM. Decreased ethanol sensitivity and tolerance development in gamma-protein kinase C null mutant mice is dependent on genetic background. Alcohol Clin Exp Res. 1999;23:387–397. [PubMed] [Google Scholar]
- Bowers BJ, Wehner JM. Ethanol consumption and behavioral impulsivity are increased in protein kinase Cgamma null mutant mice. J Neurosci. 2001;21:RC180. doi: 10.1523/JNEUROSCI.21-21-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon EP, Idzerda RL, McKnight GS. PKA isoforms, neural pathways, and behaviour: making the connection. Curr Opin Neurobiol. 1997;7:397–403. doi: 10.1016/s0959-4388(97)80069-4. [DOI] [PubMed] [Google Scholar]
- Brandon EP, Logue SF, Adams MR, Qi M, Sullivan SP, Matsumoto AM, et al. Defective motor behavior and neural gene expression in RIIbeta-protein kinase A mutant mice. J Neurosci. 1998;18:3639–3649. doi: 10.1523/JNEUROSCI.18-10-03639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappell H, Herman CP. Alcohol and tension reduction. A review. Q J Stud Alcohol. 1972;33:33–64. [PubMed] [Google Scholar]
- Choi DS, Wang D, Dadgar J, Chang WS, Messing RO. Conditional rescue of protein kinase C epsilon regulates ethanol preference and hypnotic sensitivity in adult mice. J Neurosci. 2002;22:9905–9911. doi: 10.1523/JNEUROSCI.22-22-09905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Constantinescu A, Gordon AS, Diamond I. cAMP-dependent protein kinase types I and II differentially regulate cAMP response element-mediated gene expression: implications for neuronal responses to ethanol. J Biol Chem. 2002;277:18810–18816. doi: 10.1074/jbc.M112107200. [DOI] [PubMed] [Google Scholar]
- Contet C, Rawlins JN, Deacon RM. A comparison of 129S2/SvHsd and C57BL/6JOlaHsd mice on a test battery assessing sensorimotor, affective and cognitive behaviours: implications for the study of genetically modified mice. Behav Brain Res. 2001;124:33–46. doi: 10.1016/s0166-4328(01)00231-5. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Bukstein O, Salloum I, Clark D. Alcohol and psychiatric comorbidity. Recent Dev Alcohol. 2003;16:361–374. doi: 10.1007/0-306-47939-7_24. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Cotnam CJ, Cameron AJ, Schlumbohm JP, Rhodes JS, Metten P, Wahlsten D. Strain differences in three measures of ethanol intoxication in mice: the screen, dowel and grip strength tests. Genes Brain Behav. 2003;2:201–213. doi: 10.1034/j.1601-183x.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- Davis M. Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- Dohrman DP, Chen HM, Gordon AS, Diamond I. Ethanol-induced translocation of protein kinase A occurs in two phases: control by different molecular mechanisms. Alcohol Clin Exp Res. 2002;26:407–415. [PubMed] [Google Scholar]
- Dohrman DP, Diamond I, Gordon AS. Ethanol causes translocation of cAMP-dependent protein kinase catalytic subunit to the nucleus. Proc Natl Acad Sci USA. 1996;93:10217–10221. doi: 10.1073/pnas.93.19.10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Li T-K, Lumeng L, Kinkead B, Owens MJ, Nemeroff CB. Neurontensin studies in alcohol naive, preferring and non-preferring rats. Neuroscience. 1999;93:227–236. doi: 10.1016/s0306-4522(99)00113-x. [DOI] [PubMed] [Google Scholar]
- Erwin VG, Gehle VM, Davidson K, Radcliffe RA. Confirmation of correlations and common quantitative trait loci between neurotensin receptor density and hypnotic sensitivity to ethanol. Alcohol Clin Exp Res. 2001;25:1699–1707. [PubMed] [Google Scholar]
- Erwin VG, Jones BC, Myers R. Effects of acute and chronic ethanol administration on neurotensinergic systems. Ann NY Acad Sci. 1994;739:185–196. doi: 10.1111/j.1749-6632.1994.tb19820.x. [DOI] [PubMed] [Google Scholar]
- Harris RA, McQuilkin SJ, Paylor R, Abeliovich A, Tonegawa S, Wehner JM. Mutant mice lacking the gamma isoform of protein kinase C show decreased behavioral actions of ethanol and altered function of gamma-aminobutyrate type A receptors. Proc Natl Acad Sci USA. 1995;92:3658–3662. doi: 10.1073/pnas.92.9.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings BA. cAMP mediated proteolysis of the catalytic subunit of cAMP-dependent protein kinase. FEBS Lett. 1986;196:126–130. doi: 10.1016/0014-5793(86)80226-5. [DOI] [PubMed] [Google Scholar]
- Henderson ND, Turri MG, DeFries JC, Flint J. QTL analysis of multiple behavioral measures of anxiety in mice. Behav Genet. 2004;34:267–293. doi: 10.1023/B:BEGE.0000017872.25069.44. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Raber J, McMahon T, Walter H, Sanchez-Perez AM, Olive MF, Mehmert K, Morrow AL, Messing RO. Decreased anxiety-like behavior, reduced stress hormones, and neurosteroid supersensitivity in mice lacking protein kinase Cepsilon. J Clin Invest. 2002;110:1003–1010. doi: 10.1172/JCI15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe DG, Wiley JC, McKnight GS. Molecular and behavioral effects of a null mutation in all PKA C beta isoforms. Mol Cell Neurosci. 2002;20:515–524. doi: 10.1006/mcne.2002.1119. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Menninger JA, Tabakoff B. An initial study of the relationship between platelet adenylyl cyclase activity and alcohol use disorder criteria. Alcohol Clin Exp Res. 1998;22:1057–1064. [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G, et al. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci. 1998;18:3470–3479. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Saiers JA, Pohorecky LA (1993) Observations of novel behaviors as indices of ethanol withdrawal-induced anxiety, in Advances in Biomedical Alcohol Research (Taberner PV, Badaway AA eds), pp 489–493. Pergamon, New York. [PubMed]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Werme M, Brene S, Franck J. The selective kappa-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav Brain Res. 2001;120:137–146. doi: 10.1016/s0166-4328(00)00368-5. [DOI] [PubMed] [Google Scholar]
- McKnight GS. Cyclic AMP second messenger systems. Curr Opin Cell Biol. 1991;3:213–217. doi: 10.1016/0955-0674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Bandy AL, McGroarty KK. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:175–183. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- Nagy LE, Diamond I, Gordon A. Cultured lymphocytes from alcoholic subjects have altered cAMP signal transduction. Proc Natl Acad Sci USA. 1988;85:6973–6976. doi: 10.1073/pnas.85.18.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Knapp DJ, Thiele TE. MTII-induced reduction of voluntary ethanol drinking is blocked by pretreatment with AgRP-(83–132) Neuropeptides. 2003;37:338–344. doi: 10.1016/j.npep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Messing RO, Hodge CW. Reduced operant ethanol self-administration and in vivo mesolimbic dopamine responses to ethanol in PKCepsilon-deficient mice. Eur J Neurosci. 2000;12:4131–4140. doi: 10.1046/j.1460-9568.2000.00297.x. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Low MJ, Grandy DK, Phillips TJ. Effects of a Drd2 deletion mutation on ethanol-induced locomotor stimulation and sensitization suggest a role for epistasis. Behav Genet. 2003;33:311–324. doi: 10.1023/a:1023450625826. [DOI] [PubMed] [Google Scholar]
- Pandey SC. Anxiety and alcohol abuse disorders: a common role for CREB and its target, the neuropeptide Y gene. Trends Pharmacol Sci. 2003;24:456–460. doi: 10.1016/S0165-6147(03)00226-8. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Carr LG, Heilig M, Ilveskoski E, Thiele TE. Neuropeptide Y and alcoholism: genetic, molecular, and pharmacological evidence. Alcohol Clin Exp Res. 2003a;27:149–154. doi: 10.1097/01.ALC.0000052706.21367.0E. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Mittal N. Effects of chronic ethanol intake and its withdrawal on the expression and phosphorylation of the creb gene transcription factor in rat cortex. J Pharmacol Exp Ther. 2001;296:857–868. [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H. The decreased phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats. Alcohol Clin Exp Res. 2003b;27:396–409. doi: 10.1097/01.ALC.0000056616.81971.49. [DOI] [PubMed] [Google Scholar]
- Park SK, Sedore SA, Cronmiller C, Hirsh J. Type II cAMP-dependent protein kinase-deficient Drosophila are viable but show developmental, circadian, and drug response phenotypes. J Biol Chem. 2000;275:20588–20596. doi: 10.1074/jbc.M002460200. [DOI] [PubMed] [Google Scholar]
- Parsian A, Todd RD, Cloninger CR, Hoffman PL, Ovchinnikova L, Ikeda H, Tabakoff B. Platelet adenylyl cyclase activity in alcoholics and subtypes of alcoholics. WHO/ISBRA Study Clinical Centers. Alcohol Clin Exp Res. 1996;20:745–751. doi: 10.1111/j.1530-0277.1996.tb01681.x. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Hen R, Crabbe JC. Complications associated with genetic background effects in research using knockout mice. Psychopharmacology. 1999;147:5–7. doi: 10.1007/s002130051128. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Brick J. A new method for the determination of blood ethanol levels in rodents. Pharmacol Biochem Behav. 1982;16:693–696. doi: 10.1016/0091-3057(82)90219-2. [DOI] [PubMed] [Google Scholar]
- Rodan AR, Kiger JA, Jr, Heberlein U. Functional dissection of neuroanatomical loci regulating ethanol sensitivity in Drosophila. J Neurosci. 2002;22:9490–9501. doi: 10.1523/JNEUROSCI.22-21-09490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Pandey SC. The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcohol Clin Exp Res. 2002;26:796–803. [PubMed] [Google Scholar]
- Rustay NR, Wahlsten D, Crabbe JC. Assessment of genetic susceptibility to ethanol intoxication in mice. Proc Natl Acad Sci USA. 2003;100:2917–2922. doi: 10.1073/pnas.0437273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Galvan-Rosas A, Bachtell RK, Risinger FO. High alcohol/sucrose consumption during dark circadian phase in C57BL/6J mice: involvement of hippocampus, lateral septum and urocortin-positive cells of the Edinger-Westphal nucleus. Psychopharmacology. 2003;165:296–305. doi: 10.1007/s00213-002-1284-y. [DOI] [PubMed] [Google Scholar]
- Sandi C, Borrell J, Guaza C. Involvement of kappa type opioids on ethanol drinking. Life Sci. 1988;42:1067–1075. doi: 10.1016/0024-3205(88)90562-0. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Hesselbrock V. Alcohol dependence and anxiety disorders: what is the relationship? Am J Psychiatry. 1994;151:1723–1734. doi: 10.1176/ajp.151.12.1723. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology. 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Koh MT, Pedrazzini T. Voluntary alcohol consumption is controlled via the neuropeptide Y Y1 receptor. J Neurosci. 2002;22:RC208. doi: 10.1523/JNEUROSCI.22-03-j0006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Miura GI, Marsh DJ, Bernstein IL, Palmiter RD. Neurobiological responses to ethanol in mutant mice lacking neuropeptide Y or the Y5 receptor. Pharmacol Biochem Behav. 2000a;67:683–691. doi: 10.1016/s0091-3057(00)00413-5. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci. 2000b;20:RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turri MG, Datta SR, DeFries J, Henderson ND, Flint J. QTL analysis identifies multiple behavioral dimensions in ethological tests of anxiety in laboratory mice. Curr Biol. 2001;11:725–734. doi: 10.1016/s0960-9822(01)00206-8. [DOI] [PubMed] [Google Scholar]
- Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271–281. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- Wand G, Levine M, Zweifel L, Schwindinger W, Abel T. The cAMP-protein kinase A signal transduction pathway modulates ethanol consumption and sedative effects of ethanol. J Neurosci. 2001;21:5297–5303. doi: 10.1523/JNEUROSCI.21-14-05297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Lui J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci USA. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Rust NC, Miller NS, Palmiter RD. Ethanol-associated behaviors of mice lacking norepinephrine. J Neurosci. 2000;20:3157–3164. doi: 10.1523/JNEUROSCI.20-09-03157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Michels KM (1991) Statistical Principles in Experimental Design 3rd ed. McGraw-Hill, New York.
- Yang X, Oswald L, Wand G. The cyclic AMP/protein kinase A signal transduction pathway modulates tolerance to sedative and hypothermic effects of ethanol. Alcohol Clin Exp Res. 2003;27:1220–1225. doi: 10.1097/01.ALC.0000081626.02910.19. [DOI] [PubMed] [Google Scholar]


