Abstract
Background
Neuropeptide Y (NPY) is a 36–amino acid neuromodulator that is expressed throughout the central nervous system. Recent genetic and pharmacological evidence suggests that the NPY Y1 receptor modulates ethanol intake. To further characterize the role of the Y1 receptor, we examined voluntary ethanol consumption by mice after administration of [(−)-2-[1-(3-chloro-5-isopropyloxycarbonylaminophenyl)ethylamino]-6-[2-(5-ethyl-4-methyl-1,3-tiazol-2-yl)ethyl]-4-morpholinopyridine] (compound A), a novel and selective Y1 receptor antagonist (Y1RA) that acts centrally on brain receptors when administered peripherally.
Methods
C57BL/6J mice were habituated to drinking a 10% (v/v) ethanol solution by using a two-bottle-choice procedure and were then given an intraperitoneal (ip) injection (5 ml/kg) of the Y1RA (0, 25, 50, or 75 mg/kg). In a second study, mice were given intracerebroventricular infusion of the Y1RA (0, 30, or 100 μg). Finally, we determined whether the Y1RA alters open-field locomotor activity, ethanol-induced sedation (3.8 g/kg, ip), or blood ethanol levels.
Results
Relative to control treatment, ip injection (50 and 75 mg/kg) and intracerebroventricular infusion (100 μg) of the Y1RA significantly reduced ethanol consumption and food intake without altering water drinking. However, the Y1RA did not alter open-field locomotor activity, ethanol-induced sedation, or blood ethanol levels.
Conclusions
These data indicate that acute blockade of the NPY Y1 receptor with a systemically bioavailable NPY Y1RA reduces voluntary ethanol consumption by C57BL/6J mice. These results are consistent with observations that hypothalamic infusion of NPY increases ethanol drinking by rats.
Keywords: Neuropeptide Y, Y1 Receptors, Alcohol, Voluntary Consumption, Sedation
NEUROPEPTIDE Y (NPY) is a 36–amino acid neuromodulator belonging to the PP-fold family of peptides (Berglund et al., 2003; Colmer and Wahlestedt, 1993; Dumont et al., 1992) and is expressed throughout the central nervous system (Gray and Morley, 1986). NPY is involved with a diverse set of biological functions and has been implicated in the control of food intake (Clark et al., 1984; Levine and Morley, 1984), neuronal development (Hansel et al., 2001a,b), seizure activity (Woldbye et al., 1996,1997), cardiovascular homeostasis (Pedrazzini et al., 1998), the integration of emotional behavior (Heilig et al., 1993; Heilig and Widerlov, 1995), thermogenesis (Lopez-Valpuesta et al., 1996), circadian rhythms (Biello et al., 1997; Golombek et al., 1996; Gribkoff et al., 1998; Harrington and Schak, 2000), pain modulation (Shi et al., 1999, 2001), and reproduction (Kalra et al., 1998; Kasuya et al., 1998). In the mouse, NPY acts through at least five receptor subtypes—the Y1, Y2, Y4, Y5, and Y6 receptors—all of which couple to G proteins that inhibit production of cyclic adenosine monophosphate (Palmiter et al., 1998).
In recent years, evidence has emerged that shows that NPY is also involved with neurobiological responses to ethanol and drugs of abuse (Pandey et al., 2003a; Thiele and Badia-Elder, 2003; Thiele et al., 2003a). Administration of ethanol and ethanol withdrawal alter central NPY expression in rodents (Bison and Crews, 2003; Clark et al., 1998; Ehlers et al., 1998; Kinoshita et al., 2000; Roy and Pandey, 2002; Thiele et al., 2000a), and intracerebroventricular (icv) infusion of NPY significantly attenuates ethanol withdrawal responses in Wistar rats (Woldbye et al., 2002). NPY also modulates neurobiological responses to ethanol. Voluntary ethanol consumption and resistance to the intoxicating effects of ethanol are inversely related to NPY levels in knockout and transgenic mice (Thiele et al., 1998), and a lack of NPY is associated with increased sensitivity to the locomotor stimulant effects of ethanol (Thiele et al., 2000b). However, ethanol-associated phenotypes are not consistently observed in NPY knockout mice and are dependent on the genetic background (Thiele et al., 2000b). A genetic linkage analysis conducted in F2 inter-cross progenies of selectively bred alcohol-preferring (P) and -nonpreferring (NP) rat lines identified a chromosomal region that includes the gene for the NPY precursor (Bice et al., 1998; Carr et al., 1998; but see Foroud et al., 2000). It was later found that P rats, as well as high-alcohol-drinking (HAD) rats, have low levels of NPY in the amygdala when compared with controls (Ehlers et al., 1998; Hwang et al., 1999). It is interesting to note that icv administration of NPY reduces ethanol drinking in P and HAD rats, but not in NP rats, low-alcohol-drinking (LAD) rats, or outbred Wistar rats (Badia-Elder et al., 2001, 2003; Gilpin et al., 2003; Katner et al., 2002a,b; Slawecki et al., 2000).
Evidence has implicated the NPY Y1 receptor in the modulation of ethanol consumption. The Y1 receptor is located postsynaptically and is found in brain regions that are involved with neurobiological responses to ethanol, including the hippocampus, the hypothalamus, and the amygdala (Naveilhan et al., 1998). Y1 receptor knock-out mice (Y1−/−) show increased consumption of solutions containing 3, 6, and 10% (v/v) ethanol and are less sensitive to the sedative effects of ethanol (3.5 and 4.0 g/kg), as measured by more rapid recovery from ethanol-induced sleep (Thiele et al., 2002). Recent pharmacological studies have also confirmed a role for the Y1 receptor in ethanol consumption (Kelley et al., 2001; Schroeder et al., 2003b).
The purpose of this study was to further characterize the role of the Y1 receptor in neurobiological responses to ethanol. To this end, we examined voluntary consumption of ethanol and ethanol-induced sedation in C57BL/6J mice after icv and intraperitoneal (ip) injection of [(−)-2-[1-(3-chloro-5-isopropyloxycarbonylaminophenyl)ethylamino]-6-[2-(5-ethyl-4-methyl-1,3-tiazol-2-yl)ethyl]-4-morpholinopyridine] (compound A), a novel and selective Y1 receptor antagonist (Y1RA) that acts centrally on brain receptors when administered peripherally (Kanatani et al., 2001).
MATERIALS AND METHODS
Subjects
Subjects were C57BL/6J (Jackson Laboratory, Bar Harbor, ME) mice, each weighing between 25 and 30 g at the beginning of the experiment. All mice were individually housed in plastic mouse cages with free access to standard rodent chow (Teklad, Madison WI) and water except where noted. Mice were approximately 16 weeks old at the start of the experiment. The colony room was maintained at approximately 22°C with a 12-hr/12-hr light/dark cycle. All procedures were in compliance with NIH guidelines, and the protocols were approved by the University of North Carolina Animal Care and Use Committee.
Drug Preparation
Compound A was donated by Merck & Co., Inc. (Rahway, NJ), and Banyu Pharmaceutical Co., Ltd (Tsukuba, Japan). On the basis of binding affinity data, compound A is more than 7000-fold selective for the human Y1 receptor over the human Y2 receptor and more than 4000-fold selective for the human Y1 receptor over the human Y5 receptor. Furthermore, compound A is without effect in Y1−/− mice (Kanatani et al., 2001). Hereafter, we also refer to compound A as the Y1RA. For studies involving ip injection of drug, the Y1RA was prepared in a vehicle of 0.5% carboxymethylcellulose (CMC) in sterile water as described elsewhere (Kanatani et al., 1999). For icv infusion, the Y1RA was prepared in a vehicle of 50% propylene glycol (PG) in sterile water (Kanatani et al., 2001).
Voluntary Ethanol Consumption After ip Injection of the Y1RA
Thirty-nine naive mice were acclimated to drinking from two bottles for approximately 2 weeks (continuous 24-hr access). One bottle contained water, and the other contained an ethanol solution (3% v/v for 4 days, 6% for 4 days, and 10% for 6 days). Bottle positions were changed every 2 days to control for position preference. Food was available ad libitum throughout the experiment except where noted, and body weights were recorded weekly. On the test day, mice were distributed into one of four groups based on their consumption of the 10% ethanol solution. Two hours before the beginning of the dark cycle, food, water, and ethanol were removed from the cages, and mice were weighed. Approximately 1 hr before the dark phase, mice were given an ip injection of CMC (n = 10) or a 25 mg/kg (n = 10), 50 mg/kg (n = 9), or 75 mg/kg (n = 10) dose of the Y1RA. All doses were injected in a volume of 5 ml/kg body weight. Just before the dark cycle, food, water, and 10% ethanol solution were put back in the cages. Measurements of food consumed, water consumed, and 10% ethanol consumed were collected every 2 hr up to 8 hr.
Voluntary Ethanol Consumption After icv Infusion of the Y1RA
Thirty-three naive mice were anesthetized with a solution containing ketamine (117 mg/kg) and xylazine (7.92 mg/kg) and surgically implanted with a 26-gauge cannula (Plastics One, Roanoke, VA) aimed at the left lateral ventricle by using the following stereotaxic coordinates: 0.2 mm posterior to the bregma, 1.0 mm lateral to the midline, and 2.3 mm ventral to the skull surface. After surgery, mice were allowed to recover for approximately 2 weeks. After experimental procedures, cannula placement was verified histologically. Mice were habituated to the two-bottle-choice procedure described previously and distributed into three groups equated for consumption of the 10% ethanol solution. Two hours before the beginning of the dark cycle, food, water, and ethanol were removed from the cages, and mice were weighed. One hour later, mice were given icv injection of PG (n = 10), 30 μg of Y1RA (n = 11), or 100 μg of Y1RA (n = 12). Intracerebroventricular infusions were given in a 1.0-μl volume with a 5.0-μl Hamilton syringe and infused over 1 min with a Harvard Apparatus PHD 2000 microinfusion pump (Holliston, MA). Just before the dark cycle, food, water, and 10% ethanol solution were put back in the cages. Measurements of food consumed, water consumed, and 10% ethanol consumed were collected every 2 hr for up to 8 hr.
Open-Field Locomotor Activity After ip Injection of the Y1RA
To determine whether the Y1RA could promote motor activity impairment, we measured open-field locomotor activity after ip administration of compound A. Thirty-eight mice were distributed into one of three groups equated for body weight. Mice were removed from their cages and given an ip injection of a 25 or 50 mg/kg dose of Y1RA (n = 13 per group) or an equal volume of CMC (n = 12). Twenty-five minutes after infusion, mice were individually tested for 5 min in an open-field apparatus that automatically recorded locomotor activity (Harvard Apparatus). Testing was conducted 25 min after icv infusion because the Y1RA reaches maximal brain levels within approximately 30 min after ip administration (Kanatani et al., 2001). Locomotor activity was expressed as centimeters traveled during the 5-min session.
Ethanol-Induced Sedation After ip Injection of the Y1RA
Immediately after testing in the open-field activity chamber (30 min after injection of the Y1RA), all mice used in the previous procedure were given ip injection of ethanol (3.8 g/kg; 19% w/v mixed in isotonic saline), a dose that is within a range effective for inducing sedation in C57BL/6 mice (Thiele et al., 2002, 2003b). At the onset of sedation, each mouse was placed on its back in a plastic U-shaped trough. The time (minutes) that elapsed between the ethanol injection and when the mouse could right itself onto all four paws three times within a 30-sec interval was used as the index of time to regain the righting reflex.
Blood Ethanol Concentrations After ip Injection of the Y1RA
To determine whether the Y1RA alters clearance of ethanol from blood, blood samples were collected from the mice described previously immediately after they regained their righting reflex. Mouse tails were cleaned, and sterile single-blade razors were used to make small tail-tip nicks for blood collection. Blood ethanol samples were analyzed with gas chromatographic methods described elsewhere (Knapp et al., 1993). Tail blood (6 μl) and standards (6 μl; 0 –300 mg/100 ml) were mixed with 375 μl of distilled water and 0.5 g of NaCl in 12 × 75-mm borosilicate glass culture tubes. The tubes were capped and then heated at 55°C for 10 min in a water bath, at which point 1.5 ml of headspace gas was removed with a plastic 3.0-ml syringe and injected directly into an SRI 8610C gas chromatograph (Torrance, CA) equipped with an external syringe adapter and a 1.0-ml external loading loop. The oven temperature was isothermal at 140°C and contained a Hayesep D column and a flame ionization detector. Hydrogen gas, carrier gas (also hydrogen), and internal air generator flow rates were 13.3, 25, and 250 ml/min, respectively. Peak retention time was 2 min, and the areas under the curve were analyzed with SRI PeakSimple software for Windows running on a Dell (Austin, TX) Inspiron 3500 laptop computer.
Data Analyses
All data in this report are presented as mean ± SEM. We used ANOVA for statistical examination of all data with SPSS for Windows, version 11.5 (SPSS Inc., Chicago, IL); t tests were used for planned comparisons (Winer et al., 1991). Significance was accepted at p < 0.05 (two tailed).
RESULTS
Voluntary Ethanol Consumption After ip Injection of the Y1RA
Relative to mice given ip injection of the vehicle CMC, ip injection of the selective Y1RA significantly reduced ethanol consumption up to 8 hr [F(3,35) = 6.34; p = 0.002; Fig. 1A]. Planned comparisons revealed that the 50 and 75 mg/kg doses of the Y1RA significantly reduced ethanol consumption but that the 25 mg/kg dose failed to alter ethanol drinking. None of the doses of the Y1RA tested significantly altered water drinking [F(3,35) = 2.34; p = 0.09; Fig. 1B]. Relative to CMC treatment (96.88 ± 4.62 g/kg), the 25 mg/kg (74.15 ± 5.41 g/kg), 50 mg/kg (72.15 ± 4.55 g/kg), and 75 mg/kg (76.58 ± 6.65 g/kg) doses of the Y1RA significantly reduced food intake for up to 6 hr [F(3,35) = 4.48; p = 0.009] but not at the 8-hr measurements [F(3,35) = 2.32; p = 0.09; Fig. 1C].
Fig. 1.
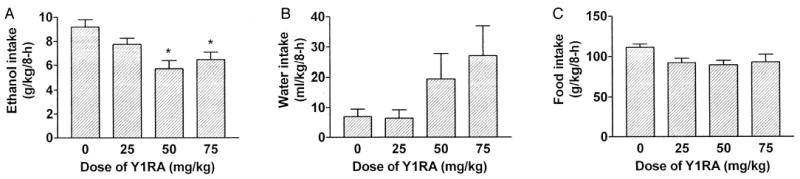
Consumption of 10% (v/v) ethanol (A), water (B), and food (C) over an 8-hr test after ip injection of the NPY Y1 receptor antagonist (Y1RA) compound A (0, 25, 50, or 75 mg/kg). All values are means ± SEM; *p < 0.05 relative to the control group.
Voluntary Ethanol Consumption After icv Infusion of the Y1RA
As seen in Fig. 2A, icv infusion of the 100-μg dose of the Y1RA significantly reduced 8-hr ethanol consumption relative to mice given icv infusion of PG [F(2,30) = 3.89; p = 0.03]. Planned comparisons confirmed that the 100-μg, but not the 30-μg, dose of the Y1RA significantly reduced ethanol consumption. It should be noted that the absolute level of ethanol consumption by control treated mice in this study was lower than consumption by controls in the previous ip injection study. Consumption differences between studies may be related to the different routes of administration or the different vehicles used. It is important to note that water consumption was not significantly altered by the icv treatment [F(2,30) = 1.74; p = 0.19; Fig. 2B]. Finally, icv administration of a 100-μg dose of the Y1RA significantly decreased 8-hr food consumption [F(2,30) = 4.23; p = 0.024], and planned comparisons confirmed this conclusion (Fig. 2C).
Fig. 2.
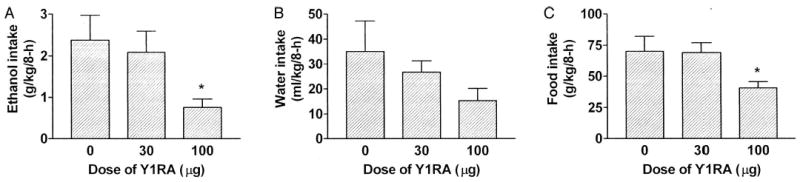
Consumption of 10% (v/v) ethanol (A), water (B), and food (C) over an 8-hr test after icv infusion of the NPY Y1 receptor antagonist (Y1RA) compound A (0, 30, or 100 μg). All values are means ± SEM; *p < 0.05 relative to the control group.
Open-Field Locomotor Activity, Ethanol-Induced Sedation, and Blood Ethanol Levels After ip Injection of the Y1RA
Relative to CMC, neither the 25 mg/kg nor the 50 mg/kg dose of the Y1RA significantly altered 5-min open-field locomotor activity when tested 25 min after ip injection [F(2,35) = 0.35; p = 0.71; Fig. 3A]. Similarly, neither dose of the Y1RA significantly altered ethanol-induced sedation after a 3.8 g/kg dose [F(2,31) = 0.64; p = 0.53; Fig. 3B]. Finally, blood ethanol levels taken immediately upon recovery of the righting reflex did not differ significantly between mice injected with CMC or either dose of the Y1RA [F(2,35) = 0.49; p = 0.64; Fig. 3C].
Fig. 3.

Open-field locomotor activity (A), latency to regain the righting reflex after ethanol injection (3.8 g/kg ip) as a measurement of sensitivity to ethanol’s sedative effects (B), and blood ethanol levels at the time the righting reflex was established (C) in mice after ip injection of the NPY Y1 receptor antagonist (Y1RA) compound A (0, 25, or 50 mg/kg). All values are means ± SEM.
DISCUSSION
Here we provide pharmacological evidence that the NPY Y1 receptor modulates ethanol consumption in mice. The ip injection of the Y1RA (50 and 75 mg/kg) selectively reduces 8-hr ethanol consumption without altering water drinking. Similarly, when given in an icv infusion, a 100-μg dose of the Y1RA significantly reduces ethanol, but not water, drinking for up to 8 hr. These results strengthen the argument that the Y1RA modulates ethanol consumption by acting centrally. Reduction of ethanol drinking caused by the Y1RA does not seem to be related to alteration of locomotor behavior or augmentation of ethanol’s sedative effects, because ip injection of this compound does not alter open-field locomotor activity or ethanol-induced sedation. Furthermore, Y1RA-induced reduction of ethanol drinking is not likely related to a shift in the rate of ethanol metabolism, because this antagonist does not cause significant changes in plasma ethanol levels. Together, these data add to a growing body of evidence that ethanol self-administration is modulated by NPY Y1 receptors (Kelley et al., 2001; Schroeder et al., 2003b; Thiele et al., 2002). Additionally, in agreement with previous data (Kanatani et al., 2001), we showed that both ip injection (25, 50, and 75 mg/kg) and icv infusion (100 μg) of the Y1RA significantly reduced short-term food intake by C57BL/6J mice.
Y1−/− mice show increased consumption of solutions containing 3, 6, and 10% (v/v) ethanol (Thiele et al., 2002). Thus, the present pharmacological data and genetic evidence support a role for the NPY Y1 receptor in the maintenance of ethanol consumption. However, there is an obvious inconsistency between paradigms; deletion of the Y1 receptor promotes increased ethanol consumption, whereas pharmacological blockade of the Y1 receptor causes a reduction of ethanol intake. Procedures used in this study are similar to those used with Y1−/− mice (Thiele et al., 2002). For example, mice were on a C57BL/6 background, a two-bottle-choice procedure with 10% ethanol was used, and mice had ad libitum access to food. Although developmental compensation by Y1−/− mice cannot be ruled out, there are other explanations for these divergent results.
First, deletion of the Y1 receptor gene in Y1−/− mice is a chronic lifelong treatment, whereas pharmacological blockade of the Y1 receptor is an acute short-term manipulation. Thus, it will be important to determine whether long-term exposure to the Y1RA can increase ethanol consumption. A second explanation is that the Y1RA reduces ethanol intake by acting at the paraventricular nucleus of the hypothalamus (PVN). It is interesting to note that PVN infusion of NPY increases ethanol drinking by Long-Evans rats, an effect that is blocked by pretreatment with the selective Y1RA BIBP-3226 (Kelley et al., 2001). More recently, PVN infusion of NPY was shown to increase ethanol drinking by selectively bred HAD rats (Gilpin et al., 2004). Because icv infusion of NPY reduces ethanol drinking in HAD rats (Badia-Elder et al., 2003), NPY must act on receptors in brain regions other than the PVN to attenuate ethanol consumption. Additional evidence that NPY signaling within the hypothalamus controls ethanol drinking is the observation that ip administration of a Y5 receptor antagonist delays the onset of ethanol-reinforced responding in mice (Schroeder et al., 2003a). The Y5 receptor is expressed mainly in the hypothalamus (Berglund et al., 2003). On the basis of these observations, we suggest that the Y1RA used in our study likely reduces ethanol drinking by acting on hypothalamic structures such as the PVN. However, disruption of NPY signaling in regions outside of the hypothalamus, such as in the nucleus accumbens (NAc) or the amygdala, likely accounts for the increased ethanol intake by Y1−/− mice (Thiele et al., 2002). In fact, Y1 receptors are expressed in the NAc (Pickel et al., 1998), high-ethanol-drinking C57BL/6J mice have significantly lower levels of NPY in the shell of the NAc when compared with low-ethanol-drinking DBA/2J mice (Misra and Pandey, 2003), and NPY modulates dopamine release in this region (Ault et al., 1998).
There is evidence that blunted NPY signaling in the amygdala increases ethanol drinking (but see Schroeder et al., 2003b). A recent report showed that amygdalar infusion of a protein kinase A inhibitor caused local reduction of NPY levels and increased ethanol drinking. Increased ethanol drinking was reduced by amygdalar coadministration of NPY (Pandey et al., 2003b). It is interesting to note that NPY does not effect ethanol consumption by rats not treated with the protein kinase A inhibitor and which have normal amygdalar NPY levels (Pandey et al., 2003b). Furthermore, P and HAD rats have low levels of NPY in the amygdala when compared with NP and LAD rats (Ehlers et al., 1998; Hwang et al., 1999), and icv administration of NPY reduces ethanol drinking in P and HAD rats but does not influence ethanol consumption in NP or LAD rats (Badia-Elder et al., 2001, 2003; Gilpin et al., 2003). Low NPY signaling in the amygdala may cause selectively bred rats to be anxious (Stewart et al., 1993), which in turn predisposes them to high ethanol drinking. Because NPY has anxiolytic properties (Heilig et al., 1989), central infusion of NPY could rescue the high ethanol drinking in selectively bred rats by reducing anxiety levels. Inconsistent with this suggestion, however, are the observations that HAD rats and high-ethanol-drinking Alko alcohol rats show normal or low anxiety-like behaviors, respectively (Badia-Elder et al., 2003; Moller et al., 1997).
One must consider the possibility that the Y1RA reduces ethanol drinking due to possible nonspecific consequences, including visceral illness, motor impairment, or anxiogenic effects (Kask et al., 1996, 1999). The effects of visceral illness or malaise seem unlikely because the Y1RA antagonist continues to significantly reduce ethanol drinking after food intake has returned to normal levels. Furthermore, water drinking is not reduced and is in fact slightly increased by ip injection of the Y1RA. Because the ethanol solution contains water and because mice treated with the Y1RA drink less ethanol, increased drinking from the water bottle by these mice likely reflects the need to maintain fluid balance. Motor impairment as an explanation for reduced ethanol drinking seems unlikely because the Y1RA does not alter locomotor activity, nor does it interact with the sedative properties of ethanol. Although possible anxiogenic effects of the Y1RA cannot be completely ruled out, one would predict that increased anxiety should uniformly attenuate all behavior, yet mice treated with the antagonist drink normal amounts of water and show normal open-field activity. Finally, it should be noted that although in vitro studies indicate that the Y1RA is highly selective (Kanatani et al., 2001), there is the possibility the Y1RA acts on other NPY receptors in vivo at high doses.
Both pharmacological and genetic data support a role for NPY and the Y1 receptor in the modulation of the sedative effects of drugs. Pretreatment with NPY augments sodium pentobarbital–, ketamine-, and ethanol-induced sedation (Naveilhan et al., 2001a,b; Thiele et al., 2003b; Yamada et al., 1996). Furthermore, Y1−/− mice are resistant to the sedative effects of ethanol and sodium pentobarbital (Naveilhan et al., 2001a; Thiele et al., 2002). Inconsistent with these previously published observations, ip injection of the Y1RA, compound A, does not alter ethanol-induced sedation. Because NPY-induced enhancement of ethanol’s sedative effects requires central administration of this peptide, it is possible that peripheral injection of the Y1RA does not gain access to the central Y1 receptors that are critical for modulating sedation. It is also possible that exogenous stimulation of NPY receptors can modulate the sedative effects of drugs, but blockade of endogenous NPY receptor signaling is not effective. At odds with the latter suggestion is the observation the Y1−/− mice, which pre-sumably have chronic blockade of normal Y1 receptor signaling, show reduced sensitivity to the sedative effects of ethanol (Thiele et al., 2002).
In summary, this study demonstrates that ip injection of the systemically bioavailable and selective Y1RA compound A reduces voluntary consumption of ethanol by C57BL/6J mice without altering water drinking or modifying the sedative effects produced by ethanol. These results are consistent with observations that hypothalamic infusion of NPY increases ethanol drinking by rats. Further experimentation is necessary to delineate the discrepancy between genetic and pharmacological research with respect to the role of Y1 receptor signaling in neurobiological responses to ethanol.
Acknowledgments
We thank Makoto Jitsuoka and Yuji Haga of Tsukuba Research Institute, Banyu Pharmaceuticals Co., Tsukuba, Japan, for preparing compound A. We also thank Donald J. Marsh for helpful discussions.
Footnotes
This work was supported by NIH Grants AA13573 and AA011605 and by Merck & Co., Inc.
References
- Ault DT, Radeff JM, Werling LL. Modulation of [3H]dopamine release from rat nucleus accumbens by neuropeptide Y may involve a sigma1-like receptor. J Pharmacol Exp Ther. 1998;284:553–560. [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Murphy JM, Li T-K. Effects of neuropeptide Y on sucrose and ethanol intake and on anxiety-like behavior in high alcohol drinking (HAD) and low alcohol drinking (LAD) rats. Alcohol Clin Exp Res. 2003;27:894 –899. doi: 10.1097/01.ALC.0000071929.17974.DA. [DOI] [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, Li T-K. Effect of neuropeptide Y (NPY) on oral ethanol intake in Wistar, alcohol-preferring (P), and -nonpreferring (NP) rats. Alcohol Clin Exp Res. 2001;25:386 –390. [PubMed] [Google Scholar]
- Berglund MM, Hipskind PA, Gehlert DR. Recent developments in our understanding of the physiological role of PP-fold peptide receptor subtypes. Exp Biol Med. 2003;228:217–244. doi: 10.1177/153537020322800301. [DOI] [PubMed] [Google Scholar]
- Bice P, Foroud T, Bo R, Castelluccio P, Lumeng L, Li T-K, Carr LG. Genomic screen for QTLs underlying alcohol consumption in the P and NP rat lines. Mamm Genome. 1998;9:949 –955. doi: 10.1007/s003359900905. [DOI] [PubMed] [Google Scholar]
- Biello SM, Golombek DA, Harrington ME. Neuropeptide Y and glutamate block each other’s phase shifts in the suprachiasmatic nucleus in vitro. Neuroscience. 1997;77:1049 –1057. doi: 10.1016/s0306-4522(96)00547-7. [DOI] [PubMed] [Google Scholar]
- Bison S, Crews FT. Alcohol withdrawal increases neuropeptide Y immunoreactivity in rat brain. Alcohol Clin Exp Res. 2003;27:1173–1183. doi: 10.1097/01.ALC.0000075827.74538.FE. [DOI] [PubMed] [Google Scholar]
- Carr LG, Foroud T, Bice P, Gobbett T, Ivashina J, Edenberg H, Lumeng L, Li T-K. A quantitative trait locus for alcohol consumption in selectively bred rat lines. Alcohol Clin Exp Res. 1998;22:884 –887. [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Clark JT, Keaton AK, Sahu A, Kalra SP, Mahajan SC, Gudger JN. Neuropeptide Y (NPY) levels in alcoholic and food restricted male rats: implications for site selective function. Regul Peptides. 1998;75–76:335–345. doi: 10.1016/s0167-0115(98)00086-x. [DOI] [PubMed] [Google Scholar]
- Colmer WF, Wahlestedt C (eds) (1993) The Biology of Neuropeptide Y and Related Peptides Humana Press, Totowa, NJ.
- Dumont Y, Martel JC, Fournier A, St.-Pierre S, Quirion R. Neuropeptide Y and neuropeptide Y receptor subtypes in brain and peripheral tissues. Prog Neurobiol. 1992;38:125–167. doi: 10.1016/0301-0082(92)90038-g. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Li T-K, Lumeng L, Hwang BH, Somes C, Jimenez P, Mathe AA. Neuropeptide Y levels in ethanol-naive alcohol-preferring and nonpreferring rats and in Wistar rats after ethanol exposure. Alcohol Clin Exp Res. 1998;22:1778 –1782. [PubMed] [Google Scholar]
- Foroud T, Bice P, Castelluccio P, Bo R, Miller L, Ritchotte A, Lumeng L, Li T-K, Carr LG. Identification of quantitative trait loci influencing alcohol consumption in the high alcohol drinking and low alcohol drinking rat lines. Behav Genet. 2000;30:131–140. doi: 10.1023/a:1001955205117. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Badia-Elder NE. The effects of neuropeptide Y (NPY) in the paraventricular nucleus of the hypothalamus (PVN) on ethanol drinking in high- (HAD1) and low-alcohol-drinking (LAD1) rats (abstract) Alcohol Clin Exp Res. 2004;28:55A. doi: 10.1097/01.alc.0000141813.27875.d5. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Li T-K, Badia-Elder NE. Neuropeptide Y reduces oral ethanol intake in alcohol-preferring (P) rats following a period of imposed ethanol abstinence. Alcohol Clin Exp Res. 2003;27:787–794. doi: 10.1097/01.ALC.0000065723.93234.1D. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Biello SM, Rendon RA, Harrington ME. Neuropeptide Y phase shifts the circadian clock in vitro via a Y2 receptor. Neuroreport. 1996;7:1315–1319. doi: 10.1097/00001756-199605170-00020. [DOI] [PubMed] [Google Scholar]
- Gray TS, Morley JE. Neuropeptide Y: anatomical distribution and possible function in mammalian nervous system. Life Sci. 1986;38:389 –401. doi: 10.1016/0024-3205(86)90061-5. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Wisialowski TA, van den Pol AN, Yocca FD. Phase shifting of circadian rhythms and depression of neuronal activity in the rat suprachiasmatic nucleus by neuropeptide Y: mediation by different receptor subtypes. J Neurosci. 1998;18:3014 –3022. doi: 10.1523/JNEUROSCI.18-08-03014.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001a;410:940 –944. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Regulation of olfactory neurogenesis by amidated neuropeptides. J Neurosci Res. 2001b;66:1–7. doi: 10.1002/jnr.1191. [DOI] [PubMed] [Google Scholar]
- Harrington ME, Schak KM. Neuropeptide Y phase advances the in vitro hamster circadian clock during the subjective day with no effect on phase during the subjective night. Can J Physiol Pharmacol 2000. 2000;78:87–92. [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heilig M, Soderpalm B, Engel JA, Widerlov E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology. 1989;98:524 –529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- Heilig M, Widerlov E. Neurobiology and clinical aspects of neuropeptide Y. Crit Rev Neurobiol. 1995;9:115–136. [PubMed] [Google Scholar]
- Hwang BH, Zhang JK, Ehlers CL, Lumeng L, Li T-K. Innate differences of neuropeptide Y (NPY) in hypothalamic nuclei and central nucleus of the amygdala between selectively bred rats with high and low alcohol preference. Alcohol Clin Exp Res. 1999;23:1023–1030. [PubMed] [Google Scholar]
- Kalra SP, Xu B, Dube MG, Moldawer LL, Martin D, Kalra PS. Leptin and ciliary neurotropic factor (CNTF) inhibit fasting-induced suppression of luteinizing hormone release in rats: role of neuropeptide Y. Neurosci Lett. 1998;240:45–49. doi: 10.1016/s0304-3940(97)00896-3. [DOI] [PubMed] [Google Scholar]
- Kanatani A, Hata M, Mashiko S, Ishihara A, Okamoto O, Haga Y, et al. A typical Y1 receptor regulates feeding behaviors: effects of a potent and selective Y1 antagonist, J-115814. Mol Pharmacol. 2001;59:501–505. doi: 10.1124/mol.59.3.501. [DOI] [PubMed] [Google Scholar]
- Kanatani A, Kanno T, Ishihara A, Hata M, Sakuraba A, Tanaka T, et al. The novel neuropeptide Y Y(1) receptor antagonist J-104870: a potent feeding suppressant with oral bioavailability. Biochem Biophys Res Commun. 1999;266:88 –91. doi: 10.1006/bbrc.1999.1750. [DOI] [PubMed] [Google Scholar]
- Kask A, Kivastik T, Rago L, Harro J. Neuropeptide Y Y1 receptor antagonist BIBP3226 produces conditioned place aversion in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:705–711. doi: 10.1016/s0278-5846(99)00029-9. [DOI] [PubMed] [Google Scholar]
- Kask A, Rago L, Harro J. Anxiogenic-like effect of the neuropeptide Y Y1 receptor antagonist BIBP3226 —antagonism with diazepam. Eur J Pharmacol. 1996;317:R3–R4. doi: 10.1016/s0014-2999(96)00838-2. [DOI] [PubMed] [Google Scholar]
- Kasuya E, Mizuno M, Watanabe G, Terasawa E. Effects of an antisense oligodeoxynucleotide for neuropeptide Y mRNA on in vivo luteinizing hormone-releasing hormone release in ovariectomized female rhesus monkeys. Regul Pept. 1998;75–76:319–325. doi: 10.1016/s0167-0115(98)00084-6. [DOI] [PubMed] [Google Scholar]
- Katner SN, Slawecki CJ, Ehlers CL. Neuropeptide Y administration into the amygdala does not affect ethanol consumption. Alcohol. 2002a;28:29 –38. doi: 10.1016/s0741-8329(02)00235-5. [DOI] [PubMed] [Google Scholar]
- Katner SN, Slawecki CJ, Ehlers CL. Neuropeptide Y administration into the third ventricle does not increase sucrose or ethanol self-administration but does affect the cortical EEG and increases food intake. Psychopharmacology. 2002b;160:146 –154. doi: 10.1007/s00213-001-0950-9. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Nannini MA, Bratt AM, Hodge CW. Neuropeptide-Y in the paraventricular nucleus increases ethanol self-administration. Peptides. 2001;22:515–522. doi: 10.1016/s0196-9781(01)00361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita H, Jessop DS, Finn DP, Coventry TL, Roberts DJ, Ameno K, Ijiri I, Harbuz MS. Acute ethanol decreases NPY mRNA but not POMC mRNA in the arcuate nucleus. Neuroreport. 2000;11:3517–3519. doi: 10.1097/00001756-200011090-00023. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Saiers JA, Pohorecky LA (1993) Observations of novel behaviors as indices of ethanol withdrawal-induced anxiety, in Advances in Biomedical Alcohol Research (Taberner PV, Badaway AA eds), pp 489 –493. Pergamon, New York. [PubMed]
- Levine AS, Morley JE. Neuropeptide Y: a potent inducer of con-summatory behavior in rats. Peptides. 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- Lopez-Valpuesta FJ, Nyce JW, Griffin-Biggs TA, Ice JC, Myers RD. Antisense to NPY-Y1 demonstrates that Y1 receptors in the hypothalamus underlie NPY hypothermia and feeding in rats. Proc R Soc Lond B Biol Sci. 1996;263:881–886. doi: 10.1098/rspb.1996.0130. [DOI] [PubMed] [Google Scholar]
- Misra K, Pandey SC. Differences in basal levels of CREB and NPY in nucleus accumbens regions between C57BL/6 and DBA/2 mice differing in inborn alcohol drinking behavior. J Neurosci Res. 2003;74:967–975. doi: 10.1002/jnr.10831. [DOI] [PubMed] [Google Scholar]
- Moller C, Wiklund L, Thorsell A, Hyytia P, Heilig M. Decreased measures of experimental anxiety in rats bred for high alcohol preference. Alcohol Clin Exp Res. 1997;21:656 –660. [PubMed] [Google Scholar]
- Naveilhan P, Canals JM, Arenas E, Ernfors P. Distinct roles of the Y1 and Y2 receptors on neuropeptide Y-induced sensitization to sedation. J Neurochem. 2001a;78:1201–1207. doi: 10.1046/j.1471-4159.2001.00534.x. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Canals JM, Valjakka A, Vartiainen J, Arenas E, Ernfors P. Neuropeptide Y alters sedation through a hypothalamic Y1-mediated mechanism. Eur J Neurosci. 2001b;13:2241–2246. doi: 10.1046/j.0953-816x.2001.01601.x. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Neveu I, Arenas E, Ernfors P. Complementary and overlapping expression of Y1, Y2 and Y5 receptors in the developing and adult mouse nervous system. Neuroscience. 1998;87:289 –302. doi: 10.1016/s0306-4522(98)00141-9. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Erickson JC, Hollopeter G, Baraban SC, Schwartz MW. Life without neuropeptide Y. Recent Prog Horm Res. 1998;53:163–199. [PubMed] [Google Scholar]
- Pandey SC, Carr LG, Heilig M, Ilveskoski E, Thiele TE. Neuropeptide Y and alcoholism: genetic, molecular, and pharmacological evidence. Alcohol Clin Exp Res. 2003a;27:149 –154. doi: 10.1097/01.ALC.0000052706.21367.0E. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H. The decreased phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats. Alcohol Clin Exp Res. 2003b;27:396 –409. doi: 10.1097/01.ALC.0000056616.81971.49. [DOI] [PubMed] [Google Scholar]
- Pedrazzini T, Seydoux J, Kunstner P, Aubert JF, Grouzmann E, Beermann F, Brunner HR. Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nat Med. 1998;4:722–726. doi: 10.1038/nm0698-722. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Beck-Sickinger AG, Chan J, Weiland HA. Y1 receptors in the nucleus accumbens: ultrastructural localization and association with neuropeptide Y. J Neurocsci Res. 1998;52:54 –68. doi: 10.1002/(SICI)1097-4547(19980401)52:1<54::AID-JNR6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Roy A, Pandey SC. The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcohol Clin Exp Res. 2002;26:796 –803. [PubMed] [Google Scholar]
- Schroeder JP, Iller KA, Hodge CW. Neuropeptide-Y Y5 receptors modulate the onset and maintenance of operant ethanol self-administration. Alcohol Clin Exp Res. 2003a;27:1912–1920. doi: 10.1097/01.ALC.0000098873.80433.BA. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Olive MF, Koenig H, Hodge CW. Intra-amygdala infusion of the NPY Y1 receptor antagonist BIBP 3226 attenuates operant ethanol self-administration. Alcohol Clin Exp Res. 2003b;27:1884 –1891. doi: 10.1097/01.ALC.0000098875.95923.69. [DOI] [PubMed] [Google Scholar]
- Shi TJ, Cui JG, Meyerson BA, Linderoth B, Hokfelt T. Regulation of galanin and neuropeptide Y in dorsal root ganglia and dorsal horn in rat mononeuropathic models: possible relation to tactile hypersensitivity. Neuroscience. 1999;93:741–757. doi: 10.1016/s0306-4522(99)00105-0. [DOI] [PubMed] [Google Scholar]
- Shi TJ, Tandrup T, Bergman E, Xu ZQ, Ulfhake B, Hokfelt T. Effect of peripheral nerve injury on dorsal root ganglion neurons in the C57 BL/6J mouse: marked changes both in cell numbers and neuropeptide expression. Neuroscience. 2001;105:249 –263. doi: 10.1016/s0306-4522(01)00148-8. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Walpole T, Ehlers CL. Increases in sucrose consumption, but not ethanol consumption, following ICV NPY administration. Pharmacol Biochem Behav. 2000;66:591–594. doi: 10.1016/s0091-3057(00)00215-x. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, Li T-K, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of alcohol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Badia-Elder NE. A role for neuropeptide Y in alcohol intake control: evidence from human and animal research. Physiol Behav. 2003;79:95–101. doi: 10.1016/s0031-9384(03)00109-4. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Cubero I, van Dijk G, Mediavilla C, Bernstein IL. Ethanol-induced c-Fos expression in catecholamine- and neuropeptide Y-producing neurons in rat brainstem. Alcohol Clin Exp Res. 2000a;24:802–809. [PubMed] [Google Scholar]
- Thiele TE, Koh MT, Pedrazzini T. Voluntary alcohol consumption is controlled via the neuropeptide Y Y1 receptor. J Neurosci. 2002;22:RC208. doi: 10.1523/JNEUROSCI.22-03-j0006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366 –369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Miura GI, Marsh DJ, Bernstein IL, Palmiter RD. Neurobiological responses to ethanol in mutant mice lacking neuropeptide Y or the Y5 receptor. Pharmacol Biochem Behav. 2000b;67:683–691. doi: 10.1016/s0091-3057(00)00413-5. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Navarro M, Sparta DR, Fee JR, Knapp DJ, Cubero I. Alcoholism and obesity: overlapping neuropeptide pathways? Neuropeptides. 2003a;37:321–337. doi: 10.1016/j.npep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Sparta DR, Fee JR, Navarro M, Cubero I. Central neuropeptide Y alters ethanol-induced sedation, but not ethanol intake, in C57BL/6 mice. Alcohol. 2003b;31:155–160. doi: 10.1016/j.alcohol.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Michels KM (1991) Statistical Principles in Experimental Design 3rd ed. McGraw-Hill, New York.
- Woldbye DP, Larsen PJ, Mikkelsen JD, Klemp K, Madsen TM, Bolwig TG. Powerful inhibition of kainic acid seizures by neuropeptide Y via Y5-like receptors. Nat Med. 1997;3:761–764. doi: 10.1038/nm0797-761. [DOI] [PubMed] [Google Scholar]
- Woldbye DP, Madsen TM, Larsen PJ, Mikkelsen JD, Bolwig TG. Neuropeptide Y inhibits hippocampal seizures and wet dog shakes. Brain Res. 1996;737:162–168. doi: 10.1016/0006-8993(96)00730-5. [DOI] [PubMed] [Google Scholar]
- Woldbye DP, Ulrichsen J, Haugbol S, Bolwig TG. Ethanol withdrawal in rats is attenuated by intracerebroventricular administration of neuropeptide Y. Alcohol Alcohol. 2002;37:318 –321. doi: 10.1093/alcalc/37.4.318. [DOI] [PubMed] [Google Scholar]
- Yamada K, Shibasaki T, Tsumori C, Imaki T, Hotta M, Wakabayashi I, Demura H. Neuropeptide Y reverses corticotropin-releasing hormone- and psychological stress-caused shortening of sodium pentobarbital-induced sleep in rats. Brain Res. 1996;725:272–275. doi: 10.1016/0006-8993(96)00405-2. [DOI] [PubMed] [Google Scholar]


