Abstract
Background: Recent studies have suggested that mouse allergen exposure and sensitization are common in urban children with asthma. The effectiveness of environmental intervention in reducing mouse allergen exposure has not been established.
Objective: To evaluate whether environmental intervention of mouse extermination and cleaning results in a reduction in mouse allergen levels.
Methods: Eighteen homes of children with positive mouse allergen skin test results and at least mild persistent asthma in urban Boston, MA, with evidence of mouse infestation or exposure were randomized in a 2:1 ratio (12 intervention and 6 control homes). The intervention homes received an integrated pest management intervention, which consisted of filling holes with copper mesh, vacuuming and cleaning, and using low-toxicity pesticides and traps. Dust samples were collected and analyzed for major mouse allergen (Mus m 1) and cockroach allergen (Bla g 1) at baseline and 1, 3, and 5 months after the intervention was started and compared with control homes.
Results: Mouse allergen levels were significantly decreased compared with control homes by the end of the intervention period at month 5 in the kitchen and bedroom (kitchen intervention, 78.8% reduction; control, 319% increase; P = .02; bedroom intervention, 77.3% reduction; control, 358% increase; P < .01; and living room intervention, 67.6% reduction; control, 32% reduction; P = .07).
Conclusions: Mouse allergen levels were significantly reduced during a 5-month period using an integrated pest management intervention.
INTRODUCTION
It is widely recognized that allergen avoidance and exposure reduction are helpful in reducing asthma morbidity in sensitized individuals.1 Recently, studies have demonstrated an extremely high prevalence of mouse allergen in inner-city homes of children with asthma.2,3 These studies also found that mouse sensitization is common in inner-city children with asthma and that there appears to be a dose-response relationship between exposure and sensitization.2,4 Although these studies did not find a relationship between mouse allergen and asthma morbidity, additional study of this potentially important inner-city allergen is clearly needed.
Although environmental intervention trials have been conducted for other allergens, such as dust mite,1,5 cat,6 and cockroach,7–10 to our knowledge, there are no published studies to date that evaluate the potential role of environmental intervention in reducing mouse allergen. The primary purpose of this study was to determine if an integrated pest management strategy could reduce mouse allergen levels in mouse-infested, inner-city homes. In addition, we sought to obtain preliminary data to determine if lung function and/or asthma symptoms could be reduced using this intervention.
METHODS
Study Homes
Eighteen children aged 6 to 18 years with a physician diagnosis of at least mild persistent asthma based on National Heart, Lung, and Blood Institute (NHLBI) guidelines who were otherwise healthy and whose parents reported mouse infestation in the home were recruited for the study. All the families were informed that the study involved a home intervention protocol to reduce mouse allergen. On recruitment, subjects were evaluated by means of a questionnaire regarding their medical history, asthma symptoms, and characteristics of the home environment. Specific questions were asked regarding mouse infestation and exposure. All subjects were taking maintenance anti-inflammatory medication, such as inhaled corticosteroids or inhaled cromolyn sodium. If the child had unstable asthma on evaluation, a 1-month period of undergoing appropriate asthma therapy as recommended by the NHLBI11 guidelines was implemented to ensure each patient had stable asthma control before enrollment. The study was approved by the Committee for Clinical Investigation of Children's Hospital, Boston, Harvard Medical School, Boston, MA, and all participants provided written, informed consent.
Subjects underwent prick-puncture skin tests to mouse, cockroach, cat, dog, dust mite (Dermatophagoides farinae and Dermatophagoides pteronyssinus), ragweed, tree (oak and maple), grass (orchard), and mold (Aspergillus fumigatus, Alternaria tenuis, and Helminthosporium savitum) extracts (Greer Laboratories, Lenoir, NC). Mouse skin test extracts were 1:20 (wt/vol). A positive skin test response to mouse allergen, defined as a wheal greater than half the diameter of the histamine control and at least 3 mm larger than the glycerin-saline control, was required for entry into the study. All subjects avoided short-acting antihistamines for 72 hours, long-acting antihistamines for 7 days, and tricyclic antidepressants for 6 weeks before skin testing.
Baseline spirometry (model PB100; Puritan Bennett Renaissance, Wilmington, MA) was also performed at study entry. On recruitment, if more than 1 child qualified in the same household, the child with the lowest baseline percentage of predicted forced expiratory volume in 1 second (FEV1) was enrolled.
On recruitment, subjects' homes were evaluated by trained personnel, including a home inspection using a protocol documenting environmental conditions and collection of dust samples for allergen analysis. Dust samples were collected using a hand-held vacuum (model BB870-AD; Orek Corporation, New Orleans, LA) with a fabric collector (Dust Collector; Johns Hopkins University, Baltimore, MD) fitted into the inlet hose of the vacuum using a standardized protocol.12 The entire floor was vacuumed in the kitchen, whereas in the living room a piece of furniture and the surrounding floor were vacuumed. In the bedroom, the bed, the bedding, and the floor adjacent to the bed were sampled. The filters were removed from the vacuum, returned to the laboratory, and stored at -20° C until they were processed. Dust samples were removed from the filters and sieved through a 0.3-μg mesh device to produce fine dust. The fine dust was weighed and a 100-mg aliquot was extracted in 2 mL of phosphate-buffered saline (pH 7.4) by rotation overnight. All dust samples were analyzed for the major mouse allergen, Mus m 1, using a sandwich enzyme-linked immunosorbent assay (ELISA) with an affinity-purified, monospecific anti-Mus m 1 antibody to determine concentration of mouse allergen. Samples were also analyzed for cockroach allergen (Bla g 1) using a noncompetitive ELISA based on a combination of polyclonal and monoclonal antibodies (Indoor Biotechnologies, Charlottesville, VA).13 The lower limit of detection in the assay used was 0.04 μg/g for Mus m 1 and 1 U/g for Bla g 1. The performance of the assay was masked to group allocation between intervention and control homes. To qualify for the study there needed to be a report of either mouse infestation in the home or Mus m 1 allergen levels of more than 0.5 μg/g of dust in any one room, which has been suggested as positive exposure in previous studies.2,4
Intervention
Eighteen households of children who fulfilled all the inclusion criteria were randomized by a computer in a 2:1 ratio (12 intervention and 6 control homes) to receive environmental intervention. The intervention used an integrated pest management strategy, which consisted of filling holes and cracks with copper mesh and caulk sealant, vacuuming with HEPA filters, cleaning surfaces with mild detergents (Pinesol or Fantastik), and educating the family on pest control measures. Tracking powder with indandione (Rozol; Lipha Chemicals Inc, New York, NY), a low-toxicity pesticide, was dusted on wall voids and pipe chases, which serve as rodent runways. Snap traps were set away from children and pets in the kitchen, concentrating on the area around the refrigerator, trash, and stove. Additional traps were applied in the living room and bedroom. The intervention was performed in a standardized fashion by professional exterminators with special training in conducting interventions for research purposes. Families were instructed to continue cleaning between visits with mild detergents. Home visits were again performed 1, 3, and 5 months after the start of the intervention, and standardized integrated pest management strategies were implemented at each of the home visits.
The control homes did not receive professional extermination, sealing of cracks and holes, tracking powder, snap traps, pest management education, vacuuming, cleaning, or other mouse allergen environmental control measures. Parents of the children in the intervention and control groups were given results of their allergy skin tests and general standard advice on environmental control measures, such as recommendations for dust mite covers for mite-allergic children and general advice on cleanliness.
Evaluation
House dust samples were collected from both the control and intervention homes at the baseline home visit and 1, 3, and 5 months after the initiation of the baseline or intervention (in the intervention group). At each home visit, a home evaluation was conducted, including a questionnaire about the home and a standardized home inspection. Data were gathered about the type of the home (detached house vs duplex or apartment), state of repair and cleanliness (extremely poor to bad vs average to good), evidence of dirty dishes in the kitchen (yes or no), evidence of cockroach or mouse infestation (yes or no), and presence of carpeting (yes or no).
Children in both the control and intervention groups came to the clinic for follow-up visits 2 and 5 months after randomization. At these visits, spirometry was performed and a questionnaire was also completed by the caretaker regarding asthma symptoms, using similar questions that have been validated previously in the National Cooperative Inner-City Asthma Study,12 such as the number of times albuterol was used in the past 2 weeks, the number of school days missed in the last 3 months, the number of days of wheezing in the past 2 weeks, and the number of nights the caregiver lost sleep due to the child's asthma during the last 2 weeks.
Statistical Analysis
The sample size of 12 intervention homes and 6 control homes was empiric in this project. However, from other similarly designed cockroach home intervention studies, this sample size appears to be sufficient to detect reduction in mouse allergen levels.8,9 We estimated that if allergen levels can be reduced with an effect size of 1.5, we would have 80% power to detect this difference (sample size estimated using nQuery Advisor version 4.0; Statistical Solutions Inc, Saugus, MA).
Summary statistics for mouse allergen levels before and after intervention were calculated for each group (intervention and control). The Wilcoxon rank-sum test was applied to compare the percent change in mouse allergen levels from baseline to 1, 3, and 5 months after start of the intervention between the intervention and control groups. Statistical significance was determined by a 2-sided P value of .05. Graphs of allergen levels vs time and a repeated-measures analysis on the base 10 logarithms of allergen levels by mixed-effects models were used to explore the trends in allergen levels over time. In addition, for the intervention group, allergen levels at 5 months after the start of the intervention were compared with baseline by the Wilcoxon signed-rank test. The Wilcoxon rank-sum test was performed to examine whether the percent change in mouse allergen levels from baseline to month 5 was related to household characteristics.
Summary statistics for FEV1 and asthma symptoms before and after intervention were computed for the intervention and control group. Change in FEV1 and asthma symptoms from baseline to 2 months and 5 months after initiation of the intervention between intervention and control groups was compared using the Wilcoxon rank-sum test.
RESULTS
Demographic Variables
All of the subjects had positive skin test results to other allergens besides mouse. Ten (83%) of the 12 intervention children and 5 (83%) of the 6 controls had 3 or more other positive skin test results (Table 1). All of the children in both the intervention and control groups took daily maintenance inhaled steroids for their asthma.
Table 1.
Demographics Comparing Intervention and Control Groups at Baseline
| Variable | Intervention | Controls |
|---|---|---|
| Abbreviation: FEV1, forced expiratory volume in 1 second. | ||
| No. of subjects | 12 | 6 |
| Median age (range), y | 9 (6–14) | 7 (6–10) |
| No. (%) of boys | 7 (58) | 2 (33) |
| Type of dwelling, No. (%) | ||
| Single | 3 (25) | 1 (17) |
| Apartment | 3 (25) | 2 (33) |
| Row or duplex | 6 (50) | 3 (50) |
| Race, No. (%) | ||
| White | 1 (8) | 1 (17) |
| Black | 8 (67) | 2 (33) |
| Hispanic | 3 (25) | 3 (50) |
| No. (%) with ≥ 3 other positive skin test results | 10 (83) | 5 (83) |
| No. (%) with positive cockroach skin test result | 5 (42) | 3 (50) |
| Median inhaled fluticasone dose (range), μg* | 198 (176–440) | 176 (176–440) |
| Percentage of predicted FEV1 (range) | 77 (70–105) | 81 (70–120) |
One child in the intervention group was taking 400 μg/d of inhaled budesonide.
Mouse Allergen Levels
Baseline mouse allergen levels ranged from 0.07 to 413.4 μg/g (Table 2). No statistically significant differences in Mus m 1 levels were indicated between control and intervention homes (kitchen, P = .57; bedroom, P = .96; living room, P = .96). The mouse allergen levels in the control homes tended to rise during the study in the kitchen and bedroom, with median levels of 25.6 μg/g in the kitchen at 5 months after baseline. In contrast, the levels in the intervention homes tended to decrease during the study, with lowest levels by month 5 in all 3 rooms. At month 5, mouse allergen levels were significantly lower than baseline in the intervention homes (P = .001 for kitchen, P = .002 for bedroom, and P < .001 for living room).
Table 2.
Summary of Mouse Allergen Levels in the Intervention and Control Groups
| Location | Allergen levels in dust, median (range), μg/g |
|||
|---|---|---|---|---|
| Baseline | Month 1 | Month 3 | Month 5 | |
| Abbreviation: BD, below detection. | ||||
| Kitchen | ||||
| Intervention | 24.1 (0.3–277.9) | 12.9 (0.2–197.6) | 7.6 (BD–223.2) | 2.8 (BD–14.1)* |
| Control | 8.2 (0.5–413.4) | 2.1 (0.3–197.2) | 14.9 (0.02–356.1) | 25.6 (1.02–51.5) |
| Bedroom | ||||
| Intervention | 5.2 (0.3–222.1) | 2.6 (0.06–283.4) | 2.9 (BD–81.7) | 2.2 (BD–52.5)* |
| Control | 8.6 (BD–37.5) | 14.3 (0.4–52.7) | 3.3 (0.1–8.6) | 24.1 (BD–515.5) |
| Living room | ||||
| Intervention | 3.6 (0.2–71.4) | 2.0 (0.07–72.0) | 2.8 (1.2–71.6) | 0.9 (BD–92.2) |
| Control | 8.0 (0.07–133.6) | 3.7 (0.4–10.8) | 16.1 (0.03–7.9) | 5.4 (0.04–185.7) |
P < .05 in the intervention homes compared with controls.
In comparing allergen levels between intervention and control homes, the percent change from baseline was statistically different between these 2 groups only by the end of the intervention period at month 5 (78.8% reduction for kitchen intervention vs 319% increase for control, P = .02; 77.3% reduction for bedroom intervention vs 358% increase for control, P < .01; 67.6% reduction for living room intervention vs 32% reduction for control, P = .07).
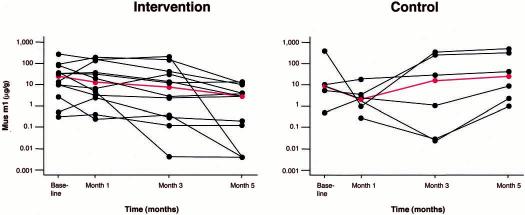
Individual mouse allergen concentrations in the kitchens are illustrated in Figure 1. At month 5, allergen levels were reduced compared with baseline in all 12 intervention homes (range, 33%–100%), whereas the levels rose in 4 of 5 kitchen samples in control homes (1 kitchen sample had insufficient dust for analysis). This pattern was also observed in the bedroom, where mouse allergen levels were reduced in 11 of 12 intervention homes (range, 11%–99.71%) by month 5 and higher in only 1 home (>121%); the allergen levels rose in all control homes. A similar but less profound difference was seen in the living room: allergen levels were reduced in 11 of the 12 intervention samples (range, 31.3%–99.8%) but rose in 4 of 6 control samples. These observed differences in the time course of mouse allergen levels were also indicated in the repeated-measures analysis in which the group-by-time interaction was statistically significant for the kitchen (P = .002) and marginally significant for the bedroom (P = .06) and living room (P = .16).
Figure 1.
Mouse allergen (Mus m 1) levels in kitchens from individual intervention and control homes. Red lines indicate the median at each time point.
Data were also analyzed to determine whether the changes in mouse allergen in the intervention homes were related to demographic or environmental variables, such as general housekeeping, type of dwelling, evidence of dirty dishes, and carpeting. No statistically significant effects on the reduction of mouse allergen levels were detected for these covariates, possibly due to the small sample size.
Report of Mice
In the intervention group, 11 of the 12 homes reported seeing mice at baseline, among which only 5 reported seeing mice by month 5. Four of the 6 control homes reported seeing mice at baseline and 2 of these 4 homes reported mice at month 5. The differences in report of mice between intervention and control homes were not statistically significant (P value ranged from .24 to >.99). The results were similar for sightings of mouse droppings (data not shown).
Cockroach Allergen Levels
Although this intervention trial was targeted to mouse allergen, we also analyzed our dust samples for cockroach allergen, because many homes with mouse allergen also have cockroach allergen.2,4 In the 12 intervention homes, baseline cockroach allergen levels were below detection (BD) in 6 homes in the kitchen (range, BD-108 U/g), 7 homes in the bedroom (range, BD-15 U/g), and 9 homes in the living room (range, BD-13 U/g). In the 6 control homes, cockroach levels were BD in all of the rooms except the living room (1.2 U/g) in one home and the kitchen (1 U/g) in another home. No analysis was performed to compare the reduction in cockroach allergen levels, because the allergen levels were BD for many of the study homes at baseline.
Symptoms
The median baseline percentage of predicted FEV1 was 77% (range, 44%–105%) for the intervention group and 81% (range, 47%–130%) for the control group. The median percentage of predicted FEV1 values for month 2 and month 5 were 83% (range, 44%–108%) and 86% (range, 43%–110%), respectively, for the intervention group and 78% (range, 42%–116%) and 71% (range, 60%–87%), respectively, for the control group. No statistically significant difference was indicated between these 2 groups (P = .96). Furthermore, there was no significant difference in the percentage of predicted FEV1 change from baseline at month 2 and month 5 between children in the intervention or control homes (P = .58 and P = .96, respectively).
For representative asthma symptoms (Table 3), no statistically significant differences were detected at baseline (P values ranged from .20 to .84). For changes from baseline, no statistically significant differences between the 2 groups were detected (P values ranged from .13 to >.99).
Table 3.
Medians (Range) of Asthma Morbidity Variables between Intervention and Control*
| Group | Baseline | Month 2 | Month 5 |
|---|---|---|---|
| Intervention | |||
| Albuterol use in past 2 weeks, d | 7 (1–14) | 4 (0–14) | 2 (0–14) |
| No. of nights when caregiver lost sleep in past 2 weeks | 1 (0–14) | 0 (0–5) | 0 (0–6) |
| No. of school days missed in past 3 months | 1 (0–5) | 2 (0–4) | 0 (0–6) |
| No. of days of wheezing in past 2 weeks | 2 (0–7) | 2 (0–6) | 1 (0–14) |
| Control | |||
| Albuterol use in past 2 weeks, d | 3.5 (2–14) | 3.5 (0–7) | 1 (0–14) |
| No. of nights when caregiver lost sleep in past 2 weeks | 1.5 (0–8) | 0.5 (0–3) | 1.5 (0–2) |
| No. of School days missed in past 3 months | 0.5 (0–2) | 1 (0–2) | 1 (0–2) |
| No. of days of wheezing in past 2 weeks | 2.5 (0–14) | 2.6 (0–6) | 2 (0–7) |
No statistically significant differences were indicated for any of these morbidity variables.
DISCUSSION
This is the first study to comprehensively evaluate the role of an intervention strategy to decrease mouse allergen in innercity homes. We showed that during a 5-month intervention period, mouse allergen settled dust samples were significantly decreased compared with controls in both the kitchen and the bedroom.
We found only one published abstract regarding mouse allergen intervention of 14 intervention and 14 control homes, using a kitchen-targeted pest management strategy. The intervention described in this abstract decreased mouse allergen levels only in the kitchen.14 The abstract did not discuss length of intervention, allergen sensitization, or morbidity data. Chen and Eggleston15 also showed that sodium hypochlorite (bleach) was effective in decreasing surface mouse allergen in the laboratory, but we have not seen any published studies looking at this in home environments.
Other studies have examined interventions to reduce other allergens in home environments. In early investigation, the effect of cat removal on allergen levels has been shown to be a slow and somewhat difficult task, taking often up to 6 months to significantly reduce levels.6 Furthermore, cockroach allergen intervention trials have shown some success with decreasing levels of this allergen in urban homes,8–10 but it is unclear whether these interventions are effective in decreasing levels enough to provide clinical benefit. Our study suggests that mouse allergen levels may take time to be effectively reduced. It took several months before allergen levels were significantly lower than baseline. The allergen, therefore, may be particularly sticky with similar properties as cat allergen.6,16 Furthermore, effective mouse extermination requires the simultaneous implementation of several different strategies, which include blocking points of entry, traps, and low-toxicity pesticides, which may help explain why it took several months to see a significant effect.
In our study of homes in Boston, many of the baseline levels for cockroach were BD. This is less than what has been seen in other inner-city environments2,4,17; however, it is difficult to make any conclusions with our sample size. Furthermore, we could not evaluate whether our mouse allergen–targeted intervention is also helpful in decreasing cockroach allergen, since many of our baseline levels were BD. Since other studies have shown that both mouse and cockroach allergen exposure often affects similar homes,2,4,17 it may be important to consider larger-scale studies that look at strategies to reduce both mouse and cockroach allergen.
With our sample size, it is not surprising that we were unable to detect any clinical differences in terms of lung function or asthma symptoms. We observed a trend toward improvement in percentage of predicted FEV1 in the intervention group (median of 86% at month 5 vs 77% at baseline), so it is possible that our sample size was too small to detect a difference where one may exist. It is also unknown what degree of mouse allergen reduction and length of time of reduction is required to improve clinical symptoms. It is also unknown whether our intervention will ultimately improve asthma symptoms. Further study with larger sample sizes and extended periods of follow-up is therefore desirable to determine the relationship between mouse allergen levels and asthma morbidity.
We conclude that an integrated pest management strategy can be effective in decreasing mouse allergen levels in innercity homes of mouse-allergic children with asthma. Questions remain as to whether such interventions can sustain lower allergen levels and if they will provide any clinical benefit. Other issues, such as cost-effectiveness of clinical benefit of such strategies, may be important, but we are encouraged that our strategy appears effective in initial reduction of allergen levels. However, based on the available data, we would recommend that inner-city children with asthma be screened for mouse sensitivity and that an integrated pest management approach be implemented whenever possible for those children with mouse sensitivity and a history suggestive of mouse allergen exposure.
ACKNOWLEDGMENTS
We thank Greer Laboratories Inc for their donation of skin testing supplies and Don Rivard for his consultative services on rodent pest management
Footnotes
This study was supported by The Medical Foundation–Deborah Munroe Noonan Memorial Fund and the Children's Hospital Community Health Benefits Research Grant. Dr. Phipatanakul is supported by grant K-23 AI 054972 from the National Institutes of Health.
REFERENCES
- 1.Platts-Mills TA, Vaughan JW, Carter MC, Woodfolk JA. The role of intervention in established allergy: avoidance of indoor allergens in the treatment of chronic allergic disease. J Allergy Clin Immunol. 2000;106:787–804. doi: 10.1067/mai.2000.110548. [DOI] [PubMed] [Google Scholar]
- 2.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. the National Cooperative Inner-City Asthma Study Group. Mouse allergen, I: the prevalence of mouse allergen in inner-city homes. J Allergy Clin Immunol. 2000;106:1070–1074. doi: 10.1067/mai.2000.110796. [DOI] [PubMed] [Google Scholar]
- 3.Stelmach I, Jerzynska J, Stelmach W, Majak P, Chew G, Kuna P. The prevalence of mouse allergen in inner-city homes. Pediatr Allergy Immunol. 2001;13:299–302. doi: 10.1034/j.1399-3038.2002.01079.x. [DOI] [PubMed] [Google Scholar]
- 4.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. the National Coooperative Inner-City Asthma Study Group. Mouse allergen, II: the relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J Allergy Clin Immunol. 2000;106:1075–1080. doi: 10.1067/mai.2000.110795. [DOI] [PubMed] [Google Scholar]
- 5.Htut T, Higenbottam TW, Gill GW, Darwin R, Anderson PB, Syed N. Eradication of house dust mite from homes of atopic asthmatic subjects: a double-blind trial. J Allergy Clin Immunol. 2001;107:55–60. doi: 10.1067/mai.2001.111240. [DOI] [PubMed] [Google Scholar]
- 6.Wood RA, Chapman MD, Adkinson NF, Jr, Eggleston PA. The effect of cat removal on allergen content in household-dust samples. J Allergy Clin Immunol. 1989;83:730–734. doi: 10.1016/0091-6749(89)90006-7. [DOI] [PubMed] [Google Scholar]
- 7.Williams LW, Reinfried P, Brenner RJ. Cockroach extermination does not rapidly reduce allergen in settled dust. J Allergy Clin Immunol. 1999;104:702–703. doi: 10.1016/s0091-6749(99)70346-5. [DOI] [PubMed] [Google Scholar]
- 8.Wood RA, Eggleston PA, Rand C, Nixon WJ, Kanchanaraksa S. Cockroach allergen abatement with extermination and sodium hypochlorite cleaning in inner-city homes. Ann Allergy Asthma Immunol. 2001;87:60–64. doi: 10.1016/S1081-1206(10)62324-1. [DOI] [PubMed] [Google Scholar]
- 9.Eggleston PA, Wood RA, Rand C, Nixon WJ, Chen PH, Lukk P. Removal of cockroach allergen from inner-city homes. J Allergy Clin Immunol. 1999;104:842–846. doi: 10.1016/s0091-6749(99)70296-4. [DOI] [PubMed] [Google Scholar]
- 10.Gergen PJ, Mortimer KM, Eggleston PA, et al. Results of the National Cooperative Inner-City Asthma Study (NCICAS) environmental intervention to reduce cockroach allergen exposure in inner-city homes. J Allergy Clin Immunol. 1999;103:501–506. doi: 10.1016/s0091-6749(99)70477-x. [DOI] [PubMed] [Google Scholar]
- 11.National Institutes of Health, National Heart, Lung, and Blood Institute . Guidelines for Diagnosis and Management of Asthma: Expert Panel Report 2. National Institutes of Health, National Heart, Lung, and Blood Institute; Bethesda, MD: 1997. pp. 45–46. [Google Scholar]
- 12.Mitchell H, Senturia Y, Gergen P, et al. Design and methods of the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24:237–252. doi: 10.1002/(sici)1099-0496(199710)24:4<237::aid-ppul3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Pollart SM, Smith TF, Morris EC, Gelber LE, Platts-Mills TA, Chapman MD. Environmental exposure to cockroach allergens: analysis with monoclonal antibody-based enzyme immunoassays. J Allergy Clin Immunol. 1991;87:505–510. doi: 10.1016/0091-6749(91)90009-d. [DOI] [PubMed] [Google Scholar]
- 14.Correa JC, Chew GL, Kinney PL. Reduction of mouse allergen in low income New York City apartments. J Allergy Clin Immunol. 2002;109:S359. [Google Scholar]
- 15.Chen P, Eggleston PA. Allergenic proteins are fragmented in low concentrations of sodium hypochlorite. Clin Exp Allergy. 2001;31:1086–1093. doi: 10.1046/j.1365-2222.2001.01127.x. [DOI] [PubMed] [Google Scholar]
- 16.Wood RA, Mudd KE, Eggleston PA. The distribution of cat and dust mite allergens on wall surfaces. J Allergy Clin Immunol. 1992;89:126–130. doi: 10.1016/s0091-6749(05)80049-1. [DOI] [PubMed] [Google Scholar]
- 17.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]