Abstract
Cdc42 is a highly conserved small GTP-binding protein that is involved in regulating morphogenesis in eukaryotes. In this study, we isolated and characterized a highly conserved Cdc42 gene from Colletotrichum trifolii (CtCdc42), a fungal pathogen of alfalfa. CtCdc42 is, at least in part, functionally equivalent to Saccharomyces cerevisiae Cdc42p, since it restores the temperature-sensitive phenotype of a yeast Cdc42p mutant. Inhibition of CtCdc42 by expression of an antisense CtCdc42 or a dominant negative form of CtCdc42 (DN Cdc42) resulted in appressorium differentiation under noninductive conditions, suggesting that CtCdc42 negatively regulates pathogenic development in this fungus. We also examined the possible linkage between CtCdc42 and Ras signaling. Expression of a dominant active Cdc42 (DA Cdc42) in C. trifolii leads to aberrant hyphal growth under nutrient-limiting conditions. This phenotype was similar to that of our previously reported dominant active Ras (DA Ras) mutant. Also consistent with our observations of the DA Ras mutant, high levels of reactive oxygen species (ROS) were observed in the DA Cdc42 mutant, and proline restored the wild-type phenotype. Moreover, overexpression of DN Cdc42 resulted in a significant decrease in spore germination, virtually no hyphal branching, and earlier sporulation, again similar to what we observed in a dominant negative Ras (DN Ras) mutant strain. Interestingly, coexpression of DA Cdc42 with DN Ras resulted in germination rates close to wild-type levels, while coexpression of DN Cdc42 with the DA Ras mutant restored the wild-type phenotype. These data suggest that CtCdc42 is positioned as a downstream effector of CtRas to regulate spore germination and pathogenic development.
The regulation of growth and development in eukaryotes generally involves members of the small G protein family of GTPases (18). Accumulating evidence has shown that, in particular, the Rho family of GTPases, including Rho, Rac, and Cdc42, are key downstream effectors in Ras-mediated signaling pathways that control organization of the actin cytoskeleton, activate kinase cascades, modulate gene expression, regulate membrane trafficking, promote growth transformation, and induce apoptosis (45, 53). Cycling between active GTP- and inactive GDP-bound states, these small G proteins act as molecular switches to integrate numerous environmental cues that regulate cellular activities. Their regulation is achieved through three classes of regulatory molecules, including guanine exchange factors, GTPase-activating proteins, and guanine nucleotide dissociation inhibitors. Guanine exchange factors facilitate the exchange of GDP, GTPase-activating proteins catalyze the intrinsic GTPase activity, and guanine nucleotide dissociation inhibitors inhibit the release of GDP from the GTPase (6, 17). Among the Rho family members, Cdc42, which is found in all eukaryotes, has garnered particular interest due to its important role in cytoskeletal reorganization (21, 31). In mammals, Cdc42p plays a key role in the formation of actin-dependent subcellular structures, such as filopodia and vinculin-containing focal complexes, in cell cycle progression, and in apoptosis (21). Cdc42p also mediates anchorage-independent growth and is necessary for Ras transformation (1, 21). In Saccharomyces cerevisiae, actin cytoskeleton rearrangement regulated by Cdc42p is required for morphological changes, including bud emergence and enlargement during the mitotic cell cycle, mating projection formation, and pseudohyphal growth (21). Recently, it has been shown that filamentous growth of S. cerevisiae is controlled by Ras2 via Cdc42p, which triggers a stress response via a mitogen-activated protein kinase (MAPK) cascade (15, 19). Overexpression of a dominant negative form of Cdc42 (DN Cdc42) inhibited Ras2-dependent filamentous growth, but expression of a dominant active form of Cdc42 (DA Cdc42) induced filamentous growth, confirming that pseudohyphal growth in S. cerevisiae is regulated by activation of Ras2 via Cdc42p (52). Similarly, polarized cell growth in Schizosaccharomyces pombe is also controlled by Ras1 through the proper function of Cdc42p (5, 35).
To date, Cdc42 homologs in several filamentous and dimorphic fungi have been isolated and characterized. The role of the Cdc42 protein in these organisms appears to be associated with growth, development, and in some cases pathogenicity, although its relative significance in actin cytoskeleton organization has varied depending on the organism. For example, in Claviceps purpurea, a biotrophic pathogen of cereals, a Cdc42 homolog (Cpcdc42) has been found to be involved in vegetative differentiation and is required for pathogenicity (41). Studies of Ashbya gossypii also showed that disruption of AgCdc42 abolished the ability to switch from isotropic to polar growth, resulting in the failure of germ tube production (47). In addition, it was found that activation of WdCdc42 in Wangiella dermatitidis induced a morphological transition of yeast-like cells to produce sclerotic bodies while repressing hyphal development (51). Moreover, CflA, a Cdc42 homolog from Penicillium marneffei, is required for proper cell shape and hyphal growth but not for dimorphic switching or asexual development (3). Comparatively, Cdc42 in Candida albicans (CaCdc42) is important for polarized growth of both the yeast and hyphal growth stages of C. albicans (33, 44), and it was suggested that the function of CaCdc42p in the dimorphic switch to bud emergence may be connected to Ras, since mRNA levels of CaCdc42p transiently increase in these processes in accordance with Ras activation (25). However, the exogenous or endogenous signals that regulate Cdc42 proteins in these fungi have not been addressed, and there is no direct evidence indicating that Cdc42 acts as a downstream filamentous fungal effector in Ras-mediated signaling pathways in a manner similar to its role in mammals and yeasts.
Colletotrichum trifolii is a fungal pathogen causing alfalfa anthracnose (13). Previous studies of this fungus have shown that the unique gene that encodes C. trifolii Ras (CtRas) plays a pivotal role in regulating fungal growth and development (8, 43). Ectopic expression of a dominant active form of CtRas (DA Ras) exhibited pleiotropic phenotypes, including rapid vegetative growth, aberrant hyphal morphology, and the inability to undergo normal development under nutrient-limiting conditions (43). Aberrant hyphal morphology of the DA Ras mutant includes curled and highly distorted hyphal tips, indicating a defect in orientation of apical growth (e.g., polarized growth), whereas wild-type C. trifolii displayed polarized linear extension of hyphal tips and branching. Moreover, this mutant forms smaller and more compact colonies than does the wild type on minimal medium, suggesting a defect in radial growth. Staining of DA Ras mutant strains with the cell wall dye calcofluor indicated that the distorted hyphal tips are more heavily stained than in normal wild-type cells, suggesting alterations in the cell wall and/or defects in cytoskeletal organization. Thus, CtRas may act as a positive regulator for cytoskeletal organization. We are therefore interested in investigating the role of Ras-coupled effector pathways in the hyphal growth and development of C. trifolii.
In this study, a Cdc42 homolog (CtCdc42) was isolated from C. trifolii and the cellular function of this small GTPase was examined, particularly with respect to CtRas. We generated several constructs, including DA Cdc42, a DN Cdc42, and an antisense Cdc42, and transferred these constructs into wild-type C. trifolii. The resulting phenotypes of these mutants were analyzed and compared with those observed in CtRas mutants (32, 43). Additionally, we created a series of double mutants of the CtRas and CtCdc42 constructs. Phenotypes of these mutants were investigated, and the contribution of Cdc42 to Ras signaling is also described. Our results suggest that CtCdc42 negatively regulates pathogenic development (appressorial development) but actively regulates spore germination and proper hyphal growth by functioning at least in part as a downstream effector of CtRas.
MATERIALS AND METHODS
Fungal strains and growth conditions.
The C. trifolii strains used in this study are listed in Table 1. C. trifolii cultures were maintained on YPSS agar (0.4% yeast extract, 2% soluble starch, 0.1% potassium phosphate, 0.05% magnesium sulfate, 1.6% agar, pH 6.8). Vegetative cultures were grown in YPSS liquid medium for 3 to 7 days with gentle agitation at room temperature (22 to 25°C), spores were obtained from liquid YPSS cultures grown for 4 to 5 days and shaken at 150 rpm, and protoplasts for fungal transformation were produced from germinated conidia grown in stationary liquid YPSS cultured for 5 to 7 days. A yeast cdc42 temperature-sensitive mutant strain (DJTD2-16A MATa cdc42-1 his4 leu2 trp1 ura3) was used as the recipient for complementation analysis. Yeast strain EGY48 (Clontech, Mountain View, CA) was used to confirm the specificity of anti-Cdc42 antibody. Escherichia coli strain DH5α (Invitrogen, Carlsbad, CA) was used for plasmid DNA transformation.
TABLE 1.
Fungal strains used in this study
| C. trifolii strain | Plasmid | Description | Reference |
|---|---|---|---|
| Wild type | Wild-type C. trifolii race 1 | 13 | |
| DA Ras mutant | Ct-RasVal2 | Dominant active Ras mutant | 43 |
| DN Ras mutant | Ct-RasT22N | Dominant negative Ras mutant | 16 |
| DA Cdc42 mutant | CtCdc42G14V | Dominant active Cdc42 mutant | This study |
| DN Cdc42-1 mutant | CtCdc42T19N | Dominant negative Cdc42 mutant 1 | This study |
| DN Cdc42-2 mutant | CtCdc42D120A | Dominant negative Cdc42 mutant 2 | This study |
| As Cdc42 mutant | CtCdc42-reverse | Antisense CtCdc42 mutant | This study |
| DA Ras DN Cdc42-1 mutant | Ct-RasVal2 + CtCdc42T19N | Double mutant overexpressing DA Ras and DN Cdc42 | This study |
| DA Ras DN Cdc42-2 mutant | Ct-RasVal2 + CtCdc42D120A | Double mutant overexpressing DA Ras and DN Cdc42 | This study |
| DA Ras As Cdc42 mutant | Ct-RasVal2 + CtCdc42-reverse | Double mutant overexpressing DA Ras and As Cdc42 | This study |
| DA Ras DA Cdc42 mutant | Ct-RasVal2 + CtCdc42G14V | Double mutant overexpressing DN Ras and DA Cdc42 | This study |
Molecular constructs and fungal transformation.
For the yeast complementation assay, a 580-bp cDNA fragment of the CtCdc42 gene was digested by BamHI and XhoI and cloned into the yeast vector pYES2 (Invitrogen, Carlsbad, CA). Expression of CtCdc42 was driven by the conditional promoter GAL1.
Site-directed mutagenesis was used to generate mutant versions of C. trifolii Cdc42 (DA Cdc42 and DN Cdc42) by Taq PCR-mediated DNA amplification. Two primers, the forward primer 5′-GTTGTTGTTGGTGACGTTGCGGTC-3′ and the reverse primer 5′-TGTCTTTCCGACCGCAACGTCACC-3′, were used to generate DA Cdc42 by replacing glycine 14 (G14) of CtCdc42 with valine. DN Cdc42 mutant 1 (DN Cdc42-1) was generated by replacing threonine 19 (T19) with asparagine; two primers, the forward primer 5′-GACGGTGCGGTCGGAAAGAACTGCCTTCTT-3′ and the reverse primer 5′-GAGCGGCCGCCAGTGTGATGGATATCTGCA-3′, were used. DN Cdc42 mutant 2 (DN Cdc42-2) was generated by replacing aspartic acid 120 (D120) with alanine; two primers, the forward primer 5′-CTCATCGTCGGTACTCAGGTGGCCTTGAGA-3′ and the reverse primer 5′-CACGCTCGGATCCTCTCTCAAGGCCACCTG-3′, were used. All mutagenized DNA fragments were amplified with Pfu polymerase (Stratagene, La Jolla, CA) and sequenced. Expression of DA Cdc42 and DN Cdc42 was driven by the constitutive Aspergillus nidulans glyceraldehyde-3-phosphate dehydrogenase (gpd) promoter from pNOM102 (39). To generate antisense expression of CtCdc42, the coding region of the cDNA of CtCdc42, a 650-bp EcoRI fragment, was end filled and ligated to pNOM102 in the opposite transcriptional direction. For selection, the hygromycin B phosphotransferase gene expression cassette from a derivative of pHA1.3 (36) or the bleomycin resistance gene expression cassette from pAMPH520 (2) was included. Polyethylene glycol-mediated fungal transformation was performed as previously described (49).
Expression of CtCdc42 in S. cerevisiae.
The pYES2 plasmid was used to express the C. trifolii Cdc42 gene behind the Gal1/10 promoter, and this construct was transformed into yeast strain DJTD2-16 by the alkali cation method (28). Synthetic dropout (SD) medium consisted of 0.67% yeast nitrogen base (Difco) and 2% glucose or 2% galactose supplemented with appropriate amino acids and nucleotide bases. Transformants expressing CtCdc42 were maintained in SD medium lacking uracil at 30°C. For temperature sensitivity testing, transformed cells were replica plated onto medium containing 2% galactose as the sole carbon source and then incubated for 4 days at 30°C or 37°C.
Measurement of intracellular oxidation levels.
Intracellular H2O2 levels in C. trifolii were monitored with the oxidant-sensitive probe dihydrorhodamine-123 (Molecular Probes). Briefly, protoplasts were generated from C. trifolii strains treated under the indicated conditions, as described previously (8). Aliquots of protoplasts were incubated with 50 μM dihydrorhodamine-123 for 20 min at room temperature, rinsed twice with phosphate-buffered saline, and then observed under an epifluorescence microscope (Zeiss Axioskop).
Microscopy.
Spore germination and hyphal morphological changes during development were observed microscopically using a Zeiss Axioskop microscope with differential inference contrast optics. Sterile microscope glass slides were dipped in molten growth medium and placed under sterile glass petri dishes. Spores of fungal strains were inoculated onto the slides, and the petri dishes were incubated in a plastic box lined with water-saturated paper towels to maintain a humid environment. Images were captured via a charge-coupled-device camera and processed using AxioVision 3.1 for Windows (AxioCam HR, Thornwood, NY).
Western analysis.
Methods to prepare total fungal proteins from different developmental stages were as described previously (7). Protein concentration was determined by the method of Bradford (4), and 20 μg of protein per lane was electrophoresed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ). After the electroblotting, the membrane was incubated for 1 h at room temperature in the blocking solution with Cdc42p primary antibodies (Santa Cruz Biotechnology, Inc.) at a 1:1,000 dilution. The secondary antibody (dilution, 1:10,000) was alkaline phosphatase conjugated anti-rabbit immunoglobulin G (Invitrogen, Carlsbad, CA). Proteins were visualized by incubating the membrane with alkaline phosphatase substrate mixture followed by a 5- to 10-min exposure to X-ray film.
Nucleotide sequence accession number.
The GenBank accession number of the CtCdc42 gene is AAK31624 (EMBL).
RESULTS
Cloning of the CtCdc42 gene and functional complementation analysis in an S. cerevisiae mutant.
A Cdc42 homolog was isolated from a wild-type strain of C. trifolii race 1 via a PCR-based library screening strategy. BLAST searches of CtCdc42 showed that the deduced amino acid sequence is 92%, 80%, and 79.5% identical to A. nidulans MODA (GenBank accession no. AAF24514), C. albicans Cdc42p (AAB69764), and S. cerevisiae Cdc42p (NP013330), respectively. Importantly, the putative CtCdc42 amino acid sequence contains all the consensus domains characteristic of Cdc42 proteins, including the GTP/GDP binding domains, effector binding site, Rho insert domain, and membrane localization domain (CAAX box [C, cysteine; A, an aliphatic amino acid; X, usually methionine or serine]). Southern analysis revealed that CtCdc42 is a single-copy gene in the C. trifolii genome (not shown).
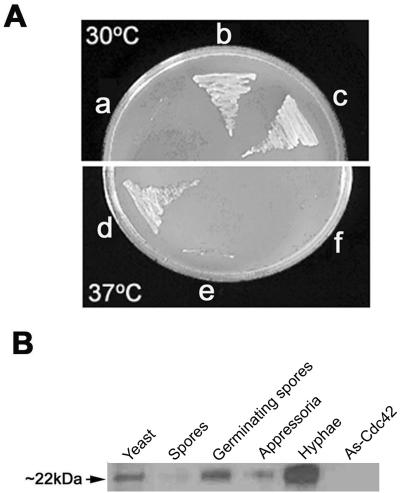
Since the putative CtCdc42 amino acid sequence is 79.5% identical to S. cerevisiae Cdc42p, we determined whether the CtCdc42 gene could functionally replace the S. cerevisiae homolog. Thus, CtCdc42 was cloned into pYES2 and conditionally expressed by a galactose-inducible promoter. A yeast Cdc42 temperature-sensitive mutant (DJTD2-16A) was transformed with the plasmid containing CtCdc42 or with an empty vector, and transformants were screened for growth at the restrictive temperature. The C. trifolii Cdc42 gene was able to rescue the temperature-sensitive growth defects of the S. cerevisiae strain lacking a functional Cdc42 gene at the restrictive temperature (Fig. 1A).
FIG. 1.
CtCdc42 restores the wild-type phenotype to a yeast mutant. (A) Complementation of the temperature-sensitive phenotype of S. cerevisiae DJTD2-16A (Cdc42 disruptant) with CtCdc42. The pYES2 vector or pYES2-CtCdc42 plasmid was transformed into S. cerevisiae DJTD2-16A. Each clone was streaked on an SD Gal plate lacking Ura and subsequently incubated at 30°C or 37°C for 3 days. a and f, recipient strains; b and e, cells transformed with the empty vector pYES2; c and d, cells transformed with pYES2-CtCdc42. (B) Developmental Western blot analysis of CtCdc42. Total protein (20 μg per lane) was isolated from spores, germinating spores, appressoria, and vegetatively growing hyphae, as described in Materials and Methods. Protein extracts from yeast strain EGY48 and the antisense Cdc42 (As-Cdc42) mutant were used as positive and negative controls, respectively. A yeast-derived Cdc42p antibody (Santa Cruz Biotechnology) was used to detect the expression levels of the CtCdc42 protein in different developmental stages.
We then tested whether antibodies raised against the S. cerevisiae Cdc42 protein can detect the homologous proteins in C. trifolii cell lysates (the yeast antibody is derived from a peptide sequence that is also highly conserved in the CtCdc42 protein). In Western blots the yeast-Cdc42p-specific antibody detected a protein of similar size (∼22 kDa) in tissues from all developmental stages of C. trifolii, including spores, germinating spores, appressoria, and mycelia (Fig. 1B). Proteins isolated from yeast strain EGY48 were used as a positive control to confirm the specificity of this antibody. The S. cerevisiae Cdc42 antibody detects C. trifolii Cdc42 in cell lysates. Moreover, we found that high levels of the CtCdc42 protein were observed in germinating conidia and growing hyphae, but relatively low levels of this protein were detected within spores and appressoria, suggesting the possible role of CtCdc42 during cell proliferation (Fig. 1B). Equal loadings of each sample were verified by direct gel staining with Coomassie blue (not shown).
Expression of DA Cdc42 in C. trifolii mimics the phenotypic alterations observed in the DA Ras mutant.
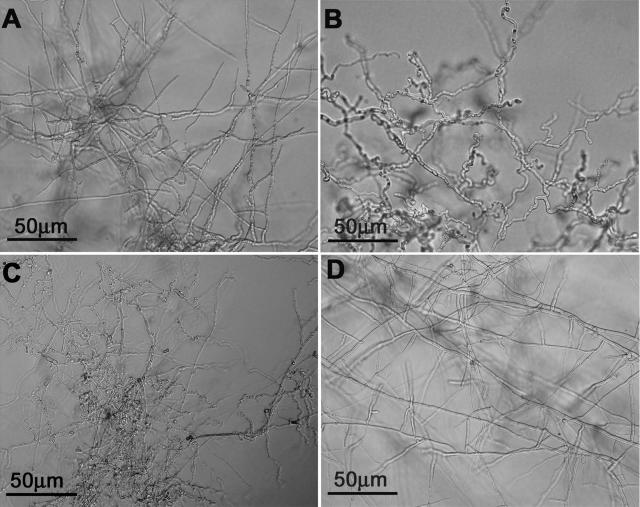
As with all GTPases, Cdc42p cycles between the active GTP-bound form and the inactive GDP-bound form. Single point mutations in the GTP/GDP binding domain (e.g., glycine 12, threonine 17, or aspartic acid 118) are known to result in a dominant active or dominant negative form (11). To investigate the functional role(s) of CtCdc42, we tested the effect of expressing DA Cdc42 following introduction into C. trifolii cells. Transformants expressing DA Cdc42 exhibited no obvious morphological changes when grown on rich medium (YPSS), and the rate of conidial germination, hyphal growth, and conidiation was similar to that of the wild type (not shown). However, after 3 to 6 days of growth on minimal medium, the DA Cdc42 mutant exhibited heavily distorted hyphal growth, which is similar to that of DA Ras (Fig. 2B and C) (43). Intriguingly, we found that the spore germination rate in the DA Cdc42 mutant is as normal as that of the wild type (Fig. 6A and B). However, like the DA Ras mutant strain (43), the DA Cdc42 mutant also failed to develop appressoria under inducing conditions in which the wild-type strain differentiated (not shown).
FIG. 2.
Expression of DA Cdc42 in C. trifolii. Images depict hyphal morphology on minimal medium. As described in Materials and Methods, a plasmid expressing DA Cdc42 was transformed into wild-type C. trifolii. Of 27 independent transformants, 23 transformants exhibited the same phenotypes and were inoculated onto minimal medium for 6 days at room temperature. Hyphal morphology was assessed by microscopy, and the picture shown is a representative of these 23 transformants. (A) Wild type. (B) DA Cdc42. (C) DA Ras. (D) DA Cdc42 mutant grown on minimal medium containing 1.6 mM proline.
FIG. 6.
Coexpression of DA Cdc42 in DN Ras mutants promotes spore germination. (A) Phenotypes of the double mutant expressing DA Cdc42 and DN Ras. Spores of the wild type (panel 1), the DA Cdc42 mutant (panel 2), the DN Ras DA Cdc42 double mutant (panel 3), and the DN Ras mutant (panel 4) were inoculated onto minimal medium supplemented with 15 mM quinic acid, and spore germination was observed by light microscopy 24 h postinoculation. (B) Relative spore germination rates in different strains. Spores from the wild type, the DA Cdc42 mutant, DN Ras mutant, and the double mutants (DN Ras DA Cdc42) were plated at about 100 spores per plate on minimal medium amended with 15 mM quinic acid, and the numbers of germinating spores were recorded after 24 h postinoculation. The percent germination rate represents the percentage of germinating spores compared to the total number of inoculated spores. Error bars represent standard derivations. Experiments were repeated three times, and representative data are shown. (C) Hyphal morphologies of different mutants. Spores of indicated strains were separately inoculated onto minimal medium and grown at room temperature for 6 days. Images depict hyphal morphologies on minimal medium. 1, wild type; 2, DA Cdc42 mutant; 3, DN Ras DA Cdc42 double mutant; 4, DN Ras mutant.
The DA Cdc42 mutant, when grown on minimal medium, expresses high levels of reactive oxygen species (ROS) that are inhibited by proline.
Previously, we have shown that C. trifolii mutants expressing DA Ras exhibited severe defects in polarized growth, aberrant hyphal morphology, and significantly reduced conidiation under nutrient-limiting growth conditions (43). Moreover, proline, when added to minimal medium at a concentration of 1.6 mM, was sufficient to restore the wild-type phenotype (32). Since the growth phenotypes of DA Cdc42 and DA Ras appear to be similar and are nutrient dependent, we asked whether proline also could restore the phenotype of DA Cdc42. Addition of proline (1.6 mM) to minimal medium resulted in phenotypic restoration of the DA Cdc42 mutant, and the wild-type hyphal phenotype was clearly observed (Fig. 2D). This finding suggests that CtCdc42 and CtRas may function in the same signaling cascade. Addition of the other 19 amino acids did not restore the phenotype of the DA Cdc42 mutant (not shown). Overexpression of wild-type CtCdc42 showed no phenotypic alterations on rich medium or minimal medium, indicating that increased levels of CtCdc42 are not overtly detrimental.
Our recent studies have revealed that high levels of ROS production contribute to the aberrant hyphal morphology observed in the DA Ras mutant under nutrient-limiting conditions and that proline restores the wild-type hyphal phenotype by functioning as a potent antioxidant to scavenge ROS (9). Since the DA Cdc42 mutant mimics phenotypic alterations observed in the DA Ras mutant and the heavily distorted hyphal phenotype is restored by proline treatment, it is reasonable to hypothesize that proline-mediated phenotypic restoration also involves changes in ROS generation. To test this, we examined intracellular ROS production in the DA Cdc42 mutant, using the ROS indicator dihydrorhodamine-123. As shown in Fig. 3, the fluorescence emission was significantly higher in DA Cdc42 mutant cells than in the wild-type strain. The effect of proline on ROS generation in the DA Cdc42 mutant was also evaluated. We found that treatment of the DA Cdc42 mutant with 1.6 mM proline dramatically reduced ROS production. These results further establish the antioxidant role of proline in removing intracellular ROS produced in fungal cells and also suggest that the CtCdc42 and CtRas proteins may operate in the same signal transduction pathway to regulate hyphal growth and development.
FIG. 3.
Proline inhibits intracellular ROS generation in the DA Cdc42 mutant when grown on minimal medium. The wild type and the DA Cdc42 mutant were grown at room temperature on minimal medium supplemented with or without proline (1.6 mM). After 6 days of incubation, protoplasts were generated from each strain and aliquots of protoplast cells were incubated with 50 μM dihydrorhodamine-123 (DHR123) and then photographed with an epifluorescence microscope. Pictures shown are representative of three independent experiments. DIC, differential inference contrast optical image. Bar, 50 μm.
Expression of DN Cdc42 significantly decreases spore germination.
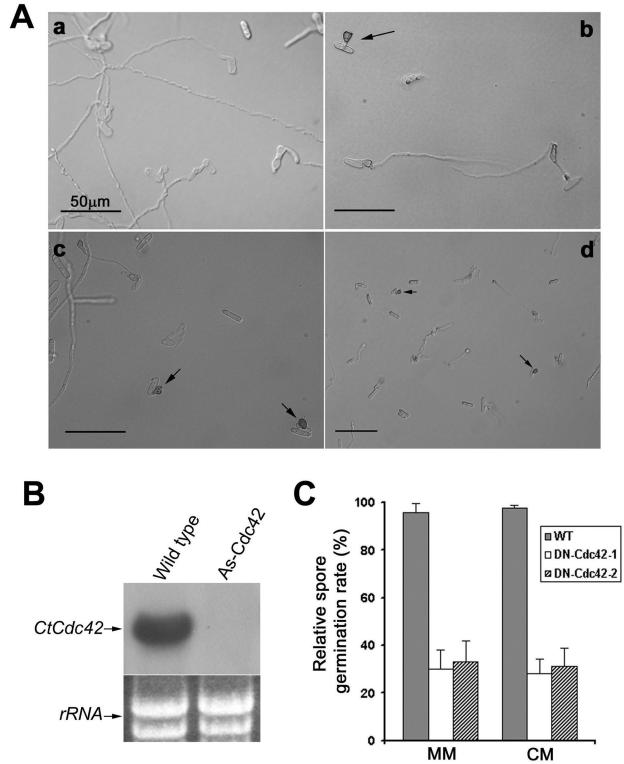
Two independent DN Cdc42 mutant strains (DN Cdc42-1 and DN Cdc42-2) were generated as described in Materials and Methods. As shown in Fig. 4A, these mutants displayed distinct hyphal phenotypes. When grown on minimal medium, DN Cdc42-1 and DN Cdc42-2 showed reduced conidial germination (30% and 33%, respectively) (Fig. 4C). Interestingly, expression of a dominant negative form of Ras (DN Ras) in minimal medium also decreased conidial germination (∼3%) (16). Moreover, we also found that conidial germination of these two mutants was decreased on rich medium (28% and 31%, respectively). Thus, these data suggest that CtCdc42 plays a key role in regulating spore germination in C. trifolii.
FIG. 4.
Phenotypic characterization of CtCdc42 mutants following inhibition of Cdc42 activity. (A) Morphological alterations of C. trifolii transformants expressing dominant negative forms of CtCdc42 or the antisense CtCdc42. Transformants with the same phenotypes (8 of DN Cdc42-1 mutants, 6 of DN Cdc42-2 mutants, and 11 of As Cdc42 mutants) were used for further studies. Pictures shown are representatives of those transformants. Spores of the wild type (a), the DN Cdc42-1 mutant (b), the DN Cdc42-2 mutant (c), and the antisense CtCdc42 mutant (d) were inoculated onto soft water agar medium (1.6%), and cell morphologies were observed with light microscopy after 24 h postinoculation. Note appressorium formation in both the DN Cdc42 and As Cdc42 mutants (arrows). (B) Northern analysis of CtCdc42 expression in the wild type and in the antisense mutant. Agar-mycelium plugs of the wild type or the As Cdc42 mutant were inoculated onto YPSS liquid medium and incubated for 3 to 6 days with constant shaking. Mycelia were harvested by filtration and washed at least three times with sterilized water to completely remove the rich medium. Cultures were then maintained in Czapek-Dox minimal medium (0.2% sodium nitrate, 0.1% potassium phosphate dibasic, 0.05% magnesium sulfate, 0.05% potassium chloride, 0.001% ferric sulfate, 2% agar) for 24 h prior to RNA extraction. Standard Northern analysis was employed using the full-length CtCdc42 cDNA as a probe. For quantification, RNA on the agarose gel was directly stained with ethidium bromide. (C) Relative spore germination rates in different strains. Spores from the wild type (WT) and the CtCdc42 mutants were plated at about 100 spores per plate on minimal medium, and the numbers of germinating spores were recorded after 24 h postinoculation. The percent germination rate represents the percentage of germinating spores compared to the total number of inoculated spores. Data are averages of three separate measurements, and representative data are shown; error bars represent standard deviations. Similar results were observed in three different experiments.
Inhibition of CtCdc42 by antisense expression promotes appressorium formation under noninducing conditions.
We attempted disruptions of the CtCdc42 gene, but viable transformants were not obtained; presumably CtCdc42 is also an essential gene in C. trifolii, similar to the CtRas gene. Thus, an alternative strategy was employed to evaluate the role of CtCdc42 in regulating cell differentiation in C. trifolii. We generated an antisense CtCdc42 mutant strain (As Cdc42) and evaluated antisense expression with respect to spore germination and appressorium formation. Both Northern and Western analyses indicated that the expression of CtCdc42 in this antisense mutant was undetectable compared to expression in the wild-type strain (Fig. 4B and 1B). Consistent with results observed in the DN Cdc42 mutants, expression of an antisense CtCdc42 in C. trifolii also decreased conidial germination by approximately 50% on rich and minimal media. These data further support the positive role of CtCdc42 in spore germination.
In C. trifolii, germinating spores develop appressoria under conditions of nutrient starvation and hard surface contact. Normally appressorium formation is not observed with fungi grown on rich medium or soft surfaces (e.g., 1.6% agar). However, we found that approximately 20% of the germinating spores in the As Cdc42 mutant developed appressoria even on soft agar (Fig. 4A, panel d). We also observed appressorium development in the DN Cdc42 mutants when the germinating spores were incubated on the soft agar (Fig. 4A, panels b and c). Appressorium formation in these mutants is as normal as that of the wild type when grown under conditions of nutrient starvation and hard surface contact (not shown). Collectively, our findings indicate that CtCdc42 acts as a negative regulator in appressorium development in C. trifolii.
Coexpression of DN Cdc42 or As Cdc42 in the DA Ras background restores wild-type hyphal morphology in minimal medium.
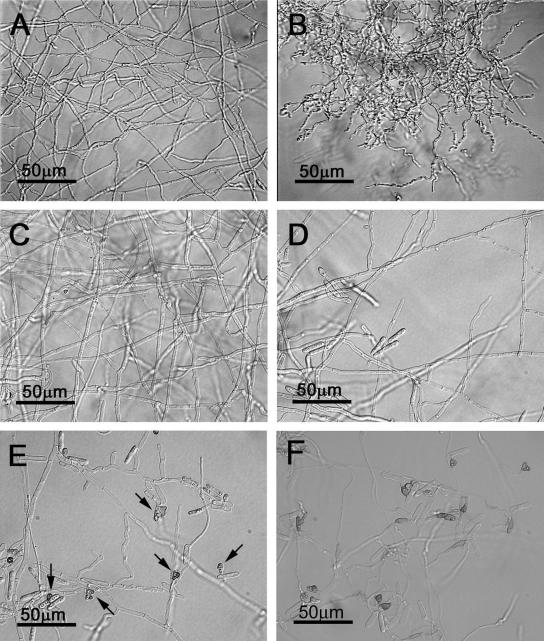
These data suggest that CtCdc42 and CtRas may be positioned in the same signal transduction pathway. To further address this possibility, we examined the effect of DN Cdc42 in the DA Ras mutant. Expression of the DN Cdc42 construct was driven by the constitutively active A. nidulans gpd promoter and confirmed by Northern analysis (not shown). Here, two versions of DN Cdc42 constructs were transformed. We examined 11 DA Ras DN Cdc42-1 double mutants from 20 drug-resistant transformants and 8 DA Ras DN Cdc42-2 double mutants from 15 drug-resistant transformants. On rich media, the double mutants (DA Ras DN Cdc42) grew and developed as does the wild type. However, when grown on minimal medium, where the DA Ras mutant displays aberrant hyphal growth (Fig. 5B), the double mutant (Fig. 5C and D) produced a wild-type hyphal phenotype, suggesting that DN Cdc42 significantly inhibited the DA Ras-induced aberrant hyphal morphogenesis. Moreover, in contrast to the DA Ras mutant, we observed that these double mutants also restored the ability to conidiate and develop appressoria (Fig. 5F).
FIG. 5.
Coexpression of DN CtCdc42 (DN Cdc42-1 and DN Cdc42-2) or antisense CtCdc42 (As Cdc42) in a DA Ras mutant leads to phenotypic restoration and appressorial formation under noninducible conditions. The double mutants were grown on minimal medium for 6 days, and the morphologies of hyphae were observed with light microscopy. To detect appressorial development, spores of two double mutants (DA Ras As Cdc42 and DA Ras DN Cdc42-1) were inoculated onto soft water agar medium (1.6%) and cell morphologies were observed with light microscopy after 24 h postinoculation. (A to D) Hyphal phenotypes after 6 days of incubation. A, wild type; B, DA Ras; C, DA Ras DN Cdc42-1; D, DA Ras DN Cdc42-2. (E and F) Development of appressoria on noninducible, soft water agar medium following 24 h of incubation. E, DA Ras As Cdc42 (arrows show the generation of appressoria); F, DA Ras DN Cdc42-1.
To confirm the above observations, we also overexpressed the antisense CtCdc42 construct (As Cdc42) in the DA Ras mutant. Expression of antisense CtCdc42 was also verified by Northern analysis. From 33 drug-resistant transformants, 25 transformants showed similar levels of hyphal growth and were used for the next study. As shown in Fig. 5E, inhibition of CtCdc42 expression in the DA Ras mutant also resulted in normal conidiation and appressorium formation. This mutant still displayed moderate hyphal distortion, suggesting that expression of As Cdc42 only partially suppressed the aberrant hyphal phenotype of the DA Ras mutant. We observed that overexpression of As Cdc42 in the DA Ras mutant also induced appressorium formation on a soft agar (1.6%) surface (Fig. 5E), further supporting the finding that CtCdc42 negatively regulates appressorium formation. Overexpression of wild-type CtCdc42 or DA Cdc42 in the DA Ras mutant failed to suppress the distorted hyphal phenotype (data not shown). Taken together, our results suggest that CtCdc42 and CtRas are positioned in the same pathway and that CtCdc42 may act as a downstream effector of CtRas.
CtCdc42 regulates spore germination and hyphal growth by distinct pathways.
To further confirm that CtCdc42 is a downstream effector of CtRas, we coexpressed DN Ras in wild-type C. trifolii with DA Cdc42. In this case, expression of DA Cdc42 was driven by the constitutive A. nidulans glyceraldehyde-3-phosphate dehydrogenase (gpd) promoter, but expression of DN Ras was driven by the conditional Neurospora crassa qa-2 promoter. The N. crassa qa-2 promoter is activated by quinic acid but repressed by glucose and other carbon sources. Twelve positive transformants from 18 drug-resistant colonies were investigated. As shown in Fig. 6A, when grown on minimal medium containing 15 mM quinic acid, DA Cdc42 strains have nearly normal spore germination rates (Fig. 6A, panel 2), while DN Ras-expressing strains barely germinate (Fig. 6A, panel 4). In the double mutant (DN Ras DA Cdc42), DN Ras was not able to revert the DA Cdc42 phenotype, consistent with CtCDC42 being downstream of CtRAS. A quantitative assay of germination rates in the various genetic backgrounds is consistent with this conclusion (Fig. 6B). However, we found that the distorted hyphal morphology shown in the DA Cdc42 mutant was restored to a wild-type-like hyphal phenotype when DN Ras was coexpressed (Fig. 6C, panel 3), similar to what we observe in the DN Ras background (Fig. 6C, panel 4). Thus, in this case, DN Ras remediates hyphal growth in the DA Cdc42 mutant. These data suggest that CtRas and CtCdc42 may be linked in the same pathway (e.g., spore germination), may be independent in the same pathway, or may be in parallel pathways (e.g., hyphal growth).
DISCUSSION
Numerous reports have shown that Cdc42p homologs play diverse roles during growth and development in eukaryotes. In fungi, the Cdc42 protein has been found to regulate morphological changes, including organization of the actin cytoskeleton and polarized growth (18, 21, 37). C. trifolii Cdc42p (CtCdc42) has significant sequence identity with other known Cdc42p proteins and contains all of the predicted domains necessary for GTPase function. Moreover, we found that CtCdc42 functionally complements the S. cerevisiae temperature-sensitive Cdc42 mutation. Thus, the high level of structural conservation suggests the functional conservation of CtCdc42 protein as well. In this study, we investigated the role of Cdc42 in cell growth regulation of C. trifolii by examining the consequences of altering the expression of CtCdc42. As a result, we found that CtCdc42 is involved in the positive regulation of spore germination and negative regulation of appressorium formation.
We observed that the inhibition of CtCdc42 by antisense expression dramatically decreased spore germination rates. In accordance with this observation, we also found that overexpression of DN Cdc42 in C. trifolii showed a significant decrease in the rate of spore germination. The involvement of Cdc42 proteins in spore germination has also been described for other fungi. For example, the C. trifolii DA Cdc42 construct, when expressed in C. purpurea, completely inhibited conidiation (41). Moreover, the Cdc42 homolog CflA in P. marneffei was found to be required for proper cell polarization and to affect the rate of conidial germination (3). Spore germination is a key step in the colonization process of a new environment by filamentous fungi. Recent studies of C. trifolii have revealed that various signaling molecules, including calcium, cyclic AMP (cAMP), calmodulin, and protein kinases, are important players in regulating spore germination (14, 46, 50). Here we described the involvement of the small GTPase Cdc42 in this process; it will be of interest to examine the relationship between Cdc42 and these other signaling components.
We found that CtCdc42 negatively regulates appressorium formation. Appressoria are structures that a number of fungal pathogens employ for attachment to the plant surface in preparation for infection of host tissue (12). The initiation, formation, and activity of the appressorium is an integral part of the infection process. Appressoria facilitate the breaching of the cuticular barrier of the plant and are essential for fungal penetration (22). In C. trifolii, appressorium development is generally induced by starvation and hard surface contact (inducible conditions) and suppressed by rich media and/or soft surfaces (noninducing conditions). Unexpectedly, we found that inhibition of Cdc42 activity by either antisense expression or a dominant negative mutation promotes appressorium formation under noninducing conditions, suggesting that CtCdc42 activity is critical for proper regulation of appressorium development in C. trifolii. To our knowledge, this is the first report of the involvement of the Cdc42 protein in appressorium development. Previous studies using the DN Ras mutant have shown that unlike under inductive conditions (hard surface), where a slight decrease of appressorium formation was observed, this mutant exhibited no appressorium formation on soft agar. Thus, it appears that CtCdc42 is integrated with other as-yet-uncharacterized pathways, in addition to the Ras signaling pathway, to regulate appressorium development. Previous studies of C. trifolii have shown that an increase in endogenous cAMP levels by using cAMP-elevating agents, such as 8-Br-cAMP, sodium fluoride, and 3-isobutyl-1-methylxanthine, also results in induction of appressorial differentiation on a noninductive surface, suggesting a possible linkage between CtCdc42 and the cAMP signaling pathway (50). Moreover, several signaling molecules in C. trifolii that are important for appressorium development have been isolated and characterized, including the catalytic subunit of PKA, calmodulin, and lipid-induced protein kinase (14, 46, 49, 50). Molecular analysis of the connection of CtCdc42 with these molecules will improve the understanding of signaling pathways involved in appressorium development in C. trifolii. The mechanism(s) of appressorium development has been intensively studied in phytopathogenic fungi, including Magnaporthe grisea and other Colletotrichum spp. The results of these studies indicated that cAMP/PKA- and MAPK-mediated signaling pathways are operative during appressorium development (10, 46, 48-50). Interestingly, disruption of MAGB, encoding an inhibitory group I subunit of a heterotrimeric G protein (Gαi), affects the appressorium formation and pathogenicity of M. grisea (26), suggesting the possibility that G protein may participate in the process of appressorium formation.
Recent studies of diverse eukaryotes have demonstrated the connection between Cdc42 and Ras signaling. In mammals, Cdc42 is involved not only in cytoskeletal reorganization but also in Ras-dependent transformation, anchorage-independent growth in soft agar, and tumor formation (38, 40); in the fission yeast S. pombe, Ras1 activates Cdc42p to regulate cell polarity and the mating pathway (30); in the budding yeast S. cerevisiae, Ras2 stimulates filamentous growth via activation of Cdc42p through the Ste 20/MAPK module (34); and in C. albicans, it was suggested that CaCdc42 may transduce the signals from CaRas1p to control the dimorphic switch and vegetative growth (20, 23). In this study, we also provided evidence to establish the linkage between Cdc42 and Ras signaling in C. trifolii. By generating DA Ras, we showed previously that Ras plays a pivotal role in regulating proper growth and development in C. trifolii, especially hyphal morphology, conidiation, and appressorium differentiation (8, 16, 43). Like the DA Ras mutant, morphological alterations, including aberrant hyphal growth and failure in conidiation and appressorium formation, were also observed in the DA Cdc42 mutant under nutrient-limiting conditions. Expression of DN Cdc42 in C. trifolii inhibits spore germination, as does DN Ras. In addition, coexpression of dominant negative CtCdc42 in the DA Ras mutant leads to suppression of aberrant hyphal morphology and also to restoration of normal conidiation. Antisense expression of CtCdc42 in DA Ras partially restored the wild-type phenotype, and coexpression of DA Cdc42 in the DN Ras mutant increased sporulation. Thus, these data suggest that CtCdc42 and CtRas may function in the same signaling cascade to control proper fungal development. Our data, however, also indicate that Cdc42 is not always downstream of Ras in this fungus, since the abnormal hyphal growth phenotype observed in DA Cdc42 was restored to that of the wild type when coexpressed with DN Ras.
The positioning of CtCDC42 downstream of CtRAS was further supported by phenotypic remediation with proline. We have previously shown that proline (1.6 mM), when added to minimal medium, is sufficient to restore the wild-type hyphal phenotype of the DA Ras mutant (32). When proline was supplied to the DA Cdc42 mutant, the wild-type phenotype was also fully restored. Recently, we proposed that proline functions, at least in part, to scavenge intracellular ROS generation induced by DA Ras and therefore inhibits the apoptosis-like programmed cell death in C. trifolii (9). Thus, we considered whether proline could restore the wild-type phenotypes of DA Cdc42 via the inhibition of intracellular ROS generation. We found that expression of dominant active Cdc42 in C. trifolii induces large amounts of ROS and that this ROS production is effectively quenched by proline. To date, the relationship between Cdc42 and ROS production in filamentous fungi has not been described.
Specific gene expression in response to nutrient deprivation is a common theme in microbial development. In fungi, nutrient starvation is a major environmental cue leading to changes in invasiveness, nutrient foraging, cell-cell interaction, and important morphological transitions (24, 29). As demonstrated in this study, we found that Cdc42, together with Ras, may be involved in sensing nutritional signals to regulate hyphal growth and development. DA Cdc42 and DA Ras, when expressed in C. trifolii, both yield similar nutrient-dependent responses. When grown on nutrient-rich medium, the growth and development of these two mutants are indistinguishable from that of the wild type. However, under nutrient deprivation conditions, both of these mutants exhibit distorted hyphal growth, virtually no sporulation, and a failure in appressorial formation. Recent studies have shown a direct association between ROS generation and nutrient sensing. For example, in plants, ROS, notably hydrogen peroxide, mediate an early root response to nutrient deficiency, suggesting a link between ROS generation and nutrient deprivation (42). Moreover, nutrient deprivation, which is associated with ischemia, a complex physiological process in the inflammatory response, involves NADPH oxidase activation and subsequent ROS generation (27). To understand how nutrient deficiency alters fungal growth and development, we measured intracellular ROS generation in these two mutants. As expected, extremely low levels of ROS production were observed in these two mutants when grown on nutrition-rich medium, as was observed with the wild-type strain. However, significant amounts of ROS were produced when these strains were transferred to nutrient deprivation conditions. Thus, ROS may play an important role in modulating the induction of gene expression in C. trifolii in response to nutrient deficiency. Our results suggest that ROS production in C. trifolii, at least in part, is regulated by the activities of small GTP-binding proteins. More importantly, ROS generation is involved in a nutrient signaling cascade, and we propose that these signals regulate changes in fungal growth and development.
Our previous data have also shown that the small GTPase Rac is involved in Ras-regulated hyphal morphogenesis in C. trifolii (8). Like CtCdc42, CtRac1 acts as a downstream effector of Ras, and its activity contributes to proper hyphal growth and development. Interestingly, coexpression of DA Rac in the DA Ras mutant fully restored the wild-type phenotype under nutrient-limiting conditions via the activation of the MAPK pathway. Since coexpression of dominant negative Cdc42 in a DA Ras mutant resulted in the same phenotype, it is possible that an antagonistic role between Rac and Cdc42 exists in C. trifolii; the balance between Rac and Cdc42 activity may be a major determinant of hyphal growth and development in this fungus. To support this hypothesis, future studies will focus on how Ras regulates Cdc42 activity and how Cdc42 affects Rac and MAPK activities.
ADDENDUM
Inoculation of alfalfa plants with either dominant negative or antisense Cdc42 strains resulted in levels of disease that were similar to wild-type strain infection.
Acknowledgments
This work was supported by the United States-Israel Binational Agriculture Research and Development Fund (BARD) grant no. 2814-96.
REFERENCES
- 1.Aspenstrom, P. 1999. The Rho GTPases have multiple effects on the actin cytoskeleton. Exp. Cell Res. 246:20-25. [DOI] [PubMed] [Google Scholar]
- 2.Austin, B., R. M. Hall, and B. M. Tyler. 1990. Optimized vectors and selection for transformation of Neurospora crassa and Aspergillus nidulans to bleomycin and phleomycin resistance. Gene 93:157-162. [DOI] [PubMed] [Google Scholar]
- 3.Boyce, K. J., M. J. Hynes, and A. Andrianopoulos. 2001. The CDC42 homolog of the dimorphic fungus Penicillium marneffei is required for correct cell polarization during growth but not development. J. Bacteriol. 183:3447-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Chang, E. C., M. Barr, Y. Wang, V. Jung, H. P. Xu, and M. H. Wigler. 1994. Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell 79:131-141. [DOI] [PubMed] [Google Scholar]
- 6.Chant, J., and L. Stowers. 1995. GTPase cascades choreographing cellular behavior: movement, morphogenesis, and more. Cell 81:1-4. [DOI] [PubMed] [Google Scholar]
- 7.Chen, C., and M. B. Dickman. 2002. Colletotrichum trifolii TB3 kinase, a COT1 homolog, is light inducible and becomes localized in the nucleus during hyphal elongation. Eukaryot. Cell 1:626-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, C., and M. B. Dickman. 2004. Dominant active Rac and dominant negative Rac revert the dominant active Ras phenotype in Colletotrichum trifolii by distinct signalling pathways. Mol. Microbiol. 51:1493-1507. [DOI] [PubMed] [Google Scholar]
- 9.Chen, C., and M. B. Dickman. 2005. Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc. Natl. Acad. Sci. USA 102:3459-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi, W., and R. A. Dean. 1997. The adenylate cyclase gene MAC1 of Magnaporthe grisea controls appressorium formation and other aspects of growth and development. Plant Cell 9:1973-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis, C. R., T. J. Richman, S. B. Deliduka, J. O. Blaisdell, C. C. Collins, and D. I. Johnson. 1998. Analysis of the mechanisms of action of the Saccharomyces cerevisiae dominant lethal cdc42G12V and dominant negative cdc42D118A mutations. J. Biol. Chem. 273:849-858. [DOI] [PubMed] [Google Scholar]
- 12.Dean, R. A. 1997. Signal pathways and appressorium morphogenesis. Annu. Rev. Phytopathol. 35:211-234. [DOI] [PubMed] [Google Scholar]
- 13.Dickman, M. B. 1988. Whole cell transformation of the alfalfa fungal pathogen Colletotrichum trifolii. Curr. Genet. 14:241-246. [Google Scholar]
- 14.Dickman, M. B., Y. S. Ya, Z. Yang, B. Adams, and C. Huang. 2004. A protein kinase from Colletotrichum trifolii is induced by plant cutin and is required for appressorium formation. Mol. Plant-Microbe Interact. 16:411-421. [DOI] [PubMed] [Google Scholar]
- 15.Gimeno, C. J., P. O. Ljungdahl, C. A. Styles, and G. R. Fink. 1992. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68:1077-1090. [DOI] [PubMed] [Google Scholar]
- 16.Ha, Y. S., S. D. Memmott, and M. B. Dickman. 2003. Functional analysis of Ras in Colletotrichum trifolii. FEMS Microbiol. Lett. 226:315-321. [DOI] [PubMed] [Google Scholar]
- 17.Hakoshima, T., T. Shimizu, and R. Maesaki. 2003. Structural basis of the Rho GTPase signaling. J. Biochem. (Tokyo) 134:327-331. [DOI] [PubMed] [Google Scholar]
- 18.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 19.Ho, J., and A. Bretscher. 2001. Ras regulates the polarity of the yeast actin cytoskeleton through the stress response pathway. Mol. Biol. Cell 12:1541-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang, L., C. M. Lee, and S. H. Shen. 2002. Functional characterization of the Candida albicans homologue of secretion-associated and Ras-related (Sar1) protein. Yeast 19:423-428. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, D. I. 1999. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63:54-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubo, Y., K. Suzuki, I. Furusawa, N. Ishida, and M. Yamamoto. 1982. Relation of appressorium pigmentation and penetration of nitrocellulose membranes by Colletotrichum lagenarium. Phytopathology 72:498-501. [Google Scholar]
- 23.Leberer, E., D. Harcus, D. Dignard, L. Johnson, S. Ushinsky, D. Y. Thomas, and K. Schroppel. 2001. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol. Microbiol. 42:673-687. [DOI] [PubMed] [Google Scholar]
- 24.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W. C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, H. 2001. Transcriptional control of dimorphism in Candida albicans. Curr. Opin. Microbiol. 4:728-735. [DOI] [PubMed] [Google Scholar]
- 26.Liu, S., and R. A. Dean. 1997. G protein alpha subunit genes control growth, development, and pathogenicity of Magnaporthe grisea. Mol. Plant-Microbe Interact. 10:1075-1086. [DOI] [PubMed] [Google Scholar]
- 27.Lopes, N. H. M., S. Vasudevan, D. Greeg, B. Selvakumar, P. Pagano, H. Kovacic, and P. J. Goldschmidt-Clermont. 2002. Rac-dependent monocyte chemoattractant protein-1 production is induced by nutrient deprivation. Circ. Res. 91:798-805. [DOI] [PubMed] [Google Scholar]
- 28.Lundblad, V. 1994. Saccharomyces cerevisiae, vol. 2. John Wiley & Sons, Inc., New York, N.Y.
- 29.Madhani, H. D., and G. R. Fink. 1998. The control of filamentous differentiation and virulence. Trends Cell Biol. 8:348-353. [DOI] [PubMed] [Google Scholar]
- 30.Marcus, S., A. Polverino, E. Chang, D. Robbins, M. H. Cobb, and M. H. Wigler. 1995. Shk1, a homolog of the Saccharomyces cerevisiae Ste20 and mammalian p65PAK protein kinases, is a component of a Ras/Cdc42 signaling module in the fission yeast Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 92:6180-6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matozaki, T., H. Nakanishi, and Y. Takai. 2000. Small G-protein networks: their crosstalk and signal cascades. Cell Signal. 12:515-524. [DOI] [PubMed] [Google Scholar]
- 32.Memmott, S. D., Y. S. Ha, and M. B. Dickman. 2002. Proline reverses the abnormal phenotypes of Colletotrichum trifolii associated with expression of endogenous constitutively active Ras. Appl. Environ. Microbiol. 68:1647-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michel, S., S. Ushinsky, B. Klebl, E. Leberer, D. Thomas, M. Whiteway, and J. Morschhauser. 2002. Generation of conditional lethal Candida albicans mutants by inducible deletion of essential genes. Mol. Microbiol. 46:269-280. [DOI] [PubMed] [Google Scholar]
- 34.Mosch, H. U., R. L. Roberts, and G. R. Fink. 1996. Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93:5352-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polverino, A., J. J. Frost, P. Yang, M. Hutchison, A. M. Neiman, M. H. Cobb, and S. Marcus. 1995. Activation of mitogen-activated protein kinase cascades by p21-activated protein kinases in cell-free extracts of Xenopus oocytes. J. Biol. Chem. 270:26067-26070. [DOI] [PubMed] [Google Scholar]
- 36.Powell, W. A., and H. C. Kistler. 1990. In vivo rearrangement of foreign DNA by Fusarium oxysporum produced linear self-replicating plasmids. J. Bacteriol. 172:3163-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pringle, J. R., E. Bi, H. A. Harkins, J. E. Zahner, C. De Virgilio, J. Chant, K. Corrado, and H. Fares. 1995. Establishment of cell polarity in yeast. Cold Spring Harbor Symp. Quant. Biol. 60:729-744. [DOI] [PubMed] [Google Scholar]
- 38.Qiu, R. G., A. Abo, F. McCormick, and M. Symons. 1997. Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol. Cell. Biol. 17:3449-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts, I. N., R. P. Oliver, P. J. Punt, and C. A. van den Hondel. 1989. Expression of the Escherichia coli beta-glucuronidase gene in industrial and phytopathogenic filamentous fungi. Curr. Genet. 15:177-180. [DOI] [PubMed] [Google Scholar]
- 40.Roux, P., C. Gauthier-Rouviere, S. Doucet-Brutin, and P. Fort. 1997. The small GTPases Cdc42Hs, Rac1 and RhoG delineate Raf-independent pathways that cooperate to transform NIH 3T3 cells. Curr. Biol. 7:629-637. [DOI] [PubMed] [Google Scholar]
- 41.Scheffer, J., C. Chen, P. Heidrich, M. B. Dickman, and P. Tudzynski. A CDC42 homologue in Claviceps purpurea is involved in vegetative differentiation and is essential for pathogenicity. Eukaryot. Cell 4:1228-1238. [DOI] [PMC free article] [PubMed]
- 42.Shin, R., and D. P. Schachtman. 2004. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc. Natl. Acad. Sci. USA 101:8827-8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Truesdell, G. M., C. Jones, T. Holt, G. Henderson, and M. B. Dickman. 1999. A Ras protein from a phytopathogenic fungus causes defects in hyphal growth polarity, and induces tumors in mice. Mol. Gen. Genet. 262:46-54. [DOI] [PubMed] [Google Scholar]
- 44.Ushinsky, S., D. Harcus, J. Ash, D. Dignard, A. Marcil, J. Morchhause, D. Thomas, M. Whiteway, and E. Leberer. 2002. CDC42 is required for polarized growth in the human pathogen Candida albicans. Eukaryot. Cell 1:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Aelst, L., and C. D'Souza-Schorey. 1997. Rho GTPases and signaling networks. Genes Dev. 11:2295-2322. [DOI] [PubMed] [Google Scholar]
- 46.Warwar, V., S. Oved, and M. B. Dickman. 2000. Antisense expression of the calmodulin gene from Colletotrichum trifolii impairs prepenetration development. FEMS Microbiol. Lett. 191:213-219. [DOI] [PubMed] [Google Scholar]
- 47.Wendland, J., and P. Philippsen. 2001. Cell polarity and hyphal morphogenesis are controlled by multiple rho-protein modules in the filamentous ascomycete Ashbya gossypii. Genetics 157:601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu, J. R. 2000. Map kinases in fungal pathogens. Fungal Genet. Biol. 31:137-152. [DOI] [PubMed] [Google Scholar]
- 49.Yang, Z., and M. B. Dickman. 1999. Molecular cloning and characterization of Ct-PKAR, a gene encoding the regulatory subunit of cAMP-dependent protein kinase in Colletotrichum trifolii. Arch. Microbiol. 171:249-256. [DOI] [PubMed] [Google Scholar]
- 50.Yang, Z., and M. B. Dickman. 1997. Regulation of cAMP and cAMP dependent protein kinase during conidial germination and appressorium formation in Colletotrichum trifolii. Physiol. Mol. Plant Pathol. 50:117-127. [Google Scholar]
- 51.Ye, X., and P. J. Szaniszlo. 2000. Expression of a constitutively active Cdc42 homologue promotes development of sclerotic bodies but represses hyphal growth in the zoopathogenic fungus Wangiella (Exophiala) dermatitidis. J. Bacteriol. 182:4941-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziman, M., D. Preuss, J. Mulholland, J. M. O'Brien, D. Botstein, and D. I. Johnson. 1993. Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol. Biol. Cell 4:1307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zohn, I. M., S. L. Campbell, R. Khosravi-Far, K. L. Rossman, and C. J. Der. 1998. Rho family proteins and Ras transformation: the RHOad less traveled gets congested. Oncogene 17:1415-1438. [DOI] [PubMed] [Google Scholar]