Abstract
The mechanism by which encephalitic flaviviruses enter the brain to inflict a life-threatening encephalomyelitis in a small percentage of infected individuals is obscure. We investigated this issue in a mouse model for flavivirus encephalitis in which the virus was administered to 6-week-old animals by the intravenous route, analogous to the portal of entry in natural infections, using a virus dose in the range experienced following the bite of an infectious mosquito. In this model, infection with 0.1 to 105 PFU of virus gave mortality in ∼50% of animals despite low or undetectable virus growth in extraneural tissues. We show that the cytolytic effector functions play a crucial role in invasion of the encephalitic flavivirus into the brain. Mice deficient in either the granule exocytosis- or Fas-mediated pathway of cytotoxicity showed delayed and reduced mortality. Mice deficient in both cytotoxic effector functions were resistant to a low-dose peripheral infection with the neurotropic virus.
Murray Valley encephalitis virus (MVE) is an Australian member of the encephalitic flaviviruses grouped into the Japanese encephalitis virus (JEV) serocomplex, which also includes West Nile virus (WNV) and St. Louis encephalitis virus (7, 37). The viruses are transmitted by mosquitoes to their normal vertebrate hosts (predominantly birds) and can cause incidental epidemic outbreaks of disease in humans. Most infections of humans with flaviviruses of the JEV serocomplex are subclinical, the estimated ratio of inapparent to apparent infection being between 200:1 and 1,000:1; however, the case fatality rate can be high (up to 50%), and life-long neuropsychological sequelae frequently occur among survivors of flaviviral encephalitis (for reviews, see references 33 and 37). Thus, an improved understanding of the pathogenesis of flaviviruses in animal models could significantly improve human health.
Mice provide an excellent animal model for flaviviral encephalitis in humans. Members of the JEV serocomplex are neurotropic in mice. When directly inoculated into the brain, they grow to high titers, causing a mostly fatal encephalomyelitis. The disease outcome following extraneural inoculation of the encephalitic flaviviruses into mice is strongly host factor dependent (for a review, see reference 35). For example, age of the animals is particularly important (11, 31, 32); mice up to 3 to 4 weeks of age are highly susceptible to a low-dose virus inoculum, whereas in older animals the peripheral injection of these flaviviruses often fails to result in morbidity or mortality over a wide dose range.
One disadvantage of the mouse model is the erratic course of infection in older mice, which requires a larger number of animals to make a reliable determination of the 50% lethal dose. A number of factors could cause the age-dependent differences in the susceptibility of mice to flavivirus infection; these include, but not exclusively, (i) higher peripheral virus yield and viremia in younger animals (32), (ii) an age-dependent difference in the permeability of the blood-brain barrier, (iii) a stronger tropism of the viruses for developing neurons (47, 48), and (iv) absence, delay, or dysfunction of innate and/or adaptive immune responses in the younger animals (52, 55, 63).
The key to controlling viremia is humoral immunity. Passive transfer of antibodies and vaccination-induced humoral immune responses can protect against lethal flavivirus challenge, including infection via the intracerebral (i.c.) route (4, 12, 22, 56). However, the kinetics of induction of humoral immunity in a primary flavivirus infection may not be sufficiently rapid to prevent neuroinvasion following peripheral virus growth and, in turn, fatal encephalitis. On the other hand, cellular antiviral immune responses are raised with a kinetics consistent with the requirement for rapid viral elimination (10, 20, 21). For example, primary WNV-immune cytotoxic T (Tc) cells are apparent at 5 days postinfection (p.i.), and the response peaks on day 7. Virus-immune Tc cells and natural killer (NK) cells have also been isolated from the brains of WNV-infected mice (26). However, it is still unclear whether these virus-induced cytolytic lymphocytes (NK/Tc cells) are necessary for the recovery from encephalitic flavivirus infection or whether they induce immunopathology, as observed in other viral models (5, 8, 38). Their role in recovery may depend on whether their function, when directed against virus-infected cells in the periphery, can prevent virus spread into the central nervous system (CNS).
Following neuroinvasion, encephalitic flaviviruses efficiently infect large numbers of neurons, and this process is accompanied by an inflammatory reaction (13, 15). The relative contributions to neuronal destruction and mortality of flaviviral infection of neurons, per se, and of the induced immunopathological events remain ambiguous (1, 24, 46).
Perforin-dependent granule exocytosis- and Fas-mediated cytotoxicity are the two major pathways causing target cell damage by cytolytic lymphocytes (14, 19). The availability of mouse strains defective in these cytotoxic effector pathways as a consequence of gene knockout or null mutations allows evaluation of the role of these two cytotoxic pathways in viral pathogenesis. Here we have applied this approach to a mouse model of flaviviral encephalomyelitis and revealed an unexpected Tc/NK-dependent pathogenicity component.
MATERIALS AND METHODS
Viruses and cells.
The MVE prototype strain MVE-1-51 (9) was used. It has been passaged 15 times in suckling mouse brain. Working stocks were prepared from either 10% suckling mouse brain homogenates in Hanks' balanced salt solution (HBSS) containing 20 mM HEPES buffer (pH 8.0) and 0.2% bovine serum albumin (HBSS-BSA) or from infected Aedes albopictus C6/36 cell culture supernatant buffered to pH 8.0. Virus was stored in single-use aliquots at −70°C. Titers of working stocks ranged from 5 × 107 to 2 × 109 Vero cell PFU/ml.
Vero cells (African green monkey kidney; obtained from the American Type Culture Collection), and the methylcholanthrene-induced fibrosarcoma cells 2R (H-2KkDb) and 5R (H-2KbDd) and L929 cells (H-2k) were maintained in Eagle's minimal essential medium (EMEM) plus nonessential amino acids and 5% fetal calf serum (FCS). C6/36 cells were grown in EMEM plus nonessential amino acids and 8% FCS at 28°C.
Animals.
C57BL/6 (B6) mice, syngeneic gene knockout mice defective in perforin (perf−/−) (17), granzymes A and B (gzmAxB−/−) (58), or granzymes A and B plus perforin (perf × gzmAxB−/−) (42), and syngeneic mutant mice with defects in the Fas receptor (lpr) (54) or Fas ligand (gld) (60) were bred under specific-pathogen-free conditions and supplied by the Animal Breeding Facilities at the John Curtin School of Medical Research, Canberra. Mice doubly deficient in the perforin and Fas pathways of cytotoxicity (perf−/− × gld) were generated by back-crossing breeders heterozygous for perforin and homozygous for the Fas ligand mutation (perf+/− × gld). This protocol was required because female perf−/− × gld mice are infertile (also reported in references 18 and 59). Weanling mice obtained from this mating were screened for homozygosity of the perforin knockout mutation and kept for experimentation. The screening was done by PCR on genomic DNA obtained by tail biopsies using a protocol described previously (58).
Plaque assay.
Virus was titrated by plaque formation on ≈80% confluent monolayers of Vero cells (≈3 × 105 cells/well) in six-well plastic tissue culture plates (Linbro Scientific Inc., Hamden, Conn.). Samples to be assayed were serially diluted in HBSS-BSA on ice, and monolayers were inoculated in duplicate with 0.1-ml aliquots of the diluted virus. Adsorption was for 1 h at 37°C in an atmosphere of 5% CO2 and 95% air with occasional shaking. An agar overlay medium (EMEM plus nonessential amino acids containing 1% Bacto-agar [Difco, Detroit, Mich.], 2% FCS, penicillin [30 μg/ml; Sigma, St. Louis, Mo.], streptomycin sulfate [50 μg/ml; Sigma], neomycin sulfate [50 μg/ml; Sigma], and amphotericin B [Fungizone; 0.25 μg/ml; Life Technologies, Rockville, Md.]; 4 ml/well) was added to the monolayer, and the cells were incubated for 72 to 96 h at 37°C. The monolayers were stained by the addition of 1.5 ml of 0.03% neutral red (BDH Chemicals, Poole, England) in HBSS, and plaques were counted 10 to 16 h later, following removal of the stain and the overlay.
For virus determination in infected mouse tissues, animals were sacrificed at a given time p.i., and tissues were aseptically removed, snap-frozen in liquid nitrogen or dry ice, and stored at −70°C. The 10% (wt/vol) tissue suspensions in ice-cold HBSS-BSA were homogenized and clarified by centrifugation (18,000 × g for 5 min at 4°C), and supernatants were stored in aliquots at −70°C prior to plaque titration. The limits of detection of virus in tissues and serum of infected mice by plaque titration were 103 PFU/g and 102 PFU/ml, respectively.
ELISA.
MVE antigen-coated 96-well microtiter plates were prepared as previously described (6). Serial twofold dilutions (50 μl/well) of antisera in phosphate-buffered saline (PBS) containing 1% BSA and 0.05% Tween 20 (Sigma; PBS/BSA/Tween) were added to the plates and incubated for 2 h at room temperature. The plates were washed four times with PBS containing 0.05% Tween 20 before the addition of 50-μl/well horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (Ig; Dako, Carpinteria, Calif.) diluted 1:1,000 in PBS/BSA/Tween for 1 h at room temperature. The wells were washed as above, 50 μl of the peroxidase substrate 2.2′-azino-di[3-ethyl-benzthiazoline sulfonate] (ABTS; Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was added per well, and the plates were kept at room temperature for ∼20 min to allow color development. The enzymatic reaction was stopped by the addition of 1% sodium dodecyl sulfate, and plates were read at 405 nm with a Molecular Dynamics microplate reader.
Optical density (OD) cutoff values were established as the mean OD of eight negative control wells containing sera from naïve mice plus 3 standard deviations (SD). OD values were considered positive if they were equal to or greater than the OD cutoff. Endpoint titers were calculated as the reciprocal of the last dilution that was positive, and the antibody titers are given as log10 values.
Chromium release assay.
The generation of secondary in vitro MVE-immune effector Tc cells, target cell infections, and 51Cr release assay were performed as described previously (27, 51). Briefly, B6 mice were immunized intraperitoneally with 5 × 106 PFU of MVE, spleens were harvested at 7 days p.i., and single-cell suspensions were prepared. One-fifth of the spleen cell suspension was infected with MVE at a multiplicity of 5 PFU/cell for 1 h, washed, and cultured with the rest of the splenocytes for 5 days. 2R and 5R target cells were infected with MVE at a multiplicity of 50 PFU/cell for 16 h prior to 51Cr labeling for 1 h and incubation with titrated numbers of effector cells for 6 h. All samples were triplicates, the standard error of the mean (SEM) was always <3%, and spontaneous release was between 10 and 20%.
RESULTS
In vitro cytotoxicity of splenocytes from H-2b mice against MVE-infected target cells.
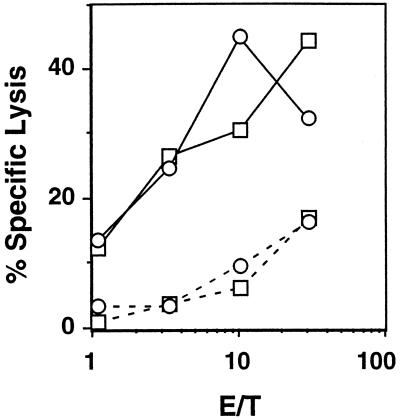
We investigated the role of the cytolytic effector functions of NK and Tc cells in a mouse model of flaviviral encephalitis. We have previously reported on the H-2k- and H-2d-restricted Tc cell responses against MVE (27, 29, 51). To verify that MVE-specific Tc cell responses are also induced in H-2b (B6) mice, secondary, in vitro-stimulated, MVE-immune splenocytes were tested against MVE-infected and uninfected 2R (Kk, Db) and 5R (Kb, Dd) target cells in 51Cr release assays. Figure 1 shows that Db- and Kb-restricted anti-MVE Tc cell responses are elicited in MVE-infected B6 mice. No MVE-specific killing of H-2-mismatched MVE-infected target cells (L929, H-2k) by the MVE-immune effector cells was observed (data not shown), showing that the Db- and Kb-restricted lysis was Tc cell mediated.
FIG. 1.
MVE infection of B6 mice elicits H-2b-restricted antiviral Tc cell responses. Lysis of 51Cr-labeled 2R (□) and 5R (○) cells infected with MVE (solid line) or left uninfected (dashed line) by secondary, in vitro-stimulated MVE-immune Tc cells from B6 mice was measured. Representative data from one of four experiments are shown. The SEM was always <3%. E/T, effector-to-target cell ratio.
Mortality caused by MVE in 6-week-old B6 mice.
Inoculation by the intravenous (i.v.) route of 6-week-old B6 mice with MVE characteristically failed to produce a dose-dependent increase in mortality. A high virus dose (≥108 Vero cell PFU) consistently resulted in 100% death, whereas virus doses in the range from 0.1 to 105 PFU killed ∼50% of the animals (Table 1). This pattern, which we also observed for the encephalitic flavivirus WNV (unpublished data), was reflected in two distinct kinetics for the average survival time (AST). Mice injected with 108 PFU of MVE died at 5 to 6 days p.i., whereas mortality caused by the lower doses occurred from days 9 to 13 p.i. (Table 1). When 102 PFU of MVE was inoculated by the i.c. route into 6-week-old B6 mice, death uniformly occurred at days 5 to 7 p.i. (see Fig. 5D).
TABLE 1.
Susceptibility of B6 mice, wild type or defective in granule exocytosis- or Fas-mediated cytotoxicity, to i.v. infection with MVE
| Dose (PFU) | Mouse strain | No./group | % Mortalitya | AST ± SEMa |
|---|---|---|---|---|
| 0.1 | Wild type | 27 | 59 | 10.3 ± 0.6 |
| perf−/− | 12 | 33 | 11.0 ± 0.7 | |
| gzmA×B−/− | 18 | 281 | 11.6 ± 0.6 | |
| perf × gzmA×B−/− | 6 | 33 | 11.0 ± 1.0 | |
| gld | 10 | 40 | 12.3 ± 1.5 | |
| lpr | 6 | 33 | 14.0 ± 01 | |
| 102 | Wild type | 70 | 46 | 11.6 ± 0.4 |
| perf−/− | 15 | 201 | 12.3 ± 2.2 | |
| gzmA×B−/− | 20 | 25 | 10.8 ± 1.5 | |
| perf × gzmA×B−/− | 19 | 32 | 12.5 ± 0.3 | |
| gld | 11 | 27 | 11.7 ± 0.7 | |
| lpr | 11 | 55 | 11.3 ± 1.1 | |
| 103 | Wild type | 9 | 56 | 12.0 ± 0.8 |
| perf−/− | 16 | 38 | 12.8 ± 0.7 | |
| 105 | Wild type | 20 | 40 | 10.9 ± 0.8 |
| perf−/− | 12 | 33 | 13.3 ± 1.01 | |
| gld | 14 | 36 | 12.8 ± 1.0 | |
| lpr | 10 | 801 | 10.8 ± 0.6 | |
| 108 | Wild type | 19 | 100 | 5.5 ± 0.2 |
| perf−/− | 10 | 100 | 6.6 ± 0.23 | |
| gzmA×B−/− | 6 | 100 | 6.2 ± 0.4 | |
| perf × gzmA×B−/− | 5 | 100 | 9.4 ± 1.43 | |
| gld | 10 | 100 | 6.6 ± 0.32 | |
| lpr | 5 | 100 | 5.8 ± 0.2 |
Statistical significance relative to groups of wild-type mice is indicated by superscript numbers as follows: 1, P ≤ 0.1; 2, P ≤ 0.05; and 3, P ≤ 0.01. Differences in mortality were assessed using Fisher's exact test, and differences in AST were assessed using the Mann-Whitney test.
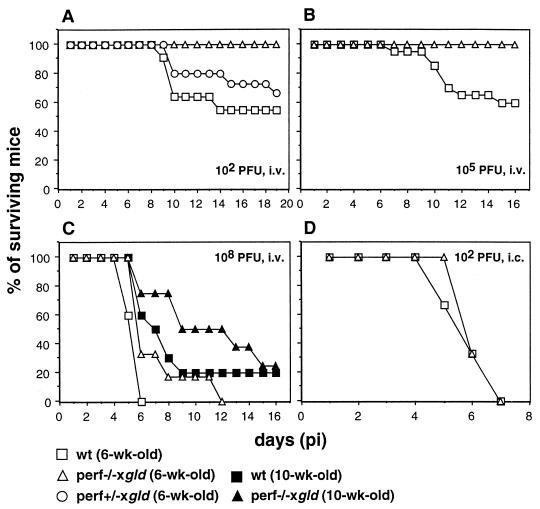
FIG. 5.
Susceptibility of perf−/− × gld mice to infection with MVE. (A) Groups of 6-week-old wild-type (wt) (n = 11), perf−/− × gld doubly deficient (n = 8), and perf+/− × gld heterozygous (n = 15) mice were infected i.v. with 102 PFU of MVE. Mortality due to lymphoproliferative disease occurred in the group of doubly deficient mice on days 9 (n = 1) and 13 (n = 3) and was excluded from the total. (B) Groups of 6-week-old wild-type (n = 20) and perf−/− × gld (n = 8) mice were infected i.v. with 105 PFU of MVE. (C) Groups of 6-week-old and 10-week-old wild-type (n = 5 and 10, respectively) and perf−/− × gld (n = 6 and 8, respectively) mice were infected i.v. with 108 PFU of MVE. (D) Time to death of 6-week-old wild-type (n = 3) and perf−/− × gld (n = 3) mice infected i.c. with 102 PFU of MVE. In all experiments, morbidity and mortality were recorded daily, and surviving mice were monitored for 21 days.
Accordingly, it appears that i.v. inoculation of 108 PFU results in early viral invasion of the CNS, with a disease outcome comparable to that following i.c. injection of the virus. The longer time to death in mice infected i.v. with the lower virus doses suggests that peripheral virus growth was required prior to the time that neuroinvasion took place. However, a 106-fold difference in the size of the initial i.v. inoculum did not result in any significant change in the AST. Animals that succumbed to high- or low-dose i.v. infections with MVE showed similar clinical signs at 1 to 2 days prior to death, which included wasting, ruffled fur, a hunched posture, and hind limb paralysis. These observations, in addition to comparable virus titers in the brains of moribund mice from groups infected i.v. with 108 PFU of MVE or the lower doses (see Fig. 3), suggested that in both cases viral encephalitis was the cause of death.
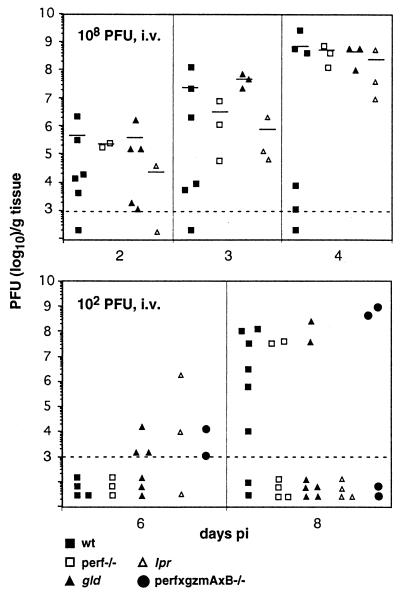
FIG. 3.
Kinetics of neuroinvasion and growth of MVE in the brain of wild-type (wt) mice and mice defective in granule exocytosis- or Fas-mediated cytotoxicity. Groups of mice (6 weeks old) were infected i.v. with 102 or 108 PFU of MVE. At the indicated times, animals from each group were sacrificed, and virus titers in the brain were determined by plaque titration. Each symbol represents an individual mouse. The lower limit of detection of virus titers was 103 PFU/g of tissue and is indicated by the dashed line. To calculate mean titers (indicated by a horizontal line), values below the limit of detection were ignored.
Role of perforin and granzymes in the pathogenesis of mice infected with MVE.
Killing of virus-infected cells by cytolytic lymphocytes via the granular exocytosis mechanism involves at least two defined components of the secreted granules, perforin and the granzymes (39). To investigate whether granular exocytosis-mediated cytotoxicity by NK/Tc cells is important in recovery of mice from infection with an encephalitic flavivirus, the outcome of MVE infection was tested in knockout mice with defects in perforin (perf−/−), granzymes A and B (gzmAxB−/−), and perforin plus the two granzymes (perf × gzmAxB−/−). Groups of B6 wild-type and knockout mice were inoculated i.v. with doses of MVE ranging from 0.1 to 108 PFU and observed for 3 weeks to record mortality and AST.
Table 1 shows that in the dose range of 0.1 to 105 PFU of MVE, mortality in the groups of perf−/−, gzmAxB−/−, and perf × gzmAxB−/− mice was always lower than that for the wild-type mice. When mortality data in this dose range for groups of perf−/− and gzmAxB−/− mice are summed, a statistically significant increase in resistance to MVE infection is found for the two strains relative to wild-type mice (P = 0.022 and 0.014, respectively; Fisher's exact test). Given the relatively small number of perf × gzmAxB−/− mice that were available for this study, the difference in additive mortality values for this knockout mouse (8 dead, 17 alive) and those for the wild-type (59 dead, 59 alive) was not statistically significant (P = 0.125). However, a significant increase in AST of perf × gzmAxB−/− (9.4 days) relative to wild-type mice (5.5 days) was noted (P = 0.009; Mann-Whitney test) in groups injected i.v. with 108 PFU of MVE (Table 1). This difference was less obvious for perf−/− and gzmAxB−/− mice, for which the AST was prolonged by ∼1 day. The comparison of the ASTs for groups of mice inoculated with the lower doses of MVE (0.1 to 105 PFU) also showed a trend towards a slightly increased AST of mice deficient in the granular exocytosis pathway of cytotoxicity relative to the wild-type mice.
In summary, the data in Table 1 indicate that granule exocytosis-mediated cytotoxicity of NK/Tc cells has no protective value in MVE infection; in fact, it appears to accelerate the fatal outcome of viral growth in the CNS and increase mortality rates.
Role of Fas-mediated cytotoxicity in the pathogenesis of mice infected with MVE.
The second major cytolytic mechanism of NK/Tc cells involves the engagement of Fas on the target cell by Fas ligand (FasL) (for a review, see reference 43). This mechanism is essential in immune regulation, demonstrated by a lymphoproliferative disease in mice with a loss-of-function mutation of the Fas gene (lpr) or a point mutation in the FasL gene (gld) (44, 45). The Fas-mediated death pathway has been implicated in the control of some viral infections (61, 62). In the absence of functional FasL expression (gld mutant mouse), the mortality inflicted in the i.v. dose range of 0.1 to 105 PFU of MVE was always less than that found for wild-type B6 mice (Table 1). In addition, the AST was significantly increased in gld mice relative to wild-type mice when 108 PFU was injected i.v. (P = 0.014). The Fas defect in lpr mice showed no consistent trend in the outcome of infection with MVE (Table 1). However, it is known that the lpr mutation is leaky and that small amounts of functional Fas are expressed in the mutant mouse (3).
Virus growth in tissues of B6 wild-type mice and mutant mice with defects in the granule exocytosis- or Fas-mediated cytotoxic effector mechanism.
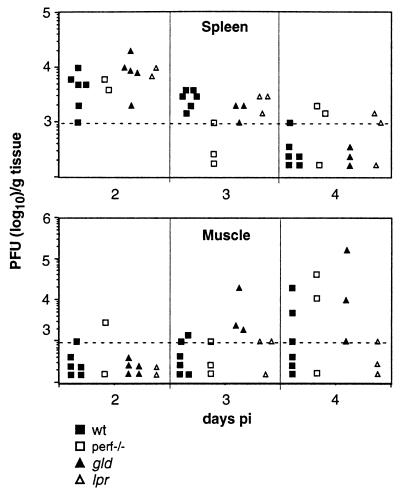
Tissues (spleen, muscle, lymph nodes, and liver) from 6-week-old B6 wild-type, perf−/−, gld, and lpr mice infected i.v. with 108 or 105 PFU of MVE were tested at 1- and 2-day intervals, respectively, for the presence of virus or viral RNA by plaque titration and reverse transcription (RT)-PCR. Consistent with earlier studies (32), we found that MVE grows poorly in extraneural tissues of mice of this age. When inoculated i.v. with 105 PFU of MVE, barely detectable virus titers were found in the spleen at 4 days p.i., and virus was cleared by 6 days p.i.; virus was not detectable in lymph nodes, muscle, liver, or blood (data not shown), although all the mice had seroconverted (Table 2). Inoculation i.v. of 108 PFU resulted in virus detection in the spleen, muscle (Fig. 2), and lymph nodes, but not the liver (data not shown).
TABLE 2.
MVE-specific antibody responses in C57B1/6 wt mice and in mutant mice deficient in granule exocytosis and/or Fas pathways of cytotoxicity
| Expt no. | Virus dose (PFU) | Mouse strain | Time (days p.i.) | No./group | % Seroconversion | Mean antibody titer,a log10 (range) |
|---|---|---|---|---|---|---|
| 1 | 0.1 | Wild type | 10 | 6 | 100 | 3.7 (2.6-4.1) |
| perf−/− | 3 | 100 | 4.0 (3.8-4.1) | |||
| gzmAxB−/− | 4 | 100 | 4.2 (4.1-4.4) | |||
| gld | 3 | 100 | 3.7 (2.6-4.4) | |||
| Wild type | 21-25 | 4 | 100 | 4.3 (3.8-4.7) | ||
| perf−/− | 3 | 100 | 4.6 (4.1-5.0) | |||
| gld | 1 | 100 | 5.0 | |||
| 102 | Wild type | 21-32 | 11 | 100 | 4.0 (2.9-4.7) | |
| perf−/− | 4 | 100 | 4.3 (4.1-4.4) | |||
| gzmAxB | 5 | 100 | 3.8 (3.5-4.1) | |||
| perf × gzmA × B−/− | 8 | 100 | 4.0 (3.8-4.1) | |||
| gld | 8 | 100 | 4.5 (3.5-5.0)b | |||
| lpr | 4 | 100 | 4.3 (3.8-4.7) | |||
| 2 | 105 | Wild type | 5 | 6 | 100 | 2.5 (2.0-2.9) |
| perf−/− × gld | 3 | 100 | 2.7 (2.6-2.9) | |||
| Wild type | 10 | 7 | 100 | 3.4 (3.2-3.5) | ||
| perf−/− × gld | 4 | 100 | 4.0 (3.8-4.4)b | |||
| Wild type | 15 | 7 | 100 | 3.7 (3.5-3.8) | ||
| perf−/− × gld | 4 | 100 | 4.1 (3.5-4.7) | |||
| Wild type | 21 | 7 | 100 | 3.8 (3.5-4.1) | ||
| perf−/− × gld | 4 | 100 | 4.5 (4.1-4.7)b | |||
| gld | 3 | 100 | 4.5 (4.4-4.7)b | |||
| perf−/− | 3 | 100 | 4.0 (3.8-4.1) |
MVE-specific antibody titers were determined by ELISA, and endpoints were calculated as described in Materials and Methods.
Statistically significant difference (P ≤ 0.05; Mann-Whitney test) relative to groups of wild-type mice.
FIG. 2.
Growth of MVE in spleen and muscle of B6 wild-type (wt) mice and mice deficient in granule exocytosis- or Fas-mediated cytotoxicity. Mice (6 weeks old) were infected i.v. with 108 PFU of MVE. At the indicated times, animals were sacrificed, and virus titers in tissues were determined by plaque titration. Each symbol represents an individual mouse. The lower limit of virus detection was 103 PFU/g tissue and is indicated by the dashed line.
No consistent difference in growth kinetics or magnitude of virus titers was apparent between wild-type mice and mice defective in NK/Tc cell cytotoxic effector mechanisms. Virus titers in the spleen were highest on day 2 and lowest on day 4 p.i.. This pattern was also found for virus titers in lymph nodes (data not shown). MVE appeared to grow in muscle from day 2 to day 4 p.i. but was not detectable in all animals. No virus was detectable in the blood (<102 PFU/ml) 2 days after i.v. injection of 108 PFU of MVE. The apparent inability of extraneural tissues to support efficient growth of MVE was confirmed by RT-PCR on samples from liver, spleen, muscle, kidney, ovaries, lymph nodes, and blood obtained from infected mice at 2, 4, 6, and 8 days p.i. (unpublished data). The sensitivity of detection of viral RNA by the RT-PCR method was estimated at ∼104 templates/reaction.
Unfortunately, these data do not permit us to say whether (i) NK/Tc cell-mediated cytotoxicity contributes to the control of virus infection in these tissues or (ii) the magnitude of virus titers in extraneural tissues is a decisive factor that determines the incidence of neuroinvasion and thus can account for the variability between individuals in the susceptibility to infection with MVE.
Kinetics of neuroinvasion and growth of MVE in the brain of wild-type mice and mutant mice defective in granule exocytosis- or Fas-mediated cytotoxicity.
Groups of 6-week-old mice were infected i.v. with either 108 or 102 PFU of MVE. Individuals were sacrificed at 2, 3, and 4 days after 108 PFU and 6 and 8 days after 102 PFU inoculations, and virus titers in the brain were determined. The dose of 108 PFU i.v. gave detectable virus titers in the brains of most animals at 2 days p.i. (ranging from 103 to 106 PFU/g), which increased to titers of 107 to 109 PFU on day 4 p.i. in the perf−/−, gld, and lpr mice and in some of the wild-type mice (Fig. 3). The mean titers of MVE in the brains of the four strains of mice were comparable, but wild-type mice showed much greater variability than the other groups on day 3 and day 4 p.i..
After inoculation i.v. of 102 PFU of MVE, low titers of virus were found on day 6 p.i. in brains of a few mice in the gld, lpr, and perf × gzmAxB−/− but not the wild-type and perf−/− groups (Fig. 3). By day 8 p.i., virus was found in six of eight wild-type mice, and titers ranged from 103 to 108 PFU/g. Members of the groups of perf−/−, gld, and perf × gzmAxB−/− mice had high brain titers (107 to 109 PFU/g) on day 8 p.i., but overall the majority of mice defective in granule exocytosis- or Fas-mediated cytotoxicity had no detectable virus in the brain, consistent with the lower mortality rates in these mice relative to wild-type mice.
Resistance to infection with MVE of mice doubly deficient in granule exocytosis- and Fas-mediated cytotoxic pathways.
The above results suggest that mice with functional defects in the granule exocytosis- or Fas-mediated pathway of cytotoxicity are less susceptible to lethal infection with MVE than wild-type mice. This implies that NK/Tc cell-mediated killing contributes to the pathogenesis of encephalitic flaviviruses in their vertebrate hosts. Given the possibility of redundancy between the two major pathways of cytotoxicity (49, 61, 62), the detrimental role of NK/Tc cells in encephalitic flaviviral disease may have been missed or underestimated in studies using mice defective in only one of the two pathways.
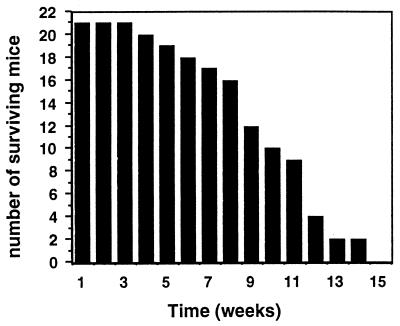
To investigate this possibility, mice defective in both cytotoxic effector mechanisms (perf−/− × gld) were generated. Consistent with earlier reports (18, 59), we found that these doubly deficient mice developed a severe autoimmune syndrome characterized by splenomegaly and lymphadenopathy, resulting in weight loss and death between the ages of 4 and 16 weeks (Fig. 4). About one-third of doubly deficient mice died between the ages of 6 and 9 weeks, but the clinical manifestations of MVE infection were distinguishable from signs of lymphoproliferative disease in these animals.
FIG. 4.
Time to death of animals in a cohort of perf−/− × gld mice.
Groups of 6-week-old perf−/− × gld and wild-type mice were infected i.v. with 102 or 105 PFU of MVE, and morbidity and mortality were recorded daily for 21 days. All animals in a group of eight perf−/− × gld mice inoculated with 105 PFU survived, in contrast to 40% mortality in the wild-type group (Fig. 5B). Similarly, fatal viral encephalitis did not occur in the group of perf−/− × gld mice infected with 102 PFU (Fig. 5A). However, 4 of 12 doubly deficient mice in this group died without displaying the characteristic clinical signs of viral encephalitis, and MVE could not be recovered from the brains of cadavers within a period of 16 h after death had occurred. Since acute lymphadenopathy was seen in all cadavers and the mortality rate was consistent with that in the uninfected control group (Fig. 4), we conclude that death was most likely due to lymphoproliferative disease.
The susceptibility to i.v. infection with 102 PFU of MVE of 6-week-old gld mice heterozygous for the perforin knockout mutation (perf+/− × gld) was marginally lower than that of wild-type mice (33 and 45%, respectively; Fig. 5A) and comparable to that of gld mice (Table 1). This is indicative of a lack of gene dose effect in the contribution of perforin on the gld background to the pathogenesis of MVE.
Infection of perf−/− × gld mice with 108 PFU of MVE by the i.v. route gave 100% mortality in 6-week-old animals and 75% mortality in 10-week-old mice (Fig. 5C). These values were comparable to those for the wild-type mice. However, the AST was longer in the doubly deficient animals relative to wild-type mice (7.3 and 5.6 days, respectively, for 6-week-old mice and 9.7 and 7.0 days, respectively, for 10-week-old mice). Intracerebral injection of 102 PFU of MVE in 6-week-old wild-type and perf−/− × gld mice resulted in 100% mortality, with comparable kinetics to death in both mouse strains (Fig. 5D).
To further substantiate that virus growth in the CNS of the doubly deficient mice was not suppressed, 6-week-old perf−/− × gld mice were infected i.v. with 108 PFU of MVE, and brain titers were determined on days 3 and 4 p.i. No difference was found between the MVE titers in the brain of perf−/− × gld mice on day 3 p.i. (3.5 × 106 to 2.5 × 108 PFU/g) and day 4 p.i. (2.8 × 109 to 4.5 × 109 PFU/g) and those found in wild-type mice (Fig. 3). Thus, in perf−/− × gld mice, neuroinvasion by MVE occurred following high-dose i.v. inoculation, and virus growth in the brain was not inhibited.
MVE-specific antibody responses in B6 wild-type mice and mutant mice deficient in granule exocytosis and/or Fas pathway of cytotoxicity.
Single vaccination of B6 mice with 106 PFU of UV-inactivated MVE does not induce MVE-specific antibodies detectable by ELISA (unpublished data). Seroconversion of mice following inoculation of smaller doses of live virus (0.1 to 105 PFU) would therefore be consistent with a productive infection. To verify that i.v. infections of mice deficient in the granule exocytosis- and/or Fas-mediated pathway of cytotoxicity with low doses of MVE resulted in virus growth in extraneural tissues, and to test whether they showed differences in the magnitude of the humoral immune response against MVE, antibody titers and incidence of seroconversion were determined.
The sera from all animals infected with MVE at 0.1 to 105 PFU were reactive against MVE antigen (Table 2). No dose-dependent difference was seen in the magnitude of the antibody response in wild-type mice. MVE-specific antibody titers in perf−/− × gld mice were four- to sevenfold higher than those in wild-type mice at 10 to 21 days p.i., although the difference in mortality between the two groups may have been a biasing factor, since only those mice that survived the virus infection until serum collection were tested. A significant increase in the magnitude of the humoral immune response against MVE was also seen in some groups of gld but not perf−/−, gzmAxB−/−, perf × gzmAxB−/−, and lpr mice relative to wild-type mice (Table 2).
DISCUSSION
We have investigated the contribution of the cytotoxic effector functions of NK/Tc cells to recovery from and/or pathogenesis of encephalitic flavivirus infection using mutant mouse strains defective in the granule exocytosis and Fas or pathway of cytotoxicity. We first established parameters in immunologically mature mice, allowing the study of pathogenesis and control of infection with MVE, a member of the JEV serocomplex. Using 6-week-old B6 mice, we found that, depending on the virus dose used for i.v. inoculation, two kinetically distinct disease processes led to fatal encephalitis. A high dose of MVE given i.v. (108 PFU) gave pathogenesis comparable to that with i.c. injection of 102 PFU with respect to the first appearance of signs of encephalitis on day 4 p.i., the AST (5 to 6 days), and disease outcome (100% mortality). This pattern is consistent with the notion that sufficient virus from a high i.v. dose can enter the brain directly from blood to establish a lethal infection without the requirement for replication in extraneural tissues.
This rapid mode of invasion of the brain contrasted with the delayed kinetics and variable incidence of virus entry into the brain when lower i.v. doses of MVE, in the range of 0.1 to 105 PFU, were used. Virus first appeared in the brain between 6 and 8 days p.i., and mortality was observed between 9 and 13 days p.i. in ∼50% of infected mice. This disease pattern suggests that the virus first replicated in extraneural tissues prior to entering the CNS and that invasion of the brain was a stochastic process unrelated to the dose of virus injected in the range of 0.1 to 105 PFU. Infection of mice with low doses of MVE rarely gave rise to detectable virus in the extraneural tissues tested and never produced a detectable secondary viremia, but all animals seroconverted, confirming productive virus infection.
The lack of any difference in AST over a 106 range of i.v. virus inocula indicates that the kinetics of neuroinvasion was surprisingly dose insensitive and that equalizing factors exist. We propose that the kinetics of induction of the antiflaviviral Tc cell response, which also displays a remarkably flat dose response at low to medium virus concentrations (20), is one such factor. In addition, the antiviral alpha/beta interferon response is most important in controlling flavivirus growth in extraneural tissues, given that adult alpha interferon receptor knockout mice rapidly succumb to an acute fulminant infection when inoculated with 102 PFU of MVE (unpublished data). The magnitude of the interferon response may inversely correlate with the virus dose inoculated.
The mechanism that allows encephalitic flaviviruses to breach the blood-brain barrier remains uncertain, but four candidate routes for CNS invasion have been canvassed (for reviews, see references 16 and 35): (i) by the neuronal route after infection of peripheral nerves; (ii) by infection of highly susceptible olfactory neurons, which are unprotected by the blood-brain barrier (36); (iii) by virus entry into vascular endothelial cells of capillaries in the brain, transcytosis, and release of virus into the brain parenchyma (25); and (iv) by diffusion of virus between capillary endothelial cells in individuals displaying leakiness of the blood-brain barrier due to factors unrelated or secondary to the viral infection (13, 23, 30). Antiviral immune responses could contribute to the fourth mechanism by inducing breakdown of the blood-brain barrier. Given the distinct modes of disease progression leading to fatal encephalitis that were observed when high (108 PFU) and lower (0.1 to 105 PFU) doses of MVE were inoculated i.v. and the concomitant differences in virus load in the vascular compartment, the possibility that encephalitic flaviviruses can breach the blood-brain barrier by a number of different mechanisms must be considered.
In addition to establishing conditions for investigating the role of the cytotoxic effector function of splenocytes in recovery or disease from MVE infections in a mouse model, we have confirmed that B6 mice mount H-2b-restricted Tc cell responses against MVE. The role of NK cells in flavivirus immunity appears to be evaded due to the upregulation of major histocompatibility complex (MHC) class I by flavivirus infection and, as a consequence, the inhibition of NK cell-mediated infected target cell lysis (28, 34, 41).
The most striking finding in this study is the demonstration that cytotoxic effector pathways of NK/Tc cells contribute to accelerated and more severe pathogenesis of encephalitic flavivirus infection. Cytotoxic effector mechanisms may be involved at two stages in the disease process: first, in the events leading to neuroinvasion, and second, in the pathology due to the inflammatory response in the brain. Both the granule exocytosis- and the Fas-mediated pathways of cytotoxicity can contribute to disease progression with potentially compensatory function in mice when one of the two mechanisms is defective. Thus, contrary to the expectation based on the established importance of Tc and NK cells and their cytolytic mechanism in control and clearance of infection in other virus models (2, 17, 39, 40, 64), doubly deficient perf−/− × gld mice displayed a greatly increased resistance to i.v. infection with MVE at virus doses which gave ∼50% mortality in wild-type mice. In support of the detrimental role of CD8+ T cells in the recovery from encephalitic flavivirus infection, we also found a significantly reduced mortality of β2-microglobulin knockout mice, which lack CD8+ T cells and expression of their restriction elements, in comparison to wild-type mice when infected with 102 PFU of MVE i.v. (data not shown).
The Fas pathway plays a critical role in lymphocyte homeostasis, which accounts for a severe autoimmune syndrome characterized by enlarged spleen and lymph nodes and lymphocyte infiltration in liver and kidney in Fas receptor (lpr)- and FasL (gld)-deficient mice (43). This disease is exacerbated in the absence of functional perforin, and, consistent with previous reports (18, 59), perf−/− × gld mice died at between 4 and 16 weeks of age. Mortality due to unchecked expansion of activated T cells in perf−/− × gld mice could be distinguished from that caused by MVE infection. Thus, we did not observe clinical signs typically associated with flaviviral encephalitis in any of the infected perf−/− × gld mice inoculated i.v. with 102 or 105 PFU of MVE. In addition, these doses did not result in virus titers detectable by plaque titration in the brains of mice that died during the virulence assays (most likely due to the lymphoproliferative syndrome) or that were sacrificed at 4 (n = 2), 6 (n = 2), and 8 (n = 4) days p.i. (data not shown). Accordingly, we conclude that in the absence of the two major cytotoxic pathways of NK/Tc cells, neuroinvasion by encephalitic flaviviruses is prevented. Presumably neuroinvasion by MVE in this model requires extraneural virus growth for several days following primary virus inoculation into the bloodstream, resembling natural infection by the bite of an infected arthropod.
What is the mechanism by which a deficiency in the two main cytotoxic effector functions prevents fatal flaviviral encephalitis? It is conceivable that cytotoxic cells disrupt the blood-brain barrier by killing infected endothelial cells lining the brain capillaries, allowing virus access into the brain parenchyma. perf−/− and gld mice display a marked decrease in vascular leakage resulting from endothelial cell injury (50), and the double deficiency in perf−/− × gld mice may entirely prevent capillary leakage due to the effector functions of cytotoxic lymphocytes. A gradation in the severity of vascular endothelium cytolysis in wild-type, perf−/−, gld, and doubly deficient mice may account for the progressive reduction in mortality following i.v. low-dose MVE infection found in these mouse strains. Thus, the key stochastic factors allowing neuroinvasion in infections with low virus doses would be whether virus infection of capillary endothelial cells in the brain and lysis of these by cytotoxic effector cells occurred.
The requirement for cytotoxic effector function in this process suggests that alternative mechanisms for neuroinvasion (infection of olfactory neurons, transcytosis, or diffusion between capillary endothelial cells) were not involved. However, in mice peripherally infected with a very large virus dose (108 PFU), it is likely that the rapid virus entry into the brain occurs by infection of olfactory neurons or diffusion across the blood-brain barrier. The encephalitic flavivirus WNV readily infects human endothelial cells, and the expression of leukocyte adhesion molecules is induced at the plasma membrane of the infected cells (57), which would thus be susceptible to NK/Tc cell-mediated attack. The kinetics of appearance of MVE in the brain (at 6 to 8 days p.i.) following i.v. infection with a low virus dose is consistent with that of the induction of the antiviral Tc cell response (20) and, in turn, Tc cell-induced damage of the cells lining the brain capillaries.
We do not have direct experimental evidence supporting the hypothesis that damage to the integrity of the blood-brain barrier due to the cytotoxic effector pathways allows encephalitic flaviviruses to invade the brain. However, we can exclude the possibility that the brains of perf−/− × gld mice were resistant to replication of the virus. When a low dose of MVE (102 PFU) was injected directly into the brain or when a high dose (108 PFU) was injected i.v., all doubly deficient animals, like the wild-type controls, developed fatal encephalitis, and virus titers in the brains of perf−/− × gld mice were comparable to those in wild-type mice. The induction of virus-specific antibodies in all infected wild-type and mutant mice confirms that infection and extraneural growth of MVE occurred in these animals.
The magnitude of the humoral immune response against MVE determined by ELISA was elevated in mice defective in the Fas receptor (gld and perf−/− × gld) relative to wild-type mice. However, given that the perf−/− × gld mice were significantly more resistant to MVE infection than gld mice, it is unlikely that antibody-mediated virus clearance accounted for the greatly reduced mortality in the former. The ELISA titers of serum samples from infected mice correlated closely with their respective in vitro neutralization activities against MVE measured in plaque reduction neutralization assays (data not shown). Unlike reports on other viral infections (53), the lack of perforin did not alter anti-MVE antibody titers.
The AST of the doubly deficient mice was prolonged by ∼2 days relative to that in the wild-type group when infected i.v. with a high virus dose (108 PFU). A similar finding was also recorded in a group of perf × gzmAxB−/− mice and to a lesser extent in groups of perf−/− and gld mice. This result suggests a second pathogenic consequence of the function of the cytolytic effector pathways of NK/Tc cells in encephalitic flavivirus disease, viz., exacerbation of the contribution of inflammatory infiltration into the CNS of infected mice to a fatal disease outcome.
In summary, this investigation shows the dependence of a neurotropic virus on the major cytotoxic effector pathways for CNS invasion following inoculation of a virus dose by a route analogous to those in natural infections. A deficiency of both the perforin- and Fas-mediated killing pathways was required for this pathogenesis component to become clearly apparent. These findings may pave the way to designing treatments for early times during flavivirus infection to prevent CNS involvement.
REFERENCES
- 1.Andrews, D. M., V. B. Matthews, L. M. Sammels, A. C. Carrello, and P. C. McMinn. 1999. The severity of Murray Valley encephalitis in mice is linked to neutrophil infiltration and inducible nitric oxide synthase activity in the central nervous system. J. Virol. 73:8781-8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanden, R. V. 1971. Mechanisms of recovery from a generalized viral infection: mousepox. II. Passive transfer of recovery mechanisms with immune lymphoid cells. J. Exp. Med. 133:1074-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booker, J. K., E. A. Reap, and P. L. Cohen. 1998. Expression and function of Fas on cells damaged by gamma-irradiation in B6 and B6/lpr mice. J. Immunol. 161:4536-4541. [PubMed] [Google Scholar]
- 4.Camenga, D. L., N. Nathanson, and G. A. Cole. 1974. Cyclophosphamide-potentiated West Nile viral encephalitis: relative influence of cellular and humoral factors. J. Infect. Dis. 130:634-641. [DOI] [PubMed] [Google Scholar]
- 5.Cannon, M. J., P. J. M. Openshaw, and B. A. Askonas. 1988. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 168:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombage, G., R. Hall, M. Pavy, and M. Lobigs. 1998. DNA-based and alphavirus-vectored immunisation with prM and E proteins elicits long-lived and protective immunity against the flavivirus Murray Valley encephalitis virus. Virology 250:151-163. [DOI] [PubMed] [Google Scholar]
- 7.De Madrid, A. T., and J. S. Porterfield. 1974. The flaviviruses (group B arboviruses): cross-neutralization study. J. Gen. Virol. 23:91-96. [DOI] [PubMed] [Google Scholar]
- 8.Doherty, P. C., and R. M. Zinkernagel. 1974. T-cell-mediated immunopathology in viral infections. Transplant. Rev. 19:89-120. [DOI] [PubMed] [Google Scholar]
- 9.French, E. L. 1952. Murray Valley encephalitis: isolation and characterization of the aetological agent. Med. J. Aust. 1:100-103. [PubMed] [Google Scholar]
- 10.Gajdosova, E., C. Oravec, and V. Mayer. 1981. Cell-mediated immunity in flavivirus infections. I. Induction of cytotoxic T lymphocytes in mice by an attenuated virus from the tick-borne encephalitis complex and its group-reactive character. Acta Virol. 25:10-18. [PubMed] [Google Scholar]
- 11.Grossberg, S. E., and W. F. Scherer. 1966. The effect of host age, virus dose and route of inoculation on inapparent infection in mice with Japanese encephalitis virus. Proc. Soc. Exp. Biol. Med. 123:118-124. [DOI] [PubMed] [Google Scholar]
- 12.Hall, R. A., T. N. H. Brand, M. Lobigs, M. Y. Sangster, M. J. Howard, and J. S. Mackenzie. 1996. Protective immune responses to the E and NS1 proteins of Murray Valley encephalitis virus in hybrids of flavivirus-resistant mice. J. Gen. Virol. 77:1287-1294. [DOI] [PubMed] [Google Scholar]
- 13.Hase, T., D. R. Dubois, and P. L. Summers. 1990. Comparative study of mouse brains infected with Japanese encephalitis virus by intracerebral or intraperitoneal inoculation. Int. J. Exp. Pathol. 71:857-869. [PMC free article] [PubMed] [Google Scholar]
- 14.Henkart, P. A. 1994. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity 1:343-346. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, R. T., D. S. Burke, M. Elwell, C. J. Leake, A. Nisalak, C. H. Hoke, and W. Lorsomrudee. 1985. Japanese encephalitis: immunocytochemical studies of viral antigen and inflammatory cells in fatal cases. Ann. Neurol. 18:567-573. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, R. T., and G. A. Mims. 1968. Pathogenesis for viral infections of the nervous system. N. Engl. J. Med. 278:84-92. [DOI] [PubMed] [Google Scholar]
- 17.Kägi, D., B. Ledermann, K. Burki, P. Seiler, B. Odermatt, K. J. Olsen, E. R. Podack, R. M. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369:31-37. [DOI] [PubMed] [Google Scholar]
- 18.Kägi, D., B. Odermatt, and T. W. Mak. 1999. Homeostatic regulation of CD8+ T cells by perforin. Eur. J. Immunol. 29:3262-3272. [DOI] [PubMed] [Google Scholar]
- 19.Kägi, D., F. Vignaux, B. Ledermann, K. Burki, V. Depraetere, S. Nagata, H. Hengartner, and P. Golstein. 1994. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 265:528-530. [DOI] [PubMed] [Google Scholar]
- 20.Kesson, A. M., R. V. Blanden, and A. Müllbacher. 1987. The primary in vivo murine cytotoxic T cell response to the flavivirus, West Nile. J. Gen. Virol. 68:2001-2006. [DOI] [PubMed] [Google Scholar]
- 21.Kesson, A. M., R. V. Blanden, and A. Müllbacher. 1988. The secondary in vitro murine cytotoxic T cell response to the flavivirus, West Nile. Immunol. Cell Biol. 66:23-32. [DOI] [PubMed] [Google Scholar]
- 22.Kimura-Kuroda, J., and K. Yasui. 1988. Protection of mice against Japanese encephalitis virus by passive administration with monoclonal antibodies. J. Immunol. 141:3606-3610. [PubMed] [Google Scholar]
- 23.Kobiler, D., S. Lustig, Y. Gozes, D. Ben-Nathan, and Y. Akov. 1989. Sodium dodecyl sulphate induces a breach in the blood-brain barrier and enables a West Nile virus variant to penetrate into mouse brain. Brain Res. 496:314-316. [DOI] [PubMed] [Google Scholar]
- 24.Lad, V. J., A. K. Gupta, M. K. Goverdhan, V. L. Ayachit, J. J. Rodrigues, and L. V. Hungund. 1993. Susceptibility of BL6 nude (congenitally athymic) mice to Japanese encephalitis virus by the peripheral route. Acta Virol. 37:232-240. [PubMed] [Google Scholar]
- 25.Liou, M. L., and C. Y. Hsu. 1998. Japanese encephalitis virus is transported across the cerebral blood vessels by endocytosis in mouse brain. Cell Tissue Res. 293:389-394. [DOI] [PubMed] [Google Scholar]
- 26.Liu, Y., R. V. Blanden, and A. Müllbacher. 1989. Identification of cytolytic lymphocytes in West Nile virus-infected murine central nervous system. J. Gen. Virol. 70:565-573. [DOI] [PubMed] [Google Scholar]
- 27.Lobigs, M., C. E. Arthur, A. Müllbacher, and R. V. Blanden. 1994. The flavivirus nonstructural protein, NS3, is a dominant source of cytotoxic T cell peptide determinants. Virology 202:195-201. [DOI] [PubMed] [Google Scholar]
- 28.Lobigs, M., R. V. Blanden, and A. Müllbacher. 1996. Flavivirus-induced up-regulation of MHC class I antigens; implications for the induction of CD8+ T-cell-mediated autoimmunity. Immunol. Rev. 152:5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lobigs, M., A. Müllbacher, and M. Pavy. 1997. The CD8+ cytotoxic T cell response to flavivirus infection. Arbovirus Res. Aust. 7:160-165. [Google Scholar]
- 30.Lustig, S., H. D. Danenberg, Y. Kafri, D. Kobiler, and D. Ben-Nathan. 1992. Viral neuroinvasion and encephalitis induced by lipopolysaccharide and its mediators. J. Exp. Med. 176:707-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacDonald, F. 1952. Murray Valley encephalitis infection in the laboratory mouse. I. Influence of age in susceptibility to infection. Aust. J. Exp. Biol. Med. Sci. 30:319-324. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald, F. 1952. Murray Valley encephalitis infection in the laboratory mouse. II. Multiplication of virus inoculated intramuscularly. Aust. J. Exp. Biol. Med. Sci. 30:325-332. [DOI] [PubMed] [Google Scholar]
- 33.Marshall, I. D. 1988. Murray Valley and Kunjin encephalitis, p. 151-189. In T. Monath (ed.), The arboviruses: epidemiology and ecology, vol. III. CRC Press, Inc., New York, N.Y. [Google Scholar]
- 34.Momburg, F., A. Müllbacher, and M. Lobigs. 2001. Modulation of transporter associated with antigen processing (TAP)-mediated peptide import into the endoplasmic reticulum by flavivirus infection. J. Virol. 75:5663-5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monath, T. P. 1986. Pathobiology of the flaviviruses, p. 375-440. In S. Schlesinger and M. Schlesinger (ed.), The Togaviridae and Flaviviridae. Plenum Press, New York, N.Y.
- 36.Monath, T. P., C. B. Cropp, and A. K. Harrison. 1983. Mode of entry of a neurotropic arbovirus into the central nervous system. Reinvestigation of an old controversy. Lab. Investig. 48:399-410. [PubMed] [Google Scholar]
- 37.Monath, T. P., and F. X. Heinz. 1996. Flaviviruses., p. 961-1034. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Strauss (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 38.Moskophidis, D., and D. Kioussis. 1998. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathological manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J. Exp. Med. 188:223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müllbacher, A., K. Ebnet, R. V. Blanden, R. T. Hla, T. Stehle, C. Museteanu, and M. M. Simon. 1996. Granzyme A is critical for recovery of mice from infection with the natural cytopathic viral pathogen, ectromelia. Proc. Natl. Acad. Sci. USA 93:5783-5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Müllbacher, A., R. T. Hla, C. Museteanu, and M. M. Simon. 1999. Perforin is essential for control of ectromelia virus but not related poxviruses in mice. J. Virol. 73:1665-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müllbacher, A., and M. Lobigs. 1995. Up-regulation of MHC class I by flavivirus-induced peptide translocation into the endoplasmic reticulum. Immunity 3:207-214. [DOI] [PubMed] [Google Scholar]
- 42.Müllbacher, A., P. Waring, R. Tha Hla, T. Tran, S. Chin, T. Stehle, C. Museteanu, and M. M. Simon. 1999. Granzymes are the essential downstream effector molecules for the control of primary virus infections by cytolytic leukocytes. Proc. Natl. Acad. Sci. USA 96:13950-13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagata, S. 1999. Fas ligand-induced apoptosis. Annu. Rev. Genet. 33:29-55. [DOI] [PubMed] [Google Scholar]
- 44.Nagata, S., and P. Golstein. 1995. The Fas death factor. Science 267:1449-1456. [DOI] [PubMed] [Google Scholar]
- 45.Nagata, S., and T. Suda. 1995. Fas and Fas ligand: lpr and gld mutations. Immunol. Today 16:39-43. [DOI] [PubMed] [Google Scholar]
- 46.Nathanson, N., and G. A. Cole. 1970. Fatal Japanese encephalitis virus infection in immunosuppressed spider monkeys. Clin. Exp. Immunol. 6:161-166. [PMC free article] [PubMed] [Google Scholar]
- 47.Ogata, A., K. Nagashima, W. W. Hall, M. Ichikawa, J. Kimura-Kuroda, and K. Yasui. 1991. Japanese encephalitis virus neurotropism is dependent on the degree of neuronal maturity. J. Virol. 65:880-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliver, K. R., and J. K. Fazakerley. 1998. Transneuronal spread of Semliki Forest virus in the developing mouse olfactory system is determined by neuronal maturity. Neuroscience 82:867-877. [DOI] [PubMed] [Google Scholar]
- 49.Parra, B., M. T. Lin, S. A. Stohlman, C. C. Bergmann, R. Atkinson, and D. R. Hinton. 2000. Contributions of Fas-Fas ligand interactions to the pathogenesis of mouse hepatitis virus in the central nervous system. J. Virol. 74:2447-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rafi, A. Q., A. Zeytun, M. J. Bradley, D. P. Sponenberg, R. L. Grayson, M. Nagarkatti, and P. S. Nagarkatti. 1998. Evidence for the involvement of Fas ligand and perforin in the induction of vascular leak syndrome. J. Immunol. 161:3077-3086. [PubMed] [Google Scholar]
- 51.Regner, M., A. Müllbacher, R. V. Blanden, and M. Lobigs. 2001. Immunogenicity of two peptide determinants in the cytolytic T cell response to flavivirus infection: inverse correlation between peptide affinity for MHC class I and T cell precursor frequency. Viral Immunol. 14:135-149. [DOI] [PubMed] [Google Scholar]
- 52.Ridge, J. P., E. J. Fuchs, and P. Matzinger. 1996. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science 271:1723-1726. [DOI] [PubMed] [Google Scholar]
- 53.Sambhara, S., I. Switzer, A. Kurichh, R. Miranda, L. Urbanczyk, O. James, B. Underdown, M. Klein, and D. Burt. 1998. Enhanced antibody and cytokine responses to influenza viral antigens in perforin-deficient mice. Cell. Immunol. 187:13-18. [DOI] [PubMed] [Google Scholar]
- 54.Samelson, L. E., W. F. Davidson, H. C. Morse III, and R. D. Klausner. 1986. Abnormal tyrosine phosphorylation on T-cell receptor in lymphoproliferative disorders. Nature 324:674-676. [DOI] [PubMed] [Google Scholar]
- 55.Sarzotti, M., D. S. Robbins, and P. M. Hoffman. 1996. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science 271:1726-1728. [DOI] [PubMed] [Google Scholar]
- 56.Schlesinger, J. J., M. Foltzer, and S. Chapman. 1993. The Fc portion of antibody to yellow fever virus NS1 is a determinant of protection against YF encephalitis in mice. Virology 192:132-141. [DOI] [PubMed] [Google Scholar]
- 57.Shen, J., S. S. T-To, L. Schrieber, and N. J. King. 1997. Early E-selectin, VCAM-1, ICAM-1, and late major histocompatibility complex antigen induction on human endothelial cells by flavivirus and comodulation of adhesion molecule expression by immune cytokines. J. Virol. 71:9323-9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simon, M. M., M. Hausmann, T. Tran, K. Ebnet, J. Tschopp, R. ThaHla, and A. Müllbacher. 1997. In vitro- and ex vivo-derived cytolytic leukocytes from granzyme A × B double knockout mice are defective in granule-mediated apoptosis but not lysis of target cells. J. Exp. Med. 186:1781-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spielman, J., R. K. Lee, and E. R. Podack. 1998. Perforin/Fas-ligand double deficiency is associated with macrophage expansion and severe pancreatitis. J. Immunol. 161:7063-7070. [PubMed] [Google Scholar]
- 60.Takahashi, T., M. Tanaka, C. I. Brannan, N. A. Jenkins, N. G. Copeland, T. Suda, and S. Nagata. 1994. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 76:969-976. [DOI] [PubMed] [Google Scholar]
- 61.Topham, D. J., R. C. Cardin, J. P. Christensen, J. W. Brooks, G. T. Belz, and P. C. Doherty. 2001. Perforin and Fas in murine gammaherpesvirus-specific CD8+ T cell control and mortality. J. Gen. Virol. 82:1971-1981. [DOI] [PubMed] [Google Scholar]
- 62.Topham, D. J., R. A. Tripp, and P. C. Doherty. 1997. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 159:5197-5200. [PubMed] [Google Scholar]
- 63.Trgovcich, J., J. F. Aronson, J. C. Eldridge, and R. E. Johnston. 1999. TNFα, interferon, and stress response induction as a function of age-related susceptibility to fatal Sindbis virus infection of mice. Virology 263:339-348. [DOI] [PubMed] [Google Scholar]
- 64.Zinkernagel, R. M. 1993. Immunity to viruses, p. 1211-1250. In W. E. Paul (ed.), Fundamental immunology, 3rd ed. Raven Press, New York, N.Y.