Abstract
Trypanosoma brucei brucei is the causative agent of nagana in cattle and can infect a wide range of mammals but is unable to infect humans because it is susceptible to the innate cytotoxic activity of normal human serum. A minor subfraction of human high-density lipoprotein (HDL) containing apolipoprotein A-I (apoA-I), apolipoprotein L-I (apoL-I), and haptoglobin-related protein (Hpr) provides this innate protection against T. b. brucei infection. This HDL subfraction, called trypanosome lytic factor (TLF), kills T. b. brucei following receptor binding, endocytosis, and lysosomal localization. Trypanosoma brucei rhodesiense, which is morphologically and physiologically indistinguishable from T. b. brucei, is resistant to TLF-mediated killing and causes human African sleeping sickness. Human infectivity by T. b. rhodesiense correlates with the evolution of a resistance-associated protein (SRA) that is able to ablate TLF killing. To examine the mechanism of TLF resistance, we transfected T. b. brucei with an epitope-tagged SRA gene. Transfected T. b. brucei expressed SRA mRNA at levels comparable to those in T. b. rhodesiense and was highly resistant to TLF. In the SRA-transfected cells, intracellular trafficking of TLF was altered, with TLF being mainly localized to a subset of SRA-containing cytoplasmic vesicles but not to the lysosome. These results indicate that the cellular distribution of TLF is influenced by SRA expression and may directly determine the organism's susceptibility to TLF.
Humans are resistant to infections by Trypanosoma brucei brucei, which can infect cattle and wild animals (4, 35, 48). This innate resistance to T. b. brucei is mediated by a subfraction of human high-density lipoprotein (HDL), termed trypanosome lytic factor (TLF), which represents <0.5% of the total serum HDL (21, 38, 39, 45, 46). The protein composition of TLF was recently elucidated by mass spectroscopy and biochemical reconstitution, and it was found to be composed of apolipoprotein A-I (apoA-I), apoL-I, and Hpr (13, 29, 43). In human serum, these proteins are colocalized within spherical particles containing phospholipids and cholesterol. When isolated from human HDL, both Hpr and apoL-I are toxic to T. b. brucei; however, reconstitution of these proteins into the same HDL particle increases the specific activity of trypanosome killing approximately 10-fold (43). In human serum, >99% of the trypanolytic activity is associated with native HDLs containing both Hpr and apoL-I. Furthermore, the specific activity of this HDL subclass for T. b. brucei killing is several hundredfold higher than those of HDLs containing Hpr or apoL-I alone (43). The synergism of Hpr and apoL-I may be critical for the sterile innate immunity humans have against T. b. brucei infection.
The cellular pathway for TLF killing of T. b. brucei requires high-affinity binding to cell surface receptors in the flagellar pocket, followed by endocytosis and routing to the lysosome (12, 19, 20, 26, 44). Interruption of trafficking to or acidification of the lysosome spares T. b. brucei from TLF killing. The biochemical events that occur within the lysosome, eventually leading to trypanosome lysis, may be complicated since multiple toxins are present in the TLF particle. Two potential mechanisms for TLF killing have been proposed. The first model proposes that within the acidic lysosome, TLF is able to accelerate the reduction of Fe3+, resulting in the formation of reactive free radicals, lipid peroxidation, and eventually, lysosomal membrane destabilization (3). The second model is based on studies with recombinant apoL-I which demonstrate that apoL-I is able to form anion channels in vitro, and when taken up by T. b. brucei, triggers depolarization of the lysosomal membrane, leading to an influx of chloride and subsequent osmotic swelling of the lysosome until the trypanosome lyses (36). The recent report that TLF can form cation-selective pores in unilamellar vesicles is consistent with a pore-forming mechanism for trypanosome killing (33). The identification of two toxins within the same native HDL raises the interesting possibility that the two proposed mechanisms may work in concert to provide the synergism observed with native and reconstituted HDL containing Hpr and apoL-I (43). Despite the uncertainties concerning the precise mechanism of killing, there is general agreement that localization of TLF to the lysosome is a prerequisite step (32, 47).
Resistance to the cytotoxic activities of normal human serum has been recognized as the key feature distinguishing T. b. brucei from the human pathogen T. b. rhodesiense. Human serum resistance in all isolates of T. b. rhodesiense coincides with the expression of the serum resistance-associated gene (SRA) (8, 9, 10, 31, 40, 51, 37, 49). SRA is a member of the variable surface glycoprotein (VSG) family of proteins in African trypanosomes, and despite having low sequence homology (<25%) with VSGs, it shares several sequence and structural features with VSGs (6, 9, 11). SRA is an expression site-associated gene in T. b. rhodesiense and is located upstream of the VSG in the active telomeric expression site (51).
The role of SRA in resistance to human serum was conclusively shown in transfection studies of T. b. brucei with SRA (50, 51). These studies were extended to show that recombinant apoL-I and SRA bind in vitro by a coiled-coil interaction between the two proteins, and this has been proposed to directly inhibit trypanosome killing by apoL-I (47). Immunofluorescence microscopy analysis of T. b. rhodesiense indicated that apoL-I and SRA colocalized to the lysosome in trypanosomes treated with apoL-I. However, other studies have shown that TLF uptake and cellular localization differed in resistant and susceptible lines of T. b. rhodesiense (19). TLF accumulation was reduced approximately sixfold in resistant trypanosomes, and the cell-associated TLF was excluded from the lysosome (19). This indicated that differences in cellular trafficking of TLF might contribute to trypanosome susceptibility to TLF killing.
Here we show that transfection of SRA into three different bloodstream-stage T. b. brucei isolates expressing different VSGs was sufficient to confer high levels of resistance to TLF and human serum. Epitope-tagged SRA also conferred TLF resistance and allowed subcellular localization of SRA to nonlysosomal vesicles predominantly located between the nucleus and the kinetoplast. In SRA-expressing cells, TLF was not routed to the lysosome, and colocalization of TLF and SRA was observed in small cytoplasmic vesicles. Based on these observations, we conclude that the association of SRA with TLF-containing endosomes results in rerouting of TLF, thus preventing lysosomal localization and trypanosome death.
MATERIALS AND METHODS
Trypanosome isolates and culture methods.
Culture-adapted monomorphic isolates of T. b. brucei MiTat 1.2 (427/221), pleomorphic BiTat 1.1 (KETRI 667), and GuTat 10.1 (TREU 927/4) cells were used throughout these studies. Bloodstream-stage cultures were grown in HMI-9 medium supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT) and 10% Serum Plus (JRH Bioscience, Lenexa, KS) (22).
Cloning of Ty epitope-tagged SRA and transfection of T. b. brucei.
The DNA sequence encoding the Ty epitope, containing the coding information for a 10-amino-acid sequence of the major structural protein of the Saccharomyces cerevisiae Ty1 virus-like particle, was inserted within the coding sequence of the SRA cDNA (accession no. AF097331). The oligomers Ty-5′ and Ty-3′ were synthesized with a HindIII overhang (Ty-5′, AGCTTGAGGTCCATACTAACCAGCATCCACTTGAC; Ty-3′, AGCTTGTCAAGTGGATCCTGGTTAGTATGGACCTC [the HindIII overhang is shown with underlining]), annealed, and cloned into a unique HindIII site at position 848 in the SRA gene. SRA, with and without the epitope tag, was PCR amplified with the Expand High Fidelity PCR system (Roche Molecular Biochemicals, Indianapolis, IN), using primers 5′SRA and 3′SRA with EcoRI sites (5′SRA, CCCGAATTCGTAACAGCAATGCCCCGAAATTCGGGC; 3′SRA, CCCGAATTCGTGAAAATTAAAACAGAAAGGCC [EcoRI sites are shown with underlining; the positions of the ATG start codon and the TTA termination codon are indicated in boldface]). Digested PCR products were cloned into the trypanosome expression vector pURAN (25). SRA was targeted to a small polycistronic transcription unit containing the neomycin resistance gene downstream. The multiple cloning site was flanked by tubulin intergenic regions to ensure accurate and efficient processing of the transcript. Prior to transfection, the vector was linearized at a unique SunI (isoschizomer of BsiW1) site of the ribosomal promoter sequence to allow efficient insertion into the endogenous rRNA gene locus (25).
For transfections of bloodstream-stage trypanosomes, cells were resuspended at 3 × 107 cells/ml in Cytomix (120 mM KCl, 150 μM CaCl2, 10 mM phosphate buffer, pH 7.6, 25 mM HEPES, pH 7.6, 5 mM MgCl2, 2 mM EGTA, pH 7.6). Cells (1.2 × 106) were mixed with 30 μg of linearized DNA and electroporated (GenePulser II; Bio-Rad Laboratories, Richmond, CA) in a 0.4-cm GenePulser cuvette (Bio-Rad 165-2088) at 1.5 kV, 25 μF, and 200 Ω. Electroporated cells were allowed to recover for 24 h in HMI-9 medium before the addition of 2.5 μg/ml Geneticin (G418 sulfate). Drug-resistant populations were cloned, grown on agarose plates containing 2 μg/ml G418, and subsequently maintained in HMI-9 medium with constant drug selection (7).
Purification of trypanolytic HDLs and in vitro lysis assays.
Normal human blood was obtained from healthy fasting donors. Lytic HDLs were purified as described previously, and aliquots were frozen at −70°C (21). One unit of lytic activity is defined as the amount of HDL needed to kill 50% of human-serum-susceptible T. b. brucei (IlTat 1.3) organisms in a standard 2-h lysis assay at 37°C (21). Typically, 0.01 μg of purified TLF provided a unit of trypanolytic activity in this in vitro assay (43). Lysis assays performed with cultured cells were extended to 4 h. The percentage of lysed cells was counted among 100 cells. Growth inhibition assays were performed by the addition of human serum (50%) to cultured bloodstream-stage trypanosomes. After 24 h, the percentage of cells surviving was determined by phase-contrast microscopy.
To estimate the growth rates of wild-type and transfected trypanosomes, cells were inoculated at 1 × 104 cells/ml in HMI-9 medium and counted daily until stationary phase was reached. The growth rate was calculated with the following equation: 1/v = [log10 2 (T − T0)]/(log10 N − log10 N0).
DNA and RNA analysis.
Genomic DNA was isolated as described previously (30). Twenty nanograms of genomic DNA was used for PCRs to examine the presence of SRA (5′ primer, CACACCTCTAAGAATCACAATAG; 3′ primer, AATTCATGAAAATGTGTTAAAG) and tubulin (5′ primer, CCGTGGCATATGGCAAG; 3′ primer, GGGGGTGCACTTTGTC) gene sequences. Southern blots were performed according to standard protocols (42). Total RNA was isolated with Trizol reagent (Roche Biochemicals, Indianapolis, IN), and 5 μg of RNA was separated in 1% formaldehyde gels and electroblotted at 15 V for 1 h, followed by 50 V for 1.5 h, onto a positively charged nylon membrane (Roche Biochemicals, Indianapolis, IN). DNA probes were labeled by the random primer method (Life Technologies-Invitrogen, Carlsbad, CA) with [32P]dCTP. The blots were hybridized in 0.9 M NaCl, 5× Denhardt's solution, 10% dextran sulfate, 5 mM EDTA, 0.1% sodium dodecyl sulfate [SDS], 40% formamide, 0.1 mg/ml salmon sperm DNA overnight at 42°C and subsequently washed at 60°C with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 1% SDS, and finally 0.1× SSC, 1% SDS at room temperature. Alternatively, the probes were labeled with AlkPhos Direct (Amersham Pharmacia, Piscataway, NJ), hybridized, and developed according to the manufacturer's description.
Analysis of SRA by SDS-PAGE and Western blotting.
Cultured cells were washed twice with phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 4.3 mM KH2PO4, 1.4 mM Na2HPO4) and then resuspended in lysis buffer (100 mM Tris, pH 8; 10 mM EDTA; 0.5% SDS) containing a protease inhibitor cocktail (Complete Mini; Roche Biochemicals, Indianapolis, IN). Freshly prepared cell lysates (equivalent to 3 × 106 cells per lane) were separated in 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gels (24). Proteins were transferred to nitrocellulose membranes (0.2-μm Protran; Schleicher and Schuell, Dassel, Germany) for 45 min at 57 V. Polyclonal antibodies against VSG 221 were added at a 1:2,000 dilution, incubated for 1 h at room temperature, and developed according to a standard protocol (5). The monoclonal antibodies against the Ty epitope (immunoglobulin G1 subtype) are specific to the epitope tag and have previously been shown to have no cross-reactivity with procyclic trypanosome proteins (2). These antibodies were incubated with protein blots overnight at a 1:5 dilution and developed with BCIP/NBT (5-bromo-4-chloro-3-indolylphosphate/Nitro Blue Tetrazolium) under standard conditions. BB2 hybridoma cells expressing antibodies against the Ty epitope were generously provided by Keith Gull, University of Oxford, United Kingdom.
Cellular localization of SRA and TLF.
Cultured bloodstream-stage trypanosomes were collected by centrifugation at 2,000 × g for 10 min, washed in PBS containing 1% glucose (PBSG), resuspended in PBS-10% FBS at 3 × 107 cells/ml, and smeared onto a microscope slide. After being air dried, cells were fixed in cold methanol for 10 min at 4°C. Cells were rehydrated in PBS-10% FBS and subsequently incubated in PBS-10% FBS with anti-Ty (1:50 dilution) for 1 h at room temperature. Slides were washed with PBS, and Alexa Fluor 488-labeled goat anti-mouse immunoglobulin G (Molecular Probes, Eugene, OR) was added at a 1:1,000 dilution in PBS-10% FBS for 1 h at room temperature. 4′,6′-Diamidino-2-phenylindole (DAPI; 2-μg/ml final concentration) was added during the secondary antibody incubation. Slides were washed two times in PBS and microscopically analyzed.
To determine whether SRA colocalized with the endoplasmic reticulum (ER), fixed cells were incubated with monoclonal anti-Ty (1:50) and polyclonal anti-BiP (1:4,000) (1). Goat anti-mouse Alexa Fluor 488-labeled and goat anti-rabbit Alexa Fluor 594-labeled antibodies were added, and slides were processed as described above. To determine SRA localization with the lysosome, cells were stained with the anti-Ty antibody as described above and then incubated with a monoclonal antibody to the lysosomal marker p67 (1:1,000) conjugated to Alexa Fluor 594 (23).
To further localize SRA with the endocytic marker tomato lectin (TL; Vector Laboratories, Burlingame, CA) (34) and TLF, we directly conjugated TL and TLF to Alexa fluorochromes. Cells were incubated with 4 μg/ml TL and/or 10 to 20 μg/ml TLF for 30 min at 37°C. After incubation, the cells were put on ice, washed, resuspended in PBSG at 4°C, smeared onto a slide, and fixed with cold methanol. For colocalization of SRA with TL or TLF, cells were stained with anti-Ty as described above. For localization of TLF and p67, cells were incubated with 50 μM chloroquine for 30 min before adding Alexa-conjugated TLF to a final concentration of 20 μg/ml. Cells were incubated for an additional 90 min before being processed and stained for p67 as described above. Trypanosomes were analyzed at a magnification of ×100 with a Zeiss fluorescence microscope, and digital images were captured with a Zeiss AxioCam video camera. The contrast and brightness of some images were adjusted and overlaid with Adobe Photoshop software.
RESULTS
Genotypic and phenotypic analysis of SRA-transfected T. b. brucei.
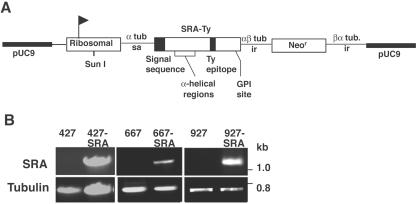
Previous studies have shown that transfection of procyclic T. b. brucei with SRA and subsequent transmission through tsetse flies resulted in bloodstream-stage T. b. brucei organisms that were resistant to normal human serum (51). The cyclical transmission of trypanosomes through tsetse flies is difficult due to the low transmission efficiency, variability in the ability of T. b. brucei isolates to infect tsetse flies, and specific developmental changes in gene expression influencing the VSG expression sites and other genes. To circumvent these problems and to directly determine if SRA and Ty epitope-tagged SRA were sufficient to confer resistance to normal human serum, three different isolates of bloodstream-stage T. b. brucei were transfected with SRA and SRA-Ty. The location of the Ty epitope within the SRA gene was not expected to disrupt the proposed N-terminal alpha-helical domains implicated in having a role in SRA resistance (Fig. 1A) (6, 47). Similar transfections of bloodstream-stage T. b. brucei with the hemagglutinin (HA) epitope fused to the amino or carboxy terminus of SRA were reported previously (50). Genomic DNAs from wild-type 427, transfected 427-SRA and 427-SRA-Ty, wild-type 667, transfected 667-SRA and 667-SRA-Ty, wild-type 927, and transfected 927-SRA and 927-SRA-Ty organisms were analyzed for the presence of the SRA gene by PCR with primers complementary to the 5′ and 3′ coding sequences of SRA. A 1.4-kb SRA product was only detected in SRA- and SRA-Ty-transfected T. b. brucei lines and not in wild-type cells (Fig. 1B). Southern blotting confirmed single-copy integration of SRA in the SRA and SRA-Ty transfectants (data not shown). Control PCRs using primers specific to the α-tubulin gene produced the expected 0.75-kb product (Fig. 1B).
FIG. 1.
Diagram of SRA gene construct and PCR analysis of transfectants. (A) Diagram of SRA gene indicating the locations of the Ty epitope tag, signal sequence, proposed α-helical domains, and a proposed glycosylphosphatidylinositol (GPI) anchor site. SRA-Ty was cloned into the transfection vector pURAN, consisting of a pUC9 backbone, the neomycin resistance gene (Neor), and sequences for targeting the rRNA promoter (ribosomal [marked with a flag]) following cleavage with the restriction enzyme SunI (25). Correct RNA processing and stability were provided by α-tubulin splice acceptor (α-tub sa) and αβ-tubulin intergenic (αβ-tub ir) sequences. The Ty epitope was cloned into the SRA gene at a unique HindIII site at 848 bp (accession no. AF097331) (2). (B) PCR amplification of SRA and tubulin genes from genomic DNAs isolated from trypanosomes transfected with pURAN vector alone (T. b. brucei 427, 667, and 927) or with pURAN containing SRA (T. b. brucei 427-SRA, 667-SRA, and 927-SRA). The PCR products for the SRA and tubulin genes run at 1.4 kb and 0.7 kb, respectively.
By light microscopy, no morphological differences were observed between SRA-transfected and wild-type cells. In addition, cells with and without SRA reached approximately the same cell densities and grew at the same rates, indicating that the expression of SRA did not influence the growth characteristics of bloodstream-stage T. b. brucei (data not shown).
Susceptibility of SRA-transfected T. b. brucei to TLF killing.
In order to determine whether transfection with SRA was sufficient to confer the serum resistance phenotype, transfected cells were incubated with increasing concentrations of TLF (Fig. 2) or normal human serum (data not shown). The three wild-type lines of T. b. brucei were highly susceptible to lysis by TLF. Following SRA transfection, the three lines of T. b. brucei showed high levels of resistance to TLF (Fig. 2). The level of serum resistance in SRA-transfected T. b. brucei was similar to that observed for human infectious T. b. rhodesiense (data not shown). No difference in the level of TLF resistance was detected between cells transfected with SRA alone and those transfected with SRA-Ty (data not shown). To confirm whether SRA-induced TLF resistance was comparable to that seen for T. b. rhodesiense, the survival of SRA-transfected T. b. brucei was examined at concentrations of TLF similar to those found in human serum (1,000 U/ml) and by incubation for 24 h under the same growth conditions in the presence of 50% human serum. The SRA-transfected T. b. brucei lines remained viable under these conditions (data not shown).
FIG. 2.
Transfection of three T. b. brucei lines with SRA confers resistance to TLF. Lysis assays were conducted with T. b. brucei transfected with empty vector (427 [▪], 667 [•], and 927 [▴]) and with SRA-Ty vector (427-SRA [□], 667-SRA [○], and 927-SRA [▵]). The percentages of trypanosomes lysed by increasing amounts of TLF under standard assay conditions were determined following a 4-h incubation at 37°C.
Early studies suggested that the VSG expressed on the surfaces of trypanosomes might influence their susceptibility to normal human serum. Each of the T. b. brucei lines used in the current studies expresses different VSG genes producing serologically distinct VSG coats. Based on the T. b. brucei isolates tested, it appears that resistance to TLF is highly dependent on SRA expression but independent of the VSG expressed on the trypanosome surface. The transfection of bloodstream-stage lines of T. b. brucei directly demonstrated that expression of the SRA gene is sufficient to confer resistance to TLF and may lead to human infectivity.
Expression of SRA mRNA in transfected cells.
SRA mRNA is an abundant transcript in T. b. rhodesiense, representing as much as 10% of the total cellular RNA (10, 18, 31). In order to determine the level of SRA mRNA in transfected cells and to compare this level with that in human infectious T. b. rhodesiense, total cellular RNA was isolated from T. b. brucei transfected with either vector alone (427) or SRA (427-SRA) and from a human-serum-resistant line of T. b. rhodesiense. Blots were hybridized with probes specific for the VSG expressed by T. b. rhodesiense (VSG-R) and the VSG-221 expressed by the T. b. brucei 427 cell line (Fig. 3A). The amount of SRA mRNA in human-serum-resistant T. b. rhodesiense was similar to the level of SRA mRNA in T. b. brucei 427-SRA (Fig. 3A). The SRA mRNA in T. b. brucei 427-SRA is approximately 460 nucleotides larger than that in T. b. rhodesiense because of additional 5′ and 3′ untranslated region sequences added to the SRA sequence in the expression vector (about 430 nucleotides) and the addition of the epitope tag within the gene (30 nucleotides). Equal RNA loading was confirmed by ethidium bromide staining of the agarose gel. These results show that while the transgenic SRA gene is expressed from the ribosomal locus in T. b. brucei 427-SRA, the expression level of the gene is similar to the endogenous expression level of SRA in human-serum-resistant T. b. rhodesiense strains.
FIG. 3.
Expression of SRA mRNA and SRA protein in transfected T. b. brucei. (A) Northern blot analysis of total cell RNA from transfected and nontransfected T. b. brucei 427. RNA samples from T. b. brucei transfected with either empty vector (427) or SRA-containing vector (427-SRA) or from human-serum-resistant T. b. rhodesiense [T. b. r. (R)] were separated in 1% agarose gels and hybridized with probes specific for the VSG mRNA from T. b. brucei 427 (VSG-221) or wild-type T. b. rhodesiense (VSG-R) and for SRA mRNA. Ethidium bromide staining of the agarose gel is shown in the top panel. (B) Protein gel and Western blot of T. b. brucei 427. The Coomassie-stained gel and Western blot analysis show results for protein extracts from T. b. brucei transfected with vector alone (427) or with SRA (427-SRA-Ty). The Western blot was probed with antibodies to the Ty epitope, and proteins of approximately 59 and 65 kDa were detected in extracts from T. b. brucei 427-SRA-Ty (arrows).
Expression of SRA in transfected T. b. brucei.
Despite high levels of SRA mRNA, the detection of SRA protein in T. b. rhodesiense and in SRA-transfected T. b. brucei has been problematic in some cases (8, 9, 51). However, visualization of SRA has been reported for T. b. rhodesiense cells by use of a polyclonal mouse antiserum raised against recombinant SRA (31). In addition, endogenous SRA in T. b. rhodesiense as well as recombinant SRA (rSRA) expressed in T. b. brucei was detected using antibodies raised against an SRA polypeptide (47). To examine SRA expression in transfected T. b. brucei, total cell lysates from 3 × 106 trypanosomes were separated by 10% SDS-PAGE and analyzed by Coomassie staining and Western blotting with monoclonal antibodies specific to the Ty tag inserted into the SRA gene. Epitope-tagged proteins, migrating at approximately 59 and 65 kDa, were detected in Western blots of cell lysates of both 427-SRA and 667-SRA but not in lysates of wild-type 427 and 667 (Fig. 3B; data for 667 and 667-SRA are not shown). The predicted size of mature SRA is approximately 38,000 Da. While the nature of the doublet is unknown, it may be the result of differences in posttranslational modification of SRA. The staining patterns observed are similar to previous Western blot results obtained with T. b. rhodesiense extracts analyzed with anti-rSRA and SRA-HA-transfected T. b. brucei probed with anti-HA (31, 50). As reported by others, we found that the detection of SRA by Western blot analysis was variable, suggesting that SRA may be highly susceptible to proteolytic degradation while cell extracts are prepared for analysis.
Cellular localization of SRA and TLF in T. b. brucei 427-SRA-Ty.
We determined the cellular localization of SRA in T. b. brucei 427-SRA-Ty by immunofluorescence microscopy, using a monoclonal antibody against the Ty epitope. This antibody is highly specific for the Ty epitope and does not react with wild-type T. b. brucei 427 proteins by either Western blotting or immunofluorescence analysis. T. b. brucei 427-SRA-Ty cells were fixed, permeabilized, and incubated with anti-Ty. SRA-Ty was visible within cytoplasmic vesicles located mainly between the kinetoplasts and the nuclei of T. b. brucei 427-SRA cells (Fig. 4C, G, and K). Only minor cell surface labeling was observed, indicating that SRA in transfected T. b. brucei is mainly localized to intracellular compartments.
FIG. 4.
Cellular localization of SRA in T. b. brucei 427-SRA-Ty. Immunofluorescence analysis was done with cells treated with antibodies to Ty epitope-tagged SRA and to the ER marker BiP (A to D), the endocytic marker TL (E to H), or the lysosomal marker p67 (I to L). The positions of the nucleus (N) and the kinetoplast (K) were visualized by staining with DAPI (blue). (A, E, and I) Cells visualized by bright-field (BF) microscopy overlaid with DAPI-stained DNA. (B) Cell stained with anti-BiP (red). (F) Cell following uptake of Alexa-conjugated TL (red). (J) Cell stained with anti-p67 (red). (C, G, and K) Cells stained for SRA with anti-Ty (green). (D, H, and L) Overlays of images of cells stained for DNA/BiP/SRA, DNA/TL/SRA, or DNA/p67/SRA, respectively.
We were concerned that the expression of SRA-Ty from the ribosomal locus in T. b. brucei might result in aggregation within the ER or targeting to the lysosome for degradation. T. b. brucei 427-SRA-Ty cells were double stained for SRA (anti-Ty) and the ER (anti-BiP), endocytic compartments (Alexa-conjugated TL), or the lysosome (anti-p67). Double labeling of T. b. brucei 427-SRA-Ty with anti-BiP and anti-Ty revealed a distinctive ER staining pattern with the BiP antibody (red), with little colocalization with SRA-Ty (green; Fig. 4A to D). To determine whether SRA was located in endosomes or the lysosome, T. b. brucei 427-SRA-Ty cells were incubated with Alexa Fluor 594-labeled TL (red) for 30 min at 37°C to allow uptake and lysosomal targeting of the ligand. Cells were then fixed and incubated with anti-Ty (green). Under these conditions, TL accumulated largely in the trypanosome lysosome (Fig. 4F), and to a limited extent, in endosomes located nearer the flagellar pocket (green; Fig. 5C and G). While SRA-Ty localized to the region between the kinetoplast and the nucleus, SRA-Ty staining did not overlap with TL staining (Fig. 4E to H). To directly address lysosomal localization, we stained cells with an antibody to p67 (red) and found that SRA-containing vesicles (green) did not significantly overlap with localization of the lysosomal membrane protein p67 (Fig. 4I to L). This pattern suggests that SRA-Ty is predominately nonlysosomal.
FIG. 5.
Cellular localization of TL and TLF taken up by wild-type T. b. brucei 427 (A to D) and T. b. brucei 427-SRA-Ty (E to H). The positions of the nucleus and the kinetoplast were visualized by staining with DAPI (blue). (A and E) Cells visualized by bright-field (BF) microscopy overlaid with DAPI-stained DNA. Cells are shown following incubation with Alexa-conjugated TLF (red) (B and F) or Alexa-conjugated TL (green) (C and G). (D and H) Overlays of images of cells stained for DNA and visualized for the appearance of Alexa-conjugated TLF and TL. The arrows in panel D indicate the sites of TLF and TL colocalization in wild-type T. b. brucei.
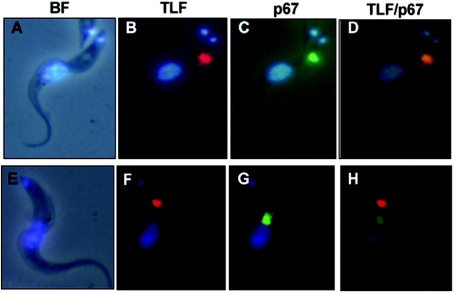
TLF killing of T. b. brucei requires lysosomal localization and subsequent acidification (20, 26). T. b. brucei 427 was incubated with TLF conjugated with Alexa Fluor 594 (red) and TL conjugated with Alexa Fluor 488 (green) for 30 min at 37°C. Under these conditions, both TLF and TL colocalized mainly to the lysosomes of wild-type cells (Fig. 5A to D). These results are consistent with previous studies that localized TLF to lysosomes of other isolates of T. b. brucei (20, 44). In contrast, TLF did not colocalize with the endocytic marker TL in T. b. brucei 427-SRA-Ty cells (Fig. 5E to H). After 30 min, small TLF-containing vesicles were seen close to the flagellar pocket (Fig. 5F).
Once we saw that TLF did not seem to be trafficking by its normal endocytic pathway in the SRA-expressing cells, we asked whether TLF could be concentrated in the lysosome in the presence of chloroquine. Previous studies have shown that chloroquine blocks lysosome acidification and prevents the degradation of endocytosed proteins (44). Cells were treated with chloroquine for 30 min before incubation with TLF for 90 min in an attempt to force TLF into the lysosome. As expected, in wild-type T. b. brucei, TLF accumulated and colocalized with the lysosomal marker p67 (Fig. 6A to D). However, in the T. b. brucei 427-SRA-Ty cells, TLF localization was quite different. TLF did not localize with p67 and accumulated in nonlysosomal vesicles (Fig. 6E to H). Similar results were obtained when non-chloroquine-treated cells were examined (data not shown). Based on these results, we conclude that the intracellular trafficking of TLF is altered in T. b. brucei 427-SRA-Ty and that the expression of SRA may prevent TLF from reaching the lysosome, the site of trypanolytic activity.
FIG. 6.
Cellular localization of TLF relative to p67 in T. b. brucei 427 (A to D) and T. b. brucei 427-SRA-Ty (E to H) in the presence of chloroquine. The positions of the nucleus and the kinetoplast were visualized by staining with DAPI (blue). (A and E) Cells visualized by bright-field (BF) microscopy overlaid with DAPI staining. (B and F) Cells incubated with Alexa-conjugated TLF (red). (C and G) Cells stained with anti-p67 (green). (D and H) Overlays of images of cells incubated with Alexa-conjugated TLF and stained for DNA and p67.
SRA and TLF colocalize in T. b. brucei 427-SRA-Ty.
The essential nature of SRA expression in human-serum-resistant T. b. brucei suggests that SRA and TLF may interact with one another directly. In fact, studies have shown that SRA directly interacts with apoL-I, one of the trypanolytic components of TLF (47). To examine whether SRA and TLF are contained in the same cellular compartments, we incubated T. b. brucei 427-SRA-Ty with Alexa Fluor 594-conjugated TLF and subsequently stained the cells for SRA using the Ty antibody. Fluorescence microscopy showed that TLF (red) and SRA-Ty (green) overlap in their subcellular distributions (Fig. 7A to H). TLF and SRA colocalized to a subpopulation of small cytosolic vesicles in SRA-transfected T. b. brucei, but neither was found in the lysosome. We observed many SRA-containing vesicles without TLF, but few vesicles containing TLF alone, without SRA, were detected (Fig. 7D and H). These findings are consistent with a proposed mechanism of resistance to TLF resulting from reduced targeting of TLF to the lysosome and with the idea that the association of SRA and TLF is required for resistance (19).
FIG. 7.
Colocalization of TLF-1 and SRA in T. b. brucei 427-SRA-Ty. The positions of the nucleus and the kinetoplast were visualized by staining with DAPI (blue) (A to H). (A and E) Cells visualized by bright-field (BF) microscopy overlaid with DAPI staining of the nucleus and kinetoplast. (B and F) Cells incubated with Alexa-conjugated TLF (red). (C and G) Cells stained with anti-Ty (green) to visualize SRA. (D and H) Overlaid images of cells treated with Alexa-conjugated TLF and stained for DNA and SRA.
DISCUSSION
Initial studies using subtractive hybridization methods suggested that human-serum-resistant and -sensitive African trypanosomes might differ by the expression of a single gene (27). This possibility was tested in an elegant series of transfection experiments, in which the SRA gene from T. b. rhodesiense was introduced into a human-serum-susceptible line of T. b. brucei and was found to be sufficient to confer resistance (51). These studies relied on transfection of an insect-borne developmental stage of T. b. brucei and subsequent transmission through the tsetse fly prior to analysis of transfectants. This strategy, though certainly successful, is both cumbersome and potentially prone to artifacts due to changes in gene expression unrelated to the transgene. In this paper and elsewhere, direct transfection of bloodstream-stage T. b. brucei with SRA was shown to render the parasites resistant to both TLF and normal human serum (50). Transfected T. b. brucei cells express the SRA transgene at high levels, resulting in steady-state amounts of the SRA mRNA that are similar to those detected in human infectious T. b. rhodesiense. Using antibodies against the Ty epitope, we examined the intracellular distribution of SRA in T. b. brucei 427-SRA-Ty and found it to be largely localized to small cytoplasmic vesicles between the flagellar pocket and the nucleus. The incubation of T. b. brucei 427-SRA-Ty with TLF resulted in the accumulation of TLF in SRA-containing vesicles, but TLF was not seen in the trypanosome lysosome. Since the acidified lysosome is the site of action for TLF, this altered intracellular trafficking may be the primary cause of TLF and human serum resistance in SRA-transfected cells.
In several studies, data have shown that while SRA mRNAs are abundant in both T. b. rhodesiense and SRA-transfected T. b. brucei, the detection of SRA has been problematic (31, 51). More recently however, antibodies to SRA have been successfully used to detect endogenous SRA in T. b. rhodesiense by immunofluorescence and rSRA expressed in T. b. brucei by Western blotting (47). The variability in SRA detection may be a consequence of rapid intracellular degradation, the secretion of newly synthesized SRA, or accelerated turnover of the protein during parasite isolation. While intracellular SRA-Ty was consistently observed by immunofluorescence microscopy, we found that the detection of Ty-tagged SRA in protein extracts was highly variable. In comparison to one study, our results with T. b. brucei 427-SRA-Ty differ from those obtained for T. b. rhodesiense in a potentially important way (31). In that study, SRA was detected both in cytoplasmic vesicles, similar in distribution to those reported here, and on the surfaces of the trypanosomes. It is possible that the cell surface reactivity was simply a consequence of cross-reactivity of the polyclonal mouse antiserum against SRA with the VSG. This is a particular concern since SRA is a member of the VSG gene family and shares characteristics with other trypanosome cell surface proteins (6, 31). Alternatively, the amounts of SRA present on the cell surface in different trypanosome lines may vary. Regardless, the significance of the cell surface-associated SRA is questionable, since little or no SRA was found on the surfaces of T. b. brucei 427-SRA-Ty cells yet they were highly resistant to both TLF-1 and human serum. Other labs have also shown that the localization of endogenous SRA in T. b. rhodesiense is primarily intracellular (47). In this paper, we showed that SRA-Ty localizes to an intracellular, nonlysosomal, vesicular location in transfected T. b. brucei.
We were concerned that high-level expression of SRA might result in aberrant intracellular localization. Transgenes are often expressed at abnormal levels due to the use of heterologous promoters. In our construct, SRA expression is driven from a constitutively active rRNA polymerase I promoter known to mediate high levels of mRNA synthesis in African trypanosomes (25, 41). The 5′ and 3′ untranslated regions were derived from tubulin sequences, which contribute posttranscriptionally to the stability of mRNAs in both bloodstream and procyclic forms. Previous studies have shown that SRA mRNA is an abundant transcript in both wild-type T. b. rhodesiense and transfected T. b. brucei, representing as much as 5 to 10% of the total mRNA (31, 51). This is comparable to the levels of VSG mRNA in African trypanosomes. We found that the amount of SRA mRNA in our transfectants was similar to the level of SRA mRNA expression in T. b. rhodesiense. Since SRA in T. b. brucei 427-SRA-Ty confers resistance to human serum and the amount of SRA mRNA is similar to that in wild-type T. b. rhodesiense, it seems likely that the SRA-containing cytosolic vesicles revealed by immunofluorescence microscopy are the primary sites of SRA activity.
The subcellular localization of SRA and how it prevents TLF from reaching the lysosome may provide a better understanding of both the function of SRA and the endocytic pathways of trypanosomes. This is in contrast to the proposed site of SRA activity being at the lysosome, where it interacts with apoL-1 (47). However, an important difference between these studies is the use of purified human HDLs containing both Hpr and apoL-1 in our studies rather than purified and recombinant apoL-1. The uptake and trafficking of native TLF and its interaction with SRA may differ from that of recombinant apoL-I. Endocytosis in trypanosomes shares many characteristics with endocytosis in other eukaryotic organisms but also exhibits several unique features, including developmental regulation, selective trafficking of proteins from the flagellar pocket, and the potential routing of resident lysosomal proteins through the flagellar pocket prior to localization to the lysosome (35). Vesicular trafficking between organelles occurs through the fusion of donor and specific acceptor membranes. This process is highly regulated and ensures proper directionality in protein sorting and packaging. Monomeric GTPases of the Rab family play a pivotal role in the control of membrane fusion and vesicle trafficking. Several T. brucei Rab proteins have now been identified that localize to specific subcellular compartments (14-17, 28, 35). The availability of antibodies to the T. b. brucei Rab proteins makes it possible to determine whether the cytosolic vesicles containing SRA and TLF are part of a vesicle recycling pathway.
Although we have shown that SRA confers human serum resistance when transfected into bloodstream-stage T. b. brucei and that SRA and TLF colocalize within cytoplasmic vesicles, the mechanism of SRA function is still unknown. One proposed mechanism is that inhibition may depend on interactions between SRA and apoL-I in the lysosome (47, 48). However, we previously showed that TLF was not targeted to the lysosome in T. b. rhodesiense, whereas a naturally occurring human-serum-sensitive variant of T. b. rhodesiense transported TLF to the lysosome prior to cell lysis (19). The distributions of TLF in T. b. rhodesiense and in T. b. brucei 427-SRA-Ty appear to be somewhat different. In contrast to the localization of TLF to small cytoplasmic vesicles in T. b. brucei 427-SRA-Ty, TLF was largely localized at or near the flagellar pocket of T. b. rhodesiense (19). Therefore, it remains possible that SRA can influence TLF uptake and lysosomal trafficking at either the flagellar pocket, where receptor-mediated endocytosis initiates, or at later steps in the endocytic pathway.
Regardless of the precise role of SRA, we have shown that SRA expression is sufficient to confer resistance to TLF and normal human serum in T. b. brucei bloodstream-stage trypanosomes. Furthermore, we have shown that SRA is responsible for the rerouting of TLF within the trypanosome endocytic pathway, with the majority of the TLF becoming associated with nonlysosomal, SRA-containing vesicles. Future experiments will address whether SRA alters TLF localization by either recycling it out of the cell or directing it to a cellular compartment where TLF degradation is accelerated. The elucidation of mechanisms of TLF resistance could lead to the development of inhibitors of the SRA-mediated resistance pathway, thereby increasing the susceptibility of T. b. rhodesiense-mediated human sleeping sickness trypanosomes to TLF and thus leading to novel treatment of the disease.
Acknowledgments
We thank Jay Bangs, University of Wisconsin, for providing culture-adapted MiTat 1.2 427/221 cells and BiP and p67 antibodies; Piet Borst, The Netherlands Cancer Institute, for the transfection vector pURAN; John Donelson, University of Iowa, for bloodstream forms of GuTat 10.1 927/4; and Philippe Bastin and Keith Gull, University of Oxford, United Kingdom, for providing BB2 hybridoma cell lines expressing Ty antibodies. We thank Sara Faulkner for help with the cultivation of trypanosomes and other members of the Hajduk laboratory for their critical discussions and helpful comments on the manuscript.
This research was supported by a grant from the NIH (AI39033) and support from the Ellison Medical Foundation.
REFERENCES
- 1.Bangs, J. D., L. Uyetake, M. J. Brickman, A. E. Balber, and J. C. Boothroyd. 1993. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. J. Cell Sci. 105:1101-1113. [DOI] [PubMed] [Google Scholar]
- 2.Bastin, P., Z. Bagherzadeh, K. R. Matthews, and K. Gull. 1996. A novel epitope tag system to study protein targeting and organelle biogenesis in Trypanosoma brucei. Mol. Biochem. Parasitol. 77:235-239. [DOI] [PubMed] [Google Scholar]
- 3.Bishop, J. R., M. Shimamura, and S. L. Hajduk. 2001. Insight into the mechanism of trypanosome lytic factor-1 killing of Trypanosoma brucei brucei. Mol. Biochem. Parasitol. 118:33-40. [DOI] [PubMed] [Google Scholar]
- 4.Black, S. J., J. R. Seed, and N. B. Murphy. 2001. Innate and acquired resistance to African trypanosomiasis. J. Parasitol. 87:1-9. [DOI] [PubMed] [Google Scholar]
- 5.Burnette, W. N. 1981. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112:195-203. [DOI] [PubMed] [Google Scholar]
- 6.Campillo, N., and M. Carrington. 2003. The origin of the serum resistance associated (SRA) gene and a model of the structure of the SRA polypeptide from Trypanosoma brucei rhodesiense. Mol. Biochem. Parasitol. 127:79-84. [DOI] [PubMed] [Google Scholar]
- 7.Carruthers, V. B., and G. A. Cross. 1992. High-efficiency clonal growth of bloodstream- and insect-form Trypanosoma brucei on agarose plates. Proc. Natl. Acad. Sci. USA 89:8818-8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Greef, C., E. Chimfwembe, J. Kihang'a Wabacha, E. Bajyana Songa, and R. Hamers. 1992. Only the serum-resistant bloodstream forms of Trypanosoma brucei rhodesiense express the serum resistance associated (SRA) protein. Ann. Soc. Belg. Med. Trop. 72:13-21. [PubMed] [Google Scholar]
- 9.De Greef, C., and R. Hamers. 1994. The serum resistance-associated (SRA) gene of Trypanosoma brucei rhodesiense encodes a variant surface glycoprotein-like protein. Mol. Biochem. Parasitol. 68:277-284. [DOI] [PubMed] [Google Scholar]
- 10.De Greef, C., H. Imberechts, G. Matthyssens, N. Van Meirvenne, and R. Hamers. 1989. A gene expressed only in serum-resistant variants of Trypanosoma brucei rhodesiense. Mol. Biochem. Parasitol. 36:169-176. [DOI] [PubMed] [Google Scholar]
- 11.Donelson, J. E. 2003. Antigenic variation and the African trypanosome genome. Acta Trop. 85:391-404. [DOI] [PubMed] [Google Scholar]
- 12.Drain, J., J. R. Bishop, and S. L. Hajduk. 2001. Haptoglobin-related protein mediates trypanosome lytic factor binding to trypanosomes. J. Biol. Chem. 276:30254-30260. [DOI] [PubMed] [Google Scholar]
- 13.Duchateau, P. N., C. R. Pullinger, R. E. Orellana, S. T. Kunitake, J. Naya-Vigne, P. M. O'Connor, M. J. Malloy, and J. P. Kane. 1997. Apolipoprotein L, a new human high density lipoprotein apolipoprotein expressed by the pancreas. J. Biol. Chem. 272:25576-25582. [DOI] [PubMed] [Google Scholar]
- 14.Field, H., B. R. Ali, T. Sherwin, K. Gull, S. L. Croft, and M. C. Field. 1999. TbRab2p, a marker for the endoplasmic reticulum of Trypanosoma brucei, localises to the ERGIC in mammalian cells. J. Cell Sci. 112:147-156. [DOI] [PubMed] [Google Scholar]
- 15.Field, H., M. Farjah, A. Pal, K. Gull, and M. C. Field. 1998. Complexity of trypanosomatid endocytosis pathways revealed by Rab4 and Rab5 isoforms in Trypanosoma brucei. J. Biol. Chem. 273:32102-32110. [DOI] [PubMed] [Google Scholar]
- 16.Field, H., and M. C. Field. 1997. Tandem duplication of rab genes followed by sequence divergence and acquisition of distinct functions in Trypanosoma brucei. J. Biol. Chem. 272:10498-10505. [DOI] [PubMed] [Google Scholar]
- 17.Field, H., T. Sherwin, A. C. Smith, K. Gull, and M. C. Field. 2000. Cell-cycle and developmental regulation of TbRAB31 localisation, a GTP-locked Rab protein from Trypanosoma brucei. Mol. Biochem. Parasitol. 106:21-35. [DOI] [PubMed] [Google Scholar]
- 18.Gibson, W., T. Backhouse, and M. Griffiths. 2002. The human serum resistance associated gene is ubiquitous and conserved in Trypanosoma brucei rhodesiense throughout East Africa. Infect. Genet. Evol. 1:207-214. [DOI] [PubMed] [Google Scholar]
- 19.Hager, K. M., and S. L. Hajduk. 1997. Mechanism of resistance of African trypanosomes to cytotoxic human HDL. Nature 385:823-826. [DOI] [PubMed] [Google Scholar]
- 20.Hager, K. M., M. A. Pierce, D. R. Moore, E. M. Tytler, J. D. Esko, and S. L. Hajduk. 1994. Endocytosis of a cytotoxic human high density lipoprotein results in disruption of acidic intracellular vesicles and subsequent killing of African trypanosomes. J. Cell Biol. 126:155-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajduk, S. L., D. R. Moore, J. Vasudevacharya, H. Siqueira, A. F. Torri, E. M. Tytler, and J. D. Esko. 1989. Lysis of Trypanosoma brucei by a toxic subspecies of human high density lipoprotein. J. Biol. Chem. 264:5210-5217. [PubMed] [Google Scholar]
- 22.Hirumi, H., and K. Hirumi. 1989. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 75:985-989. [PubMed] [Google Scholar]
- 23.Kelley, R. J., D. L. Alexander, C. Cowan, A. E. Balber, and J. D. Bangs. 1999. Molecular cloning of p67, a lysosomal membrane glycoprotein from Trypanosoma brucei. Mol. Biochem. Parasitol. 98:17-28. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 25.Ligtenberg, M. J., W. Bitter, R. Kieft, D. Steverding, H. Janssen, J. Calafat, and P. Borst. 1994. Reconstitution of a surface transferrin binding complex in insect form Trypanosoma brucei. EMBO J. 13:2565-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorenz, P., P. E. Barth, W. Rudin, and B. Betschart. 1994. Importance of acidic intracellular compartments in the lysis of Trypanosoma brucei brucei by normal human serum. Trans. R. Soc. Trop. Med. Hyg. 88:487-488. [DOI] [PubMed] [Google Scholar]
- 27.Matthyssens, G., C. De Greef, P. Kronenberger, E. Wittouck, N. Van Meirvenne, E. Magnus, and R. Hamers. 1987. Presented at the Molecular Strategies of Parasitic Invasion. UCLA Symposia on Molecular and Cellular Biology New Series, New York, N.Y.
- 28.Mauricio de Mendonca, S. M., J. L. Nepomuceno da Silva, N. Cunha e-Silva, W. de Souza, and U. Gazos Lopes. 2000. Characterization of a Rab11 homologue in Trypanosoma cruzi. Gene 243:179-185. [DOI] [PubMed] [Google Scholar]
- 29.McEvoy, S. M., and N. Maeda. 1988. Complex events in the evolution of the haptoglobin gene cluster in primates. J. Biol. Chem. 263:15740-15747. [PubMed] [Google Scholar]
- 30.Medina-Acosta, E., and G. A. M. Cross. 1993. Rapid isolation of DNA from trypanosomatid protozoa using a simple “mini-prep” procedure. Mol. Biochem. Parasitol. 59:327-330. [DOI] [PubMed] [Google Scholar]
- 31.Milner, J. D., and S. L. Hajduk. 1999. Expression and localization of serum resistance associated protein in Trypanosoma brucei rhodesiense. Mol. Biochem. Parasitol. 104:271-283. [DOI] [PubMed] [Google Scholar]
- 32.Molina Portela, M. P., J. Raper, and S. Tomlinson. 2000. An investigation into the mechanism of trypanosome lysis by human serum factors. Mol. Biochem. Parasitol. 110:273-282. [DOI] [PubMed] [Google Scholar]
- 33.Molina Portela, M. P., E. B. Lugli, E. Recio-Pinto, and J. Raper. 2005. Trypanosome lytic factor, a subclass of high-density lipoprotein, forms cation-selective pores in membranes. Mol. Biochem. Parasitol. 144:218-226. [DOI] [PubMed] [Google Scholar]
- 34.Nolan, D. P., M. Geuskens, and E. Pays. 1999. N-linked glycans containing linear poly-N-acetyllactosamine as sorting signals in endocytosis in Trypanosoma brucei. Curr. Biol. 9:1169-1172. [DOI] [PubMed] [Google Scholar]
- 35.Overath, P., and M. Engstler. 2004. Endocytosis, membrane recycling and sorting of GPI-anchored proteins: Trypanosoma brucei as a model system. Mol. Microbiol. 53:735-744. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Morga, D., B. Vanhollebeke, F. Paturiaux-Hanocq, D. P. Nolan, L. Lins, F. Homble, L. Vanhamme, P. Tebabi, A. Pays, P. Poelvoorde, A. Jacquet, R. Brasseur, and E. Pays. 2005. Apolipoprotein L-I promotes trypanosome lysis by forming pores in lysosomal membranes. Science 309:469-472. [DOI] [PubMed] [Google Scholar]
- 37.Radwanska, M., M. Chamekh, L. Vanhamme, F. Claes, S. Magez, E. Magnus, P. De Baetselier, P. B. Uscher, and E. Pays. 2002. The serum resistance-associated gene as a diagnostic tool for the detection of Trypanosoma brucei rhodesiense. Am. J. Trop. Med. Hyg. 67:684-690. [DOI] [PubMed] [Google Scholar]
- 38.Raper, J., M. P. Portela, E. Lugli, U. Frevert, and S. Tomlinson. 2001. Trypanosome lytic factors: novel mediators of human innate immunity. Curr. Opin. Microbiol. 4:402-408. [DOI] [PubMed] [Google Scholar]
- 39.Rifkin, M. R. 1978. Identification of the trypanocidal factor in normal human serum: high density lipoprotein. Proc. Natl. Acad. Sci. USA 75:3450-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rifkin, M. R., C. De Greef, A. Jiwa, F. R. Landsberger, and S. Z. Shapiro. 1994. Human serum-sensitive Trypanosoma brucei rhodesiense: a comparison with serologically identical human serum-resistant clones. Mol. Biochem. Parasitol. 66:211-220. [DOI] [PubMed] [Google Scholar]
- 41.Rudenko, G., P. A. Blundell, A. Dirks-Mulder, R. Kieft, and P. Borst. 1995. A ribosomal DNA promoter replacing the promoter of a telomeric VSG gene expression site can be efficiently switched on and off in T. brucei. Cell 83:547-553. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Shiflett, A. M., J. R. Bishop, A. Pahwa, and S. L. Hajduk. 2005. Human high density lipoproteins are platforms for the assembly of multi-component innate immune complexes. J. Biol. Chem. 270:32578-32585. [DOI] [PubMed] [Google Scholar]
- 44.Shimamura, M., K. M. Hager, and S. L. Hajduk. 2001. The lysosomal targeting and intracellular metabolism of trypanosome lytic factor by Trypanosoma brucei brucei. Mol. Biochem. Parasitol. 115:227-237. [DOI] [PubMed] [Google Scholar]
- 45.Smith, A. B., J. D. Esko, and S. L. Hajduk. 1995. Killing of trypanosomes by the human haptoglobin-related protein. Science 268:284-286. [DOI] [PubMed] [Google Scholar]
- 46.Tytler, E. M., D. R. Moore, M. A. Pierce, K. M. Hager, J. D. Esko, and S. L. Hajduk. 1995. Reconstitution of the trypanolytic factor from components of a subspecies of human high-density lipoproteins. Mol. Biochem. Parasitol. 69:9-17. [DOI] [PubMed] [Google Scholar]
- 47.Vanhamme, L., F. Paturiaus-Hanocq, P. Poelvoorde, D. P. Nolan, L. Lins, J. VanDen Abbeele, A. Pays, P. Tebabi, H. Van Xong, A. Jacquet, N. Moguilevsky, M. Dieu, J. P. Kane, P. deBaetselier, R. Brasseur, and E. Pays. 2003. Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature 422:83-87. [DOI] [PubMed] [Google Scholar]
- 48.Vanhamme, L., and E. Pays. 2004. The trypanosome lytic factor of human serum and the molecular basis of sleeping sickness. Int. J. Parasitol. 34:887-898. [DOI] [PubMed] [Google Scholar]
- 49.Welburn, S. C., K. Picozzi, E. M. Fevre, P. G. Coleman, M. Odiit, M. Carrington, and I. Maudlin. 2001. Identification of human-infective trypanosomes in animal reservoir of sleeping sickness in Uganda by means of serum-resistance-associated (SRA) gene. Lancet 358:2017-2019. [DOI] [PubMed] [Google Scholar]
- 50.Wang, J., U. Bohme, and G. A. M. Cross. 2003. Structural features affecting variant surface glycoprotein expression in Trypanosoma brucei. Mol. Biochem. Parasitol. 128:135-145. [DOI] [PubMed] [Google Scholar]
- 51.Xong, H. V., L. Vanhamme, M. Chamekh, C. E. Chimfwembe, J. Van Den Abbeele, A. Pays, N. Van Meirvenne, R. Hamers, P. De Baetselier, and E. Pays. 1998. A VSG expression site-associated gene confers resistance to human serum in Trypanosoma rhodesiense. Cell 95:839-846. [DOI] [PubMed] [Google Scholar]