Abstract
Chitin in the cyst wall of Entamoeba histolytica is made by two chitin synthases (Chs), one of which is unique (EhCHS-1) and one of which resembles those of insects and nematodes (EhCHS-2). EhCHS-1 is deposited chitin in the lateral wall of transformed Saccharomyces cerevisiae Chs mutants, independent of accessory proteins (Chs4p to Chs7p) required by yeast Chs3p.
Chitin, the β1,4-linked polymer of N-acetylglucosamine (GlcNAc), is present in fungi, insects, nematodes, and Entamoeba species (reviewed in references 17, 18, and 21). Chitin synthases (Chs) are highly variable proteins which contain a conserved catalytic domain bordered by multiple transmembrane helices (TMHs) (18, 21). Phylogenetic analyses have found support for two major divisions (or families) of Chs (17, 18). Division I includes oomycete Chs and fungal Chs classes I to III, while division II includes fungal Chs classes IV and V along with insect and nematode Chs. In Saccharomyces cerevisiae, different pools of chitin are produced from cytosolic UDP-GlcNAc by three specialized chitin synthases (Chs1p to Chs3p) that differ in optimum pH, cation dependence, zymogenic behavior, and susceptibility to inhibitors (reviewed in references 5, 8, 15, and 17). Chs1p acts as a repair enzyme at the time of cytokinesis; Chs2p makes the chitin in the primary septum that separates mother and daughter cells, while Chs3p makes the bulk of cellular chitin, including the chitin along the lateral cell wall and in the ring that forms at the base of an emerging bud. Chs3p requires several accessory proteins either for its catalytic activity (Chs4p), trafficking, and targeting to the plasma membrane (Chs5p and Chs6p) or for exit from the endoplasmic reticulum (Chs7p) (17).
The infectious form of Entamoeba histolytica, the protist that causes amebic colitis and liver abscesses, is a cyst that contains chitin in its wall (1, 2). Because E. histolytica does not encyst when grown axenically in vitro, most laboratory studies of encystation use the reptilian parasite Entamoeba invadens, which can easily be induced to encyst in vitro by osmotic shock, glucose deprivation, or a combination of the two (4, 19, 24). The most abundant protein in the E. invadens cyst wall is Jacob, a lectin with five unique chitin-binding domains that cross-link chitin fibrils (14). E. invadens also has an encystation-specific chitinase that contains a unique chitin-binding domain that is distinct from those of the Jacob lectin (11, 23).
Encystation of E. invadens can be blocked by the addition of the Chs inhibitors polyoxin D and nikkomycin (3). Das and Gillin have previously studied two E. invadens Chs activities, described as “soluble” and “particulate” because they were associated with the supernatant or pellet, respectively, resulting from high-speed (100,000 × g) centrifugation of cell extracts (9). Campos-Góngora et al. have previously determined the primary structures of two E. histolytica Chs: EhCHS-1, which is 642 amino acids long with 7 predicted TMHs, and EhCHS-2, which is 980 amino acids long with 17 predicted TMHs (7). mRNAs encoding E. invadens Chs (EiCHS-1 and EiCHS-2) are each up-regulated during encystation, paralleling increases in chitin synthase activity and chitin formation during encystation (7, 9). We also found that EiCHS-1 and EiCHS-2 mRNA levels increase during encystation, while EhCHS-1, but not EhCHS-2, mRNAs were made by cultured E. histolytica trophozoites (data not shown).
The present study attempted to answer three additional questions concerning chitin synthases of entamoebae. First, what are the origins of EhCHS-1 and EhCHS-2? Do they represent gene duplications by entamoebae or do they share common ancestry with Chs of fungi, insects, or nematodes? Second, can we complement S. cerevisiae Chs mutants with EhCHS-1 and EhCHS-2? If so, is the amebic Chs function dependent upon accessory proteins of yeast (Chs4p to Chs7p)? Third, what are the catalytic properties of an Entamoeba Chs expressed in the yeast mutant?
EhCHS-1 is unique, while EhCHS-2 belongs to a clade of insect and nematode chitin synthases.
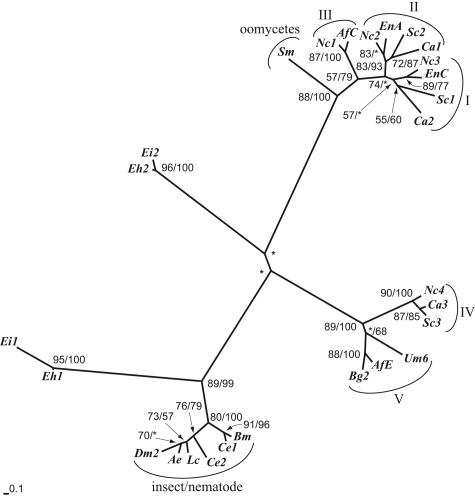
Four inferences were derived from our phylogenetic analysis of the relationship of the Entamoeba Chs to representative Chs from fungi, insects, and nematodes (Fig. 1) (10, 12, 13, 20, 22). First, gene duplication, if it occurred in Entamoeba, occurred long before the divergence of E. histolytica and E. invadens. EhCHS-1 and EiCHS-1 were monophyletic, as were EhCHS-2 and EiCHS-2, but there was no support for a clade containing all of the Entamoeba Chs. Second, there was strong support for a clade containing Entamoeba CHS-2 and insect and nematode Chs. Like insect and nematode Chs, EhCHS-2 contains numerous TMHs (17 in total) on both sides of the catalytic domain as well as a conserved octapeptide (CATMYHET) that is N terminal to the catalytic domain (7, 25). Third, the relationship of Entamoeba CHS-1 to other Chs was not resolved in this analysis, suggesting that EhCHS-1 is unique. However, the number of TMHs (7) in EhCHS-1 (but not the exact arrangement) is similar to those observed in many fungal Chs (6, 7). With a predicted length of 642 amino acids, EhCHS-1 is also significantly shorter than other reported Chs, which range between 738 and 1,869 amino acids in length (18). Fourth, our analysis did not find support for the two divisions of Chs that were previously identified (17, 18). There was strong bootstrap support for a clade containing oomycetes and fungal Chs classes I to III as well as a clade containing fungal Chs classes IV and V. However, the relationship between these two fungal clades and the mixed clade containing Entamoeba CHS-2 and insect and nematode Chs was not well resolved, suggesting that there may be three families or divisions of eukaryotic Chs.
FIG. 1.
Phylogenetic analysis of the relationship of Entamoeba chitin synthases to those of fungi (classes I to V), oomycetes, insects, and nematodes. Amino acid sequences of the conserved catalytic domain of chitin synthases (corresponding to positions 306 to 469 of EhCHS-1) were aligned using CLUSTAL W (22). Phylogenetic relationships were inferred using a distance matrix generated by TREE-PUZZLE (20) under the Dayhoff model (10), with the inclusion of observed amino acid frequencies, estimated proportion of invariant sites, and estimation of among-site variation for the remaining sites using a gamma distribution. The optimal tree was inferred using the Fitch-Margoliash algorithm (13) with global rearrangements and 100 random-addition replicates. Bootstrap values obtained using the 100 resampled data sets under the same model for distance and PHYLIP's PROTPARS program for parsimony (12), respectively, are shown at the relevant nodes. Asterisks mark nodes with bootstrap values below 50%. The scale bar indicates the estimated sequence divergence per unit branch length. Abbreviations for entamoebae: Eh, E. histolytica chs1 and chs2 (7); Ei, E. invadens chs1 (GenBank accession number AY737008) and chs2 (accession number AY737007). Abbreviations for fungi: Af, Aspergillus fumigatus C (accession number JC6015) and E (accession number S78102); Bg, Blumeria graminis 2 (accession number AAF05595); Ca, Candida albicans 1 (accession number T18220), 2 (accession number S20538), and 3 (accession number P30573); En, Emericella nidulans A (accession number JC2314) and C (accession number A59054); Nc, Neurospora crassa 1 (accession number 1805248A), 2 (accession number T47246), 3 (accession number CAC28596), and 4 (accession number S61886); Sc, Saccharomyces cerevisiae 1 (accession number A23944), 2 (accession number S45167), and 3 (accession number S45879); Um, Ustilago maydis 6 (AAB84285). Abbreviation for oomycetes: Sm, Saprolegnia monoica (AAC49743). Abbreviations for insects: Ae, Aedes aegypti (accession number AAF34699); Dm, Drosophila melanogaster 2 (accession number NP_524209); Lc, Lucilia cuprina (accession number AAG09712). Abbreviations for nematodes: Bm, Brugia malayi (accession number AAG49219); Ce, Caenorhabditis elegans 1 (accession number CAA96688) and 2 (accession number AAB71283).
EhCHS-1 functions in budding yeast independent of accessory proteins.
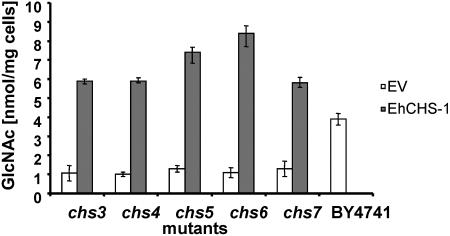
To test the function of the E. histolytica Chs, an S. cerevisiae chs1/chs3 double mutant strain created in the BY4741 background was transformed with a multicopy pYES2.1-TOPO plasmid (Invitrogen Corp., Carlsbad, CA) containing either EhCHS-1 or EhCHS-2 under the control of a galactose-inducible promoter. A c-myc sequence and stop codon were included in the reverse PCR primer used to amplify the Entamoeba genes for cloning. Yeast transformants were grown in minimal synthetic medium without uracil supplemented with 2% glucose to an optical density at 600 nm of 1.5 to 2. Protein expression was induced by switching to synthetic medium containing 2% galactose. After 2 to 6 h of induction, mRNAs of both EhCHS-1 and EhCHS-2 were detected in the transformed yeast by reverse transcription-PCR, and their respective protein products were detected by immunoblotting with an anti-c-myc antibody. For reasons that are not clear, EhCHS-1, but not EhCHS-2, was found to be functional in this system. Chitin was detected by calcofluor white M2R staining in cells transformed by the EhCHS-1 plasmid but not the EhCHS-2 plasmid or the empty vector. Chitin deposition by EhCHS-1 appeared to be restricted to the lateral cell wall; no chitin was detected as a ring in the bud scars of mother cells (Fig. 2). Chitin levels in cells expressing EhCHS-1 were also assayed directly using a modified Morgan-Elson method adapted to 96-well plates (6). After 6 h of induction, the amount of chitin deposited in the cell wall reached a maximum of 8.5 ± 1.7 nmol GlcNAc/mg cells, which was 2 to 2.5 times higher than the amount of chitin in the wild-type parent strain (3.9 ± 0.4 nmol GlcNAc/mg cells). In order to determine if EhCHS-1, which is clearly functional in S. cerevisiae without any auxiliary Entamoeba proteins, uses any of the yeast accessory proteins (Chs4p to Chs7p) for its activity or trafficking, chitin levels were assayed in yeast Chs deletion mutations (chs3, chs4, chs5, chs6, and chs7) in the BY4741 background (Research Genetics, Invitrogen Corp., Carlsbad, CA) transformed with EhCHS-1::pYES2.1-TOPO. Figure 3 shows that EhCHS-1 made virtually the same amount of chitin regardless of which background was used, suggesting that the yeast Chs3 accessory proteins are not required for EhCHS-1 activity.
FIG. 2.
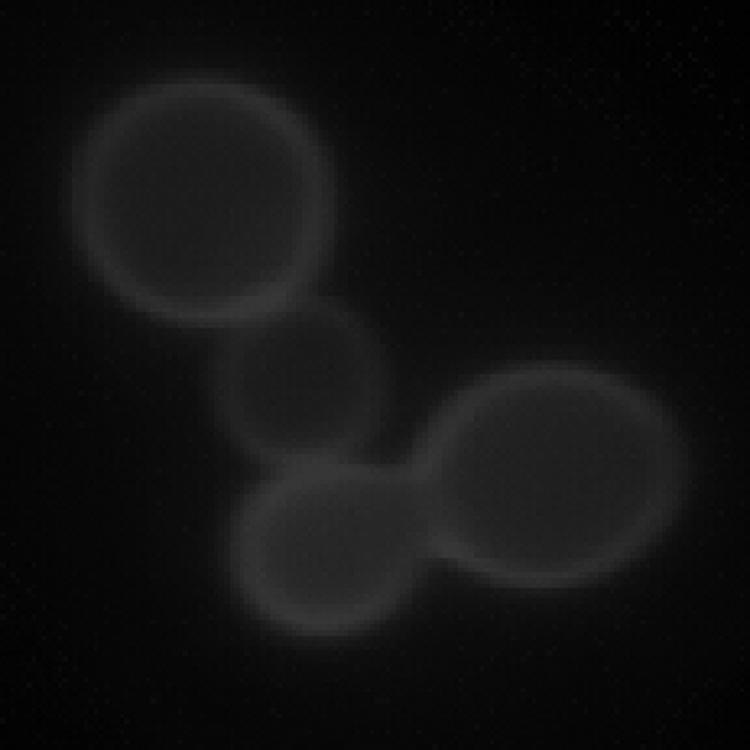
Calcofluor white M2R staining of an S. cerevisiae chs1/chs3 mutant expressing E. histolytica CHS-1::pYES2.1-TOPO.
FIG. 3.
Chitin content of S. cerevisiae chitin synthase mutants expressing E. histolytica CHS-1::pYES2.1-TOPO. Cells transformed with empty vector (EV) were used as a negative control. The chitin content of wild-type yeast cells (BY4741) is shown for comparison.
Catalytic properties of EhCHS-1.
The catalytic properties of EhCHS-1 expressed in the chs1/chs3 double mutant were examined using a previously described colorimetric assay for Chs activity in cell extracts of S. cerevisiae (16). Cells were induced for 6 h in synthetic medium containing 2% galactose, lysed in the presence of a fungal protease inhibitor cocktail, and subjected to high-speed centrifugation (55,000 × g) over a 10% (wt/wt) sucrose cushion to prepare a total membrane fraction for the assays. The basic assay reaction mixture contained 100 mM Tris-HCl, pH 8, 10 mM Mg2+, and 10 mM UDP-GlcNAc. Reactions were performed for 1 h at 30°C. Pretreatment of membranes with trypsin was not found to have a significant effect on EhCHS-1 activity, so we performed our assays with untreated membranes. This allowed us to independently assay EhCHS-1 activity in the presence of yeast Chs2p, which has negligible activity unless membranes are pretreated with trypsin. The total specific activity of EhCHS-1 (27.8 ± 2.2 nmol GlcNAc/h/mg protein) in the membrane fraction of the chs1/chs3 mutant was slightly higher than Chs3 activity in wild-type S. cerevisiae (18.5 ± 4 nmol GlcNAc/h/mg protein), probably due to a higher level of protein expression. No Chs activity could be detected in the supernatant after high-speed centrifugation, so crude extracts (1,500 × g, supernatant) were used for subsequent experiments (pH optimum, divalent cation preference, digitonin treatment, and Km measurement). Table 1 shows a comparison of the enzymatic properties of EhCHS-1 with the three Chs activities of S. cerevisiae and the “soluble” and “particulate” activities of E. invadens (8, 9). EhCHS-1 was found to be most active at a slightly basic pH (7.5 to 8.0) in the presence of Mg2+. EhCHS-1 activity did not increase when membranes were pretreated with trypsin but did increase when 0.1% digitonin was included in the assay mixture. The apparent Km of EhCHS-1 was 2 mM for UDP-GlcNAc. The pH optimum of EhCHS-1 was more similar to that of the “particulate” form of E. invadens Chs than that of the “soluble” form. However, EhCHS-1 differed from both reported E. invadens Chs activities in certain other characteristics, such as divalent cation preference and apparent Km. In the future, we hope to clone and express full-length E. invadens chitin synthase genes (for which only partial sequences are known) in our yeast heterologous expression system to allow for better comparisons between Chs activity measured in yeast and endogenous Chs activity.
TABLE 1.
Comparison of the enzymatic properties of Entamoeba histolytica CHS-1 with the three chitin synthase activities of Saccharomyces cerevisiae (Chs1p to Chs3p)a and the “soluble” and “particulate” activities of E. invadensb
| Property | Chs1p | Chs2p | Chs3p | E. invadens “soluble” | E. invadens “particulate” | EhCHS-1 |
|---|---|---|---|---|---|---|
| pH optimum | 6.5 | 8.0 | 7.5-8.0 | 6.0 | 7.0-7.5 | 7.5-8.0 |
| Divalent cation preference | Mg2+ | Co2+ | Ni2+, Co2+ | Mn2+, Co2+ | Mn2+ | Mg2+ |
| Trypsinization effect | Required | Required | Stimulatory | Inhibitory | Inhibitory | No effect |
| Digitonin effect | Stimulatory | No effect | Inhibitory | Stimulatory | Stimulatory | Stimulatory |
| Km (mM) | 0.5 | 0.8 | 0.6-0.8 | 0.35 | 0.23 | 2 |
Significance.
While EhCHS-2 resembles chitin synthases of insects and nematodes, EhCHS-1 is distinct from all previously described chitin synthases. To our knowledge, this is the first example of heterologous expression of a nonfungal Chs in budding yeast, and we and others have numerous unreported failures to express Chs of even closely related fungi in S. cerevisiae. Because EhCHS-1 is functionally independent of S. cerevisiae accessory proteins, the Entamoeba Chs appears to move by a bulk flow transport system to the plasma membrane, where, by itself, EhCHS-1 synthesizes and extrudes chitin polymer to the cell wall. Presently, we are creating chimeras of EhCHS-1 and S. cerevisiae Chs3p in order to determine those parts of yeast Chs that interact with the accessory proteins.
Acknowledgments
This work was supported in part by National Institutes of Health grants AI44070 (to J.C.S.) and GM31318 (to P.W.R.) as well as NSF grant IBN-0316963 (to C.A.S.).
We are grateful to Barbara Osmond of the Massachusetts Institute of Technology (Cambridge, MA) for providing the S. cerevisiae chs1/chs3 double mutant.
REFERENCES
- 1.Arroyo-Begovich, A., and A. Carabez-Trejo. 1982. Location of chitin in the cyst wall of Entamoeba invadens with colloidal gold tracers. J. Parasitol. 68:253-258. [PubMed] [Google Scholar]
- 2.Arroyo-Begovich, A., A. Carabez-Trejo, and J. Ruiz-Herrera. 1980. Identification of the structural component in the cyst wall of Entamoeba invadens. J. Parasitol. 66:735-741. [PubMed] [Google Scholar]
- 3.Avron, B., R. M. Deutsch, and D. Mirelman. 1982. Chitin synthesis inhibitors prevent cyst formation by Entamoeba trophozoites. Biochem. Biophys. Res. Commun. 108:815-821. [DOI] [PubMed] [Google Scholar]
- 4.Avron, B., T. Stolarsky, A. Chayen, and D. Mirelman. 1986. Encystation of Entamoeba invadens IP-1 is induced by lowering the osmotic pressure and depletion of nutrients from the medium. J. Protozool. 33:522-525. [DOI] [PubMed] [Google Scholar]
- 5.Bulawa, C. E. 1993. Genetics and molecular biology of chitin synthesis in fungi. Annu. Rev. Microbiol. 47:505-534. [DOI] [PubMed] [Google Scholar]
- 6.Bulik, D. A., M. Olczak, H. A. Lucero, B. C. Osmond, P. W. Robbins, and C. A. Specht. 2003. Chitin synthesis in Saccharomyces cerevisiae in response to supplementation of growth medium with glucosamine and cell wall stress. Eukaryot. Cell 2:886-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campos-Góngora, E., F. Ebert, U. Willhoeft, S. Said-Fernandez, and E. Tannich. 2004. Characterization of chitin synthases from Entamoeba. Protist 155:323-330. [DOI] [PubMed] [Google Scholar]
- 8.Choi, W. J., and E. Cabib. 1994. The use of divalent cations and pH for the determination of specific yeast chitin synthetases. Anal. Biochem. 219:368-372. [DOI] [PubMed] [Google Scholar]
- 9.Das, S., and F. D. Gillin. 1991. Chitin synthase in encysting Entamoeba invadens. Biochem. J. 280:641-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dayhoff, M. O., R. M. Schwartz, and B. C. Orcutt. 1978. Atlas of protein sequencing and structure, vol. 5, suppl. 3. National Biomedical Research Foundation, Silver Spring, Md.
- 11.de la Vega, H., C. A. Specht, C. E. Semino, P. W. Robbins, D. Eichinger, D. Caplivski, S. Ghosh, and J. Samuelson. 1997. Cloning and expression of chitinases of Entamoebae. Mol. Biochem. Parasitol. 85:139-147. [DOI] [PubMed] [Google Scholar]
- 12.Felsenstein, J. 1989. PHYLIP—phylogeny inference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 13.Fitch, W. M., and E. Margoliash. 1967. Construction of phylogenetic trees. Science 155:279-284. [DOI] [PubMed] [Google Scholar]
- 14.Frisardi, M., S. K. Ghosh, J. Field, K. Van Dellen, R. Rogers, P. Robbins, and J. Samuelson. 2000. The most abundant glycoprotein of amebic cyst walls (Jacob) is a lectin with five Cys-rich, chitin-binding domains. Infect. Immun. 68:4217-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henar Valdivieso, M., A. Duran, and C. Roncero. 1999. Chitin synthases in yeast and fungi. EXS 87:55-69. [DOI] [PubMed] [Google Scholar]
- 16.Lucero, H. A., M. J. Kuranda, and D. A. Bulik. 2002. A nonradioactive, high throughput assay for chitin synthase activity. Anal. Biochem. 305:97-105. [DOI] [PubMed] [Google Scholar]
- 17.Roncero, C. 2002. The genetic complexity of chitin synthesis in fungi. Curr. Genet. 41:367-378. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Herrera, J., J. M. Gonzalez-Prieto, and R. Ruiz-Medrano. 2002. Evolution and phylogenetic relationships of chitin synthases from yeasts and fungi. FEMS Yeast Res. 1:247-356. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez, L., V. Enea, and D. Eichinger. 1994. Identification of a developmentally regulated transcript expressed during encystation of Entamoeba invadens. Mol. Biochem. Parasitol. 67:125-135. [DOI] [PubMed] [Google Scholar]
- 20.Strimmer, K., and A. Von Haeseler. 1996. Quartet puzzling: a quartet maximum-likelihood method for reconstructing tree topologies. Mol. Biol. Evol. 16:964-969. [Google Scholar]
- 21.Tellam, R. L., T. Vuocolo, S. E. Johnson, J. Jarmey, and R. D. Pearson. 2000. Insect chitin synthase cDNA sequence, gene organization and expression. Eur. J. Biochem. 267:6025-6043. [DOI] [PubMed] [Google Scholar]
- 22.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Dellen, K., S. K. Ghosh, P. W. Robbins, B. Loftus, and J. Samuelson. 2002. Entamoeba histolytica lectins contain unique 6-Cys or 8-Cys chitin-binding domains. Infect. Immun. 70:3259-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vazquezdelara-Cisneros, L. G., and A. Arroyo-Begovich. 1984. Induction of encystation of Entamoeba invadens by removal of glucose from the culture medium. J. Parasitol. 70:629-633. [PubMed] [Google Scholar]
- 25.Zhu, Y. C., C. A. Specht, N. T. Dittmer, S. Muthukrishnan, M. R. Kanost, and K. J. Kramer. 2002. Sequence of a cDNA and expression of the gene encoding a putative epidermal chitin synthase of Manduca sexta. Insect Biochem. Mol. Biol. 32:1497-1506. [DOI] [PubMed] [Google Scholar]