Abstract
The akuA gene encoding the Ku70 component of the nonhomologous end-joining machinery was deleted in the opportunistic pathogen Aspergillus fumigatus. No obvious phenotype could be assessed for the corresponding mutant strain but relative frequencies of homologous recombination were increased as deduced from targeting the laccase-encoding abr2 gene.
Filamentous fungi represent valuable and versatile model systems in the biology of eukaryotic organisms. With an advanced molecular biology and increasing numbers of complete genome sequences publicly available, they have become highly suitable for basic and applied research on topics such as cellular biology, genetics, signal transduction, and differentiation (2). Genes identified in higher eukaryotes may be studied in a filamentous host expressing the fungal orthologue. To gain a very first hint on the cellular function of a given gene product, the generation of a corresponding null mutant in any fungal model organism is of high benefit.
Targeting and the replacement of gene loci in filamentous fungi are supported by the cellular machinery that accomplishes recombination and DNA repair. Especially the rate of homologous recombination in a given host determines its utility in knockout approaches using marker modules that are flanked by homologous stretches of the gene locus to be replaced (1). A cellular feature that tempers homologous recombination in fungi is the nonhomologous end-joining pathway, as it was recently illustrated by the works of Inoue and coworkers for the filamentous fungus Neurospora crassa (10).
Integration events mediated by nonhomologous end joining do not rely on homologous sites and therefore attenuate homologous recombination, which results in decreased frequencies of the desired gene knockout in a given transformation experiment with a suitable gene replacement cassette. In humans, the direct ligation of DNA strands in the nonhomologous end-joining process is mediated by the heterodimeric Ku protein and the ligase IV-XRCC4 complex, and homologues of the Ku70 and Ku80 subunits were identified in vertebrates, insects, plants, and fungi (4).
Recently, an advance was made in the molecular biology of the ascomycetous model organism N. crassa by evaluating the effectiveness of gene targeting in a genetic background lacking the nonhomologous end-joining pathway (10): in strains deleted for the Ku70- and Ku80-encoding genes mus-51 and mus-52, respectively, the relative frequency of homologous recombination is significantly increased, as assayed by integration of exogenous DNA at various target sites for gene replacements. Correspondingly, the lengths of homologous sequences flanking the marker gene and the targeting frequency strongly correlate in a way that as little as 500 bp resulted in 90% homologous integration; however, shorter regions of 100 bp did not yield a high percentage of correct transformants.
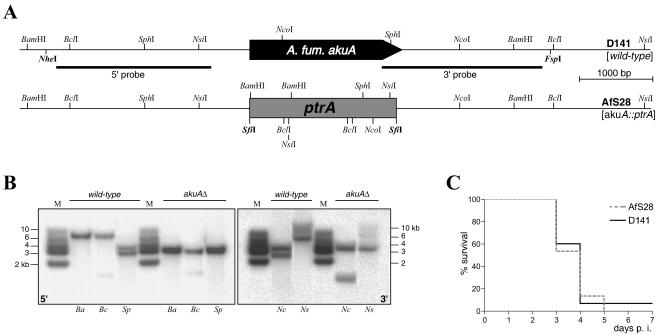
To estimate the influence of the nonhomologous end-joining pathway on gene targeting in the opportunistic pathogen Aspergillus fumigatus, we screened the annotated genome for deduced sequences similar to either of the Ku subunits. Two loci could be identified that had been assigned GenBank accession numbers XM_748769 (Ku70) and XM_744304 (Ku80). The gene orthologous to the Ku70 subunit-encoding gene mus-51 of N. crassa was chosen for further studies. The coding sequence of the encoding akuA locus (for Aspergillus Ku) was removed from the genome of the clinical isolate D141 (13) by gene replacement with a dominant ptrA marker cassette (8) (Fig. 1A) following the protocol of Kämper (5). For that purpose, flanking sequences were amplified by PCR from plasmid pME3001, which contains the A. fumigatus akuA genomic locus as a 6.8-kb NheI/FspI fragment in pBluescriptII KS [XbaI/EcoRV]. This plasmid had been isolated by screening a plasmid library containing genomic DNA fragments of D141 by colony hybridization with a suitable probe.
FIG. 1.
Aspergillus fumigatus genome includes a ku70 orthologue. (A) Structure of the genomic A. fumigatus akuA gene and the deleted akuA::ptrA locus of strain AfS28. Recognition sites for restriction enzymes as employed in Southern analyses are indicated; the positions of 5′- and 3′-specific probes are given as black bars. (B) Autoradiographs from Southern membranes. Genomic DNA from strains D141 (wild type) and AfS28 (akuAΔ) was subjected to digestion with enzymes BamHI (Ba), BclI (Bc), SphI (Sp), NcoI (Ni), and NsiI (Ns), and separated by agarose gel electrophoresis. After blotting and immobilization, membranes were hybridized with a radioactively labeled 5′-specific (left panel) or 3′-specific (right panel) probe as specified in panel A; M indicates the DNA size standard with fragments' lengths given on either edge. (C) Assessment of strain AfS28 in an insect virulence model. Groups of 15 larvae of the greater wax moth Galleria mellonella were infected with 8 × 106 conidia from D141 and AfS28, respectively, by injection of a 20-μl conidial suspension in saline supplemented with 10 μg/ml rifampin in the left last proleg. Larvae were kept in the dark at 30°C and inspected on a daily basis for motility and melanization.
Among the transformants selected on pyrithiamine-containing medium, one isolate could be identified in Southern analyses (14) that, after hybridizing with 5′- and 3′-specific probes, displayed patterns as expected from in silico calculations (Fig. 1B). In comparison to its wild-type progenitor, the A. fumigatus akuA::ptrA deletion strain AfS28 did not display any deviations with respect to vegetative growth, sporulation capacities, nutritional requirements, pigmentation, and sensitivity towards phleomycin (not shown). The latter point is of special interest, as such sensitivity has been described for the N. crassa mutant strain deleted of its mus-51 gene (10).
To assess the pathogenicity of the A. fumigatus mutant in a simplified virulence model, spores of AfS28 and D141 were injected into cohorts of larvae of the greater wax moth Galleria mellonella (6, 9) (purchased from Fauna Topics GmbH, Germany). Infected animals were kept at 30°C and inspected daily for casualties or signs of melanization. Whereas control groups of larvae pricked with 20 μl of saline containing 10 μg/ml of rifampin stayed healthy throughout the experiment, a high proportion of larvae that had been challenged with 8 × 106 freshly harvested conidia of D141 died within 7 days postinfection (Fig. 1C). Interestingly, conidia of strain AfS28 did not display any virulence reduction in this insect model compared to the wild-type isolate D141 as deduced from statistical analyses of the survival curves by the log rank test (P = 0.73).
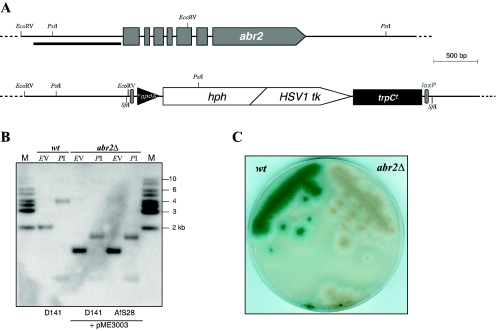
To estimate the relative rate of homologous integration in this genetic background, the laccase-encoding gene abr2 was targeted for deletion (Fig. 2A). The Abr2 protein contributes to the synthesis of the A. fumigatus conidial pigment, and therefore strains lacking this enzymatic activity display brownish conidia, in contrast to gray-green ones of wild-type strains (15). As a genetic marker, a derivative of a recently described module was used (7): in this cassette (pME3002), expression of a bifunctional loxP-hygroR/tk marker conferring resistance to hygromycin as well as sensitivity to nucleoside analogues is driven by a truncated version (position −260 from the transcriptional start site) of the Aspergillus nidulans gpdA promoter (11). Additionally, incompatible SfiI restriction sites allowing the construction of suitable replacement cassettes by ligation-mediated PCR sandwich the whole marker. Constructs targeting the abr2 locus of A. fumigatus were assembled by amplifying flanking 5′ and 3′ regions from genomic DNA by PCR and, after digestion, ligating them to the marker module of pME3002 by SfiI sites that had been incorporated into the priming oligonucleotides (Fig. 2A). From this template (pME3003), versions of replacement cassettes were amplified, in which homologous flanking regions of 1,500 bp, 1,000 bp, 500 bp, and 100 bp are present on either site of the selection marker.
FIG. 2.
Targeting the A. fumigatus abr2 locus to assess frequencies of homologous recombination in a nonhomologous end-joining-deficient background. (A) Genomic architecture of the A. fumigatus abr2 locus before and after replacement with the marker cassette of pME3003. The black bar gives the position of the hybridization probe in Southern experiments with restriction sites used as indicated. (B) Autoradiograph of Southern hybridization experiment with transformants derived from D141 and AfS28, respectively. Genomic DNA prepared from the specified strains was digested with restriction enzymes EcoRV (EV) or PstI (PI); M stands for the DNA size standard with fragments' sizes indicated. (C) Appearance of an A. fumigatus abr2Δ strain (right) with its brownish conidia in comparison to the wild-type progenitor (left) displaying the typical gray-green spore pigmentation.
When the complete replacement cassette from pME3003 was transformed into the recipient strain AfS28, a high proportion of the transformants displayed the brownish color phenotype as expected from deletion of the abr2 locus (Fig. 2C). Correlation of this phenotype with the corresponding genotype was confirmed by Southern hybridizations on genomic DNA of selected transformants (Fig. 2B). Compared to transformations employing the wild-type isolate D141 as the recipient, the frequency of correct integrations was increased in the akuA::ptrA genetic background: of 42 primary AfS28 transformants, 40 (95%) displayed the color phenotype, whereas from the pool of D141 picks, 22% (7 of 32) did so.
A breakdown of the flanking regions to 1,500 bp or 1,000 bp did not significantly alter the high frequency of homologous integration in the akuAΔ background, whereas when using replacement cassettes with 500 bp or 100 bp, a drop in the percentage of correct transformants was evident (Table 1). This correlated with a decline of transformation efficiencies, which can be explained by the lack of ectopic integration events and therefore a reduced pool of primary transformants.
TABLE 1.
Relative frequencies of homologous recombination (HR) in wild-type and akuAΔ genetic backgrounds of A. fumigatus as estimated from targeted replacement of the abr2 locusa
| Strain | % HR at arm length:
|
||||
|---|---|---|---|---|---|
| ∼2.0 kb | 1.5 kb | 1.0 kb | 0.5 kb | 0.1 kb | |
| Wild type | 22 | 14 | 10 | 2 | 0 |
| akuAΔ | 95 | 96 | 96 | (84) | (75) |
Frequencies were assessed from transformation experiments with strains D141 and AfS28 as recipients; transformation protoplasts were mixed with 10 μg of DNA to achieve polyethylene glycol-mediated fusion (12). Percentages were calculated from the proportion of brownish colonies among the primary transformant pool by scoring 50 randomly chosen colonies in total; values in parentheses were estimated from fewer transformants (19 and 4 for 0.5 and 0.1 kb, respectively) and therefore have to be considered statistically insignificant.
To validate the usefulness of the akuAΔ genetic background in gene targeting further, we aimed at gene replacement of an additional locus, the akuA::ptrA locus itself (our unpublished results). This was done with the intention to exchange the deletion marker in AfS28 with a more suitable one, the loxP-hph/tk marker module of pME3002. The flanking regions of akuA were ligated to this module as outlined above, and the corresponding replacement cassette (pME3007) was transformed into AfS28. Among the hygromycin-resistant transformants, a high percentage (23 out of 24, equaling 96%) displayed a pyrithiamine-sensitive phenotype (not shown), indicative of proper replacement of the deletion marker in AfS28. This high proportion is in sharp contrast to the low yield when targeting the akuA gene in the wild-type background of D141, where 1 isolate out of 20 transformants had been identified as a correct deletion mutant.
Altogether, we were able to validate the convenience of an A. fumigatus strain deficient in the nonhomologous end-joining pathway for targeted integration of replacement cassettes into the fungal genome. For future studies in the postgenomic era of Aspergillus molecular biology (3), this genetic background might prove useful for comprehensive deletion analyses with the aim to elucidate the specific virulence traits of this opportunistic pathogen; however, upcoming experiments in animal models will have to address the general value of this genetic background. Moreover, the possibility of a synthetic interaction of the akuAΔ allele and a deleted locus has to be taken into account in forthcoming gene targeting experiments. One suitable way to counteract this lies in reconstituting the akuAΔ locus via retransformation with a wild-type copy of the akuA gene, as the asexual nature of A. fumigatus impedes back-crossing approaches.
Acknowledgments
We thank Verena Grosse for outstanding technical assistance and all other members of the department for general support; Stephan Seiler is thanked for an informative hint, and initial efforts of Inga V. Behrens are acknowledged.
Funding was received from the Deutsche Forschungsgemeinschaft via its Priority Program SPP1160 (KR 2294/1-1), the Fonds der Chemischen Industrie, and the Volkswagenstiftung.
REFERENCES
- 1.Bird, D., and R. Bradshaw. 1997. Gene targeting is locus dependent in the filamentous fungus Aspergillus nidulans. Mol. Gen. Genet. 255:219-225. [DOI] [PubMed] [Google Scholar]
- 2.Borkovich, K. A., L. A. Alex, O. Yarden, M. Freitag, G. E. Turner, N. D. Read, S. Seiler, D. Bell-Pedersen, J. Paietta, N. Plesofsky, M. Plamann, M. Goodrich-Tanrikulu, U. Schulte, G. Mannhaupt, F. E. Nargang, A. Radford, C. Selitrennikoff, J. E. Galagan, J. C. Dunlap, J. J. Loros, D. Catcheside, H. Inoue, R. Aramayo, M. Polymenis, E. U. Selker, M. S. Sachs, G. A. Marzluf, I. Paulsen, R. Davis, D. J. Ebbole, A. Zelter, E. R. Kalkman, R. O'Rourke, F. Bowring, J. Yeadon, C. Ishii, K. Suzuki, W. Sakai, and R. Pratt. 2004. Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol. Mol. Biol. Rev. 68:1-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brakhage, A. A., and K. Langfelder. 2002. Menacing mold: the molecular biology of Aspergillus fumigatus. Annu. Rev. Microbiol. 56:433-455. [DOI] [PubMed] [Google Scholar]
- 4.Critchlow, S. E., and S. P. Jackson. 1998. DNA end-joining: from yeast to man. Trends Biochem. Sci. 23:394-398. [DOI] [PubMed] [Google Scholar]
- 5.Kämper, J. 2004. A PCR-based system for highly efficient generation of gene replacement mutants in Ustilago maydis. Mol. Genet. Genomics 271:103-110. [DOI] [PubMed] [Google Scholar]
- 6.Kavanagh, K., and E. P. Reeves. 2004. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 28:101-112. [DOI] [PubMed] [Google Scholar]
- 7.Krappmann, S., Ö. Bayram, and G. H. Braus. 2005. Deletion and allelic exchange of the Aspergillus fumigatus veA locus via a novel recyclable marker module. Eukaryot. Cell 4:1298-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubodera, T., N. Yamashita, and A. Nishimura. 2000. Pyrithiamine resistance gene (ptrA) of Aspergillus oryzae: cloning, characterization and application as a dominant selectable marker for transformation. Biosci. Biotechnol. Biochem. 64:1416-1421. [DOI] [PubMed] [Google Scholar]
- 9.Maerker, C., M. Rohde, A. A. Brakhage, and M. Brock. 2005. Methylcitrate synthase from Aspergillus fumigatus. FEBS J. 272:3615-3630. [DOI] [PubMed] [Google Scholar]
- 10.Ninomiya, Y., K. Suzuki, C. Ishii, and H. Inoue. 2004. Highly efficient gene replacements in Neurospora strains deficient for nonhomologous end-joining. Proc. Natl. Acad. Sci. USA 101:12248-12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Punt, P. J., M. A. Dingemanse, A. Kuyvenhoven, R. D. Soede, P. H. Pouwels, and C. A. van den Hondel. 1990. Functional elements in the promoter region of the Aspergillus nidulans gpdA gene encoding glyceraldehyde-3-phosphate dehydrogenase. Gene. 93:101-109. [DOI] [PubMed] [Google Scholar]
- 12.Punt, P. J., and C. A. van den Hondel. 1992. Transformation of filamentous fungi based on hygromycin B and phleomycin resistance markers. Methods Enzymol. 216:447-457. [DOI] [PubMed] [Google Scholar]
- 13.Reichard, U., S. Buttner, H. Eiffert, F. Staib, and R. Rüchel. 1990. Purification and characterisation of an extracellular serine proteinase from Aspergillus fumigatus and its detection in tissue. J. Med. Microbiol. 33:243-251. [DOI] [PubMed] [Google Scholar]
- 14.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503-517. [DOI] [PubMed] [Google Scholar]
- 15.Tsai, H. F., M. H. Wheeler, Y. C. Chang, and K. J. Kwon-Chung. 1999. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 181:6469-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]