Abstract
The genus Hafnia, a member of the family Enterobacteriaceae, consists of gram-negative bacteria that are occasionally implicated in both intestinal and extraintestinal infections in humans. Despite the fact that the genus currently contains only a single species (H. alvei), more extensive phylogenetic depth (two or more species) is apparent based upon DNA relatedness and 16S rRNA gene sequencing studies. Hafnia causes a variety of systemic infections, including septicemia and pneumonia; however, its role as a gastrointestinal pathogen is controversial. Many of the data supporting a role for hafniae as enteric pathogens were incorrectly attributed to this genus rather than to the actual pathogen, Escherichia albertii. There are numerous gaps in our understanding of this genus, including ecologic habitats and population genetics, disease-producing role in animals, phenetic and genetic methods useful in distinguishing genomospecies within the H. alvei complex, and bona fide pathogenicity factors.
INTRODUCTION
The genus Hafnia is one of more than 40 genera that currently comprise the family Enterobacteriaceae. Although Møller originally described this genus in 1954 (89), the legitimacy of this group was constantly challenged over the next two decades, often being referred to by such synonyms as “Enterobacter alvei” “Enterobacter aerogenes subsp. hafniae” and “Enterobacter hafniae” (www.bacterio.cict.fr/h/hafnia.html). Subsequent DNA-DNA relatedness investigations conducted by Don Brenner and the Centers for Disease Control and Prevention (CDC) conclusively established that these bacteria represented a unique taxon within the family and that at least two or more DNA groups existed within the genus (20, 21, 131).
Although this genus is over 50 years old, very little is known about these organisms in regard to the role(s) they may play as both human and veterinary pathogens. In one sense, these bacteria appear to be “orphan enterobacteria” in search of a medical and public health identity. Hafnia is fairly often isolated from clinical material, yet with the exception of its documented role as a rare cause of bacteremia (31, 104, 105), its relationship to other clinical infections is questionable. In fact, data from the early 1990s suggesting that hafniae were true enteropathogens (4, 5) now turn out to have been incorrectly attributed to hafniae rather than to the actual pathogen, Escherichia albertii (63, 69). The possible role of Hafnia in bacterial gastroenteritis is presently unknown, although one recent case report suggests a possible role in enteritis (see Addendum).
Over the past quarter of a century, there have been few studies that have systematically looked at the role of these bacteria in clinical microbiology. Greipsson and Priest (56) published the only taxonomic study on this genus in 1983, and Günthard and Pennekamp (59) reported on the clinical significance of the only large series of extraintestinal isolates. Recently there have been a number of studies describing and characterizing multiple genomospecies within the genus, phylogenetic relatedness, clinical infections, pathogenicity, and biochemical characteristics of genomovars. This review attempts to systematically review the pertinent literature on this genus, discuss current controversies surrounding these bacteria, and shed some light on the potential role these “orphan enterobacteria” may play in human disease.
HISTORICAL PERSPECTIVES
It is the subject of some speculation and debate as to when organisms currently in the genus Hafnia were first isolated. In 1919, L. Bahr worked on a bacterium that he designated “Bacillus paratyphi-alvei,” an organism reputedly pathogenic for bees but not mice or guinea pigs (121). One of Bahr's apparently authentic “Bacillus paratyphi-alvei” strains (referred to as “Paratyphus alvei”) was subsequently characterized in 1954 as belonging to a new group of enteric bacteria for which Møller coined the name “Hafnia group” during a systematic investigation of amino acid decarboxylase patterns among members of the family Enterobacteriaceae (89). Some groups subsequently questioned the legitimacy of this name in light of the fact that Bahr's strains differed in some biochemical characteristics from those described by Møller. However, Møller considered that Bahr's strain should be regarded as the type species of Hafnia, and he suggested the name Hafnia alvei.
There are no extant cultures of Bahr's strains. However, in the early 1940s Stuart and colleagues (135) biochemically characterized a large group of paracolon bacteria. Within the group named “Paracolon Aerobacter,” two divisions were recognized based upon the results of indole-methyl red-Voges-Proskauer-citrate assays. Division 2 contained seven distinct biotypes, the largest of which was referred to as type 32011 (35 cultures). Type 32011 and related types had properties generally consistent with inclusion in the genus Hafnia, being indole, lactose, and sucrose negative; aerogenic; and Voges-Proskauer (VP) positive. Type 32011 strains were subsequently characterized by several independent groups, including Edwards and Ewing (29), who confirmed that the biochemical characteristics of type 30211 were identical or nearly identical to those reported for the “Hafnia group” by Møller and others.
From the 1940s until the mid- to late 1970s, when nomenclature and classification issues surrounding this taxa were resolved, H. alvei was known by a number of different names, including “Paracolon Aerobacter,” “Paracolobactrum aerogenoides,” Bacillus cadaveris, the Hafnia group, Enterobacter alvei, and Enterobacter hafniae (16, 120, 121). For one brief period of time it was even relegated to subspecies rank within Enterobacter aerogenes (32). However, subsequent taxonomic investigations demonstrated that it belonged to a unique lineage within the Enterobacteriaceae and deserved genus (species) rank (see “NOMENCLATURE, TAXONOMY, AND CLASSIFICATION” below). The name Hafnia alvei appears in the Approved Lists of Bacterial Names, and the type strain is ATCC 13337T (129). Three of the oldest extant cultures of H. alvei are ATCC 13337T (Stuart's type 32011 = NCTC 8105 = NCDC 434-68), ATCC 11604 (“Paracolobactrum aerogenoides” = 3565 [Conn 3565], Stuart's type 32011) of Borman et al. (16), and NCTC 6578 of Gale and Epps (43).
NOMENCLATURE, TAXONOMY, AND CLASSIFICATION
Nomenclature
The specific epithet in the name Hafnia alvei is derived from the Latin noun alveus, meaning beehive, with “alvei” meaning “of a beehive.” Ewing (33) questioned the epithet “alvei,” stating that the name implied that these bacteria had something to do with bees or beehives although they did not. However, H. alvei has been recovered on occasion from the intestines of honeybees (Apis mellifera) as well as from honey, and several of these strains are included in the BCCM (Brussels, Belgium) collection (125). The genus name Hafnia is the historical name (Havn) for the city of Copenhagen, Denmark (122).
Biochemical and Numerical Taxonomy Studies
Prior to the advent of molecular biology, bacterial nomenclature and taxonomy were dependent primarily upon characters or phenotypes of cultures expressed under defined conditions. In their most sophisticated form, such analyses employed the principles of Adansonian taxonomy (numerical taxonomy or numerical phenetic analysis), but often studies simply compared large data sets of biochemical characteristics between two apparently different groups. In that vein, members of the family Enterobacteriaceae (17-19, 60, 75, 119) were grouped into tribes consisting of genera with similar characteristics. The tribe Klebsiellae contained four such genera (Klebsiella, Enterobacter, Hafnia, and Serratia), with most but not all of the tribe's members sharing a number of phenotypic features. These features included formation of acetylmethylcarbinol (VP), growth on Simmon's citrate agar and in potassium cyanide broth, urea negativity (48 h), and failure to produce hydrogen sulfide (33).
Few studies have dealt with the genus Hafnia by employing numerical taxonomy principles. Bascomb and coinvestigators (12) studied members of the then-tribe Klebsiellae, including 15 strains of H. alvei. By using three different methods of numerical classification, all H. alvei strains were found to form a separate and distinct branch with a rank similar to that of the tribe Klebsiellae. Johnson et al. (73) studied 384 strains in the family Enterobacteriaceae for 216 independent characters, including morphological, biochemical, and physiologic traits; 10 of these enteric isolates were H. alvei. Based upon simple matching (SM) and Jaccard (SJ) coefficients, H. alvei strains were found to cluster independently of other groups, joining members of the tribe Klebsiellae at 78% similarity. Those authors concluded that this justified the status of this taxon as an independent genus rather than being placed within the genus Enterobacter. The only study to specifically look at Hafnia by use of numerical taxonomic principles was that of Greipsson and Priest (56), who studied 47 strains from the United Kingdom, Japan, France, and the United States by using 101 different characters. All 47 strains exhibited high similarity values (>87%) by SM coefficient when average-linkage cluster analysis was used with the type strain NCTC 8105T (ATCC 13337T), located near the center of the phenon. These results support previous conclusions reached by Johnson et al. (73) that all H. alvei isolates reside within a single phenon or cluster.
DNA Relatedness
Despite the general conclusions drawn from several phenotypic studies regarding the homogeneity of Hafnia isolates, other studies provided indirect evidence that genetic diversity exists within this nomenspecies. A study by Gavini and colleagues (45) identified two distinct Hafnia phenons, designated I1 and I2, in their analysis of 32 strains originating from feces and different water sources. These two clusters could not be unambiguously separated from one other, although they differed in the frequency of certain characters, including production of acetylmethylcarbinol at 37°C and utilization of malonate and d-tartrate. Another clue suggesting diversity within H. alvei involves the moles percent G+C content of hafniae (66). Two very different G+C contents for Hafnia have been reported; the more common estimate is 48.7 mol%, while a higher value of 52.6 to 57.7 mol% has also been published (56).
The first direct evidence proving the existence of more than one DNA group within Hafnia alvei was reported by Steigerwalt and coinvestigators (131) as part of a study of DNA relatedness between Enterobacter and Serratia species. In that study, using H. alvei (“E. hafniae”) CDC 4360-67 as the source of labeled DNA, two distinct DNA groups could be discerned, one highly (85% to 100%) related to CDC 4360-87 and one less (51% to 55%) related; the latter contained the type strain of H. alvei, ATCC 13337T. H. alvei was found to be only 16% to 24% related to other members of the Enterobacteriaceae by DNA-DNA hybridization in that study. In subsequent publications, Brenner (20, 21) reported the existence of two or more hybridization groups (HGs) within H. alvei, and this has been confirmed in subsequent studies (47). Furthermore, Brenner (21) found that the type strain of Obesumbacterium proteus (ATCC 12841T) is 75% related to DNA group 2 (in some studies called DNA group 1) of H. alvei and is 52% related to the type strain of H. alvei, ATCC 13337T. Thus, a valid type strain for O. proteus does not exist. Table 1 lists reference strains and their vernacular designations from surveys that studied both H. alvei DNA groups (HGs). By convention, the type strain for the nomenspecies (ATCC 13337T) and related reference strains should be classified as DNA group 1 (HG1), since this group represents H. alvei sensu stricto. DNA group 2 (HG2, unnamed) is represented by several reference strains, including ATCC 29927. CDC 4360-67 (DNA group 2) was not retained in the permanent culture collection of CDC and is no longer available as a reference strain for DNA group 2 (HG2) (C. O'Hara, personal communication).
TABLE 1.
Recognized DNA groups in the genus Hafniaa
| Reference | No. of groups detected | Proposed DNA group nomenclature for H. alveib
|
|
|---|---|---|---|
| DNA group 1 | DNA group 2 | ||
| 131 | 2 | DNA group 2 (ATCC 13337 = NCTC 8105) | DNA group 1 (CDC 4360-67) |
| 20 | 3c | DNA group 1 (CDC 5632-72 = ATCC 29926); O. proteus (CDC 4296-74) | DNA group 2 (CDC 4510-73 = ATCC 29927) |
| 21 | 3d | NGe | NG |
| 33 | 2 | DNA group 2 (ATCC 13337) | DNA group 1 (CDC 4360-67) |
| 47 | 2 | HG1 (CDC 5632-72) | HG2 (CDC 4510-73) |
Based upon DNA relatedness studies.
Based upon ATCC 1337T representing DNA group 1.
DNA group 3, represented by strain CDC 329-73, was subsequently determined to be Yokenella regensburgei (20).
HG3 was resistant to Hafnia-specific bacteriophage and was more closely related to Salmonella-Escherichia-Citrobacter-Enterobacter than to Hafnia.
NG, not given.
Other recent studies employing different techniques also support the division of the genus Hafnia into two or more groups. 16S rRNA sequencing of 22 H. alvei isolates revealed two clusters, one containing 5 strains (including ATCC 29926) that exhibited 0% to 0.19% sequence divergence from ATCC 13337T (DNA group 1) and a second cluster (DNA group 2) of 17 strains that exhibited 0% to 0.09% sequence divergence from ATCC 29927 (0.95% to 1.04% divergence from ATCC 13337T) (71). A subsequent study of 52 Hafnia strains by the same group again found two DNA groups that were clearly distinguishable using 16S rRNA gene sequencing (70). An Australian study in 2003 investigated the genetic structures of 161 Hafnia isolates by multilocus enzyme electrophoresis at12 different loci (98). As in the previous study, two populations were detected, one consisting of 83 strains (including ATCC 13337T and ATCC 29926) and a second group of 81 isolates (including ATCC 29927). These studies indicate that 16S rRNA sequencing and multilocus enzyme electrophoresis also detect HGs that were previously recognizable only through DNA pairing studies.
Phylogenetic and Related Studies
Only a limited number of phylogenetic investigations have included one or more strains of H. alvei. The utility of 16S rRNA sequencing to study the family Enterobacteriaceae has been questioned due to the high intrafamily DNA relatedness values and limitations on the resolution of 16S rRNA for this group based upon the number of informative sites (27, 130). Spröer et al. (130) found that the type strain of H. alvei exhibited 99.5% sequence similarity to the type strain for O. proteus, DSM 2777T (ATCC 12841T), supporting previous observations by Brenner (21) regarding the DNA relatedness of these two species. In that study, the nearest neighbors to H. alvei were two distinct Serratia clusters containing nine species. Dauga (27) also looked at the phylogenetic position of H. alvei ATCC 13337T relative to other Enterobacteriaceae. By using the neighbor-joining method, H. alvei was rooted by the tribe Proteeae (containing the genera Proteus, Providencia, and Morganella); by maximum-likelihood analysis, H. alvei clustered with Serratia, while by the distance method its nearest neighbors were the Escherichia-Salmonella-Citrobacter group. A 2003 study employing only a few enteric genera found the closest relative to H. alvei to be Klebsiella pneumoniae based upon 16S rRNA gene sequencing (139).
Hedegaard and colleagues (62) investigated the utility of partial sequencing of the infB gene (encoding translation initiation factor 2) as a phylogenetic tool for studying 39 Enterobacteriaceae strains. By using a clinical strain of H. alvei and the distance matrix method, the closest neighbors to hafniae were found to be members of the tribe Proteeae. Phylogenetic investigations using gyrB gene (encoding the ATPase domain of DNA gyrase) and protein sequence data yielded mixed results (27). Dauga (27) noted that there was atypical codon usage (third-codon G+C content) in the gyrB gene of H. alvei relative to other Enterobacteriaceae, which suggested foreign acquisition of this gene. If this is true, analysis of the gyrB sequences would be indicative only of the origin of the gene and not the species. Wertz et al. (139) studied five housekeeping gene and 16S rRNA gene sequences in an analysis of a limited series of species in the Enterobacteriaceae. Cladograms generated by maximum-likelihood analysis found that H. alvei's nearest neighbor was Serratia plymuthica for four genes (groEL, gyrA, pgi, and ompA), while by gapA sequencing it was Enterobacter cloacae and by 16S rRNA analysis K. pneumoniae. Phylogenetic data to date are inconclusive regarding where the genus belongs. One study on the evolution of aromatic amino acid biosynthesis genes and enzymes suggests clustering of H. alvei with Edwardsiella, Yersinia enterocolitica, and Proteus vulgaris (2). However, to date phylogenetic data regarding where the genus should be rooted are inconclusive.
EPIDEMIOLOGY
Environmental Distribution
Most standard microbiology texts list mammals, birds, reptiles, fish, soil, water, sewage, and foods as sources from which hafniae can be recovered (35, 36). However, there are surprisingly little hard data in the literature on this topic, and no systematic environmental surveys on the genus Hafnia have been published. Most of the data regarding the environmental distribution of hafniae come from investigations on the frequency, incidence, concentration, and distribution of members of the family Enterobacteriaceae. Host and geographical factors appeared to influence the thermal niche of H. alvei isolated from Australian mammals in one study (97).
The gastrointestinal tracts of animals, and in particular mammals, appear to be a very common ecologic habitat for hafniae. Paleomicrobiology investigations have identified H. alvei originating from intestinal mass samples and sediment collected from 12,000-year-old mastodon remains in Michigan and Ohio (110). In a study of 642 Australian mammals, Gordon and FitzGibbon (53) found that H. alvei was the third most common enteric species identified, following Escherichia coli and E. cloacae. The isolation of H. alvei was significantly associated with recovery from marsupial carnivores and murid rodents. Hafniae have also been recovered sporadically from pack animal manure samples collected from national park trails (28) and from 7% of grizzly and black bear specimens tested (51). Among avian species, H. alvei has been frequently isolated from birds of prey, including falcons, owls, and turkey vultures; even high-altitude alpine accentors that have virtually no contact with humans have had Hafnia isolated from them, at frequencies ranging from 3% to 16% (9, 78, 142). Other sources for H. alvei include reptiles (snakes and skinks), invertebrates, insects, fish, and bats (24, 52, 98).
Food products commonly yield hafniae. Meats, including minced and vacuum-packed beef and pork products, ground beef chubs, and retail packages, harbor H. alvei (82, 113). One Finnish study found almost 50% of all enteric isolates recovered from refrigerated meats to be H. alvei (113). A subsequent study identified 34% of minced meat samples, 14% of milk and cream products, and 12% of freshwater fish as containing hafniae (44). Counts of H. alvei in these samples were as high as 7.5 × 1010 to 8.0 × 1010 CFU/g (82). Some isolates of H. alvei recovered from albacore produced histamine, although the relevance of this finding to scrombroid poisoning is unknown (76). Other consumables that are reported to yield H. alvei include cheese and honey (90). Vegetables do not appear to be a frequent reservoir for these bacteria, although H. alvei was recovered from nine Finnish and imported vegetable samples in one study (100). There are only a few anecdotal reports describing the isolation of hafniae from water (98).
Disease States Associated with H. alvei
There are only a limited amount of data presently available on disease states associated with H. alvei. There are two well-described outbreaks of H. alvei infection associated with the poultry industry. Real et al. (108) described an outbreak of disease in commercial laying hens in Spain, characterized by loss of appetite, decreased egg productivity, catarrhal enteritis, and fulminant septicemia. Prominent histopathologic features of the disease included hepatosplenomegaly, multifocal nectrotizing hepatitis, and splenitis. H. alvei was recovered in pure culture from infected tissues at necropsy. A very similar disease presentation could be reproduced using wild-type H. alvei isolated from ill hens experimentally infected by either the intraperitoneal or oral route. In 2004, a large outbreak of H. alvei disease in 12-week-old pullets was reported from Italy (103). These pullets initially suffered from anorexia, depression, ruffled feathers, and diarrhea. The overall mortality rate was 20.7%. Autopsy findings were remarkably similar to those observed by Real and colleagues (108), including catarrhal enteritis and hepatosplenomegaly. Again, the disease could be experimentally reproduced in healthy pullets challenged with the infective strain.
H. alvei has been implicated in a variety of other animal infections. H. alvei has been reported as the cause of an epizootic outbreak of septicemia in rainbow trout in Bulgaria (47). Hafnia was recovered in pure culture from parenchymal organs during the acute phase of disease and was also isolated from necrotic tissues later in the course of infection. A 1963 French publication describes an epizootic outbreak of disease in white laboratory rats 3 to 8 weeks old (8). A number of the rats were lethargic and died unexpectedly. Autopsy demonstrated splenic hypertrophy (four to five times normal size) with congestion in visceral organs, including the brain and cerebellum. H. alvei was recovered from samples of organ tissues and heart blood. This strain possessed phenotypic properties that are consistent with inclusion in the genus, except that these were VP negative (≥85% of H. alvei isolates are positive) and produced acid from dulcitol (<1% of H. alvei isolates are positive). Other animal diseases linked to Hafnia infection include caprine pneumonia (127) and equine abortion (91).
Ecologic Structure
The first evidence suggesting that there may be a nonrandom association of distinct genetic populations of H. alvei with specific ecologic niches comes from a recent study by Okada and Gordon (98). In that investigation, genetic group 2 strains (corresponding to DNA group 2 in Table 1) were predominantly associated with freshwater fish, while strains from genetic group 1 (corresponding to DNA group 1 in Table 1) were associated with birds, frogs, invertebrates, and water isolates. Both groups were equally represented in mammals and reptiles.
Clinical Frequency, Outbreaks, and Nosocomial Infections
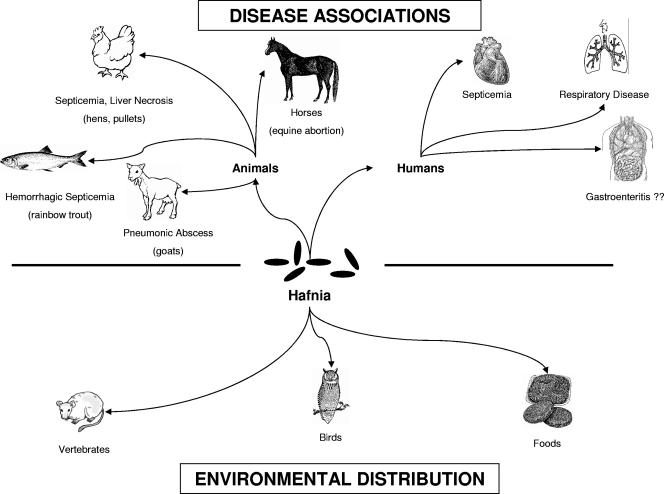
H. alvei is an uncommon human pathogen, although this species has received increased attention from the medical community over the past decade due to its possible association with gastroenteritis (Fig. 1) (see “CLINICAL SIGNIFICANCE” below). Hafniae are commonly recovered from oropharyngeal specimens, from the gastrointestinal tract, less often from blood, and from a variety of other anatomic sites, including tissue, urine, and catheters (59, 138). In a study of 17 Hafnia alvei (“Enterobacter hafniae”) isolates recovered by the Mayo Clinic from 1968 to 1970, only 5 isolates (29%) were judged to be clinically significant (138). In all five of these cases, H. alvei was determined to be a secondary pathogen (respiratory, n = 2; abscess, n = 3). Overall, the mean age of persons infected/colonized with H. alvei was 52.9 years, with a male/female ratio of 1:1.1. Slightly over 50% of all isolates were acquired via a nosocomial route, including all five associated with infection (138). In only two instances was H. alvei recovered in pure culture, and in neither case was the strain clinically significant. A 1996 study by Günthard and Pennekamp (59) reviewed 80 H. alvei isolates isolated from 61 patients over a 30-month period (1992 to 1995). As in the previous report, the mean age of all patients was 53.5 years; however, almost two-thirds of the patients were male. In only 6 of these 61 patients (9.8%) was H. alvei was thought to be the etiologic agent of the disease, three of which were proven cases (septicemia, n = 2; peritonitis, n = 1) and three of which were probable cases (septicemia, n = 2; pneumonia, n = 1).
FIG. 1.
Schematic representation of major disease associations associated with the genus Hafnia and their environmental distribution.
There are no well-described outbreaks of disease caused by H. alvei in which the epidemiologic, clinical, and laboratory correlates are overwhelmingly in support of an unquestionable role for hafniae in these illnesses. Table 2 lists five outbreaks of gastrointestinal disease, affecting between 2 and 72 individuals, in which H. alvei was suspected as the possible etiologic agent. Two Canadian outbreaks of gastroenteritis that occurred 1 month apart in the same institution in inpatients suggest a possible role for hafniae in the disease process. However, only 32% of the patients had H. alvei recovered in large numbers from their stools, and one symptomatic person continued to grow large numbers of hafniae from feces even after the person became asymptomatic (107). A report from Russia describes the possible association of H. alvei with diarrhea in 72 hospitalized persons and provides some microbiologic and immunologic data to support this hypothesis; however, a number of questions remain (54). These include a short incubation period, unusual symptoms (possibly neurotoxicosis), and the presence of rotavirus in at least some of these samples (54). Clearly, more definitive work in this area needs to be performed.
TABLE 2.
Reputed outbreaks of disease caused by H. alvei
| Outbreak no. (reference) | Yr | Location | No. ill | Symptoms | Persons affected | Comments |
|---|---|---|---|---|---|---|
| 1 (134) | 1943 | United States | 28 | GEa | Inpatients and staff | Biotype 32011 (“H. alvei”) isolated from a patient, cream-filled pastry, and milk; milk was the implicated vehicle |
| 2 (61) | 1957 | Japan | 2 | GE | 9-yr-old girl and 6-yr-old brother | Strains with identical biochemical and serologic properties |
| 3 (106, 107) | 1979 | Canada | 15 | GE | Inpatients | H. alvei (n = 5) as pure or predominant growth; convalescent phase, 4/5 negative for hafniae; all strains biotype 1, serotype O3:H− |
| 4 (106, 107) | 1979 | Canada | 25 | GE | Inpatients | H. alvei (n = 8) as pure or predominant growth; strains biotype 1, serotype O3:H− |
| 5 (54)b | 2003 | Russia | 72 | GE, neurotoxicosis | Inpatients | H. alvei St+ isolated; present in high concn in acute phase, disappearance in convalescent phase; rise in antibody titers to H. alvei; rotavirus (n = 8)c |
GE, gastroenteritis.
Article in Russian, only English abstract translation available.
St+, heat-stable enterotoxin.
Since H. alvei is presently an uncommon human pathogen, incidence data such as those derived from the National Nosocomial Infection Surveillance System for many enteric pathogens are not available (112). From three separate studies, the percentage of documented H. alvei nosocomial infections ranged from 38% to 59%, although the absolute number of cases was relatively small (8 to 80) and they spanned a number of years (59, 105, 138).
CLINICAL SIGNIFICANCE
Historical Anecdotes Regarding the Association of Hafniae with Human Disease
There are numerous narrative comments in the medical and scientific literature regarding the potential pathogenicity of H. alvei. In most instances these comments center on presumed associations between this bacterium and diarrheal disease. In 1943, Stuart and Rustigian (134) reported on one outbreak of mild to acute gastroenteritis that involved a fairly sizeable number of persons in Rhode Island, with the common thread of consumption of milk or milk-related products from the same supplier. The implicated organism was biotype 32011 (“H. alvei”) (Table 2). In the same study, the authors also described two apparent laboratory-acquired gastrointestinal infections, in a research student and in a glassware technician, both of whom handled biotype 32011. Other reports similar to this have surfaced from time to time, such as the case reports of Harada et al. (61) in 1957 describing the isolation of H. alvei from two siblings, who developed diarrhea 3 weeks apart. However, most of these data are extremely sketchy, and Matsumoto (85) in 1963 stated that the pathogenicity of Hafnia, at least for humans, was not well clarified.
The first credible report linking H. alvei to human illness came from a 1967 case study by Jennis and McCarthy (72). A 58-year-old man who underwent multiple medical procedures for a duodenal ulcer developed Hafnia bacteremia with fever (40.5°C) and hypotension (80/50) 6 days after surgery. Two blood culture specimens were positive for H. alvei, and he was treated with oxytetracycline, to which he responded favorably. Two years later, a second case of H. alvei bacteremia was described; this was in another 58-year-old male with suspected myocardial infarction (31). H. alvei was repeatedly isolated from blood cultures for over a month despite multiple antibiotic regimens, leading to the suspicion of a hidden nidus of infection. No such focus was ever identified, and it was postulated that the bacteremia continually seeded from the genitourinary tract and retention catheter. These two publications provided strong evidence that H. alvei could cause extraintestinal infections. A study from the Mayo Clinic in 1971 confirmed that hafniae could cause secondary illnesses associated with wounds/abscesses and respiratory problems, including bronchopneumonia (138).
To date there have been three reports describing a series of cases involving infections or asymptomatic colonization with H. alvei; in each of these studies the number of patients ranged from 8 to 61 (59, 105, 138). In one investigation of 17 patients where most hafniae isolated were judged to be commensals, 59% of all isolates were determined to be have been transmitted nosocomially (138), while in a smaller study of 8 patients, most or all of whom had true H. alvei infections, 63% of the infections were found to be community acquired (105). Most individuals (63% to 93%) colonized or infected with hafniae have had underlying diseases, the most common of which are cancer (in particular hematologic malignancies), surgery, trauma, chronic or acute pulmonary disease, cirrhosis/hepatitis, and pancreatitis. In the largest series of cases, 66% of the patients were intubated, including all those from whom H. alvei was recovered from the respiratory tree (59). Two studies found that 37% to 55% of persons colonized/infected with Hafnia had received antibiotics prior to the isolation of this microorganism. No deaths attributable to H. alvei were recorded in any of these three studies.
From 12% to 75% of clinical specimens yield H. alvei in pure culture (59, 105, 138). Sites most likely to yield hafniae as the sole pathogens include urine and blood. When found as part of mixed microbial flora, they are often associated with other enteric bacteria, staphylococci, streptococci, and yeast (59). When hafniae have been isolated in pure growth or as predominant flora, there is a better correlation with disease status (e.g., bronchopneumonia) than when H. alvei is recovered in small numbers (138).
Bacteremia
There are no national figures on the incidence or number of cases of H. alvei bacteremia in the United States. A 2004 publication from the United Kingdom reports the number of cases of Hafnia bacteremia varying from 19 to 42 per year between 2001 and 2003 (6). If one compares the 2002 population of the United Kingdom (roughly 59 million) to that of the United States (287 million), this translates into somewhere between 92 and 204 cases of Hafnia bacteremia occurring in the United States annually, and this no doubt is an underestimate given the lack of an active surveillance system for this uncommon pathogen.
There have been relatively few cases of Hafnia bacteremia reported in the medical literature. Of those cases, 18 were published between 1967 and 2004 with enough data to characterize the series to some extent, although many of these reports lack laboratory information, including methods of identification of hafniae and biochemical characteristics of the infective strain. Of the 18 cases reported in Table 3, 12 occurred in adults, 4 in pediatric patients (2 to 13 years of age), and 2 in neonates (8 to 20 days old). Neonatal infections may be linked to frequent carriage of hafniae by women as part of the vaginal flora prior to birth (92). The symptoms associated with Hafnia sepsis are typical of gram-negative septicemias in general and include fever (38.6 to 40.5°C), occasional chills, and abdominal pain (14, 23, 26, 31, 37, 57, 72, 87, 105, 145). Diarrhea, either during or preceding the septic crisis, is not a consistent feature of H. alvei bacteremia but has been reported on several occasions. Fecal samples have been described as being bloody, melenic, or tarry in consistency (26, 31, 48, 104, 105). Additional laboratory studies, when reported, include an elevated level of C-reactive protein (6.8 to 37.3 mg/dl) and leukocytosis (14,200 to 32,600 mm3) (23, 26, 37, 57, 105). A majority of these blood-borne infections occur in males (71%) and are community acquired. The times of first positive Hafnia blood cultures in hospitalized patients have ranged from 1 to 41 days after admission. Half of all bacteremic patients were immunocompromised, with malignancies, liver disease, or human immunodeficiency virus (HIV) infection being the most common underlying conditions responsible for their impaired immune status. Several persons have also developed Hafnia bacteremia subsequent to liver transplants (10), and in one case Hafnia bacteremia occurred in a soldier following a gunshot wound and surgery (14). Two cases of pneumatosis intestinalis accompanying H. alvei bacteremia have also been described, one in a 6-year-old boy with nonlymphocytic leukemia (145) and another in a 20-day-old infant with necrotizing enterocolitis (48).
TABLE 3.
Reported cases of Hafnia bacteremia in the literature
| Case no. | Yr | Age (yr)/sexa | Underlying condition(s)b | Immunocompromised | Acquisitionc | Reference |
|---|---|---|---|---|---|---|
| 1 | 1967 | 58/M | None | No | N | 72 |
| 2 | 1969 | 58/M | None | No | NN | 31 |
| 3 | 1971 | 69/M | Diabetes, chronic pyelonephritis | No | N | 87 |
| 4 | 1975 | 13/F | None | No | N | 55 |
| 5 | 1977 | 20/M | Trauma (gunshot) | No | NN | 14 |
| 6 | 1987 | 6/M | Nonlymphocytic leukemia | Yes | NN | 145 |
| 7 | 1988 | 20 days/M | Low birth weight, premature | Yes | NN | 48 |
| 8 | 1991 | 63/M | Lung cancer | Yes | N | 84 |
| 9 | 1996 | 8/M | HIV+ | Yes | NN | 26 |
| 10 | 1996 | 37/M | Acute pancreatitis | No | N | 59 |
| 11 | 1996 | 49/F | Sigmoid diverticulitis | No | N | 59 |
| 12 | 1997 | 61/F | Biliary cirrhosis | Yes | N | 10 |
| 13 | 1997 | 2/M | Autoimmune hepatitis | Yes | NN | 10 |
| 14 | 1997 | 54/F | HIV+ | Yes | NN | 37 |
| 15 | 2000 | 79/F | Liver cirrhosis, cholangiocarcinoma | Yes | NN | 105 |
| 16 | 2000 | 73/? | Recklinghausen syndrome | No | NN | 57 |
| 17 | 2003 | 69/M | Stromal tumor | No | NN | 104 |
| 18 | 2004 | 8 days/M | None | Yes | NN | 23 |
M, male; F, female.
Known at time of admission or diagnosis. HIV+, HIV positive.
N, nosocomial; NN, nonnosocomial.
Although all 18 H. alvei septicemia cases listed in Table 3 appear to have resolved successfully with medical intervention, including one case with septic shock and disseminated intravascular coagulation (84), an unusual finding in several reports was the chronic nature of the bacteremia. In addition to the report of Englund (31), cited above, Grajwer and associates (55) reported a second case of chronic polymicrobic bacteremia involving H. alvei in a healthy 13-year-old girl. H. alvei was isolated from seven blood culture bottles (six pure culture) 6 weeks after clearance of her initial septic episode. Her infection resolved, but only after a prolonged course of treatment that included aminoglycosides and an antipseudomonal penicillin. A third case of chronic septicemia has been reported by Conte et al. (26); this was in an HIV-positive 8-year-old boy who had two separate bacteremic episodes 18 days apart that were apparently caused by the same strain. The authors could find no apparent reason for the septic relapse after appropriate antimicrobial chemotherapy for the initial episode.
Most cases of H. alvei bacteremia are monomicrobic, although rare instances of polymicrobic septicemia involving Pantoea agglomerans (55) and Enterococcus faecalis (10) have been reported; the number of positive blood cultures in each case report ranged from one to seven. Besides blood, H. alvei has been recovered from other anatomic sites in approximately 33% of cases, including hepatic abscesses, pancreatic pseudocyst fluid, sputum, pleural fluid, feces, and a central venous catheter (10, 37, 48, 59). Although the source of H. alvei bacteremias is unknown in most instances, the presumed origin of infections is the gastrointestinal or respiratory tract.
Hafnia alvei (“Enterobacter hafnia”) meningitis in a 1-year-old Iranian child with intermittent fevers of 1 month in duration has been reported (88). A cerebrospinal fluid tap grew H. alvei (no blood culture results were reported), and she was treated with gentamicin, chloramphenicol, and tetracycline. Although she started to improve, she died of nosocomially acquired Pseudomonas aeruginosa sepsis.
Gastroenteritis
A controversial issue.
H. alvei has a checkered past regarding its possible or reputed association with diarrheal disease. This association is chronicled in early publications from the onset of the discovery of type 32011 in the mid-1940s through the 1950s, including anecdotal stories linking this genus to cases of gastrointestinal disease (61, 134). By the 1960s, interest in hafniae as enteric pathogens had essentially waned, although sporadic mention of their possible role in diarrhea occasionally surfaced, such as in a report by Ratnam (107) describing two Canadian outbreaks of gastroenteritis associated with H. alvei.
In 1991, renewed interest in hafniae as bona fide enteropathogens was reawakened by a report of Albert et al. (4) describing diarrhea thought to be caused by H. alvei in a 9-month-old girl. This strain elicited diarrhea in rabbits and was found to contain the attaching-and-effacing gene (eae) of enteropathogenic E. coli. Other reports by the same authors followed, describing similar H. alvei strains linked to diarrhea and possessing the eae gene (5). These publications further triggered a series of case histories (109, 141) documenting Hafnia as a cause of acute gastroenteritis, with each of these studies citing the prior work of Albert et al. (4, 5), and a number of other studies and reviews cited accumulating evidence regarding the reputed pathogenicity of H. alvei (59).
Although the strains described by Albert et al. were initially identified as H. alvei by using the API 20E strip, subsequent studies by independent research teams suggested that these strains might not in fact belong to this genus. These conclusions were reached on the basis of 16S rRNA gene sequencing, the failure of the isolates described by Albert et al. to be lysed by a Hafnia-specific bacteriophage, and other atypical biochemical properties (see “LABORATORY ISOLATION AND IDENTIFICATION” below) (65, 69, 114). Further study indicated that the strains described by Albert et al. belonged to the genus Escherichia (69), and a proposal to include them in the genus Escherichia as a new species, E. albertii, was formally made by Huys and associates in 2003 (63).
Is H. alvei an enteropathogen?
Given the fact that the most credible recent information supporting the case for Hafnia as an enteric pathogen was actually based upon studies performed on strains that are not hafniae but rather members of the genus Escherichia, where does this leave us with isolates of H. alvei sensu stricto?
The single best indicator of the enteropathogenicity of any organism is the isolation of only that bacterium from multiple patient samples during the acute phase of an outbreak. When such isolates are related to one another, and hopefully to strains recovered from a vehicle of infection such as consumable products, the incriminating evidence becomes even stronger. In the case of H. alvei, several outbreaks attributed to this microorganism have been described (Table 2), but none of these completely meet the definition listed above. For the outbreaks reported to date, the best and most detailed report is that of Ratnam and others (106, 107). In support of the pathogenicity of these strains were the recovery of H. alvei as predominant or heavy pure growth from 13 of 23 stools (57%) involved in these two outbreaks, the fact that strains from both outbreaks possessed the same biotype (biotype 1) and serotype (O3:HNM), and the fact that of over 1,000 stools subsequently tested after these outbreaks, only one yielded H. alvei. However, in this study, no virulence factor was identified and there was no human immune response recorded or pathological data provided to corroborate culture results, and subsequent studies by an another laboratory testing reference strains from each outbreak found that they belonged to different genomospecies (71). Food and food handlers were the suspected sources, but this could not be proven.
Not much is known regarding the carrier state, although it is known to exist. In 1963, Matsumoto (85) screened 1,913 stools from asymptomatic inhabitants of Nagano Prefecture in Japan and found that 251 of these persons (13%) carried H. alvei. These numbers crossed all age groups and occupations. However, very different conclusions were drawn from a more recent study of Finnish travelers to Morocco, where no H. alvei was detected in the stools of 321 healthy persons (115). In that same study, H. alvei was recovered from 2% to 16% of persons with diarrhea in two distinct settings. However, a majority of these H. alvei isolates (57%) were recovered in conjunction with other recognized enteropathogens (Salmonella, Campylobacter, Aeromonas, or Shigella), thus limiting the conclusions that could be drawn from this survey.
Apart from these observations, there are few additional data regarding the possible enteropathogenicity of hafniae. Several case reports have been published (109, 141), and resolution of symptoms was accompanied by eradication or significant reduction of H. alvei in the gastrointestinal tract. However, antimicrobial therapy prescribed to eliminate Hafnia could also have been effective against other possible bacterial agents. Several potential virulence factors (other than eae) which could play a role in gastroenteritis have been also described, but either these reports are very old, the factor has only been found in a few strains, or the activity cannot be faithfully reproduced (see “PATHOGENICITY” below). Thus, at present, there are very few epidemiologic, clinical, and laboratory data to support Hafnia as a cause of gastroenteritis. This does not mean that hafniae are not enteric pathogens, just that much more work and evidence need to be accumulated to support such a position.
Respiratory Tract Infections
Next to the gastrointestinal tract, hafniae are most commonly recovered from respiratory secretions such as sputum, tracheal and bronchial aspirates, nasal smears, and bronchoalveolar lavage fluid and from the mouth and throat (59, 138). The vast majority of these isolates are not clinically significant. Washington et al. (138) isolated eight strains of H. alvei over a 2-year period at the Mayo Clinic. Of these eight isolates, only two (25%), from sputum and trachea, were determined to be clinically relevant as secondary pathogens; both were associated with cases of pneumonia, with one of these accompanied by pulmonary abscesses. Both of these significant isolates were the predominant flora and were associated with two fatal cases of bronchopneumonia. Günthard and Pennekamp (59) reported similar findings, where eight respiratory isolates were found to represent colonization rather than infection. Of seven H. alvei respiratory isolates recovered at a community hospital in New York City from 1989 to 1992, only one patient had Hafnia-associated disease (77). That singular case involved a 68-year-old female with chronic obstructive pulmonary disease and a left lower lung infiltrate with cardiomegaly and renal failure. H. alvei was recovered from her sputum and in pure culture from a fiberoptic bronchoscopy performed with a protected sheath brush. She was treated with aztreonam but subsequently died, and it is unclear how much hafniae contributed to her eventual demise. Collectively, these seven patients had a mean age of 60 years and had a variety of underlying diseases, including chronic obstructive pulmonary disease, lung cancer, and HIV infection. A case of nosocomial pneumonia due to Hafnia in a person on mechanical ventilation for 12 days duration has also been reported from Switzerland; H. alvei was again recovered in pure culture from a bronchoscopy specimen (40).
Miscellaneous Infections
H. alvei has been reported to be a rare cause of a variety of other extraintestinal infections. The analysis of these case reports is complicated by two facts. First, hafniae are often recovered from such syndromes as part of a mixed infection, and it is difficult to assess how much H. alvei contributes to the overall disease process. Second, in other instances there is only circumstantial evidence at best that Hafnia caused the illness or syndrome in question. Often this circumstantial evidence involves the recovery of these bacteria in large concentrations from gastrointestinal samples. Whether such a circumspect analysis is appropriate is unclear.
Although wounds would be thought to be common sites from which to recover hafniae, there are surprisingly few publications in the literature on this topic (40). Berger et al. (14) recovered H. alvei along with P. aeruginosa from a nosocomial wound infection of a 19-year-old soldier who had sustained a gunshot wound. What if any role hafniae played in the infectious process is unclear. H. alvei has also been isolated from a psoas abscess, but this gram-negative bacterium was isolated simultaneously with Streptococcus bovis (105). In a series of 17 cases of Hafnia colonization/infection, 3 were associated with wounds or abscesses (138). In all three cases they were thought to be secondary pathogens, although again they were recovered as part of a mixed microbial flora. Detailed information on these cases was not provided. The only convincing case of a wound infection attributed to H. alvei was reported by Augustin and Cunha (7) and involved a 44-year-old male who sustained a penetrating trauma to his right buttock by carpet nails. A subsequent abscess developed, and 50 ml of serosanguinous fluid grew a pure culture of H. alvei. H. alvei has infrequently been linked to hepatobiliary disease, including two cases of hepatic abscess (mixed cultures and bacteremia after liver transplantation) (10). Two cases of cholecystitis associated with Hafnia infection have also been published (105, 144). In one of these cases, that of a 67-year-old woman who apparently developed biliary disease after handling fish, hafniae were recovered from the infected gall bladder in small numbers in comparison to the predominant Aeromonas. Most recently, a single case of spontaneous bacterial peritonitis caused by H. alvei in a 60-year-old Saudi man with underlying mesothelioma has been reported (3).
The urinary tract occasionally harbors hafniae. Ramos and Dámaso (105) isolated H. alvei in pure culture from urine specimens of three different patients and felt they were clinically significant, while Washington et al. isolated hafniae in small numbers from a similar number of persons without signs of disease and determined that they were commensals (138). Septic arthritis associated with the recovery of both H. alvei and Klebsiella oxytoca from joint fluid of a 29-year-old woman after anterior cruciate ligament reconstruction has been reported (83). A case of reactive arthritis of greater than 10 weeks in duration has been linked to H. alvei enteritis (93). Although this pathogen was recovered in large numbers from feces, the joint fluid, blood, and urine were culture negative; the role of hafniae in this illness is debatable. Hafnia endophthalmitis has been reported in two instances. One was as part of a coinfection with Salmonella enterica subsp. arizonae (22). In this specific case, involving a 55-year-old Hispanic woman, the source of infection appeared to be snake powder from Mexico used to season food. A second case has recently been reported from Spain (118). In both instances, visual perception was lost despite aggressive medical intervention.
PATHOGENICITY
Understanding of which virulence factors play a role in the pathogenicity of Hafnia in regard to both human and animal infections is presently in its infancy. The limited amount of research in this area is most likely attributable to the low frequency of occurrence of this species in human illnesses and the fact that there are no clear-cut disease syndromes specifically associated with H. alvei. A further obstacle has been the unfortunate association of the eae gene with enteritis-producing strains that were thought to be H. alvei (4) but that were determined years later to actually be members of a new species, E. albertii (63).
What is presently known regarding Hafnia pathogenicity can be looked at from two perspectives, that is, virulence factors potentially operative in extraintestinal infections and those restricted primarily to the intestine. With all of these data there are numerous “gaps” in information, and many of the original findings date back 2 decades or more, where the description of strains and techniques is often incomplete.
Lethality studies, such as determinations of 50% lethal dose, to compare the overt pathogenicity of H. alvei to those of other members of the family, including E. coli, Shigella, and Salmonella, have not been performed. There is some sketchy and anecdotal information that hafniae can produce morbidity and mortality in certain animal models. One study found that 60% to 100% of rainbow trout injected subcutaneously and white mice and guinea pigs injected intravenously with aliquots of broth cultures containing hafniae died within 24 h (mice) to 10 days (fish); mice injected subcutaneously or orally challenged did not die (46). Unfortunately, the inoculum used in these experiments was not quantified, although the challenge dose was likely very high. One other study mentions that mice injected intrapertioneally with hafniae did not succumb to infection (91).
Approximately 35% to 55% of H. alvei strains are resistant to the bactericidal activity of human or bovine serum (67, 102). The classical pathway appears to be the major route by which serum-susceptible strains are killed; preincubation of bovine serum with H. alvei O-specific polysaccharide reduces or eliminates the bactericidal activity (67). Iron-scavenging mechanisms can also play important roles in pathogenesis. H. alvei strains produce siderophores as detected by a universal chemical assay (71, 102). One study found that the H. alvei siderophore was of neither the hydroxamate (aerobactin) nor the catechol (enterobactin) type (67). The siderophores of H. alvei can, however, take up ferric enterobactin and ferrichrome via an E. coli FepA-like outer membrane protein (119). Other factors described for hafniae, which may or may not play a role in their pathogenicity, include mannose-sensitive and mannose-resistant (MR/K, type 3 fimbria) hemagglutinins (102). Enzymatic properties associated with some pathogenic bacteria, including hemolysin, protease, elastase, and lecithinase activities, have not been detected in hafniae (71).
As early as the 1950s some strains of H. alvei were thought to be enteropathogenic, based upon results in animal studies. Sakazaki (120) found that 15 H. alvei strains produced enteritis in the ligated rabbit intestine model. However, no credible data evolved over the next 30 years. Numerous studies in the 1990s confirmed the fact that true H. alvei did not contain the eae gene as had been previously reported (65, 69), and in fact one Spanish study found all 102 H. alvei strains tested to be eae negative by both PCR and dot blot analysis (126). Other fecal isolates associated with outbreaks of gastroenteritis have uniformly been negative for heat-stable and heat-labile enterotoxins, invasiveness, and production of Vero cell cytotoxins (106).
Approximately 70% of Hafnia strains carry one or no plasmids (128). However, plasmid-bearing strains often harbor very large extrachromosomal elements ranging from 128 to 256 kb (128). Not much is known regarding what genes or functions are encoded by these plasmids, although atypical properties, such as lactose fermentation, have been attributed to plasmid carriage by rare strains (81). Recently, two small plasmids designated pAlvA and pAlvB were found to encode bacteriocins, or alveicins (140). These bacteriocin-carrying plasmids are very small (5.1 to 5.2 kb), and the protein products produced from these genes range from 358 to 408 amino acids in size. The alveicins are active only against their own species; about 15% of H. alvei strains screened appear to produce alveicins.
LABORATORY ISOLATION AND IDENTIFICATION
Cultural Characteristics
Not much attention has been given to optimizing cultural conditions for the specific recovery of hafniae. This can be attributed mostly to two facts: the lack of any substantial evidence that H. alvei is indeed a bona fide enteropathogen and the infrequent occurrence of hafniae as primary or secondary pathogens in other highly contaminated specimens, such as the respiratory tract and wounds. Even classic reference texts in the field of microbiology deal little with the isolation and cultural characteristics of H. alvei (86, 121, 122). Hafnia grows in media containing 2% to 5% NaCl, over a pH range of 4.9 to 8.25, and over thermal gradients of 4°C to 44°C (56); the optimum temperature for growth has been reported as 35°C (89). Some investigations have found growth at 44°C to be variable (73). There is general agreement that almost 100% of Hafnia strains grow on MacConkey, Hektoen enteric, eosin-methylene blue, and xylose-lysine-deoxycholate agars, all of which are differential to moderately selective media (56, 123, 124). For other media, such as desoxycholate-citrate agar (DCA), there are conflicting data, in that Greipson and Priest (56) reported that all 47 strains they tested grew on DCA, while Sakazaki (123) found that DCA was inhibitory for many hafniae. Regarding more inhibitory selective media, from 25% to 60% of strains fail to grow on Salmonella-Shigella (SS) agar, while 75% to 100% of isolates are inhibited on brilliant green medium (68, 73).
Classic strains of H. alvei are lactose and sucrose negative and as such appear as nonfermenting colonies on enteric isolation media. On moderately selective agars, they typically appear as large, smooth, convex, translucent colonies of 2 to 3 mm in diameter with an entire edge (68, 123); some may exhibit an irregular border. Several media have been employed on rare occasions to recover hafniae from fecal specimens. Using SS agar, Matsumoto (85) was able to isolate H. alvei from 13% of the 1,913 stools tested in 1961; this number probably represents a minimum value, since SS agar is known to be inhibitory to many Hafnia strains. Sakazaki (121) took advantage of the inability of hafniae to ferment d-sorbitol to recover H. alvei from 42% of 598 stools by using desoxycholate-lactose-sucrose-sorbitol agar, while comparable recovery rates on MacConkey agar were only 2%. H. alvei has also been isolated on sorbitol-MacConkey agar, a medium designed for the isolation E. coli O157:H7 (38). For environmental samples such as food, both violet red bile lactose and fecal coliform agars have been successfully used to isolate hafniae from consumables, including cheese and sausage (56, 80). Enrichment broths in which some but not all hafniae grow include selenite and tetrathionate broths (123).
Several additional cultural characteristics of H. alvei bear mentioning. Because H. alvei strains are H2S negative, they can occasionally be mistaken for H2S-negative Salmonella on media such as enteric agar (123). Similarly, some hafniae also produce red or pink colonies on xylose-lysine-desoxycholate agar (121). Some Hafnia strains or variants are lactose positive, a trait that in some instances has been determined to be plasmid mediated (81). Atypical H. alvei colonies on violet red bile lactose and fecal coliform agars have also been described (80).
Identification
The genus Hafnia consists of strains that conform to general characteristics for inclusion in the family Enterobacteriaceae, being gram-negative, peritrichously flagellated rods that are oxidase negative, are nonsporulating, produce nitrate reductase, and are positive for enterobacterial common antigen. They are facultatively anaerobic, producing acid with or without gas from the metabolism of d-glucose and other carbohydrate or carbohydrate-like compounds (35). Major distinguishing phenotypic features of the Hafnia complex include being indole negative, lysine and ornithine decarboxylase positive, arginine dihydrolase negative, and H2S and d-sorbitol negative, with production of acid from the fermentation of d-glucose, l-arabinose, and l-rhamnose. Most strains are also VP positive and are motile (35). Strains that deviate from this ideal phenotype do exist but are uncommon (70, 120). Often they can be mistaken for H2S-negative Salmonella on primary plating media or can be confused with/misidentified as either Enterobacter or Serratia. On occasion, members of the genus Hafnia can also be mistaken for E. coli or Yokenella regensburgei (123). Tests useful in distinguishing H. alvei from Y. regensburgei include VP and glycerol fermentation (both of which are positive for hafniae) and acid production from melibiose (which is negative for hafniae) (79, 133).
Temperature can play a prominent role in phenotype expression in H. alvei. Strains typically incubated at lower (22°C) rather than higher (35°C) temperatures are more likely to produce acetylmethylcarbinol, form gas, and be more actively motile (89, 120, 123), while conversely, the methyl red test is positive at elevated temperatures. Early studies suggested that Hafnia strains were H2S positive (89), although these strains are invariably H2S negative on traditional screening media such as TSI (35). As with many Enterobacteriaceae that are H2S negative on TSI, H. alvei strains are moderately to weakly H2S positive on thiosulfate-containing media such as gelatin-cysteine-thiosulfate medium at 48 h (unpublished data).
Commercial systems.
Most recent published studies involving the identification of Enterobacteriaceae on commercial systems have had little trouble identifying H. alvei. These systems include both MicroScan conventional and rapid panels (11, 42, 94, 116) and bioMérieux Vitek GNI+ and Vitek 2 systems (41, 42, 95). In five of these studies, 61 of 62 isolates (98%) were correctly identified as H. alvei (11, 41, 42, 94, 116). The single exception was an atypical strain that was esculin positive and l-rhamnose negative and that was identified as a rare biotype on MicroScan conventional panels (116). A sixth study conducted by the CDC found that 6 of 10 H. alvei strains yielded excellent identifications on Vitek ID-GNB, 3 of 10 strains produced a probability of a good identification, and 1 isolate was unidentified (95). An earlier study by the CDC found that four of nine H. alvei strains were identified as inactive E. coli by Vitek GNI+ (96). The converse situation can also occur, where species such as Salmonella enterica subsp. arizonae and Y. regensburgei have been misidentified as hafniae (96). A more recent observation concerns the recently described species E. albertii. Originally identified as H. alvei on the API 20E system by Albert et al. (4), this observation has subsequently been confirmed by other groups, and such misidentifications occur not only on the API 20E but also on Vitek and MicroScan rapid panels (1). Conventional biochemical tests are needed to distinguish between these two taxa at present (see “Conventional methods” below).
Agreement between commercially available systems and conventional biochemical formats varies with each test. Rodríguez et al. (116) found that there was generally good agreement between MicroScan Combo Negative type IS panels, conventional biochemical test results, and recorded character traits in Bergey's Manual of Systematic Bacteriology (122) for most phenotypic results. However major differences were noted in test correlation between commercial and conventional formats for o-nitrophenyl-β-d-galactopyranoside (47.6%), urease (23.8%), VP (14.2%), and citrate (4.7%). For arginine dihydrolase, correlation of assays improved from 80.9% to 100% when test results were read manually rather than by using the WalkAway.
Conventional methods.
Despite the fact that most strains of H. alvei can be easily identified using commercial systems, conventional biochemical test results can be necessary under a number of circumstances. These include when probability of a correct identification is low, when H. alvei strains with two or more atypical properties (e.g., VP negative and urease positive) are identified, when hafniae are isolated from an anatomic site not traditionally associated with this species (e.g., blood), for unusual disease syndromes linked to Hafnia infection, for chronic or recurring illnesses, and for publication purposes. One study assessing the reproducibility of results from 22 laboratories in the United Kingdom to identify a series of enteric bacteria in a multipoint identification scheme found H. alvei to be one of the most difficult organisms to identify, with 37% of strains reported with either an incorrect identification or no identification at all (143).
Specialized tests. (i) Hafnia-specific phage.
In 1967, Guinée and Valkenburg (58) described a lytic bacteriophage that was specific for the genus Hafnia. Phage 1672 lysed all 100 Hafnia strains tested against it and none of over 200 other enteric bacteria, including Salmonella, Citrobacter, Enterobacter, Klebsiella, and Serratia strains. The test is performed by inoculating a 24-h broth culture onto the surface of a nutrient agar plate and, after removal of excess fluid, adding a drop of bacteriophage suspension to the plate. After overnight incubation at 37°C, a clear lytic zone surrounded by growth is recorded as positive (123). This is an excellent test to definitively identify members of the genus Hafnia and has been used extensively in strain characterization (71, 108). Phage 1672 is available from the American Type Culture Collection (Manassas, Va.) (catalogue no. 51873-B1). The propagating host strain for this phage is H. alvei ATCC 51873 (HER 1272 [1672]).
(ii) GAD.
The assay for glutamate decarboxylase (GAD) activity can be performed in a single-tube format, as described by Rice and colleagues (111). A change in color from blue to yellow is considered to be a positive test. Escherichia species, including E. coli and E. albertii, are GAD positive, while hafniae are GAD negative (71, 111).
(iii) LPA.
Among enteric bacteria, strong l-prolineaminopeptidase (LPA) activity is detectable only in H. alvei, Serratia marcescens, and Serratia liquefaciens (25, 34). The assay can be performed in tube or microtiter format or using commercially available kits (Aminopeptidase Wee-Tab; Key Scientific Products, Round Rock, Tex.) (71). This assay needs to be read within 30 min to 1 h, as weak LPA activity can be detected in other species upon prolonged incubation (34). LPA is useful in distinguishing hafniae from E. cloacae and related groups and from some Serratia species.
Biotypes. (i) Biotype 1 strains.
Farmer and colleagues at CDC defined biotype 1 strains in 1985 as “H. alvei biogroup 1” or “H. alvei—brewery biogroups” (36). These strains are also synonymous with the previous epithet name Obesumbacterium proteus and the H. alvei biogroup 1 strains listed in Table 1 of reference 35 (J. J. Farmer, personal communication). Biogroup 1 strains have not been recovered from clinical specimens (36). Major phenotypic differences distinguishing “brewery strains” from typical H. alvei (Table 1 of reference 35), which includes both DNA groups, are listed in Table 4.
TABLE 4.
Distinguishing features of H. alvei and H. alvei biogroup 1 strains
| Test | Result fora:
|
|
|---|---|---|
| H. alveib | H. alvei biogroup 1c | |
| Methyl red | Variable (40) | + (85) |
| Ornithine decarboxylase | + (98) | Variable (45) |
| Motility | + (85) | − (0) |
| Growth in KCN | + (95) | − (0) |
| d-Glucose (gas) | + (98) | − (0) |
| Acid from: | ||
| l-Arabinose | + (95) | − (0) |
| l-Rhamnose | + (97) | − (0) |
| Glycerol | + (95) | − (0) |
Data are from reference 35. Numbers in parentheses indicate percent positive for indicated character. +, positive; −, negative.
Includes DNA groups 1 and 2.
Brewery strains (Obesumacterium proteus biogroup 1).
(ii) Barbe biotypes.
In 1969, Barbe described two biotypes of H. alvei based upon fermentation of d-arabinose and salicin and on esculin and arbutin hydrolysis (doctoral thesis cited in reference 21). These two biotypes did not correlate completely with the two known DNA hybridization groups within the H. alvei complex. The number of Barbe biotypes has subsequently been expanded to four by other investigators in a phenotypic and genotypic analysis of 73 H. alvei strains (71). Types 1 and 2 predominate (Table 5).
TABLE 5.
Modified Barbe biotypesa
| Test | Result for Barbe biotype:
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Esculin | + | + | + | − |
| Arbutin | − | + | + | − |
| Salicin | − | + | + | − |
| d-Arabinose | + | − | + | − |
Adapted from reference 71.
Distinguishing H. alvei complex from Escherichia albertii.
There has been considerable controversy in the literature regarding the enteropathogenicity of H. alvei, based upon the initial misidentification of E. albertii strains as H. alvei (see “Gastroenteritis” in “CLINICAL SIGNIFICANCE” above). The two groups share a number of phenotypic features, including lack of indole production, inability to ferment lactose or d-sorbitol, and similar antimicrobial susceptibility profiles (69). This controversy took over 10 years to resolve with the publication of a new species, E. albertii (63). Several groups have investigated these and similar strains either prior to or after the naming of E. albertii. These phenotypic and molecular tests are presented in Table 6.
TABLE 6.
Phenotypic and genotypic features useful in distinguishing H. alvei sensu stricto and E. albertii
| Property type | Testa | Result forb:
|
|
|---|---|---|---|
| H. alvei | E. albertii | ||
| Phage lysis | φ1672 | + (100) | − (0) |
| Biochemical | KCN | + (98) | − (0) |
| VP | + (100) | − (0) | |
| GAD | − (0) | + (100) | |
| Glycerol (24 h) | + (96) | − (0) | |
| l-Rhamnose (24 h) | + (51) | − (0) | |
| d-Xylose | − (100) | + (0) | |
| Enzymatic | Chitinase (6 h) | + (99) | − (5) |
| Hydroxyproline amidase | + (100) | − (0) | |
| Tripeptidase | + (100) | − (0) | |
| Proline deaminase | + (100) | + (0) | |
| Molecular | phoE | − (0) | + (100) |
| eae | − (0) | + (100) | |
| cdt | − (0) | + (100) | |
| PFGE typical ladder (XbaI) | − (0) | + (100) | |
cdt, cytolethal distending toxin gene; eae, E. coli attaching-and-effacing gene; phoE, outer membrane protein gene; PFGE, pulsed-field gel electrophoresis.
Phenotypic and genotypic separation of H. alvei DNA groups 1 and 2.
One of the difficult challenges that microbiologists face is trying to develop biochemical tests that can readily distinguish most strains of DNA group 1 (H. alvei sensu stricto) from DNA group 2 (unnamed Hafnia species) isolates. While the initial studies by Steigerwalt et al. (131) in 1976 detected two distinct hybridization groups within the genus, no differential tests to separate these genomospecies were noted. The first true breakthrough in this area involves the defining publication by Don Brenner (21), where a number of biochemical characteristics were found to be preferentially associated with either DNA group 1 or 2. These tests included motility at 24 h, hydrolysis of arbutin, fermentation of salicin or inulin, and utilization of sodium acetate. In that study, Brenner noted that these two groups could be distinguished from one another by a series of tests but that no single test was completely discriminatory, although motility at 24 h was the best predictor of DNA group (DNA group 1, 9% positive; DNA group 2, 100% positive). Furthermore, Barbe's biotypes (see above) did not correlate perfectly with these two DNA relatedness groups (21).
There were no major studies on this subject for over 20 years. In 2002, a study conducted by the Microbial Diseases Laboratory found that 22 Hafnia isolates could be assigned to their correct DNA relatedness groups by 16S rRNA gene sequencing (71). Biochemical characterization of these strains showed that while motility did tend to correlate with genomospecies (DNA group 1, 20% positive; DNA group 2, 71% positive), as in the study by Brenner (21), a better differential test appeared to be malonate utilization (DNA group 1, 100% positive; DNA group 2, 17% positive). Okada and Gordon (97) subsequently confirmed the discriminatory value of the malonate test in their ecologic study of hafniae in Australia. These collective data were reinforced by a 2005 study in which malonate and several other tests were found to be useful in separating these two relatedness groups (70). The proposal was also made in latter study to recognize four biogroups with H. alvei sensu stricto (DNA group 1), based upon the fermentation of d-arabinose and salicin as well as esculin hydrolysis. Table 7 provides cumulative data from several studies on the best tests currently available for separation of H. alvei DNA groups 1 and 2.
TABLE 7.
Selected phenotypic tests useful in distinguishing H. alvei DNA groups 1 and 2
| Test | Result fora:
|
|||
|---|---|---|---|---|
| DNA group 1 (H. alvei sensu stricto)
|
DNA group 2 (Hafnia unnamed species)
|
|||
| Phenotype | % Positive | Phenotype | % Positive | |
| Motility (24 h) | V | 9-33 | V | 61-100 |
| Utilization of: | ||||
| Acetate | − | 9 | V | 71 |
| Citrate | V | 59-70 | V | 31-80 |
| Malonate | + | 96-100 | V | 14-22 |
| Methyl red | + | 100 | V | 29 |
| Hydrolysis of: | ||||
| Arbutin | V | 45 | − | 0 |
| Esculin | V | 20-52 | −b | 0-57 |
| Fermentation of: | ||||
| d-Arabinose | − | 13 | V | 79 |
| Inulin | − | 9 | V | 71 |
| Salicin | V | 45-52 | − | 0 |
| Enzymatic | ||||
| p-n-p-β-Galactoside | V | 53 | + | 88 |
| p-n-p-N-Acetylglucosaminide | V | 63 | + | 98 |
Data are from references 21, 70, 71, and 98. Not all tests were performed using identical procedures in each study. +, >85% positive; −, <15% positive; V, variable (15% to 85% positive).
Two of three studies reported DNA group 2 strains to be 0% positive, so the negative phenotype is listed rather than the variable character.
Molecular identification.
No specific probes or assays have been developed to detect the genus Hafnia. H. alvei strains do react with oligonucleotide probes developed to identify members of the family Enterobacteriaceae. These probes target specific regions of the small-subunit (16S) or large-subunit (23S) rRNA gene (15, 99).
Serology
The immunochemistry of Hafnia lipopolysaccharide (LPS) is extremely complicated. All H. alvei LPS appears to contain glucose, glucosamine, heptose, and 3-deoxyoctulosonic acid (117). Some LPS also contains other amino sugars or carbohydrates such as mannose, galactose, galactosamine, and mannosamine. The core oligosaccharide structure of some strains consists of an identical hexasaccharide structure composed of two d-glucose residues, three ld-heptose residues, and one 3-deoxyoctulosonic acid residue. Other strains have an additional core fragment (117). There is extensive serologic and immunologic diversity in this genus, and active research continues in this arena (74).
Two formal serologic typing schemes for H. alvei have been published. Riichi Sakazaki and colleagues at the National Institute of Health in Tokyo, Japan, pioneered the older of these two schemes. This scheme officially recognized 68 somatic (“O”) and 34 flagellar (“H”) types (123, 124). Unfortunately, this typing system is no longer available because of the loss of many test strains for specific O- and H-antigen groups (123). A second, independent scheme proposed by the Laboratory of Opportunistic Enteric Pathogens at the Mechnikov Research Institute for Vaccines and Sera in Moscow, Russia, recognizes 39 O and 36 H types (13, 117). The availability of this second typing scheme is unknown, and we are unaware of any recently published studies reporting Hafnia serotypes or serogroups. Some Hafnia antigens are shared with other enteric groups, potentially producing serologic cross-reactions. These groups include Salmonella, Shigella, E. coli, Citrobacter freundii, and E. cloacae (123, 124, 137). Some H. alvei strains have been reported to agglutinate in O157 antisera (123).
Several other antigens in hafniae have been described. Several studies have reported that some H. alvei strains produce K antigens which may actually be a slime antigen (124). Other strains produce the alpha antigen described by Stamp and Stone (30). This antigen consists of a complex of three factors, and Hafnia strains that carry this antigen either contain all three or two of these three factors. Other enteric groups, such as Providencia, occasionally carry the alpha antigen as well.
ANTIMICROBIAL SUSCEPTIBILITY
Analogous to the situation described above regarding pathogenicity, there are virtually no studies available in which a large collection of Hafnia isolates have been studied for their susceptibility to different classes of antimicrobial compounds. The lone exception to this is a 2005 study by Stock et al. (132), in which 76 H. alvei isolates were investigated for their susceptibility to 69 antibiotics or drugs. The general pattern that emerges from this study is that hafniae are typically susceptible to carbapenems, monobactams, chloramphenicol, quinolones, aminoglycosides, and antifolates (e.g., trimethoprim-sulfamethoxazole) and resistant to penicillin, oxacillin, and amoxicillin plus clavulanic acid. Susceptibility to tetracyclines and cephalosporins is variable, with most H. alvei strains being susceptible to broad-spectrum and “fourth-generation” cephalosporins (cefipime) but often resistant to narrow- and expanded-spectrum compounds. A 2000 Spanish study involving enteric pathogens also found that 32 strains of H. alvei were universally susceptible to all quinolones (including gemifloxacin and grepafloxacin), cefotaxime, gentamicin, co-trimoxazole, and naladixic acid (39); 78% of the strains in that study were susceptible to doxycycline.
Some H. alvei strains produce both low-level inducible cephalosporinases (ceftazidime susceptible) and high-level constitutive cephalosporinase activity that is resistant to ceftazidime (50, 101, 136). These activities are chromosomally encoded cephalosporinases belonging to Bush group 1 β-lactamases (Ambler class C). The ampC gene of one H. alvei strain exhibits 94% amino acid sequence homology to a plasmid-borne cephalosporinase of K. pneumoniae (49). Unrelated H. alvei isolates show 85% to 100% amino acid identity in their AmpC sequences.
CONCLUSIONS
There are many unanswered questions regarding the genus Hafnia. Clearly, the most important issue concerns the frequency and type of human infections associated with this genus. If at a future date credible evidence linking this species to diarrheal disease surfaces, and given the frequency of occurrence of H. alvei in gastrointestinal specimens, its relative importance as a human pathogen may be significantly enhanced. Another unanswered question concerns the taxonomy of this genus. The naming of DNA group 2 and identification of useful tests to separate this genomospecies from H. alvei sensu stricto (DNA group 1) should lead to a better appreciation of whether there are major differences between these two species in disease presentation, environmental distribution, and pathogenicity (70). We presently know very little regarding the distribution of hafniae in environmental samples, their ecologic niches other than the gastrointestinal tract, and the ability of the H. alvei complex to incite disease in animals, fish, and other wildlife. Recent work from Australia, however, suggests that significant differences in environmental distribution do occur (98). Finally, a variety of antimicrobial resistance mechanisms have been recently identified in sporadic H. alvei isolates (50, 101). At present the frequency of these resistotypes is low, but if widespread dissemination should occur, treatment of systemic H. alvei infections could become problematic.
ADDENDUM
Since the submission of this review, we have recovered a strain of H. alvei from two stool specimens of an 11-year-old girl with probable hemolytic uremic syndrome (C. Crandall, S. L. Abbott, Y. Q. Zhao, W. Probert, and J. M. Janda, Infection, in press). This strain produces a cytolytic toxin that is active on Vero cells and is morphologically indistinguishable from Shiga toxin (Stx). However, this toxin appears to be less potent and antigenically distinct, as its activity is not neutralizable when the strain is preincubated with antisera directed against both Stx1 and Stx2.
REFERENCES
- 1.Abbott, S. L., J. O'Connor, T. Robin, B. L. Zimmer, and J. M. Janda. 2003. Biochemical properties of a newly described Escherichia species, Escherichia albertii. J. Clin. Microbiol. 41:4852-4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad, S., W. G. Weisburg, and R. A. Jensen. 1990. Evolution of aromatic amino acid biosynthesis and application to the fine-tuned phylogenetic positioning of enteric bacteria. J. Bacteriol. 172:1051-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akcam, F. Z., M. Isler, O. R. Tarhan, and H. E. Eroglu. 2005. Spontaneous bacterial peritonitis due to Hafnia alvei in a patient with peritoneal mesothelioma. Saudi Med. J. 26:151-153. [PubMed] [Google Scholar]
- 4.Albert, M. J., K. Alam, M. Islam, J. Montanaro, A. S. M. H. Rahman, K. Haider, M. A. Hossain, A. K. M. G. Kibriya, and S. Tzipori. 1991. Hafnia alvei, a probable cause of diarrhea in humans. Infect. Immun. 59:1507-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert, M. J., S. M. Faraque, M. Ansaruzzaman, M. M. Islam, K. Haider, K. Alam, I. Kabir, and R. Robins-Browne. 1992. Sharing of virulence-associated properties at the phenotypic and genotypic levels between enteropathogenic Escherichia coli and Hafnia alvei. J. Med. Microbiol. 37:310-314. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous. 2004. Klebsiella, Enterobacter, Serratia, and Citrobacter spp. bacteraemia, England, Wales, and Northern Ireland: 2003. CPR Wkly. 14:1-6. [Online.] www.hpa.org.uk/cdr/PDFfiles/2004/bact_2104.pdf. [Google Scholar]
- 7.Augustin, E. T., and B. A. Cunha. 1995. Buttock abscess due to Hafnia alvei. Clin. Infect. Dis. 20:1426. [DOI] [PubMed] [Google Scholar]
- 8.Balmus, G., I. Samuel, and L. Mirza. 1963. Research on a severe Hafnia epizootic in young laboratory rats. Rev. Pathol. Gen. Physiol. Clin. 63:573-580. [PubMed] [Google Scholar]
- 9.Bangaert, R. L., A. C. Ward, E. H. Stauber, B. R. Cho, and P. R. Widders. 1988. A survey of the aerobic bacteria in the feces of captive raptors. Avian Dis. 32:53-62. [PubMed] [Google Scholar]
- 10.Barry, J. W., E. A. Dominguez, D. J. Boken, and L. C. Preheim. 1997. Hafnia alvei infection after liver transplantation. Clin. Infect. Dis. 24:1263-1264. [DOI] [PubMed] [Google Scholar]
- 11.Bascomb, S., S. L. Abbott, J. D. Bobolis, D. A. Bruckner, S. J. Connell, S. K. Cullen, M. Daugherty, D. Glenn, J. M. Janda, S. J. Lentsch, D. Lindquist, P. B. Mayhew, D. M. Nothaft, J. R. Skinner, G. B. Williams, J. Wong, and B. L. Zimmer. 1997. Multicenter evaluation of the MicroScan gram-negative identification type 3 panel. J. Clin. Microbiol. 35:2531-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bascomb, S., S. P. Lapage, W. R. Wilcox, and M. A. Curtis. 1971. Numerical classification of the tribe Klebsiellae. J. Gen. Microbiol. 66:279-295. [DOI] [PubMed] [Google Scholar]
- 13.Baturo, A. P., and V. P. Raginskaya. 1978. Antigenic scheme for the hafniae. Int. J. Syst. Bacteriol. 28:126-127. [Google Scholar]
- 14.Berger, S. A., S. C. Edberg, and R. S. Klein. 1977. Enterobacter hafniae infection; report of two cases and review of the literature. Am. J. Med. Sci. 273:101-105. [PubMed] [Google Scholar]
- 15.Bohnert, J., B. Hübner, and K. Botzenhart. 2000. Rapid identification of Enterobacteriaceae using a novel 23S rRNA-targeted oligonucleotide probe. Int. J. Hyg. Environ. Health 203:77-82. [DOI] [PubMed] [Google Scholar]
- 16.Borman, E. K., C. A. Stuart, and K. M. Wheeler. 1944. Taxonomy of the family Enterobacteriaceae. J. Bacteriol. 48:351-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Böttger, E. K., M. Jürs, T. Barrett, K. Wachsmuth, S. Metzger, and D. Bitter-Suermann. 1987. Qualitative and quantitative determination of enterobacterial common antigen (ECA) with monoclonal antibodies: expression of ECA by two Actinobacillus species. J. Clin. Microbiol. 25:377-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouvet, O. M. M., P. Lenormand, E. Ageron, and P. A. D. Grimont. 1995. Taxonomic diversity of anaerobic glycerol dissimilation in the Enterobacteriaceae. Res. Microbiol. 146:279-290. [DOI] [PubMed] [Google Scholar]
- 19.Branca, G., C. Galasso, G. Cornaglia, R. Fontana, and G. Satta. 1996. A simple method to detect bacteriolytic enzymes produced by Enterobacteriaceae. Microbios 86:205-212. [PubMed] [Google Scholar]
- 20.Brenner, D. J. 1977. Characterization and clinical identification of Enterobacteriaceae by DNA hybridization. Prog. Clin. Pathol. 7:71-117. [PubMed] [Google Scholar]
- 21.Brenner, D. J. 1981. Introduction to the family Enterobacteriaceae, p. 1105-1127. In M. P. Starr, H. Stolp, H. G. Trüper, and H. G. Schlegel (ed.), The prokaryotes: a handbook on habitats, isolation, and identification of bacteria. Springer-Verlag, Berlin, Germany.
- 22.Caravalho, J., Jr., V. M. McMillan, R. B. Ellis, and A. Betancourt. 1990. Endogenous endophthalmitis due to Salmonella arizonae and Hafnia alvei. South. Med. J. 83:325-327. [DOI] [PubMed] [Google Scholar]
- 23.Cassanova-Román, M., A. Sanchez-Porto, and M. Casanova-Bellido. 2004. Late-onset neonatal sepsis due to Hafnia alvei. Scand. J. Infect. Dis. 36:70-71. [DOI] [PubMed] [Google Scholar]
- 24.Cassel-Beraud, A. M., and C. Richard. 1988. Étude de la flore intestinale aérobie de microchéiroptères Chaerephon pumila a Madagascar. Bull. Soc. Path. Exot. Filiales. 81:806-810. (In French.) [PubMed] [Google Scholar]
- 25.Chagla, A. H., A. A. Borczyk, J. E. Aldom, S. D. Rosa, and D. D. Cole. 1993. Evaluation of the l-pyrrolidonyl-β-naphthylamide hydrolysis test for differentiation of members of the families Enterobacteriaceae and Vibrionaceae. J. Clin. Microbiol. 31:1946-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conte, M., E. Castagnola, P. Venzano, L. Tasso, and R. Giacchino. Bacteremia caused by Hafnia alvei in a human immunodeficiency virus-infected child. Pediatr. Infect. Dis. J. 15:182-183. [DOI] [PubMed]
- 27.Dauga, C. 2002. Evolution of the gyrB gene and the molecular phylogeny of Enterobacteriaceae: a model molecule for molecular systematic studies. Int. J. Syst. Evol. Microbiol. 52:531-547. [DOI] [PubMed] [Google Scholar]
- 28.Derlet, R. W., and J. R. Carlson. 2002. An analysis of human pathogens found in horse/mule manure along the John Muir Trail in Kings Canyon and Sequoia and Yosemite national parks. Wild. Environ. Med. 13:113-118. [DOI] [PubMed] [Google Scholar]
- 29.Edwards, P. R., and W. H. Ewing. 1955. Identification of Enterobacteriaceae. Burgess Publishing Company, Minneapolis, Minn.
- 30.Emslie-Smith, A. H. 1961. Hafnia alvei strains possessing the alpha antigen of Stamp and Stone. J. Pathol. Bacteriol. 81:534-536. [DOI] [PubMed] [Google Scholar]
- 31.Englund, G. W. 1969. Persistent septicemia due to Hafnia alvei. Am. J. Clin. Pathol. 51:717-719. [DOI] [PubMed] [Google Scholar]
- 32.Ewing, W. H. 1963. An outline of nomenclature for the family Enterobacteriaceae. Int. Bull. Bacteriol. Nomencl. Taxon. 13:95-99. [Google Scholar]
- 33.Ewing, W. H. 1986. Identification of Enterobacteriaceae. Elsevier, New York, N.Y.
- 34.Fanghänel, S., R. Reissbrodt, and H. Giesecke. 1991. l-Prolineaminopeptidase activity as a tool for identification and differentiation of Serratia marcescens, Serratia liquefaciens and Hafnia alvei strains. Zentralbl. Bakteriol. 275:11-15. [DOI] [PubMed] [Google Scholar]
- 35.Farmer, J. J., III. 2003. Enterobacteriaceae: introduction and identification, p. 636-653. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Tolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, D.C.
- 36.Farmer, J. J., III, B. R. Davis, F. W. Hickman-Brenner, A. McWhorter, G. P. Huntley-Carter, M. A. Ashbury, C. Riddle, H. G. Wathen-Grady, C. Elias, G. R. Fanning, A. G. Steigerwalt, C. M. O'Hara, G. K. Morris, P. B. Smith, and D. J. Brenner. 1985. Biochemical identification of new species and biogroups of Enterobacteriaceae isolated from clinical specimens. J. Clin. Microbiol. 21:46-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fazal, B. A., J. E. Justman, G. S. Turett, and E. E. Telzak. 1997. Community-acquired Hafnia alvei infection. Clin. Infect. Dis. 24:527-528. [DOI] [PubMed] [Google Scholar]
- 38.Feng, P. 1995. Escherichia coli O157:H7: novel vehicles of infection and emergence of phenotypic variants. Emerg. Infect. Dis. 1:47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernández-Roblas, R., F. Cabria, J. Esteban, J. C. López, I. Gadea, and F. Soriano. 2000. In vitro activity of gemifloxacin (SB-265805) compared with 14 other antimicrobials against intestinal pathogens. J. Antimicrob. Chemother. 46:1023-1027. [DOI] [PubMed] [Google Scholar]
- 40.Frick, T., M. Kunz, M. Vogt, and M. Turina. 1990. Nosocomial infection with Hafnia alvei: report of two cases and literature review. Schweiz. Rundschau Med. (Praxis) 79:1092-1094. [PubMed] [Google Scholar]
- 41.Funke, G., and P. Funke-Kissling. 2004. Evaluation of the new VITEK 2 card for identification of clinically relevant gram-negative rods. J. Clin. Microbiol. 42:4067-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Funke, G., D. Monnet, C. deBernardis, A. von Graevenitz, and J. Freney. 1998. Evaluation of the VITEK 2 system for rapid identification of medically relevant gram-negative rods. J. Clin. Microbiol. 36:1948-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gale, E. F., and H. M. R. Epps. 1943. Lysine decarboxylase: preparation of enzyme and coenzyme. Nature 152:327-328. [Google Scholar]
- 44.Gamage, S. D., J. B. Luchansky, and S. C. Ingram. 1998. Pulsed-field gel electrophoresis typing of Hafnia alvei isolated from chub-packed and retail ground beef. Lett. Appl. Microbiol. 26:105-109. [DOI] [PubMed] [Google Scholar]
- 45.Gavini, F., C. Ferragut, B. Lefebvre, and H. Leclerc. 1976. Étude taxonomique d-entérobactéries appartenant ou apparentées au genre Enterobacter. Ann. Microbiol. (Inst. Pasteur) 127B:317-335. [PubMed] [Google Scholar]
- 46.Gelev, I., and E. Gelev. 1988. A new species of fish-pathogenic bacterium antigenically related to classical brucellae. Zentralbl. Bakteriol. Hyg. A 269:1-6. [DOI] [PubMed] [Google Scholar]
- 47.Gelev, I., E. Gelev, A. G. Steigerwalt, G. P. Carter, and D. J. Brenner. 1990. Identification of the bacterium associated with haemorrhagic septicaemia in rainbow trout as Hafnia alvei. Res. Microbiol. 141:573-576. [DOI] [PubMed] [Google Scholar]
- 48.Ginsberg, H. G., and J. P. Goldsmith. 1988. Hafnia alvei septicemia in an infant with necrotizing enterocolitis. J. Perinatol. 8:122-123. [PubMed] [Google Scholar]
- 49.Girlich, D., T. Naas, S. Bellais, L. Poirel, A. Karim, and P. Nordmann. 2000. Biochemical-genetic characterization and regulation of expression of an ACC-1-like chromosome-borne cephalosporinase from Hafnia alvei. Antimicrob. Agents Chemother. 44:1470-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girlich, D., T. Naas, S. Bellais, L. Poirel, A. Karim, and P. Nordmann. 2000. Heterogeneity of AmpC cephalosporinases of Hafnia alvei clinical isolates expressing inducible or constituitive ceftazidime resistance phenotypes. Antimicrob. Agents Chemother. 44:3220-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goatcher, L. J., M. W. Barrett, R. N. Coleman, A. W. L. Hawley, and A. Qureshi. 1987. A study of predominant aerobic microflora of black bears (Ursus americanus) and grizzly bears (Ursus arctos) in northwestern Alberta. Can. J. Microbiol. 33:949-954. [DOI] [PubMed] [Google Scholar]
- 52.Goldstein, E. J. C., E. O. Agyare, A. E. Vagvolgyi, and M. Halpern. 1981. Aerobic bacterial flora of garter snakes: development of normal flora and pathogenic potential for snakes and humans. J. Clin. Microbiol. 13:954-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gordon, D. M., and F. FitzGibbon. 1999. The distribution of enteric bacteria from Australian mammals: host and geographical effects. Microbiology 145:2663-2671. [DOI] [PubMed] [Google Scholar]
- 54.Gracheva, N. M., N. I. Leont'eva, V. M. Bondarenko, S. V. Fialkina, G. N. Konovalova, O. S. Partin, I. T. Shcherbakov, A. I. Solov'eva, and T. A. Blokhina. 2003. Clinical and microbiological features of acute enteric mixed infection caused by Hafnia alvei and rotavirus. Zh. Mikrobiol. Epidemiol. Immunolbiol. 2003(March-April):62-65. (In Russian.) [PubMed] [Google Scholar]
- 55.Grajwer, L. A., D. Mukhopadhyay, and B. J. Grossman. 1975. Chronic polymicrobial bacteremia. Clin. Pediatr. 14:280-283. [DOI] [PubMed] [Google Scholar]
- 56.Greipsson, S., and F. G. Priest. 1983. Numerical taxonomy of Hafnia alvei. Int. J. Syst. Bacteriol. 33:470-475. [Google Scholar]
- 57.Guichenez, P. E. Oziol, Z. Joomaye, J. Marcciano, G. Guichenez, and J. Y. Ygout. 2000. Septicémie à Hafnia alvei rélélant un pyocholécyste compliqué d'abcès hépatique chez immunocompetent. Presse Méd. 29:1704. (In French.) [PubMed] [Google Scholar]
- 58.Guinée, P. A. M., and J. J. Valkenburg. 1968. Diagnostic value of a Hafnia-specific bacteriophage. J. Bacteriol. 96:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Günthard, H., and A. Pennekamp. 1996. Clinical significance of extraintestinal Hafnia alvei isolates from 61 patients and review of the literature. Clin. Infect. Dis. 22:1040-1045. [DOI] [PubMed] [Google Scholar]
- 60.Hamana, K. 1996. Distribution of diaminopropane and acetylspermidine in Enterobacteriaceae. Can. J. Microbiol. 42:107-114. [DOI] [PubMed] [Google Scholar]
- 61.Harada, K., K. Shimizu, and T. Matsuyama. 1957. Hafnia isolated from men. Gunma J. Med. Sci. 6:109-112. [Google Scholar]
- 62.Hedegaard, J., S. A. de A. Steffensen, N. Nørskov-Lauritsen, K. K. Mortensen, and H. U. Sperling-Petersen. 1999. Identification of Enterobacteriaceae by partial sequencing of the gene encoding translation initiation factor 2. Int. J. Syst. Bacteriol. 49:1531-1538. [DOI] [PubMed] [Google Scholar]
- 63.Huys, G., M. Cnockaert, J. M. Janda, and J. Swings. 2003. Escherichia albertii sp. nov., a diarrhoeagenic species isolated from stool specimens of Bangladeshi children. Int. J. Syst. Evol. Microbiol. 53:807-810. [DOI] [PubMed] [Google Scholar]
- 64.Hyma, K. E., D. W. Lacher, A. M. Nelson, A. C. Bumbaugh, J. M. Janda, N. A. Strockbine, V. B. Young, and T. S. Whittam. 2005. Evolutionary genetics of a new pathogenic Escherichia species: Escherichia albertii and related Shigella boydii strains. J. Bacteriol. 187:619-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ismaili, A., B. Bourke, J. C. S. de Azavedo, S. Ratnam, M. A. Karmali, and P. M. Sherman. 1996. Heterogeneity in phenotypic and genotypic characteristics among strains of Hafnia alvei. J. Clin. Microbiol. 34:2973-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Izard, D., C. Ferragut, F. Gavini, and H. Leclerc. 1978. Variations of the moles percent guanine plus cytosine within a group of Enterobacteriaceae belonging or related to the genus Enterobacter. Int. J. Syst. Bacteriol. 28:449-452. [Google Scholar]
- 67.Jakowski, S., S. Rowiński, A. Cisowska, and A. Gamian. 1996. The sensitivity of Hafnia alvei strains to the bactericidal effect of serum. FEMS Immunol. Med. Microbiol. 13:59-64. [DOI] [PubMed] [Google Scholar]
- 68.Janda, J. M., and S. L. Abbott. 1998. The enterobacteria. Lippincott-Raven, Philadelphia, Pa.
- 69.Janda, J. M., S. L. Abbott, and M. J. Albert. 1999. Prototypal diarrheagenic strains of Hafnia alvei are actually members of the genus Escherichia. J. Clin. Microbiol. 37:2399-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Janda, J. M., S. L. Abbott, S. Bystrom, and W. S. Probert. 2005. The genus Hafnia: identification of two distinct hybridization groups based upon 16S rRNA gene sequencing and phenotypic methods. J. Clin. Microbiol. 43:3320-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Janda, J. M., S. L. Abbott, S. Khashe, and W. Probert. 2002. Phenotypic and genotypic properties of the genus Hafnia. J. Med. Microbiol. 51:575-580. [DOI] [PubMed] [Google Scholar]
- 72.Jennis, F., and S. W. McCarthy. 1967. Hafnia: an unusual cause of post-operative gram-negative bacteriaemia. Med. J. Aust. 1:286-287. [DOI] [PubMed] [Google Scholar]
- 73.Johnson, R., R. R. Colwell, R. Sakazaki, and K. Tamura. 1975. Numercial taxonomy study of the Enterobacteriaceae. Int. J. Syst. Bacteriol. 25:12-37. [Google Scholar]
- 74.Katzenellenbogen, E., N. A. Kocharova, G. V. Zatonsky, A. S. Shashkov, A. Korzeniowska-Kowal, A. Gamian, M. Bogulska, and Y. A. Knirel. 2005. Structure of the O-polysaccharide of Hafnia alvei strain PCM 1189 that has hexa- to octasaccharide repeating units owing to incomplete glucosylation. Carbohydr. Res. 340:263-270. [DOI] [PubMed] [Google Scholar]
- 75.Kharat, A. S. 2001. Phenotypic variability of β-glucoside utilization and its correlation to pathogenesis process in a few enteric bacteria. FEMS Microbiol. Lett. 199:241-246. [DOI] [PubMed] [Google Scholar]
- 76.Kim, S. H., K. G. Field, M. T. Morrissey, R. J. Price, C. I. Wei, and H. An. 2001. Source and identification of histamine-producing bacteria from fresh and temperature-abused albacore. J. Food Prot. 64:1035-1044. [DOI] [PubMed] [Google Scholar]
- 77.Klapholz, A., K.-D. Lennsau, B. Huang, W. Talavera, and J. F. Boyle. 1994. Hafnia alvei: respiratory tract isolates in a community hospital over a three-year period and a literature review. Chest 105:1098-1100. [DOI] [PubMed] [Google Scholar]
- 78.Kmet', J. T. V. 2003. Susceptibility of Enterobacteriaceae from the alpine accentor Prunella collaris. Acta Vet. Brno 72:285-288. [Google Scholar]
- 79.Kosako, Y., R. Sakazaki, G. P. Huntley-Carter, and J. J. Farmer III. 1987. Yokenella regensburgei and Koserella trabulsii are subjective synonyms. Int. J. Syst. Bacteriol. 37:127-129. [Google Scholar]
- 80.Leclercq, A., C. Wanegue, and P. Baylac. 2002. Comparison of fecal coliform agar and violet red bile lactose agar for fecal coliform enumeration in foods. Appl. Environ. Microbiol. 68:1631-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.LeMinor, L., and C. Coynault. 1976. Dèterminisme plasmidique du caractère atypique lactose positif de Enterobacter hafniae et de Proteus morganii. Ann. Microbiol. (Inst. Pasteur) 127A:213-221. [PubMed] [Google Scholar]
- 82.Lindberg, A.-M., Å. Ljungh, S. Ahrné, S. Löfdahl, and G. Molin. 1998. Enterobacteriaceae found in high numbers in fish, minced meat and pasteurized milk or cream and the presence of toxin encoding genes. Int. J. Food Microbiol. 39:11-17. [DOI] [PubMed] [Google Scholar]
- 83.Lutz, B., R. Ratard, D. Dodson, J. M. Malecki, A. C. Morse, S. Wiersma, and D. Perrotta. 2001. Septic arthritis following anterior cruciate ligament reconstruction using tendon allografts—Florida and Louisiana, 2000. Morb. Mortal. Wkly. Rep. 50:1081-1083. [PubMed] [Google Scholar]
- 84.Masaki, H., M. Ide, S. Mochinaga, Y. Kawachi, K. Fukuda, N. Tsukamoto, K. Watanabe, A. Takahashi, T. Nagatake, and K. Matsumoto. 1991. Hafnia alvei septicemia with shock and DIC in an adult with postoperative lung cancer. Kansenshogaku Zasshi. 65:1205-1210. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 85.Matsumoto, H. 1963. Studies on the Hafnia isolated from normal human. Jpn. J. Microbiol. 7:105-114. [PubMed] [Google Scholar]
- 86.Mcbee, M. E., and D. B. Schauer. 2004. The genus Hafnia. In M. Dworkin (ed.), The prokaryotes: an evolving electronic resource for the microbiological community. Release 3.17. [Online.] http://141.150.157.117:8080/prokPUB/index.htm. Springer-Verlag, New York, N.Y.
- 87.Mobley, D. F. 1971. Hafnia septicemia. South. Med. J. 64:505-506. [DOI] [PubMed] [Google Scholar]
- 88.Mojtabee, A., and A. Siadati. 1978. Enterobacter hafnia meningitis. J. Pediatr. 93:1062-1063. [DOI] [PubMed] [Google Scholar]
- 89.Møller, V. 1954. Distribution of amino acid decarboxylases in Enterobacteriaceae. Acta Pathol. Microbiol. Scand. 35:259-277. [DOI] [PubMed] [Google Scholar]
- 90.Morales, P., E. Fernández-Garcia, and M. Nuñez. 2003. Caseinolysis in cheese by Enterobacteriaceae strains of dairy origin. Lett. Appl. Microbiol. 37:410-414. [DOI] [PubMed] [Google Scholar]
- 91.Mukherjee, S. R., A. M. Das, V. L. Paranjape, and S. R. Marwah. 1986. Hafnia alvei isolated from an equine aborted foetus. Indian. J. Vet. Med. 6:101-102. [Google Scholar]
- 92.Nakaya, T., S. Hanada, and Y. Yagami. 1990. Implications of maternal blood endotoxins and preterm labor. Int. J. Feto-Maternal Med. 3:196-204. [Google Scholar]
- 93.Newmark, J. J., W. N. Hobbs, and B. E. Wilson. 1994. Reactive arthritis associated with Hafnia alvei enteritis. Arthritis Rheum. 37:960-962. [DOI] [PubMed] [Google Scholar]
- 94.O'Hara, C. M., and J. M. Miller. 2000. Evaluation of the MicroScan Rapid Neg ID3 panel for identification of Enterobacteriaceae and some common gram-negative nonfermenters. J. Clin. Microbiol. 38:3577-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O'Hara, C. M., and J. M. Miller. 2003. Evaluation of the Vitek 2 ID-GNB assay for identification of members of the family Enterobacteriaceae and other nonenteric gram-negative bacilli and comparison with Vitek GNI+ card. J. Clin. Microbiol. 41:2096-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O'Hara, C. M., G. L. Westbrook, and J. M. Miller. 1997. Evaluation of Vitek GNI+ and Becton Dickinson Microbiology Systems Crystal E/NF identification systems for identification of members of the family Enterobacteriaceae and other gram-negative, glucose-fermenting and non-glucose-fermenting bacilli. J. Clin. Microbiol. 35:3269-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Okada, S., and D. M. Gordon. 2001. Host and geographical factors influence the thermal niche of enteric bacteria isolated from native Australian mammals. Mol. Ecol. 10:2499-2513. [DOI] [PubMed] [Google Scholar]
- 98.Okada, S., and D. M. Gordon. 2003. Genetic and ecological structure of Hafnia alvei in Australia. Syst. Appl. Microbiol. 26:585-594. [DOI] [PubMed] [Google Scholar]
- 99.Ootsubo, M., T. Shimizu, R. Tanaka, T. Sawabe, K. Tajima, M. Yoshimizu, Y. Ezura, T. Ezaki, and H. Oyaizu. 2002. Oligonucleotide probe for detecting Enterobacteriaceae by in situ hybridization. J. Appl. Microbiol. 93:60-68. [DOI] [PubMed] [Google Scholar]
- 100.Österblad, M., O. Pensala, M. Peterzéns, H. Heleniusc, and P. Huovinen. 1999. Antimicrobial susceptibility of Enterobacteriaceae isolated from vegetables. J. Antimicrob. Chemother. 43:503-509. [DOI] [PubMed] [Google Scholar]
- 101.Piddock, L. J. V., R. N. Walters, Y.-F. Jin, H. L. Turner, D. M.Gascoyne-Binzi, and P. M. Hawkey. 1997. Prevalence and mechanism of resistance to ‘third-generation’ cephalosporins in clinically relevant isolates of Enterobacteriaceae from 43 hospitals in the UK, 1990-1991. J. Antimcrob. Chemother. 39:177-187. [DOI] [PubMed] [Google Scholar]
- 102.Podschun, R., A. Fischer, and U. Ullmann. 2001. Characterisation of Hafnia alvei isolates from human clinical extra-intestinal specimens: haemagglutinins, serum resistance and siderophore synthesis. J. Med. Microbiol. 50:208-214. [DOI] [PubMed] [Google Scholar]
- 103.Proietti, P. C., F. Passamonti, M. P. Franciosini, and G. Asdrubali. 2004. Hafnia alvei infection in pullets in Italy. Avian Pathol. 33:200-204. [DOI] [PubMed] [Google Scholar]
- 104.Puicini, C., S. Roth, E. Bernard, F. Vandenbos, J.-L. Bernard, and P. Dellamonica. 2003. Bactériémie à Hafnia alvei avec abcès hépatique et musculaire. Presse Méd. 32:1026-1027. (In French.) [PubMed] [Google Scholar]
- 105.Ramos, A., and D. Dámaso. 2000. Extraintestinal infection due to Hafnia alvei. Eur. J. Clin. Microbiol. Infect. Dis. 19:708-710. [DOI] [PubMed] [Google Scholar]
- 106.Ratnam, S. 1991. Etiologic role of Hafnia alvei in human diarrheal illness. Infect. Immun. 59:44-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ratnam, S., R. W. Butler, S. March, S. Parsons, P. Clarke, A. Bell, and K. Hogan. 1979. Enterobacter hafniae—Newfoundland. Can. Dis. Wkly. Rep. 5:231-232. [Google Scholar]
- 108.Real, F., A. Fernández, F. Acosta, B. Acosta, P. Castro, S. Déniz, and J. Orós. 1997. Septicemia associated with Hafnia alvei in laying hens. Avian Dis. 41:741-747. [PubMed] [Google Scholar]
- 109.Reina, J., J. Hervas, and N. Borrell. 1993. Acute gastroenteritis caused by Hafnia alvei in children. Clin. Infect. Dis. 16:443. [DOI] [PubMed] [Google Scholar]
- 110.Rhodes, A. N., J. W. Urbance, H. Youga, H. Corlew-Newman, C. A. Reddy, M. J. Klug, J. M. Tiedje, and D. C. Fisher. 1998. Identification of bacterial isolates obtained from intestinal contents associated with 12,000-year-old mastodon remains. Appl. Environ. Microbiol. 64:651-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rice, E. W., C. H. Johnson, M. E. Dunnigan, and D. J. Reasoner. 1993. Rapid glutamate decarboxylase assay for detection of Escherichia coli. Appl. Environ. Microbiol. 59:4347-4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Richards, M. J., J. R. Edwards, D. H. Culver, R. P. Gaynes, and the National Nosocomial Infection Surveillance System. 2000. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 21:510-515. [DOI] [PubMed] [Google Scholar]
- 113.Ridell, J., and H. Korkeala. 1997. Minimum growth temperatures of Hafnia alvei and other Enterobacteriaceae isolated from refrigerated meat determined with a temperature gradient incubator. Int. J. Food Microbiol. 35:287-292. [DOI] [PubMed] [Google Scholar]
- 114.Ridell, J., A. Siitonen, L. Paulin, O. Lindroos, H. Korkeala, and M. J. Albert. 1995. Characterization of Hafnia alvei by biochemical tests, random amplified polymorphic DNA PCR, and partial sequencing of 16S rRNA gene. J. Clin. Microbiol. 33:2372-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ridell, J., A. Siitonen, L. Paulin, L. Mattila, H. Korkeala, and M. J. Albert. 1994. Hafnia alvei in stool specimens from patients with diarrhea and healthy controls. J. Clin. Microbiol. 32:2335-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rodríguez, L. A., J. Vivas, C. S. Gallardo, F. Acosta, L. Babbeyto, and F. Real. 1999. Identification of Hafnia alvei with MicroScan WalkAway system. J. Clin. Microbiol. 37:4186-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Romanowska, E. 2000. Immunochemical aspects of Hafnia alvei O antigens. FEMS Immunol. Med. Microbiol. 27:219-225. [DOI] [PubMed] [Google Scholar]
- 118.Ruiz-Moreno, J. M., J. L. Alió, and F. de la Hoz. 2001. Delayed-onset postoperative endophthalmitis caused by Hafnia alvei. Eur. J. Ophthalmol. 11:189-192. [DOI] [PubMed] [Google Scholar]
- 119.Rutz, J. M. T. Abdullah, S. P. Singh, V. I. Kalve, and P. E. Klebba. 1991. Evolution of the ferric enterobactin receptor in gram-negative bacteria. J. Bacteriol. 173:5964-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sakazaki, R. 1961. Studies on the Hafnia group of Enterobacteriaceae. Jpn. J. Med. Sci. Biol. 14:223-241. [DOI] [PubMed] [Google Scholar]
- 121.Sakazaki, R. 1981. The genus Hafnia, p. 1181-1186. In M. P. Starr, H. Stolp, H. G. Trüper, A. Balows, and H. G. Schlegel (ed.), The prokaryotes: a handbook on habitats, isolation, and identification of bacteria. Springer-Verlag, Berlin, Germany.
- 122.Sakazaki, R. 1984. Hafnia, p. 484-486. In N. R. Krieg, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams & Wilkins, Baltimore, Md. [Google Scholar]
- 123.Sakazaki, R. 2005. Genus Hafnia Møller 1954, p. 681-685. In D. Brenner, N. Krieg, J. T. Staley, and G. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed. Springer, New York, N.Y.
- 124.Sakazaki, R., and K. Tamura. 1991. The genus Hafnia, p. 2816-2821. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes: a handbook on the biology of bacteria ecophysiology, isolation, and identification, applications. Springer-Verlag, Berlin, Germany.
- 125.Salimov, R. M. 1978. Study of the Hafnia isolated from honey. Veterinariia 1978(April):44-46. (In Russian.) [PubMed] [Google Scholar]
- 126.Seral, C., F. J. Castillo, M. T. Llorente, M. Varea, A. Clavel, M. C. Rubio, and R. Gómez-Lus. 2001. The eaeA gene is not found in Hafnia alvei from patients with diarrhea in Aragón, Spain. Int. Microbiol. 4:81-82. [DOI] [PubMed] [Google Scholar]
- 127.Sharma, R. K., B. R. Boro, and P. Borah. 1991. Incidence of caprine pneumonia and associated bacterial species. Indian J. Anim. Sci. 61:54-55. [Google Scholar]
- 128.Sherley, M., D. M. Gordon, and P. J. Collignon. 2003. Species differences in plasmid carriage in the Enterobacteriaceae. Plasmid 49:79-85. [DOI] [PubMed] [Google Scholar]
- 129.Skerman, V. B. D., V. McGowan, and P. H. A. Sneath. 1980. Approved lists of bacterial names. Int. J. Syst. Bacteriol. 30:225-420. [Google Scholar]
- 130.Spröer, C., U. Mendrock, J. Swiderski, E. Lang, and E. Stackebrandt. 1999. The phylogenetic position of Serratia, Buttiauxella and some other genera of the family Enterobacteriaceae. Int. J. Syst. Evol. Bacteriol. 49:1433-1438. [DOI] [PubMed] [Google Scholar]
- 131.Steigerwalt, A., G. R. Fanning, M. A. Fife-Asbury, and D. J. Brenner. 1976. DNA relatedness among species of Enterobacter and Serratia. Can. J. Microbiol. 22:121-137. [DOI] [PubMed] [Google Scholar]
- 132.Stock, I., M. Rahman, K. J. Sherwood, and B. Wiedemann. 2005. Natural antibiotic susceptibility patterns and biochemical identification of Escherichia albertii and Hafnia alvei strains. Diagn. Microbiol. Infect. Dis. 51:151-163. [DOI] [PubMed] [Google Scholar]
- 133.Stock, I., K. J. Sherwood, and B. Wiedemann. 2004. Antimicrobial susceptibility patterns, β-lactamases, and biochemical identification of Yokenella regensburgei. Diagn. Microbiol. Infect. Dis. 48:5-15. [DOI] [PubMed] [Google Scholar]
- 134.Stuart, C. A., and R. Rustigian. 1943. Further studies on one type of Paracolon organism. Am. J. Public Health 33:1323-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stuart, C. A., K. M. Wheeler, R. Rustigian, and A. Zimmerman. 1943. Biochemical and antigenic relationships of the paracolon bacteria. J. Bacteriol. 45:101-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thomson, K. S., C. C. Sanders, and J. A. Washington II. 1993. Ceftazidime resistance in Hafnia alvei. Antimicrob. Agents Chemother. 37:1375-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Oye, E., and G. Ghysels. 1961. Antigenic relationship between Hafnia and Shigella flexneri. J. Bacteriol. 82:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Washington, J. A., II, R. J. Birk, and R. E. Ritts, Jr. 1971. Bacteriologic and epidemiologic characteristics of Enterobacter hafniae and Enterobacter liquefaciens. J. Infect. Dis. 124:379-386. [DOI] [PubMed] [Google Scholar]
- 139.Wertz, J. E., C. Goldstone, D. M. Gordon, and M. A. Riley. 2003. A molecular phylogeny of enteric bacteria and implications for a bacterial species concept. J. Evol. Biol. 16:1236-1248. [DOI] [PubMed] [Google Scholar]
- 140.Wertz, J. E., and M. A. Riley. 2004. Chimeric nature of two plasmids of Hafnia alvei encoding bacteriocins alveicins A and B. J. Bacteriol. 186:1598-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Westblom, T. U., and T. W. Milligan. 1992. Acute bacterial gastroenteritis caused by Hafnia alvei. Clin. Infect. Dis. 14:1271-1272. [DOI] [PubMed] [Google Scholar]
- 142.Winsor, D. K., A. P. Bloebaum, and J. J. Mathewson. 1981. Gram-negative, aerobic, enteric pathogens among intestinal microflora of wild turkey vultures (Cathartes aura) in West Central Texas. Appl. Environ. Microbiol. 42:1123-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Winstanley, T. G., D. I. Limb, P. F. Wheat, and C. D. Nicol. 1993. Multipoint identification of Enterobacteriaceae: report of the British Society for Microbial Technology collaborative study. J. Clin. Pathol. 46:637-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wiström, J., T. Myrnäs, C. Lundgrren, and T. Monsen. 1998. A case of acute cholecystitis due to Aeromonas sobria and Hafnia alvei from northern Europe. Clin. Microbiol. Infect. 4:607-609. [DOI] [PubMed] [Google Scholar]
- 145.Yeager, A. M., M. E. Kanof, S. S. Kramer, B. Jones, R. Saral, A. M. Lake, and G. W. Santos. 1987. Pneumatosis intestinalis in children after allogeneic bone marrow transplantation. Pediatr. Radiol. 17:18-22. [DOI] [PubMed] [Google Scholar]