Abstract
The surface levels of major histocompatibility complex class I antigens are diminished on tumorigenic adenovirus type 12 (Ad12)-transformed cells, enabling them to escape from immunosurveillant cytotoxic T lymphocytes (CTLs). This is due to the down-regulation of the class I transcriptional enhancer, in which there is strong binding of the repressor COUP-TFII and lack of binding of the activator NF-κB. Even though NF-κB (p65/p50) translocates to the nuclei of Ad12-transformed cells, it fails to bind to DNA efficiently due to the hypophosphorylation of the p50 subunit. In this study, tumor necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β) were shown to promote degradation of the NF-κB cytoplasmic inhibitor IκBα and permit the nuclear translocation of a phosphorylated form of NF-κB that is capable of binding DNA. Interestingly, when Ad12-transformed cells were treated with TNF-α or IL-1β, class I gene transcription substantially increased when transcriptional repression by COUP-TFII was blocked. This indicates that in cytokine-treated Ad12-transformed cells, COUP-TFII is able to repress activation of class I transcription by newly nucleus-localized NF-κB. Our results suggest that Ad12 likely employs a “fail-safe” mechanism to ensure that the transcription of class I genes remains tightly repressed under various physiological conditions, thus providing tumorigenic Ad12-transformed cells with a means of escaping CTL recognition and lysis.
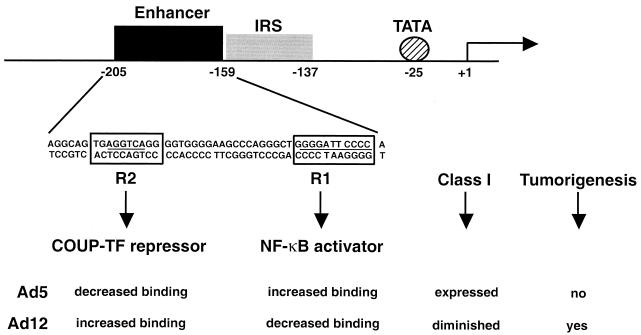
Cell surface major histocompatibility complex (MHC) class I antigen levels are significantly diminished in tumorigenic adenovirus type 12 (Ad12)-transformed cells compared to levels in nontumorigenic Ad5-transformed cells, and this effect is solely regulated by Ad12 E1A (6, 34, 40). In Ad12-transformed cells, the repression of all class I genes occurs at the level of transcription (2, 8). The class I promoter elements consist of a canonical TATA box, an interferon response sequence, and a 47-bp enhancer (19). As shown in Fig. 1, the class I enhancer has a consensus binding site (R1) for the transcription activator NF-κB and a second binding site (R2) for nuclear hormone receptor family members, including the transcription repressor COUP-TFII. In Ad12-transformed cells, binding of NF-κB to the R1 site is diminished (1, 26) while binding of COUP-TFII to the R2 site is elevated (27). The converse occurs in Ad5-transformed cells (1, 26, 27), which results in a major reduction in class I transcription and expression of cell surface class I antigens on Ad12-transformed cells. The low levels of class I antigen contribute to the tumorigenic potential of Ad12-transformed cells by allowing them to evade detection and lysis by cytotoxic T lymphocytes (CTLs).
FIG. 1.
COUP-TFII and NF-κB binding to the MHC class I enhancer affects class I transcription and tumorigenesis in adenovirus-transformed cells. MHC class I transcription is diminished in Ad12- compared to that in Ad5-transformed cells, which contributes to their tumorigenic potential. In Ad12-transformed cells, binding of the repressor COUP-TF to the R2 site is increased and binding of the activator NF-κB to the R1 site of the class I enhancer is decreased. Bent arrow, transcriptional start site. IRS, IFN response sequence.
Recent findings have provided insight into how COUP-TFII functions as a repressor of class I transcription in Ad12-transformed cells (37, 38). COUP-TFII binds strongly to the R2 site of the class I enhancer as a homodimer and associates with the nuclear corepressor (N-CoR) and histone deacetylase (HDAC) (37, 38), which is known to repress transcription by maintaining chromatin in a condensed conformation (4, 37, 38). This repressive effect of COUP-TFII can be relieved by the HDAC inhibitor trichostatin A (TSA) (38). COUP-TFII may also repress gene transcription through interacting with the preinitiation complex component TFIIB (14, 33).
Recent studies have also revealed why NF-κB fails to bind DNA in Ad12-transformed cells. In the classical regulatory pathway, the NF-κB heterodimer, consisting of p50 (NF-κB1) and p65 (RelA), is retained in the cytoplasm by IκB (11, 18). After IκB becomes phosphorylated by an IκB kinase complex in response to a variety of stimuli, including UV, mitogens, cytokines, and bacterial and viral products (42), it is ubiquitinated and subsequently degraded by the 26S proteasome. NF-κB is no longer arrested in the cytoplasm and is able to translocate to the nucleus, where it binds DNA promoters and stimulates transcription of arrays of genes involved in immune, antiapoptotic, developmental, and other physiological responses (3, 25, 32). Ad12-transformed cells are unusual in that NF-κB (p65/p50) translocates to the nucleus but is unable to bind DNA (26). In Ad12-transformed cells, it was recently discovered that the nuclear p50 subunit is hypophosphorylated, which accounts for the diminished DNA binding activity of NF-κB (22).
Here we inquired whether in Ad12-transformed cells, NF-κB inducers are able to stimulate an active form of nuclear NF-κB which can bind DNA and stimulate class I transcription. We show that two cytokines, tumor necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β), can induce newly nucleus-localized NF-κB, which is phosphorylated and capable of binding DNA. However, these cytokines were able to significantly stimulate class I gene transcription only when COUP-TFII repression was relieved.
MATERIALS AND METHODS
Cell lines.
Cell lines transformed with Ad5 (DP5-2) and Ad12 (12-1) were derived from Hooded Lister rats (15, 16). Cells were grown in minimal essential medium α (Gibco-BRL) supplemented with 10% fetal bovine serum (HyClone), 2 mM l-glutamine, 100 U of penicillin per ml, 0.1 mg of streptomycin per ml, and 2.5 μg of amphotericin B (Fungizone) per ml.
Reagents.
Trichostatin A (TSA) was purchased from Biomol. Recombinant rat TNF-α, IL-1β, IL-2, and human platelet-derived growth factor (PDGF) were purchased from Biosource. Recombinant rat gamma interferon (IFN-γ) and phorbol myristate acetate (PMA) were purchased from Gibco. Lipopolysaccharide (LPS) was from Sigma. MG-132 was purchased from Calbiochem.
Nuclear- and cytoplasmic-extract preparation.
Nuclear and cytoplasmic extracts were prepared as previously described (22). Both nuclear and cytoplasmic extracts were dialyzed for 1 h at 4°C in Shapiro's buffer D (20 mM HEPES [pH 7.9], 20% glycerol, 100 mM KCl, 0.2 mM EDTA, 0.2 mM EGTA, 2 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 0.5 mg of leupeptin per liter, 0.7 mg of pepstatin A per liter). For experiments involving calf intestinal alkaline phosphatase (CIP) treatment, nuclear extracts were dialyzed in buffer D without EDTA and EGTA.
Western blotting and antisera.
Western blotting was performed as previously described (22). Anti-p50 antibody 1157, anti-p65 antibody 1226, and anti-IκB-α antibody 751 were kindly provided by Nancy Rice. Monoclonal β-actin antibody (AC-15) was purchased from Sigma.
Electrophoretic mobility shift assay (EMSA).
A double-spaced oligonucleotide probe (5′ tcgAGGGCTGGGGATTCCCCATCTg 3′; bases in lowercase are overhung ends that do not anneal to the other strand) of the MHC class I R1 enhancer site, which has a recognition sequence for NF-κB, was labeled with [α-32P]dCTP (3,000 Ci/mmol; New England Nuclear) and Klenow enzyme (Boehringer Mannheim). DNA band-shifts were analyzed as previously described (20).
Two-dimensional (2-D) electrophoresis.
Proteins from nuclear extracts were separated with a pH isoelectric gradient of 3.5 to 10 in the first dimension and a 10% acrylamide gel in the second dimension (Kendrick Labs). Proteins were transferred to polyvinylidene difluoride membranes and probed by Western blotting.
Total RNA preparation and Northern blotting.
12-1 cells were treated for 48 h with either TNF-α (10 ng/ml) or IL-1β (5 ng/ml) alone or in combination with TSA (150 nM). Total RNA was extracted with an RNeasy (Qiagen) kit by following the manufacturer's instructions. A mouse MHC class I Kk gene cDNA sequence was used as the probe for Northern blotting and was labeled with [α-32P]dCTP by random priming. Twenty micrograms of total RNA was hybridized with an MHC class I probe according to standard procedures.
Cell transfection and CAT assays.
Transfections of 12-1 cells were performed with GeneJammer transfection reagent (Stratagene) according to the manufacturer's instructions. Chloramphenicol acetyltransferase (CAT) assays were performed essentially as described previously (9, 27).
RESULTS
TNF-α and IL-1β increase nuclear translocation of NF-κB through degradation of IκBα.
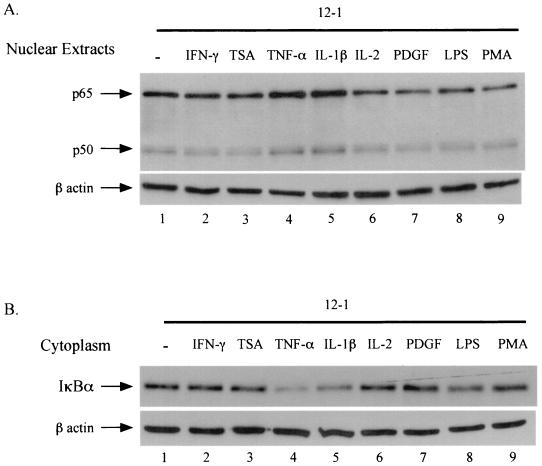
Ad12- and Ad5-transformed cells have similar levels of nuclear NF-κB. However, unlike in Ad5-transformed cells, nuclear Ad12 NF-κB is hypophosphorylated and fails to bind to DNA efficiently (22). In order to further understand this phenomenon in Ad12-transformed cells, we investigated whether there are inducers which can stimulate NF-κB nuclear translocation, DNA binding activity, and transactivation. The Ad12-transformed cell line 12-1 was used since it is extensively studied and is representative of all our Ad12-transformed cell lines (20, 21, 27). The 12-1 cells were treated with TNF-α, IL-1β, IL-2, PDGF, LPS, and PMA under conditions previously described for these well-studied NF-κB inducers (31). Nuclear extracts were prepared from treated 12-1 cells and assayed for nuclear NF-κB protein levels by Western blotting. As shown in Fig. 2A, of the NF-κB inducers tested, only TNF-α (lane 4) and IL-1β (lane 5) produced increases, albeit modest, in the constitutive levels of both p65 and p50 nuclear proteins (compare lane 1 with lanes 4 and 5). By comparison, the inducers IL-2 (lane 6), PDGF (lane 7), LPS (lane 8), and PMA (lane 9) failed to alter the levels of nuclear p65/p50 NF-κB. In order to substantiate the result shown in Fig. 2A, the corresponding cytoplasmic fractions were examined for accompanying decreases in IκBα protein levels, since NF-κB inducers stimulate the nuclear translocation of NF-κB through degradation of cytoplasmic IκB. As expected, the levels of IκBα from TNF-α- and IL-1β-treated 12-1 cells were reduced (Fig. 2B, compare lane 1 with lanes 4 and 5) whereas the levels of IL-2, PDGF, and PMA were unchanged (lanes 6, 7, and 9). LPS showed a marginal decrease in cytoplasmic IκB (lane 8). IFN-γ and TSA are known to stimulate MHC class I expression through mechanisms not involving NF-κB nuclear translocation and DNA binding (5, 17, 38, 39). This fact is consistent with the results seen in Fig. 2 (lanes 2 [IFN-γ] and 3 [TSA]), in which it can be seen that treatment with IFN-γ or TSA failed to change the levels of nuclear NF-κB and cytoplasmic IκB.
FIG. 2.
TNF-α and IL-1β increase nuclear translocation of NF-κB through degradation of IκBα in Ad12-transformed cells. The Ad12-transformed cell line 12-1 was treated with rat IFN-γ (25 U/ml, 30 min), TSA (150 nM, 120 min), rat TNF-α (10 ng/ml, 30 min), rat IL-1β (5 ng/ml, 30 min), rat IL-2 (5 g/ml, 120 min), human PDGF (5 ng/ml, 120 min), LPS (10 μg/ml, 120 min), and PMA (1 μg/ml, 30 min). Nuclear and cytoplasmic extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by Western blotting. Monoclonal β-actin antibody recognizes a 42-kDa protein, β-actin, and proves the presence of equal amounts of β-actin protein. Shown are Western blots of nuclear extracts probed with p50, p65, and β-actin antibodies (A) and cytoplasmic extracts probed with IκBα and β-actin antibody (B).
NF-κB DNA binding activity is dramatically increased by cytokine treatment.
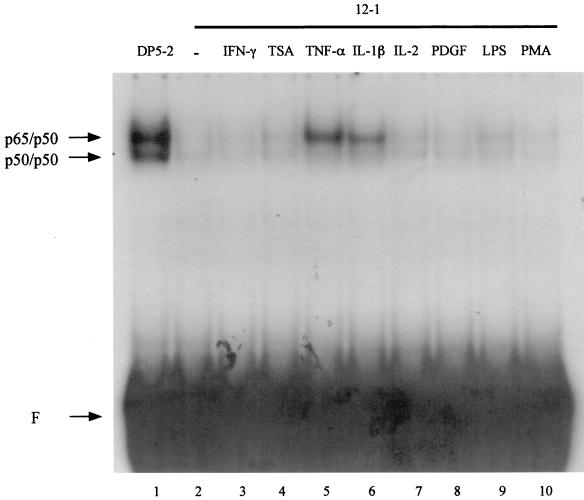
Although a significant amount of both the p65 and p50 subunits of NF-κB reside in the nuclei of 12-1 cells, NF-κB complexes fail to bind to DNA efficiently (1, 21, 26, 29, 30). This can be observed by comparing DNA band-shifts obtained by using a labeled NF-κB oligonucleotide probe with nuclear extracts from 12-1 cells and Ad5 DP5-2-transformed cells. Clearly, as seen from the EMSA results in Fig. 3, nuclear extracts from 12-1 cells, which have levels of nuclear NF-κB comparable to those in DP-2 cells (26), exhibited negligible DNA binding activity (compare lanes 1 and 2). We asked whether treatment of 12-1 cells with TNF-α and IL-1β, which led to an increase in the protein levels of nuclear p65 and p50 (Fig. 2), would also result in an increase in the DNA binding activity of NF-κB. An EMSA analysis of 12-1 nuclear extracts showed that TNF-α and IL-1β treatment dramatically increased the DNA binding activity of NF-κB (Fig. 3, lanes 5 and 6). By contrast, there was no change in DNA binding activity in response to treatment with IFN-γ, TSA, IL-2, PDGF, or PMA (Fig. 3, lanes 3, 4, 7, 8, and 10, respectively), consistent with their inability to affect the levels of nuclear p65 and p50 (Fig. 2A).
FIG. 3.
TNF-α and IL-1β dramatically increase NF-κB DNA binding in Ad12-transformed cells. 12-1 cells were treated by using the same conditions as those described for Fig. 2. Nuclear extracts were analyzed by EMSA for NF-κB DNA binding activity to the R1 probe. Positions for p50/p50 homodimers and p65/p50 heterodimers are indicated. F, free probe.
It is apparent that the dramatic increase of NF-κB DNA binding activity by TNF-α and IL-1β (Fig. 3, compare lane 2 with lanes 5 and 6) exceeds the relatively modest increase in nuclear p50 and p65 protein levels (Fig. 2A, compare lane 1 with lanes 4 and 5). This paradox might be explained if the increased levels of nuclear p50 and p65 induced by TNF-α or IL-1β constitute a new pool of NF-κB with an elevated DNA binding affinity. An alternative explanation may be that the preexisting population of nuclear NF-κB, which is unable to bind to DNA efficiently, becomes activated following TNF-α and IL-1β treatment.
Cytokine-induced DNA binding activity of NF-κB is attributed to newly nucleus-translocated NF-κB.
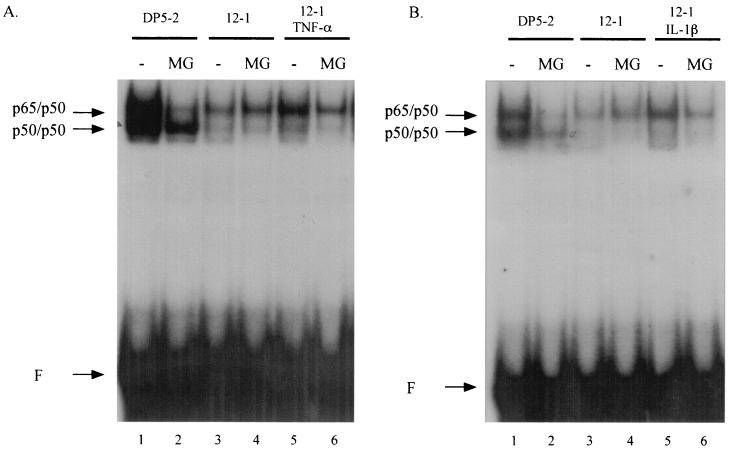
To distinguish whether the increase in DNA binding activity caused by TNF-α and IL-1β is derived from the preexisting pool of nuclear NF-κB or from the newly nucleus-localized NF-κB, we blocked the nuclear translocation of NF-κB with MG-132. MG-132 is a well-characterized 26S proteasome inhibitor which prevents degradation of cytoplasmic IκB following its phosphorylation and ubiquitination. As a consequence of this inhibitory action by MG-132, NF-κB remains complexed with IκB and its nuclear translocation is precluded (7). 12-1 cells were pretreated with MG-132 for 1 h prior to the addition of TNF-α and IL-1β. Nuclear extracts were subsequently prepared and analyzed for NF-κB DNA binding activity by EMSA. Pretreatment with MG-132 was found to substantially reduce the NF-κB DNA binding activity induced by TNF-α (Fig. 4A, compare lanes 5 and 6) and IL-1β (Fig. 4B, compare lanes 5 and 6). These results demonstrate that the increase in DNA binding activity induced by TNF-α and IL-1β in 12-1 cells is attributed to newly nucleus-localized NF-κB and that the preexisting nuclear pool of NF-κB remained incapable of binding DNA efficiently. Interestingly, the constitutively high level of NF-κB DNA binding activity in Ad5-transformed DP5-2 cells was dramatically reduced upon treatment with MG-132 (Fig. 4, compare lanes 1 and lanes 2). This result indicates that the high level of NF-κB DNA binding activity in Ad5-transformed cells is maintained through continuous IκB degradation and NF-κB nuclear translocation.
FIG. 4.
The increase in the DNA binding activity of NF-κB by TNF-α and IL-1β is attributed to newly nucleus-translocated NF-κB. DP5-2 and 12-1 cells were pretreated with 50 μg of MG-132 per ml for 1 h. 12-1 cells were then treated with TNF-α (A) or IL-1β (B). Nuclear extracts were analyzed by EMSA for NF-κB DNA binding activity to the R1 probe. Positions for p50/p50 homodimers and p65/p50 heterodimers are indicated. F, free probe.
DNA binding of cytokine-induced NF-κB requires phosphorylation.
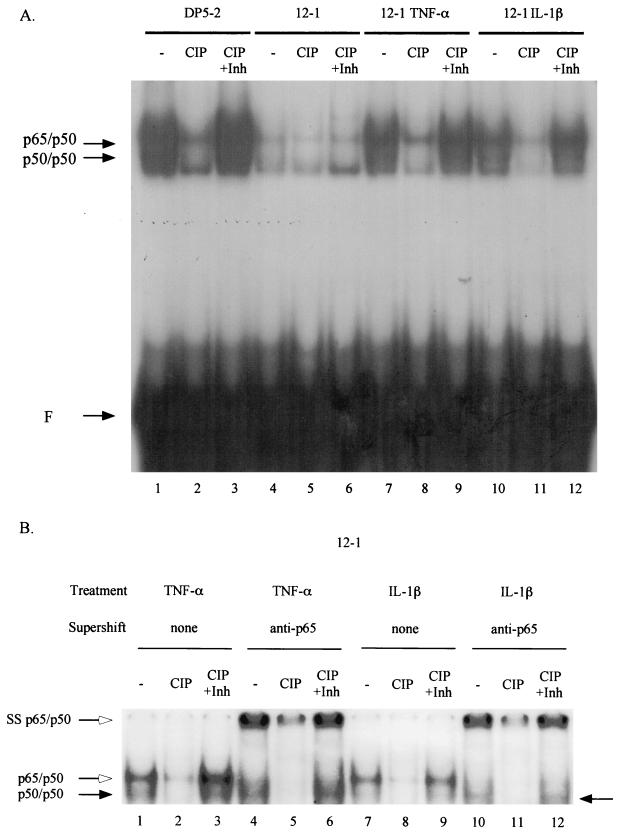
It was previously shown that treatment with TNF-α or IL-1β leads to hyperphosphorylation of both the NF-κB p50 and p65 subunits (24, 36, 41). Earlier work had shown that phosphorylation of the NF-κB p50 subunit is required for efficient DNA binding in both Ad12-transformed cells (22) and Jurkat cells (23). We inquired whether the increased DNA binding activity of the newly nucleus-localized NF-κB following TNF-α and IL-1β treatment is dependent on NF-κB phosphorylation. Nuclear extracts from TNF-α- and IL-1β-treated 12-1 cells were incubated with CIP in the presence or absence of phosphatase inhibitors and then analyzed by EMSA for NF-κB DNA binding activity. As shown in Fig. 5A, CIP treatment greatly reduced the NF-κB DNA binding activity of nuclear extracts from TNF-α- and IL-1β-treated 12-1 cells (compare lane 7 with lane 8 and lane 10 with lane 11). Phosphatase inhibitors completely negated the effects of CIP (lanes 9 and 12). These results demonstrate that in Ad12-transformed cells, the form of NF-κB that translocates to the nucleus following induction by TNF-α and IL-1β is phosphorylated, a modification which enables it to bind to DNA with an efficiency far greater than that of the preexisting nuclear pool of unphosphorylated NF-κB. In the same fashion, CIP treatment also diminished NF-κB DNA binding activity of DP5-2 nuclear extracts (compare lanes 1 to 3).
FIG. 5.
The increase in DNA binding activity of NF-κB by TNF-α and IL-1β requires phosphorylation of NF-κB. (A) 12-1 cells were either untreated or treated with TNF-α or IL-1β. Nuclear extracts from untreated and cytokine-treated 12-1 cells were prepared in buffer D without EDTA and EGTA. The extracts were either mock treated (lanes 1, 4, 7, and 10) or treated with CIP for 1 h in the absence (lanes 2, 5, 8, and 11) or in the presence (lanes 3, 6, 9, and 12) of phosphatase inhibitors (1 mM Na3VO4, 10 mM NaF, 0.5 mM EDTA) and then analyzed by EMSA for NF-κB DNA binding activity to the R1 probe. Positions for p50/p50 homodimers and p65/p50 heterodimers are indicated. F, free probe; Inh, inhibitor. (B) Anti-p65 antibody 1226 was used to supershift p65/p50 heterodimer-DNA complex. The position for supershifted p65/p50 heterodimers is indicated (SS p65/p50).
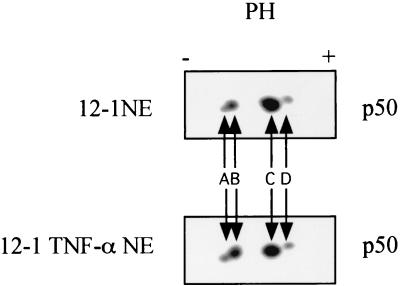
We previously showed that the NF-κB p50 subunit is hypophosphorylated and responsible for the decreased NF-κB DNA binding in Ad12-transformed cells (22). We examined whether the cytokines induce NF-κB DNA binding, in part, through the phosphorylation of p50. This suggestion was supported by 2-D gel analysis that showed an increase in the most negatively charged form of p50 in nuclear extracts derived from TNF-α-treated Ad12-transformed cells (Fig. 6, compare spots A and B). To clearly demonstrate the specific contribution of p50 phosphorylation to DNA binding upon cytokine induction, we supershifted the p65/p50 heterodimer-DNA complex to better reveal the p50/p50 homodimer-DNA complex. As shown in Fig. 5B, CIP treatment greatly reduced the p50/p50 DNA binding activities in nuclear extracts from TNF-α- and IL-1β-treated 12-1 cells (compare lane 4 with lane 5 and lane 10 with lane 11). Phosphatase inhibitors completely negated the effects of CIP on p50/p50 DNA binding (lanes 6 and 12). These results indicate that cytokine-induced NF-κB DNA binding depends, at least partially, on the phosphorylation of the p50 subunit. Our results are in accord with those of Li et al., in whose work PMA-induced p50 phosphorylation was shown to be important for the binding of NF-κB in Jurkat cells (23).
FIG. 6.
2-D electrophoresis reveals that cytokine treatment of Ad12-transformed cells increases the levels of the most negatively charged species of p50. Nuclear extracts from untreated and TNF-α-treated 12-1 cells were resolved by 2-D electrophoresis, transferred to PVDF membranes, and probed with p50 antibody 1157. There are four major p50 species revealed by Western blotting, indicated as A, B, C, and D.
In Ad12-transformed cells, MHC class I transcription is stimulated by TNF-α and IL-1β when COUP-TF repression is relieved.
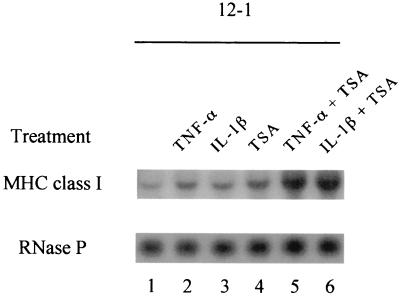
In Ad12-transformed cells, class I transcription is down-regulated as a consequence of decreased binding of the activator NF-κB and increased binding of the repressor COUP-TFII to the class I enhancer (1, 26, 27). Since treatment of Ad12-transformed cells with TNF-α or IL-1β greatly enhanced NF-κB DNA binding activity, it was essential to investigate whether these cytokines would also increase class I gene transcription. A Northern blot analysis of RNAs from cytokine-treated 12-1 cells was probed with a mouse MHC class I gene. As shown in Fig. 7, the levels of class I mRNA in 12-1 cells (lane 1), which are known to be low (12, 13, 35), were only slightly increased (∼1.8-fold) by TNF-α and IL-1β treatment (lanes 2 and 3). This moderate increase in class I transcription was surprising in light of the strong NF-κB DNA binding activities induced by these cytokines. One explanation to account for this disparity may be that COUP-TFII, which binds to the R2 site on the class I enhancer, maintains its repressive effect on class I transcription even when NF-κB is induced by cytokines. We have recently shown that COUP-TFII mediates transcriptional repression through its association with HDAC and that treatment of Ad12-transformed cells with TSA, an inhibitor of HDAC activity, was able to relieve COUP-TFII repression, resulting in a moderate increase in cell surface class I expression in the absence of NF-κB activation (38). Consistent with this finding, treatment of 12-1 cells with TSA resulted in a modest increase in the level of class I transcription (∼2.5-fold) (Fig. 7, lane 4). Importantly, when TNF-α and IL-1β were used in combination with TSA, the levels of class I transcripts were significantly increased (approximately sixfold) (lanes 5 and 6). This result strongly suggests that in Ad12-transformed cells, class I transcription stimulation by cytokine-induced NF-κB requires the relief of COUP-TFII repression.
FIG. 7.
TNF-α and IL-1β stimulate MHC class I transcription in Ad12-transformed cells when COUP-TFII repression is relieved by TSA. 12-1 cells were treated for 48 h with either TNF-α or IL-1β alone or in combination with TSA. Total RNA was extracted and analyzed by Northern blot analysis by using the MHC class I gene as the probe. The blot was reprobed with RNase P to confirm that the samples contained equivalent amounts of RNA.
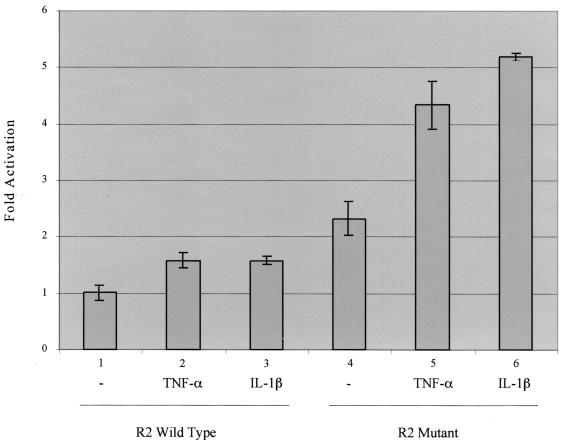
To confirm that elimination of COUP-TFII repression is needed in order for cytokine-induced NF-κB to stimulate class I transcription, we employed a different strategy. Rather than using TSA to relieve COUP-TF repression, we prevented COUP-TF from binding to an artificial class I promoter by mutating the R2 sites. 12-1 cells were transfected with a reporter plasmid containing the NF-κB R1 binding site and either the COUP-TFII R2 binding site [p(R2/R12)2CAT] or a mutated R2 site [p(R2m/R12)2CAT]. Transfected cells were treated with TNF-α or IL-1β. As seen in Fig. 8, the plasmid promoter containing the wild-type R2 site was only marginally stimulated by each cytokine (compare lanes 2 and 3 with lane 1). Compared to the wild-type promoter, the transcription activity of the plasmid promoter containing the mutated R2 site was 2.3-fold greater (compare lane 4 with lane 1). TNF-α and IL-1β treatment led to a pronounced increase in the transcription activity of the R2 mutant plasmid by 4.3- and 5.2-fold, respectively. Taken together with the Northern blot results (Fig. 7), our results indicate that COUP-TFII can repress both nonactivated and activated class I transcription.
FIG. 8.
NF-κB induced by TNF-α and IL-1β activates transcription from class I enhancer R1 elements when COUP-TFII repression is eliminated by mutation of R2 elements. 12-1 cells were transfected with a reporter plasmid containing the class I enhancer NF-κB R1 binding site and either the COUP-TFII wild-type R2 binding site [p(R2/R12)2CAT] or a mutated R2 binding site [p(R2m/R12)2CAT]. After 24 h, cells were treated with TNF-α or IL-1β for another 24 h, after which cell extracts were analyzed for CAT activity. Results presented here are the averages of results of duplicate transfections and are representative of two independent experiments.
DISCUSSION
In Ad12-transformed cells, MHC class I transcription is down-regulated through strong DNA binding activity of repressor COUP-TFII and weak binding of activator NF-κB to the class I enhancer. This results in reduced levels of cell surface class I antigens and contributes to the tumorigenic potentials of these transformed cells by enabling them to escape cytotoxic-T-lymphocyte detection and lysis. In this study, we have investigated the effects of various NF-κB inducers on NF-κB nuclear translocation, DNA binding activity, and MHC class I transcription in Ad12-transformed cells. Among the inducers tested, TNF-α and IL-1β were found to stimulate IκBα degradation, NF-κB nuclear translocation, and DNA binding activity. These inducers were capable of substantially enhancing MHC class I transcription only when COUP-TF repression was relieved. Reasons why other NF-κB inducers (IL-2, PDGF, LPS, and PMA) failed to activate NF-κB in Ad12-transformed cells may be the lack of a receptor or the blocking of a component of the signal transduction pathway.
Most of the research on NF-κB regulation has focused on its nuclear translocation. NF-κB is sequestered in the cytoplasm by IκB and translocates into the nucleus upon the phosphorylation, ubiquitination, and subsequent degradation of IκB (7, 18, 25). The Ad12-transformed cells are unusual in that there is a substantial amount of NF-κB (p65/p50) present in the nucleus, yet NF-κB is unable to bind to DNA. We have recently discovered that this lack of binding of NF-κB (p65/p50) to DNA is due to hypophosphorylation of the p50 subunit (22). This novel means of regulating NF-κB DNA binding through p50 phosphorylation is distinct from the control of NF-κB transactivation through phosphorylation of p65 (10, 28, 36, 43). In this study, treatment of Ad12-transformed cells with TNF-α and IL-1β, in addition to inducing IκBα degradation and NF-κB nuclear translocation, induced the phosphorylation of this population of newly nucleus-localized NF-κB. This new pool of nucleus-localized and phosphorylated NF-κB possessed increased DNA binding ability, while the preexisting pool of hypophosphorylated NF-κB remained unable to bind to DNA. Our study suggests that in addition to NF-κB nuclear translocation through IκB degradation, phosphorylation of the NF-κB p50 subunit is a second regulatory mechanism for NF-κB activation. It remains to be determined how Ad12 E1A can mediate stimulation of NF-κB nuclear translocation yet impede NF-κB phosphorylation.
Importantly, even though TNF-α and IL-1β induced significant increases in NF-κB DNA binding, MHC class I gene transcription was not substantially activated. This finding suggested that the COUP-TFII repressor could prevent newly activated NF-κB from stimulating class I transcription. Indeed, when the repressive effect of COUP-TFII was relieved either by using TSA to block HDAC activity or by using a mutated R2 site to eliminate COUP-TFII DNA binding, we witnessed a pronounced activation of class I transcription by NF-κB following cytokine treatment. Consistent with the increase in class I transcription, fluorescence-activated cell sorter analysis revealed that cell surface class I antigen levels were significantly elevated by treating Ad12-transformed cells with TSA in combination with TNF-α or IL-1β (unpublished data).
How might tumorigenic Ad12-transformed cells biologically benefit from the collateral effects of repressive COUP-TFII and hypophosphorylated inactive NF-κB in down-regulating MHC class I transcription? It is possible that tumorigenic Ad12 employs a “fail-safe” mechanism to ensure that the transcription of class I genes remains strongly down-regulated under various physiological conditions. In the event that NF-κB becomes activated by inducers, such as the cytokines TNF-α and IL-1β, COUP-TFII will still maintain repression of class I transcription and thus ensure the survival of the tumorigenic Ad12-transformed cells in vivo. Conversely, it is interesting to speculate that under certain physiological conditions, COUP-TFII fails to occupy the R2 site, in which case the inability of the hypophosphorylated NF-κB to bind DNA will ensure that class I transcription remains down-regulated.
Acknowledgments
We thank Nancy Rice for p50, p65, and IκB-α antibodies and Diane Griffin for mouse MHC class I Kk gene cDNA.
This work was supported by a grant from the National Institutes of Health (CA29797) to R.P.R.
REFERENCES
- 1.Ackrill, A. M., and G. E. Blair. 1989. Nuclear proteins binding to an enhancer element of the major histocompatibility class I promoter: differences between highly oncogenic and nononcogenic adenovirus-transformed rat cells. Virology 172:643-646. [DOI] [PubMed] [Google Scholar]
- 2.Ackrill, A. M., and G. E. Blair. 1988. Regulation of major histocompatibility class I gene expression at the level of transcription in highly oncogenic adenovirus transformed rat cells. Oncogene 3:483-487. [PubMed] [Google Scholar]
- 3.Chen, Z., J. Hagler, V. J. Palombella, F. Melandri, D. Scherer, D. Ballard, and T. Maniatis. 1995. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 9:1586-1597. [DOI] [PubMed] [Google Scholar]
- 4.Davie, J. R., and V. A. Spencer. 2000. Signal transduction pathways and the modification of chromatin structure. Prog. Nucleic Acid Res. Mol. Biol. 65:299-340. [DOI] [PubMed] [Google Scholar]
- 5.Drew, P. D., G. Franzoso, K. G. Becker, V. Bours, L. M. Carlson, U. Siebenlist, and K. Ozato. 1995. NF kappa B and interferon regulatory factor 1 physically interact and synergistically induce major histocompatibility class I gene expression. J. Interferon Cytokine Res. 15:1037-1045. [DOI] [PubMed] [Google Scholar]
- 6.Eager, K. B., J. Williams, D. Breiding, S. Pan, B. Knowles, E. Appella, and R. P. Ricciardi. 1985. Expression of histocompatibility antigens H-2K, -D, and -L is reduced in adenovirus-12-transformed mouse cells and is restored by interferon gamma. Proc. Natl. Acad. Sci. USA 82:5525-5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epinat, J. C., and T. D. Gilmore. 1999. Diverse agents act at multiple levels to inhibit the Rel/NF-κB signal transduction pathway. Oncogene 18:6896-6909. [DOI] [PubMed] [Google Scholar]
- 8.Friedman, D. J., and R. P. Ricciardi. 1988. Adenovirus type 12 E1A gene represses accumulation of MHC class I mRNAs at the level of transcription. Virology 165:303-305. [DOI] [PubMed] [Google Scholar]
- 9.Ge, R., A. Kralli, R. Weinmann, and R. P. Ricciardi. 1992. Down-regulation of the major histocompatibility complex class I enhancer in adenovirus type 12-transformed cells is accompanied by an increase in factor binding. J. Virol. 66:6969-6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh, S. 1999. Regulation of inducible gene expression by the transcription factor NF-κB. Immunol. Res. 19:183-189. [DOI] [PubMed] [Google Scholar]
- 11.Gilmore, T. D. 1999. The Rel/NF-κB signal transduction pathway: introduction. Oncogene 18:6842-6844. [DOI] [PubMed] [Google Scholar]
- 12.Huvent, I., C. Cousin, A. Kiss, M. T. Baroni de Moraes, C. Bernard, and J. C. D'Halluin. 1997. Downregulation of major histocompatibility complex class I expression and susceptibility to natural killer cells in cells transformed with the oncogenic adenovirus 12 are regulated by different E1A domains. Cancer Detect. Prev. 21:12-21. [PubMed] [Google Scholar]
- 13.Huvent, I., C. Cousin, A. Kiss, C. Bernard, and J. C. D'Halluin. 1996. Susceptibility to natural killer cells and down regulation of MHC class I expression in adenovirus 12 transformed cells are regulated by different E1A domains. Virus Res. 45:123-134. [DOI] [PubMed] [Google Scholar]
- 14.Ing, N. H., J. M. Beekman, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1992. Members of the steroid hormone receptor superfamily interact with TFIIB (S300-II). J. Biol. Chem. 267:17617-17623. [PubMed] [Google Scholar]
- 15.Jelinek, T., and F. L. Graham. 1992. Recombinant human adenoviruses containing hybrid adenovirus type 5 (Ad5)/Ad12 E1A genes: characterization of hybrid E1A proteins and analysis of transforming activity and host range. J. Virol. 66:4117-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jelinek, T., D. S. Pereira, and F. L. Graham. 1994. Tumorigenicity of adenovirus-transformed rodent cells is influenced by at least two regions of adenovirus type 12 early region 1A. J. Virol. 68:888-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, D. R., and J. S. Pober. 1994. HLA class I heavy-chain gene promoter elements mediating synergy between tumor necrosis factor and interferons. Mol. Cell. Biol. 14:1322-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karin, M. 1999. How NF-κB is activated: the role of the IκB kinase (IKK) complex. Oncogene 18:6867-6874. [DOI] [PubMed] [Google Scholar]
- 19.Kimura, A., A. Israel, O. Le Bail, and P. Kourilsky. 1986. Detailed analysis of the mouse H-2Kb promoter: enhancer-like sequences and their role in the regulation of class I gene expression. Cell 44:261-272. [DOI] [PubMed] [Google Scholar]
- 20.Kralli, A., R. Ge, U. Graeven, R. P. Ricciardi, and R. Weinmann. 1992. Negative regulation of the major histocompatibility complex class I enhancer in adenovirus type 12-transformed cells via a retinoic acid response element. J. Virol. 66:6979-6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kushner, D. B., D. S. Pereira, X. Liu, F. L. Graham, and R. P. Ricciardi. 1996. The first exon of Ad12 E1A excluding the transactivation domain mediates differential binding of COUP-TF and NF-kappa B to the MHC class I enhancer in transformed cells. Oncogene 12:143-151. [PubMed] [Google Scholar]
- 22.Kushner, D. B., and R. P. Ricciardi. 1999. Reduced phosphorylation of p50 is responsible for diminished NF-κB binding to the major histocompatibility complex class I enhancer in adenovirus type 12-transformed cells. Mol. Cell. Biol. 19:2169-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, C. C., R. M. Dai, E. Chen, and D. L. Longo. 1994. Phosphorylation of NF-KB1-p50 is involved in NF-kappa B activation and stable DNA binding. J. Biol. Chem. 269:30089-30092. [PubMed] [Google Scholar]
- 24.Li, C. C., M. Korner, D. K. Ferris, E. Chen, R. M. Dai, and D. L. Longo. 1994. NF-kappa B/Rel family members are physically associated phosphoproteins. Biochem. J. 303:499-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, Y. C., K. Brown, and U. Siebenlist. 1995. Activation of NF-kappa B requires proteolysis of the inhibitor I kappa B-alpha: signal-induced phosphorylation of I kappa B-alpha alone does not release active NF-kappa B. Proc. Natl. Acad. Sci. USA 92:552-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, X., R. Ge, and R. P. Ricciardi. 1996. Evidence for the involvement of a nuclear NF-κB inhibitor in global down-regulation of the major histocompatibility complex class I enhancer in adenovirus type 12-transformed cells. Mol. Cell. Biol. 16:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, X., R. Ge, S. Westmoreland, A. J. Cooney, S. Y. Tsai, M. J. Tsai, and R. P. Ricciardi. 1994. Negative regulation by the R2 element of the MHC class I enhancer in adenovirus-12 transformed cells correlates with high levels of COUP-TF binding. Oncogene 9:2183-2190. [PubMed] [Google Scholar]
- 28.Madrid, L. V., C. Y. Wang, D. C. Guttridge, A. J. Schottelius, A. S. Baldwin, Jr., and M. W. Mayo. 2000. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-κB. Mol. Cell. Biol. 20:1626-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meijer, I., A. J. Boot, G. Mahabir, A. Zantema, and A. J. van der Eb. 1992. Reduced binding activity of transcription factor NF-kappa B accounts for MHC class I repression in adenovirus type 12 E 1-transformed cells. Cell. Immunol. 145:56-65. [DOI] [PubMed] [Google Scholar]
- 30.Nielsch, U., S. G. Zimmer, and L. E. Babiss. 1991. Changes in NF-kappa B and ISGF3 DNA binding activities are responsible for differences in MHC and beta-IFN gene expression in Ad5- versus Ad12-transformed cells. EMBO J. 10:4169-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pahl, H. L. 1999. Activators and target genes of Rel/NF-κB transcription factors. Oncogene 18:6853-6866. [DOI] [PubMed] [Google Scholar]
- 32.Palombella, V. J., O. J. Rando, A. L. Goldberg, and T. Maniatis. 1994. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell 78:773-785. [DOI] [PubMed] [Google Scholar]
- 33.Sagami, I., S. Y. Tsai, H. Wang, M. J. Tsai, and B. W. O'Malley. 1986. Identification of two factors required for transcription of the ovalbumin gene. Mol. Cell. Biol. 6:4259-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrier, P. I., R. Bernards, R. T. Vaessen, A. Houweling, and A. J. van der Eb. 1983. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. Nature 305:771-775. [DOI] [PubMed] [Google Scholar]
- 35.Shemesh, J., R. Rotem-Yehudar, and R. Ehrlich. 1991. Transcriptional and posttranscriptional regulation of class I major histocompatibility complex genes following transformation with human adenoviruses. J. Virol. 65:5544-5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sizemore, N., S. Leung, and G. R. Stark. 1999. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-κB p65/RelA subunit. Mol. Cell. Biol. 19:4798-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smirnov, D. A., S. Hou, X. Liu, E. Claudio, U. K. Siebenlist, and R. P. Ricciardi. 2001. Coup-TFII is up-regulated in adenovirus type 12 tumorigenic cells and is a repressor of MHC class I transcription. Virology 284:13-19. [DOI] [PubMed] [Google Scholar]
- 38.Smirnov, D. A., S. Hou, and R. P. Ricciardi. 2000. Association of histone deacetylase with COUP-TF in tumorigenic Ad12-transformed cells and its potential role in shut-off of MHC class I transcription. Virology 268:319-328. [DOI] [PubMed] [Google Scholar]
- 39.Ten, R. M., V. Blank, O. Le Bail, P. Kourilsky, and A. Israel. 1993. Two factors, IRF1 and KBF1/NF-kappa B, cooperate during induction of MHC class I gene expression by interferon alpha beta or Newcastle disease virus. C. R. Acad. Sci. Ser. III 316:496-501. [PubMed] [Google Scholar]
- 40.Vasavada, R., K. B. Eager, G. Barbanti-Brodano, A. Caputo, and R. P. Ricciardi. 1986. Adenovirus type 12 early region 1A proteins repress class I HLA expression in transformed human cells. Proc. Natl. Acad. Sci. USA 83:5257-5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, D., S. D. Westerheide, J. L. Hanson, and A. S. Baldwin, Jr. 2000. Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J. Biol. Chem. 275:32592-32597. [DOI] [PubMed] [Google Scholar]
- 42.Whiteside, S. T., and A. Israel. 1997. I kappa B proteins: structure, function and regulation. Semin. Cancer Biol. 8:75-82. [DOI] [PubMed] [Google Scholar]
- 43.Zhong, H., H. SuYang, H. Erdjument-Bromage, P. Tempst, and S. Ghosh. 1997. The transcriptional activity of NF-κB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89:413-424. [DOI] [PubMed] [Google Scholar]