Abstract
Alternative foldings are an inherent property of RNA and a ubiquitous problem in scientific investigations. To a living organism, alternative foldings can be a blessing or a problem, and so nature has found both, ways to harness this property and ways to avoid the drawbacks. A simple and effective method employed by nature to avoid unwanted folding is the modulation of conformation space through post-transcriptional base modification. Modified nucleotides occur in almost all classes of natural RNAs in great chemical diversity. There are about 100 different base modifications known, which may perform a plethora of functions. The presumably most ancient and simple nucleotide modifications, such as methylations and uridine isomerization, are able to perform structural tasks on the most basic level, namely by blocking or reinforcing single base-pairs or even single hydrogen bonds in RNA. In this paper, functional, genomic and structural evidence on cases of folding space alteration by post-transcriptional modifications in native RNA are reviewed.
INTRODUCTION
RNA consists of four major and a number of minor bases, the latter being generated post-transcriptionally by chemical modification of the four major ones, or in exceptional cases, by base replacement (1). The four major bases dispose of a matched set of hydrogen bond acceptors and donors, enabling the mutual complementary recognition in Watson–Crick pairs which are known as the basis of the genetic code. These interactions are quite strong and govern RNA folding in such a predominant and predictable way, that the pattern of Watson–Crick base pairs is perceived as a structural level in its own right and thus named secondary structure. RNA also undergoes interactions through hydrogen bonds on their so called Hoogsteen and sugar edges, giving rise to sophisticated 3D structures, referred to as tertiary structure (2,3). The combination of a low diversity of elemental units and the high strength of interaction makes it difficult for a given sequence to specify a unique secondary and tertiary structure. These circumstances are also known as ‘the RNA folding problem’, since they result in a multitude of alternative conformations of similar energy for almost any given RNA sequence. The ensemble of possible foldings of a given RNA is referred to as its folding space (4) or conformation space (5,6), even though the exact meaning of these terms in the literature differs depending on the authors. For the purpose of this review, the terms folding and folding space will be applied to issues of secondary, tertiary or global three-dimensional structure, thus not comprising local changes in the 3D structure without changes in base pairing.
In comparison with proteins, RNA displays a paucity of primary sequence information which may be partially remedied by post-transcriptional introduction of nucleotide modifications. Modified nucleotides perform a large number of functions (7,8), some of which are yet to be discovered. One particular function of modified nucleotides, namely the stabilization of functional RNA structures, is the object of this review. In the majority of the investigated cases, unmodified RNA would fold into a secondary and tertiary structure closely resembling that of the fully modified RNA. In those cases, the stabilizing effect of nucleotide modification mostly results in augmented thermal stability and reduced dynamics. Under certain circumstances, however, the presence of one or several modified nucleotides can induce an alternative folding, often comprising significant alterations in the secondary structure.
Since all function is mediated by structure on some level, a clean differentiation between functional and structural modifications is not possible, especially considering the numerous interactions with other molecules encountered, e.g. by tRNA. However, for the purpose of this review, modifications that have been shown to affect molecular interaction by direct contact with other molecules will be addressed as functional, while those for which structural changes extending beyond the local conformation have been described will be addressed as structural (vide infra). Note that this differentiation is necessarily incomplete and does not preclude a placement of a modification in both categories.
Alternative foldings
The laws of thermodynamics dictate that at ambient or body temperature several alternative structures of a given RNA sequence must coexist in significant proportions, provided the difference in their Gibbs free energies is small enough and a thermodynamic equilibrium is achieved. The proportions of two given foldings are determined according to their difference in free Gibbs energy: ΔΔG = R•T•ln (K), where ΔΔG is the difference in free Gibbs energies of both conformations, R is the universal gas constant, T is the absolute temperature in Kelvin, and K is the ratio of both conformations. If no equilibrium is achieved on a biologically significant timescale, at least one of the RNA conformations can be regarded as kinetically trapped and may therefore be called metastable (9,10). The trapping of metastable structures in kinetically stabilized minima might conceivably be prevented by RNA chaperones. By analogy to protein chaperones, RNA chaperones would be expected to interact with the RNA co-transcriptionally, to prevent misfolding in the first place. However, RNA polymerase produces RNA chains faster than the ribosome produces protein chains (11,12) and models for RNA chaperone activities are discussed to act on fully synthesized misfolded RNAs, rather than on nascent polymer chains (9,13). Excitingly though, in the rapidly evolving field of RNA switches, there are indications that small molecules might interact co-transcriptionally with RNA, thereby directing its folding pathway in a chaperon-like manner (14). RNA switches are structural RNAs with two alternative structures, one of which can be induced to prevail upon binding of a small molecule. Most reported RNA switches are regulatory elements in mRNAs which interact with metabolites related to the enzymatic activity of the proteins encoded in the respective mRNA (15,16). The concentration of metabolic ligands in a biological environment may thus have a drastic effect on the prevailing structure and corresponding function of an RNA. While it is unambiguous that small molecules do interact strongly with RNA switches to influence their conformation for regulatory purposes, recent evidence points to this influence being exerted through kinetic rather than thermodynamic control of the resulting conformation (14,17). Interestingly enough, different metabolites involved in nucleotide modification appear to be regulated by riboswitches (17,18). It is also noteworthy that a regulatory function of a post-transcriptional modification in a eukaryotic RNA has been reported (19).
The coexistence of several alternative structures of an RNA might conceivably present drawbacks to an organism, if only one of the structures has the desired biological activity. If, however, that activity has to be regulated, stabilization of either structure is a possible, and, importantly, simple regulation mechanism.
A number of recent papers suggest or directly report stabilization of a functionally relevant conformation by post-transcriptional nucleotide modification (20). Several such cases concern tRNAs (20–23), but a case of conformational fixation by base modification has also been suggested in ribosomal RNA (24). In at least one case, it could be shown that regulation of the steady state level of RNA can be achieved through modulation of modification activity (20). In what follows, these cases will be reviewed, bearing in mind a general scenario for regulation of RNA expression through nucleotide modification.
Impact of nucleotide modifications on local structure
From a biosynthetic point of view, the roughly 100 known different RNA nucleotide modifications can be easily divided according to their complexity (1,25). There are simple chemical transformations, such as addition of a methyl group or bond isomerization of uridine to yield pseudouridine, and there are more complex multistep transformations involving the action of several enzymes in a defined order. The synthesis of dihydrouridine in a redox reaction from uridine may hold something like a middle ground. The chemically simple modifications are, not surprisingly, also those which are wider spread. Not only are they found in many if not all classes and types of RNAs, but also in all kingdoms and probably in all species. Arguably, they may be the most ancient of nucleotide modifications and their modes of action relatively simple.
Since a detailed discussion of the electronic and steric effects of nucleoside modifications on base stacking, base pairing and sugar pucker is given by Davis (26), only a few effects are mentioned here. The stabilizing effect of isomerization of uridine to pseudouridine (Ψ) has been suggested to arise from improved stacking (27,28) or from the additional hydrogen bond donor that pseudouridine has to offer compared to uridine (29). Although inspection of tRNA crystal structures revealed no direct involvement of the N1-H of Ψ in hydrogen bonds with other nucleotides, electron density suggesting a Ψ-coordinated water molecule, as well as molecular dynamics simulations indicate an improved definition of the hydration sphere around this site. Thus, the N1-H of Ψ may form a bridge with adjacent phosphate residues, mediated by a stably coordinated water molecule (29–32). Methyl groups, such as in m5C, m1Ψ and T, increase base-stacking due to their hydrophobicity and because they augment the polarizability (27,33). Methyl groups may also induce structural changes by increasing steric encumbrance (34), or by blocking hydrogen bonds, e.g. at Watson–Crick positions (21,35,36). The ability of modified bases to affect hybridization has successfully been used in microarray hybridization screens for the detection of modified nucleotides (37). Methylations at the 2′-OH favour a 3′endo-conformation of the ribose (26), block sugar edge interactions and change the hydration sphere around the oxygen (31,32). They also increase stability against hydrolysis by bases and nucleases. Modifications may also change the acceptor/donor pattern of the base, e.g. in m3C. The formation of m1A simultaneously blocks a Watson–Crick position in the adenosine and introduces a positive charge to the nucleoside (35,38). Such positive charge, which has also been observed in m7G (38), modulates the electron density in the aromatic purine system and thus influences the strength of hydrogen bonds. It may conceivably promote ionic interactions with the negatively charged phosphates in the backbone. Reduction of uridine to dihydrouridine has been found to change the sugar pucker (33). Structural changes induced by modifications may be compounded through the creation of additional binding pockets, e.g. for magnesium ions, which in turn may enhance stability of the new structure (33,39).
Transfer RNA is a treasure chest of information
Most knowledge on modified nucleotides comes from studies of tRNA. Historically, tRNAs were the first ‘small’ RNAs discovered, and their function as translator molecules sustains a high level of continued scientific interest. The effect of nucleotide modification on RNA structure was studied with a variety of biophysical and biochemical methods including NMR, UV-melting curves, structural probing and X-ray crystallography. In tRNAs, which are among the most strongly modified RNAs, most nucleotide modification sites are clustered in two sites, one in the structural core of the L-shaped 3D structure and one in the anticodon domain (40).
Truncated anticodon-stem–loops (ASLs or ACSLs), which usually comprise the 5 bp of the anticodon stem and 7 nt of theanticodon loop, are readily accessible through solid phase synthesis and their small size facilitates assignment of NMR signals. Studies on ASLs have demonstrated important alterations of the anticodon loop structure induced by nucleotide modification, which in turn entail diverse functional effects. As there appears to be no direct evidence of structural cross-talk that would translate modification-induced structural changes from the tRNA core to the anticodon or vice versa, the structure of ASLs is generally assumed to be very similar to that of the corresponding region of the respective full length tRNAs. This has been validated by comparison of NMR structures of ASLs with the X-ray structures of full size tRNAs (6,41–43). Further validation for the use of ASLs as model systems comes from their activity in ribosome binding assays (44).
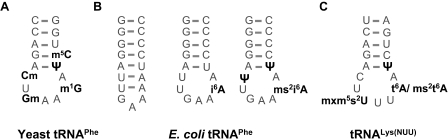
Studies of Ψ39 in ASLs have significantly contributed to the understanding of the role of Ψ in RNA structure (27,28). NMR studies of several modification isoforms of the yeast tRNAPhe ASL included incorporation of Ψ39 in one case (27), and of Cm32, Gm34 m5C40, and m1G37, the natural precursor of the hypermodified wybutosine base found at position 37 of the fully matured native tRNA (6) (Figure 1A). NMR studies on an ASL of Escherichia coli tRNAPhe revealed a hairpin structure including 7 bp and a three base loop. The expected ASL structure including a 7 base loop with a typical U-turn was only discovered after enzymatic introduction of i6A37 (45,46) (Figure 1B). Thus, modification of A37 to i6A37 is responsible for a change in secondary structure, while leaving the hydrogen bond capacity pattern of A37 unchanged. The thermodynamic effects of the i6A and similar modifications have also been studied after chemical modification of a non-natural precursor, which had been incorporated into the RNA by solid phase synthesis (34,47). The ASL of the tRNALys(NUU) isoacceptor (Figure 1C) has been the object of several studies (28,42,44,48–50), employing different combinations of Ψ39, s2U34, mnm5s2U34 (in E.coli) mcm5U34 or mcm5s2U34 (in human), t6A37 or ms2t6A37. The hypermodifications at positions 34 and 37 are of special interest since they were reported to play major roles in aminoacylation identity [references in (51)], codon recognition, frameshifting (52–54) misreading (55), and the annealing process of the human to the HIV RNA genome as a primer for reverse transcription (56,57). It is noteworthy that these modifications, which, according to the above definition, fall into the functional category, appear to confer the classical U-turn structure to anticodon loop, whose structure otherwise suffers from weak stacking of the four subsequent uridines (41,49). Anticodon loop nucleotide modifications and hypermodifications and their functions have been expertly reviewed by several authors (52,58). The particular importance of modifications in positions 34 and 37 is underscored by the fact that the major fraction of the chemically diversified ‘zoo’ of about 100 nt modifications is found there (7,25,58).
Figure 1.
Anticodon stem loops (ASLs) of well studied classical tRNAs. (A) Yeast tRNAPhe including m1G37, the natural precursor of the hypermodified wybutosine base found at position 37 of the fully matured native tRNA (compare Figure 2). (B) E.coli tRNAPhe ASL modification isoforms. The unmodified ASL displays (left) a hairpin structure with a small loop of three nucleotides and two extra base pairs U32-A38 and U33-A37. Introduction of i6A as sole nucleotide modification leads two the formation of the classical ASL-stem loop structure including a U-turn (middle). An ASL corresponding to that of the native tRNAPhe would include Ψ32, Ψ39 and ms2i6A modification (right). (C) tRNALys(NUU) from E.coli contains mnms2U (x = m) and t6A, while the tRNA from human cytosol contains mcms2U (x = c) and ms2t6A.The human mitochondrial tRNALys has a similar stem and an identical loop sequence (compare Figure 5) and contains τm5U (x = τ).
In stark contrast, the vast majority of modifications found in the core are of the biosynthetically simple type (methylated nucleotides, pseudouridines and dihydrouridines). A large body of data suggests primarily structural roles for these simple modifications. Structural investigations were applied mostly to full-length molecules as opposed to truncated molecules, such as ASLs. While avoiding possible artifacts arising from truncation, this advantage is partially offset by experimental problems of synthetic and analytical nature. Assignment of NMR signals and the subsequent model building is more complicated for 76mer-tRNAs than for 17mer-ASLs. Also, the solid-phase synthesis of a full-length tRNA has only recently been reported (59), and limitations in yield make the synthesis of sufficient amounts of modified and partially modified tRNAs a challenge.
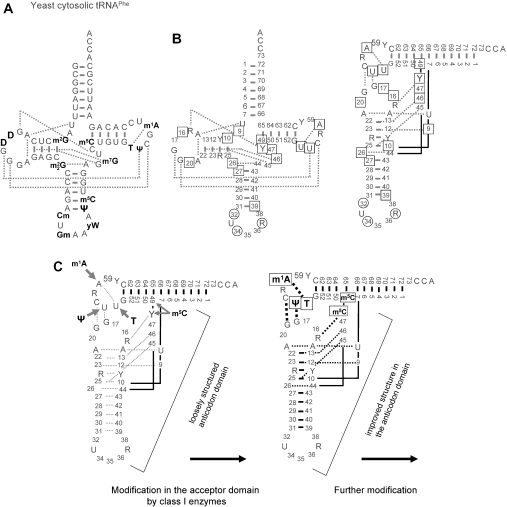
Early work, conducted by comparison of tRNA from wild-type and mutant strains lacking certain modification activities (60), was severely limited by the availability of the respective mutant strains. Therefore, the introduction by Uhlenbeck of in vitro transcription with T7-RNA polymerase as the standard method to produce tRNA transcripts was a milestone (61). This technique produced a large number of studies in which unmodified transcripts were compared to native tRNAs isolated from living cells. These studies showed some common features of the ensemble of base modification. First exemplified with tRNAPhe from yeast (Figure 2A), lower conformational flexibility, improved thermal stability and improved aminoacylation parameters were illustrated as the paramount advantages that come with being modified. Interestingly, these effects seemed to be mediated by increased magnesium binding affinity in the modified tRNA, and the lack of modification in in vitro transcripts could be compensated by an increased concentration of magnesium ions (61–63). Similar observations were made for other classical tRNAs such as tRNAVal (64–68), tRNAPhe (69) and tRNAGln (30) from E.coli, and for yeast tRNAAsp (70). In general, at high concentrations of magnesium ions transcripts behaved very similar to native tRNA, but differences were clearly observed at low concentrations. As one important consequence, the majority of functional studies, especially in vitro aminoacylation, are still performed at magnesium ion concentrations an order of magnitude above physiological value [references in (51)]. In some cases, at low magnesium ion concentrations misfolding was observed as a consequence of a lack of modification.
Figure 2.
(A) Secondary structure of yeast tRNAPhe. Modified nucleotides are in bold. Tertiary interactions are indicated by dotted lines. (B) General architecture of classical elongator tRNAs. On the cloverleaf structure on the left, conserved residues important for elements of tertiary structure are indicated. R stands for conserved purine residues and Y stands for conserved pyrimidine residues. Frequently modified positions [>25%; according to reference (121)] in the anticodon are highlighted by circles. Frequently modified positions elsewhere in the tRNA are boxed. Tertiary interactions are indicated by dotted lines. In the representation on the right, acceptor and anticodon domains are arranged in an L-shape according to the 3D structure. (C) Reinforcement hypothesis. On the left, the unmodified tRNA forms a stable acceptor domain and a loosely structured anticodon domain (weak interactions are symbolized by grey dotted lines). Class I modification enzymes act on the acceptor domain and introduce modifications like T54, Ψ55, m1A58, m5C48, m5C49 and/or others, as indicated by arrows. These modifications stabilize the T-loop structure and reinforce tertiary interactions with the D-loop. The formerly loose structure of the anticodon domain is thus better defined (symbolized by black lines) and now allows better substrate recognition by other modification enzymes.
Promotion of correct tRNA folding by nucleotide modification may occur either through direct reinforcement of structural features or through facilitation of magnesium ion binding. While the latter mechanism is in agreement with a number of reports [detailed discussion is given by Agris (33)], exceptions are reported (69). Similarly, while restrictions of conformational dynamics due to modified nucleotides are a recurrent observation made in several systems [recent examples in (6,49)] exceptions showing opposite effects are noted [e.g. (45,46)].
Early genetic approaches were aimed at analyzing the fitness of mutant bacteria containing genes for non-functional modification enzymes. Thus, in bacteria devoid of one single modification activity at a time, a number of activities for nucleotide modification were found to be non-essential, with many of these showing weak or cryptic phenotypes (52,71). The more obvious phenotypes were mostly attributed to failures in codon recognition and frame shifting due to lack of functional modifications at positions 34 and 37 in the anticodon loop [reviewed in (54,72)].
The low apparent impact is in sharp contrast with the fact that about 1% of the E.coli genome is involved in post-transcriptional base modification (72), with estimates in yeast being similar (73). Only lately have more severe phenotypes of core modifications been described (74–76). One might conclude that the ubiquitous tRNAs are so heavily used, that stabilization against degradation by modification outweighs the effort of upregulated de novo synthesis. This consideration applies regardless of the mechanism by which modifications produce metabolic stability and would be consistent with tRNA being the most heavily modified of all RNAs, even if ‘functional’ anticodon modifications are omitted from the above discussion.
tRNA tertiary structure and reinforcement
The structure of yeast tRNAPhe contains an acceptor domain composed of the acceptor stem, D-arm and D-loop, and an anticodon domain, composed of T-stem, T-loop, anticodon stem and anticodon loop. The 3D L-shaped structure, as known from X-ray crystallography, is stabilized by tertiary interactions, which are indicated by dotted lines in Figure 2A and B. These features, which are common to almost all cytosolic and some mitochondrial tRNAs, are mediated by a set of conserved and semi-conserved residues shown in Figure 2B. Various studies report that the tertiary interactions between the D-loop and the T-loop are the first to unravel upon heating, and that the first domain to unfold is the D-domain [e.g. (77,78)]. Modified nucleotides in the core of the tRNA, which would be addressed as ‘structural’, stabilize the entire tRNA structure, and do so at least in part by reinforcing these tertiary interactions. Thus, tRNAMet from E.coli lacking T54 displayed decreased thermal stability in NMR experiments (60). A reinforcement of interactions of D- and T-domains was lately demonstrated by Nobles et al. (79) to be effected by modified bases m5C49, T54 and Ψ55 in the 3′half of yeast tRNAPhe. These modifications, which are frequently found in many other tRNAs, are effected by enzymes which recognize tRNA substructures, e.g. the T-stem and loop, even when the rest of the substrate is disordered (40,80–82). As opposed to these enzymes, which correspond to class I modification enzymes in the categorization proposed by Grosjean et al. (40), enzymes requiring an intact tRNA architecture for substrate recognition belong to class II. Using microinjection of yeast tRNATyr precursors into Xenopus oocytes, it was demonstrated that the typical class I type modifications m1A58, T54, Ψ55 and m5C49 occur on substrates containing intron and 5′leader sequences before any modification in the D-loop or stem take place (83,84). Only after intron removal were modifications at positions 34 and 37 produced.
There may be a general order of modification events in classical tRNA transcripts, where the D-domain is initially not well structured and the first modifications only occur in the adequately structured T-domain. These modifications may then aid in the structuring of the D-domain and enable its subsequent modification (Figure 2C). The fact that many enzymes effecting modification in the D-domain, for example m2G10 or , act on otherwise unmodified transcripts in vitro (85,86) might be perceived as a certain contradiction. However, in vitro conditions frequently include high concentrations of magnesium ions, which, by being elevated by as much as an order of magnitude, may emulate the effect of base modification and thus stabilize the D-domain, as has been discussed above. Also, the exact degree of structuring of the D-domain in vivo is not known and is certain to vary among tRNA species. Slight deviations from the hypothesis presented in Figure 2C have been shown, e.g. in Pyrococcus abyssi. Constantinesco et al. (87) have found some class I characteristics of the methyltransferase in the formation of m2G10. However, the same methyltransferase behaves like a class II enzyme in the second methylation step of the same nucleotide, leading to the formation of . Recently Urbonavicius et al. (88) have shown very similar properties for the methyltransferase from the same organism. Significantly, only the second methylation step requires the presence of magnesium ions, presumably to induce correct 3D substrate tRNA conformation.
In summary, it has gradually become clear that only very few single modifications have an outstanding impact on the structure of cytosolic elongator tRNAs. Rather, every single modification appears to contribute a certain measure of structural stabilization, thereby improving functionality and likely the lifetime of classical tRNA.
A cytosolic tRNA with deviations from the classical pattern
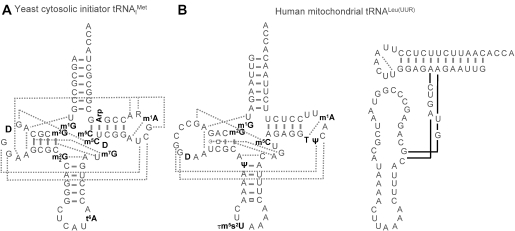
Recently, Anderson et al. (74,75) have discovered by a genetic approach an accelerated degradation of yeast initiator which was unmodified at position A58. Methylation at N1-A58 does allow normal expression of the initiator tRNA, while lack of methylation leads to a temperature-sensitive, non viable phenotype, which may be rescued through overexpression of the initiator tRNA. Importantly, lack of m1A58 in elongator tRNAs does not restrict either their expression or their stability. Presumably due to its particular function, yeast initiator tRNA has a particular sequence, deviating from the classic pattern in a number of nucleotides which ensure proper classic folding. The ensuing 3D structure, as determined by X-ray crystallography (89), sets it apart from all elongator tRNAs in yeast. As illustrated in Figure 3A, patterns of tertiary interactions in the T-loop and between T- and D-loop are altered. In particular, m1A58 is engaged in an interaction with A54, which strongly resembles the corresponding interaction with T54 in elongator tRNAs. A likely scenario implies a weakened or nonexistent A58–A54 interaction in the unmodified T-loop of the initiator tRNA as compared to m1A58-A54. Disruption of N1-A58 methylation activity of the Trm6/Trm61 protein complex abolishes the only class I modification in the T-loop of this tRNA (75). It is thus tempting to speculate that the reinforcement mechanism of T-loop–D-loop interactions, as outlined above, might break down as a consequence. This might give rise to a non-functional tRNA structure, leading to tRNA degradation, while methylation at N1-A58 would induce correct functional structure, and thus promote escape from premature degradation. The rather outstanding importance of m1A58 would thus be explained by the lack of other class I modifications, namely T54 and pseudouridine 55 in the T-loop of yeast initiator .
Figure 3.
Slight deviations from the classical tRNA structure. (A) Secondary structure of initiator from yeast. Modified nucleotides are in bold. Tertiary interactions are indicated by dotted lines. Note the absence of T54 and Ψ55 and the additional tertiary interactions between the A20 and the T-loop as compared to tRNAPhe. R denotes a purine at position 59 of for which sequence data in the literature are contradictory (96,89). (B) Human mitochondrial tRNALeu(UUR). The secondary structure of the native tRNA including all modified bases is shown on the left. Modified nucleotides are in bold and tertiary interactions are indicated by dotted lines. The loose structure of the anticodon domain of the unmodified transcript (98) is shown on the right. Modified nucleotides are abbreviated according to references (96,122).
This scenario implies the existence of a kind of structural proofreading mechanism for tRNAs, which has indeed been experimentally supported by the discovery of genetic interaction between the TRM6/TRM61 genes coding for the N1-A58-methyltransferase and genes coding for proteins of the nuclear exosome and a poly(A)-polymerase. These findings by the Anderson group (20) suggest a polyadenylation-dependent pathway of degradation of tRNAs by the exosome. Indeed, such a pathway has recently been characterized as structure-dependent in yeast (90). A somewhat similar pathway might exist in E.coli (91) although no proof of correlation to tRNA nucleotide modification has emerged yet.
A mitochondrial tRNA with classical aspects
Sequence and structure of animal mitochondrial (mt) tRNAs are known for their deviations from the classical prototype tRNAs. Several lines of evidence suggest that with the loss of classical structure and the introduction of a bias in nucleotide composition (92), the role of modified nucleotides for the maintenance of the L-shaped 3D tRNA structure increases in mitochondrial tRNA as compared with the cytosolic counterparts. These structural effects can reach such importance that certain mitochondrial tRNAs are entirely non-functional when devoid of modified nucleotides. An impressive demonstration of the differential susceptibility of cytosolic and mitochondrial tRNAs to lack of nucleotide modification has recently been published. A missense mutation in the human gene for pseudouridin synthase 1 (PUS1) ablates formation of pseudouridines at positions 27 and 28 (and probably others) in cytosolic as well as in mitochondrial tRNAs (93,94). While cytosolic protein synthesis is largely intact, the lack of modification in mitochondrial tRNAs causes the mitochondrial protein synthesis to stall and results in a human mitochondrial myopathy.
Human mitochondrial tRNALeu(UUR) is one of the most investigated mitochondrial tRNAs, because of its involvement in the pathogenesis of a number of mitochondrial diseases (95). This tRNA is actually the one with the most classic attributes in animal mitochondrial genomes (Figure 3B). A sequence alignment of 30 mammalian mitochondrial tRNALeu(UUR) shows the conservation of all major tertiary interactions known from tRNAPhe and other classical tRNAs (92). Also, mt tRNALeu(UUR) contains one of the highest numbers of modified nucleotides found in any animal mitochondrial tRNA so far (96), among them eight structural modifications (m1G9, m2G10, D20, Ψ27, m5C48, T54, Ψ55 and m1A58) assumed to reinforce tertiary interactions. Because of its involvement in mitochondrial myopathies, the structural and functional properties of tRNALeu(UUR) have been studied by several groups (97–101). Owing to the unavailability of native material, especially such carrying pathogenic points mutations, all studies have been carried out using RNAs which were synthesized by transcription in vitro and thus devoid of modified nucleotides. Transcripts were found to be aminoacylatable with preparations of pure recombinant mitochondrial leucyl-tRNA synthetase, but the structure was found to be ill defined (98). While the acceptor domain behaved as the expected in structural probing experiments, the anticodon domain was found to be highly accessible to several modifying agents and nucleases (98) (Figure 3B). The fact that the anticodon-domain is generally less stable than the acceptor domain has already been mentioned and applies to mt tRNALeu(UUR) to a degree that is higher than usual. The apparent looseness of this anticodon domain is in keeping with the low structural stability of animal mt tRNA transcripts in general. In accordance with the reinforcement mechanism hypothesis displayed in Figure 2C, in vitro modification assays of tRNALeu(UUR) transcripts with mitochondrial protein extracts show preferential modification by class I enzymes in the acceptor domain. As opposed to the transcript, the modified native tRNA forms a well-defined structure in the anticodon domain as well [(102); M. Helm, unpublished data].
The Kelley laboratory has studied the tendency of unmodified transcripts of human mt tRNALeu(UUR) to dimerize in vitro (103,104). Dimerization is observed upon introduction of a mutation at position 3243 of the human mitochondrial genome, one of the most frequent pathogenic mutations and commonly known as the MELAS mutation. This mutation causes an A→G transition in the middle of a stretch of nucleotides identified by the authors as a self-complementary dimerization interface. The experimentally determined Kd of 150 ± 30 nM is in a range that might well allow such dimerization to occur in vivo. Interestingly, the dimerization interface is located in the D-domain, in proximity to two methylated guanosines, m1G9 and m2G10 and a dihydrouridine at position 20, of which m2G10, was indeed reported to be affected by the very MELAS mutation investigated (23). These modifications can be expected to have a stabilizing influence on the tertiary structure of monomeric tRNA and might thus affect dimerization. Inversely, dimerized transcripts might escape modification and thus account for lowered steady-state levels or processing artifacts attributed to the MELAS mutation at 3243 (95).
Alternative secondary structures in tRNAs
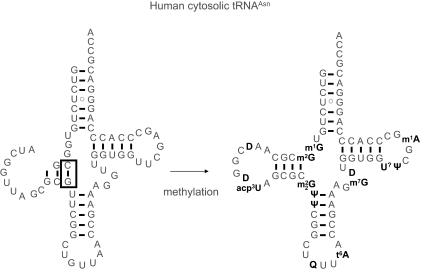
A number of tRNA sequences are known (96) which cannot be unambiguously fitted into the standard cloverleaf pattern shown in Figure 2B, raising the question how their structures can be recognized by the many components in the protein biosynthesis machinery. Based on computer simulations (105) different structural organization patterns have been identified that allow maintenance of the overall L-shape despite the apparent incompatibility. In these alternative foldings, helices which are shortened or extended by one or two base pairs are compensated for by corresponding changes in the second helix of the respective domain. The number of base pairs in each domain thus remains constant, and the different spatial orientation of closing base pairs in the helices is compensated for by changes in the length of the connector regions formed by nucleotides 8–9, 26 and the variable loop in classical tRNA (compare Figure 2B). Screening tRNA sequences, Steinberg and Cedergren (21) have found a correlation between the presence of “ and alternative tRNA foldings. They proposed that might prevent misfolding of tRNA by prohibiting the formation of base pairs, as shown in Figure 4. Interestingly, base pairs would still be allowed. Although these calculations lack experimental verification, they may be regarded as the first prototype of a rearrangement of secondary structure induced by a single modified nucleotide.
Figure 4.
Rearrangements on the secondary structure level induced by double methylation of human cytosolic tRNAAsn. The calculated structure of the unmodified transcript on the left side features an aberrant D-stem without G10, but including G26. Double methylation on N6 of G26 impedes its Watson–Crick pairing with cytidines and thus renders the base pair G26-C11, which is highlighted by a box, impossible. The fully modified tRNAAsn may thus adopt the classical cloverleaf structure as shown on the right. U? denotes an unknown modified uridine, likely a derivative of ribothymidine, at position 54.
Transfer RNAs from animal mitochondria are known for their strong deviations from the standard secondary structure pattern, which sometimes include the substitution of an entire D-or T-domain with short replacement loops. A survey in mammalian mitochondria has evidenced the loss of the classical T-loop structure in the majority of tRNA species (92). Concomitant with the loss of the structural signature of the T-arm, which presented an important recognition signal for several class I modification enzymes, the corresponding modifications in the acceptor domain are also considerably less abundant (96). It is thus clear that folding and structure formation must rely on different pathways and principles than in classical tRNAs. The animal mitochondrial tRNALeu(UUR) with all its attributes of a classical tRNA is thus rather an exception among mitochondrial tRNAs.
Human mt tRNALys is an example of a tRNA having lost all recognizable D-loop/T-loop interactions. Its sequence is altogether poorly conserved (92). An in vitro transcript of the corresponding tRNA gene adopts an extended hairpin structure rather than a cloverleaf, as has been determined by structural probing (Figure 5). The native tRNALys isolated from human mitochondria contains five types of base modifications in six positions and does fold into the expected cloverleaf, illustrating that the secondary structure and the ensuing 3D shape depend strongly on the presence of modified nucleotides (22). A closer inspection of the unusual structure of the transcript revealed an extension of the normally 7 bp long acceptor stem by three additional base pairs, which are incompatible with the presence of an m1A at position 9 in the native tRNA. Nitrogen 1 in adenosines is involved in standard Watson–Crick pairing, but blocked by a methyl group in m1A. A chimeric tRNALys with m1A9 as sole modified nucleotide did indeed adopt the expected cloverleaf folding (35). While the cloverleaf shape was readily aminoacylated, the extended hairpin structure was inactive in aminoacylation experiments in vitro (106). Analysis of post-transcriptional modification events in vitro indicated that further introduction of m2G at position 10 and pseudouridines at positions 27 and 28 depended strongly on the cloverleaf structure and thus on previous synthesis of m1A at position 9 (102). Thus, a post-transcriptional modification by a single methyl group controls biological function by means of controlling a structural rearrangement. While an analysis of mechanistic aspects of the modification process (13,107) would go beyond the scope of this review, it is clear that the insights coming from such studies might consolidate kinetic and thermodynamic aspects of alternative RNA structures and thus be of high interest. The above example impressively illustrates how a single methyl group can bring about a conformational rearrangement by breaking one single Watson–Crick base pair. Such a base pair contributes roughly 12 kJ/mol to the free Gibbs energy, and its neutralization may thus bring about a large change in the equilibrium constant K. It is tempting to speculate that such a mechanism may be used for regulation of activity and stability of the respective tRNA. The recent evidence of involvement of tRNA modification in a structure-specific degradation pathway (20,90) might indeed be indicative of a more general and widespread mechanism for tRNA maintenance, which has thus far gone unnoticed. As is typical for animal mitochondrial tRNAs, mt tRNALys carries relatively few modified nucleotides, compared to its cytosolic counterpart (96). As already suggested by the differential impact of defective PUS1 (93,94) on mitochondrial and cytosolic tRNAs, the relative importance of a single modification may be much bigger in mitochondrial tRNAs. Similar importance of nucleotide modification in mitochondrial tRNAs is indeed suggested by the fact that unmodified transcripts are mostly poor substrates for their cognate synthetases (97,108–110). That a strong impact of a single modification on secondary structure has been observed in a mitochondrial tRNA rather than in a cytosolic one is, therefore, probably not a coincidence, even though the clarity of it might have been a stroke of luck.
Figure 5.
Methylation-induced rearrangement of human mitochondrial tRNALys. (A) Cloverleaf secondary structure of the fully modified human mitochondrial tRNALys. (B) The extended hairpin secondary structure of the unmodified transcript of human mitochondrial tRNALys (left) is converted to the cloverleaf by methylation on N1-A9, as evidenced in a chimeric tRNA containing m1A9 as single modified nucleotide (right). The methylation prevents an A9-U64 base pair (boxed) in the extended hairpin structure on the left. Modified nucleotides in bold are abbreviated according to references (96,122).
Other RNAs
Pseudouridine is a ubiquitous and abundant modified nucleotide which was found to improve RNA stability in a number of cases including rRNA from the small ribosomal subunit (111,112). In eukaryotic U2 snRNA, the extra hydrogen bond of a conserved pseudouridine significantly alters the branch-site architecture, with important consequences for splicing activity. A dynamic equilibrium of a nonfunctional conformation, in which the adenosine 24 is stacked within a helix, and a functional extrahelical conformation of the same adenosine is thought to occur in the unmodified snRNA. The pseudouridine lowers the free Gibbs energy enough to shift the equilibrium towards the functional extrahelical conformation (113,114). Ribosomal RNA, especially in eukaryotes, contains many modified bases, although its large size depresses the overall ratio to unmodified bases. The large majority of the nucleotide modifications consists of pseudouridines and 2′-OH ribose methylations. The process of modification and the modification enzymes themselves are involved in the complicated and highly coordinated process of rRNA processing and assembly in the nucleolus, including post-transcriptional modifications involved in regulatory processes (115). Since rRNA exists and functions as a ribonucleoprotein (RNP), the situation becomes both simpler and more complicated than in tRNA. It becomes simpler for the RNA to adopt its functional structure, because permanent association to a protein with potential for ‘structure induction’ and/or ‘structure capture’ (13) provides a multitude of pathways to overcome the RNA folding problem without the need for nucleotide modification. However, for the scientist it is challenging to investigate and define a native and functional RNA structure, because by definition the naked RNA of an RNP cannot adopt it. A number of modified nucleotides in rRNA stand out, because they are highly conserved and neither pseudouridines nor ribose methylations. A possible role of such particular nucleotide modifications has been hinted at by the Micura laboratory. A highly conserved stem–loop structure in helix 45 of the small ribosomal subunit, with an equally high conserved methylation pattern situated in a tetraloop was investigated (24,116). These four base methylations are effected by a single enzyme and are accompanied by other base methylations in some species [reviewed in (115)]. Höbartner et al. (24) could show the potential of these methylations to shift a dynamic equilibrium of alternative structures towards the biologically active tetraloop structure, as illustrated in Figure 6. Using several short oligoribonucleotides derived from the stem–loop structure, thermodynamic parameters were determined by NMR and UV-melting experiments These investigations were based on insights from a groundwork study on conformation space restriction by naturally occurring base methylations (24,36,117). Several groups have recently shown the potential of base modifications in problems of fundamental research. Using custom-made modifications they were able to release a previously restricted RNA conformation into an extended conformation space by chemical (118) or photochemical (119) removal of the modification in situ. Such applications are extremely useful for studying RNA rearrangements (116).
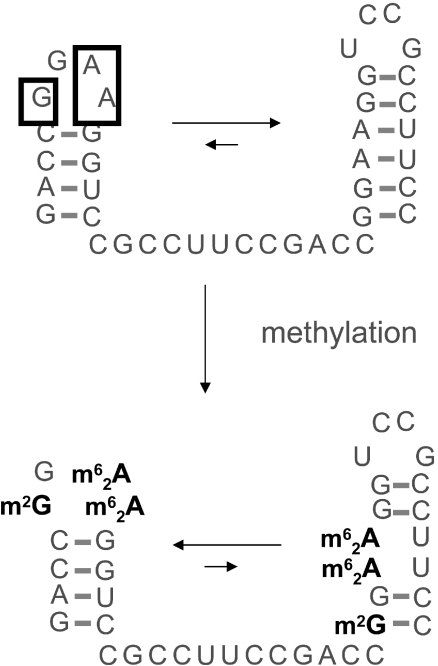
Figure 6.
Possible influence of a conserved methylation pattern in rRNA. An unmodified oligoribonucleotide derived from helix 45 of a bacterial small subunit rRNA shows two alternative conformations in equilibrium, with K around 3, favouring the structure shown on the upper right. Introduction of several methyl groups restricts conformation space and results in the equilibrium being shifted almost completely to the structure shown on the lower left. Modified nucleotides are abbreviated according to reference (24).
CONCLUSION AND OUTLOOK
Because of the extensive literature available on RNA nucleotide modification, this review has focused on such cases where significant structural changes of RNA conformation were observed as a consequence of nucleotide modification. It is important to understand the impact of nucleotide modification on RNA structure, since structure mediates all RNA function. This underscores once more the artificial character of the distinction between ‘functional’ and ‘structural’ modifications. These terms had initially been defined in this review in order to separate functional effects, mediated by direct contact of modified nucleotides with other molecules, from effects mediated by modification-induced conformational changes. As has become obvious, most evidence for a role of nucleotide modification in RNA folding has come from studies of tRNA, which maintain their structure without the help of proteins. Evidence is accumulating that thermodynamic and metabolic stabilization of classical tRNA is performed by the ensemble of ‘structural’ modifications, but that the effect of any single modification is not critical. This is in agreement with the fact that critical importance of single modifications have so far only been documented for tRNAs which deviate at least to some extent from the classical pattern shown in Figure 2B. One of these, initiator from yeast, has opened a new area of research, which is likely to show that expression and stability of tRNA may be more strictly controlled by modification events than hitherto assumed. Transfer RNAs, as the most heavily modified RNAs, thus still have a lot of secrets left to be discovered, while the structural impact of nucleotide modification in other RNAs is only starting to unveil.
The example of rRNA (116) illustrates well the problems one has to face when trying to investigate a modification-induced structural rearrangement in a large RNA, which actually has little physiological relevance when devoid of proteins. The X-ray structures of the ribosomal subunits have indeed revealed that the role of ribosomal proteins is mainly one of structural support for the RNA (67,120). One might thus speculate that nucleotide modification as an ancient remedy for RNA misfolding has been replaced to a certain degree by proteins, especially in those RNAs which today function as RNP.
At a time were small RNAs with new functions are discovered on a daily basis, the recent discovery of the involvement of pseudouridine formation in gene regulation might represent the tip of an iceberg (19), and post-transcriptional modification may turn out to be a factor of more general importance in gene expression than hitherto assumed. And why should modification-induced structural rearrangement not be involved? Advances in chemical synthesis of modified nucleotides and the rapidly progressing identification of enzymes involved in RNA modification will certainly lead to more detailed insights in the near future.
Acknowledgments
The author acknowledges funding by the DFG grant HE 3397/3-1. The author would like to thank Henri Grosjean, Jaunius Urbonavicius, Roberto Fiammengo and the referees for extremely helpful comments on such a vast topic. The author apologizes to those colleagues whose work could not be cited here. The Open Access publication charges for this article were waived by Oxford University Press.
Conflict of interest statement. None declared.
REFERENCES
- 1.Garcia G.A., Goodenough-Lashua D.M. Mechanisms of RNA-modifying and -editing enzymes. In: Grosjean H., Benne R., editors. Modification and Editing of RNA. Washington DC: ASM Press; 1998. pp. 135–168. [Google Scholar]
- 2.Leontis N.B., Westhof E. Geometric nomenclature and classification of RNA base pairs. RNA. 2001;7:499–512. doi: 10.1017/s1355838201002515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leontis N.B., Stombaugh J., Westhof E. The non-Watson–Crick base pairs and their associated isostericity matrices. Nucleic Acids Res. 2002;30:3497–3531. doi: 10.1093/nar/gkf481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sczyrba A., Kruger J., Mersch H., Kurtz S., Giegerich R. RNA-related tools on the Bielefeld Bioinformatics server. Nucleic Acids Res. 2003;31:3767–3770. doi: 10.1093/nar/gkg576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flamm C., Fontana W., Hofacker I.L., Schuster P. RNA folding at elementary step resolution. RNA. 2000;6:325–338. doi: 10.1017/s1355838200992161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuart J.W., Koshlap K.M., Guenther R., Agris P.F. Naturally-occurring modification restricts the anticodon domain conformational space of tRNA(Phe) J. Mol. Biol. 2003;334:901–918. doi: 10.1016/j.jmb.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 7.Grosjean H., Benne R., editors. Modification and Editing of RNA. Washington, DC: ASM Press; 1998. [Google Scholar]
- 8.Grosjean H., editor. Fine-Tuning of RNA Functions by Modification and Editing. Berlin Heidelberg: Springer; 2005. [Google Scholar]
- 9.Herschlag D. RNA chaperones and the RNA folding problem. J. Biol. Chem. 1995;270:20871–20874. doi: 10.1074/jbc.270.36.20871. [DOI] [PubMed] [Google Scholar]
- 10.Woodson S.A. Recent insights on RNA folding mechanisms from catalytic RNA. Cell Mol. Life Sci. 2000;57:796–808. doi: 10.1007/s000180050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makarova O.V., Makarov E.M., Sousa R., Dreyfus M. Transcribing of Escherichia coli genes with mutant T7 RNA polymerases: stability of lacZ mRNA inversely correlates with polymerase speed. Proc. Natl Acad. Sci. USA. 1995;92:12250–12254. doi: 10.1073/pnas.92.26.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pape T., Wintermeyer W., Rodnina M.V. Complete kinetic mechanism of elongation factor Tu-dependent binding of aminoacyl-tRNA to the A site of the E.coli ribosome. EMBO J. 1998;17:7490–7497. doi: 10.1093/emboj/17.24.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorsch J.R. RNA chaperones exist and DEAD box proteins get a life. Cell. 2002;109:797–800. doi: 10.1016/s0092-8674(02)00804-8. [DOI] [PubMed] [Google Scholar]
- 14.Wickiser J.K., Winkler W.C., Breaker R.R., Crothers D.M. The speed of RNA transcription and metabolite binding kinetics operate an FMN riboswitch. Mol. Cell. 2005;18:49–60. doi: 10.1016/j.molcel.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 15.Winkler W.C., Breaker R.R. Genetic control by metabolite-binding riboswitches. Chembiochem. 2003;4:1024–1032. doi: 10.1002/cbic.200300685. [DOI] [PubMed] [Google Scholar]
- 16.Mandal M., Breaker R.R. Gene regulation by riboswitches. Nature Rev. Mol. Cell Biol. 2004;5:451–463. doi: 10.1038/nrm1403. [DOI] [PubMed] [Google Scholar]
- 17.Sudarsan N., Wickiser J.K., Nakamura S., Ebert M.S., Breaker R.R. An mRNA structure in bacteria that controls gene expression by binding lysine. Genes Dev. 2003;17:2688–2697. doi: 10.1101/gad.1140003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkler W.C., Nahvi A., Sudarsan N., Barrick J.E., Breaker R.R. An mRNA structure that controls gene expression by binding S-adenosylmethionine. Nature Struct. Biol. 2003;10:701–707. doi: 10.1038/nsb967. [DOI] [PubMed] [Google Scholar]
- 19.Zhao X., Patton J.R., Davis S.L., Florence B., Ames S.J., Spanjaard R.A. Regulation of nuclear receptor activity by a pseudouridine synthase through posttranscriptional modification of steroid receptor RNA activator. Mol. Cell. 2004;15:549–558. doi: 10.1016/j.molcel.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 20.Kadaba S., Krueger A., Trice T., Krecic A.M., Hinnebusch A.G., Anderson J. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev. 2004;18:1227–1240. doi: 10.1101/gad.1183804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinberg S., Cedergren R. A correlation between N2-dimethylguanosine presence and alternate tRNA conformers. RNA. 1995;1:886–891. [PMC free article] [PubMed] [Google Scholar]
- 22.Helm M., Brule H., Degoul F., Cepanec C., Leroux J.P., Giege R., Florentz C. The presence of modified nucleotides is required for cloverleaf folding of a human mitochondrial tRNA. Nucleic Acids Res. 1998;26:1636–1643. doi: 10.1093/nar/26.7.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helm M., Florentz C., Chomyn A., Attardi G. Search for differences in post-transcriptional modification patterns of mitochondrial DNA-encoded wild-type and mutant human tRNALys and tRNALeu(UUR) Nucleic Acids Res. 1999;27:756–763. doi: 10.1093/nar/27.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Höbartner C., Ebert M.O., Jaun B., Micura R. Two-state conformation equilibria and the effect of nucleobase methylation. Angew. Chem. Int. Ed. 2002;41:605–609. [Google Scholar]
- 25.Rozenski J., Crain P.F., McCloskey J.A. The RNA Modification Database: 1999 update. Nucleic Acids Res. 1999;27:196–197. doi: 10.1093/nar/27.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis D.R. Biophysical and conformational properties of modified nucleosides in RNA (nuclear magnetic resonance studies) In: Grosjean H., Benne R., editors. Modification and Editing of RNA. Washington DC: 1998. pp. 85–102. [Google Scholar]
- 27.Yarian C.S., Basti M.M., Cain R.J., Ansari G., Guenther R.H., Sochacka E., Czerwinska G., Malkiewicz A., Agris P.F. Structural and functional roles of the N1- and N3-protons of psi at tRNA's position 39. Nucleic Acids Res. 1999;27:3543–3549. doi: 10.1093/nar/27.17.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durant P.C., Davis D.R. Stabilization of the anticodon stem-loop of tRNALys,3 by an A+-C base-pair and by pseudouridine. J. Mol. Biol. 1999;285:115–131. doi: 10.1006/jmbi.1998.2297. [DOI] [PubMed] [Google Scholar]
- 29.Charette M., Gray M.W. Pseudouridine in RNA: what, where, how, and why. IUBMB Life. 2000;49:341–351. doi: 10.1080/152165400410182. [DOI] [PubMed] [Google Scholar]
- 30.Arnez J.G., Steitz T.A. Crystal structure of unmodified tRNA(Gln) complexed with glutaminyl-tRNA synthetase and ATP suggests a possible role for pseudo-uridines in stabilization of RNA structure. Biochemistry. 1994;33:7560–7567. doi: 10.1021/bi00190a008. [DOI] [PubMed] [Google Scholar]
- 31.Auffinger P., Westhof E. Rules governing the orientation of the 2′-hydroxyl group in RNA. J. Mol. Biol. 1997;274:54–63. doi: 10.1006/jmbi.1997.1370. [DOI] [PubMed] [Google Scholar]
- 32.Auffinger P., Westhof E. Effects of Pseudouridylation on tRNA Hydration and Dynamics. In: Grosjean H., Benne R., editors. Modification and Editing of RNA. Washington: ASM Press; 1998. pp. 103–112. [Google Scholar]
- 33.Agris P.F. The importance of being modified: roles of modified nucleosides and Mg2+ in RNA structure and function. Prog. Nucleic Acid Res. Mol. Biol. 1996;53:79–129. doi: 10.1016/s0079-6603(08)60143-9. [DOI] [PubMed] [Google Scholar]
- 34.Kierzek E., Kierzek R. The thermodynamic stability of RNA duplexes and hairpins containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines. Nucleic Acids Res. 2003;31:4472–4480. doi: 10.1093/nar/gkg633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helm M., Giege R., Florentz C. A Watson–Crick base-pair-disrupting methyl group (m1A9) is sufficient for cloverleaf folding of human mitochondrial tRNALys. Biochemistry. 1999;38:13338–13346. doi: 10.1021/bi991061g. [DOI] [PubMed] [Google Scholar]
- 36.Micura R., Pils W., Hobartner C., Grubmayr K., Ebert M.O., Jaun B. Methylation of the nucleobases in RNA oligonucleotides mediates duplex-hairpin conversion. Nucleic Acids Res. 2001;29:3997–4005. doi: 10.1093/nar/29.19.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiley S.L., Jackman J., Babak T., Trochesset M., Morris Q.D., Phizicky E., Hughes T.R. Detection and discovery of RNA modifications using microarrays. Nucleic Acids Res. 2005;33:e2. doi: 10.1093/nar/gni002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agris P.F., Sierzputowska-Gracz H., Smith C. Transfer RNA contains sites of localized positive charge: carbon NMR studies of [13C]methyl-enriched Escherichia coli and yeast tRNAPhe. Biochemistry. 1986;25:5126–5131. doi: 10.1021/bi00366a022. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y., Sierzputowska-Gracz H., Guenther R., Everett K., Agris P.F. 5-Methylcytidine is required for cooperative binding of Mg2+ and a conformational transition at the anticodon stem-loop of yeast phenylalanine tRNA. Biochemistry. 1993;32:10249–10253. doi: 10.1021/bi00089a047. [DOI] [PubMed] [Google Scholar]
- 40.Grosjean H., Edqvist J., Straby K.B., Giegé R. Enzymatic formation of modified nucleosides in tRNA: dependence on tRNA architecture. J. Mol. Biol. 1996;255:67–85. doi: 10.1006/jmbi.1996.0007. [DOI] [PubMed] [Google Scholar]
- 41.Benas P., Bec G., Keith G., Marquet R., Ehresmann C., Ehresmann B., Dumas P. The crystal structure of HIV reverse-transcription primer tRNA(Lys,3) shows a canonical anticodon loop. RNA. 2000;6:1347–1355. doi: 10.1017/s1355838200000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stuart J.W., Gdaniec Z., Guenther R., Marszalek M., Sochacka E., Malkiewicz A., Agris P.F. Functional anticodon architecture of human tRNALys3 includes disruption of intraloop hydrogen bonding by the naturally occurring amino acid modification, t6A. Biochemistry. 2000;39:13396–13404. doi: 10.1021/bi0013039. [DOI] [PubMed] [Google Scholar]
- 43.Kim S.H., Suddath F.L., Quigley G.J., McPherson A., Sussman J.L., Wang A.H., Seeman N.C., Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974;185:435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- 44.Yarian C., Marszalek M., Sochacka E., Malkiewicz A., Guenther R., Miskiewicz A., Agris P.F. Modified nucleoside dependent Watson–Crick and wobble codon binding by tRNALysUUU species. Biochemistry. 2000;39:13390–13395. doi: 10.1021/bi001302g. [DOI] [PubMed] [Google Scholar]
- 45.Cabello-Villegas J., Tworowska I., Nikonowicz E.P. Metal ion stabilization of the U-turn of the A37 N6-dimethylallyl-modified anticodon stem–loop of Escherichia coli tRNAPhe. Biochemistry. 2004;43:55–66. doi: 10.1021/bi0353676. [DOI] [PubMed] [Google Scholar]
- 46.Cabello-Villegas J., Winkler M.E., Nikonowicz E.P. Solution conformations of unmodified and A(37)N(6)-dimethylallyl modified anticodon stem-loops of Escherichia coli tRNA(Phe) J. Mol. Biol. 2002;319:1015–1034. doi: 10.1016/S0022-2836(02)00382-0. [DOI] [PubMed] [Google Scholar]
- 47.Kierzek E., Kierzek R. The synthesis of oligoribonucleotides containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines via post-synthetic modification of precursor oligomers. Nucleic Acids Res. 2003;31:4461–4471. doi: 10.1093/nar/gkg632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agris P.F., Guenther R., Ingram P.C., Basti M.M., Stuart J.W., Sochacka E., Malkiewicz A. Unconventional structure of tRNA(Lys)SUU anticodon explains tRNA's role in bacterial and mammalian ribosomal frameshifting and primer selection by HIV-1. RNA. 1997;3:420–428. [PMC free article] [PubMed] [Google Scholar]
- 49.Durant P.C., Bajji A.C., Sundaram M., Kumar R.K., Davis D.R. Structural effects of hypermodified nucleosides in the Escherichia coli and human tRNALys anticodon loop: the effect of nucleosides s2U, mcm(5)u, mcm5S2U, mnm5S2U, t6A, and ms2t6A. Biochemistry. 2005;44:8078–8089. doi: 10.1021/bi050343f. [DOI] [PubMed] [Google Scholar]
- 50.Sundaram M., Durant P.C., Davis D.R. Hypermodified nucleosides in the anticodon of tRNA(Lys) stabilize a canonical U-turn structure. Biochemistry. 2000;39:15652. doi: 10.1021/bi005120y. [DOI] [PubMed] [Google Scholar]
- 51.Giege R., Sissler M., Florentz C. Universal rules and idiosyncratic features in tRNA identity. Nucleic Acids Res. 1998;26:5017–5035. doi: 10.1093/nar/26.22.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bjork G.R., Durand J.M., Hagervall T.G., Leipuviene R., Lundgren H.K., Nilsson K., Chen P., Qian Q., Urbonavicius J. Transfer RNA modification: influence on translational frameshifting and metabolism. FEBS Lett. 1999;452:47–51. doi: 10.1016/s0014-5793(99)00528-1. [DOI] [PubMed] [Google Scholar]
- 53.Urbonavicius J., Qian Q., Durand J.M., Hagervall T.G., Bjork G.R. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 2001;20:4863–4873. doi: 10.1093/emboj/20.17.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Namy O., Lecointe F., Grosjean H., Rousset J.-P. Translational Recoding and RNA Modification. In: Grosjean H., editor. Fine-Tuning of RNA Functions by Modification and Editing. Berlin Heidelberg: Springer; 2005. pp. 309–340. [Google Scholar]
- 55.Hagervall T.G., Pomerantz S.C., McCloskey J.A. Reduced misreading of asparagine codons by Escherichia coli tRNALys with hypomodified derivatives of 5-methylaminoamethyl-2-thiouridine in the wobble position. J. Mol. Biol. 1998;284:33–42. doi: 10.1006/jmbi.1998.2162. [DOI] [PubMed] [Google Scholar]
- 56.Isel C., Lanchy J.M., Le Grice S.F., Ehresmann C., Ehresmann B., Marquet R. Specific initiation and switch to elongation of human immunodeficiency virus type 1 reverse transcription require the post-transcriptional modifications of primer tRNA3Lys. EMBO J. 1996;15:917–924. [PMC free article] [PubMed] [Google Scholar]
- 57.Tisne C., Rigourd M., Marquet R., Ehresmann C., Dardel F. NMR and biochemical characterization of recombinant human tRNA(Lys)3 expressed in Escherichia coli: identification of posttranscriptional nucleotide modifications required for efficient initiation of HIV-1 reverse transcription. RNA. 2000;6:1403–1412. doi: 10.1017/s1355838200000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agris P.F. Decoding the genome: a modified view. Nucleic Acids Res. 2004;32:223–238. doi: 10.1093/nar/gkh185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Persson T., Kutzke U., Busch S., Held R., Hartmann R.K. Chemical synthesis and biological investigation of a 77-mer oligoribonucleotide with a sequence corresponding to E.coli tRNA(Asp) Bioorg. Med. Chem. 2001;9:51–56. doi: 10.1016/s0968-0896(00)00218-2. [DOI] [PubMed] [Google Scholar]
- 60.Davanloo P., Sprinzl M., Watanabe K., Albani M., Kersten H. Role of ribothymidine in the thermal stability of transfer RNA as monitored by proton magnetic resonance. Nucleic Acids Res. 1979;6:1571–1581. doi: 10.1093/nar/6.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sampson J.R., Uhlenbeck O.C. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc. Natl Acad. Sci. USA. 1988;85:1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hall K.B., Sampson J.R., Uhlenbeck O.C., Redfield A.G. Structure of an unmodified tRNA molecule. Biochemistry. 1989;28:5794–5801. doi: 10.1021/bi00440a014. [DOI] [PubMed] [Google Scholar]
- 63.Maglott E.J., Deo S.S., Przykorska A., Glick G.D. Conformational transitions of an unmodified tRNA: implications for RNA folding. Biochemistry. 1998;37:16349–16359. doi: 10.1021/bi981722u. [DOI] [PubMed] [Google Scholar]
- 64.Chu W.C., Horowitz J. 19F NMR of 5-fluorouracil-substituted transfer RNA transcribed in vitro: resonance assignment of fluorouracil-guanine base pairs. Nucleic Acids Res. 1989;17:7241–7252. doi: 10.1093/nar/17.18.7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Derrick W.B., Horowitz J. Probing structural differences between native and in vitro transcribed Escherichia coli valine transfer RNA: evidence for stable base modification-dependent conformers. Nucleic Acids Res. 1993;21:4948–4953. doi: 10.1093/nar/21.21.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yue D., Kintanar A., Horowitz J. Nucleoside modifications stabilize Mg2+ binding in Escherichia coli tRNA(Val): an imino proton NMR investigation. Biochemistry. 1994;33:8905–8911. doi: 10.1021/bi00196a007. [DOI] [PubMed] [Google Scholar]
- 67.Kintanar A., Yue D., Horowitz J. Effect of nucleoside modifications on the structure and thermal stability of Escherichia coli valine tRNA. Biochimie. 1994;76:1192–1204. doi: 10.1016/0300-9084(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 68.Vermeulen A., McCallum S.A., Pardi A. Comparison of the global structure and dynamics of native and unmodified tRNAval. Biochemistry. 2005;44:6024–6033. doi: 10.1021/bi0473399. [DOI] [PubMed] [Google Scholar]
- 69.Serebrov V., Vassilenko K., Kholod N., Gross H.J., Kisselev L. Mg2+ binding and structural stability of mature and in vitro synthesized unmodified Escherichia coli tRNAPhe. Nucleic Acids Res. 1998;26:2723–2728. doi: 10.1093/nar/26.11.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perret V., Garcia A., Puglisi J., Grosjean H., Ebel J.P., Florentz C., Giege R. Conformation in solution of yeast tRNA(Asp) transcripts deprived of modified nucleotides. Biochimie. 1990;72:735–743. doi: 10.1016/0300-9084(90)90158-d. [DOI] [PubMed] [Google Scholar]
- 71.Niederberger C., Graub R., Costa A., Desgres J., Schweingruber M.E. The tRNA N2,N2-dimethylguanosine-26 methyltransferase encoded by gene trm1 increases efficiency of suppression of an ochre codon in Schizosaccharomyces pombe. FEBS Lett. 1999;464:67–70. doi: 10.1016/s0014-5793(99)01679-8. [DOI] [PubMed] [Google Scholar]
- 72.Björk G.R. Genetic dissection of synthesis and function of modified nucleosides in bacterial transfer RNA. Prog. Nucleic Acid Res. Mol. Biol. 1995;50:263–338. doi: 10.1016/s0079-6603(08)60817-x. [DOI] [PubMed] [Google Scholar]
- 73.Hopper A.K., Phizicky E.M. tRNA transfers to the limelight. Genes Dev. 2003;17:162–180. doi: 10.1101/gad.1049103. [DOI] [PubMed] [Google Scholar]
- 74.Anderson J., Phan L., Cuesta R., Carlson B.A., Pak M., Asano K., Bjork G.R., Tamame M., Hinnebusch A.G. The essential Gcd10p-Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev. 1998;12:3650–3662. doi: 10.1101/gad.12.23.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anderson J., Phan L., Hinnebusch A.G. The Gcd10p/Gcd14p complex is the essential two-subunit tRNA(1-methyladenosine) methyltransferase of Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA. 2000;97:5173–5178. doi: 10.1073/pnas.090102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alexandrov A., Grayhack E.J., Phizicky E.M. tRNA m7G methyltransferase Trm8p/Trm82p: evidence linking activity to a growth phenotype and implicating Trm82p in maintaining levels of active Trm8p. RNA. 2005;11:821–830. doi: 10.1261/rna.2030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coutts S.M., Gangloff J., Dirheimer G. Conformational transitions in tRNA Asp (brewer's yeast). Thermodynamic, kinetic, and enzymatic measurements on oligonucleotide fragments and the intact molecule. Biochemistry. 1974;13:3938–3948. doi: 10.1021/bi00716a019. [DOI] [PubMed] [Google Scholar]
- 78.Filimonov V.V., Privalov P.L., Glangloff J., Dirheimer G. A calorimetric investigation of melting of tRNAAsp from brewer's yeast. Biochim. Biophys. Acta. 1978;521:209–216. doi: 10.1016/0005-2787(78)90263-0. [DOI] [PubMed] [Google Scholar]
- 79.Nobles K.N., Yarian C.S., Liu G., Guenther R.H., Agris P.F. Highly conserved modified nucleosides influence Mg2+-dependent tRNA folding. Nucleic Acids Res. 2002;30:4751–4760. doi: 10.1093/nar/gkf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gu X., Santi D.V. The T-arm of tRNA is a substrate for tRNA (m5U54)-methyltransferase. Biochemistry. 1991;30:2999–3002. doi: 10.1021/bi00226a003. [DOI] [PubMed] [Google Scholar]
- 81.Gu X., Ivanetich K.M., Santi D.V. Recognition of the T-arm of tRNA by tRNA (m5U54)-methyltransferase is not sequence specific. Biochemistry. 1996;35:11652–11659. doi: 10.1021/bi9612125. [DOI] [PubMed] [Google Scholar]
- 82.Becker H.F., Motorin Y., Sissler M., Florentz C., Grosjean H. Major identity determinants for enzymatic formation of ribothymidine and pseudouridine in the T psi-loop of yeast tRNAs. J. Mol. Biol. 1997;274:505–518. doi: 10.1006/jmbi.1997.1417. [DOI] [PubMed] [Google Scholar]
- 83.Nishikura K., De Robertis E.M. RNA processing in microinjected Xenopus oocytes. Sequential addition of base modifications in the spliced transfer RNA. J. Mol. Biol. 1981;145:405–420. doi: 10.1016/0022-2836(81)90212-6. [DOI] [PubMed] [Google Scholar]
- 84.Maden B.E.H. Intracellular locations of RNA-modifying enzymes. In: Grosjean H., Benne R., editors. Modification and Editing of RNA. Washington, DC: ASM Press; 1998. pp. 421–440. [Google Scholar]
- 85.Constantinesco F., Motorin Y., Grosjean H. Transfer RNA modification enzymes from Pyrococcus furiosus: detection of the enzymatic activities in vitro. Nucleic Acids Res. 1999;27:1308–1315. doi: 10.1093/nar/27.5.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Armengaud J., Urbonavicius J., Fernandez B., Chaussinand G., Bujnicki J.M., Grosjean H. N2-methylation of guanosine at position 10 in tRNA is catalyzed by a THUMP domain-containing, S-adenosylmethionine-dependent methyltransferase, conserved in Archaea and Eukaryota. J. Biol. Chem. 2004;279:37142–37152. doi: 10.1074/jbc.M403845200. [DOI] [PubMed] [Google Scholar]
- 87.Constantinesco F., Motorin Y., Grosjean H. Characterisation and enzymatic properties of tRNA(guanine 26, N (2), N (2))-dimethyltransferase (Trm1p) from Pyrococcus furiosus. J. Mol. Biol. 1999;291:375–392. doi: 10.1006/jmbi.1999.2976. [DOI] [PubMed] [Google Scholar]
- 88.Urbonavicius J., Armengaud J., Grosjean H. Identity elements required for enzymatic formation of N2, N2-dimethylguanosine from N2-monomethylated derivative and ist possible role in avoiding alternative conformations in archaeal tRNA. J. Mol. Biol. 2006 doi: 10.1016/j.jmb.2005.12.087. in press. [DOI] [PubMed] [Google Scholar]
- 89.Basavappa R., Sigler P.B. The 3 A crystal structure of yeast initiator tRNA: functional implications in initiator/elongator discrimination. EMBO J. 1991;10:3105–3111. doi: 10.1002/j.1460-2075.1991.tb07864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vanacova S., Wolf J., Martin G., Blank D., Dettwiler S., Friedlein A., Langen H., Keith G., Keller W. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol. 2005;3:e189. doi: 10.1371/journal.pbio.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Z., Reimers S., Pandit S., Deutscher M.P. RNA quality control: degradation of defective transfer RNA. EMBO J. 2002;21:1132–1138. doi: 10.1093/emboj/21.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Helm M., Brule H., Friede D., Giege R., Pütz J., Florentz C. Search for characteristic structural features of mammalian mitochondrial tRNAs. RNA. 2000;6:1356–1379. doi: 10.1017/s1355838200001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bykhovskaya Y., Casas K., Mengesha E., Inbal A., Fischel-Ghodsian N. Missense mutation in pseudouridine synthase 1 (PUS1) causes mitochondrial myopathy and sideroblastic anemia (MLASA) Am. J. Hum. Genet. 2004;74:1303–1308. doi: 10.1086/421530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Patton J.R., Bykhovskaya Y., Mengesha E., Bertolotto C., Fischel-Ghodsian N. Mitochondrial myopathy and sideroblastic anemia (MLASA): missense mutation in the pseudouridine synthase 1 (PUS1) gene is associated with the loss of tRNA pseudouridylation. J. Biol. Chem. 2005;280:19823–19828. doi: 10.1074/jbc.M500216200. [DOI] [PubMed] [Google Scholar]
- 95.Florentz C., Sohm B., Tryoen-Toth P., Pütz J., Sissler M. Human mitochondrial tRNAs in health and disease. Cell Mol. Life Sci. 2003;60:1356–1375. doi: 10.1007/s00018-003-2343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sprinzl M., Vassilenko K.S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 2005;33:D139–D140. doi: 10.1093/nar/gki012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bullard J.M., Cai Y.C., Spremulli L.L. Expression and characterization of the human mitochondrial leucyl-tRNA synthetase. Biochim. Biophys. Acta. 2000;1490:245–258. doi: 10.1016/s0167-4781(99)00240-7. [DOI] [PubMed] [Google Scholar]
- 98.Sohm B., Frugier M., Brule H., Olszak K., Przykorska A., Florentz C. Towards understanding human mitochondrial leucine aminoacylation identity. J. Mol. Biol. 2003;328:995–1010. doi: 10.1016/s0022-2836(03)00373-5. [DOI] [PubMed] [Google Scholar]
- 99.Wittenhagen L.M., Roy M.D., Kelley S.O. The pathogenic U3271C human mitochondrial tRNA(Leu(UUR)) mutation disrupts a fragile anticodon stem. Nucleic Acids Res. 2003;31:596–601. doi: 10.1093/nar/gkg131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roy M.D., Wittenhagen L.M., Vozzella B.E., Kelley S.O. Interdomain communication between weak structural elements within a disease-related human tRNA. Biochemistry. 2004;43:384–392. doi: 10.1021/bi035711z. [DOI] [PubMed] [Google Scholar]
- 101.Zagryadskaya E.I., Kelley S.O. Combinatorial analysis of loop nucleotides in human mitochondrial tRNALeu(UUR) Biochemistry. 2005;44:233–242. doi: 10.1021/bi0489560. [DOI] [PubMed] [Google Scholar]
- 102.Helm M., Attardi G. Nuclear control of cloverleaf structure of human mitochondrial tRNA(Lys) J. Mol. Biol. 2004;337:545–560. doi: 10.1016/j.jmb.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 103.Wittenhagen L.M., Kelley S.O. Dimerization of a pathogenic human mitochondrial tRNA. Nature Struct. Biol. 2002;9:586–590. doi: 10.1038/nsb820. [DOI] [PubMed] [Google Scholar]
- 104.Roy M.D., Wittenhagen L.M., Kelley S.O. Structural probing of a pathogenic tRNA dimer. RNA. 2005;11:254–260. doi: 10.1261/rna.7143305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Steinberg S., Gautheret D., Cedergren R. Fitting the structurally diverse animal mitochondrial tRNAsSer to common three-dimensional constraints. J. Mol. Biol. 1994;236:982–989. doi: 10.1016/0022-2836(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 106.Sissler M., Helm M., Frugier M., Giege R., Florentz C. Aminoacylation properties of pathology-related human mitochondrial tRNA(Lys) variants. RNA. 2004;10:841–853. doi: 10.1261/rna.5267604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ishitani R., Nureki O., Nameki N., Okada N., Nishimura S., Yokoyama S. Alternative tertiary structure of tRNA for recognition by a posttranscriptional modification enzyme. Cell. 2003;113:383–394. doi: 10.1016/s0092-8674(03)00280-0. [DOI] [PubMed] [Google Scholar]
- 108.Bullard J.M., Cai Y.C., Demeler B., Spremulli L.L. Expression and characterization of a human mitochondrial phenylalanyl-tRNA synthetase. J. Mol. Biol. 1999;288:567–577. doi: 10.1006/jmbi.1999.2708. [DOI] [PubMed] [Google Scholar]
- 109.Tolkunova E., Park H., Xia J., King M.P., Davidson E. The human lysyl-tRNA synthetase gene encodes both the cytoplasmic and mitochondrial enzymes by means of an unusual alternative splicing of the primary transcript. J. Biol. Chem. 2000;275:35063–35069. doi: 10.1074/jbc.M006265200. [DOI] [PubMed] [Google Scholar]
- 110.Yokogawa T., Shimada N., Takeuchi N., Benkowski L., Suzuki T., Omori A., Ueda T., Nishikawa K., Spremulli L.L., Watanabe K. Characterization and tRNA recognition of mammalian mitochondrial seryl-tRNA synthetase. J. Biol. Chem. 2000;275:19913–19920. doi: 10.1074/jbc.M908473199. [DOI] [PubMed] [Google Scholar]
- 111.Meroueh M., Grohar P.J., Qiu J., SantaLucia J., Jr, Scaringe S.A., Chow C.S. Unique structural and stabilizing roles for the individual pseudouridine residues in the 1920 region of Escherichia coli 23S rRNA. Nucleic Acids Res. 2000;28:2075–2083. doi: 10.1093/nar/28.10.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sumita M., Desaulniers J.P., Chang Y.C., Chui H.M., Clos L., II, Chow C.S. Effects of nucleotide substitution and modification on the stability and structure of helix 69 from 28S rRNA. RNA. 2005;11:1420–1429. doi: 10.1261/rna.2320605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Newby M.I., Greenbaum N.L. A conserved pseudouridine modification in eukaryotic U2 snRNA induces a change in branch-site architecture. RNA. 2001;7:833–845. doi: 10.1017/s1355838201002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Greenbaum N.L. Role of a conserved pseudouridine in U2 snRNA on the structural and electrostatic features of the splicosomal pre-mRNA branch site. In: Grosjean H., editor. Fine-Tuning of RNA Functions by Modification and Editing. Berlin Heidelberg: Springer; 2005. pp. 205–218. [Google Scholar]
- 115.Lafontaine D.L.J., Tollervey D. Regulatory aspects of rRNA modifications and pre-rRNA processing. In: Grosjean H., Benne R., editors. Modification and Editing of RNA. Washington, DC: ASM Press; 1998. pp. 281–288. [Google Scholar]
- 116.Micura R., Hobartner C. On secondary structure rearrangements and equilibria of small RNAs. Chem. Biochem. 2003;4:984–990. doi: 10.1002/cbic.200300664. [DOI] [PubMed] [Google Scholar]
- 117.Hobartner C., Micura R. Bistable secondary structures of small RNAs and their structural probing by comparative imino proton NMR spectroscopy. J. Mol. Biol. 2003;325:421–431. doi: 10.1016/s0022-2836(02)01243-3. [DOI] [PubMed] [Google Scholar]
- 118.Hobartner C., Mittendorfer H., Breuker K., Micura R. Triggering of RNA secondary structures by a functionalized nucleobase. Angew Chem. Int. Ed. Engl. 2004;43:3922–3925. doi: 10.1002/anie.200460068. [DOI] [PubMed] [Google Scholar]
- 119.Wenter P., Furtig B., Hainard A., Schwalbe H., Pitsch S. Kinetics of photoinduced RNA refolding by real-time NMR spectroscopy. Angew Chem. Int. Ed. Engl. 2005;44:2600–2603. doi: 10.1002/anie.200462724. [DOI] [PubMed] [Google Scholar]
- 120.Ramakrishnan V. Ribosome structure and the mechanism of translation. Cell. 2002;108:557–572. doi: 10.1016/s0092-8674(02)00619-0. [DOI] [PubMed] [Google Scholar]
- 121.Auffinger P., Westhof E. Appendix 5: Location and distribution of modified nucleotides in tRNA. In: Grosjean H., Benne R., editors. Modification and Editing of RNA. Washington DC: 1998. pp. 569–577. [Google Scholar]
- 122.Suzuki T., Wada T., Saigo K., Watanabe K. Taurine as a constituent of mitochondrial tRNAs: new insights into the functions of taurine and human mitochondrial diseases. EMBO J. 2002;21:6581–6589. doi: 10.1093/emboj/cdf656. [DOI] [PMC free article] [PubMed] [Google Scholar]