Abstract
We have previously identified and characterized a heme/hemoglobin receptor, HmuR, in Porphyromonas gingivalis. To analyze the conserved amino acid residues of HmuR that may be involved in hemin/hemoprotein binding and utilization, we constructed a series of P. gingivalis A7436 hmuR mutants with amino acid replacements and characterized the ability of these mutants to utilize hemin and hemoproteins. Site-directed mutagenesis was employed to introduce mutations H95A, H434A, H95A-H434A, YRAP420-423YAAA, and NPDL442-445NAAA into HmuR in both P. gingivalis and Escherichia coli. Point mutations at H95 and H434 and in the NPDL motif of HmuR resulted in decreased binding to hemin, hemoglobin, and human serum albumin-hemin complex. Notably, mutations of these conserved sites and motifs led to reduced growth of P. gingivalis when human serum was used as the heme source. Analysis using a three-dimensional homology model of HmuR indicated that H95, H434, and the NPDL motif are present on apical or extracellular loops of HmuR, while the YRAP motif is present on the barrel wall. Taken together, these results support a role for H95, H434, and the NPDL motif of the P. gingivalis HmuR protein in heme binding and utilization of serum hemoproteins and the HmuR YRAP motif in serum hemoprotein utilization.
Iron, an essential nutrient for most pathogenic microorganisms, plays a pivotal role in microbial metabolism, toxicity, and pathogenesis. Iron-containing proteins not only take part in electron transport, but also participate in other enzymatic reactions which are required for bacterial survival. Despite the abundance of iron in nature, it can be a limiting nutrient for bacteria within their environmental niches. This is due in part to the complete sequestration of free iron by host iron-binding proteins (19, 54, 55). Within the human host, the majority of iron is found in the form of heme (a term used herein to denote either the ferrous or ferric form of iron protoporphyrin IX), which is the preferable iron source for bacterial growth (47, 51).
In vivo, heme is bound by heme-binding proteins such as hemoglobin, myoglobin, hemopexin, albumin, and cytochromes (8, 15). Most heme sources used by gram-negative pathogens are either too large to pass through the porin channels (>600 Da) or too scarce to be accumulated by passive diffusion (2, 54). To survive within the iron-limited environment of the host, microorganisms have developed elaborate systems for iron utilization (for reviews, see references 19 and 55). These include (i) the production of specific outer membrane receptors, which bind iron- or heme-containing complexes directly, (ii) the release of low-molecular-weight iron chelators, known as siderophores, which bind iron for uptake (36, 45, 56), and (iii) the production and secretion of heme-binding proteins called hemophores that bind heme and subsequently deliver heme to the outer membrane receptors for transport (21, 32, 46).
Porphyromonas gingivalis, a black-pigmented gram-negative anaerobic bacterium, has been implicated as a major etiological agent in the development and progression of chronic periodontitis (24). The majority of genes required for the de novo porphyrin biosynthetic pathway are absent in P. gingivalis and the bacterium must acquire protoporphyrin IX from the environment (31). P. gingivalis is capable of utilizing a broad range of heme-containing compounds such as hemoglobin, hemoglobin-bound haptoglobin, hemin-bound hemopexin, and hemin-saturated serum albumin (5, 53). Hemin, hemoglobin, and serum albumin utilization in P. gingivalis requires the participation of the cysteine proteases, referred to as gingipains (11, 30), including the lysine-specific gingipain Kgp and arginine-specific gingipain RgpA. Different portions of Kgp and RgpA can bind hemoglobin, hemin, and protoporphyrin IX (1, 14, 29, 40, 48). Gingipains have also been demonstrated to degrade host iron- and heme-containing proteins, including hemoglobin, hemopexin, haptoglobin, and transferrin (6, 33, 53). Kgp and RgpA may function both in a catalytic capacity and as hemophore-like proteins to capture heme and deliver it to an outer membrane receptor (41).
In addition to gingipains, P. gingivalis possesses additional outer membrane proteins which are utilized for the binding and transport of hemin (4, 20) and binding of hemoglobin (1, 18, 50). Several putative TonB-dependent outer membrane receptors have been described recently. These include Tlr (TonB-linked receptor) (52), IhtA (iron heme transport) (13), and HemR (hemin-regulated receptor) (26). However, none of these receptors has been characterized by detailed mutational analysis.
We have previously described a P. gingivalis outer membrane receptor, HmuR (hemin utilization receptor), which is utilized for both hemin and hemoglobin binding and hemin transport (41, 49, 50). The HmuR protein exhibits amino acid sequence homology to TonB-dependent receptors involved in heme, vitamin B12 or iron siderophore transport in other bacteria (49). A P. gingivalis hmuR isogenic mutant was found to exhibit a decreased ability to bind hemin and hemoglobin and impaired growth when hemin or hemoglobin was used as the sole iron source (49, 50). Escherichia coli cells overexpressing P. gingivalis HmuR as well as purified recombinant HmuR (rHmuR) were also demonstrated to bind hemin, hemoglobin, and serum albumin-hemin complex (41, 49).
To define the specific amino acid residues of HmuR involved in heme utilization in P. gingivalis, we constructed a series of hmuR mutants by site-directed mutagenesis and performed functional analyses of these mutants in both P. gingivalis and E. coli backgrounds. In addition, a three-dimensional homology model of the HmuR protein has been constructed for structural analysis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The P. gingivalis and E. coli strains used in this study are indicated in Table 1. P. gingivalis wild-type strain A7436 was maintained on anaerobic blood agar (ABA; Remel, Lenexa, KS) plates. P. gingivalis hmuR mutant strains WS1, WS21, WS22, WS23, WS24, WS25, and WS26 were maintained on ABA plates supplemented with 1 μg of erythromycin per ml. All P. gingivalis plates were incubated at 37°C in an anaerobic chamber (Coy Laboratory Products, Ann Arbor, MI) with 85% N2, 5% H2, and 10% CO2 for 3 to 5 days. Following incubation at 37°C, cultures were inoculated into Anaerobe Broth MIC (AB; DIFCO, Detroit, MI) and incubated at 37°C under anaerobic conditions for 24 h. E. coli strains were maintained in Luria-Bertani (LB) medium (DIFCO, Detroit, MI) or minimal M9 medium supplemented with appropriate antibiotics.
TABLE 1.
Bacterial strains
| Species and strain | Relevant genotype | Source or reference |
|---|---|---|
| P. gingivalis | ||
| A7436 | Wild type | Laboratory collection |
| WS1 | A7436, hmuR::ermF, Ermr | 49 |
| WS21 | A7436, hmuRH95A, Ermr | This study |
| WS22 | A7436, hmuRH434A, Ermr | This study |
| WS23 | A7436, hmuRH95A,H434A, Ermr | This study |
| WS24 | A7436, hmuRYRAP420-423YAAA, Ermr | This study |
| WS25 | A7436, hmuRNPDL442-445NAAA, Ermr | This study |
| WS26 | A7436 containing ermF cloned downstream of the hmuR gene, Ermr | This study |
| E. coli | ||
| XL10-Gold | Tetr Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac Hte [F′ proAB laclqZΔM15 Tn10 (Tetr) Amy Camr] | Stratagene |
| TOP10F′ | F′ [laclq Tn10(Tetr)] mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 deoR araD139 (ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| BL21(DE3)/pLysS | F−ompT hsdSB (rB− mB−) gal dcm (DE3)(pLysS) Camr | Invitrogen |
Conserved amino acid residue analysis.
A BLAST analysis of the HmuR protein sequence was performed against the NCBI conserved domain database (Fig. 1). Homologous proteins were aligned with the HmuR protein by performing ClustalW multiple sequence alignment analysis.
FIG. 1.
Conserved amino acid residues in the C-terminal region of P. gingivalis HmuR compared with representative hemoglobin, hemoglobin-haptoglobin, heme, vitamin B12 and siderophore receptors. Conserved amino acids between receptors and the consensus sequence are indicated in boldface letters. The positions of the amino acids were numbered according to the codons from the NCBI database. Pg, P. gingivalis; Hd, Haemophilus ducreyi; Hi, Haemophilus influenzae; Vv, Vibrio vulnificus; Vc, Vibrio cholerae; Sd, Shigella dysenteriae; Ye, Yersinia enterocolitica; Nm, Neisseria meningitidis; Sm, Serratia marcescens; Ec, Escherichia coli.
Construction and isolation of hmuR site-directed mutants of E. coli and P. gingivalis.
To introduce mutations into the hmuR gene, site-directed mutagenesis was performed using an ExSite PCR-based site-directed mutagenesis kit (Stratagene, CA) following the manufacturer's instructions. The plasmid pTO2 (pCRT7/CT-TOPO containing the hmuR gene with the signal peptide sequence) (Table 2) (49) served as the template for PCR amplification. The following site-directed mutations were introduced into the hmuR gene using different primer sets: I, H95/A (CAT/GCT; hmuRH95A); II, H434/A (CAT/GCT; hmuRH434A); I+II, H95/A+H434/A (CAT/GCT and CAT/GCT; hmuRH95A, H434A); III, YRAP420-423/YAAA (TATCGTGCCCCC/TATGCT GCCGCC; hmuRYRAP420-423YAAA); and IV, NPDL442-445/NAAA (AATCCGGATTTG/AATGCGGCTGCG; hmuRNPDL442-445NAAA).
TABLE 2.
Plasmids used in this study
| Plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| pGEM-3Zf(−) | Ampr | Promega |
| pTO2 | pCRT7/CT-TOPO containing the hmuR gene with the signal peptide sequence, Ampr | 49 |
| pTO2-I | pCRT7/CT-TOPO containing the hmuRH95A gene with the signal peptide sequence, Ampr | This study |
| pTO2-II | pCRT7/CT-TOPO containing the hmuRH434A gene with the signal peptide sequence, Ampr | This study |
| pTO2-I+II | pCRT7/CT-TOPO containing hmuRH95A,H434A gene with the signal peptide sequence, Ampr | This study |
| pTO2-III | pCRT7/CT-TOPO containing the hmuRYRAP420-423YAAA gene with the signal peptide sequence, Ampr | This study |
| pTO2-IV | pCRT7/CT-TOPO containing the hmuRNPDL442-445NAAA gene with the signal peptide sequence, Ampr | This study |
| pWS1 | pGEM-3Zf(−) containing 485-bp N-terminal region of the hmuR gene and ermF cassette within the PstI site of the hmuR gene | 49 |
| pI | pGEM-3Zf(−) containing hmuRH95A, ermF, and DSR | This study |
| pII | pGEM-3Zf(−) containing the C-terminal region of the hmuRH434A gene, ermF, and DSR | This study |
| pI + II | pGEM-3Zf(−) containing hmuRH95A,H434A, ermF, and DSR | This study |
| pIII | pGEM-3Zf(−) containing the C-terminal region of the hmuRYRAP420-423YAAA gene, ermF, and DSR | This study |
| pIV | pGEM-3Zf(−) containing the C-terminal region of the hmuRNPDL442-445NAAA gene, ermF, and DSR | This study |
ermF cassette, Bacteroides fragilis ermF encoding erythromycin resistance gene; DSR, downstream region (254 bp) of the hmuR gene.
To construct the H95 and H434 double mutation in hmuR gene, the pCRT7/CT-TOPO plasmid with the H95A or H434A mutation was first digested with BstXI or BclI, respectively, and then the 970-bp hmuR fragment containing H434A was cloned into the pCRT7/CT-TOPO plasmid containing the hmuR H95A mutation (data not shown). For vector construction, the downstream region (DSR) of hmuR, encompassing a 254-bp fragment of the open reading frame encoding a putative Mg/Co chelatase, was PCR amplified from P. gingivalis A7436 genomic DNA and cloned into pGEM3zf(−) (Promega, Madison, WI) at the PstI and HindIII sites. This fragment was introduced to improve homologous recombination of the hmuR gene together with an antibiotic cassette in P. gingivalis. Then the Bacteroides fragilis ermF gene encoding erythromycin resistance was cut out from the pWS1 plasmid (Table 2) (49) and cloned into the pGD plasmid at the PstI site, yielding the pGED plasmid. The final pGEM3zf(−) constructs containing specific mutations of the hmuR gene, ermF cassette, and the downstream region of the hmuR gene were verified by PCR and DNA sequencing. These constructs were linearized by NdeI digestion and introduced into P. gingivalis A7436 by electroporation (49). Transformants were selected on ABA plates supplemented with 1 μg/ml erythromycin. Positive colonies containing the mutated hmuR gene were screened by PCR and verified by DNA sequencing and Southern blot analysis. For overexpression of recombinant HmuR protein, pCRT7/CT-TOPO plasmids containing the hmuR gene with specific mutations (Table 2) were transformed into E. coli BL21(DE3)/pLysS (Invitrogen, Carlsbad, CA).
Expression of rHmuR in E. coli cells.
E. coli BL21(DE3)/pLysS transformants containing the hmuR gene, hmuR gene with point mutations, or vector alone were prepared using the pCRT7/CT-TOPO expression vector (Invitrogen, Carlsbad, CA) according to standard transformation procedures. Expression of rHmuR (containing the native signal peptide sequence at the N terminus and the V5-6His tag at the C terminus) in BL21(DE3)/pLysS cells was induced by the addition of isopropylthiogalactopyranoside (IPTG) (0.5 mM). Cells were then harvested, resuspended in phosphate-buffered saline (PBS) and adjusted to an optical density at 660 nm (OD660) of 1.0.
Outer membrane fractions of E. coli strains were prepared as described previously (49); 20 μg of protein from each preparation was solubilized in Laemmli sample buffer under reducing conditions (in the presence of dithiothreitol) and separated in 12% polyacrylamide gels in the presence of sodium dodecyl sulfate (SDS). Transfer of proteins onto nitrocellulose membranes was carried out in 30 mM CAPS buffer, pH 11, containing 10% methanol. Proteins were probed with monoclonal anti-V5-horseradish peroxidase antibodies (Invitrogen, Carlsbad, CA) and visualized by chemiluminescence staining (Pierce, Rockford, IL).
Binding of hemin to E. coli cells expressing rHmuR with specific site-directed mutations.
E. coli BL21(DE3)/pLysS cells expressing recombinant wild-type HmuR or HmuR with point mutations (containing the native signal peptide sequence at the N terminus and the V5-6His tag at the C terminus), as well as cells harboring the vector alone were grown in M9 medium and harvested after IPTG (0.5 mM) induction. Cells and hemin solutions were prepared as described previously (41). The binding of hemin to whole E. coli cells was determined by a decrease of absorbance of the supernatant of the samples compared to the control sample containing only hemin (380 nm). Binding to E. coli cells expressing HmuR site-directed mutants was then compared with E. coli cells expressing wild-type HmuR, which was set arbitrarily at 100%. The saturation of hemin binding was determined as described previously (41) and data were further analyzed by nonlinear regression and two-way analysis of variance (ANOVA) using GraphPad software.
Binding of hemin and hemoglobin to P. gingivalis cells.
P. gingivalis wild-type (A7436) and mutant (WS1, WS21, WS22, WS23, WS24, WS25, and WS26) strains were grown in AB overnight at 37°C under anaerobic conditions. Bacteria were then inoculated into basal medium (BM: 15 g of Trypticase peptone, 5 g of yeast extract, 0.5 g cysteine, 0.5 mg menadione per liter) supplemented with hemin (5 mg per liter) and incubated at 37°C under anaerobic conditions for 24 h. These cultures were depleted of stored heme by passage three times in BM. After the third passage (OD660 of 0.5), cultures were centrifuged (room temperature, 9,000 × g, 10 min), washed, and resuspended in PBS. The final OD660 was adjusted to 1.0 in PBS (pH 7.4). Then, 800 μl of each cell suspension was mixed with 200 μl hemoglobin (final concentration, 4 μM) or hemin (final concentration, 10 μM). Samples were then incubated at 37°C for 1 h and centrifuged (as described above), and the OD380 (hemin) or OD400 (hemoglobin) of the supernatant was determined. Samples containing only hemoglobin or hemin diluted in PBS were incubated under the same conditions and served as controls.
Hemin and hemoglobin binding to P. gingivalis whole cells was determined by comparison of the decrease of the supernatant absorbance of the P. gingivalis hmuR mutants to that of the wild-type strain, which was arbitrarily set as 100% (49). Three independent experiments were performed in duplicate.
Growth analysis of the P. gingivalis hmuR mutants in the presence of various heme sources.
P. gingivalis wild-type (A7436) and mutant (WS1, WS21, WS22, WS23, WS24, WS25, and WS26) strains were grown in AB overnight at 37°C under anaerobic conditions and depleted of heme as described above. The cultures were then centrifuged (room temperature, 9,000 × g, 10 min), washed and resuspended in PBS to a final OD660 of 1.0. These suspensions were used to inoculate BM alone or BM supplemented with 1.5 μM hemin, 4 μM hemoglobin, and 10% human serum (preincubated with 0, 1.5 μM or 50 μM hemin). Bacterial growth was monitored by measuring OD at 660 nm (Beckman DU7500 spectrophotometer) at different time points. Three independent experiments were performed in duplicate. Gram stains were performed every 24 h to verify the purity of P. gingivalis cultures. Ratios represented the growth (OD value) of P. gingivalis hmuR mutant strains cultured in different heme sources at different time points compared with that of the wild-type strain, which was arbitrarily set as 1. Data from three independent experiments were expressed as means ± standard deviations and were analyzed by two-way ANOVA using GraphPad Prism 4.0 software. A P of <0.01 was considered significant.
Native gel analysis.
Free heme and heme associated with serum proteins were detected by the presence of yellow bands (the color of heme) and UV-detectable bands (which light up heme groups) following native polyacrylamide gel electrophoresis (PAGE) (34). For native PAGE analysis, 10-μl samples were mixed with an equal volume of 2× native sample buffer containing 62.5 mM Tris-HCl (pH 6.8), 40% glycerol, and 0.01% bromophenol blue (Bio-Rad, Hercules, CA), loaded onto a 12% polyacrylamide-Tris-HCl gel, and electrophoresed using running buffer without sodium dodecyl sulfate at 70 V for 3 h. The gel was then observed under UV light. The appearance of a band that corresponds to human serum albumin (HSA) was considered evidence for HSA-hemin (HSA-Hm) complex formation.
ELISA detection of serum albumin-hemin complex bound to P. gingivalis cells.
The serum albumin-hemin complex was prepared by incubating equal molar ratios of hemin and human serum albumin at 37°C for 1 h (41). Nonbound hemin was removed by dialysis at 4°C. Formation of hemoprotein complexes was confirmed by native gel analysis and by a spectrophotometric UV method (observed Soret peak: Hm, 380 nm; HSA-Hm, 404 nm; no Soret peak for HSA apoprotein) (22). P. gingivalis cells were depleted of heme as described above, washed with PBS, and then fixed with 3% formaldehyde and resuspended in carbonate-bicarbonate buffer (pH 9.6). Immunol 4Hbx enzyme-linked immunosorbent assay (ELISA) plates (Dynex, Chantilly, VA) were coated with 100 μl of cells (OD660 of 0.3) and incubated at 4°C overnight. After blocking with TTBS (Tris-buffered saline containing 0.05% Tween), HSA-Hm complexes were then applied to P. gingivalis cells and incubated at 37°C for 1 h. Anti-HSA monoclonal antibodies (Antibody Shop, Gentofte, Denmark) and alkaline phosphatase-conjugated anti-mouse immunoglobulin G (Sigma, St. Louis, MO) were added to the samples. Nonspecific binding was washed out with TTBS three times (5 min) between the steps. The plates were developed with p-nitrophenyl phosphate (Sigma, St. Louis, MO) and read at 405 nm. The amount of HSA bound to P. gingivalis was determined from a standard curve of HSA protein.
Molecular modeling.
Crystal structures of two homologous E. coli proteins, FepA (7), the ferric enterobactin receptor, and BtuB (10), the cobalamin (vitamin B12) receptor, were used as templates in the homology modeling of the HmuR protein. Structure-based amino acid sequence alignment between BtuB (PDB code 1NQE) and FepA (PDB code 1FEP) was obtained using the SAL server (27) followed by visual inspection and manual realignment using the computer program O (25). A protein segment from residues 43 to 553 of HmuR was also aligned to the BtuB and FepA sequences by BLAST. A manual realignment was performed to reduce gaps. The homology model of HmuR was then generated and refined by the amino acid sequence alignment-based three-dimensional structure-modeling program MODELLER (37). The DeepView/Swiss-Pdb viewer was used for model analysis (23).
RESULTS
Construction of site-directed hmuR mutants.
Comparison of the amino acid sequences of HmuR and several heme/hemoglobin and siderophore receptors revealed that HmuR contains highly conserved motifs which include the invariant histidine residues (H95 and H434), as well as the FRAP (in HmuR YRAP) and NPNL (in HmuR NPDL) amino acid boxes (Fig. 1), all of which may be involved in heme utilization. These conserved residues and motifs were selected for analysis.
Site-directed mutagenesis was utilized to introduce the following specific mutations into the P. gingivalis hmuR gene (Fig. 2): H95A (WS21), H434A (WS22), H95A-H434A (WS23), YRAP420-423YAAA (WS24), and NPDL442-445NAAA (WS25). A P. gingivalis ermF control strain (WS26), which contains the same ermF cassette as the mutants but an intact hmuR gene, was used as a control. The frequency of isolating mutants (containing the correct hmuR gene sequence) from erythromycin-resistant colonies was approximately 20%. The desired mutations in the hmuR gene were verified by PCR, DNA sequencing, and Southern blot analysis (see the supplemental material), which indicated a single copy of the hmuR gene in the P. gingivalis chromosome in both the wild-type and mutant strains.
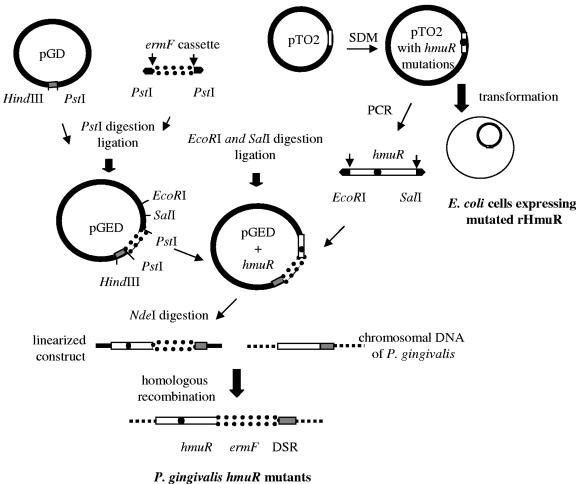
FIG. 2.
Construction of site-directed hmuR mutants in P. gingivalis and E. coli. The site-directed mutants of hmuR were constructed as described in Materials and Methods. The mutated hmuR gene was either introduced into the P. gingivalis chromosome or transformed into E. coli for overexpression. pGD, pGEM3zf(−) plasmid with the downstream region of the hmuR gene; pGED, pGEM3zf(-) plasmid with ermF cassette and DSR.
Growth analysis of P. gingivalis hmuR site-directed mutants.
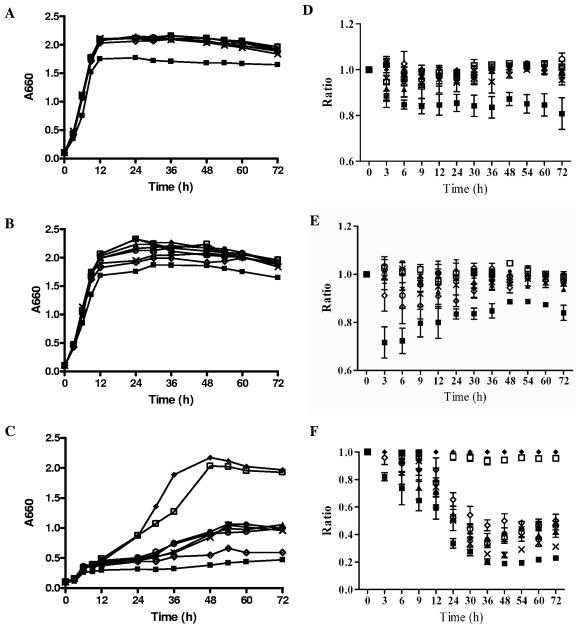
Our previous studies established that HmuR is specific for the uptake of organic heme sources, such as hemin and hemoglobin, but not inorganic iron (49). To examine the ability of the hmuR mutants to grow with different heme-containing sources, growth analysis was performed using hemin, hemoglobin, and human serum. P. gingivalis wild-type strain A7436 and the hmuR site-directed mutant strains were depleted of heme and then inoculated with different heme sources. WS1, a hmuR isogenic mutant with the hmuR gene disrupted by the erythromycin cassette, and WS26, which contains the ermF cassette but an intact hmuR gene, were used as controls. BM without an added heme source did not support the growth of P. gingivalis (data not shown), indicating good starvation of heme in these bacterial cells. When cells were grown in BM supplemented with hemin (Fig. 3A and D) or hemoglobin (Fig. 3B and E), no differences between the hmuR site-directed mutants and the wild-type strain were observed. Only the growth of the P. gingivalis WS1 strain, containing the inactive hmuR gene, was decreased, which was in agreement with our previous studies (49, 50). When human serum, which simulates the in vivo environment, was used as the sole heme source, all the hmuR mutants exhibited significant growth defects compared to the wild-type strain (Fig. 3C and F). This defect was not due to the presence of ermF in these mutants since the WS26 strain, which contains the intact hmuR gene and ermF cassette, grew similarly to the wild-type strain.
FIG. 3.
Growth of P. gingivalis hmuR mutant strains in media with hemin (A and D), hemoglobin (B and E), or human serum (C and F) as the heme source. P. gingivalis wild-type strain A7436 and the hmuR site-directed mutant strains were depleted of heme, resuspended in BM, and then inoculated into (A and D) BM supplemented with 1.5 μM hemin, (B and E) BM supplemented with 4 μM hemoglobin, (C and F) BM containing 10% human serum, or BM alone (data not shown). Bacterial growth was monitored by measuring the absorbance at 660 nm of the cultures at the indicated time points. Panels A to C: representative growth of three independent experiments with similar trends. Panels D to F: relative growth of the hmuR mutants compared to that of the wild-type strain. Ratios (y axis) represent the growth (OD value) of P. gingivalis hmuR mutant strains cultured in BM supplemented with different heme sources at different time points compared with that of the wild-type strain, which was arbitrarily set as 1. Data from three independent experiments were expressed as means ± standard deviations and were analyzed by two-way ANOVA. ⧫, A7436, P. gingivalis wild-type strain; ▪, WS1, P. gingivalis hmuR::ermF; ▴, WS21, P. gingivalis hmuRH95A; •, WS22, P. gingivalis hmuRH434A; ×, WS23, P. gingivalis hmuRH95A,H434A; ○, WS24, P. gingivalis hmuRYRAP420-423YAAA; ⋄, WS25, P. gingivalis hmuRNPDL442-445NAAA; □, WS26, P. gingivalis ermF control.
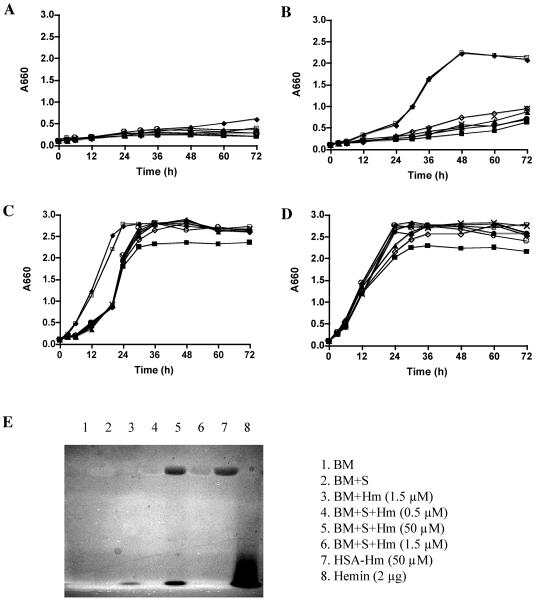
To further examine utilization of serum heme sources by P. gingivalis strains, additional growth analysis was performed using human serum supplemented with different concentrations of hemin (Fig. 4A to D). BM alone cannot support the growth of P. gingivalis cells (Fig. 4A). When serum was used as the sole heme source without addition of free hemin, all the hmuR mutants exhibited significant growth defects compared to the wild-type strain (Fig. 4B). When hemin at a low concentration (1.5 μM) was added to human serum, the P. gingivalis hmuR mutant strains demonstrated reduced growth in the early and exponential growth phase (Fig. 4C), but not in the stationary phase. When hemin at a high concentration (50 μM) was added to the human serum, the P. gingivalis hmuR mutant strains exhibited growth kinetics similar to that observed with the wild-type and ermF control strains (Fig. 4D).
FIG. 4.
Growth of P. gingivalis hmuR mutant strains in media containing human serum supplemented with different concentrations of hemin. P. gingivalis wild-type strain A7436 and the hmuR site-directed mutant strains were depleted of heme and inoculated into BM alone (A) or BM containing 10% human serum supplemented with 0 (B), 1.5 μM (C), or 50 μM (D) of hemin for growth analysis as described in Materials and Methods. The free and complexed forms of heme in different media were examined by native gel analysis and detected by UV light (E). ⧫, A7436, P. gingivalis wild-type strain; ▪, WS1, P. gingivalis hmuR::ermF; ▴, WS21, P. gingivalis hmuRH95A; •, WS22, P. gingivalis hmuRH434A; ×, WS23, P. gingivalis hmuRH95A,H434A; ○, WS24, P. gingivalis hmuRYRAP420-423YAAA; ⋄, WS25, P. gingivalis hmuRNPDL442-445NAAA; □, WS26, P. gingivalis ermF control.
Results from a native gel analysis (Fig. 4E) indicated that heme is present in complex form with serum proteins when a lower concentration of hemin (1.5 μM) was added to serum, while heme is present in both free form and complex form (with human serum albumin) when a higher concentration of hemin (50 μM) was added to serum. The observed deficiencies of the hmuR mutants to utilize serum hemoproteins at low heme concentration indicates the involvement of HmuR and its conserved residues in serum hemoprotein utilization.
Binding of hemin, hemoglobin, and albumin-hemin complex to P. gingivalis hmuR site-directed mutants.
Next, we evaluated the effect of the HmuR point mutations on hemin, hemoglobin, and albumin-hemin binding to P. gingivalis mutants. Spectrophotometric assays were used to determine the amount of hemin or hemoglobin removed from the solution by the cells. For heme, only the hmuR isogenic mutant (WS1) exhibited reduced hemin binding compared to the wild-type strain; none of the hmuR site-directed mutants demonstrated significant hemin binding deficiency (data not shown).
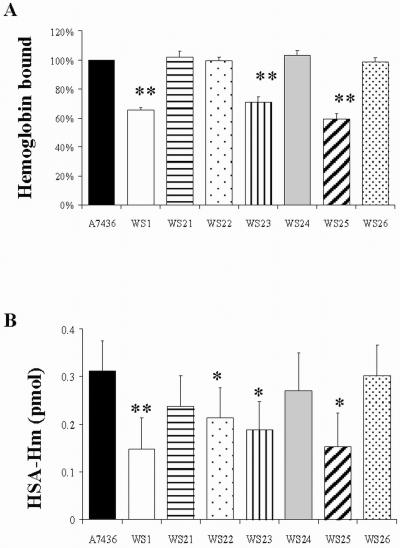
Binding of hemoglobin showed a different pattern (Fig. 5A). As expected, the hmuR isogenic mutant (WS1) demonstrated less hemoglobin binding than the wild-type strain, indicating the involvement of HmuR in binding of P. gingivalis with hemoglobin. The two mutants with single histidine mutations hmuRH95A (WS21) and hmuRH434A (WS22), did not show reduced binding. However, the strain with the double histidine mutation hmuRH95A,H434A (WS23), did show 30% less hemoglobin binding than the wild-type strain (A7436). In addition, the P. gingivalis hmuRNPDL442-445NAAA (WS25) mutant cells exhibited 38% reduced hemoglobin binding from the wild-type strain (A7436) and ermF control strain (WS26). The hmuRYRAP420-423YAAA (WS24) mutant strain did not exhibit significant differences in hemoglobin binding from the wild-type strain.
FIG. 5.
Binding of hemoglobin (A) and human serum albumin-hemin complex (B) to P. gingivalis A7436 and mutant whole cells. P. gingivalis cells depleted of heme were used for either the spectrophotometric hemoglobin binding assay (A) or the ELISA HSA-Hm binding assay (B) as described in Materials and Methods. Data were analyzed using Student's t test and are shown as the mean ± standard deviation from at least three independent experiments, each performed in duplicate. **, P < 0.01; *, P < 0.05 (mutant strains versus wild-type strain). A7436, P. gingivalis wild-type strain; WS1, P. gingivalis hmuR::ermF; WS21, P. gingivalis hmuRH95A; WS22, P. gingivalis hmuRH434A; WS23, P. gingivalis hmuRH95A,H434A; WS24, P. gingivalis hmuRYRAP420-423YAAA; WS25, P. gingivalis hmuRNPDL442-445NAAA; WS26, P. gingivalis ermF control.
We also examined the binding of the most abundant serum hemoprotein, HSA-Hm, to P. gingivalis cells by ELISA. Binding of HSA-Hm to P. gingivalis cells was calculated by subtracting the background absorbance of control samples that contained P. gingivalis cells alone but no added proteins. As shown in Fig. 5B, the hmuRH434A (WS22), hmuRH95A,H434A (WS23), and hmuRNPDL442-445NAAA (WS25) mutants showed reduced binding (P < 0.05), while the hmuRYRAP420-423YAAA (WS24) mutant did not. Compared with the wild-type P. gingivalis strain, the hmuR isogenic mutant WS1 displayed reduced ability to bind HSA-Hm complex (P < 0.01).
Hemin binding to E. coli expressing HmuR.
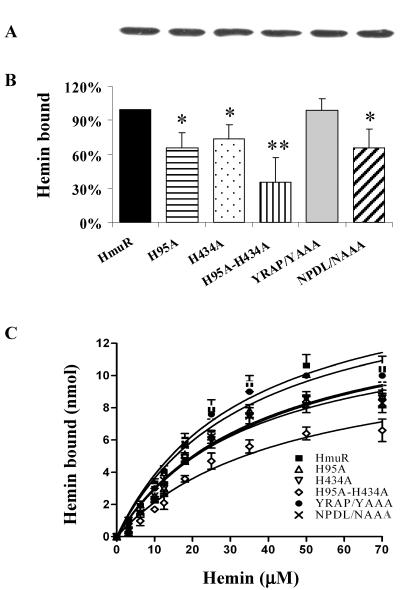
The results from the above binding experiments indicated the presence of more than one heme binding/uptake system in P. gingivalis. To separate the effect of changes in a single protein from the background of other heme/hemoprotein uptake systems, we introduced the HmuR protein containing specific mutations into an E. coli background. Binding of hemin to E. coli cells overexpressing rHmuR was examined by spectrophotometric assay as described previously (41). Specific hemin binding was calculated by subtracting the hemin bound to the cells harboring the vector alone from that bound to the cells expressing rHmuR. The protein expression levels determined in outer membrane fractions were comparable for all strains studied (Fig. 6A). When the assay was performed with 10 μM hemin, we found that E. coli cells with the rHmuR mutations H95A, H434A, and H95A-H434A exhibited decreased hemin binding, with 34%, 26%, and 65% less hemin bound, respectively, than by the wild-type rHmuR cells (Fig. 6B). E. coli cells expressing HmuR with the NPDL amino acid motif replaced by NAAA showed lower hemin binding (34% less hemin bound), but replacement of YRAP by YAAA did not result in a significant change in binding (Fig. 6B).
FIG. 6.
Binding of hemin (B and C) to E. coli cells expressing wild-type HmuR and HmuR with site-directed mutations. The level of rHmuR expression (A) was determined by SDS-PAGE and Western blot analysis of outer membrane fractions as described in Materials and Methods. The binding of hemin (10 μM hemin in B) to whole E. coli cells was determined by a decrease of absorbance of the supernatant of the samples compared to the control sample containing only hemin. Binding to E. coli cells expressing HmuR site-directed mutants was then compared with that to E. coli cells expressing wild-type HmuR, which was set arbitrarily at 100%. Five independent experiments were performed in triplicate. Data are means ± standard deviation. *, P < 0.05; **, P < 0.01 for E. coli expressing HmuR with point mutations versus E. coli harboring wild-type HmuR. Binding of hemin as a function of concentration (C) was determined as described previously (41).
A more complete picture of hemin binding was obtained by running spectrophotometric assays at a series of hemin concentrations (Fig. 6C). Compared with the E. coli cells expressing wild-type HmuR, E. coli cells expressing HmuR with H95A, H434A, H95A-H434A, and NPDL/NAAA replacements exhibited an overall reduced hemin binding (two-way ANOVA, P < 0.0001), indicating the involvement of these conserved residues in binding of hemin to HmuR. E. coli cells containing the vector alone bound hemin nonspecifically with low efficiency. This binding did not show saturation, and the transformed data clustered around the origin (data not shown).
DISCUSSION
In this study, we defined conserved HmuR residues and motifs that are involved in heme utilization by constructing site-directed hmuR mutants in both P. gingivalis and E. coli and characterizing these mutants by functional analysis. It is well established that histidines are common axial ligands for heme (9, 44). Both hemoproteins and outer membrane receptors contain histidines that can bind heme (3, 42). In Yersinia enterocolitica, two histidine residues (H128 and H461) seem to be involved in heme utilization by the heme receptor HemR (3). Our results indicate that in E. coli cells overexpressing mutated rHmuR proteins, both histidine 95 and histidine 434 are involved in the binding of hemin to HmuR. In addition, the conserved NPDL motif seems to be involved in hemin binding, while the YRAP motif is not required. Binding of hemin was observed in the order wild-type ≈ YRAP/YAAA > NPDL/NAAA ≈ H95A ≈ H434A > H95A-H434A. In contrast to the observations in E. coli, P. gingivalis site-directed hmuR mutants did not show reduced hemin binding compared to the wild-type strain. The mutants also had a normal growth phenotype when hemin was utilized as the sole heme source. Even the removal of HmuR entirely in strain WS1 reduced the growth of P. gingivalis by only about 20%, indicating the presence of other heme uptake systems (i.e., HemR, IhtA, and Tlr) in P. gingivalis (39, 50). Expression of these alternative uptake systems may complement the deficiency in heme binding by the mutated HmuR protein, allowing normal growth with hemin as a heme source.
Analysis of hemoglobin binding to P. gingivalis mutants indicated that both the H95 and H434 residues and the NPDL motif may be involved in binding of hemoglobin to HmuR. Although some reduced binding was observed in these mutants, no significant deficiency was observed in growth when hemoglobin was utilized as the sole heme source. These results indicate that reduced heme binding in itself may not be sufficient to effect bacterial growth significantly. In addition, expression of other hemoglobin utilization proteins may complement the hemoglobin binding deficiency. In a previous study, we found that expression of the hmuR, kgp, and rgpA genes is coordinately regulated for maximum heme uptake (35). When hemoglobin binding is compromised in the hmuR mutants, the expression of the gingipains may complement the hemoglobin binding deficiency and contribute to the sustained growth.
In human serum, heme is present not in its free form, but in the form of complexes with serum heme-binding proteins such as albumin, hemopexin, and lipoproteins (8, 28, 38). Among these, albumin is the most abundant serum protein and the bound heme is present in an albumin-hemin complex. Previous studies in our laboratory have shown that rHmuR can bind with HSA-Hm (41). Analysis of the mutants constructed in this study demonstrated somewhat reduced HSA-Hm binding by the H434A, H95A-H434A, and NPDL/NAAA P. gingivalis mutants. The binding deficiencies of these P. gingivalis mutants to HSA-Hm could contribute to the reduced growth of the mutants in serum. Compared with wild-type P. gingivalis (A7436), the hmuR isogenic mutant (WS1) exhibited reduced binding, but substantial HSA-Hm binding was still observed. This indicates the presence of additional proteins in P. gingivalis that are involved in HSA-Hm binding, which are not able to replace HmuR for the utilization of serum hemoproteins when P. gingivalis is grown in the presence of serum.
Gingival crevicular fluid is similar in composition to human serum, which contains iron sources such as heme-albumin complex and transferrin (12). The concentration of heme in normal serum is to our knowledge not well known but presumably very low and cannot support the growth of the P. gingivalis hmuR mutants. When hemin at a low concentration (1.5 μM) is added to human serum, heme is present in complex with serum hemoproteins. Our data show that this amount of heme cannot be utilized efficiently by the hmuR mutants in the early and exponential phase of growth. The growth deficiency of the hmuR mutant strains was not observed in the stationary phase of growth. We have shown previously that gingipains are expressed by P. gingivalis during growth in medium containing human serum (35). In addition, P. gingivalis gingipains can degrade serum hemoproteins for heme utilization (53). Expression of gingipains in the early phase of growth, which can release free heme from serum hemoproteins, allows utilization of free hemin by these mutants in the late phase of growth. When hemin at a high concentration (50 μM) is added to human serum, no growth deficiency of the hmuR mutants is observed. The presence of free hemin can support the growth of hmuR mutants, presumably via other free heme uptake pathways in P. gingivalis.
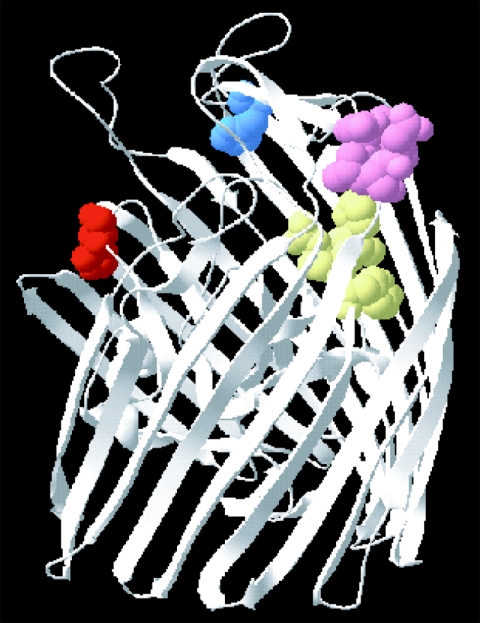
In summary, results from mutational analysis indicate that all of the analyzed residues of HmuR seem to be important in serum hemoprotein utilization. While histidines 95 and 434 as well as the NPDL motif are involved in the binding of heme/hemoproteins to HmuR, the YRAP motif is not involved in direct binding. This could be explained by the homology model of HmuR. Similar to the other ligand-gated transmembrane proteins such as FepA (7), FecA (16), FhuA (17), and BtuB (10), the HmuR homology model demonstrated a beta-barrel structure with two distinct domains (Fig. 7). The first domain is a beta barrel with long loops on the extracellular side and short turns on the periplasmic side. The second domain is a globular amino-terminal domain that folds into the barrel pore. The conserved H95 residue is present on a loop of the globular domain, close to the membrane-spanning portion of the protein; the H434 residue is present on an extracellular loop of the beta barrel. The YRAP motif resides on a strand of the barrel wall and the NPDL motif is on one of the extracellular loops of the barrel. Previous crystallographic studies have shown that the apical or extracellular loops function for ligand binding in TonB-dependent receptors such as FepA (7), FhuA (17) and BtuB (10). The presence of H95, H434, and the NPDL motif on the loops of HmuR indicated their probable function in direct binding, which is supported by the mutational analysis. The YRAP motif is almost entirely buried, indicating that it is not important for heme/hemoprotein binding. This is supported by the observations that none of the studies in either P. gingivalis or E. coli showed significant alteration of heme (hemin) or hemoproteins (hemoglobin and HSA-Hm) binding upon mutation of the YRAP motif. However, mutation of the YRAP motif to YAAA did result in significantly reduced growth of P. gingivalis in serum. These observations, and the homology model, suggest a role for the YRAP motif in another aspect of heme uptake (i.e., heme transport) by HmuR. Similar observations have been made in other receptor proteins such as TbpA (57) and HmbR (43).
FIG. 7.
Conserved residue/motif analysis of HmuR by three-dimensional modeling. The three-dimensional homology model of HmuR was constructed using the crystal structure of FepA (ferric enterobactin receptor of E. coli) and BtuB (cobalamin receptor of E. coli), two proteins homologous to HmuR. The figure shows the conserved residues and motifs of HmuR. The conserved residues are presented in different colors: H95, red; H434, blue; NPDL, pink; and YRAP, yellow.
Taken together, the data indicate that HmuR binds with hemin and hemoproteins, but may function mainly as a receptor to utilize heme from serum hemoproteins. The HmuR conserved residues H95A and H434A and the NPDL and YRAP motifs are involved in the utilization of serum hemoproteins by P. gingivalis. The significant defect of the hmuR mutant strain (WS1) in utilizing serum heme sources for growth suggests that in P. gingivalis, HmuR is essential for serum hemoprotein utilization rather than the utilization of free hemin or hemoglobin. This is important for bacteria to survive and proliferate in the early phase of periodontal disease, when no intensive bleeding providing P. gingivalis with an excess of heme occurs.
Supplementary Material
Acknowledgments
This study was supported by Public Health Service grant DE09161 from the National Institute of Dental and Craniofacial Research (C.A.G.), grant 3 P05A 113 24 from the State Committee for Scientific Research (KBN), Poland (T.O.), and AI45883 from the National Institutes of Health (D.W.D.).
We thank Yusuke Takahashi and Aneta Sroka for scientific and technical discussions and Michael T. Baer and Jeremy Derrick for critical review of the manuscript.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Amano, A., M. Kuboniwa, K. Kataoka, K. Tazaki, E. Inoshita, H. Nagata, H. Tamagawa, and S. Shizukuishi. 1995. Binding of hemoglobin by Porphyromonas gingivalis. FEMS Microbiol. Lett. 134:63-67. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. [DOI] [PubMed] [Google Scholar]
- 3.Bracken, C. S., M. T. Baer, A. Abdur-Rashid, W. Helms, and I. Stojiljkovic. 1999. Use of heme-protein complexes by the Yersinia enterocolitica HemR receptor: histidine residues are essential for receptor function. J. Bacteriol. 181:6063-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bramanti, T. E., and S. C. Holt. 1993. Hemin uptake in Porphyromonas gingivalis: Omp26 is a hemin-binding surface protein. J. Bacteriol. 175:7413-7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bramanti, T. E., and S. C. Holt. 1991. Roles of porphyrins and host iron transport proteins in regulation of growth of Porphyromonas gingivalis W50. J. Bacteriol. 173:7330-7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brochu, V., D. Grenier, K. Nakayama, and D. Mayrand. 2001. Acquisition of iron from human transferrin by Porphyromonas gingivalis: a role for Arg- and Lys-gingipain activities. Oral Microbiol. Immunol. 16:79-87. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan, S. K., B. S. Smith, L. Venkatramani, D. Xia, L. Esser, M. Palnitkar, R. Chakraborty, D. van der Helm, and J. Deisenhofer. 1999. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol. 6:56-63. [DOI] [PubMed] [Google Scholar]
- 8.Camejo, G., C. Halberg, A. Manschik-Lundin, E. Hurt-Camejo, B. Rosengren, H. Olsson, G. I. Hansson, G. B. Forsberg, and B. Ylhen. 1998. Hemin binding and oxidation of lipoproteins in serum: mechanisms and effect on the interaction of LDL with human macrophages. J. Lipid Res. 39:755-766. [PubMed] [Google Scholar]
- 9.Chakraborti, A. S. 2003. Interaction of porphyrins with heme proteins-a brief review. Mol. Cell. Biochem. 253:49-54. [DOI] [PubMed] [Google Scholar]
- 10.Chimento, D. P., R. J. Kadner, and M. C. Wiener. 2003. The Escherichia coli outer membrane cobalamin transporter BtuB: structural analysis of calcium and substrate binding, and identification of orthologous transporters by sequence/structure conservation. J. Mol. Biol. 332:999-1014. [DOI] [PubMed] [Google Scholar]
- 11.Curtis, M. A., H. K. Kuramitsu, M. Lantz, F. L. Macrina, K. Nakayama, J. Potempa, E. C. Reynolds, and J. Aduse-Opoku. 1999. Molecular genetics and nomenclature of proteases of Porphyromonas gingivalis. J. Periodont. Res. 34:464-472. [DOI] [PubMed] [Google Scholar]
- 12.Curtis, M. A., J. A. Sterne, S. J. Price, G. S. Griffiths, S. K. Coulthurst, J. M. Wilton, and N. W. Johnson. 1990. The protein composition of gingival crevicular fluid sampled from male adolescents with no destructive periodontitis: baseline data of a longitudinal study. J. Periodont. Res. 25:6-16. [DOI] [PubMed] [Google Scholar]
- 13.Dashper, S. G., A. Hendtlass, N. Slakeski, C. Jackson, K. J. Cross, L. Brownfield, R. Hamilton, I. Barr, and E. C. Reynolds. 2000. Characterization of a novel outer membrane hemin-binding protein of Porphyromonas gingivalis. J. Bacteriol. 182:6456-6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeCarlo, A. A., M. Paramaesvaran, P. L. Yun, C. Collyer, and N. Hunter. 1999. Porphyrin-mediated binding to hemoglobin by the HA2 domain of cysteine proteinases (gingipains) and hemagglutinins from the periodontal pathogen Porphyromonas gingivalis. J. Bacteriol. 181:3784-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans, R. W., J. B. Crawley, C. Joannou, and N. Sharma. 1999. Iron proteins, p. 27-86. In J. J. Bullen and E. Griffiths (ed.), Iron and infection. John Wiley & Sons, Chichester, England.
- 16.Ferguson, A. D., R. Chakraborty, B. S. Smith, L. Esser, D. van der Helm, and J. Deisenhofer. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295:1715-1719. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson, A. D., E. Hofmann, J. W. Coulton, K. Diederichs, and W. Welte. 1998. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282:2215-2220. [DOI] [PubMed] [Google Scholar]
- 18.Fujimura, S., Y. Shibata, K. Hirai, and T. Nakamura. 1996. Binding of hemoglobin to the envelope of Porphyromonas gingivalis and isolation of the hemoglobin-binding protein. Infect. Immun. 64:2339-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genco, C. A., and D. W. Dixon. 2001. Emerging strategies in microbial haeme capture. Mol. Microbiol. 39:1-11. [DOI] [PubMed] [Google Scholar]
- 20.Genco, C. A., B. M. Odusanya, and G. Brown. 1994. Binding and accumulation of hemin in Porphyromonas gingivalis are induced by hemin. Infect. Immun. 62:2885-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghigo, J. M., S. Letoffe, and C. Wandersman. 1997. A new type of hemophore-dependent heme acquisition system of Serratia marcescens reconstituted in Escherichia coli. J. Bacteriol. 179:3572-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grinberg, L. N., P. J. O'Brien, and Z. Hrkal. 1999. The effects of heme-binding proteins on the peroxidative and catalytic activities of hemin. Free Radic. Biol. Med. 27:214-219. [DOI] [PubMed] [Google Scholar]
- 23.Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714-2723. [DOI] [PubMed] [Google Scholar]
- 24.Holt, S. C., L. Kesavalu, S. Walker, and C. A. Genco. 1999. Virulence factors of Porphyromonas gingivalis. Periodontol. 2000 20:168-238. [DOI] [PubMed] [Google Scholar]
- 25.Jones, T. A. 1978. A graphics model building and refinement system for macromolecules. J. Appl. Crystallogr. 11:268-272. [Google Scholar]
- 26.Karunakaran, T., T. Madden, and H. Kuramitsu. 1997. Isolation and characterization of a hemin-regulated gene, hemR, from Porphyromonas gingivalis. J. Bacteriol. 179:1898-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kihara, D., and J. Skolnick. 2003. The PDB is a covering set of small protein structures. J. Mol. Biol. 334:793-802. [DOI] [PubMed] [Google Scholar]
- 28.Kongshaug, M. 1992. Distribution of tetrapyrrole photosensitizers among human plasma proteins. Int. J. Biochem. 24:1239-1265. [DOI] [PubMed] [Google Scholar]
- 29.Kuboniwa, M., A. Amano, and S. Shizukuishi. 1998. Hemoglobin-binding protein purified from Porphyromonas gingivalis is identical to lysine-specific cysteine proteinase (Lys-gingipain). Biochem. Biophys. Res. Commun. 249:38-43. [DOI] [PubMed] [Google Scholar]
- 30.Kuramitsu, H. K. 1999. Proteases of Porphyromonas gingivalis: what don't they do? Oral Microbiol. Immunol. 13:263-270. [DOI] [PubMed] [Google Scholar]
- 31.Kusaba, A., T. Ansai, S. Akifusa, K. Nakahigashi, S. Taketani, H. Inokuchi, and T. Takehara. 2002. Cloning and expression of a Porphyromonas gingivalis gene for protoporphyrinogen oxidase by complementation of a hemG mutant of Escherichia coli. Oral Microbiol. Immunol. 17:290-295. [DOI] [PubMed] [Google Scholar]
- 32.Letoffe, S., F. Nato, M. E. Goldberg, and C. Wandersman. 1999. Interactions of HasA, a bacterial haemophore, with haemoglobin and with its outer membrane receptor HasR. Mol. Microbiol. 33:546-555. [DOI] [PubMed] [Google Scholar]
- 33.Lewis, J. P., J. A. Dawson, J. C. Hannis, D. Muddiman, and F. L. Macrina. 1999. Hemoglobinase activity of the lysine gingipain protease (Kgp) of Porphyromonas gingivalis W83. J. Bacteriol. 181:4905-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, M., and B. Lei. 2005. Heme transfer from streptococcal cell surface protein Shp to HtsA of transporter HtsABC. Infect. Immun. 73:5086-5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, X., A. Sroka, J. Potempa, and C. A. Genco. 2004. Coordinate expression of the Porphyromonas gingivalis lysine-specific gingipain proteinase, Kgp, arginine-specific gingipain proteinase, RgpA, and the heme/hemoglobin receptor, HmuR. Biol. Chem. 385:1049-1057. [DOI] [PubMed] [Google Scholar]
- 36.Loomis, L. D., and K. N. Raymond. 1991. Solution equilibria of enterobactin and metal enterobactin complexes. Inorg. Chem. 30:906-911. [Google Scholar]
- 37.Marti-Renom, M. A., A. C. Stuart, A. Fiser, R. Sanchez, F. Melo, and A. Sali. 2000. Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 29:291-325. [DOI] [PubMed] [Google Scholar]
- 38.Miller, Y. I., and N. Shaklai. 1999. Kinetics of hemin distribution in plasma reveals its role in lipoprotein oxidation. Biochim. Biophys. Acta 1454:153-164. [DOI] [PubMed] [Google Scholar]
- 39.Nelson, K. E., R. D. Fleischmann, R. T. DeBoy, I. T. Paulsen, D. E. Fouts, J. A. Eisen, S. C. Daugherty, R. J. Dodson, A. S. Durkin, M. Gwinn, D. H. Haft, J. F. Kolonay, W. C. Nelson, T. Mason, L. Tallon, J. Gray, D. Granger, H. Tettelin, H. Dong, J. L. Galvin, M. J. Duncan, F. E. Dewhirst, and C. M. Fraser. 2003. Complete genome sequence of the oral pathogenic bacterium Porphyromonas gingivalis strain W83. J. Bacteriol. 185:5591-5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto, K., K. Nakayama, T. Kadowaki, N. Abe, D. B. Ratnayake, and K. Yamamoto. 1998. Involvement of a lysine-specific cysteine proteinase in hemoglobin adsorption and heme accumulation by Porphyromonas gingivalis. J. Biol. Chem. 273:21225-21231. [DOI] [PubMed] [Google Scholar]
- 41.Olczak, T., D. W. Dixon, and C. A. Genco. 2001. Binding specificity of the Porphyromonas gingivalis heme and hemoglobin receptor HmuR, gingipain K, and gingipain R1 for heme, porphyrins, and metalloporphyrins. J. Bacteriol. 183:5599-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paoli, M., B. F. Anderson, H. M. Baker, W. T. Morgan, A. Smith, and E. N. Baker. 1999. Crystal structure of hemopexin reveals a novel high-affinity heme site formed between two beta-propeller domains. Nat. Struct. Biol. 6:926-931. [DOI] [PubMed] [Google Scholar]
- 43.Perkins-Balding, D., M. T. Baer, and I. Stojiljkovic. 2003. Identification of functionally important regions of a haemoglobin receptor from Neisseria meningitidis. Microbiology 149:3423-3435. [DOI] [PubMed] [Google Scholar]
- 44.Poulos, T. L. 1996. Ligands and electrons and haem proteins. Nat. Struct. Biol. 3:401-403. [DOI] [PubMed] [Google Scholar]
- 45.Raymond, K. N., and E. A. Dertz. 2004. Biochemical and physical properties of siderophores, p. 3-17. In J. H. Crosa, A. R. Mey and S. M. Payne (ed.), Iron transport in bacteria. American Society for Microbiology, Washington, D.C.
- 46.Rossi, M. S., J. D. Fetherston, S. Letoffe, E. Carniel, R. D. Perry, and J. M. Ghigo. 2001. Identification and characterization of the hemophore-dependent heme acquisition system of Yersinia pestis. Infect. Immun. 69:6707-6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rouault, T. A. 2004. Pathogenic bacteria prefer heme. Science 305:1577-1578. [DOI] [PubMed] [Google Scholar]
- 48.Shi, Y., D. B. Ratnayake, K. Okamoto, N. Abe, K. Yamamoto, and K. Nakayama. 1999. Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis. Construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J. Biol. Chem. 274:17955-17960. [DOI] [PubMed] [Google Scholar]
- 49.Simpson, W., T. Olczak, and C. A. Genco. 2000. Characterization and expression of HmuR, a TonB-dependent hemoglobin receptor of Porphyromonas gingivalis. J. Bacteriol. 182:5737-5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simpson, W., T. Olczak, and C. A. Genco. 2004. Lysine-specific gingipain K and heme/hemoglobin receptor HmuR are involved in heme utilization in Porphyromonas gingivalis. Acta Biochim. Pol. 51:253-262. [PubMed] [Google Scholar]
- 51.Skaar, E. P., M. Humayun, T. Bae, K. L. DeBord, and O. Schneewind. 2004. Iron-source preference of Staphylococcus aureus infections. Science 305:1626-1628. [DOI] [PubMed] [Google Scholar]
- 52.Slakeski, N., S. G. Dashper, P. Cook, C. Poon, C. Moore, and E. C. Reynolds. 2000. A Porphyromonas gingivalis genetic locus encoding a heme transport system. Oral Microbiol. Immunol. 15:388-392. [DOI] [PubMed] [Google Scholar]
- 53.Sroka, A., M. Sztukowska, J. Potempa, J. Travis, and C. A. Genco. 2001. Degradation of host heme proteins by lysine- and arginine-specific cysteine proteinases (gingipains) of Porphyromonas gingivalis. J. Bacteriol. 183:5609-5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wandersman, C., and P. Delepelaire. 2004. Bacterial iron sources: from siderophores to hemophores. Annu. Rev. Microbiol. 58:611-647. [DOI] [PubMed] [Google Scholar]
- 55.Wandersman, C., and I. Stojiljkovic. 2000. Bacterial heme sources: the role of heme, hemoprotein receptors and hemophores. Curr. Opin. Microbiol. 3:215-220. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto, S., N. Okujo, T. Yoshida, S. Matsuura, and S. Shinoda. 1994. Structure and iron transport activity of vibrioferrin, a new siderophore of Vibrio parahaemolyticus. J. Biochem. (Tokyo) 115:868-874. [DOI] [PubMed] [Google Scholar]
- 57.Yost-Daljev, M. K., and C. N. Cornelissen. 2004. Determination of surface-exposed, functional domains of gonococcal transferrin-binding protein A. Infect. Immun. 72:1775-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.