Abstract
Natural killer (NK) cells are considered to be key players in the early innate responses to protozoan infections, primarily indirectly by producing gamma interferon (IFN-γ) in response to cytokines, like interleukin 12 (IL-12). We demonstrate that live, as well as heat-inactivated, tachyzoites of Neospora caninum, a Toxoplasma-like protozoan, directly trigger production of IFN-γ from purified, IL-2-activated bovine NK cells. This response occurred independently of IL-12 but was increased by the addition of the cytokine. A similar IFN-γ response was measured in cocultures of NK cells and N. caninum-infected autologous fibroblasts. However, no NK cell-derived IFN-γ response was detected when cells were cultured with soluble antigens from the organism, indicating that intact tachyzoites or nonsoluble components are necessary for NK cell triggering. Furthermore, N. caninum-infected autologous fibroblasts had increased susceptibility to NK cell cytotoxicity compared to uninfected fibroblasts. This cytotoxicity was largely mediated by a perforin-mediated mechanism. The activating receptor NKp46 was involved in cytotoxicity against fibroblasts but could not explain the increased cytotoxicity against infected targets. Interestingly, N. caninum tachyzoites were able to infect cultured NK cells, in which tachyzoites proliferated inside parasitophorous vacuoles. Together, these findings underscore the role of NK cells as primary responders during a protozoan infection, describe intracellular protozoan infection of NK cells in vitro for the first time, and represent the first functional study of purified bovine NK cells in response to infection.
The intracellular apicomplexan parasite Neospora caninum was first detected in dogs in 1984 (4) and is now recognized as a major worldwide cause of abortion and congenital infection in cattle (15). The organism is closely related to Toxoplasma gondii and was only recently clearly distinguished from other related coccidia (14). A number of mammalian species can act as intermediate hosts. It is uncertain whether N. caninum can infect humans, but serological evidence of human infection has been reported (43), and nonhuman primates have been experimentally infected, causing disease that had remarkable similarities to congenital toxoplasmosis in humans (17).
N. caninum infection incites a typical cell-mediated immunity, which is important for the host defense but which may also be involved in the pathogenesis of fetal rejection (19). The importance of gamma interferon (IFN-γ) for protection against N. caninum has been well documented in mouse models (1, 22, 28) and bovine tissue cultures (20, 47). The source of this proinflammatory cytokine during bovine neosporosis has been attributed to CD4+ T lymphocytes (26). Parasite-specific cytotoxic T lymphocytes, predominantly of the CD4+ phenotype, have been demonstrated in N. caninum-infected cattle (38). While these and most other reports on neosporosis immunity provide insight into the acquired phase of immunity, little is known about innate immune mechanisms.
Natural killer (NK) cells are known to play a key role in host resistance against several important protozoa, particularly in the early course of infection. Several studies have shown that NK cells can kill protozoon-infected cells, as well as extracellular protozoan organisms, but the biological significance of NK cell cytotoxicity in vivo is debated (23, 33). The most critical NK cell response to protozoa is considered to be cytokine production, most importantly IFN-γ, promoting a Th1-type adaptive response. The monokine interleukin 12 (IL-12) has been regarded as necessary for IFN-γ production by NK cells during infections with intracellular pathogens, including protozoa (3, 44, 48). However, recent reports have shown IL-12-independent direct stimulation of human NK cells by live protozoa (29) and protozoan antigens (2).
The role of natural killer (NK) cells in bovine neosporosis has not been investigated to date. Recently, NK cells in cattle were characterized, and a method for isolation based on the highly NK cell-specific receptor NKp46 was described (39, 40). The purpose of the present study was to investigate NK cell functions during bovine neosporosis, using an in vitro host-parasite model. We demonstrate IL-12-independent IFN-γ production by IL-2-activated NK cells following direct stimulation by N. caninum tachyzoites, as well as by N. caninum-infected target cells. We also show that NK cells killed N. caninum-infected autologous, as well as allogeneic, fibroblasts from healthy animals. The activating NKp46 receptor was involved in killing of fibroblasts but did not appear to be responsible for the increased susceptibility of N. caninum-infected targets. Finally, we demonstrate that N. caninum tachyzoites are able to infect bovine NK cells in vitro, which to our knowledge is the first description of an experimental infection of NK cells with a protozoan parasite.
MATERIALS AND METHODS
Animals.
NK cells and fibroblasts were isolated from healthy Norwegian Red cattle. Specifically, four unrelated 3- to 6-month-old male calves were used for autologous NK cell-fibroblast experiments. All animals were from herds free of Neospora infection and tested seronegative for antibodies to N. caninum by an enzyme-linked immunosorbent assay (ELISA) as previously described (5).
Culture of N. caninum and preparation of tachyzoites.
N. caninum tachyzoites of the NC-Liverpool isolate were propagated in Vero cell monolayers in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U penicillin, 100 μg of streptomycin, and 0.25 μg of fungizone per ml (all Invitrogen). Tachyzoite-infected cells were harvested after 3 to 4 days in culture using a rubber policeman and counted in a hemocytometer using trypan blue exclusion; passage numbers used were between 50 and 65.
Purification of tachyzoites was performed as previously described (6). Briefly, cell debris was removed by centrifuging cells on a 30% Percoll (Amersham Biosciences) gradient at 2,000 × g for 10 min. The purified tachyzoites were washed twice and resuspended in phosphate-buffered saline (PBS) at 107 tachyzoites/ml and used immediately. Vero cells were similarly purified and applied as controls to exclude interference of residual cell material. Heat-inactivated tachyzoites were obtained by incubation at 56°C for 50 min; the efficiency of inactivation was verified by the inability to reinfect Vero cell cultures.
Soluble antigens were obtained by sonicating purified tachyzoites on ice 10 times for 10 seconds each time. The debris was spun down at 13,000 × g for 15 min, and the supernatant was passed through a 0.22-μm filter to remove residual particulate material. The protein concentration was determined by measuring the optical density at 280 nm. Purified sonicated Vero cells were used as controls. The biological activity of sonicated tachyzoites was confirmed by the ability to induce IFN-γ production from CD4+ cells from N. caninum-infected animals (unpublished results).
Culture of bovine NK cells.
Bovine NK cells were isolated and cultured as previously described (39). Briefly, peripheral blood mononuclear cells were positively selected using a monoclonal antibody (MAb) against NKp46 (AKS1) and immunomagnetic anti-mouse panimmunoglobulin G beads (Dynal, Oslo, Norway) and cultured in medium containing 100 U/ml bovine recombinant IL-2 (rIL-2) (produced in our laboratory). The beads were magnetically removed after spontaneous detachment on days 2 and 3, and the cells were cultured for 7 to 10 days, with medium added as necessary.
The cultured NK cells were confirmed to be 97 to 99% NKp46+ on the day of use, employing anti-NKp46 MAb (AKS1) and other relevant leukocyte markers in flow cytometry as previously described (39).
Culture and infection of bovine fibroblasts.
Primary cultures of dermal fibroblasts established from dermal-punch biopsies were maintained in minimum essential medium with Earle’s salts supplemented as described for Vero cell medium at 37°C in 5% CO2. Fibroblasts of passages 4 to 8 were infected with N. caninum tachyzoites at a multiplicity of infection of 4:1.
An intracellular flow-cytometric method was established to assess the level of N. caninum infection of fibroblasts. Infected or uninfected fibroblasts were washed in PBS and nonenzymatically detached with prewarmed Cell Dissociation solution (Sigma-Aldrich) in 37°C for 15 min. Detached cells were washed twice in PBS and centrifuged at 400 × g for 5 min, and 1 × 105 to 2 × 105 cells per well were placed in 96-well plates. Permeabilization was performed by incubating the wells with 250 μl Cytofix/Cytoperm solution (BD Biosciences) for 20 min on ice and washing them twice in 200 μl Perm/Wash solution (BD Biosciences). The cells were incubated for 30 min on ice with MAb 5B6 (VMRD, Pullman, WA), which recognizes the gp65 antigen on N. caninum, at 10 μg/ml in Perm/Wash solution and washed twice. Finally, the cells were incubated for 30 min with phycoerythrin-conjugated secondary antibody (Southern Biotech) and washed twice. Control labeling was performed with secondary antibodies only. The cells were suspended in FACS Flow solution and analyzed in a FACSCalibur flow cytometer equipped with CellQuest software (all BD Biosciences), with gating for viable cells.
Production of IFN-γ by NK cells.
IL-2-activated NK cells were cocultured for 24 h in triplicates of 105 in NK medium, with either of the following preparations: N. caninum-infected fibroblasts, using uninfected fibroblasts as controls; purified live or heat-inactivated tachyzoites, using uninfected Vero cells similarly purified as controls; or sonicated tachyzoites, using medium or Vero cell sonicates as controls. To assess the ability of MAb ligation of the NKp46 receptor to trigger IFN-γ production, NK cells were cultured at 105 cells per well in MaxiSorp plates (NUNC, Denmark) precoated overnight with anti-NKp46 MAb in 0.05 M carbonate buffer, pH 9.6. In some cases, bovine rIL-12, kindly provided by Jayne Hope (Institute of Animal Health, Compton, United Kingdom), was added to the cultures.
IFN-γ production in the supernatants was assessed using an ELISA for bovine IFN-γ (Bovine IFN-γ EASIA; Biosource, Nivelles, Belgium) according to the manufacturer's instructions. Concentrations were calculated from a standard curve made using purified bovine IFN-γ, kindly provided by Stephen Jones (Pfizer, Melbourne, Australia).
NK cell cytotoxicity assays.
The cytotoxic capacity of IL-2-activated bovine NK cells was tested in a standard 51Cr release assay as described previously (39). Briefly, adherent fibroblast targets were dissociated as described above and incubated for 1 hour with 100 μCi Na51CrO4 (Amersham Biosciences). NK cells and 51Cr-labeled fibroblasts were counted in a hemocytometer using trypan blue exclusion and cocultured in triplicate at 37°C for 4 hours; the supernatant was harvested using the Supernatant Collection System (Molecular Devices, Sunnyvale, CA), and radioactivity was measured in a gamma counter (Wizard 1470; Wallac, Finland). Specific lysis was calculated on the basis of the following ratio: (sample release − spontaneous release)/(total release − spontaneous release). Spontaneous release of up to 30% was allowed in this study. Lytic units (LU) were calculated as the capacity to kill 20% of targets contained within 107 effectors, as described by Bryant and colleagues (11).
In blocking experiments, the effector cells were preincubated either for 30 min with 1 μg/ml anti-NKp46 or anti-CD8 MAb as a control or for 2 hours with 10 nM concanamycin A (CMA) (Sigma). The viability of CMA-incubated NK cells was assessed by incubating the cells with propidium iodide for 15 min at room temperature, followed by analysis of propidium iodide uptake in a flow cytometer.
Infection of NK cells with N. caninum.
IL-2-activated NK cells were incubated for 14 to 36 h with purified N. caninum tachyzoites at a multiplicity of infection of 1:1. The NK cells were then assayed for intracellular parasites by flow cytometry, as described above. To visualize intracellular parasites microscopically, cytospins were prepared by centrifuging 5 × 104 NK cells in 50 μl PBS onto poly-l-lysine-coated glass slides at 80 × g for 5 min. The slides were immediately fixed in methanol for 5 min, air dried, and stained with a 1:20 dilution of Giemsa stain (Sigma-Aldrich) in water for 20 to 30 min. Finally, the slides were washed in water buffered to pH 7.0 (3 mM sodium phosphate). Intracellular tachyzoite detection in NK cells was performed as described for fibroblasts, omitting the detachment step.
Statistical analysis.
Statistical analysis of differential LU values from cytotoxicity assays was carried out with the Wilcoxon matched pairs signed rank sum test, using JMP 5.1 statistical software (SAS Institute Inc., Cary, NC).
RESULTS
In vitro infection of fibroblasts with N. caninum tachyzoites.
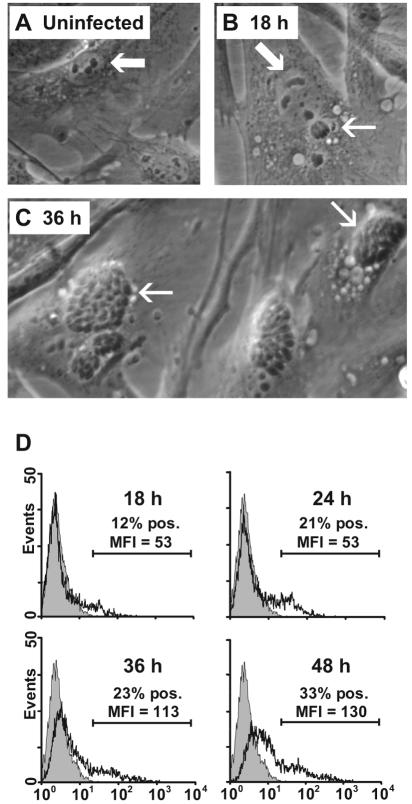
Primary bovine fibroblasts (Fig. 1A) were infected with tachyzoites of N. caninum (Fig. 1B and C). Microscopic examination indicated that the fibroblasts were efficiently infected, intracellular parasites being clearly detectable from approximately 18 h of exposure (Fig. 1B) and widespread at 36 h (Fig. 1C). After 48 h of exposure, many cells were lysing, releasing tachyzoites into the medium.
FIG. 1.
Microscopic and flow-cytometric detection of N. caninum infection of bovine primary dermal fibroblast cultures at different time points. (A) In uninfected fibroblasts, the cell morphology reflects the tissue origin with an ovoid, large, and light nucleus with evident nucleoli (arrow). (B) After 18 h of infection, a single tachyzoite within a parasitophorous vacuole (thin arrow) is seen separate from a cell nucleus (wide arrow). (C) After 36 h of infection, fibroblasts contain numerous groups of tachyzoites (arrows). (D) Flow-cytometric intracellular detection of N. caninum antigens in permeabilized fibroblasts labeled with the parasite-specific MAb 5B6 and a fluorochrome-conjugated secondary MAb (open histograms). The shaded histograms represent labeled uninfected fibroblasts; secondary MAb controls were similar (not shown). The results shown are from fibroblast culture of one animal representative of four repeated twice with similar results. MFI, mean fluorescence intensity; pos., positive.
The level of infection was assessed by intracellular labeling with the parasite-specific MAb 5B6 and flow cytometry. In cultures of fibroblasts incubated with tachyzoites for 18 to 24 h, between 10 and 20% of the cells were labeled positive, increasing to 25 to 35% after 36 to 48 h (Fig. 1D). The mean fluorescence intensity values of positively gated cells had increased at 36- and 48-h infection times. The percentage of positive cells in the flow analysis agreed well with the proportion of cells containing visible parasite bodies, while the mean fluorescence intensity of positive cells corresponded to the number of parasite bodies per infected cell, in agreement with similar techniques described with T. gondii-infected cells (34). Based on these findings, tachyzoites infected for 24 to 48 h were included in the subsequent experiments.
N. caninum-infected fibroblasts induce IFN-γ production from NK cells.
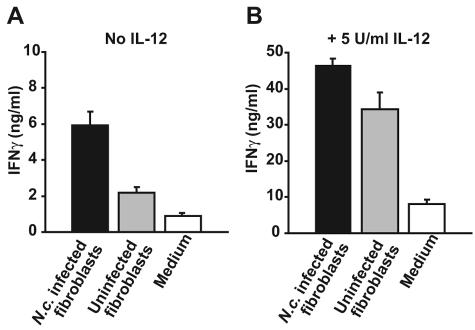
To investigate the role of NK cell production of IFN-γ during N. caninum infection, IL-2-activated NK cells were cultured with N. caninum-infected autologous fibroblasts at a ratio of 10:1. Following 24 h of coculture, a marked IFN-γ response was seen: a twofold increase compared to uninfected fibroblasts and sixfold compared to NK monocultures (Fig. 2A). When bovine rIL-12 was added at the start of the coculture, a large rise in IFN-γ in all cultures was observed; infected cells induced 1.4-fold-higher IFN-γ production than uninfected cells and around six-fold higher than NK monocultures (Fig. 2B). Individual differences in the size of the response were seen, but the pattern was consistent in repeated assays, including allogeneic NK cells from two additional animals (not shown). Thus, an increased production of IFN-γ by NK cells exposed to N. caninum-infected fibroblasts was observed, both in the presence and absence of IL-12.
FIG. 2.
IFN-γ production from NK cells cocultured with N. caninum (N.c.)-infected fibroblasts. (A) IFN-γ in supernatants of IL-2-activated NK cells cocultured for 24 h at a 10:1 rate with autologous fibroblasts infected for 36 h (filled bars), uninfected fibroblasts (shaded bars), or medium alone (open bars) were measured by ELISA. The results shown are means + SEM of two animals analyzed in triplicate, representative of three experiments. (B) As for panel A in the presence of 5 U/ml bovine rIL-12. Note the different values on the y axis. The results shown are means + SEM of two animals analyzed in triplicate representative of two experiments.
Purified live and heat-inactivated N. caninum tachyzoites induce IFN-γ production from NK cells.
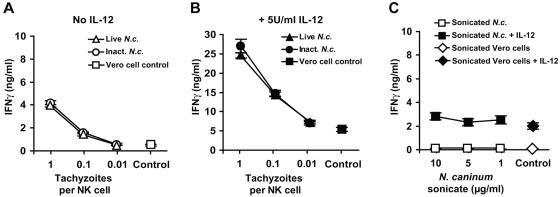
The ability of free N. caninum tachyzoites to induce IFN-γ production from NK cells was investigated by incubating IL-2-activated NK cell cultures for 24 h with tachyzoites at different parasite-to-cell ratios, with or without the addition of IL-12. Live and heat-inactivated tachyzoites repeatedly induced an equal extent of IFN-γ production from NK cells, in a dose-response manner, (Fig. 3A). Addition of IL-12 resulted in a 6- to 10-fold increase in all cultures, while the relative rise by live and heat-inactivated tachyzoites compared to controls was maintained (Fig. 3B). As with infected fibroblasts, some individual differences were seen, but tachyzoites consistently induced an IFN-γ response above that with controls both in the presence and absence of IL-12. Soluble antigens (sonicate) of N. caninum at various concentrations did not elicit any significant increase in IFN-γ production from IL-2-activated NK cell cultures, in either the presence or absence of IL-12 (Fig. 3C). Medium controls and sonicate of Vero cells, corresponding to the maximum possible contamination, gave similar results.
FIG. 3.
IFN-γ production from NK cells exposed to live, heat-inactivated, or sonicated N. caninum tachyzoites. (A) IFN-γ in supernatants of IL-2-activated NK cells exposed for 24 h to Percoll-purified live tachyzoites (triangles), heat-inactivated tachyzoites (56°C for 50 min; circles), or Percoll-purified uninfected Vero cell controls, corresponding to the greatest possible contamination (squares). (B) As for panel A in the presence of 5 U/ml bovine rIL-12. Note the different values on the y axis. The results shown are means ± SEM of two animals analyzed in triplicate representative of five individual experiments, in two of which inactivated tachyzoites were included. (C) IFN-γ levels in NK culture supernatants in the presence of sonicate of N. caninum at the indicated concentrations (squares) or sonicated, Percoll-purified Vero cells as controls (diamonds), in the absence (open) or presence (filled) of 5 U/ml bovine rIL-12. The results shown are means ± SEM of four animals analyzed in triplicate representative of two individual experiments.
These findings indicated that N. caninum tachyzoites were able to directly stimulate bovine IL-2-activated NK cells to produce IFN-γ, both independently of IL-12 and when this cytokine was present. The IFN-γ response was unaffected by heat inactivation of the tachyzoites but was completely absent when soluble antigens from N. caninum were used.
Infection of fibroblasts with N. caninum augments cytotoxicity by NK cells.
The capacity of NK cells to kill N. caninum-infected target cells was assessed by incubating autologous fibroblasts with tachyzoites for 24 to 48 h, followed by a 4-hour 51Cr release assay with IL-2-activated NK cells. Infected fibroblasts were significantly more efficiently killed than uninfected fibroblasts (Fig. 4). Calculating LU (11) for all assays, the mean (± standard error of the mean [SEM]) cytotoxic increase in animals no. 1 to 4 attributed to target cell infection were 72.1% (± 25%), 5.8% (± 28%), 252.2% (± 74%), and 67.7% (± 45%), respectively. The average increase in LU of all performed assays and animals, weighted by animal, was 73.2% (P < 0.001). Cytotoxicity in an allogeneic setting was assayed with five additional effector animals using the same targets; all effectors killed infected fibroblasts more efficiently than uninfected ones and to a degree similar to that with autologous effectors (not shown).
FIG. 4.
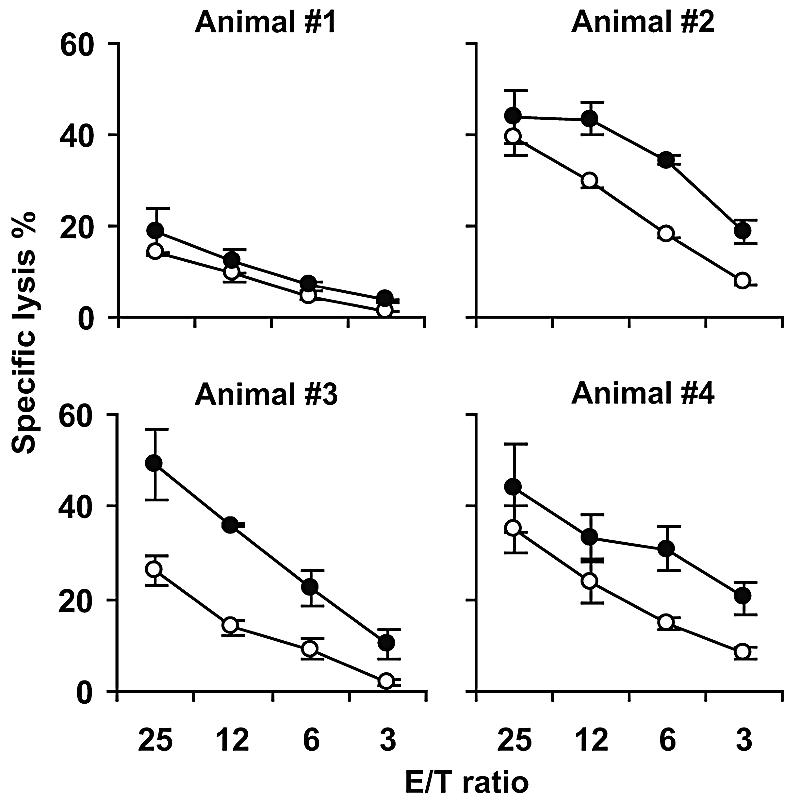
NK cell killing of autologous N. caninum-infected fibroblasts in a 4-h 51Cr release assay. The NK cell killing of autologous fibroblasts infected for 24 h (closed circles) increased compared to uninfected control targets (open circles). The results shown are means ± standard deviations of triplicates from one representative experiment performed four to six times per animal. Allogeneic killing was also performed with NK cell cultures from five additional animals with similar results (not shown). E/T, effector-to-target-cell ratio.
In some cases, NK cells were preincubated for 2 hours with 10 nM CMA to selectively inhibit perforin-based cytotoxicity (21). This reduced the cytotoxicity against both infected and uninfected fibroblasts by about 90% (not shown). Six hours of treatment with CMA affected neither spontaneous lysis of fibroblasts nor the viability of NK cells, as measured by propidium iodide uptake in flow cytometry, indicating that the inhibition was not due to a general cell-toxic effect.
Taken together, an enhanced, perforin-mediated NK cytotoxicity against N. caninum-infected fibroblasts was repeatedly observed.
Involvement of the NKp46 receptor in fibroblast-induced NK activation.
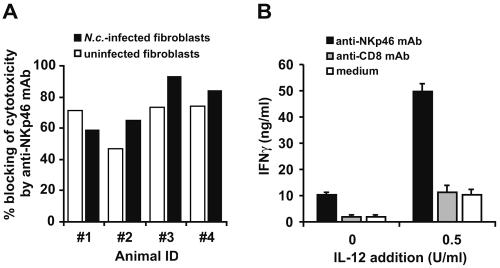
The difference in IFN-γ production by NK cells cultured with uninfected fibroblasts and NK cells cultured alone (Fig. 2A and B) and the finding that even uninfected fibroblasts were susceptible to NK cell cytotoxicity (Fig. 4) indicated that fibroblasts could activate NK cells even in the absence of infection. To assess whether this activation was mediated through direct cell contact or by soluble factors, supernatants from uninfected fibroblasts passed through a 0.22-μm filter were added to NK cell cultures and incubated for 24 h. No IFN-γ production could be detected (not shown), indicating that the observed activation required cell-to-cell contact.
To assess if binding of the NKp46 on fibroblasts was involved in contact-dependent activation, anti-NKp46 MAb was added in cytotoxicity assays, efficiently inhibiting killing of fibroblasts (Fig. 5A). However, no clear difference in the degree of MAb inhibition was observed between N. caninum-infected and uninfected fibroblasts. Addition of MAbs against CD8 did not result in any blocking (not shown).
FIG. 5.
Involvement of the NKp46 receptor in the activation of bovine NK cells. (A) Percent blocking of NK cell cytotoxicity (LU) by 1 μg/ml anti-NKp46 MAb, compared to medium alone, against fibroblasts infected for 36 h with N. caninum (closed bars) or uninfected (open bars). The results are expressed as means of triplicate samples from four individual animals, representative of one out of two experiments. ID, identifier. (B) IFN-γ production by NK cells (2 × 106/ml) incubated in IL-2-containing medium for 24 h in plastic wells coated with anti-NKp46 MAb (filled bars), anti-CD8 MAb (shaded bars), or medium alone (open bars) with or without the addition of 0.5 U/ml bovine rIL-12. The results shown are means + SEM of two animals analyzed in triplicate representative of two experiments.
The possible involvement of an NKp46 ligand for IFN-γ production was assessed indirectly using surface-bound anti-NK MAbs, mimicking a ligand under cross-linking conditions. NK cells were incubated for 24 h in plastic wells coated with anti-NKp46 MAb. A strong IFN-γ response ensued, potentiated by addition of IL-12 (Fig. 5B). Control MAb against CD8, confirmed by flow cytometry to be present on a large proportion of NK cells, did not elicit any such response. Incubation of NK cells with anti-NKp46 MAbs in solution did not elicit IFN-γ production (not shown).
N. caninum tachyzoites are found inside NK cells following in vitro exposure.
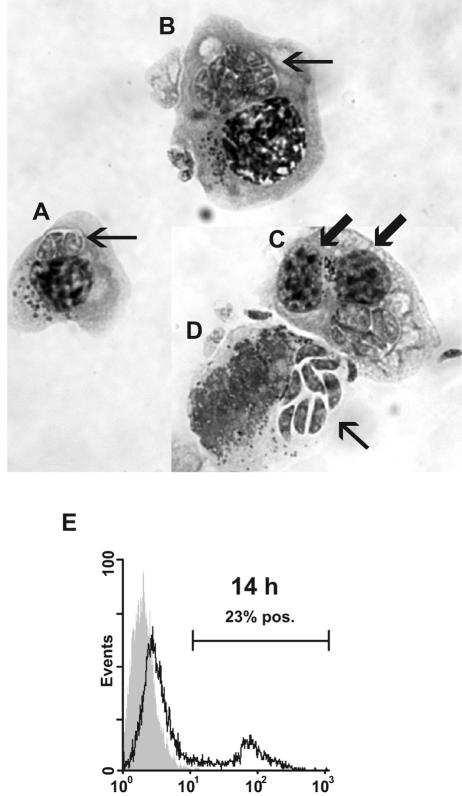
The observation that intact tachyzoites could induce IFN-γ from purified NK cells while sonicates did not inspired a closer microscopic investigation of N. caninum-stimulated NK cells. NK cells cocultured with tachyzoites for 24 h tended to form aggregates in the culture wells. Cytospin preparations of exposed NK cell cultures revealed the presence of parasitic bodies located in parasitophorous vacuoles inside the cytoplasm of many NK cells (Fig. 6A and B). Several infected cells appeared binucleated (Fig. 6C). Using intracellular staining with an N. caninum-specific MAb, N. caninum antigen was detected inside 14 to 35% of exposed cells at 14 h postinfection (Fig. 6E) and in up to 90% of cells at 36 h (data not shown). Microscopic evaluation showed that the number of tachyzoites inside each infected NK cell increased during this period, indicating that the tachyzoites were proliferating intracellularly. In conclusion, NK cells were not only stimulated, but also infected by the parasites after in vitro exposure to live N. caninum tachyzoites.
FIG. 6.
Giemsa-stained cytospin preparation of N. caninum-infected bovine NK cells. (A) The arrow points at two tachyzoites in a parasitic vacuole. (B) NK cell harboring several tachyzoites (arrow). (C) Binucleated N. caninum-infected NK cell. The arrows point at the two nuclei. (D) Free N. caninum tachyzoites (arrow) emerging from lysed NK cell. (E) Flow-cytometric histogram of infected NK cells permeabilized and labeled with the parasite-specific MAb 5B6 and a fluorochrome-conjugated secondary MAb. The shaded histograms represent labeled uninfected NK cells; secondary controls were similar (not shown). The results shown are from NK cell cultures of one animal representative of two; the experiment was repeated twice with similar results. pos., positive.
DISCUSSION
This study aimed to investigate bovine NK cell responses during N. caninum infection, using an in vitro cell culture model. The main findings were that both live and heat-inactivated tachyzoites, as well as infected fibroblasts, stimulated NK cells to produce IFN-γ, independently of but also increased by IL-12; that soluble proteins did not elicit such a response; and that infected fibroblasts had increased susceptibility to NK cell cytotoxicity. Additionally, we observed that NK cells were infected with N. caninum under these experimental conditions, with tachyzoites multiplying inside NK cells.
The effector cells used here were virtually uniformly NKp46 positive and free of contaminating cells, like CD14+ cells or αβ or γδ T cells, as described previously (39). The finding that live purified N. caninum tachyzoites were able to trigger IFN-γ production from pure IL-2-activated NK cells, in the absence of other cells and without the addition of cytokines like IL-12, appears to be in conflict with the established view that IFN-γ production from NK cells requires IL-12 (3, 44, 48). However, our finding largely parallels that of Nylen and colleagues, who recently reported NK cell production of IFN-γ triggered by live Leishmania promastigotes (29). IL-12-independent NK-cell stimulation has also been reported in IL-12-deficient mice during infections with Trypanosoma (45) and in bacterial infections, such as with Helicobacter (49) and Listeria (10). While activation of NK cells may occur through several alternative mechanisms, yet to be fully explored, one conceivable mechanism would be the engagement of pattern-recognizing receptors, like Toll-like receptors, recently reported to be expressed on NK cells (35). Notably, stimulation of human NK cells by Leishmania-derived glycosylphosphatidylinositol (GPI) molecules through Toll-like receptor 2 was recently reported (2). GPI structures have also been characterized in N. caninum (18). In the present study, intact glycolipids, like GPIs, were likely to be absent from the sonicate, suggesting a possible explanation for the inability of this preparation to elicit an IFN-γ response by NK cells.
Purified NK cells from the cow have not previously been studied for cytotoxic activity in response to infection. In general, the most established view is that during protozoan infections, NK cells act by releasing cytokines, while cytotoxicity plays a less significant role, as reviewed by Scharton-Kersten et al. (33). However, as mentioned in that review, the main tool for assessing the cytolytic significance of NK cells in vivo has been mouse models. Interestingly, all but one publication found on NK cytotoxicity against human target cells infected with protozoa demonstrated increased NK cell cytotoxicity (8, 9, 27, 30, 32, 41). The one negative report found, on Plasmodium (42), has been criticized for methodology in the context of NK cells (27). The present observation that N. caninum-infected fibroblasts were killed to a larger extent than uninfected fibroblasts parallels findings seen with other protozoans in human NK cells, providing a contribution to the discussion on whether NK cytotoxocity is important in protozoan infections. The biological relevance of these findings remains to be clarified.
The NKp46 receptor has been reported to play a central role in NK cell cytotoxicity against various target cells (36, 46). Ligands for this receptor have not been identified, although studies have indicated an association between the receptor and viral hemagglutins, as well as heparan sulfate proteoglycans (7, 25). We have previously reported that an anti-NKp46 MAb can block killing of conventional targets, as well as augment killing (in a redirected lysis of FcR-bearing targets) (39). In the present study, we found that cell-to-cell contact was necessary for NK activation and that the NKp46 receptor mediated a major part of the cytotoxicity against both infected and uninfected fibroblasts, indicating the expression of an NKp46 ligand on the fibroblasts but not explaining the increased killing of N. caninum-infected fibroblasts. Antibody ligation of the NKp46 receptor potently induced IFN-γ production by the NK cells, as seen with human NK cells (37), suggesting by analogy one possible mechanism for the IFN-γ response caused by uninfected fibroblasts.
Since an NKp46 ligand was apparently not responsible for the N. caninum-caused rise in NK cell cytotoxicity, explanations could be sought within the known activation pathways for NK cells, e.g., recognition of pathogen-derived structures expressed on infected cells, downregulation of major histocompatibility complex (MHC) class 1 receptors (3), upregulation of stress-associated ligands for activating NK receptors (24), or combinations of any of these. In the present study, an observation made while performing assays for the intracellular detection of N. caninum antigens was that the parasite-specific MAb to a certain extent also bound to the surfaces of infected fibroblasts (results not shown). It is not known whether this was due to MHC class 1 presentation, which might be relevant, since very recent studies have suggested NK cell activation through recognition of MHC class 1 molecules specifically on virus-infected cells (13).
The almost total inhibition of target lysis caused by concanamycin A parallels what has previously been observed in cytotoxic T lymphocytes during bovine neosporosis (38). However, in both cases, inhibited killing of uninfected, as well as infected, targets was seen with this agent. These findings demonstrate that perforin generally plays a prominent role in bovine lymphocyte cytotoxicity, regardless of infection. The role of the other major known NK cell cytotoxic mechanism, engagement of death receptors (e.g., Fas/CD95), remains to be studied in bovine cells.
Interestingly, the NK cells themselves became infected during culture with the parasites, as demonstrated microscopically and in intracellular flow-cytometric analysis. We found that N. caninum-infected NK cells proliferated well and that the tachyzoites multiplied inside the NK cells. N. caninum is known to infect a wide range of cell types (15), but to our knowledge, this is the first report of infection of NK cells with an intracellular parasite. T. gondii can infect lymphocytes under experimental conditions (12). The theileriae, another group of apicomplexan protozoans, target leukocytes as their main host cells during infection of the vertebrate. Infection of NK cells with Theileira annulata has been suspected, but not verified (16, 31). In preliminary investigations of peripheral blood NK cells from five calves experimentally intravenously infected with N. caninum, we could not demonstrate infected NK cells during the acute phase (results not shown). While further studies of various tissue compartments, as well as different stages of infection, would be required to draw conclusions about any role of Neospora-infected NK cells in vivo, the finding that experimental infection is possible should open up a perspective in which NK cells should not only be regarded as effector cells, but also as potential targets.
The results presented here represent the first study of interactions between NK cells and N. caninum. Besides providing a step forward in the understanding of immune response to the organism in cattle, the findings that NK cell response does not require IL-12 and that NK cells preferentially kill infected target cells contribute to the basic understanding of innate immunity during protozoan infections. Finally, the observation that this protozoan organism can infect NK cells would be an interesting topic for further investigation.
Acknowledgments
We thank Inger Austrheim Heffernan and Grethe M. Johansen for their work on cytokine assays and NK cell isolation and Jorun Tharaldsen for support. Furthermore, we thank Camilla Björkman (Swedish University of Agricultural Sciences, Uppsala, Sweden) for her kind donation of parasites and key assistance in parasite methodology, Jayne C. Hope (Institute for Animal Health, Compton, United Kingdom) for providing recombinant bovine IL-12, and Stephen Jones (Pfizer, Melbourne, Australia) for providing recombinant bovine IFN-γ. Finally, we thank Erik Dissen (Institute of Basic Medical Sciences, University of Oslo) for critical reading of the manuscript.
This work was carried out under grant support from the Norwegian Research Council.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Baszler, T. V., M. T. Long, T. F. McElwain, and B. A. Mathison. 1999. Interferon-gamma and interleukin-12 mediate protection to acute Neospora caninum infection in BALB/c mice. Int. J. Parasitol. 29:1635-1646. [DOI] [PubMed] [Google Scholar]
- 2.Becker, I., N. Salaiza, M. Aguirre, J. Delgado, N. Carrillo-Carrasco, L. G. Kobeh, A. Ruiz, R. Cervantes, A. P. Torres, N. Cabrera, A. Gonzalez, C. Maldonado, and A. Isibasi. 2003. Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Mol. Biochem. Parasitol. 130:65-74. [DOI] [PubMed] [Google Scholar]
- 3.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 4.Bjerkas, I., S. F. Mohn, and J. Presthus. 1984. Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z. Parasitenkd. 70:271-274. [DOI] [PubMed] [Google Scholar]
- 5.Bjorkman, C., O. J. Holmdahl, and A. Uggla. 1997. An indirect enzyme-linked immunoassay (ELISA) for demonstration of antibodies to Neospora caninum in serum and milk of cattle. Vet. Parasitol. 68:251-260. [DOI] [PubMed] [Google Scholar]
- 6.Bjorkman, C., A. Lunden, J. Holmdahl, J. Barber, A. J. Trees, and A. Uggla. 1994. Neospora caninum in dogs: detection of antibodies by ELISA using an iscom antigen. Parasite Immunol. 16:643-648. [DOI] [PubMed] [Google Scholar]
- 7.Bloushtain, N., U. Qimron, A. Bar-Ilan, O. Hershkovitz, R. Gazit, E. Fima, M. Korc, I. Vlodavsky, N. V. Bovin, and A. Porgador. 2004. Membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NKp30 and NKp46. J. Immunol. 173:2392-2401. [DOI] [PubMed] [Google Scholar]
- 8.Bouyou-Akotet, M. K., S. Issifou, J. F. Meye, M. Kombila, E. Ngou-Milama, A. J. Luty, P. G. Kremsner, and E. Mavoungou. 2004. Depressed natural killer cell cytotoxicity against Plasmodium falciparum-infected erythrocytes during first pregnancies. Clin. Infect. Dis. 38:342-347. [DOI] [PubMed] [Google Scholar]
- 9.Brodskyn, C. I., A. Barral, V. Boaventura, E. Carvalho, and M. Barral-Netto. 1997. Parasite-driven in vitro human lymphocyte cytotoxicity against autologous infected macrophages from mucosal leishmaniasis. J. Immunol. 159:4467-4473. [PubMed] [Google Scholar]
- 10.Brombacher, F., A. Dorfmuller, J. Magram, W. J. Dai, G. Kohler, A. Wunderlin, K. Palmer-Lehmann, M. K. Gately, and G. Alber. 1999. IL-12 is dispensable for innate and adaptive immunity against low doses of Listeria monocytogenes. Int. Immunol. 11:325-332. [DOI] [PubMed] [Google Scholar]
- 11.Bryant, J., R. Day, T. L. Whiteside, and R. B. Herberman. 1992. Calculation of lytic units for the expression of cell-mediated cytotoxicity. J. Immunol. Methods 146:91-103. [DOI] [PubMed] [Google Scholar]
- 12.Chai, J. Y., J. Kook, S. M. Guk, Y. P. Chang, and C. K. Yun. 1997. Experimental infection of murine splenic lymphocytes and granulocytes with Toxoplasma gondii RH tachyzoites. Korean J. Parasitol. 35:79-85. [DOI] [PubMed] [Google Scholar]
- 13.Desrosiers, M. P., A. Kielczewska, J. C. Loredo-Osti, S. G. Adam, A. P. Makrigiannis, S. Lemieux, T. Pham, M. B. Lodoen, K. Morgan, L. L. Lanier, and S. M. Vidal. 2005. Epistasis between mouse Klra and major histocompatibility complex class I loci is associated with a new mechanism of natural killer cell-mediated innate resistance to cytomegalovirus infection. Nat. Genet. 37:593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubey, J. P., B. C. Barr, J. R. Barta, I. Bjerkas, C. Bjorkman, B. L. Blagburn, D. D. Bowman, D. Buxton, J. T. Ellis, B. Gottstein, A. Hemphill, D. E. Hill, D. K. Howe, M. C. Jenkins, Y. Kobayashi, B. Koudela, A. E. Marsh, J. G. Mattsson, M. M. McAllister, D. Modry, Y. Omata, L. D. Sibley, C. A. Speer, A. J. Trees, A. Uggla, S. J. Upton, D. J. Williams, and D. S. Lindsay. 2002. Redescription of Neospora caninum and its differentiation from related coccidia. Int. J. Parasitol. 32:929-946. [DOI] [PubMed] [Google Scholar]
- 15.Dubey, J. P., and D. S. Lindsay. 1996. A review of Neospora caninum and neosporosis. Vet. Parasitol. 67:1-59. [DOI] [PubMed] [Google Scholar]
- 16.Forsyth, L. M., L. A. Jackson, G. Wilkie, A. Sanderson, C. G. Brown, and P. M. Preston. 1997. Bovine cells infected in vivo with Theileria annulata express CD11b, the C3bi complement receptor. Vet. Res. Commun. 21:249-263. [DOI] [PubMed] [Google Scholar]
- 17.Ho, M. S., B. C. Barr, A. F. Tarantal, L. T. Lai, A. G. Hendrickx, A. E. Marsh, K. W. Sverlow, A. E. Packham, and P. A. Conrad. 1997. Detection of Neospora from tissues of experimentally infected rhesus macaques by PCR and specific DNA probe hybridization. J. Clin. Microbiol. 35:1740-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howe, D. K., A. C. Crawford, D. Lindsay, and L. D. Sibley. 1998. The p29 and p35 immunodominant antigens of Neospora caninum tachyzoites are homologous to the family of surface antigens of Toxoplasma gondii. Infect. Immun. 66:5322-5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Innes, E. A., A. G. Andrianarivo, C. Bjorkman, D. J. Williams, and P. A. Conrad. 2002. Immune responses to Neospora caninum and prospects for vaccination. Trends Parasitol. 18:497-504. [DOI] [PubMed] [Google Scholar]
- 20.Innes, E. A., W. R. Panton, J. Marks, A. J. Trees, J. Holmdahl, and D. Buxton. 1995. Interferon gamma inhibits the intracellular multiplication of Neospora caninum, as shown by incorporation of 3H uracil. J. Comp. Pathol. 113:95-100. [DOI] [PubMed] [Google Scholar]
- 21.Kataoka, T., N. Shinohara, H. Takayama, K. Takaku, S. Kondo, S. Yonehara, and K. Nagai. 1996. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J. Immunol. 156:3678-3686. [PubMed] [Google Scholar]
- 22.Khan, I. A., J. D. Schwartzman, S. Fonseka, and L. H. Kasper. 1997. Neospora caninum: role for immune cytokines in host immunity. Exp. Parasitol. 85:24-34. [DOI] [PubMed] [Google Scholar]
- 23.Korbel, D. S., O. C. Finney, and E. M. Riley. 2004. Natural killer cells and innate immunity to protozoan pathogens. Int. J. Parasitol. 34:1517-1528. [DOI] [PubMed] [Google Scholar]
- 24.Lanier, L. L. 2005. NK cell recognition. Annu. Rev. Immunol. 23:225-274. [DOI] [PubMed] [Google Scholar]
- 25.Mandelboim, O., N. Lieberman, M. Lev, L. Paul, T. I. Arnon, Y. Bushkin, D. M. Davis, J. L. Strominger, J. W. Yewdell, and A. Porgador. 2001. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 409:1055-1060. [DOI] [PubMed] [Google Scholar]
- 26.Marks, J., A. Lunden, D. Harkins, and E. Innes. 1998. Identification of Neospora antigens recognized by CD4+ T cells and immune sera from experimentally infected cattle. Parasite Immunol. 20:303-309. [DOI] [PubMed] [Google Scholar]
- 27.Mavoungou, E., A. J. Luty, and P. G. Kremsner. 2003. Natural killer (NK) cell-mediated cytolysis of Plasmodium falciparum-infected human red blood cells in vitro. Eur. Cytokine Netw. 14:134-142. [PubMed] [Google Scholar]
- 28.Nishikawa, Y., K. Tragoolpua, N. Inoue, L. Makala, H. Nagasawa, H. Otsuka, and T. Mikami. 2001. In the absence of endogenous gamma interferon, mice acutely infected with Neospora caninum succumb to a lethal immune response characterized by inactivation of peritoneal macrophages. Clin. Diagn. Lab. Immunol. 8:811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nylen, S., K. Maasho, K. Soderstrom, T. Ilg, and H. Akuffo. 2003. Live Leishmania promastigotes can directly activate primary human natural killer cells to produce interferon-gamma. Clin. Exp. Immunol. 131:457-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orago, A. S., and C. A. Facer. 1991. Cytotoxicity of human natural killer (NK) cell subsets for Plasmodium falciparum erythrocytic schizonts: stimulation by cytokines and inhibition by neomycin. Clin. Exp. Immunol. 86:22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preston, P. M., C. G. Brown, and R. L. Spooner. 1983. Cell-mediated cytotoxicity in Theileria annulata infection of cattle with evidence for BoLA restriction. Clin. Exp. Immunol. 53:88-100. [PMC free article] [PubMed] [Google Scholar]
- 32.Saha, A., G. Chakrabarti, S. Sen, and S. Bandyopadhyay. 1999. Leishmania donovani parasites interact with gamma/delta+ human peripheral blood T cells and induce susceptibility to NK cell-mediated lysis. Scand. J. Immunol. 50:588-595. [DOI] [PubMed] [Google Scholar]
- 33.Scharton-Kersten, T. M., and A. Sher. 1997. Role of natural killer cells in innate resistance to protozoan infections. Curr. Opin. Immunol. 9:44-51. [DOI] [PubMed] [Google Scholar]
- 34.Shin, E. H., S. B. Kim, H. W. Nam, E. T. Han, J. H. Park, H. J. Ahn, and J. Y. Chai. 2004. Use of monoclonal antibodies for flow cytometric detection of intracellular Toxoplasma gondii in murine splenic lymphocytes. J. Parasitol. 90:161-166. [DOI] [PubMed] [Google Scholar]
- 35.Sivori, S., M. Falco, C. M. Della, S. Carlomagno, M. Vitale, L. Moretta, and A. Moretta. 2004. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc. Natl. Acad. Sci. USA 101:10116-10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sivori, S., D. Pende, C. Bottino, E. Marcenaro, A. Pessino, R. Biassoni, L. Moretta, and A. Moretta. 1999. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur. J. Immunol. 29:1656-1666. [DOI] [PubMed] [Google Scholar]
- 37.Sivori, S., M. Vitale, L. Morelli, L. Sanseverino, R. Augugliaro, C. Bottino, L. Moretta, and A. Moretta. 1997. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J. Exp. Med. 186:1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staska, L. M., T. C. McGuire, C. J. Davies, H. A. Lewin, and T. V. Baszler. 2003. Neospora caninum-infected cattle develop parasite-specific CD4+ cytotoxic T lymphocytes. Infect. Immun. 71:3272-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storset, A. K., S. Kulberg, I. Berg, P. Boysen, J. C. Hope, and E. Dissen. 2004. NKp46 defines a subset of bovine leukocytes with natural killer cell characteristics. Eur. J. Immunol. 34:669-676. [DOI] [PubMed] [Google Scholar]
- 40.Storset, A. K., I. O. Slettedal, J. L. Williams, A. Law, and E. Dissen. 2003. Natural killer cell receptors in cattle: a bovine killer cell immunoglobulin-like receptor multigene family contains members with divergent signaling motifs. Eur. J. Immunol. 33:980-990. [DOI] [PubMed] [Google Scholar]
- 41.Subauste, C. S., L. Dawson, and J. S. Remington. 1992. Human lymphokine-activated killer cells are cytotoxic against cells infected with Toxoplasma gondii. J. Exp. Med. 176:1511-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theander, T. G., B. J. Andersen, B. K. Pedersen, S. Jepsen, I. C. Bygbjerg, L. Hviid, P. B. Larsen, and A. Kharazmi. 1988. Cell-mediated immunity to Plasmodium falciparum infection: evidence against the involvement of cytotoxic lymphocytes. Scand. J. Immunol. 28:105-111. [DOI] [PubMed] [Google Scholar]
- 43.Tranas, J., R. A. Heinzen, L. M. Weiss, and M. M. McAllister. 1999. Serological evidence of human infection with the protozoan Neospora caninum. Clin. Diagn. Lab. Immunol. 6:765-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trinchieri, G. 1995. Natural killer cells wear different hats: effector cells of innate resistance and regulatory cells of adaptive immunity and of hematopoiesis. Semin. Immunol. 7:83-88. [DOI] [PubMed] [Google Scholar]
- 45.Une, C., J. Andersson, and A. Orn. 2003. Role of IFN-alpha/beta and IL-12 in the activation of natural killer cells and interferon-gamma production during experimental infection with Trypanosoma cruzi. Clin. Exp. Immunol. 134:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westgaard, I. H., S. F. Berg, J. T. Vaage, L. L. Wang, W. M. Yokoyama, E. Dissen, and S. Fossum. 2004. Rat NKp46 activates natural killer cell cytotoxicity and is associated with FcɛRIγ and CD3ζ. J. Leukoc. Biol. 76:1200-1206. [DOI] [PubMed] [Google Scholar]
- 47.Yamane, I., H. Kitani, T. Kokuho, T. Shibahara, M. Haritani, T. Hamaoka, S. Shimizu, M. Koiwai, K. Shimura, and Y. Yokomizo. 2000. The inhibitory effect of interferon gamma and tumor necrosis factor alpha on intracellular multiplication of Neospora caninum in primary bovine brain cells. J. Vet. Med. Sci. 62:347-351. [DOI] [PubMed] [Google Scholar]
- 48.Yap, G. S., and A. Sher. 1999. Cell-mediated immunity to Toxoplasma gondii: initiation, regulation and effector function. Immunobiology 201:240-247. [DOI] [PubMed] [Google Scholar]
- 49.Yun, C. H., A. Lundgren, J. Azem, A. Sjoling, J. Holmgren, A. M. Svennerholm, and B. S. Lundin. 2005. Natural killer cells and Helicobacter pylori infection: bacterial antigens and interleukin-12 act synergistically to induce gamma interferon production. Infect. Immun. 73:1482-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]