Abstract
Cattle were vaccinated with an adenovirus expressing the mycobacterial antigen 85A (rAd85A), with Mycobacterium bovis BCG followed by rAd85A heterologous boosting, or with rAd85A followed by BCG boosting. BCG/rAd85A resulted in the highest direct gamma interferon responses. Cultured enzyme-linked immunospot assay analysis demonstrated that memory responses were induced by all three protocols but were strongest after BCG/rAd85A and rAd85A/BCG vaccination.
Over the last 2 decades, there has been a steady rise in the incidence of bovine tuberculosis (bTB) in cattle in Great Britain (7), and the development of a cattle bTB vaccine is considered the best option for its control (7). Mycobacterium bovis bacillus Calmette-Guerin (BCG) is associated with variable efficacy both in humans and in cattle, and improving its efficacy is a priority (3, 6). Heterologous prime-boost strategies have been developed to improve its efficacy (2, 8, 9, 13). In particular, application of the recombinant modified vaccinia virus Ankara strain expressing the mycobacterial protective antigen 85A (Ag85A) (Rv3804c) has shown promise in small-animal models of human tuberculosis (5, 19), and human phase I clinical trials of BCG vaccination followed by MVA85A boosting are under way (9, 10).
Recently, vaccination with a replication-deficient recombinant adenovirus expressing Ag85A (rAd85A) protected mice from M. tuberculosis infection (12, 17). To assess this viral vaccine in cattle, calves were vaccinated with rAd85A in heterologous prime-boost scenarios together with BCG to determine its immunogenicity in this natural bTB target species. rAd85A was prepared as described previously (17), and groups of five calves (ca. 6-month-old Holstein females) were vaccinated with (i) rAd85A (109 PFU/0.5 ml, delivered intramuscularly) at week 0 and BCG Pasteur (Staten Serum Institute, Copenhagen, Denmark; 106 CFU/1 ml, delivered subcutaneously) at week 6, (ii) BCG Pasteur at week 0 and rAd85A at week 6, or (iii) rAd85A at weeks 0 and 3. These boosting intervals were chosen because rAd85A (12, 17) and BCG (16) trigger peak responses at different times postvaccination (1 to 2 weeks and 3 to 5 weeks, respectively), and the intention was not to perform the boost during peak responses, but at a time when effector immune responses had decreased substantially.
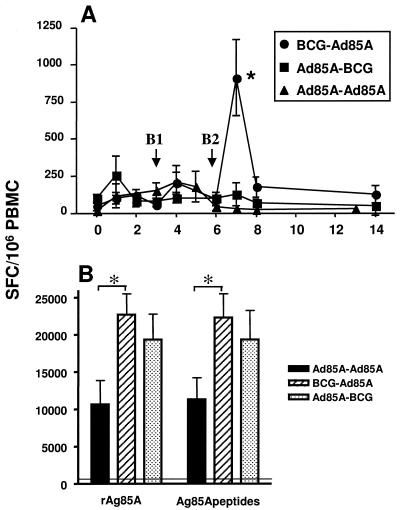
Peripheral blood mononuclear cells (PBMC) were prepared and cultured (14, 15) to establish the numbers of ex vivo gamma interferon (IFN-γ)-secreting cells (spot-forming cells [SFC]) in enzyme-linked immunospot (ELISPOT) assays (14) after in vitro stimulation with rAg85A (5 μg/ml; Lionex, Braunschweig, Germany) or bovine tuberculin purified protein derivative (10 μg/ml; Veterinary Laboratories Agency-Weybridge). The results shown in Fig. 1A demonstrate that all vaccination protocols resulted in the induction of T cells specific for Ag85A. Priming vaccination with rAd85A resulted in rAg85A-specific responses peaking at weeks 1 to 3 for the rAd85-rAd85A and rAd85A-BCG groups (254 ± 131 and 155 ± 50 SFC/106 PBMC, respectively). Similar response levels against rAg85A were observed after BCG priming of the BCG-rAd85A group. Although BCG-induced IFN-γ response peaks may vary between experiments, the responses of these BCG-vaccinated calves were not significantly different from the results of earlier BCG vaccination experiments (16). In addition, the response kinetic was identical to previous results obtained after a single BCG vaccination, which resulted in peak responses between weeks 3 and 5 followed by a decline in responses towards prevaccination levels between weeks 6 and 8 postvaccination (16). Thus, the responses described up to week 6, i.e., immediately before boosting of the BCG prime group, were identical to those observed previously following a single BCG vaccination (BCG-alone group) and were expected to further decline after week 6 if the cattle were not boosted.
FIG. 1.
IFN-γ responses after vaccination. (A) Kinetics of ex vivo, direct responses after stimulation of PBMC (2 × 105/well) with rAg85A (5 μg/ml). Results are depicted as mean net SFC (SFC with stimulant minus SFC of medium controls) ± standard errors of the means (SEM) for five cattle per group. *, P < 0.05 compared to the other two groups (one-way analysis of variance followed by the Tukey-Kramer multiple-comparison postanalysis test). Circles, BCG-rAd85A (n = 5); squares, rAd85A-BCG (n = 5); triangles, rAd85A-rAd85A (n = 5). Priming vaccination was done at week 0. B1, rAd85A boost of rAd85A-rAd85A group; B2, BCG or rAd85A boost of BCG-rAd85A or rAd85A-BCG group, respectively. Positive responses were recorded when the SFC with stimulant minus the SFC of medium controls was >50 SFC/106 PBMC and more than the prevaccination values. (B) Cultured ELISPOT responses established 8 weeks after booster injections. PBMC were stimulated with rAg85A and IL-2 for 13 days, and IFN-γ ELISPOT assays were performed by stimulating cultured cells with rAg85A (5 μg/ml) or a peptide cocktail derived from Ag85A (6 μg/ml of each peptide). Cultures were performed in the presence of autologous macrophages as a source of antigen-presenting cells. Medium control values were subtracted, and data are presented as means of groups of five animals ± SEM. Statistical differences between groups were evaluated using unpaired, two-tailed t tests. Differences between the BCG-rAd85A and rAd85A-rAd85A groups did not reach statistical significance. The horizontal line shows peptide-stimulated cultured ELISPOT results for PBMC from a group of six unvaccinated control animals (SFC/106 cells, 480 ± 480).
Boosting BCG-primed calves with rAd85A resulted in statistically significant anamnestic IFN-γ responses compared to preboost peak levels (Fig. 1A) (five of five calves; mean preboost BCG-alone peak value at week 4, 206 ± 67 SFC/106 PBMC; mean postboost peak value, 914 ± 255 SFC/106 PBMC; P = 0.028). This enhanced ex vivo response peaked at 1 week post-rAd85A infection (week 7) and was significantly different from those of the other two groups at this time point (P = 0.011 for comparison to postboost peak response at week 7 with rAd85A-BCG and at week 4 with rAd85A-rAd85A). Ex vivo responses contracted over the following weeks (Fig. 1A). Boosting the animals in the rAd85A-rAd85A group with rAd85A at week 3 resulted in enhanced responses compared to preboost peak levels in two of five animals (mean level, 212 ± 111 SFC/106 PBMC) (Fig. 1A), peaking 1 week after the boost. Boosting rAd85A-primed cattle with BCG resulted in enhanced rAg85A responses in three of five animals compared to the levels at the time of boosting at week 6 (100 ± 39 SFC/106 PBMC at week 6 versus 127 ± 80 SFC/106 PBMC after the boost) (Fig. 1A) and never exceeded the IFN-γ levels observed after rAd85A priming. Responses after ex vivo stimulation with bovine purified protein derivative were also determined and confirmed the results obtained with rAg85A (not shown). One mechanism for the failure of BCG to recall rAd85A-primed responses, or even to induce stronger responses on its own, could be due to Ad85A-induced preexisting immunity to BCG. A similar mechanism has been postulated to explain why exposure to environmental mycobacteria results in immunity to cross-reacting antigens that limits BCG multiplication and protective immunity (1). It is also possible that rAd85A is a weak immunogen in cows, being poor at priming immune responses but capable of boosting existing responses.
Measuring ex vivo IFN-γ responses most likely assesses effector cells and effector memory T-cell responses, yet recent studies of viral and parasitic infections in mice and humans have suggested that central memory responses rather than effector memory responses correlate with pathogen clearance and protection (11, 18). However, reagents for labeling central memory responses in cattle, e.g., bovine CCR7+ cells, are unavailable. Therefore, we developed a cultured ELISPOT system to investigate long-term central memory responses in cattle. Although central memory T cells have not been formally defined for cattle, this assay has been shown to assess such responses in other species, including humans (4). Cultured ELISPOT analysis was performed at 14 weeks postpriming, when no significant ex vivo responses were found compared to prevaccination levels (Fig. 1A), by stimulating 2 × 106 PBMC/ml with the rAg85 protein (2 μg/ml). Recombinant human interleukin-2 (IL-2) (to 10 U/ml; Sigma Poole, Great Britain) was added to the cultures on days 5 and 8. On days 10 and 12, half of the supernatant was replaced with IL-2-free medium. On day 13, 2 × 104 cells/well were added to ELISPOT plates and incubated together with rAg85A (5 mg/ml) or an Ag85A peptide cocktail (peptides 1, 3, 4, 6, 8, 11, 16, 17, 18, 19, 21, 22, and 23 [16]; 6 mg/ml of each peptide). Large numbers of memory cells were found after vaccination with all three protocols (Fig. 1B). However, rAd85A-rAd85A vaccination resulted in the weakest cultured ELISPOT responses (rAg85A stimulation, 10,650 ± 1,270 SFC/106 cells) compared to BCG-rAd85A and rAd85A-BCG (22,720 ± 2,780 and 19,380 ± 3,430 SFC/106 cells, respectively), although only the responses of the BCG-rAd85A group were statistically significantly larger than those of the rAd85A-rAd85A-vaccinated calves (P < 0.05) (Fig. 1B). While the cultured ELISPOT responses observed for the BCG-rAd85A-vaccinated calves were predictably the strongest, rAd85A-BCG vaccination also resulted in recall responses comparable to those for BCG-rAd85A vaccination (Fig. 1B). Thus, despite the absence of strong ex vivo IFN-γ responses following rAd85A-rAd85A or rAd85A-BCG vaccination, all three vaccination protocols resulted in strong recall memory responses. In a pilot experiment, BCG-rAg85A-vaccinated calves were also found to be protected from a virulent M. bovis challenge, and the extent of protection correlated positively with the level of the cultured ELISPOT response for three individual calves (data not shown). This is in agreement with our recent data applying recombinant fowlpox virus or MVA expressing the same antigen to cattle vaccination, where the BCG-MVA vaccine combination also proved to be the most immunogenic (16).
It was reported recently that intranasal rAd85A delivery protected mice significantly against M. tuberculosis (17). Therefore, we intranasally boosted three additional subcutaneously BCG-vaccinated calves with rAd85A at week 7 (109 PFU/4 ml, 2 ml/nostril). BCG vaccination resulted in modest blood-based ex vivo IFN-γ responses to a pool of Ag85A peptides at the time of intranasal boosting with rAd85A. This response was boosted by intranasal rAd85A vaccination, peaking at 2 weeks postboost (Fig. 2A, week 9). Enhanced Ag85A-specific central memory responses were also demonstrable by cultured ELISPOT analysis after intranasal boosting with rAd85A (Fig. 2B).
FIG. 2.
Intranasal boosting with rAd85A results in increased ex vivo (A) and cultured ELISPOT (B) responses. (A) PBMC were prepared before the rAd85A boost (week 7, black bar) and at week 9 (gray bar) of the experiment and stimulated with a pool of immunodominant Ag85A-derived peptides. Results are expressed as mean SFC/106 PBMC ± SEM (n = 3). (B) SFC were determined after culture with rAg85A for the cultured ELISPOT protocol at week 7 (black bar) and week 10 (gray bar) of the experiment. ELISPOT assays were performed 13 days after culture initiation, using the synthetic peptide cocktail used for panel A in the absence of additional antigen-presenting cells. Results are expressed as mean SFC/106 cells ± SEM (n = 3). *, P < 0.05 (unpaired t test).
In conclusion, the results reported here demonstrate that a heterologous prime-boost vaccination schedule based on BCG and systemic or mucosal vaccination with an adenovirus expressing Ag85A induced strong cellular immune responses in cattle and that its assessment in large-scale protective efficacy studies is warranted.
Acknowledgments
This study was funded by the Department for Environment, Food and Rural Affairs, United Kingdom. K.H. was supported by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
Editor: J. L. Flynn
REFERENCES
- 1.Brandt, L., J. Feino Cunha, A. Weinreich Olsen, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng, C. G., U. Palendira, C. Demangel, J. M. Spratt, A. S. Malin, and W. J. Britton. 2001. Priming by DNA immunization augments protective efficacy of Mycobacterium bovis bacillus Calmette-Guerin against tuberculosis. Infect. Immun. 69:4174-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fine, P. E. 1989. The BCG story: lessons from the past and implications for the future. Rev. Infect. Dis. 11(Suppl. 2):S353-S359. [DOI] [PubMed] [Google Scholar]
- 4.Godkin, A. J., H. C. Thomas, and P. J. Openshaw. 2002. Evolution of epitope-specific memory CD4(+) T cells after clearance of hepatitis C virus. J. Immunol. 169:2210-2214. [DOI] [PubMed] [Google Scholar]
- 5.Goonetilleke, N. P., H. McShane, C. M. Hannan, R. J. Anderson, R. H. Brookes, and A. V. Hill. 2003. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J. Immunol. 171:1602-1609. [DOI] [PubMed] [Google Scholar]
- 6.Hewinson, R. G., H. M. Vordermeier, and B. M. Buddle. 2003. Use of the bovine model of tuberculosis for the development of improved vaccines and diagnostics. Tuberculosis 83:119-130. [DOI] [PubMed] [Google Scholar]
- 7.Krebs, J. R. 1997. Bovine tuberculosis in cattle and badgers. Ministry of Agriculture, Fisheries and Food Publications, London, United Kingdom.
- 8.McShane, H., R. Brookes, S. C. Gilbert, and A. V. Hill. 2001. Enhanced immunogenicity of CD4+ T-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect. Immun. 69:681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McShane, H., A. A. Pathan, C. R. Sander, N. P. Goonetilleke, H. A. Fletcher, and A. V. Hill. 2005. Boosting BCG with MVA85A: the first candidate subunit vaccine for tuberculosis in clinical trials. Tuberculosis (Edinburgh) 85:47-52. [DOI] [PubMed] [Google Scholar]
- 10.McShane, H., A. A. Pathan, C. R. Sander, S. M. Keating, S. C. Gilbert, K. Huygen, H. A. Fletcher, and A. V. Hill. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 10:1240-1244. [DOI] [PubMed] [Google Scholar]
- 11.Reece, W. H., M. Pinder, P. K. Gothard, P. Milligan, K. Bojang, T. Doherty, M. Plebanski, P. Akinwunmi, S. Everaere, K. R. Watkins, G. Voss, N. Tornieporth, A. Alloueche, B. M. Greenwood, K. E. Kester, K. P. McAdam, J. Cohen, and A. V. Hill. 2004. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat. Med. 10:406-410. [DOI] [PubMed] [Google Scholar]
- 12.Santosuosso, M., X. Zhang, S. McCormick, J. Wang, M. Hitt, and Z. Xing. 2005. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J. Immunol. 174:7986-7994. [DOI] [PubMed] [Google Scholar]
- 13.Skinner, M., B. M. Buddle, N. Wedlock, D. Keen, G. W. de Lisle, R. E. Tascon, J. C. Ferraz, D. B. Lowrie, P. J. Cockle, H. M. Vordermeier, and R. G. Hewinson. 2003. A DNA prime-BCG boost vaccination strategy in cattle induces protection against bovine tuberculosis. Infect. Immun. 71:4901-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simmons, and R. G. Hewinson. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vordermeier, H. M., P. C. Cockle, A. Whelan, S. Rhodes, N. Palmer, D. Bakker, and R. G. Hewinson. 1999. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin. Diagn. Lab. Immunol. 6:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vordermeier, H. M., S. G. Rhodes, G. Dean, N. Goonetilleke, K. Huygen, A. V. Hill, R. G. Hewinson, and S. C. Gilbert. 2004. Cellular immune responses induced in cattle by heterologous prime-boost vaccination using recombinant viruses and bacille Calmette-Guerin. Immunology 112:461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, J., L. Thorson, R. W. Stokes, M. Santosuosso, K. Huygen, A. Zganiacz, M. Hitt, and Z. Xing. 2004. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J. Immunol. 173:6357-6365. [DOI] [PubMed] [Google Scholar]
- 18.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225-234. [DOI] [PubMed] [Google Scholar]
- 19.Williams, A., G. J. Hatch, S. O. Clark, K. E. Gooch, K. A. Hatch, G. A. Hall, K. Huygen, T. H. Ottenhoff, K. L. Franken, P. Andersen, T. Mark Doherty, S. H. Kaufmann, L. Grode, P. Seiler, C. Martin, B. Gicquel, S. T. Cole, P. Brodin, A. S. Pym, W. Dalemans, J. Cohen, Y. Lobet, N. Goonetilleke, H. McShane, A. Hill, T. Parish, D. Smith, N. G. Stoker, D. B. Lowrie, G. Kallenius, S. Svenson, A. Pawlowski, K. Blake, and P. D. Marsh. 2005. Evaluation of vaccines in the EU TB Vaccine Cluster using a guinea pig aerosol infection model of tuberculosis. Tuberculosis (Edinburgh) 85:29-38. [DOI] [PubMed] [Google Scholar]