Abstract
The 190-kDa merozoite surface protein 1 (MSP-1) of Plasmodium falciparum, an essential component in the parasite's life cycle, is a primary candidate for a malaria vaccine. Rabbit antibodies elicited by the heterologously produced MSP-1 processing products p83, p30, p38, and p42, derived from strain 3D7, were analyzed for the potential to inhibit in vitro erythrocyte invasion by the parasite and parasite growth. Our data show that (i) epitopes recognized by antibodies, which inhibit parasite replication, are distributed throughout the entire MSP-1 molecule; (ii) when combined, antibodies specific for different regions of MSP-1 inhibit in a strictly additive manner; (iii) anti-MSP-1 antibodies interfere with erythrocyte invasion as well as with the intraerythrocytic growth of the parasite; and (iv) antibodies raised against MSP-1 of strain 3D7 strongly cross-inhibit replication of the heterologous strain FCB-1. Accordingly, anti-MSP-1 antibodies appear to be capable of interfering with parasite multiplication at more than one level. Since the overall immunogenicity profile of MSP-1 in rabbits closely resembles that found in sera of Aotus monkeys immunized with parasite-derived MSP-1 and of humans semi-immune to malaria from whom highly inhibiting antigen-specific antibodies were recovered, we consider the findings reported here to be relevant for the development of MSP-1-based vaccines against malaria.
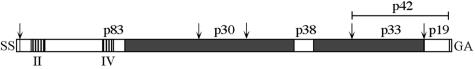
The merozoite surface protein 1 (MSP-1) complex of Plasmodium falciparum constitutes a major component at the surface of the erythrocyte (RBC)-invading form of the parasite (21). It originates from an approximately 190-kDa glycosylphosphatidylinositol (GPI)-anchored precursor, which is proteolytically processed during merozoite maturation, yielding in a first step four major fragments, p83, p30, p38, and p42, that remain, however, noncovalently associated (31). This complex interacts with further surface proteins of the parasite such as—again processed forms of—MSP-6 (48) and MSP-7 (36, 44). Before reinvasion of new RBCs, a secondary proteolytic event cleaves p42 into p33 and the approximately 10-kDa GPI-anchored C terminus, designated p19 (4). This portion of MSP-1, which contains two epidermal growth factor (EGF)-like domains, is transferred into the newly infected RBC, while the rest of the complex is shed from the parasite's surface (3). Analyses of the primary structure of MSP-1 from different clones of P. falciparum have revealed that several regions are highly conserved, whereas others appear to be dimorphic, permitting classification of strains into the K1 or MAD20 family. In addition, there are two small blocks of higher sequence variation (32, 46) (Fig. 1).
FIG. 1.
Schematic outline of MSP-1D. The precursor of MSP-1D is a protein comprising 1,720 amino acids, including a 20-amino-acid signal sequence (SS) and a signal for anchoring the protein at the cellular surface via a GPI moiety (GA). The precursor is processed into four major products, p83, p30, p38, and p42, as indicated by arrows. In a second proteolytic step, p42 is cleaved into p33 and p19. Primary sequences of MSP-1 from different strains follow distinct patterns of conservation. As indicated, there are regions of high conservation (white) and regions that are essentially dimorphic (grey), classifying the molecule as a member of either the K1 or the MAD20 family (32, 46). In addition, there are two short oligomorphic areas, block II and block IV (46). MSP-1D of strain 3D7 belongs to the MAD20 family, with the exception of block II, which is K1-like.
There is good evidence that MSP-1 plays an essential role in the parasite's life cycle and that it is crucially involved in the RBC invasion process. For example, preventing the proteolytic cleavage that generates p19 inhibits invasion of RBCs in vitro (5). Moreover, results indicating direct interactions between MSP-1 and the RBC surface have been reported (14, 34), suggesting that MSP-1 may play a role in early interactions between the parasite and RBCs, thus being possibly involved in the RBC invasion process at more than one level. Finally, attempts to genetically inactivate the msp-1 gene failed (35), underlining its essential role. All these findings make MSP-1 a most interesting target for interfering with the infectious cycle of the parasite, and there is ample evidence in support of MSP-1 as a prime candidate for a vaccine against malaria. Indeed, MSP-1 is a target of the human immune response, and numerous seroepidemiological studies have revealed associations between reduced susceptibility to clinical malaria and humoral responses against various regions of the molecule (6, 8, 11, 38-40, 47). Furthermore, immunization of Aotus monkeys with MSP-1 isolated from parasites induces high levels of protection against lethal challenges with parasites (42; H. Bujard et al., unpublished data), and partial (17, 43) or full (7) protection in the primate model was also reported for various MSP-1-derived recombinant protein preparations. Important information was collected from the mouse Plasmodium yoelii model, in which immunization with native MSP-1 (13) and with recombinant protein (24) conferred not only protection but also passive transfer of a monoclonal antibody (30).
Some studies revealed a particularly interesting role for epitopes located within the two EGF-like domains of the p19 processing fragment at the C terminus of MSP-1 (Fig. 1), as recombinant proteins containing these domains, when used as vaccines, were protective in mice and in primates (1, 7, 9, 10, 18-20, 29, 38). Moreover, monoclonal antibodies targeting specific conformational epitopes within these domains were shown to inhibit not only in vitro RBC invasion by the parasite (2) but also processing of p42 into p33 and p19 (5), thereby indicating that this proteolytic cleavage is an essential step in the infectious cycle of blood stage parasites. These findings have moved the C-terminal portion (p42 and p19) of MSP-1 to the center of interest, also as a candidate for a malaria vaccine. Interestingly, Guevara Patino et al. (15) have also identified so-called blocking antibodies that can prevent the interaction of inhibiting antibodies with their respective epitopes, thus allowing cleavage of p42 and consequently invasion of RBCs to proceed. Blocking antibodies, which were also identified in some human sera, were shown to bind not only within p19 but also in other regions of MSP-1, such as conserved domains of p83 (15). Clearly, as proposed, the induction of blocking antibodies would represent a novel mechanism of immune evasion; with respect to the development of an MSP-1-based vaccine, it would therefore seem advisable to restrict the effective antigen to p19, preferably in a modified version that induces exclusively inhibiting but not blocking antibodies (49).
On the other hand, considering the situation in vivo, the effect of blocking antibodies depends on how efficiently they compete with inhibitory antibodies, which in turn is a function of a number of thermodynamic and kinetic parameters that are difficult to quantitatively assess. In this context, it is interesting to note that the successful immunization of rodents (23) and primates (7) with recombinant p19 or p42 preparations indicates an effective competition of invasion-inhibitory antibodies with at least p19-specific blocking activities. The same appears to hold true for protective immunizations with full-size MSP-1 of mice (22) and primates (42; Bujard et al., unpublished data), again suggesting that blocking antibodies, possibly elicited by other parts of MSP-1, are not sufficiently effective at least under these conditions of immunization. Finally, it is not clear to what extent invasion-inhibiting antibodies specifically elicited by the p19 portion of MSP-1 contribute to the overall protective humoral response in semi-immune individuals and which increment of protection would be lost by the antagonism of respective blocking antibodies.
Interestingly, seroepidemiological studies aimed at uncovering associations between a reduced risk for clinical malaria and antibody responses towards various regions of MSP-1 have not led to a coherent picture, even when differences in the various experimental approaches are reconciled. They suggest rather that MSP-1 harbors, in addition to p19, further regions capable of eliciting protective immune responses.
In following up the latter question, we have examined antibodies specific for different regions of MSP-1 for the potential to interfere with the multiplication of P. falciparum in vitro. We have raised in rabbits antibodies against the four major processing products of MSP-1 of P. falciparum strain 3D7, henceforth referred to as MSP-1D, and tested their potential to inhibit in vitro parasite growth and RBC invasion. We show that epitopes eliciting highly inhibitory antibodies are distributed throughout the MSP-1 molecule and that combinations of such antibodies interfere with parasite multiplication in a strictly additive mode. Furthermore, antibodies raised against processing fragments of MSP-1D effectively cross-inhibit parasites of the FCB-1 strain, a representative of the K1 prototype. As the overall antigenicity profiles of MSP-1 in rabbits, monkeys, and humans closely resemble each other, the outcome of this study should be of interest for the development of malaria vaccines based on MSP-1.
MATERIALS AND METHODS
MSP-1 and MSP-1 processing fragments.
All MSP-1D-derived proteins were produced in Escherichia coli from coding sequences synthesized analogously as described previously (37). They contain N-terminal hexahistidine fusions and were purified as described by Kauth et al. (26). Processing fragment p42, which contains six disulfide bridges within its two EGF-like domains, was prepared and characterized as described in detail by Epp et al. (12). Full-size MSP-1 was assembled from its two “halves,” p83/30 and p38/42 (26). MSP-6 (48) and MSP-7 (36) were heterologously produced in E. coli as glutathione S-transferase fusions. All MSP-1D fragments, as well as MSP-6 and MSP-7, used in this study are more than 90% pure (26) and contain comparable amounts of endotoxins, ranging from 250 to 750 endotoxin units per 100 μg of protein, according to the Limulus amoebocyte lysate test (data not shown). MSP-1F from merozoites was isolated from synchronized late-schizont cultures of P. falciparum strain FCB-1, essentially as described by Siddiqui et al. (42).
Generation of MSP-1-specific antibodies.
Groups of three rabbits were immunized at Charles River Laboratories, Kisslegg, Germany, according to the standard procedure of the company. Accordingly, 100 μg of each of the four purified MSP-1D fragments, p83, p30, p38 and p42, was administered in Freund's complete adjuvant (CFA) for priming, followed by three boosts—on days 28, 42, and 56—with the same amount of protein in Freund's incomplete adjuvant. Serum samples were withdrawn prior to each immunization, and total sera were collected 2 weeks after the last boost.
Aotus lemurinus griseimembra monkeys were immunized with MSP-1F. Between 40 and 50 μg of protein was administered in 50% CFA for priming, followed by boosts containing the same amount of protein in 25% and 10% CFA. Serum samples were withdrawn prior to each immunization (day 0, priming; day 28, first boost; day 56, second boost) and 2 weeks after the last immunization. The full Aotus study will be reported elsewhere.
Purification of rabbit serum.
Sera from rabbits of each group were pooled and purified by Squarix, Marl, Germany, according to the standard procedures of the company. The same protocol was used in our laboratory to purify immunoglobulins of further rabbit sera and of human sera. In brief, sera were heat inactivated for 30 min at 56°C and centrifuged for 30 min at 5,000 rpm. Immunoglobulins were precipitated by slow addition of 29.2 g ammonium sulfate per 100 ml serum, and the mixture was kept for 24 h at 4°C. The precipitate was pelleted by centrifugation (60 min, 4000 × g, 4°C); redissolved in 60% of the original volume of phosphate-buffered saline (PBS), pH 7.4, containing 0.05% sodium azide; dialyzed five times against 5 liters of the same buffer; and kept for 12 to 16 h at 4°C. The resulting solution was cleared by centrifugation (60 min, 4000 × g, 4°C) and by subsequent filtration through a 0.45-μm membrane (Millipore) before it was stored at 4°C. Some anti-p30 and anti-p38 preparations were redissolved in PBS, reconstituting the original serum concentration.
Purification of antibodies by antigen affinity chromatography.
The antigens p83, p30, p38, and p42 were immobilized on HYDRA-R activated affinity resin (Squarix GmbH, Marl, Germany), according to the standard procedures of the company. In brief, the recombinant proteins were concentrated by a 10-kDa ultrafiltration unit (Millipore) and exhaustively dialyzed against coupling buffer (4 M urea, 0.05% sodium borate, pH 9.0) before they were incubated with the resin for 48 h at room temperature. After the addition of blocking buffer (4 M urea, 0.2 M ethanolamine, pH 9.2), the suspension was incubated for 12 h further at room temperature and then transferred to a chromatography system (ÄKTA; Amersham Pharmacia) and processed at 4°C. The column was washed extensively with coupling buffer, distilled water, PBS-sodium azide, and all of the buffers that were used in the following chromatography procedures.
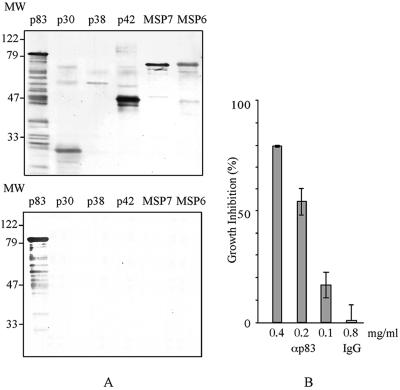
For purification of human antibodies, p83 (5 mg/ml) was dialyzed against coupling buffer (6 M urea, 50 mM sodium borate, pH 8.5) and subsequently incubated with HYDRA-R activated affinity resin for 48 h at room temperature. The resin was washed with the same buffer, blocked with 6 M urea-0.2 M ethanolamine (pH 9.2) for 24 h at room temperature, and transferred to the chromatography system. Proteins, covalently coupled to the resin, were refolded by a linear gradient replacing the blocking buffer with 1 M arginine-50 mM Tris (pH 8.5). A second linear gradient exchanged the refolding buffer for PBS, pH 7.4. For both gradients, a flow rate of 0.2 column volumes per minute over 24 h was applied. The resin was thoroughly washed with all buffers used in the subsequent chromatography procedures. The serum was loaded onto the column with a flow rate of 0.5 cm/min. After being washed with PBS, pH 7.4, the antibodies were eluted by 75 mM glycine (pH 2.8), 0.5 M NaCl, and PBS (pH 7.4) plus 1 M NaCl. The acidic eluates were immediately neutralized by 0.1 volumes of 1 M Tris, pH 8.0. All fractions were pooled, and the antibodies were precipitated by ammonium sulfate as described above. The pellet was dissolved in PBS, pH 7.4, and extensively dialyzed against the same buffer. All affinity-purified antibody preparations were examined in Western blots, as shown for the human anti-p83 preparation (see Fig. 7A).
FIG. 7.
Inhibition of parasite multiplication by human anti-p83 antibodies. Antibodies specific for p83 were isolated via antigen affinity chromatography from serum of a semi-immune blood donor from Nouna, Burkina Faso. (A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% polyacrylamide) followed by Western blot analysis of serum (top) and of purified anti-p83 antibodies (bottom). Serum and antibodies were probed against recombinant p83, p30, p38, p42, MSP-7, and MSP-6. MW, molecular weight (in thousands). (B) Inhibition of parasite replication by anti-p83 (αp83) antibodies at the concentrations indicated (measured by the LDH assay); human IgG served as a control. Error bars show standard deviations.
Immunological methods.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting were performed as described previously (37).
An enzyme-linked immunosorbent assay was performed as follows. Microtiter plates (96 well) were coated with recombinant protein by overnight incubation with 0.1 ml of 100 nM protein in PBS at 4°C. Plates were washed once with TBST (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.05% Tween 20) before they were incubated for 1 h at room temperature with blocking buffer (TBST containing 1% milk powder). Sera to be examined were diluted serially with blocking buffer and incubated for 2 h at room temperature. After three washes with TBST, the appropriate secondary antibodies (Sigma, Munich, Germany), diluted as recommended by the supplier, were added and the mixture was incubated for 1 h at room temperature. The substrate p-nitrophenyl-phosphate (1 mg/ml in 0.96% [vol/vol] diethanolamine [pH 9.5] and 1 mM MgCl2) was added, and the mixture was incubated for 1 h at room temperature in the dark. The reaction was stopped with 0.1 ml of 2 M NaOH, and the absorbance was measured at 405 nm.
Human and Aotus antibodies were detected with a goat anti-human immunoglobulin G (IgG)-alkaline phosphatase conjugate (Promega) diluted at 1:3,000, whereas rabbit antibody titers were measured with a goat anti-rabbit IgG-alkaline phosphatase conjugate (Sigma) diluted at 1:2,500.
Antibody titers were calculated at the serum dilutions that produced an absorbance (optical density at 405 nm [OD405]) of 1.0 for human, rabbit, and monkey sera.
Culturing of parasites.
P. falciparum parasites were grown under standard conditions (16) at 37°C, 5% CO2, 3% O2, and 95% humidity, until most of the parasites reached schizont stage. Magnetic cell separation columns (MACS; Miltenyi Biotech) were used to purify schizonts. After the column was placed in the magnetic field, the matrix was blocked by washing with preheated buffer A (PBS [pH 7.4]-0.5% bovine serum albumin, 37°C). Plasmodium cultures were suspended and applied to the column. After being washed with preheated buffer A, the column was removed from the magnetic field and schizonts were eluted with culture medium. Centrifugation (5 min, 1,500 rpm) yielded small black pellets consisting of infected RBCs (iRBCs), of which 90% were in the schizont stage. The pellets were dissolved in culture medium having the desired hematocrit (1%), and parasitemia was adjusted to 0.3%.
Assays for inhibition of parasite replication.
The potential of sera and antibody preparations to inhibit parasite multiplication was monitored (i) by measuring parasite-specific lactate dehydrogenase (LDH) activity and (ii) by monitoring the development of cultured parasites via flow cytometry (16).
Prior to use in inhibition assays, sera and antibody preparations, including all preparations used as references and as controls, were preadsorbed on type A human RBCs, whereby 1 ml of antibody solution was incubated with 50 μl of RBCs (>90% hematocrit) for 1 h. After RBCs were pelleted by centrifugation, the supernatants were dialyzed together against 100 volumes of RPMI medium.
LDH assay.
Sera were examined for their potency in inhibiting P. falciparum replication by measuring LDH levels in late-trophozoite/early-schizont-stage parasites, according to a protocol developed by C. A. Long (33). Plasmodium strains 3D7 and FCB-1 were synchronized by magnetic cell separation as described above. Cultures were adjusted to 0.3% parasitemia with human type A RBCs at a final hematocrit of 1%. The final assay volume of cultures was 100 μl, containing between 5 and 40% (vol/vol) serum or antigen affinity-purified antibody preparations. In standard assays, sera or antigen affinity-purified antibodies were added in a volume of 20 μl. For monitoring of the concentration dependencies of sera or antibodies or of the additive effects of sera specific for different fragments, sera or antibody preparations were supplied to the assay mixture in a total volume of 40 μl, which contained the respective amount of antibody preparation (5 to 40 μl) adjusted to a final volume of 40 μl with medium whenever required. Corresponding volumes of preimmune rabbit sera or immunoglobulins, as well as sera from rabbits immunized with the ClpB protein from E. coli in Freund's adjuvant, served as controls. P. falciparum 3D7 and FCB-1 were assayed after 40 and 48 h, respectively. Inhibition was measured at 650 nm and calculated as follows: percent inhibition = 100% − (ODimmune serum − ODRBC/ODpreimmune serum − ODRBC) × 100.
Flow cytometric assay.
P. falciparum 3D7 was synchronized by magnetic cell separation columns as described above. Cultures were adjusted to 0.3% parasitemia with human type A RBCs at a final hematocrit of 1% containing 20% (vol/vol) serum or immunoglobulin preparation. Negative controls were as described above. For following the time course of parasite multiplication, independently incubated samples were harvested at 4-h intervals up to 44 h and assayed as follows. Cells were resuspended in 0.1 ml of PBS, pH 7.4, containing 0.05% glutaraldehyde and incubated for 20 h at 4°C. For DNA staining, 10 μg/ml of propidium iodide in PBS, pH 7.4, was added to the fixed preparation, which was kept for another hour in the dark. Parasitemia was detected via flow cytometry (FACScan; Becton-Dickinson) according to DNA content. iRBCs were visualized via “density dot blotting” with FL3 as the x axis (red fluorescence) and FL2 as the y axis (orange fluorescence). Inhibition was calculated as follows: percent inhibition = 100% − (Pimmune serum − PRBC)/(Ppreimmune serum − PRBC) × 100, where P is parasitemia. In all inhibition experiments, antibody preparations to be examined for their inhibitory potential were assayed together with reference and control antibodies prepared under identical biochemical and temporal conditions.
Microscopic analysis of parasite cultures.
Samples of parasite cultures were prepared as thin films on microscopic glass slides before they were fixed for 2 min in methanol and stained for 10 min in Giemsa stain (Sigma), air dried, mounted with Mowiol, and covered with a coverslip. The preparations were examined by light microscopy with a 100× oil immersion objective lens.
RESULTS
Inhibition of parasite replication in vitro by MSP-1D-specific rabbit antibodies.
All rabbit sera used in this study were subjected to ammonium sulfate precipitation, and the precipitate was redissolved in PBS as described in Materials and Methods. For simplicity, the latter preparations are designated serum or sera in contrast to antigen affinity-purified antibodies.
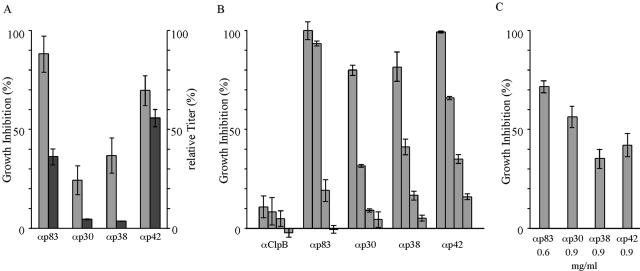
Rabbits immunized with purified p83, p30, p38, and p42 yielded sera which reproducibly differed in fragment-specific antibody titers, indicating regions of significantly differing immunogenicity within MSP-1D. Accordingly, p42 appears to be the most immunogenic protein, followed by p83, p30, and p38 (Fig. 2A). When these sera were examined for the potential to inhibit growth of P. falciparum 3D7 in vitro, as monitored by the LDH assay, they all inhibited significantly parasite replication (Fig. 2A), and when inhibition is related to fragment-specific antibody titers, sera obtained by immunization with p30 and p38 are particularly effective. As expected, the observed inhibition was concentration dependent, as shown in Fig. 2B. By contrast, no inhibition was found with serum obtained from rabbits immunized with purified ClpB protein from E. coli using the same immunization protocol. To unequivocally prove that the inhibitory activity is due to MSP-1D-specific antibodies, aliquots of the four sera were subjected to affinity chromatography on immobilized p83, p30, p38, or p42. The eluted material, consisting of more than 90% fragment-specific IgG, again inhibited efficiently parasite growth in vitro (Fig. 2C). The finding that the relative inhibitory efficacy of the antigen affinity-purified fragment-specific antibodies does not quantitatively correlate with the inhibition seen with the respective sera (Fig. 2A) is not unexpected, as, e.g., loss of high-affinity antibody populations and partial inactivation of antibodies is frequently a consequence of the purification procedures applied here.
FIG. 2.
Inhibition of parasite growth by anti-MSP-1 antibodies. Growth of P. falciparum strain 3D7 was monitored via the activity of parasite-specific LDH. (A) Equal amounts of rabbit serum containing anti-p83 (αp83), anti-p30 (αp30), anti-p38 (αp38), and anti-p42 (αp42) antibodies of the relative titers indicated were assayed. Light columns, growth inhibition; dark columns, antibody titers. (B) Dependency of growth inhibition on serum concentration, which was lowered twofold, from 40 to 5%, in consecutive steps. Rabbit serum raised against E. coli ClpB served as a control. (C) Growth inhibition of antibodies purified by antigen affinity chromatography. The final concentrations of the antibodies in the assay are indicated. Error bars show standard deviations.
Together, these results demonstrate that all four major processing products of MSP-1D elicit in rabbits antibodies that are capable of strongly interfering with the replication of homologous parasites in vitro.
Antibodies elicited by MSP-1D also inhibit growth of P. falciparum strain FCB-1.
Fragments p83, p38, and p42 contain sequences that are highly conserved among different P. falciparum strains. By contrast, p30 is located within a region considered to be dimorphic. It was therefore of interest to examine to what extent antibodies directed toward MSP-1D would inhibit growth of parasites that belong to the alternate prototypic family, K1, such as the FCB-1 strain. Accordingly, 3D7 parasites were replaced by parasites of the FCB-1 strain in our inhibition assays. Surprisingly, antibodies raised against each of the four processing products of MSP-1D inhibited the multiplication of both the FCB-1 and the 3D7 strains with almost the same efficiencies (Fig. 3). These results, which are particularly remarkable with respect to antibodies specific for p30, clearly indicate that epitopes capable of eliciting cross-protective antibodies are located also in so-called dimorphic regions.
FIG. 3.
Cross-inhibition of parasites representing the two dimorphic families by anti-MSP-1D antibodies. Growth of P. falciparum 3D7 (dark columns) and FCB-1 (light columns) was monitored in the presence of anti-p83 (αp83), anti-p30 (αp30), anti-p38 (αp38), and anti-p42 (αp42) antibodies via the LDH assay. (A) Growth inhibition by rabbit serum. (B) Growth inhibition by antigen affinity-purified antibodies. Error bars show standard deviations.
Inhibition of parasite replication by antibodies targeting different domains of MSP-1 is additive and affects different levels of parasite growth.
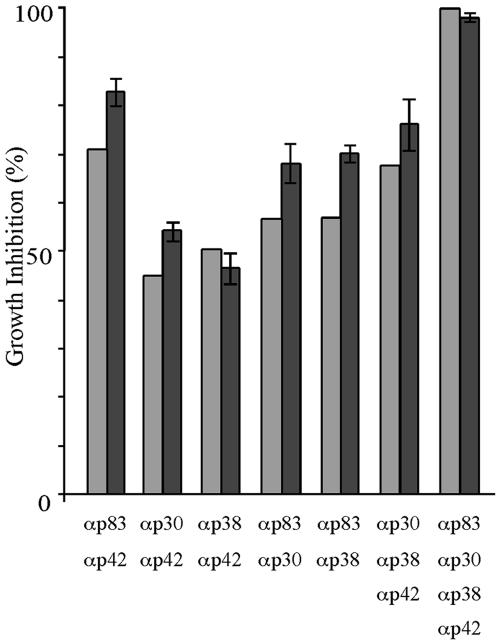
The above results, which show that parasite replication can be inhibited by antibodies targeting different domains of MSP-1D, raise questions concerning the mechanisms through which such antibodies act. It was therefore of particular interest to examine whether antibodies binding to different parts of MSP-1 would, when combined, have an additive, possibly synergistic effect on inhibition or, considering the hypothesis of immune evasion via blocking antibodies (15), would interfere with inhibitory activities. As a blocking mechanism was described in the context of inhibitory antibodies directed toward the p19 processing fragment, we first combined sera raised against p42 with p83, p30, or p38. As shown in Fig. 4, all three combinations inhibit parasite replication, as would be expected by simple addition of the individual inhibitory activities. Corresponding results were obtained with all of the other assayed combinations (Fig. 4). These results exclude a significant role of blocking antibodies under our experimental conditions.
FIG. 4.
Inhibition of parasite growth by antibodies (α) directed towards different regions of MSP-1 (p83, p30, p38, and p42). Rabbit sera specific for the four processing fragments were diluted to give suboptimal inhibition of parasite growth before the indicated combinations were composed and assayed for growth inhibition by the LDH assay. Antibodies were added to the assay mixtures in a volume of 40 μl containing 10 μl of each serum, as indicated, and whenever necessary adjusted to 40 μl with medium. Light columns, inhibition calculated from the expected contribution of the individual antibody preparations; dark columns, inhibition measured. Error bars show standard deviations.
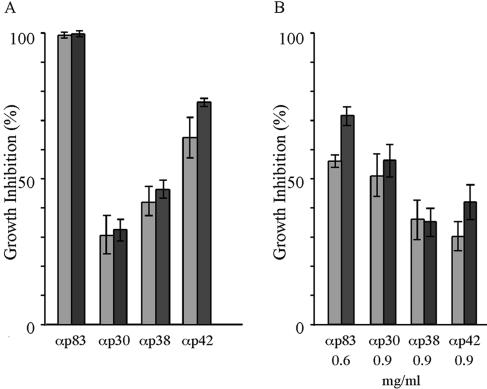
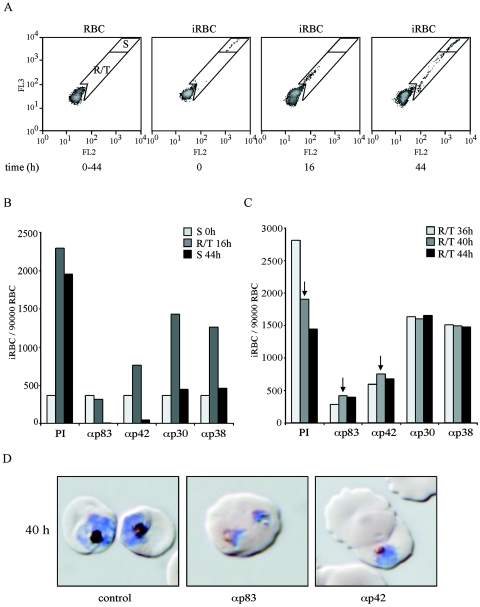
To gain an initial insight into how antibodies targeted to different regions of MSP-1D may inhibit parasite replication, we monitored the time course of parasite multiplication in synchronized cultures in the presence and absence of anti-MSP-1 antibodies by flow cytometric measurement of the DNA content of iRBCs, as well as by microscopy. As the disappearance of iRBCs from such cultures is a measure of invasion inhibition, our data demonstrate that all of our serum preparations specific for p83, p30, p38, and p42 are effective at this level (Fig. 5A and B). Thus, invasion of RBCs by the parasite can be efficiently prevented by antibodies binding to various domains of MSP-1. Analyzing the flow cytometric pattern in more detail reveals yet another intriguing feature. We found a substantial fraction of RBCs which had been successfully invaded by the parasite in the presence of inhibiting anti-MSP-1 antibodies. In these RBCs, however, the development of the parasite is severely hampered: in controls, the numbers of ring and trophozoite stage iRBCs decrease over time due to the transition into the schizont stage, but the population of iRBCs in the presence of inhibiting antibodies remains constant over the same period, indicating blocked or highly retarded development of the intraerythrocytic parasite (Fig. 5B and C). This is most clearly seen for anti-p83 and anti-p42 antibodies which, in the particular experiments shown, inhibit overall parasite multiplication by 80 to 95%. The same phenomenon is observed also with anti-p30 and anti-p38 antibodies even under conditions of lower overall inhibition (Fig. 5C). This intracellular inhibition has been verified also with antigen affinity-purified anti-p83 and anti-p42 antibodies (unpublished data). Microscopic inspection of such parasite cultures reveals iRBCs containing parasites which resemble previously described nonviable crisis forms (45), as shown in Fig. 5D.
FIG. 5.
Time course of inhibition of parasite replication in the presence of anti-MSP-1 antibodies. Synchronized schizont cultures were assayed with rabbit sera specific for the four MSP-1D processing fragments p83, p30, p38, and p42. The numbers of iRBCs and their relative DNA content were determined at 4-h intervals by flow cytometry. (A) Overview of the experimental approach. Left to right: flow cytometric pattern of uninfected RBCs; infected, synchronized RBC cultures (iRBC) showing the position of schizonts (gate S) at time zero; iRBCs at 16 h, demonstrating the position of ring stage parasites and early trophozoites (gate R/T); iRBCs at 44 h, where a substantial fraction is again in schizont stage while, due to the diminishing synchronization of the culture, late trophozoites are still present as new ring stage parasites reappear. The latter ring stage parasites are not seen when full inhibition by antibodies prevents the transition from intact trophozoites to schizonts (see below). (B) Time course of parasite multiplication in the presence of preimmune serum (PI) or of serum raised against (α) p83, p42, p30, or p38, as indicated; iRBCs per 90,000 RBCs were quantified at different time points by using the gates as shown in panel A. Light-grey columns, starting cultures containing exclusively schizonts (S); medium-grey columns, ring stage parasites and early trophozoites (R/T) at 16 h; dark-grey columns, schizonts (S) at 44 h. (C) Fates of ring stage parasites and trophozoites at different time points in the presence of preimmune serum (PI) or of sera raised against the four MSP-1 subunits as indicated. Light-grey, medium-grey, and dark-grey columns show the numbers of iRBCs per 90,000 RBCs at 36, 40, and 44 h. Arrows indicate the cultures from which the microscopic analyses shown in panel D are taken. (D) Microscopy of parasites cultured for 40 h in the absence and presence of inhibiting antibodies. Samples were taken from the experiment represented by panels B and C, as indicated by arrows in panel C. Parasites were stained with Giemsa. Early schizonts are detected in the presence of preimmune serum (control), whereas typical crisis forms which have stopped development are seen in the presence of anti-p83 (αp83) and anti-p42 (αp42) serum. The morphologies shown are representative of the parasites detected under the conditions described.
Together, these results demonstrate that invasion of RBCs by parasites can be inhibited by antibodies targeting epitopes located in different regions of MSP-1D. They indicate furthermore that MSP-1-specific antibodies are capable of interfering not only with the RBC invasion process but also with the intraerythrocytic development of the parasite.
Immunogenicity profile of MSP-1.
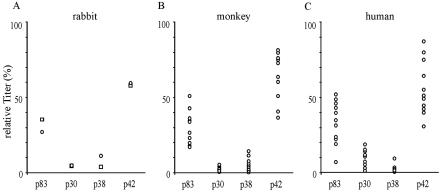
Rabbits immunized with the individual processing fragments of MSP-1D yield sera that differ largely in antibody titers, revealing a characteristic immunogenicity profile for MSP-1D. Consistently, p42 was found to be the most immunogenic compound, followed by p83, p30, and p38, whereas the titers for the two latter proteins usually do not differ significantly (Fig. 2A and 6A). Actual titers were between 9 × 105 for anti-p42 and 5 × 104 for anti-p38 antibodies under the immunization conditions used. This basic immunogenicity profile was observed irrespective of whether rabbits were immunized with individual fragments of MSP-1D (Fig. 2A) or with the full-size MSP-1D assembled from its two halves, i.e., p83/30 and p38/42 (Fig. 6A). The same hierarchy of antibody titers is found also in sera from Aotus monkeys immunized with MSP-1 isolated from merozoites of P. falciparum strain FCB-1 (Bujard et al., unpublished data) and in sera of semi-immune individuals living in an area of Burkina Faso, West Africa, where malaria is hyperendemic (Fig. 6B and C).
FIG. 6.
Immunogenicity of MSP-1 in rabbits, Aotus monkeys, and humans semi-immune to malaria. Antibodies specific for p83, p30, p38, and p42 were quantified in the various sera via an enzyme-linked immunosorbent assay yielding the fragment-specific titers shown. (A) Rabbits (n = 2) immunized with full-size MSP-1D assembled from p83/30 plus p38/42. The animals were primed with 100 μg of MSP-1D in CFA followed by two 100-μg boosts in Freund's incomplete adjuvant. (B) Aotus lemurinus griseimembra monkeys (n = 10) were immunized with 50 μg of MSP-1F isolated from parasites in CFA followed by two boosts of 50 μg of MSP-1F in Freund's incomplete adjuvant. (C) Human sera were obtained from 11 blood donors of the District Hospital of Nouna, Burkina Faso.
This obvious similarity between the immunogenicity profiles of MSP-1 in different mammalian systems raises the question of whether MSP-1-specific antibodies from semi-immune individuals would inhibit parasite replication in vitro as do the corresponding rabbit antibodies. Accordingly, in a first experiment we have purified by affinity chromatography anti-p83 antibodies from the serum of a semi-immune individual (Fig. 7A). These human antibodies inhibit parasite replication in vitro as efficiently as corresponding preparations from rabbit serum (Fig. 7B).
DISCUSSION
Clinical symptoms caused by P. falciparum infections are consequences of the asexual erythrocytic stages of the malaria parasite, during which merozoites invade and—upon multiplication—emerge from RBCs in repetitive cycles. Immune responses directed toward antigens of the blood stage parasite, which interfere with this infectious cycle, are believed to play an essential role in the control of parasitemia, leading to reduction or abrogation of clinical manifestations. Accordingly, MSP-1, which is crucially involved in the invasion process of RBCs by merozoites, is considered a prime candidate for a vaccine directed toward blood stage malaria.
With respect to MSP-1-based vaccine development, major attention has been paid in the past to the C-terminal part of the molecule, as delineated by p42 and p19, for reasons alluded to above. At the same time, we know little about the immunogenicity of other parts of MSP-1, particularly about the potentially protective nature of antibodies elicited by epitopes located outside the well-studied C-terminal portion of the molecule. In the work described here, we have examined the in vitro neutralizing potential of antibodies specific for the four major processing fragments of MSP-1D by monitoring inhibition of parasite growth and RBC invasion. If one accepts that the in vitro assays, as used here, describe valid correlates for the in vivo functions of antibodies, our results strongly suggest that protective antibodies are elicited by epitopes distributed throughout the MSP-1 molecule.
Antibodies specific for the major subunits of the MSP-1D complex were raised in rabbits using highly purified preparations of p83, p30, p38, and p42 capable of assembling into the full-size complex, as described previously (26). The resulting sera were first examined for the potential to inhibit growth of 3D7 parasites in vitro, as monitored by the LDH assay. Interestingly, all sera clearly interfered with parasite multiplication, as did antibodies purified from such sera via antigen affinity chromatography. Obviously, MSP-1 encodes numerous epitopes which elicit antibodies that, based on the criteria of our experimental systems, efficiently inhibit parasite replication in vitro. Remarkably, antibodies elicited by the apparently least-immunogenic regions of MSP-1, as delineated by p30 and p38, were highly effective.
Major regions of MSP-1, and in particular the area covered by p30, are dimorphic in nature (32, 46). It therefore appeared likely that a sizeable fraction of the inhibitory effect observed with our rabbit sera would be strain specific. We thus examined the extent to which antibodies raised against subunits of MSP-1D would cross-inhibit growth of the FCB-1 strain, which, with the exception of block II (Fig. 1) (46), is a typical representative of the K1 parasite family. Interestingly, a high degree of cross-inhibition was observed, indicating that a considerable portion of the effective epitopes is encoded in conserved regions. This result was particularly unexpected for antibodies elicited by p30. However, a more detailed comparative analysis between the amino acid sequences of the 3D7 and the FCB-1 strains reveals numerous conserved short runs of amino acids that could well serve as linear epitopes eliciting cross-reactive antibodies. In addition, data to be reported elsewhere indicate that p30 of MSP-1D and MSP-1F can form tertiary structures which are functionally homologous and thus could possibly also give rise to shared conformational epitopes. Finally, as shown by an immunofluorescence assay, our p30-specific antibodies stain both 3D7 and FCB-1 parasites (unpublished data).
We next examined the effect of combining antibodies targeting different regions of MSP-1 on growth inhibition. As it was of particular interest to see whether sera obtained by immunization with p83, p30, or p38 would contain so-called blocking antibodies, which would interfere with the inhibitory capacity of p42-specific antibodies, respective combinations were assayed first, followed by various other mixtures. All combinations of antibodies examined showed additive inhibitory effects on parasite growth, leaving no room for a net effect of blocking antibodies, which nevertheless may be contained within the various sera.
With the exception of invasion-inhibiting antibodies that function through the prevention of p42 processing (5), we have no information on how antibodies interfere with merozoite replication in vitro. To gain initial insights, we studied, via flow cytometry and microscopy, the time course of parasite replication in vitro in the presence and absence of MSP-1D-specific antibodies. It is evident from our data that all four antibody preparations efficiently prevent RBC invasion, demonstrating that antibodies which associate with MSP-1D outside of the p19 domain are capable of incapacitating merozoites for invasion. It will be interesting to explore whether some of these antibodies also act via prevention of p42 processing or whether their inhibitory effect is based on a different mechanism. Such mechanisms are conceivable, considering that MSP-1 interacts with further surface proteins of the parasite and that it has also been implicated in initial associations between the parasite and the RBC surface. Accordingly, antibodies associating with different domains of this large and abundant surface compound may be expected to affect the parasite's invasion process at several levels.
Our findings that anti-MSP-1D antibodies interfere also with the intraerythrocytic development of the parasite are particularly intriguing, as they may signal an inhibitory effect on parasites that have escaped antibody-mediated surveillance of invasion. The intraerythrocytic parasites that develop, e.g., in the presence of anti-MSP-1 antibodies resemble crisis forms in morphology (45). The generation of such nonviable intraerythrocytic forms of the parasite in the context of protective antibodies was discussed previously (25). As this interesting phenomenon may have important implications for vaccine development, presently we are attempting to quantify the contribution of the intracellular inhibition of parasite development by antibodies to overall interference with parasite multiplication, particularly with respect to antibody preparations targeting different regions of MSP-1.
One might argue that results obtained with antibodies raised in rabbits and using Freund's adjuvant do not reflect sufficiently the properties of antibody populations induced in humans living in areas in which malaria is endemic. However, when the immunogenicity profiles of MSP-1, i.e., the relative titers of antibodies specific for the four major processing fragments of MSP-1, were determined in rabbit sera as described here, in sera from Aotus monkeys immunized with MSP-1F isolated from parasites and administered with CFA, or in sera from semi-immune adults of West Africa exposed to natural infections, they closely resembled each other. Obviously, this rather coarse correlation requires a more refined analysis. We have therefore begun to isolate MSP-1D fragment-specific antibodies from the sera of individuals who are semi-immune to malaria by antigen-specific affinity chromatography, and indeed an initial preparation of human anti-p83 antibodies inhibits efficiently parasite replication in vitro, corroborating the relevance of our study in the rabbit system.
The results reported here raise questions and permit the drawing of some conclusions. For example, it will be interesting to examine whether invasion-inhibiting antibodies binding outside of p42 also prevent p42 processing or whether they act via a different mechanism. Furthermore, several modes of action can be conceived as to how MSP-1-specific antibodies may retard or block intraerythrocytic development of the parasite and apparently induce nonviable crisis forms, of which some are experimentally amenable in a straightforward manner.
On the other hand, our data strongly support the view that, with respect to MSP-1-based malaria vaccines, the entire molecule should be taken into account. It appears evident that MSP-1 is a carrier of a large number of B-cell epitopes capable of eliciting antibodies that efficiently inhibit parasite multiplication. Among these epitopes, a sufficient number is apparently encoded in conserved regions of the protein to provide potential cross-protection among parasite strains belonging to either of the two prototypic families, K1 and MAD20. Moreover, as we have to assume that under natural conditions RBC invasion by merozoites is a rapid process (41), it appears advantageous to induce, via vaccination, sets of antibodies that interfere with invasion at more than one level and which, in addition, may retard or block intraerythrocytic parasite development.
Finally, as MSP-1 is initially synthesized in the infected hepatocyte, where its gene is apparently transcribed within the first 24 h (50), a presentation of MSP-1 epitopes via major histocompatibility complex class I complexes may make infected hepatocytes vulnerable to cytotoxic T-cell attack. This reasoning is supported by the findings of Krzych and coworkers (28), who showed that lymphocytes from human volunteers immunized with radiation-attenuated sporozoites proliferate upon stimulation with MSP-1-derived peptides. Moreover, Kawabata et al. (27) have reported that adoptive transfer of MSP-1-specified CD8+ T cells results in protection in the mouse P. yoelii system. Should an MSP-1-specified cytotoxic T-cell response indeed be effective at the liver stage of the parasite, it can be safely assumed that full-size MSP-1 harbors numerous epitopes that will be presented by the various major histocompatibility complex class I complexes.
A vaccine based on the full-size MSP-1 may thus be considered multivalent, which, due to the complexity of the approximately 190-kDa protein, provides a multitude of epitopes for humoral and cellular responses, parameters that are also favorable for overcoming the possible genetic restrictions of vaccinees and for preventing the development of resistance.
Acknowledgments
We are grateful to David Haynes for advice in setting up the invasion inhibition assays and flow cytometric monitoring, to John M. Gonzalez for help in the Aotus studies, and to Bernd Bukau for kindly providing anti-ClpB rabbit serum. We gratefully acknowledge the excellent cooperation of blood donors of the District Hospital of Nouna, Burkina Faso, in particular, A. Barry, J. Bassolé, G. Coulibaly, N. Dakuyo, F. Dembélé, P. Dembélé, R. Dehoun, D. Konaté, F. Ouarmé, M. Oui, and A. Wibiga. We thank Nestor Dembélé (CRSN), Adama Compaoré (CRSN), and Silke Druffel-Augustin for diligent technical assistance; Christian Mengede (Squarix) for advice; and Sibylle Reinig for help in preparing the manuscript.
This work was supported by special research funds from the State of Baden-Wuerttemberg, by the Deutsche Forschungsgemeinschaft (SFB 544), and by Vakzine Projekt Management GmbH, Braunschweig, Germany.
We have no conflicting financial interest.
Editor: J. L. Flynn
REFERENCES
- 1.Ahlborg, N., I. T. Ling, W. Howard, A. A. Holder, and E. M. Riley. 2002. Protective immune responses to the 42-kilodalton (kDa) region of Plasmodium yoelii merozoite surface protein 1 are induced by the C-terminal 19-kDa region but not by the adjacent 33-kDa region. Infect. Immun. 70:820-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackman, M. J., H. G. Heidrich, S. Donachie, J. S. McBride, and A. A. Holder. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 172:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackman, M. J., and A. A. Holder. 1992. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133 as a noncovalently associated complex with other fragments of the MSP1. Mol. Biochem. Parasitol. 50:307-315. [DOI] [PubMed] [Google Scholar]
- 4.Blackman, M. J., I. T. Ling, S. C. Nicholls, and A. A. Holder. 1991. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol. Biochem. Parasitol. 49:29-33. [DOI] [PubMed] [Google Scholar]
- 5.Blackman, M. J., T. J. Scott-Finnigan, S. Shai, and A. A. Holder. 1994. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J. Exp. Med. 180:389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavanagh, D. R., D. Dodoo, L. Hviid, J. A. L. Kurtzhals, T. G. Theander, B. D. Akanmori, S. Polley, D. J. Conway, K. Koram, and J. S. McBride. 2004. Antibodies to the N-terminal block 2 of Plasmodium falciparum merozoite surface protein 1 are associated with protection against clinical malaria. Infect. Immun. 72:6492-6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, S. P., S. E. Case, W. L. Gosnell, A. Hashimoto, K. J. Kramer, L. Q. Tam, C. Q. Hashiro, C. M. Nikaido, H. L. Gibson, C. T. Lee-Ng, P. J. Barr, B. T. Yokota, and G. S. Hut. 1996. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect. Immun. 64:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conway, D. J., D. R. Cavanagh, K. Tanabe, C. Roper, Z. S. Mikes, N. Sakihama, K. A. Bojang, A. M. Oduola, P. G. Kremsner, D. E. Arnot, B. M. Greenwood, and J. S. McBride. 2000. A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat. Med. 6:689-692. [DOI] [PubMed] [Google Scholar]
- 9.Darko, C. A., E. Angov, W. E. Collins, E. S. Bergmann-Leitner, A. S. Girouard, S. L. Hitt, J. S. McBride, C. L. Diggs, A. A. Holder, C. A. Long, J. W. Barnwell, and J. A. Lyon. 2005. The clinical-grade 42-kilodalton fragment of merozoite surface protein 1 of Plasmodium falciparum strain FVO expressed in Escherichia coli protects Aotus nancymai against challenge with homologous erythrocytic-stage parasites. Infect. Immun. 73:287-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan, A. F., M. J. Blackman, and D. C. Kaslow. 2000. Vaccine efficacy of recombinant Plasmodium falciparum merozoite surface protein 1 in malaria-naive, -exposed, and/or -rechallenged Aotus vociferans monkeys. Infect. Immun. 68:1418-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan, A. F., J. A. Chappel, P. A. Burghaus, J. S. Morris, J. S. McBride, A. A. Holder, D. C. Kaslow, and E. M. Riley. 1995. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP1(19), the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect. Immun. 63:456-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epp, C., C. W. Kauth, H. Bujard, and R. Lutz. 2003. Expression and purification of Plasmodium falciparum MSP-1(42): a malaria vaccine candidate. J. Chromatogr. B 786:61-72. [DOI] [PubMed] [Google Scholar]
- 13.Freeman, R. R., and A. A. Holder. 1983. Characteristics of the protective response of BALB/c mice immunized with a purified Plasmodium yoelii schizont antigen. Clin. Exp. Immunol. 54:609-616. [PMC free article] [PubMed] [Google Scholar]
- 14.Goel, V. K., X. Li, H. Chen, S. C. Liu, A. H. Chishti, and S. S. Oh. 2003. Band 3 is a host receptor binding merozoite surface protein 1 during the Plasmodium falciparum invasion of erythrocytes. Proc. Natl. Acad. Sci. USA 100:5164-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guevara Patino, J. A., A. A. Holder, J. S. McBride, and M. J. Blackman. 1997. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 186:1689-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haynes, J. D., J. K. Moch, and D. S. Smoot. 2002. Erythrocytic malaria growth or invasion inhibition assays with emphasis on suspension culture GIA. Methods Mol. Med. 72:535-554. [DOI] [PubMed] [Google Scholar]
- 17.Herrera, S., M. A. Herrera, B. L. Perlaza, Y. Burki, P. Caspers, H. Dobeli, D. Rotmann, and U. Certa. 1990. Immunization of Aotus monkeys with Plasmodium falciparum blood-stage recombinant proteins. Proc. Natl. Acad. Sci. USA 87:4017-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirunpetcharat, C., D. Stanisic, X. Q. Liu, J. Vadolas, R. A. Strugnell, R. Lee, L. H. Miller, D. C. Kaslow, and M. F. Good. 1998. Intranasal immunization with yeast-expressed 19 kDa carboxyl-terminal fragment of Plasmodium yoelii merozoite surface protein-1 (yMSP119) induces protective immunity to blood stage malaria infection in mice. Parasite Immunol. 20:413-420. [DOI] [PubMed] [Google Scholar]
- 19.Hirunpetcharat, C., J. H. Tian, D. C. Kaslow, N. van Rooijen, S. Kumar, J. A. Berzofsky, L. H. Miller, and M. F. Good. 1997. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J. Immunol. 159:3400-3411. [PubMed] [Google Scholar]
- 20.Hirunpetcharat, C., P. Vukovic, X. Q. Liu, D. C. Kaslow, L. H. Miller, and M. F. Good. 1999. Absolute requirement for an active immune response involving B cells and Th cells in immunity to Plasmodium yoelii passively acquired with antibodies to the 19-kDa carboxyl-terminal fragment of merozoite surface protein-1. J. Immunol. 162:7309-7314. [PubMed] [Google Scholar]
- 21.Holder, A. A. 1988. The precursor to major merozoite surface antigens: structure and role in immunity. Prog. Allergy 41:72-97. [PubMed] [Google Scholar]
- 22.Holder, A. A., and R. R. Freeman. 1981. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature 294:361-364. [DOI] [PubMed] [Google Scholar]
- 23.Holder, A. A., R. R. Freeman, and C. I. Newbold. 1983. Serological cross-reaction between high molecular weight proteins synthesized in blood schizonts of Plasmodium yoelii, Plasmodium chabaudi and Plasmodium falciparum. Mol. Biochem. Parasitol. 9:191-196. [DOI] [PubMed] [Google Scholar]
- 24.Holder, A. A., R. R. Freeman, and S. C. Nicholls. 1988. Immunization against Plasmodium falciparum with recombinant polypeptides produced in Escherichia coli. Parasite Immunol. 10:607-617. [DOI] [PubMed] [Google Scholar]
- 25.Jensen, J. B., M. T. Boland, J. S. Allan, J. M. Carlin, J. A. Vande Waa, A. A. Divo, and M. A. Akood. 1983. Association between human serum-induced crisis forms in cultured Plasmodium falciparum and clinical immunity to malaria in Sudan. Infect. Immun. 41:1302-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kauth, C. W., C. Epp, H. Bujard, and R. Lutz. 2003. The merozoite surface protein 1 complex of human malaria parasite Plasmodium falciparum: interactions and arrangements of subunits. J. Biol. Chem. 278:22257-22264. [DOI] [PubMed] [Google Scholar]
- 27.Kawabata, Y., H. Udono, K. Honma, M. Ueda, H. Mukae, J. Kadota, S. Kohno, and K. Yui. 2002. Merozoite surface protein 1-specific immune response is protective against exoerythrocytic forms of Plasmodium yoelii. Infect. Immun. 70:6075-6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krzych, U., J. A. Lyon, T. Jareed, I. Schneider, M. R. Hollingdale, D. M. Gordon, and W. R. Ballou. 1995. T lymphocytes from volunteers immunized with irradiated Plasmodium falciparum sporozoites recognize liver and blood stage malaria antigens. J. Immunol. 155:4072-4077. [PubMed] [Google Scholar]
- 29.Kumar, S., W. Collins, A. Egan, A. Yadava, O. Garraud, M. J. Blackman, J. A. Guevara Patino, C. Diggs, and D. C. Kaslow. 2000. Immunogenicity and efficacy in Aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect. Immun. 68:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majarian, W. R., T. M. Daly, W. P. Weidanz, and C. A. Long. 1984. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J. Immunol. 132:3131-3137. [PubMed] [Google Scholar]
- 31.McBride, J. S., and H. G. Heidrich. 1987. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol. Biochem. Parasitol. 23:71-84. [DOI] [PubMed] [Google Scholar]
- 32.Miller, L. H., T. Roberts, M. Shahabuddin, and T. F. McCutchan. 1993. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1). Mol. Biochem. Parasitol. 59:1-14. [DOI] [PubMed] [Google Scholar]
- 33.Miura, K., H. Zhou, O. V. Muratova, A. Miles, L. H. Miller, A. Saul, and C. A. Long. Development and standardization of an in vitro Plasmodium falciparum growth inhibition assay utilizing measurement of lactate dehydrogenase (LDH) activity. Submitted for publication.
- 34.Nikodem, D., and E. Davidson. 2000. Identification of a novel antigenic domain of Plasmodium falciparum merozoite surface protein-1 that specifically binds to human erythrocytes and inhibits parasite invasion, in vitro. Mol. Biochem. Parasitol. 108:79-91. [DOI] [PubMed] [Google Scholar]
- 35.O'Donnell, R. A., A. Saul, A. F. Cowman, and B. S. Crabb. 2000. Functional conservation of the malaria vaccine antigen MSP-119 across distantly related Plasmodium species. Nat. Med. 6:91-95. [DOI] [PubMed] [Google Scholar]
- 36.Pachebat, J. A., I. T. Ling, M. Grainger, C. Trucco, S. Howell, D. Fernandez-Reyes, R. Gunaratne, and A. A. Holder. 2001. The 22 kDa component of the protein complex on the surface of Plasmodium falciparum merozoites is derived from a larger precursor, merozoite surface protein 7. Mol. Biochem. Parasitol. 117:83-89. [DOI] [PubMed] [Google Scholar]
- 37.Pan, W., E. Ravot, R. Tolle, R. Frank, R. Mosbach, I. Turbachova, and H. Bujard. 1999. Vaccine candidate MSP-1 from Plasmodium falciparum: a redesigned 4917 bp polynucleotide enables synthesis and isolation of full-length protein from Escherichia coli and mammalian cells. Nucleic Acids Res. 27:1094-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perraut, R., L. Marrama, B. Diouf, C. Sokhna, A. Tall, P. Nabeth, J. F. Trape, S. Longacre, and O. Mercereau-Puijalon. 2005. Antibodies to the conserved C-terminal domain of the Plasmodium falciparum merozoite surface protein 1 and to the merozoite extract and their relationship with in vitro inhibitory antibodies and protection against clinical malaria in a Senegalese village. J. Infect. Dis. 191:264-271. [DOI] [PubMed] [Google Scholar]
- 39.Riley, E. M., S. J. Allen, J. G. Wheeler, M. J. Blackman, S. Bennett, B. Takacs, H. J. Schonfeld, A. A. Holder, and B. M. Greenwood. 1992. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 14:321-337. [DOI] [PubMed] [Google Scholar]
- 40.Riley, E. M., S. Morris-Jones, M. J. Blackman, B. M. Greenwood, and A. A. Holder. 1993. A longitudinal study of naturally acquired cellular and humoral immune responses to a merozoite surface protein (MSP1) of Plasmodium falciparum in an area of seasonal malaria transmission. Parasite Immunol. 15:513-524. [DOI] [PubMed] [Google Scholar]
- 41.Saul, A. 1987. Kinetic constraints on the development of a malaria vaccine. Parasite Immunol. 9:1-9. [DOI] [PubMed] [Google Scholar]
- 42.Siddiqui, W. A., L. Q. Tam, K. J. Kramer, G. S. Hui, S. E. Case, K. M. Yamaga, S. P. Chang, E. B. Chan, and S. C. Kan. 1987. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. USA 84:3014-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh, S., M. C. Kennedy, C. A. Long, A. J. Saul, L. H. Miller, and A. W. Stowers. 2003. Biochemical and immunological characterization of bacterially expressed and refolded Plasmodium falciparum 42-kilodalton C-terminal merozoite surface protein 1. Infect. Immun. 71:6766-6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stafford, W. H., B. Gunder, A. Harris, H. G. Heidrich, A. A. Holder, and M. J. Blackman. 1996. A 22 kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol. Biochem. Parasitol. 80:159-169. [DOI] [PubMed] [Google Scholar]
- 45.Taliaferro, W. H., and L. G. Taliaferro. 1944. The effect of immunity on the asexual reproduction of Plasmodium brasilianum. J. Infect. Dis. 75:1-32. [Google Scholar]
- 46.Tanabe, K., M. Mackay, M. Goman, and J. G. Scaife. 1987. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 195:273-287. [DOI] [PubMed] [Google Scholar]
- 47.Tolle, R., K. Fruh, O. Doumbo, O. Koita, M. N′Diaye, A. Fischer, K. Dietz, and H. Bujard. 1993. A prospective study of the association between the human humoral immune response to Plasmodium falciparum blood stage antigen gp190 and control of malarial infections. Infect. Immun. 61:40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trucco, C., D. Fernandez-Reyes, S. Howell, W. H. Stafford, T. J. Scott-Finnigan, M. Grainger, S. A. Ogun, W. R. Taylor, and A. A. Holder. 2001. The merozoite surface protein 6 gene codes for a 36 kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol. Biochem. Parasitol. 112:91-101. [DOI] [PubMed] [Google Scholar]
- 49.Uthaipibull, C., B. Aufiero, S. E. Syed, B. Hansen, J. A. Guevara Patino, E. Angov, I. T. Ling, K. Fegeding, W. D. Morgan, C. Ockenhouse, B. Birdsall, J. Feeney, J. A. Lyon, and A. A. Holder. 2001. Inhibitory and blocking monoclonal antibody epitopes on merozoite surface protein 1 of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 307:1381-1394. [DOI] [PubMed] [Google Scholar]
- 50.Wang, Q., S. Brown, D. S. Roos, V. Nussenzweig, and P. Bhanot. 2004. Transcriptome of axenic liver stages of Plasmodium yoelii. Mol. Biochem. Parasitol. 137:161-168. [DOI] [PubMed] [Google Scholar]