Abstract
Inducible costimulator (ICOS) is expressed on activated T cells and plays a key role in sustaining and enhancing the effector function of CD4 T cells. Given the function of this molecule in sustaining T-cell responses, we reasoned that ICOS might play an important role in a prolonged infection model, such as Salmonella infection of mice. To test this hypothesis, wild-type (WT) and ICOS-deficient (ICOS−/−) mice were infected systemically with a Salmonella enterica serovar Typhimurium strain expressing the chicken ovalbumin gene (Salmonella-OVA). ICOS−/− mice exhibited greater splenomegaly than WT mice and showed delayed bacterial clearance. The acquired immune response in this model was slow to develop. Maximal T-cell responses to Salmonella-OVA were detected at 3 weeks postinfection in both WT and ICOS−/− mice. CD4 T-cell-dependent gamma interferon production and a class switch to immunoglobulin G2a were severely reduced in ICOS−/− mice. ICOS−/− mice also exhibited a substantial defect in antigen-specific CD8 T-cell responses. In vitro, the effect of anti-ICOS on CD8 T-cell division was greater when CD8 T cells rather than CD4 T cells expressed ICOS, suggesting that the in vivo effects of ICOS on CD8 T cells could be direct. Taken together, these studies show that ICOS plays a critical role in control of Salmonella infection in mice, with effects on antibody, Th1, and CD8 T-cell responses.
Salmonella enterica serovar Typhimurium causes a disease in mice that is similar to typhoid fever in humans (28). Upon infection, the bacteria quickly spread to the spleen and liver. Innate immune responses play a major role in the initial stages of infection. Eventually, adaptive immune responses develop, resulting in clearance of S. enterica serovar Typhimurium. CD4 T cells have been shown to be the principal cell type involved in clearing the bacteria, but CD8 T cells and antibodies also contribute (15, 28, 34, 40). Costimulation is important for this response, as shown by the failure of CD28−/− mice to control Salmonella infection (29). However, the effect of inducible costimulatory molecules in Salmonella infection has not been addressed previously.
Inducible costimulator (ICOS) is a member of the CD28/CTLA-4 family. It is expressed on activated T cells, and there is slightly higher expression on CD4 T cells than on CD8 T cells (16). The ICOS ligand, ICOS-L (also known as B7RP-1, B7h, GL-50, or LICOS), is expressed on B cells and macrophages (1, 5, 6, 41, 48). The effects of ICOS-ICOS-L interactions have been studied by blocking the interaction using ICOS-immunoglobulin (Ig) or a neutralizing anti-ICOS antibody and, more recently, by using ICOS−/− or ICOS-L−/− mice (8, 23, 24, 42). The initial studies suggested that ICOS is primarily involved in Th2 responses, but other studies revealed an additional role for ICOS in Th1 responses (3, 8, 11, 18, 24, 30, 31, 38, 42, 45). In the absence of ICOS signaling, no serum IgE was detected, and there were fewer and smaller germinal centers, as well as defects in antibody class switching, T-cell proliferation, and cytokine production (8, 24, 42).
While CD28 is important in the priming of T-cell responses, ICOS appears to play a role later in the response, maintaining effector T-cell responses (10, 39). The finding that ICOS is important at later times than CD28 prompted us to examine the role of ICOS in a prolonged infection model, such as systemic infection of mice with Salmonella. In order to monitor CD4 and CD8 responses to infection, we used an S. enterica serovar Typhimurium strain expressing chicken ovalbumin (OVA) (Salmonella-OVA). The immune response to Salmonella-OVA in mice was dependent on ICOS for IgG2a responses, as well as for Th1 and CD8 T-cell responses. In addition, ICOS−/− mice showed pronounced splenomegaly and increased bacterial loads at late times compared to wild-type (WT) mice. Thus, ICOS appears to be an important mediator of immune responses in this model of prolonged intracellular bacterial infection.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from Charles River Breeding Laboratories (St.-Constant, Quebec, Canada). (C57BL/6 × Sv129) F1 mice were purchased from Jackson Laboratories (Bar Harbor, ME). ICOS−/− mice with the C57BL/6 background (n = 9) were generated in the laboratory of Tak Mak at the Ontario Cancer Institute, Toronto, Canada (42), and were bred in the University of Toronto facility.
Antibodies.
The anti-CD3-producing hybridoma 145-2C11 was provided by J. Bluestone (University of Chicago, Chicago, IL). The anti-CD28-secreting hybridoma 37.51 was provided by J. Allison (University of California, Berkeley). The anti-ICOS antibody C398.4A (37) is available from the laboratory of U.D. (Novara, Italy). Anti-hamster IgG and hamster IgG were purchased from Sigma-Aldrich (St. Louis, MO).
Analysis of Nramp status of mice.
A functional Nramp1 allele is one of the major determinants of a macrophage's ability to control bacteria at early stages of infection (44). C57BL/6 and Sv129 mice have different Nramp1 genotypes, resulting in different 50% lethal doses, kinetics of the immune response, and bacterial loads (19, 36). ICOS−/− mice were generated from Sv129-derived embryonic stem cells and back-crossed into the C57BL/6 background (42). Because of the close proximity of the ICOS and Nramp genes, ICOS−/− mice were typed by PCR and found to have the Nramp1r phenotype (data not shown). The Nramp-1 genotype was determined by PCR as described previously (2). Briefly, a fragment was amplified from the DNA of C57BL/6, (C57BL/6 × Sv129) F1, and ICOS−/− mice using primers 5′-CCCCCATCTATGTTATCACCC-3′ and 5′-CAATGGTGATCAGTACAGCG-3′. The product amplified from the wild-type allele contained an HhaI site, which was absent from the inactive mutant form. The PCR product was precipitated with 2.5 volumes of ethanol, redissolved in 20 μl of HhaI buffer (New England Biolabs), and incubated at 37°C for 1 h with 10 U of HhaI. The DNA was then analyzed on a 1.5% agarose gel. The wild-type allele gave a 266-bp fragment, which was readily distinguished from the 285-bp fragment obtained from the null allele (data not shown). Because ICOS−/− mice with the Sv129 background were not available, we used the back-crossed ICOS−/− mice with the C57BL/6 background together with (C57BL/6 × Sv129) F1 controls that had the dominant Nramp1r phenotype.
To further validate this choice of a control strain, we compared the immune responses of (C57BL/6 × Sv129) F1 mice to the immune responses of C57BL/6 mice in our model. Adaptive immune responses developed with the same kinetics in the C57BL/6 and WT strains, with the peak responses at day 21, and the magnitudes of the responses were similar, although the CD57BL/6 mice exhibited marginally but consistently smaller responses than the F1 mice. We also compared ICOS−/− mice with C57BL/6 mice in some experiments and observed decreased responses in ICOS−/− mice compared to C57BL/6 mice in all experiments (data not shown). Based on these initial findings, we used the F1 mice (Nramp matched) as the control strain for the studies described here.
Bacterial infection of mice.
S. enterica serovar Typhimurium strain x4550 containing plasmid pYA3149-OVA was obtained from Marc Jenkins (University of Minnesota, Minneapolis) (7). The presence of the chicken ovalbumin gene in bacteria was confirmed by PCR, and the expression of OVA was confirmed by Western blot analysis (data not shown). S. enterica serovar Typhimurium was grown overnight in Luria-Bertani (LB) medium, washed twice in phosphate-buffered saline (PBS), frozen, and stored at −80°C. For infection, aliquots were thawed and grown in LB medium at 37°C overnight with aeration. The concentration of bacteria was determined by determining the optical density at 600 nm (OD600) (0.1 OD600 unit was equivalent to 2 × 108 bacteria/ml), and 105 bacteria in 200 μl of PBS were injected intraperitoneally (i.p.) into 6- to 8-week-old C57BL/6, (C57BL/6 × Sv129) F1, and ICOS−/− mice. For determination of bacterial burdens in organs, mice were killed at various times. Livers and spleens were homogenized in PBS, serial dilutions of homogenates were plated on LB agar plates, and colonies were counted after overnight incubation at 37°C.
Flow cytometry.
For tetramer staining, cell suspensions were prepared in PBS-2% fetal calf serum (FCS)-0.01% sodium azide on ice. Cells were surface stained with one or more of the following antibodies: allophycocyanin (APC)-conjugated anti-mouse CD8, APC-conjugated anti-mouse CD4, phycoerythrin (PE)-conjugated anti-mouse Mac-1, fluorescein isothiocyanate-conjugated anti-mouse CD62L, and PE-labeled tetramers consisting of murine class I major histocompatibility complex (MHC) molecule H-2Kb, β2-microglobulin, and the chicken ovalbumin peptide SIINFEKL (Beckman Coulter, Fullerton, CA). For each experiment, appropriate isotype control monoclonal antibodies (mAb) were used.
For intracellular gamma interferon (IFN-γ) staining, spleen cell suspensions were restimulated in culture medium (RPMI-10% FCS with antibiotics and 2-mercaptoethanol) for 6 h at 37°C with 1 μM SIINFEKL peptide and GolgiStop (BD PharMingen, San Diego, CA). Cells were harvested, resuspended in PBS-2% FCS-azide, and surface stained with PE-conjugated anti-CD8 and fluorescein isothiocyanate-conjugated anti-CD62L as described above. Following surface staining, cells were fixed in a Cytofix/Cytoperm solution (BD PharMingen) and then stained with APC-conjugated anti-mouse IFN-γ diluted in 1× perm/wash solution (BD PharMingen). Samples were analyzed using a FACSCalibur and the FlowJo software (Tree Star Inc., Ashland, OR)
Cytotoxicity assay.
Mice were infected with 105 S. enterica serovar Typhimurium x4550(pYA3149-OVA) cells as described above. Splenocytes were harvested after 3 weeks, resuspended to a concentration of 107 cells/ml, depleted of adherent cells, and incubated with 51Cr-labeled EL4 cells that had previously been pulsed with 50 μM SIINFEKL peptide. Serial threefold dilutions of antigen-specific effectors (referred to as dilution of the standard culture) were assayed for anti-OVA-specific cytotoxic T-lymphocyte (CTL) activity against the EL4 targets. After 5 h, 70 μl of supernatant was transferred onto 96-well Luma plates (Perkin-Elmer, Mississauga, Ontario, Canada), and counts were obtained with a Topcount scintillation counter (Canberra Packard). The maximum spontaneous release was determined with wells that contained 1% sodium dodecyl sulfate or medium alone. The percentage of specific lysis was calculated as follows: (experimental 51Cr release − spontaneous 51Cr release)/(maximum 51Cr release − spontaneous 51Cr release) × 100.
Detection of Salmonella-specific antibodies.
S. enterica serovar Typhimurium-specific antibodies were determined for IgM, IgG1, and IgG2a isotypes. Each well of 96-well plates was coated with 106 heat-killed (56°C, 1 h) S. enterica serovar Typhimurium x4550(pYA3149-OVA) cells in PBS at 4°C overnight. The plates were blocked with 3% skim milk in PBS for 2 h at 37°C. Fivefold serial dilutions of serum in PBS-1% skim milk were added to wells and incubated overnight at 4°C. After washing in PBS-0.1% Tween 20, horseradish peroxidase-conjugated anti-isotype antibodies (Caltag Laboratories, Burlingame, CA) were added for 2 h at 37°C. Following washing, H2O2 and 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) (Sigma-Aldrich, St. Louis, MO) were added in citrate phosphate buffer (pH 5.0), and color development was measured after 20 min at OD405.
Cytokine assays.
Spleen cells (5 × 106 cells) were incubated with 250 nM MHC class II-restricted peptide (OVA323-339) or 106 heat-killed Salmonella cells for 96 h. Supernatants were obtained, and the levels of IFN-γ were measured. An enzyme-linked immunosorbent assay (ELISA) was performed with diluted supernatants from cultures using pairs of anti-murine IFN-γ mAb purchased from BD PharMingen, which were used according to the manufacturer's instructions, and values were converted to ng/ml based on comparison with a recombinant standard.
Lymphocyte purification.
For T-cell isolation, adherent cells were depleted by incubation at 37°C in polystyrene tissue culture plates (Falcon, Becton Dickinson Labware). Total T cells or CD4 and CD8 T cells from naïve C57BL/6 and ICOS−/− mice were isolated by depletion of unwanted subsets using T-cell purification columns (Cedarlane Laboratories, Hornby, Ontario, Canada).
CFSE proliferation assay for the costimulatory function of ICOS.
T cells were stained with 5-(and 6-)carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) as previously described (22). In brief, cells were resuspended in PBS at a concentration of 5 × 107 cells/ml. CFSE was added to the cell suspension at a final concentration of 2.5 μM and incubated for 10 min at 37°C. Cells were washed twice in PBS-10% FCS and recounted. Then 1 ×106 total T cells or CD4 and CD8 T cells (as indicated below) were cultured in 24-well flat-bottom plates (Falcon, Becton Dickinson Labware) that had previously been coated with antibodies as follows. The plates were first coated with anti-hamster IgG (10 μg/ml) at 37°C for 2 h. Then the plates were washed with PBS, incubated with a mixture of anti-ICOS mAb (5 μg/ml) or hamster IgG (5 μg/ml) and anti-CD3 mAb (0.5 μg/ml), and incubated at 37°C for 2 h. For stimulation with anti-CD3 and anti-CD28, the wells were coated with 1 μg/ml of anti-CD3 and 10 μg/ml of anti-CD28 overnight. Cells were incubated for 2 to 3 days (as indicated below) and were analyzed by flow cytometry.
Statistical analysis.
Where indicated below, a statistical analysis was done with the unpaired two-tailed Student t test with 95% confidence intervals.
RESULTS
Splenomegaly in ICOS−/− mice.
To analyze the role of ICOS in the response to a prolonged infection with an intracellular bacterial pathogen, (C57BL/6 × Sv129) F1 and ICOS−/− mice were infected i.p. with 105 S. enterica serovar Typhimurium x4550(pYA3149-OVA) (Salmonella-OVA) cells. F1 mice were chosen in order to allow matching of the Nramp1 status of the mice (see Materials and Methods) (29). We used the ovalbumin-transfected strain of Salmonella as this made it possible to use MHC tetramers to monitor CD8 T-cell responses to the bacterially delivered antigen (see below). Although Salmonella normally infects via the gastrointestinal route, the organism can escape from the intestine and cause potentially fatal systemic infections (12). To mimic this systemic infection process, we used i.p. infection of mice. This resulted in dissemination of bacteria to the liver and spleen, as well as the development of immune responses in the spleen, the site of immune responses to blood-borne pathogens.
At several times postinfection, mice were sacrificed, and the numbers of macrophages, CD4, and CD8 T cells in the spleen were determined. By day 14 postinfection, both groups of mice had developed splenomegaly, which was greatly exaggerated in ICOS−/− mice. The spleens of infected ICOS−/− mice were up to twofold larger (by weight) than those of infected control mice (data not shown). At days 14 and 21 postinfection, the ICOS−/− mice had greater numbers of CD11b+ macrophages than control infected mice (Fig. 1, bottom panel) (P < 0.05). By day 36, the numbers of macrophages in the spleens of both groups of mice had decreased to the level in naïve mice (Fig. 1, bottom panel), which correlated with the decrease in spleen size of the ICOS−/− mice. The numbers of CD4 and CD8 T cells at day 21 were also significantly increased in ICOS−/− mice (Fig. 1, top and middle panels) (P < 0.05).
FIG. 1.
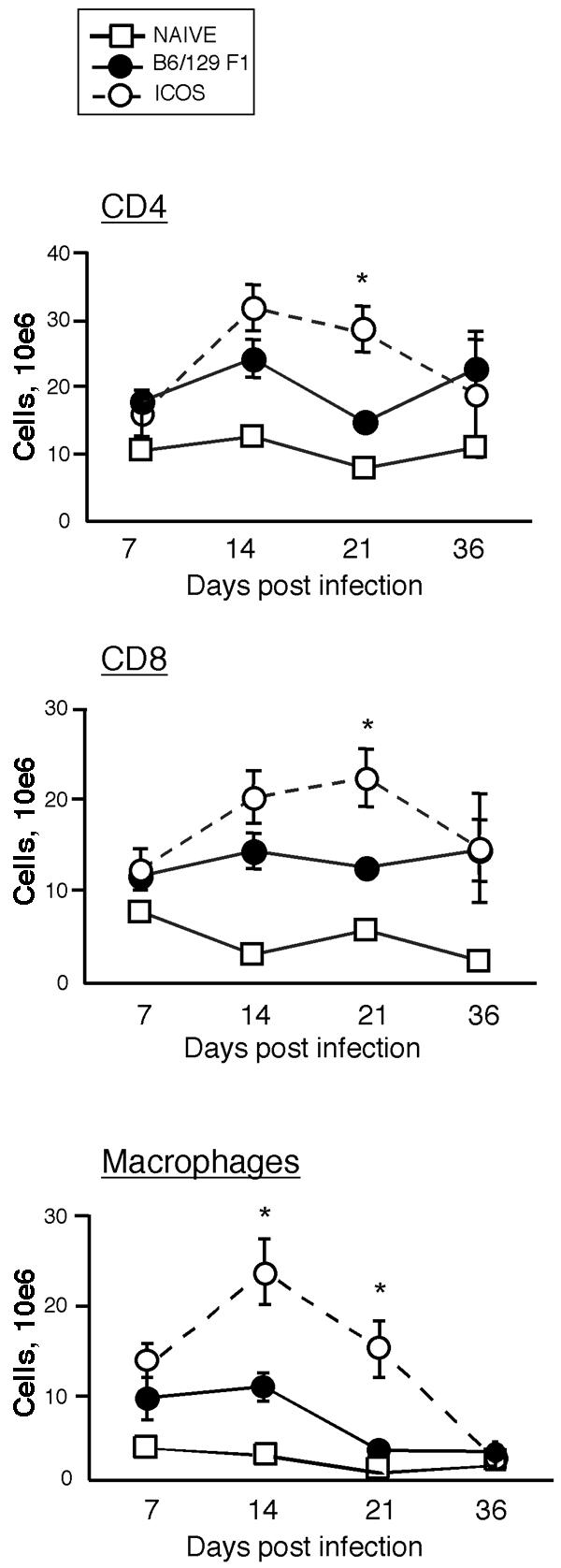
Increased numbers of macrophages and T cells in the spleens of ICOS−/− mice. ICOS−/− and (C57BL/6 × Sv129) F1 mice were infected i.p. with 105 S. enterica serovar Typhimurium x4550(pYA3149-OVA) cells and sacrificed on days 7, 14, 21, and 36 postinfection. Cells were isolated from the spleen, counted, stained with mAb to CD8, CD4, and Mac-1 (Cd11b), and analyzed by flow cytometry. The graphs show the total numbers of CD4 T cells, CD8 T cells, and macrophages as a function of time postinfection. Each symbol indicates the average for three mice, and the error bars indicate standard errors of the means. The data are representative of two complete experiments at all times, with additional experiments at day 21. An asterisk indicates that the P value is <0.05.
CD4 T-cell responses to Salmonella-OVA are decreased in ICOS−/− mice.
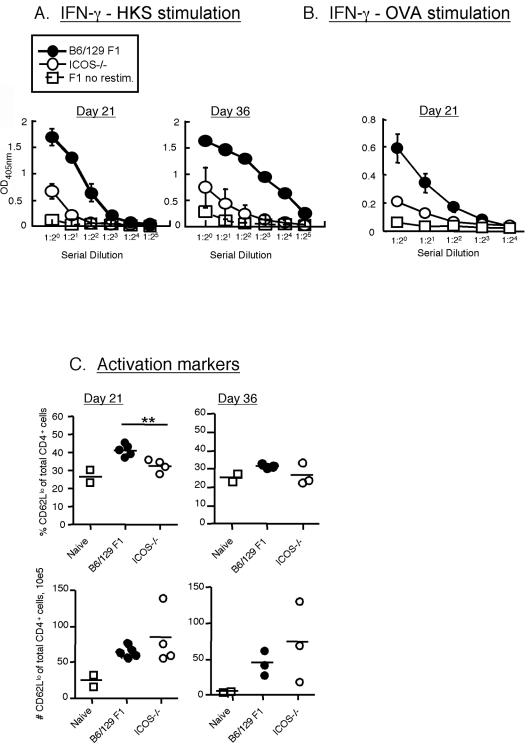
To assess the role of ICOS in the antigen-specific CD4 T-cell response to Salmonella, we restimulated splenocytes from Salmonella-OVA-immunized (C57BL/6 × Sv129) F1 and ICOS−/− mice with the MHC class II restricted OVA epitope peptide (OVA323-339) or with heat-killed Salmonella and measured the IFN-γ production by an ELISA as described in Materials and Methods. Stimulation with heat-killed Salmonella results in antigen accessing the MHC class II processing pathway for activation of CD4 T-cell responses. Although splenocytes from infected ICOS−/− mice produced detectable levels of IFN-γ under either stimulation condition, the levels were significantly lower than the levels produced by (C57BL/6 × Sv129) F1 mice (Fig. 2A and B). This profound defect in IFN-γ production by CD4 T cells from ICOS−/− mice was observed whether we used restimulation with heat-killed Salmonella or OVA peptide restimulation (Fig. 2B). Thus, ICOS plays an important role in the IFN-γ response of CD4 T cells responding to Salmonella epitopes, as well as OVA epitopes carried by Salmonella. We also assayed production of interleukin-4 by the CD4 T cells, but the levels were below the limits of detection (data not shown).
FIG. 2.
ICOS−/− mice have a defect in CD4 T-cell responses to Salmonella. ICOS−/− and (C57BL/6 × Sv129) F1 mice were infected as described in the legend to Fig. 1 and were sacrificed at days 7, 14, 21, and 36. Results are shown only for days 21 and 36, as on days 7 and 14 we did not detect any antigen-specific T-cell response (data not shown). Spleen cells were incubated for 96 h with heat-killed Salmonella (HKS) (A) or with 250 nM OVA323-339 MHC class II-restricted peptide (OVA) (B). IFN-γ production was measured by an ELISA as described in Materials and Methods. The data are the averages ± standard errors of the means for five individual mice. The data are representative of two similar experiments on day 36 and three experiments on day 21. IFN-γ levels (in pg/ml) were calculated based on a recombinant standard. The means ± standard deviations for the plots are as follows: for the response to heat-killed Salmonella on day 21 for WT and ICOS−/− mice, 17.02 ± 1.99 ng/ml and 3.02 ± 1.02 ng/ml, respectively; for the response to heat-killed Salmonella on day 36 for WT and ICOS−/− mice, 50.89 ± 4.4 ng/ml and 5.36 ± 2.56 ng/ml, respectively; for the response to OVA peptide on day 21 for WT and ICOS−/− mice, 3.65 ± 1.64 ng/ml and 0.23 ± 0.08 ng/ml, respectively. (C) Numbers of activated CD4 T cells. Cells were isolated from the spleen, counted, stained with mAb to CD4 and CD62L, and analyzed by flow cytometry. Each data point represents a single mouse, and the averages are indicated by lines. The data are representative of two separate experiments on day 36 and three experiments on day 21. Two asterisks indicate that the P value is <0.005.
The decrease in antigen-specific IFN-γ production by CD4 T cells from ICOS−/− mice could have been due to either a decreased level of IFN-γ in each activated T cell or decreased numbers of activated T cells or both. As indicated above, the numbers of CD4 T cells in the spleens of infected ICOS−/− mice were actually greater than the numbers of such cells in the spleens of infected WT mice. As downregulation of CD62L is one of the markers of T-cell activation, we examined expression of CD62L on WT and ICOS−/− CD4 T cells. We observed a significant decrease in the percentage of CD62Llow (activated) CD4 T cells in ICOS−/− mice. However, conversion of these frequencies to total numbers of activated T cells in the ICOS−/− mice revealed that the increase in overall CD4 T-cell numbers compensated for the lower proportion of these cells (2C). These data suggest that ICOS−/− CD4 T cells are activated but fail to produce IFN-γ in response to antigen. Thus, ICOS plays an important role in induction of IFN-γ by Salmonella-specific CD4 T cells, which likely contributes to the delayed clearance of Salmonella-OVA.
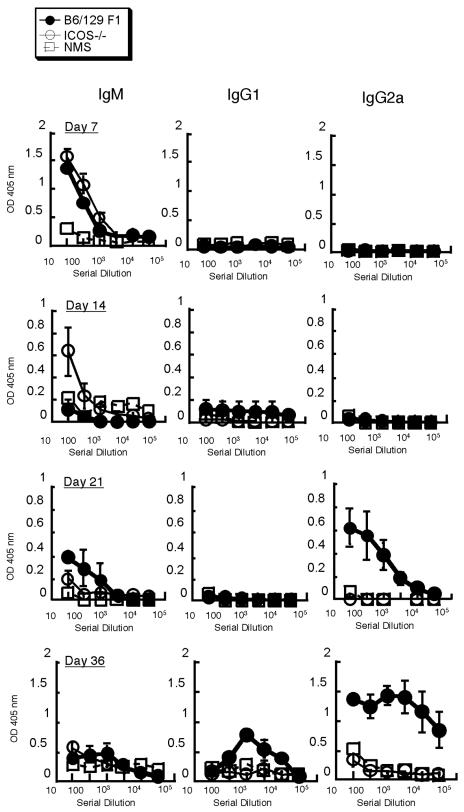
Impaired antibody class switch to IgG2a in ICOS−/− mice in response to S. enterica serovar Typhimurium.
To analyze the role of ICOS in the antibody response to Salmonella, we examined sera from infected (C57BL/6 × Sv129) F1 and ICOS−/− mice and compared them to sera from naïve mice at different times (Fig. 3). High levels of Salmonella-specific IgM were detected in both groups of infected mice in the first week postinfection. At the later times, the levels of IgM decreased slightly, but they remained above the background level throughout the response. IgG2a was not detectable until day 21 postinfection. At this time the IgG2a levels in ICOS−/− mice were significantly lower than those in the (C57BL/6 × Sv129) F1 control. High IgG2a levels were maintained in (C57BL/6 × Sv129) F1 mice, which is consistent with a Salmonella-induced Th1 response. The IgG2a levels remained low in ICOS−/− mice on day 36 postinfection (Fig. 3). No significant levels of IgG1 were detected at any time examined. Taken together, our data show that IgM responses to S. enterica serovar Typhimurium are normal in ICOS−/− mice but that an isotype switch to IgG2a is severely impaired.
FIG. 3.
ICOS−/− mice exhibit impaired IgG2a isotype class switching in response to Salmonella-OVA. ICOS−/− and (C57BL/6 × Sv129) F1 mice were infected as described in the legend to Fig. 1, and sera were taken on days 7, 14, 21, and 36 postinfection. The isotypes of the antibodies specific for S. enterica serovar Typhimurium x4550(pYA3149-OVA) from the serum samples were determined by an ELISA as described in Materials and Methods. The data are averages ± standard errors of the means for three to five individual mice at each time and are representative of three similar experiments. NMS, normal mouse serum.
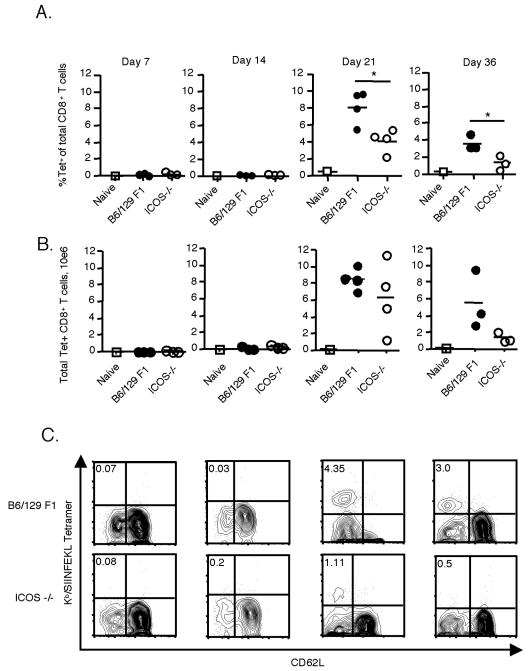
ICOS−/− mice have a defect in antigen-specific CD8 responses.
To examine whether ICOS plays a role in CD8 T-cell responses to Salmonella-OVA, infected (C57BL/6 × Sv129) F1 and ICOS−/− mice were sacrificed at different times postinfection, and spleen cells were analyzed to determine the number of antigen-specific cells using fluorescence-labeled Kb/SIINFEKL tetramers. Expansion of antigen-specific CD8 T cells was not detectable in infected mice until 3 weeks after infection (Fig. 4A and B). At 21 days after infection, ICOS−/− mice had a significantly lower percentage of antigen-specific cells. This defect was also evident on day 36 (Fig. 4A and B). Conversion of the percentages to total numbers of tetramer-positive T cells per spleen based on cell recovery revealed a similar trend, with reduced overall antigen-specific CD8 T-cell numbers in ICOS−/− mice in all experiments.
FIG. 4.
Antigen-specific CD8 T-cell responses are diminished in the absence of ICOS. ICOS−/− and (C57BL/6 × Sv129) F1 mice were infected as described in the legend to Fig. 1 and were sacrificed on days 7, 14, 21, and 36 postinfection. (A) Cells were isolated from the spleen, counted, stained with mAb to CD8, CD62L, and H-2Kb/OVA 257-264 tetramers (Tet), and analyzed by flow cytometry. Each symbol represents a single mouse, and the averages are indicated by lines. (B) Numbers of tetramer-positive CD8 T cells, calculated from the data in panel A. The data in panels A and B are data from a single experiment and are representative of two experiments (with additional experiments on day 21) performed with an average of three mice per group. (C) Examples of flow cytometry plots for days 7, 14, 21, and 36 following Salmonella-OVA infection. The events were gated on live CD8+ T cells. The numbers in the upper left quadrants indicate the percentages of CD8 T cells that stained with a tetramer. An asterisk indicates that the P value is <0.05.
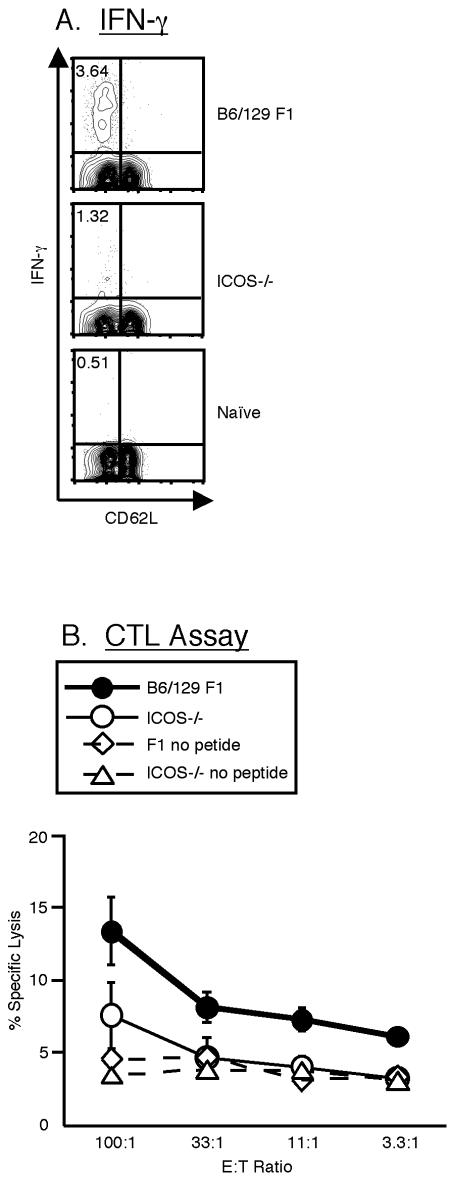
In order to assess the effector function of the CD8 T cells following Salmonella-OVA infection, splenocytes from the infected mice were analyzed by intracellular staining for the production of IFN-γ after 6 h of restimulation with the SIINFEKL peptide (Fig. 5A). As observed with tetramer-positive cells, following restimulation with the peptide no IFN-γ-producing CD8 T cells were observed during the first 2 weeks of infection (data not shown). At day 21 postinfection, ICOS−/− mice had detectable IFN-γ-producing CD8 T cells, but the frequency was significantly lower than that observed in (C57BL/6 × Sv129) F1 mice (Fig. 5A). Similar results were obtained at day 36 (data not shown).
FIG. 5.
ICOS is required for normal CD8 T-cell function in response to Salmonella-OVA. ICOS−/− and (C57BL/6 × Sv129) F1 mice were infected i.p. as described in the legend to Fig. 1 and were sacrificed on day 21. (A) Cells were restimulated with the SIINFEKL peptide for 6 h in the presence of GolgiStop and then stained with mAb to CD8 and CD62L before intracellular staining for IFN-γ. The events were gated on live CD8+ T cells. The values are the median values from a representative experiment, and the numbers in the upper left quadrants indicate the percentages of CD8 T cells that were stained with anti-IFN-γ. The means (%) ± standard errors of the means for the groups are as follows: for WT mice, 4.43 ± 0.86; for ICOS−/− mice, 2.28 ± 0.93; and for naïve mice, 0.40 ± 0.13. The data are representative of four experiments performed with an average of three mice per group. An asterisk indicates that the P value is <0.05. (B) Ex vivo CTL assay. Cells were incubated with 51Cr-labeled EL4 lymphoma cells pulsed with the SIINFEKL peptide, and specific lysis was measured at four ratios of effectors to target cells (E:T ratio). The data are means ± standard errors of the means for five individual mice and are representative of two separate experiments.
Cytotoxic activity is another indicator of CD8 effector function and may play a role in controlling S. enterica serovar Typhimurium infection by killing cells harboring the bacteria. We analyzed the ability of CD8 T cells from infected mice to lyse SIINFEKL-pulsed syngeneic targets on day 21 postinfection in a direct ex vivo chromium release assay. We used the direct ex vivo assay rather than a restimulation assay so that the results would more closely reflect the in vivo situation. This probably accounts for the overall low level of cytotoxicity. Nevertheless, it is clear that splenocytes from (C57BL/6 × Sv129) F1 mice were able to kill targets at levels significantly higher than the levels observed for splenocytes from naïve mice. In contrast, splenocytes from ICOS−/− mice showed only a low level of killing, which was reduced to background levels at lower effector-to-target cell ratios (Fig. 5B). This observation probably reflects the decreased number of antigen-specific CD8 T cells in ICOS−/− mice, as shown by tetramer staining. Thus, the quantitative defect in CD8 T-cell expansion in ICOS−/− mice may result in a reduced ability of CD8 T cells to clear an S. enterica serovar Typhimurium infection.
Agonistic anti-ICOS antibody can provide direct costimulation of CD8 T cells.
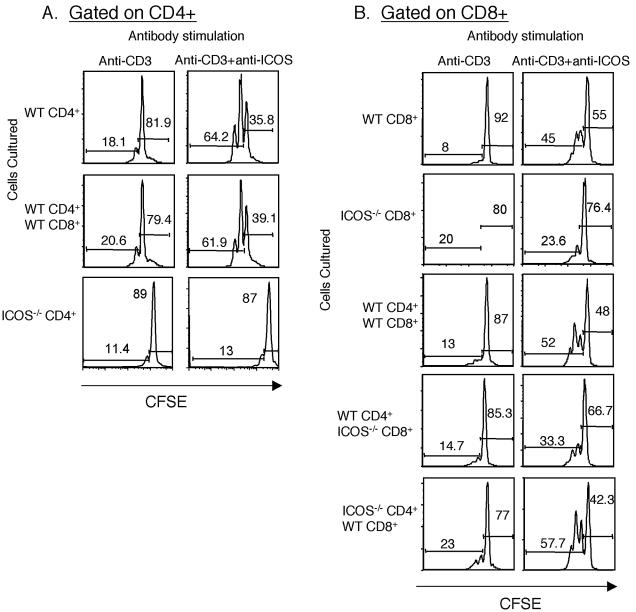
Limited data are available on the direct stimulation of CD8 T cells by ICOS (46). In order to confirm that ICOS can directly stimulate CD8 T cells, we purified CD4 and CD8 T cells from wild-type and ICOS−/− mice and plated them separately onto plates coated with either anti-CD3 plus anti-ICOS or anti-CD3 plus hamster IgG. After 48 h of incubation, a greater proportion of wild-type CD4 T cells that had been stimulated with anti-CD3 plus anti-ICOS than of cells that had been stimulated with anti-CD3 plus control Ig alone divided (Fig. 6A, top panels). Similar effects on cell division of CD4 T cells were observed in the presence or absence of CD8 T cells (Fig. 6A, top and middle panels). As expected, ICOS−/− CD4+ T cells failed to respond to anti-ICOS stimulation (Fig. 6A, bottom panels). Approximately 45% of wild-type CD8 T cells that were plated onto wells coated with anti-CD3 plus anti-ICOS showed evidence of cell division (Fig. 6B, top right panel), whereas ICOS-deficient CD8 T cells failed to show increased cell division in response to anti-ICOS. Thus, ICOS stimulation on CD8 T cells can provide a direct costimulatory signal, leading to cell division.
FIG. 6.
Anti-ICOS mAb can costimulate CD8 T cells in the absence of CD4 T-cell help. CD4 or CD8 T cells were isolated from wild type and ICOS−/− naïve mice, labeled with CFSE, and plated onto antibody-coated wells as indicated at the top. The purity of the CD4 and CD8 T cells was routinely 99% or more. (A) Division of CFSE-labeled CD4 T cells. Cells were stimulated with either anti-CD3 plus hamster IgG or with anti-CD3 plus anti-ICOS, as indicated at the top. Unstimulated cells showed no cell division. All events shown were gated on live CD4+ cells. The T cells used in the culture are indicated on the left. The graphs show CFSE loss in WT or ICOS−/− CD4 T cells cultured under the conditions indicated at the top. The numbers on the histograms indicate the percentages of cells in the undivided or divided populations. (B) Division of CFSE-labeled CD8 T cells alone (top panels) or in the presence of CD4 T cells from wild-type or ICOS−/− mice, as indicated on the left. T cells were stimulated with anti-CD3 plus hamster IgG or anti-CD3 plus anti-ICOS, as indicated at the top. All events were gated on live CD8+ cells. The numbers are the percentages of cells which have or have not divided. The results of this experiment are representative of two similar experiments analyzed at 48 h. The data were also analyzed at 72 h, with similar results.
CD8 T-cell defects in ICOS−/− mice could be caused by the absence of ICOS on CD8 T cells or by the lack of help from poorly activated CD4 T cells. To examine the indirect effects of ICOS stimulation of CD4 T cells on CD8 T-cell responses, we compared the CD8 T-cell responses to anti-CD3 plus anti-ICOS in cultures in which only the CD4 T cells expressed ICOS and in cultures in which only the CD8 T cells expressed ICOS (Fig. 6B). Addition of WT CD4 T cells to WT CD8 T cells had little or no effect on ICOS-dependent CD8 T-cell division, as the response to ICOS stimulation was 5.6-fold greater in the absence of CD4 T cells and about 4-fold greater in the presence of CD4 T cells compared to the response with anti-CD3 alone (Fig. 6B, top and third rows of panels). Addition of WT CD4 T cells to ICOS-deficient CD8 T cells had a modest effect on CD8 T-cell division (Fig. 6B, second and fourth rows of panels). Although small, the increase was reproducible in two independent experiments at two times each, suggesting that ICOS-deficient CD8 T cells can receive help from ICOS-sufficient CD4 T cells. There was also similar enhancement of CD8 T-cell division in response to ICOS−/− CD4 T cells. Taken together, these data suggest that in this in vitro culture system, direct effects of ICOS on CD8 T cells are more substantial than indirect effects on CD8 T-cell division.
ICOS−/− mice exhibit delayed clearance of Salmonella-OVA in the spleen and liver.
The data in Fig. 1 through 5 indicate that ICOS influences IFN-γ production by CD4 T cells, a class switch to IgG2a, and CD8 T-cell responses to Salmonella-OVA. To determine whether these defects have an outcome in terms of controlling infection, bacterial titers in spleens and livers of infected mice were determined at different times postinfection (Fig. 7). ICOS−/− mice had significantly increased bacterial titers at days 14 and 21 in both the spleen and liver. By day 36, there were no detectable bacteria in the WT mice, but a low level of Salmonella cells persisted in the organs of the knockout mice (1,200 ± 260 CFU/spleen and 80 ± 15 CFU/liver) (Fig. 7). The delayed clearance of Salmonella by ICOS−/− mice suggests that ICOS has a role in controlling Salmonella infection in mice.
FIG. 7.

ICOS−/− mice show delayed clearance of Salmonella-OVA. Mice were infected with Salmonella-OVA as described in the legend to Fig. 1. At different times, three to five mice per group were sacrificed, and the bacterial loads in the spleens and livers were determined and expressed as the number of CFU/organ. The data are means ± standard errors of the means. The data are representative of two similar experiments with additional experiments on day 21. An asterisk indicates that the P value is <0.05. At day 36, there were few or no bacteria in WT spleens or livers (10 ± 15 CFU/liver and 20 ± 17 CFU/spleen), whereas in ICOS−/− mice there were 1,200 ± 260 CFU/spleen and 80 ± 15 CFU/liver.
DISCUSSION
In this study we examined the role of ICOS in the immune response to Salmonella-OVA. We found that the optimal immune response to S. enterica serovar Typhimurium requires the presence of ICOS. ICOS−/− mice had an impaired ability to control Salmonella infection and exhibited defects in IFN-γ production by CD4 T cells, as well as a deficiency in the antibody isotype switch to IgG2a, indicating that the Th1 response in these mice was defective. Furthermore, we found that CD8 T-cell responses were impaired in ICOS−/− mice and that ICOS signaling on CD8 T cells can induce cell division, independent of CD4 T-cell help.
At the early times, the numbers of bacteria in the spleens and livers of ICOS−/−mice were not significantly different from the numbers of bacteria in the WT controls (data not shown). During this stage of the response, it is the innate immune response that controls the bacteria. However, starting at day 14 postinfection, the bacterial titers in ICOS−/− mice were significantly greater than those in the wild-type controls (Fig. 7). Although spleens from WT mice contained no detectable bacteria at day 36 postinfection, a low level of infection was still detected in the spleens and livers of ICOS−/− mice at this time. This suggests that ICOS is necessary for optimal control of Salmonella infection in mice. We also observed pronounced splenomegaly, which was greater in ICOS−/− mice. Macrophages were abundant in the spleens of infected mice at days 14 and 21 postinfection, accounting at least partially for the increase in spleen size (Fig. 1). The increased number of macrophages in the spleen may provide a niche for Salmonella replication in the spleen and account for the slight increase in bacterial numbers in the spleens at day 21 compared to day 14 (Fig. 7).
The numbers of activated CD4 T cells in the spleens ICOS−/− mice were equal to the numbers of activated CD4 T cells in the spleens of WT mice, but the cells were defective in IFN-γ production (Fig. 2). Although ICOS can directly influence CD4 T-cell division when it is tested in isolation (Fig. 6), in vivo other costimulatory molecules, such as CD28, likely contribute to CD4 T-cell expansion. Therefore, the finding that ICOS−/− T cells are activated in response to Salmonella infection but are impaired in IFN-γ production indicates the critical role of ICOS in CD4 T-cell-mediated IFN-γ production in this model. Thus, the present study adds to emerging data from several infectious disease models showing that ICOS can contribute to Th1 responses, as well as Th2 responses (3, 4, 11, 18, 30, 31, 38, 45).
Figures 2 and 4 show that the ICOS−/− mice had lower levels of antigen-specific CD4 and CD8 T cells in their spleens. Paradoxically, Fig. 1 shows that the ICOS−/− mice actually accumulated greater numbers of CD4 and CD8 T cells. This implies that non-antigen-specific, nonactivated T cells are recruited in greater numbers to the spleens of Salmonella-infected ICOS−/− mice than to the spleens of WT mice. The prolonged presence of Salmonella in ICOS−/− mice may result in greater or more prolonged activation of the innate immune system, leading to higher levels of inflammatory signals. The increased inflammatory signals appear to lead to increased recruitment of leukocytes (including lymphocytes and macrophages), independent of their direct recognition of antigens, thereby accounting for greater splenomegaly in the ICOS−/− mice.
The profound defect in IFN-γ production by CD4 T cells in ICOS-deficient mice probably has an effect on the ability of macrophages to kill intracellular bacteria. IFN-γ production by CD4 T cells is critical in the control of Salmonella by macrophages (9, 17, 32, 35). Indeed, recent evidence has shown that IFN-γ is critical for producing a prolonged NO burst in the macrophages, which in turn blocks the ability of Salmonella to interfere with phagosome maturation (25). Thus, the defect in IFN-γ production by ICOS-deficient CD4 T cells likely plays a significant role in the delayed bacterial clearance in this model.
Although the acquired immune response to Salmonella in ICOS−/− mice was severely impaired, by day 36 only a few colonies were detected in the spleens and livers of ICOS-deficient mice, suggesting that the residual immune response is eventually able to eliminate most of the bacteria. Figures 2, 4, and 5 show that although reduced, the number of antigen-specific CD4 and CD8 T cells and the IFN-γ response are still clearly above the background values, likely explaining the ability of the ICOS-deficient mice to eventually control Salmonella infection.
We used (C57BL/6 × Sv129) F1 mice as the control WT strain in our experiments in order to match the Nramp resistance status of the ICOS−/− mice. As discussed in Materials and Methods, although the (C57BL/6 × Sv129) F1 mice are not a perfect genetic match for ICOS−/− mice, we observed the same trend of decreased responses in ICOS−/− mice regardless of whether we used (C57BL/6 × Sv129) F1 or C57BL/6 mice as controls. Thus, while early events in bacterial infection are likely influenced by the Nramp status of mice, the late readouts used in this study (adaptive immunity and bacterial clearance at days 14 to 36) appeared to be relatively independent of the Nramp status of the mice. Thus, it is likely that the deficiencies observed in adaptive immune responses in ICOS-deficient mice were due to the absence of ICOS rather than to other genetic differences.
While it is clear that CD4 T cells and IFN-γ are major contributors to protection against Salmonella infection, there is also evidence of a role for CD8 T cells. For example, MHC class I-deficient β2 M−/− mice show increased susceptibility to Salmonella infection (21). In the present study, we observed a significant CD8 T-cell response to OVA delivered by Salmonella in WT mice, whereas ICOS−/− mice showed a defect in OVA-specific CD8 T-cell numbers between day 21 and day 36. This defect was also reflected in the percentage of IFN-γ-producing CD8 T cells, as well as in the cytotoxicity of the CD8 T cells. In contrast to the results obtained with this model, ICOS has only a limited, transient effect on CD8 T-cell numbers late in the primary response to influenza, and there is no defect in CTL function in response to influenza or lymphocytic choriomeningitis virus infection (3). The greater effect of ICOS on the CD8 T-cell response to Salmonella compared to the effects observed in viral infection models might reflect the prolonged time course of the response to Salmonella compared to the responses in acute viral infection models or may reflect the different host cells infected by Salmonella and the viral pathogens.
Support for the involvement of ICOS in CD8 T-cell responses comes from several models (3, 13, 20, 30, 33, 45, 46). However, in most studies it has been difficult to distinguish direct effects of ICOS from indirect effects due to CD4 T-cell help. In support of a direct effect of ICOS, ectopic expression of ICOS-L in a tumor cell line can costimulate rejection by CD8 T cells in the absence of CD4 T cells (20, 46). In the present study, we showed that anti-ICOS mAb can provide costimulation to CD8 T cells directly in the absence of CD4 T-cell help. In fact, direct stimulation of CD8 T cells with anti-CD3 plus anti-ICOS proved to be better than ICOS costimulation provided indirectly by CD4 T cells (Fig. 6). Thus, the decreased CD8 T-cell responses to Salmonella-OVA in ICOS−/− mice may have been due to both direct and indirect effects.
The adaptive immune response to Salmonella-OVA was slow to develop. We examined CD4, CD8, and antibody responses on days 7, 14, 21, and 36 postinfection and observed no detectable antigen-specific T-cell or IgG response until day 21. This delay in generation of T-cell-dependent immunity could have been due to interference with antigen processing and presentation by Salmonella. In macrophages, Salmonella resides inside vesicles which fail to acquire lysosomal hydrolytic enzymes or reactive oxygen intermediates, effectively allowing Salmonella to survive inside the cell (14). This has been attributed to the ability of Salmonella to interfere with endosome maturation (26). In addition, Salmonella prevents trafficking of MHC class II molecules to the surface of the cell, leading to defects in antigen presentation (27). Thus, in macrophages, Salmonella interference with phagosome maturation and MHC class II surface expression, both of which are required for the effective processing and presentation of antigens, likely results in bacteria that remain hidden from the acquired immune system until sufficient shed material or dead cells accumulate to be taken up and cross-presented by dendritic cells (47). Recently, a similar delay in activation of OVA-specific T cells in vivo was reported in a study in which OVA was delivered by Mycobacerium bovis BCG, and peak detection of OVA257-264-specific T cells was observed at day 21 (43). Thus, delayed appearance of acquired immune responses due to interference with antigen presentation appears to be a common feature of intracellular bacteria such as Salmonella and mycobacteria, which cause persistent or prolonged infections.
In summary, our results suggest that ICOS is an important mediator of immune control of Salmonella infection in mice. ICOS plays a critical role in IFN-γ production by Salmonella-specific CD4 T cells and is essential for generation of Salmonella-specific IgG2a antibodies. Moreover, ICOS is required for maximal CD8 T-cell responses to Salmonella-OVA and is capable of exerting direct effects on CD8 T cells. Together, these effects of ICOS likely contribute to the delayed control of systemic Salmonella infection in ICOS−/− mice.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research to T.H.W. and by a grant from the Associazione Italiana Ricerca Cancro (AIRC, Milan, italy) to U.D.
We thank Tak W. Mak for supplying ICOS−/− mice, Marc Jenkins for providing S. enterica serovar Typhimurium x4550 (pY3149), and Jennifer Gommerman for critical reading of the manuscript.
Editor: J. L. Flynn
REFERENCES
- 1.Bellamy, R. 1999. The natural resistance-associated macrophage protein and susceptibility to intracellular pathogens. Microbes Infect. 1:23-27. [DOI] [PubMed] [Google Scholar]
- 2.Bernheiden, M., J. M. Heinrich, G. Minigo, C. Schutt, F. Stelter, M. Freeman, D. Golenbock, and R. S. Jack. 2001. LBP, CD14, TLR4 and the murine innate immune response to a peritoneal Salmonella infection. J. Endotoxin Res. 7:447-450. [PubMed] [Google Scholar]
- 3.Bertram, E. M., A. Tafuri, A. Shahinian, V. S. Chan, L. Hunziker, M. Rechner, P. S. Ohashi, T. W. Mak, and T. H. Watts. 2002. Role of ICOS versus CD28 in anti-viral immunity. Eur. J. Immunol. 32:3376-3385. [DOI] [PubMed] [Google Scholar]
- 4.Bonhagen, K., O. Liesenfeld, M. J. Stadecker, A. Hutloff, K. Erb, A. J. Coyle, M. Lipp, R. A. Kroczek, and T. Kamradt. 2003. ICOS+ Th cells produce distinct cytokines in different mucosal immune responses. Eur. J. Immunol. 33:392-401. [DOI] [PubMed] [Google Scholar]
- 5.Brodie, D., A. V. Collins, A. Iaboni, J. A. Fennelly, L. M. Sparks, X. N. Xu, P. A. van der Merwe, and S. J. Davis. 2000. LICOS, a primordial costimulatory ligand? Curr. Biol. 10:333-336. [DOI] [PubMed] [Google Scholar]
- 6.Canonne-Hergaux, F., S. Gruenheid, G. Govoni, and P. Gros. 1999. The Nramp1 protein and its role in resistance to infection and macrophage function. Proc. Assoc. Am. Physicians 111:283-289. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Z. M., and M. K. Jenkins. 1999. Clonal expansion of antigen-specific CD4 T cells following infection with Salmonella typhimurium is similar in susceptible (Itys) and resistant (Ityr) BALB/c mice. Infect. Immun. 67:2025-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, C., A. E. Juedes, U.-A. Temann, S. Shresta, J. P. Allison, N. H. Ruddle, and R. A. Flavell. 2001. ICOS co-stimulatory receptor is essential for T cell activation and function. Nature 409:97-101. [DOI] [PubMed] [Google Scholar]
- 9.Eckmann, L., and M. F. Kagnoff. 2001. Cytokines in host defense against Salmonella. Microbes Infect. 3:1191-1200. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalo, J. A., J. Tian, T. Delaney, J. Corcoran, J. B. Rottman, J. Lora, A. Al-Garawi, R. Kroczek, J. C. Gutierrez-Ramos, and A. J. Coyle. 2001. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nat. Immunol. 2:597-604. [DOI] [PubMed] [Google Scholar]
- 11.Greenwald, R. J., A. J. McAdam, D. Van der Woude, A. R. Satoskar, and A. H. Sharpe. 2002. Cutting edge: inducible costimulator protein regulates both Th1 and Th2 responses to cutaneous leishmaniasis. J. Immunol. 168:991-995. [DOI] [PubMed] [Google Scholar]
- 12.Guiney, D. G. 2005. The role of host cell death in Salmonella infections. Curr. Top. Microbiol. Immunol. 289:131-150. [DOI] [PubMed] [Google Scholar]
- 13.Harada, H., A. D. Salama, M. Sho, A. Izawa, S. E. Sandner, T. Ito, H. Akiba, H. Yagita, A. H. Sharpe, G. J. Freeman, and M. H. Sayegh. 2003. The role of the ICOS-B7h T cell costimulatory pathway in transplantation immunity. J. Clin. Investig. 112:234-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashim, S., K. Mukherjee, M. Raje, S. K. Basu, and A. Mukhopadhyay. 2000. Live Salmonella modulate expression of Rab proteins to persist in a specialized compartment and escape transport to lysosomes. J. Biol. Chem. 275:16281-16288. [DOI] [PubMed] [Google Scholar]
- 15.Hess, J., C. Ladel, D. Miko, and S. H. Kaufmann. 1996. Salmonella typhimurium aroA− infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J. Immunol. 156:3321-3326. [PubMed] [Google Scholar]
- 16.Hutloff, A., A. M. Dittrich, K. C. Beier, B. Eljaschewitsch, R. Kraft, I. Anagnostopoulos, and R. A. Kroczek. 1999. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397:263-265. [DOI] [PubMed] [Google Scholar]
- 17.Kagaya, K., K. Watanabe, and Y. Fukazawa. 1989. Capacity of recombinant gamma interferon to activate macrophages for Salmonella-killing activity. Infect. Immun. 57:609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopf, M., A. J. Coyle, N. Schmitz, M. Barner, A. Oxenius, A. Gallimore, J. C. Gutierrez-Ramos, and M. F. Bachmann. 2000. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J. Exp. Med. 192:53-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lalmanach, A. C., A. Montagne, P. Menanteau, and F. Lantier. 2001. Effect of the mouse Nramp1 genotype on the expression of IFN-gamma gene in early response to Salmonella infection. Microbes Infect. 3:639-644. [DOI] [PubMed] [Google Scholar]
- 20.Liu, X., X. F. Bai, J. Wen, J. X. Gao, J. Liu, P. Lu, Y. Wang, P. Zheng, and Y. Liu. 2001. B7H costimulates clonal expansion of, and cognate destruction of tumor cells by, CD8+ T lymphocytes in vivo. J. Exp. Med. 194:1339-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo, W. F., H. Ong, E. S. Metcalf, and M. J. Soloski. 1999. T cell responses to Gram-negative intracellular bacterial pathogens: a role for CD8+ T cells in immunity to Salmonella infection and the involvement of MHC class Ib molecules. J. Immunol. 162:5398-5406. [PubMed] [Google Scholar]
- 22.Lyons, A. B., and C. R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171:131-137. [DOI] [PubMed] [Google Scholar]
- 23.Mak, T. W., A. Shahinian, S. K. Yoshinaga, A. Wakeham, L. M. Boucher, M. Pintilie, G. Duncan, B. U. Gajewska, M. Gronski, U. Eriksson, B. Odermatt, A. Ho, D. Bouchard, J. S. Whorisky, M. Jordana, P. S. Ohashi, T. Pawson, F. Bladt, and A. Tafuri. 2003. Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell-dependent B cell responses. Nat. Immunol. 4:765-772. [DOI] [PubMed] [Google Scholar]
- 24.McAdam, A. J., R. J. Greenwald, M. A. Levin, T. Chernova, N. Malenkovich, V. Ling, G. J. Freeman, and A. H. Sharpe. 2001. ICOS is critical for CD40-mediated antibody class switching. Nature 409:102-105. [DOI] [PubMed] [Google Scholar]
- 25.McCollister, B. D., T. J. Bourret, R. Gill, J. Jones-Carson, and A. Vazquez-Torres. 2005. Repression of SPI2 transcription by nitric oxide-producing, IFNγ-activated macrophages promotes maturation of Salmonella phagosomes. J. Exp. Med. 202:625-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meresse, S., O. Steele-Mortimer, B. B. Finlay, and J. P. Gorvel. 1999. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO J. 18:4394-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell, E. K., P. Mastroeni, A. P. Kelly, and J. Trowsdale. 2004. Inhibition of cell surface MHC class II expression by Salmonella. Eur. J. Immunol. 34:2559-2567. [DOI] [PubMed] [Google Scholar]
- 28.Mittrucker, H. W., and S. H. Kaufmann. 2000. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 67:457-463. [DOI] [PubMed] [Google Scholar]
- 29.Mittrucker, H. W., A. Kohler, T. W. Mak, and S. H. Kaufmann. 1999. Critical role of CD28 in protective immunity against Salmonella typhimurium. J. Immunol. 163:6769-6776. [PubMed] [Google Scholar]
- 30.Mittrucker, H. W., M. Kursar, A. Kohler, D. Yanagihara, S. K. Yoshinaga, and S. H. Kaufmann. 2002. Inducible costimulator protein controls the protective T cell response against Listeria monocytogenes. J. Immunol. 169:5813-5817. [DOI] [PubMed] [Google Scholar]
- 31.Miyahira, Y., H. Akiba, S. H. Ogawa, T. Ishi, S. Watanabe, S. Kobayashi, T. Takeuchi, T. Aoki, K. Tezuka, R. Abe, K. Okumura, H. Yagita, and N. Watanabe. 2003. Involvement of ICOS-B7RP-1 costimulatory pathway in the regulation of immune responses to Leishmania major and Nippostrongylus brasiliensis infections. Immunol. Lett. 89:193-199. [DOI] [PubMed] [Google Scholar]
- 32.Monack, D. M., D. M. Bouley, and S. Falkow. 2004. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNgamma neutralization. J. Exp. Med. 199:231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura, Y., Y. Yasunami, M. Satoh, E. Hirakawa, H. Katsuta, J. Ono, M. Kamada, S. Todo, T. Nakayama, M. Taniguchi, and S. Ikeda. 2003. Acceptance of islet allografts in the liver of mice by blockade of an inducible costimulator. Transplantation 75:1115-1118. [DOI] [PubMed] [Google Scholar]
- 34.Nauciel, C. 1990. Role of CD4+ T cells and T-independent mechanisms in acquired resistance to Salmonella typhimurium infection. J. Immunol. 145:1265-1269. [PubMed] [Google Scholar]
- 35.Ottenhoff, T. H., D. Kumararatne, and J. L. Casanova. 1998. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol. Today 19:491-494. [DOI] [PubMed] [Google Scholar]
- 36.Plant, J., and A. A. Glynn. 1976. Genetics of resistance to infection with Salmonella typhimurium in mice. J. Infect. Dis. 133:72-78. [DOI] [PubMed] [Google Scholar]
- 37.Redoglia, V., U. Dianzani, J. M. Rojo, P. Portoles, M. Bragardo, H. Wolff, D. Buonfiglio, S. Bonissoni, and C. A. Janeway, Jr. 1996. Characterization of H4: a mouse T lymphocyte activation molecule functionally associated with the CD3/T cell receptor. Eur. J. Immunol. 26:2781-2789. [DOI] [PubMed] [Google Scholar]
- 38.Rutitzky, L. I., E. Ozkaynak, J. B. Rottman, and M. J. Stadecker. 2003. Disruption of the ICOS-B7RP-1 costimulatory pathway leads to enhanced hepatic immunopathology and increased gamma interferon production by CD4 T cells in murine schistosomiasis. Infect. Immun. 71:4040-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharpe, A. H., and G. J. Freeman. 2002. The B7-CD28 superfamily. Nat. Rev. Immunol. 2:116-126. [DOI] [PubMed] [Google Scholar]
- 40.Sinha, K., P. Mastroeni, J. Harrison, R. D. de Hormaeche, and C. E. Hormaeche. 1997. Salmonella typhimurium aroA, htrA, and aroD htrA mutants cause progressive infections in athymic (nu/nu) BALB/c mice. Infect. Immun. 65:1566-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swallow, M. M., J. J. Wallin, and W. C. Sha. 1999. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity 11:423-432. [DOI] [PubMed] [Google Scholar]
- 42.Tafuri, A., A. Shahinian, F. Bladt, L.-M. Boucher, D. Bouchard, V. S. F. Chan, G. Duncan, B. Odermatt, A. Ho, A. Itie, T. Horan, J. S. Whoriskey, T. Pawson, J. M. Penninger, P. S. Ohashi, and T. W. Mak. 2001. ICOS is essential for effective T-helper-cell responses. Nature 409:105-109. [DOI] [PubMed] [Google Scholar]
- 43.van Faassen, H., R. Dudani, L. Krishnan, and S. Sad. 2004. Prolonged antigen presentation, APC-, and CD8+ T cell turnover during mycobacterial infection: comparison with Listeria monocytogenes. J. Immunol. 172:3491-3500. [DOI] [PubMed] [Google Scholar]
- 44.Vidal, S., M. L. Tremblay, G. Govoni, S. Gauthier, G. Sebastiani, D. Malo, E. Skamene, M. Olivier, S. Jothy, and P. Gros. 1995. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J. Exp. Med. 182:655-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villegas, E. N., L. A. Lieberman, N. Mason, S. L. Blass, V. P. Zediak, R. Peach, T. Horan, S. Yoshinaga, and C. A. Hunter. 2002. A role for inducible costimulator protein in the CD28-independent mechanism of resistance to Toxoplasma gondii. J. Immunol. 169:937-943. [DOI] [PubMed] [Google Scholar]
- 46.Wallin, J. J., L. Liang, A. Bakardjiev, and W. C. Sha. 2001. Enhancement of CD8+ T cell responses by ICOS/B7h costimulation. J. Immunol. 167:132-139. [DOI] [PubMed] [Google Scholar]
- 47.Winau, F., S. H. Kaufmann, and U. E. Schaible. 2004. Apoptosis paves the detour path for CD8 T cell activation against intracellular bacteria. Cell. Microbiol. 6:599-607. [DOI] [PubMed] [Google Scholar]
- 48.Yoshinaga, S. K., J. S. Whoriskey, S. D. Khare, U. Sarmiento, J. Guo, T. Horan, G. Shih, M. Zhang, M. A. Coccia, T. Kohno, A. Tafuri-Bladt, D. Brankow, P. Campbell, D. Chang, L. Chiu, T. Dai, G. Duncan, G. S. Elliott, A. Hui, S. M. McCabe, S. Scully, A. Shahinian, C. L. Shaklee, G. Van, T. W. Mak, et al. 1999. T-cell co-stimulation through B7RP-1 and ICOS. Nature 402:827-832. [DOI] [PubMed] [Google Scholar]