Abstract
A vaccine against enterotoxigenic Escherichia coli (ETEC) is needed to prevent diarrheal illness among children in developing countries and at-risk travelers. Two live attenuated ETEC strains, PTL002 and PTL003, which express the ETEC colonization factor CFA/II, were evaluated for safety and immunogenicity. In a randomized, double-blind, placebo-controlled trial, 19 subjects ingested one dose, and 21 subjects ingested two doses (days 0 and 10) of PTL-002 or PTL-003 at 2 × 109 CFU/dose. Anti-CFA/II mucosal immune responses were determined from the number of antibody-secreting cells (ASC) in blood measured by enzyme-linked immunospot assay, the antibody in lymphocyte supernatants (ALS) measured by enzyme-linked immunosorbent assay (ELISA), and fecal immunoglobulin A (IgA) levels determined by ELISA. Time-resolved fluorescence (TRF) ELISA was more sensitive than standard colorimetric ELISA for measuring serum antibody responses to CFA/II and its components, CS1 and CS3. Both constructs were well tolerated. Mild diarrhea occurred after 2 of 31 doses (6%) of PTL-003. PTL-003 produced more sustained intestinal colonization than PTL-002 and better IgA response rates: 90% versus 55% (P = 0.01) for anti-CFA/II IgA-ASCs, 55% versus 30% (P = 0.11) for serum anti-CS1 IgA by TRF, and 65% versus 25% (P = 0.03) for serum anti-CS3 IgA by TRF. Serum IgG response rates to CS1 or CS3 were 55% in PTL-003 recipients and 15% in PTL-002 recipients (P = 0.02). Two doses of either strain were not significantly more immunogenic than one. Based on its superior immunogenicity, which was comparable to that of a virulent ETEC strain and other ETEC vaccine candidates, PTL-003 will be developed further as a component of a live, oral attenuated ETEC vaccine.
Enterotoxigenic Escherichia coli (ETEC) are a significant cause of diarrhea in developing countries, resulting in 400,000 deaths annually in children under the age of 5 years (27). In addition, ETEC are the most common cause of traveler's diarrhea. Colonization factor antigens (CFAs) and enterotoxins are important ETEC virulence factors and attractive antigens for inclusion in ETEC vaccines. CFAs are fimbrial adhesins that promote attachment to intestinal epithelium. The most common CFAs are CFA/I; the CFA/II family, which includes coli surface (CS) antigens CS1, CS2 and CS3; and the CFA/IV family composed of CS4, CS5, and CS6 (14, 26). In addition, ETEC strains produce heat-labile toxin (LT) and/or heat-stable toxin (ST), diarrheagenic enterotoxins. Evidence indicates that anti-CFA and anti-LT immune responses play important roles in mediating protection against ETEC disease (20, 21).
Live, toxin-free mutants may be effective oral vaccines. A spontaneous mutant, E1392/75-2A, which lost ST and LT toxins but continues to express CFA/II, provided 75% protection against challenge with an LT+, ST+, CFA/II+ strain (12, 21). However, E1392/75-2A was associated with diarrhea in ca. 15% of vaccinees, indicating that further attenuation was needed to produce a prototype vaccine for ETEC. Therefore, following attenuation strategies that were successful for Salmonella strains (4-6), specific genes were deleted, including aro genes important in the biosynthesis of aromatic amino acids and chorismate, which is required for folate synthesis; ompC and ompF, which code for outer membrane proteins; and ompR, which regulates expression of ompC and ompF. The new vaccine constructs, PTL-002 (ΔaroC ΔompR) and PTL-003 (ΔaroC ΔompC ΔompF) were immunogenic when given intranasally to mice. In an open-label phase I trial 27 subjects received either PTL-002 or PTL-003 as a single oral dose of 5 × 107, 5 × 108, or 5 × 109 CFU. No serious adverse events occurred, although six subjects experienced mild to moderate gastrointestinal side effects. The 11 subjects who received the highest dose of either strain all showed mucosal immune responses, as measured by immunoglobulin A (IgA) anti-CFA/II reactivity in the antibody in lymphocyte supernatant (ALS) assay (24).
The present study was designed as a double-blind, placebo-controlled trial to further assess the safety and immunogenicity of PTL-002 and PTL-003 and to select the most appropriate construct for further development as a candidate ETEC vaccine.
MATERIALS AND METHODS
Preparation of the vaccine strains PTL-002 and PTL-003.
Construction and characterization of the two live attenuated vaccine candidate strains, PTL002 and PTL003, has been described (24). Both were constructed from the toxin-negative ETEC strain, E1392/75-2A. In addition to being ST, LT, and EAST1 negative, PTL002 and PTL003 have a deletion mutation of the aroC gene, making their growth dependent on aromatic amino acids. In addition, PTL002 has a deletion of the ompR gene, whereas in PTL003 the ompC and ompF genes are deleted. Both strains maintain a small plasmid found naturally in their parent (E1392/75-2A) that confers resistance to streptomycin. The vaccine candidates were administered to volunteers as a suspension, freshly prepared from a lawn of bacteria grown overnight at 35 to 37°C on CFA agar. The lawn was harvested in sterile phosphate-buffered saline (PBS) and, based on the optical density at 600 nm, the concentration of bacterial cells was adjusted to 2 × 109 CFU per ml.
Immunization of volunteers and assessment of vaccine safety and immunogenicity.
In a double-blind fashion 40 healthy subjects, aged 18 to 50, were randomized to one of six groups to receive vaccine or placebo on days 0 and 10. A total of 24 subjects were randomized to receive two doses of vaccine (PTL-002/PTL-002 or PTL-003/PTL-003, n = 12 per group), and 16 were randomized to receive one dose of vaccine (PTL-002/placebo, PTL-003/placebo, placebo/PTL-002, or placebo/PTL-003, n = 4 per group). Subjects fasted for 90 min before and after dosing. Volunteers were immunized by drinking 200 ml of CeraVacx buffer (Cera Products, Inc., Jessup, MD; rice solids, 7.0 g; sodium bicarbonate, 2 g; trisodium citrate, 0.5 g in 200 ml of water) containing 1 ml of the adjusted vaccine suspension. Placebo recipients received 200 ml of CeraVacx buffer alone.
For 7 days after each dose, subjects recorded symptoms on diary cards. Peripheral blood mononuclear cells (PBMC), serum, and fecal samples were obtained on days 0, 7, 10, 17, 24, and 38 to assess local and systemic immune responses. Fecal samples were obtained for culture on days 0, 3, 6, 10, 11, 16, 20, and 24. Subjects still excreting a vaccine strain on day 24 received ciprofloxacin 500 mg orally twice daily for six doses.
Isolation of PTL-002 and PTL-003 strains from stools.
Samples were plated onto MacConkey agar and on MacConkey agar containing streptomycin (25 μg/ml). Five to ten colonies were taken from the streptomycin-containing plates and plated onto Luria agar, a complete growth medium, and onto Davis media, a minimal medium which lacks aromatic metabolites. Colonies growing on complete growth media but not on minimal media were confirmed to be the vaccine strain by agglutination with CFA/II specific antiserum. The detection limit for vaccine strains is estimated to be 30 CFU/g of stool.
Preparation of CFA/II antigens.
Bacteria cultured overnight on CFA agar were incubated in PBS at 65°C for 25 min to release pili. Pili were isolated as previously reported (10, 24).
Detection of anti-CFA/II ASCs.
PBMC were isolated from peripheral blood samples by Ficoll-Hypaque gradient centrifugation, and anti-CFA/II-specific IgA and IgG antibody-secreting cells (IgA/IgG-ASCs) were determined by using an enzyme-linked immunospot assay as previously described (1, 17). A positive ASC response was defined as a ≥2-fold increase over the baseline (day 0) value when the baseline value was ≥0.5/106 PBMC. If the baseline value was <0.5/106 PBMC, a response was defined as >1.0 spots/106 PBMC.
Measurement of anti-CFA/II-specific antibody in ALS, in serum, and in fecal extracts by colorimetric ELISA.
The ALS assay was performed as previously described (3). PBMC were isolated from peripheral blood samples by Ficoll-Hypaque gradient centrifugation, and the cell concentration was adjusted to 107 PBMC/ml in complete RPMI medium. Then, 1-ml aliquots were cultured in 24-well tissue culture plates for 48 h, and the supernatants were harvested and stored frozen at −20°C until assayed for anti-CFA IgG and IgA antibodies by enzyme-linked immunosorbent assay (ELISA). IgA and IgG antibodies in serum and ALS and IgA antibodies in fecal extracts were measured by ELISA as previously described (1, 9). Samples were threefold serially diluted in duplicate in microtiter plates, and endpoint titers were determined as the reciprocal interpolated dilutions giving an A405 of 0.4 above the background. Specific fecal IgA titers were expressed as ELISA units per microgram of total IgA. A ≥2-fold increase in IgA or IgG titers between samples was considered a significant response (1, 9).
Measurement of anti-CS1 and CS3-specific antibody in serum by ELISA utilizing time-resolved fluorescence (TRF).
A modification of the method of Suonpaa et al. (19) was used. Briefly, wells of 96-well EIA plates (Costar 3590) were coated with 50 μl of CS antigen diluted in carbonate-bicarbonate coating buffer to 2 μg/ml and incubated at 37°C for 1 h. Plates were washed three times with PBS-0.05% Tween 20 and blocked with 200 μl of nonfat dried milk/well diluted to 5% in PBS-Tween. After incubation for 1 h at 37°C, the plates were washed three times, and serial dilutions of serum or ALS samples were added (50 μl/well). After a further 1-h incubation at 37°C, the plates were washed, and 50 μl of anti-human IgA-biotin (Southern Biotech, Birmingham, AL) was added/well for 1 h at 37°C. The plates were washed three times, and 50 μl of Europium-labeled streptavidin (Wallac) diluted in Wallac assay buffer was added/well for an additional 1 h at 37°C. After three washes, the fluorescent signal was generated by addition of Wallac enhancement solution (100 μl/well) and shaking for 5 min. Time-resolved fluorescence was measured on a Wallac Victor 2 Multilabel counter. The data were fitted to a four-parameter logistic equation, and endpoint titers were calculated by using XLfit software (IDBS). A twofold increase in TRF titer postinfection over baseline was considered a positive response.
RESULTS
Double-blind randomization and administration of vaccine.
Forty subjects were randomly assigned to one of six groups to receive vaccine (PTL-002 or PTL-003) or a placebo (CeraVacx buffer alone) on days 0 and 10. All volunteers received the first dose of vaccine or placebo, and 37 received the second dose. The three who missed the second dose were in the groups assigned to receive PTL-002/PTL-002, PTL-003/PTL-003, and PTL-002/placebo. An additional subject, assigned to the PTL-002/PTL-002 group, dropped out of the study after receiving the second dose of vaccine. Since no data were collected from this subject after the second vaccine dose, his results are included with the PTL-002/placebo group. The data are, therefore, available for all 40 subjects after 1 dose of vaccine, for 10 after a second dose of PTL-002, and for 11 following a second dose of PTL-003 (Table 1). Of the 20 subjects who received one or two doses of PTL-002, 15 were female, 17 were Caucasian, and 2 were African American, and the mean age was 29 years. The 20 PTL-003 recipients included 15 females; 11 subjects were Caucasian, 5 subjects were African American, and the mean age was 35 years.
TABLE 1.
Number of subjects administered a live, attenuated ETEC vaccine strain (PTL-002 or PTL-003) or placebo on days 0 and 10a
| No. of subjects | Treatment on day: |
|
|---|---|---|
| 0 | 10 | |
| 10 | PTL-002 | PTL-002 |
| 11 | PTL-003 | PTL-003 |
| 4 | Placebo | PTL-002 |
| 4 | Placebo | PTL-003 |
| 6 | PTL-002 | Placebo or not dosedb |
| 5 | PTL-003 | Placebo or not dosedc |
The placebo was CeraVacx buffer alone.
Includes two subjects who were not dosed on day 10 and one who received PTL-002 on day 10 but did not return for any additional visits.
Includes one subject who was not dosed on day 10.
Vaccine strains PTL-002 and PTL-003 were well tolerated.
During the 7 days after each dose, the rates of symptoms after ingestion of PTL-002 and PTL-003 were not significantly different from those after ingestion of placebo. The incidences of loose stools (10, 13, and 22%, respectively) and abdominal pain or cramps (23, 7, and 17%, respectively) were similar. Two subjects had three or more unformed stools in 24 h and, therefore, met the study definition of diarrhea. One subject received two doses of PTL-003 and had three loose stools on day 11, 1 day after her second dose. This subject excreted PTL-003 from day 10 through day 16. A second subject received PTL-003, followed by placebo, and had four loose stools 9 days after ingestion of PTL-003. All six stool samples obtained between days 6 and 24 grew PTL-003. Even though these two subjects excreted PTL-003 on multiple days, each had loose stools on only 1 day. One subject in the placebo group vomited; no subject reported fever (Table 2).
TABLE 2.
Incidence of gastrointestinal and general symptoms reported by subjects during the 7 days after ingestion of PTL-002, PTL-003, or placebo on days 0 and 10a
| Symptoms | Incidence (%) |
P (PTL-002/ PTL-003 vs placebo)d | ||
|---|---|---|---|---|
| PTL-002 (n = 30)b | PTL-003 (n = 31)b | Placebo (n = 18)c | ||
| Unformed stools | 3 (10) | 4 (13) | 4 (22) | 0.4/0.4 |
| Diarrheae | 0 (0) | 1 (3) | 0 (0) | 1.0/1.0 |
| Appetite loss | 0 (0) | 1 (3) | 0 (0) | 1.0/1.0 |
| Abdominal cramps | 7 (23) | 2 (7) | 3 (17) | 0.7/0.3 |
| Vomiting | 0 (0) | 0 (0) | 1 (6) | 1.0/1.0 |
| Fever (T > 100.4°F) | 0 (0) | 0 (0) | 0 (0) | 1.0/1.0 |
| Malaise | 3 (10) | 2 (7) | 0 (0) | 0.3/0.5 |
| Lightheadedness | 3 (10) | 3 (10) | 0 (0) | 0.3/0.3 |
| Headache | 5 (17) | 6 (19) | 3 (17) | 1.0/1.0 |
| Muscle aches | 1 (3) | 0 (0) | 1 (6) | 1.0/0.3 |
Volunteers ingested PTL-002 or PTL-003 in 200 ml of CeraVacx buffer. The placebo was CeraVacx buffer alone.
Of the 32 planned PTL-002 dosings, one subject was lost to follow-up after dose 1 and a second did not receive the second dose. Of the 32 planned PTL-003 dosings, one subject did not receive the second dose.
In addition to the 16 planned placebo dosings, this group includes two subjects who did not receive a second dose of PTL-002 or PTL-003 but returned for subsequent follow-up visits.
Vaccine recipients were compared to the placebo group by Fisher's exact test.
Diarrhea was defined as ≥3 unformed stools in a 24-h period. One subject had three loose stools 1 day after his second dose of PTL-003. Another subject had four loose stools 9 days after his only dose of PTL-003 and is not included in the table since diarrhea was not within 7 days of dosing.
Fecal excretion of PTL-003 was more prolonged than PTL-002.
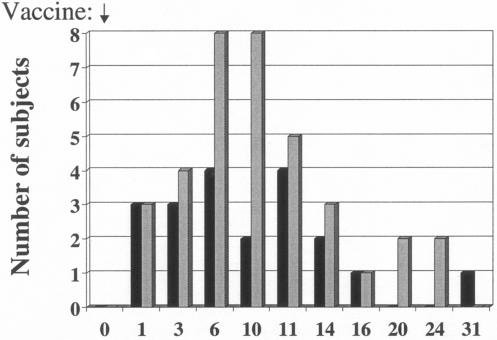
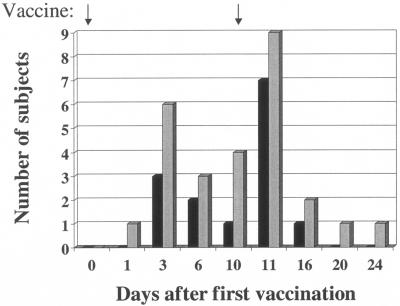
For the 10 to 11 days after the first or only dose of vaccine, 36 of 59 (61%) stool samples from PTL-003 recipients grew the vaccine strain compared to only 15 of 58 (26%) samples from PTL-002 recipients (P < 0.005 as determined by χ2 analysis). Of the 11 subjects who received two doses of PTL-003, 9 had positive stool cultures for the vaccine strain after the initial dose and the other two had positive stool cultures for the vaccine strain after the second dose. Of the 10 volunteers who gave stool samples after receiving two doses of PTL-002, 6 had positive cultures after the first dose and an additional 3 had positive cultures after the second dose (Table 3). Overall, fecal shedding was documented at least once in 36 of 40 subjects: 19 of 20 (95%) of those receiving PTL-003 and 17 of 20 (85%) of those receiving PTL-002. The time course of fecal shedding is shown in Fig. 1 and 2.
TABLE 3.
Serum IgA titers, number of IgA-ASCs, and number of positive stool cultures in subjects given one or two doses of attenuated ETEC strains PTL-002 or PTL-003 on days 0 and 10a
| Subject | PTL vaccine regimen Day 0/ day 10 | Anti-CS1 IgA titer on day: |
Anti-CS3 IgA titer on day: |
IgA-ASCs/106 PBMC on day: |
No. of positive stool culturesb at days: |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 10 | 17 | 24 | 38 | 0 | 7 | 10 | 17 | 24 | 38 | 0 | 7 | 10 | 17 | 1 to 10 | 11 to 25 | ||
| 33 | 002/— | 39 | 40 | 70 | 75 | 48 | 53 | 89 | 99 | 125 | 121 | 92 | 74 | 0.0 | 1.0 | 0.0 | 0.0 | 0 | 3 |
| 38 | 002/— | <20 | <20 | <20 | <20 | <20 | <20 | 86 | 118 | 119 | 96 | 101 | 132 | 0.3 | 2.0 | 0.0 | 0.0 | 2 | 2 |
| 44 | 002/— | <20 | <20 | NS | NS | NS | NS | <20 | <20 | NS | NS | NS | NS | 0.0 | 0.0 | 0.0 | NS | 0 | 0 |
| 59 | 002/— | <20 | <20 | <20 | <20 | <20 | <20 | 39 | 40 | 48 | 39 | 48 | 40 | 0.0 | 3.0 | 0.7 | 0.3 | 1 | 0 |
| 62 | 002/— | 221 | 281 | 339 | 573 | 468 | 490 | 392 | 654 | 872 | 757 | 545 | 434 | 1.7 | 101.0 | 43.7 | 10.0 | 1 | 0 |
| 69 | 002/— | <20 | <20 | <20 | <20 | <20 | 27 | 29 | 30 | 31 | 30 | 0.7 | 0.3 | NS | 0.3 | 1 | 0 | ||
| 31 | —/002 | <20 | 35 | 33 | 51 | 51 | 37 | 58 | 59 | 50 | 76 | 77 | 59 | 0.0 | 0.0 | 0.0 | 0.7 | 0 | 0 |
| 40 | —/002 | 32 | 114 | 49 | 76 | 33 | 79 | 279 | 43 | 259 | 271 | 470 | 607 | 0.0 | 0.3 | 0.0 | 1.0c | 0 | 3 |
| 50 | —/002 | 23 | <20 | <20 | <20 | <20 | 29 | 33 | 32 | 30 | 38 | 36 | 36 | 0.0 | 0.0 | 0.3 | 1.0 | 0 | 1 |
| 54 | —/002 | 83 | 55 | 78 | 39 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | 0.7 | 0.0 | 0.0 | 0.7 | 0 | 2 |
| 30 | 002/002 | 575 | 528 | 680 | 701 | 670 | 874 | 478 | 539 | 586 | 740 | 631 | 695 | 0.0 | 1.7 | 0.0 | 5.7 | 1 | 0 |
| 41 | 002/002 | <20 | <20 | <20 | <20 | <20 | <20 | 303 | 768 | 1,188 | 1,132 | 1,098 | 1,091 | 0.3 | 81.0 | 16.7 | 1.0 | 0 | 1 |
| 42 | 002/002 | 72 | 136 | 346 | 363 | 248 | 101 | 48 | 44 | 41 | 59 | 53 | 48 | 0.0 | 15.3 | 2.3 | 2.0 | 1 | 2 |
| 46 | 002/002 | 128 | 255 | 455 | 565 | 390 | 295 | 130 | 199 | 297 | 292 | 249 | 201 | 0.0 | 9.6 | 9.0 | 0.0 | 1 | 1 |
| 48 | 002/002 | <20 | <20 | <20 | <20 | <20 | <20 | 44 | 38 | 54 | 44 | 45 | 54 | 0.0 | 0.7 | 0.0 | 0.0 | 0 | 1 |
| 52 | 002/002 | 47 | 514 | 409 | 276 | 183 | 102 | 480 | 1,011 | >1,280 | >1,280 | 1,251 | 1,128 | 5.0 | 28.7 | 8.3 | 14.7 | 1 | 2 |
| 53 | 002/002 | 29 | 30 | 25 | 29 | 30 | 21 | 151 | 144 | 156 | 152 | 151 | 149 | 5.0 | 6.7 | 0.3 | 6.0 | 1 | 1 |
| 60 | 002/002 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | 21 | <20 | <20 | 0.0 | 0.3 | 0.0 | 0.0 | 1 | 0 |
| 64 | 002/002 | <20 | <20 | <20 | <20 | <20 | <20 | 394 | 377 | 443 | 498 | 478 | 490 | 0.0 | 0.0 | 0.0 | 1.3 | 0 | 0 |
| 71 | 002/002 | 24 | 20 | 20 | 24 | 22 | <20 | 40 | 58 | 73 | 72 | 77 | 76 | 0.0 | 1.0 | 1.3 | 1.7 | 0 | 1 |
| 34 | 003/— | 57 | 39 | 44 | 52 | 39 | 67 | 180 | 156 | 214 | 194 | 151 | 156 | 0.0 | 8.3 | 2.0 | 0.0 | 3 | 0 |
| 37 | 003/— | 37 | 34 | 39 | 41 | 104 | 120 | 77 | 106 | 132 | 219 | 292 | 210 | 0.0 | 3.3 | 4.3 | 45.7 | 2 | 4 |
| 45 | 003/— | 26 | 24 | 23 | 100 | 80 | 73 | 73 | 249 | 489 | 1,074 | 570 | 215 | 0.0 | 175.3 | 41.3 | 6.0 | 3 | 2 |
| 57 | 003/— | <20 | 25 | <20 | 153 | 121 | 73 | 31 | 288 | 325 | 626 | 277 | 64 | 0.0 | 22.0 | 43.3 | 13.0 | 3 | 1 |
| 65 | 003/— | 32 | 32 | <20 | 59 | <20 | <20 | 70 | 614 | 1,158 | 645 | 602 | 346 | 0.3 | 60.3 | 12.3 | NS | 3 | 1 |
| 32 | —/003 | <20 | 31 | 21 | 44 | 148 | 163 | 509 | 424 | 377 | 583 | 1,217 | 580 | 2.7 | 1.7 | 1.0 | 28.7 | 0 | 4 |
| 39 | —/003 | <20 | <20 | <20 | <20 | 792 | 1,051 | 84 | 39 | 93 | 93 | 344 | 178 | 3.0 | 14.0 | 4.3 | 83.0 | 0 | 4 |
| 66 | —/003 | <20 | <20 | 39 | 32 | <20 | <20 | 203 | 197 | 439 | 361 | 490 | 245 | 0.0 | 0.0 | 0.0 | 2.3 | 0 | 3 |
| 68 | —/003 | <20 | <20 | <20 | <20 | <20 | NS | 314 | 104 | 140 | 182 | 362 | NS | 0.0 | 0.0 | 0.7 | 6.0 | 0 | 0 |
| 35 | 003/003 | 39 | 39 | 40 | 40 | 40 | 40 | 74 | 71 | 70 | 73 | 74 | 80 | 0.0 | 1.3 | 0.3 | 0.3 | 1 | 2 |
| 36 | 003/003 | <20 | 39 | 60 | <20 | 27 | <20 | 464 | 680 | 517 | 543 | 568 | 607 | 0.0 | 0.0 | 0.0 | 0.0 | 1 | 1 |
| 43 | 003/003 | 31 | 34 | 28 | 57 | 131 | 64 | 108 | 116 | 102 | 156 | 223 | 112 | 0.0 | 0.0 | 0.0 | 10.0 | 2 | 1 |
| 47 | 003/003 | 89 | 114 | 98 | 141 | 149 | 138 | 276 | 295 | 250 | 303 | 546 | 527 | 0.0 | 0.0 | 0.0 | 11.0 | 0 | 1 |
| 49 | 003/003 | 53 | 39 | 90 | 57 | 61 | 37 | 192 | 267 | 507 | 252 | 285 | 118 | 0.3 | 9.6 | 8.7 | 2.0 | 1 | 1 |
| 51 | 003/003 | 260 | 460 | 897 | 907 | 1,205 | 812 | 122 | 517 | 629 | 879 | 1,121 | 933 | 0.3 | 70.0 | 38.6 | 6.7 | 2 | 0 |
| 55 | 003/003 | <20 | <20 | 39 | 272 | 130 | 84 | 63 | 1,118 | 1,155 | 993 | 314 | 151 | 0.0 | 51.7 | 29.3 | 7.7 | 3 | 1 |
| 56 | 003/003 | <20 | <20 | <20 | <20 | <20 | <20 | 36 | 152 | 224 | 144 | 80 | 117 | 0.0 | 15.7 | 0.7 | 6.7 | 0 | 1 |
| 58 | 003/003 | 109 | 115 | 125 | 146 | 252 | 207 | 935 | 748 | 1094 | 1,038 | 837 | 999 | 0.7 | 62.7 | 30.3 | 46.3 | 1 | 0 |
| 67 | 003/003 | <20 | 27 | <20 | <20 | 99 | 61 | 22 | 31 | <20 | 51 | 1,108 | 159 | 0.0 | 0.0 | 0.0 | 23.0 | 1 | 4 |
| 70 | 003/003 | <20 | <20 | <20 | <20 | <20 | <20 | 27 | 29 | 32 | 29 | 30 | 29 | 0.0 | 0.0 | 0.0 | 0.0 | 2 | 1 |
Boldface subject numbers indicate responders in the anti-CS1 or CS3 IgA antibody assay or the IgA-ASC assay. Boldface antibody titers or ASC numbers indicate response (≥2-fold increase for serum antibody and ≥2-fold increase and >1.0 for ASCs). NS, no show.
Stool samples were obtained for culture on 4 days from days 0 to 10 and 4 days from days 11 to 25.
Responder day 38 (ASC-3.7). (This is the only subject with a response at day 38 only.)
FIG. 1.
Number of subjects excreting PTL-002 and PTL-003 vaccine strains after a single dose of vaccine on day 0. Bars: ▪, PTL002 (n= 10); ░⃞, PTL003 (n = 9).
FIG. 2.
Number of subjects excreting PTL-002 and PTL-003 vaccine strains following a dose of vaccine on day 0 and day 10. Bars: ▪, PTL002 (n = 10); ░⃞, PTL003 (n = 9).
Eight subjects were still excreting the vaccine strain on day 24. Of these, five had been dosed on day 10, but three (two in the PTL-003/placebo group and one in the PTL-002/placebo group) had not been dosed since day 0. In addition, one subject in the placebo/PTL-002 group dosed on day 10 had a positive stool culture on day 11, followed by negative cultures until day 41 when a culture was positive. After treatment with ciprofloxacin, all stool cultures were negative for PTL-002 and PTL-003.
Mucosal immune responses to CFA/II, measured by ASC, ALS, and fecal IgA, were greater among PTL-003 recipients than among PTL-002 recipients.
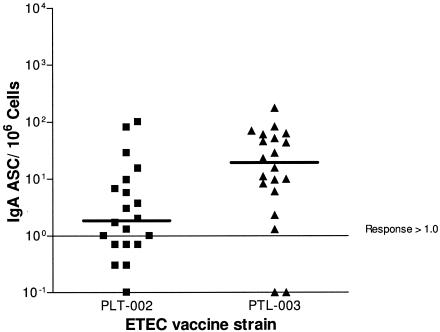
Of all subjects who received one or two doses of vaccine, 18 of 20 (90%) who ingested PTL-003 mounted an IgA-ASC response compared to 11 of 20 (55%) who received PTL-002 (P = 0.01) (Tables 3 and 4). Furthermore, the median peak number of IgA-ASCs was substantially higher for PTL-003 recipients (30/106 PBMC; range, 1.3 to 175) compared to PTL-002 recipients (4/106 PBMC; range, 1.3 to 101) (P < 0.05 by the median test) (Fig. 3). In addition, PTL-003 recipients had higher IgG-ASC response rates (50% versus 30%). Response rates by the ALS assay were lower than those determined by the ASC assay but were, nonetheless, higher among PTL-003 than PTL-002 recipients (75% versus 45% for IgA ALS and 40% versus 20% for IgG ALS). In the ASC and ALS assays, among the 21 subjects who received a second dose of vaccine, there were several new responders after the second dose (Tables 3 and 4). Among the 32 IgA- or IgG-ASC measurements following a placebo dose (before the day 10 dose in the placebo/PTL-002 or placebo/PTL-003 groups), there was one response. There were no ALS responses after a placebo dose. Total fecal IgA levels were sufficient in 26 subjects to measure specific anti-CFA/II IgA: 2 of 12 PTL-002 recipients (17%) and 4 of 14 PTL-003 recipients (29%) had twofold increases in titer (data not shown).
TABLE 4.
Number of subjects with mucosal immune responses to CFA/IIa after one or two oral doses of attenuated ETEC strain PTL-002 or PTL-003
| Assay | Immunoglobulin | Vaccine | No. of respondersb (%) after one dose | No. of new responders (%) after second dosec | Total no. of responders (%) after one or two doses | Pd |
|---|---|---|---|---|---|---|
| ASC | IgA | PTL-002 | 10/20 (50) | 1/4 (25) | 11/20 (55) | 0.01 |
| PTL-003 | 15/20 (75) | 3/5 (60) | 18/20 (90) | |||
| IgG | PTL-002 | 6/20 (30) | 0/7 (0) | 6/20 (30) | 0.20 | |
| PTL-003 | 9/20 (45) | 1/7 (14) | 10/20 (50) | |||
| ALS | IgA | PTL-002 | 9/20 (45) | 0/5 (0) | 9/20 (45) | 0.06 |
| PTL-003 | 11/20 (55) | 4/7 (57) | 15/20 (75) | |||
| IgG | PTL-002 | 3/20 (15) | 1/8 (13) | 4/20 (20) | 0.17 | |
| PTL-003 | 6/20 (30) | 2/9 (22) | 8/20 (40) |
The response in the ASC assay is ≥2-fold increase over the baseline and >1.0 spots/106 PBMC. The response in the ALS assay is ≥2-fold increase over the baseline.
Responders/total number of subjects in group (%).
Subjects in the two-dose group who did not respond to the first dose but did respond to the second dose given on day 10/all subjects in the two-dose group who did not respond to the first dose (%).
Chi-square or Fisher's exact test comparison of total responders to PTL-002 versus total responders to PTL-003.
FIG. 3.
Peak number of CFA/II-specific IgA-ASC per 106 PBMC in volunteers immunized with PTL-002 or PTL-003 with one or two doses of PTL-002 or PTL-003. The bar represents the median value.
Systemic immune responses, measured by serum IgA and IgG antibody responses to CFA/II, CS1, and CS3, were greater among PTL-003 recipients than PTL-002 recipients.
Antibody to CFA/II measured by ELISA showed IgA responses in 7 of 20 subjects (35%) versus 0 of 20 subjects (P < 0.01) and IgG responses in 3 of 20 (15%) versus 0 of 20 subjects given PTL-003 and PTL-002, respectively. The more sensitive TRF assay was used to measure antibody to CS1 and CS3. By this assay 15 of 20 PTL-003 recipients (75%) and 7 of 20 PTL-002 recipients (35%) had an IgA response to one or both antigens (P = 0.03), and 55 and 15%, respectively, had IgG responses (P = 0.02) (Tables 3 and 5). All subjects classified as responders in the ELISA were also responders in the TRF assay. Among the 22 subjects with a serum IgA response by TRF, two failed to show an IgA-ASC response (Table 3). Serum antibody response rates were not significantly different between the one-dose and two-dose regimens (Table 5). Of the 64 IgA or IgG measurements by TRF after a placebo dose (before the day 10 dose in the placebo/PTL-002 or placebo/PTL-003 groups), 2 showed a twofold increase over baseline.
TABLE 5.
Serum antibody responses to CS1 or CS3 measured by TRF ELISA after one or two doses of PTL-002 or PTL-003a
| Antibody | Vaccine | No. of respondersb (%) |
Total no. of respondersb (%) | Pc | |
|---|---|---|---|---|---|
| One-dose group | Two-dose group | ||||
| Anti-CS1 | |||||
| IgA | PTL-002 | 3/10 (30) | 3/10 (30) | 6/20 (30) | 0.11 |
| PTL-003 | 5/9 (56) | 6/11 (55) | 11/20 (55) | ||
| IgG | PTL-002 | 0/10 (0) | 2/10 (20) | 2/20 (10) | 0.66 |
| PTL-003 | 1/9 (11) | 3/11 (27) | 4/20 (20) | ||
| Anti-CS3 | |||||
| IgA | PTL-002 | 2/10 (20) | 3/10 (30) | 5/20 (25) | 0.03 |
| PTL-003 | 7/9 (78) | 6/11 (55) | 13/20 (65) | ||
| IgG | PTL-002 | 0/10 (0) | 2/10 (20) | 2/20 (10) | 0.03 |
| PTL-003 | 4/9 (44) | 5/11 (45) | 9/20 (45) | ||
| Anti-CS1 or anti-CS3 | |||||
| IgA | PTL-002 | 3/10 (30) | 4/10 (40) | 7/20 (35) | 0.03 |
| PTL-003 | 7/9 (78) | 8/11 (73) | 15/20 (75) | ||
| IgG | PTL-002 | 0/10 (0) | 3/10 (30) | 3/20 (15) | 0.02 |
| PTL-003 | 4/9 (44) | 7/11 (64) | 11/20 (55) | ||
Antibody levels were measured by time-resolved fluorescence. Response was defined as ≥2-fold increase compared to preimmune levels.
Number of subjects with a response/total number of subjects in group (%).
Chi-square or Fisher's exact test comparison of total responders to PTL-002 versus total responders to PTL-003.
DISCUSSION
The fimbrial CFAs were identified as potential targets of ETEC vaccines in the 1970s (13). Initial attempts at oral vaccination with purified CFAs were thwarted by gastric enzymes which reduced the antigenicity of CFAs (7). Microencapsulation and administration of CFAs via intestinal tubes prevented degradation in the stomach and resulted in 30% protection against challenge (22).
Difficulties with production and delivery of purified CFAs stimulated interest in killed vaccines and live attenuated vaccines. A formalin-inactivated whole-cell vaccine produced by SBL Vaccin, Stockholm, Sweden, contains a variety of strains with many of the common CFAs and the B subunit of cholera toxin (CT), which is structurally similar to, and gives cross-protection against, ETEC LT. This vaccine was immunogenic in several adult and pediatric populations (1, 9, 11, 17, 18, 25). In a study of 685 U.S. citizens traveling to Mexico and Guatemala, the vaccine protected against moderate to severe ETEC illness (protective efficacy of 77%, P = 0.04) but not against mild ETEC diarrhea (D. A. Sack, J. Shimko, O. Torres, A. Karnell, I. Nyquist, B. Gustafsson, A. L. Bourgeois, O. Newton, and A. Svennerholm, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. G-1217D, 2002). A second larger study of travelers to Latin America corroborated these results. (A. L Bourgeois, J. Halpern, B. Gustafasson, et al., Abstr. 45th Annual ICACC, abstr. G-408, 2005). Unfortunately, when the vaccine was field-tested in 314 Egyptian children aged 6 to 18 months, the protective efficacy was only 20% (S. J. Savarino, R. Abu-Elyazeed, M. Rao, R. Frenck, I. Abdel-Messih, E. Hall, S. Putnam, H. El-Mohamady, T. Wierzba, K. Kamal, P. Moyer, B. Morsy B, A. Svennerholm, Y. Lee, and J. Clemens, Abstr. 6th Annu. Conf. Vaccine Res. Natl. Found. Infect. Dis., abstr. 02-A-43-NFID, 2003). Since this vaccine contains whole cells and CT, it is difficult to assess the role of the CFAs in protecting travelers from moderate to severe ETEC illness.
Another method of delivering CFAs to the small intestine is via live, attenuated bacteria. Attenuated Salmonella and Shigella strains have been genetically engineered to express ETEC CFAs, often with the B subunit or a mutant form of LT or CT (2, 8, 15). The immunogenicity of these strains has been demonstrated in animal models. In addition, live, attenuated ETEC strains have been studied in human trials. Administration of a toxin-positive ETEC strain protected against subsequent rechallenge with the same strain (13). Furthermore, administration of a toxin-negative strain, E1392/75-2A, protected against challenge with a toxin-positive strain homologous only for CFAs (12, 21).
The current randomized, double-blind trial assessed the safety and immunogenicity of two attenuated ETEC strains, PTL-002 and PTL-003, which were derived from strain E1392/75-2A. The aroC gene was deleted to form both strains. In addition, ompC and ompF were deleted to generate PTL-003; and ompR, part of a two-component regulatory system, which regulates the expression of ompC, ompF, and other genes, was deleted to generate PTL-002 (24). The ompR mutation of PTL-002 is, therefore, more pleiotropic than the ompC and ompF mutations of PTL003 and probably more attenuating.
As suggested by these attenuation strategies, PTL-003 caused more sustained colonization than PTL-002. PTL-003 was isolated more than twice as frequently as PTL-002. The duration of fecal excretion by the two vaccine constructs was somewhat longer than reported for other live attenuated enteric vaccine candidates, e.g., CVD908-htrA (23). In the present study the streptomycin-resistant marker carried by the vaccine strains made the detection of organisms in stool samples very sensitive and specific. Since quantitative cultures were not done, the level of shedding was not determined.
In previous studies with the parent strain and a pilot study with PTL-002 and PTL-003, diarrhea occurred in 15 to 20% of subjects (21, 24). Administration of these strains in sodium bicarbonate may have caused some loose stools. In the present study the vaccine strains were administered in CeraVacx, a rice-based oral rehydration solution. Given in this buffer, the vaccine strains were well tolerated and were associated with mild diarrhea in only 2 of 40 subjects. CeraVacx was chosen as the buffer for the current study based on a comparison of three buffers for delivery of Peru-15, a live oral cholera vaccine. Vibriocidal antibody titers were higher when Peru-15 was given in CeraVacx compared to a standard bicarbonate-ascorbic acid buffer or Alka-Seltzer (16). In the previous PTL-002/003 pilot study five subjects ingested PTL-002 and six ingested PTL-003 as a single dose of 5 × 109 CFU in bicarbonate buffer. None of these subjects developed IgA or IgG serum anti-CFA/II responses by ELISA (D. A. Sack, unpublished results); however, all had significant increases in IgA titers in the ALS assay (24). In the present study, which utilized the same immunologic assays performed in the same laboratory as the pilot study, a slightly lower dose (2 × 109 CFU) of PTL-002 or PTL-003 given in CeraVacx resulted in similar mucosal responses but higher serum antibody responses to CFA/II measured by ELISA. Even though two doses of vaccine were given to 21 of the 40 subjects in the current study, serologic responses were similar for those in the two-dose and those in the one-dose groups. Further studies are needed to determine whether carbohydrate-based buffers will improve the tolerability and immunogenicity of live vaccines.
The more persistent excretion of PTL-003 correlates with its greater immunogenicity compared to PTL-002. For neither strain was a second dose on day 10 clearly beneficial in eliciting more serologic responses or greater increases in titer. There were, however, several new mucosal antibody responders noted in the ASC and ALS assays after the second dose. A larger study would be necessary to determine whether a second dose is beneficial.
PTL-003 induced IgA-ASC and serum IgA responses comparable to those elicited by two other prototype vaccines: a whole-cell, formalin-inactivated vaccine comprising five ETEC strains and CTB (1, 11) and a CFA/II vaccine encapsulated in biodegradable polymer microspheres (22). Anti-CFA/II IgA-ASC response rates were 90% for PTL-003 compared to 76% (anti-CS1 or CS3 IgA-ASCs) and 50% for the other two vaccine candidates, respectively. In addition, immune responses induced by PTL-003 were similar to those observed after challenge with 109 CFU of E24377A, a virulent CS1+, CS3+, LT+, ST+ ETEC strain (unpublished data). PTL-003 compared to E24377A elicited similar IgA-ASC response rates (90% versus 90%) and serum IgA response rates to CFA/II measured by standard colorimetric ELISA (35% versus 30%). In the TRF assay response rates to CS3 were similar (65% versus 55%), but response rates to CS1 were lower for PTL-003 compared to E245377A (55% versus 100%).
The evaluation of the comparative immunogenicity of the PTL-002 and PTL-003 vaccine constructs in this trial provided an opportunity for the first time to directly compare ALS and TRF to the more standard ASC and ELISAs for their relative abilities to quantify mucosal and serum antibody responses to ETEC antigens. The proportion of ALS responders was slightly lower than those detected by the ASC assay for both the IgA and IgG isotypes (Table 4). This discrepancy may have resulted from the shorter, 48-h incubation time used for the ALS assay in the present study. In subsequent studies longer ALS incubation times of 72 or 96 h produced better concordance between these two tests. Additional studies comparing TRF with the colorimetric ELISA corroborated results in this report, indicating that TRF is more sensitive for detecting anti-CFA responses after infection or immunization with ETEC strains (2a).
One limitation of this trial is the small number of subjects in each group. Although the size of the trial was adequate to demonstrate that PTL-003 was more immunogenic than PTL-002, it was not large enough to determine whether a second dose of vaccine was beneficial. In addition, the trial provided no information on the durability of the immune response beyond 1 month. Since immune correlates of protection against ETEC illness have not been determined, we do not know if the strong CFA response elicited by PTL-003 would protect against a virulent ETEC strain. However, based on the greater immunogenicity of the PTL-003 construct, this strain has been selected for further development as a prototype ETEC vaccine. A “proof of concept” challenge study is planned in which volunteers will be vaccinated with the PTL-003 construct and subsequently challenged with a fully virulent ETEC strain homologous for CFAs.
Acknowledgments
This study was supported by Acambis Research, Ltd., Cambridge, United Kingdom.
We thank Patricia Maples for clinical coordination of this trial.
Editor: J. D. Clements
REFERENCES
- 1.Ahren, C., M. Jertborn, and A. M. Svennerholm. 1998. Intestinal immune responses to an inactivated oral enterotoxigenic Escherichia coli vaccine and associated immunoglobulin A responses in blood. Infect. Immun. 66:3311-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, E. M., Z. Altboum, G. Losonsky, and M. M. Levine. 2003. Immune responses elicited against multiple enterotoxigenic Escherichia coli fimbriae and mutant LT expressed in attenuated Shigella vaccine strains. Vaccine 21:333-340. [DOI] [PubMed] [Google Scholar]
- 2a.Carpenter, C. M., E. R. Hall, R. Randall, R. McKenzie, F. Cassels, N. Diaz, N. Thomas, P. Bedford, M. Darsley, C. Gewert, C. Howard, R. B. Sack, D. A. Sack, H. S. Chang, G. Gomes, and A. L. Bourgeois. 7September2005, posting date. Comparison of the antibody in lymphocyte supernatant (ALS) and ELISPOT assays for detection of mucosal immune responses to antigens of enterotoxigenic Escherichia coli in challenged and vaccinated volunteers. Vaccine [Online.] doi: 10.1016/j.vaccine.2005.07.022. [DOI] [PubMed]
- 3.Chang, H. S., and D. A. Sack. 2001. Development of a novel in vitro assay (ALS assay) for evaluation of vaccine-induced antibody secretion from circulating mucosal lymphocytes. Clin. Diagn. Lab. Immunol. 8:482-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatfield, S. N., C. J. Dorman, C. Hayward, and G. Dougan. 1991. Role of ompR-dependent genes in Salmonella typhimurium virulence: mutants deficient in both ompC and ompF are attenuated in vivo. Infect. Immun. 59:449-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatfield, S. N., N. Fairweather, I. Charles, D. Pickard, M. Levine, D. Hone, M. Posada, R. A. Strugnell, and G. Dougan. 1992. Construction of a genetically defined Salmonella typhi Ty2 aroA, aroC mutant for the engineering of a candidate oral typhoid-tetanus vaccine. Vaccine 10:53-60. [DOI] [PubMed] [Google Scholar]
- 6.Dorman, C. J., S. Chatfield, C. F. Higgins, C. Hayward, and G. Dougan. 1989. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect. Immun. 57:2136-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans, D. G., D. Y. Graham, and D. J. Evans, Jr. 1984. Administration of purified colonization factor antigens (CFA/I, CFA/II) of enterotoxigenic Escherichia coli to volunteers: response to challenge with virulent enterotoxigenic Escherichia coli. Gastroenterology 87:934-940. [PubMed] [Google Scholar]
- 8.Giron, J. A., J. G. Xu, C. R. Gonzalez, D. Hone, J. B. Kaper, and M. M. Levine. 1995. Simultaneous expression of CFA/I and CS3 colonization factor antigens of enterotoxigenic Escherichia coli by ΔaroC, ΔaroD Salmonella typhi vaccine strain CVD 908. Vaccine 13:939-946. [DOI] [PubMed] [Google Scholar]
- 9.Hall, E. R., T. F. Wierzba, C. Ahren, M. R. Rao, S. Bassily, W. Francis, F. Y. Girgis, M. Safwat, Y. J. Lee, A. M. Svennerholm, J. D. Clemens, and S. J. Savarino. 2001. Induction of systemic antifimbria and antitoxin antibody responses in Egyptian children and adults by an oral, killed enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine. Infect. Immun. 69:2853-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hess, S., F. J. Cassels, and L. K. Pannell. 2002. Identification and characterization of hydrophobic Escherichia coli virulence proteins by liquid chromatography-electrospray ionization mass spectrometry. Anal. Biochem. 302:123-130. [DOI] [PubMed] [Google Scholar]
- 11.Jertborn, M., C. Ahren, and A. M. Svennerholm. 2001. Dose-dependent circulating immunoglobulin A antibody-secreting cell and serum antibody responses in Swedish volunteers to an oral inactivated enterotoxigenic Escherichia coli vaccine. Clin. Diagn. Lab. Immunol. 8:424-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine, M. M. 1986. Fimbriae (pili) as vaccines, p. 143-145. In D. L. Lark (ed.), Protein-carbohydrate interactions in biological systems: the molecular biology of microbial pathogenicity. Academic Press, Ltd., London, United Kingdom.
- 13.Levine, M. M., D. R. Nalin, D. L. Hoover, E. J. Bergquist, R. B. Hornick, and C. R. Young. 1979. Immunity to enterotoxigenic Escherichia coli. Infect. Immun. 23:729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranallo, R. T., C. P. Fonseka, F. Cassels, J. Srinivasan, and M. M. Venkatesan. 2005. Construction and characterization of bivalent Shigella flexneri 2a vaccine strains SC608(pCFAI) and SC608(pCFAI/LTB) that express antigens from enterotoxigenic Escherichia coli. Infect. Immun. 73:258-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sack, D. A., J. Shimko, R. B. Sack, J. G. Gomes, K. MacLeod, D. O'Sullivan, and D. Spriggs. 1997. Comparison of alternative buffers for use with a new live oral cholera vaccine, Peru-15, in outpatient volunteers. Infect. Immun. 65:2107-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savarino, S. J., E. R. Hall, S. Bassily, F. M. Brown, F. Youssef, T. F. Wierzba, L. Peruski, N. El-Masry, M. Safwat, M. Rao, H. Mohamady, R. Abu-Elyazeed, A. Naficy, A. M. Svennerholm, M. Jertborn, Y. J. Lee, J. D. Clemens, et al. 1999. Oral, inactivated, whole-cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine: results of the initial vaccination in children. J. Infect. Dis. 179:107-114. [DOI] [PubMed] [Google Scholar]
- 18.Savarino, S. J., F. Brown, E. R. Hall, S. Bassily, F. Youssef, T. Wierzba, L. Peruski, N. El-Masry, M. Safwat, M. Rao, M. Jertborn, A. M. Svennerholm, Y. J. Lee, and J. D. Clemens. 1998. Safety and immunogenicity of an oral, killed enterotoxigenic Escherichia coli cholera toxin B subunit vaccine in Egyptian adults. J. Infect. Dis. 177:796-799. [DOI] [PubMed] [Google Scholar]
- 19.Suonpaa, M., E. Markela, T. Stahlberg, and I. Hemmila. 1992. Europium-labeled streptavidin as a highly sensitive universal label. Indirect time-resolved immunofluorometry of FSH and TSH. J. Immunol. Methods 149:247-253. [DOI] [PubMed] [Google Scholar]
- 20.Svennerholm, A. M., C. Ahren, and M. Jertborn. 1997. Vaccines against enterotoxigenic Escherichia coli infections. I. Oral inactivated vaccines against enterotoxigenic Escherichia coli, p. 865-873. In M. M. Levine, G. C. Woodrow, J. B. Kaper, and G. S. Cobon (ed.), New generation vaccines, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 21.Tacket, C. O., and M. M. Levine. 1997. Vaccines against enterotoxigenic Escherichia coli infections. II. Live oral vaccines and subunit (purified fimbriae and toxin subunit vaccines), p. 875-883. In M. M. Levine, G. C. Woodrow, J. B. Kaper, and G. S. Cobon (ed.), New generation vaccines, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 22.Tacket, C. O., R. H. Reid, E. C. Boedeker, G. Losonsky, J. P. Nataro, H. Bhagat, and R. Edelman. 1994. Enteral immunization and challenge of volunteers given enterotoxigenic Escherichia coli CFA/II encapsulated in biodegradable microspheres. Vaccine 12:1270-1274. [DOI] [PubMed] [Google Scholar]
- 23.Tacket, C. O., M. B. Sztein, S. S. Wasserman, G. Losonsky, K. L. Kotloff, T. L. Wyant, J. P. Nataro, R. Edelman, J. Perry, P. Bedford, D. Brown, S. Chatfield, G. Dougan, and M. M. Levine. 2000. Phase 2 clinical trial of attenuated Salmonella enterica serovar Typhi oral live vector vaccine CVD 908-htrA in U.S. volunteers. Infect. Immun. 68:1196-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner, A. K., T. D. Terry, D. A. Sack, P. Londono-Arcila, and M. J. Darsley. 2001. Construction and characterization of genetically defined aro omp mutants of enterotoxigenic Escherichia coli and preliminary studies of safety and immunogenicity in humans. Infect. Immun. 69:4969-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenneras, C., F. Qadri, P. K. Bardhan, R. B. Sack, and A. M. Svennerholm. 1999. Intestinal immune responses in patients infected with enterotoxigenic Escherichia coli and in vaccinees. Infect. Immun. 67:6234-6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf, M. K., D. N. Taylor, E. C. Boedeker, K. C. Hyams, D. R. Maneval, M. M. Levine, K. Tamura, R. A. Wilson, and P. Echeverria. 1993. Characterization of enterotoxigenic Escherichia coli isolated from U.S. troops deployed to the Middle East. J. Clin. Microbiol. 31:851-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. 1999. New frontiers in the development of vaccines against enterotoxigenic (ETEC) and enterohaemorrhagic (EHEC) E. coli infections. Wkly. Epidemiol. Rec. 74:98-101. [PubMed] [Google Scholar]