More than 100 years after the discovery of the tubercle bacillus by Robert Koch (43) and approximately 50 years after the introduction of effective antimycobacterial therapeutic agents (3, 4, 46), tuberculosis (TB) continues to be a leading cause of morbidity and mortality worldwide (18, 81). The bacillus Calmette-Guérin (BCG) vaccine, in use since the 1920s, protects young children against disseminated TB, but it does not prevent the development of pulmonary TB, which is the most prevalent and contagious form of the disease (2). In recent years many efforts have focused on the identification of new vaccine candidates against Mycobacterium tuberculosis. These vaccine candidates include live attenuated, DNA, and subunit vaccines (reviewed in reference 16). However, the BCG vaccine and most vaccine candidates against TB are thought to protect the host by augmenting cell-mediated immunity to control mycobacterial replication after the infection has already been established. This mechanism of protection differs substantially from that of most vaccines currently used in humans against many pathogens. Such vaccines confer protection by eliciting protective antibody (Ab) responses, which in turn are thought to eliminate the infecting inoculum (61). For many decades, the scientific paradigm was that Ab plays little or no role in the host defense against M. tuberculosis. Several factors played a role in forming this paradigm: the theory that the immune response to a particular microorganism was either cell mediated or Ab mediated, the view that host defense against intracellular microorganisms was primarily a function of cell-mediated immunity, and the belief that intracellular pathogens were not accessible to Abs. In addition, great difficulty was encountered by investigators trying to demonstrate natural protective Ab responses to M. tuberculosis (reviewed in references 27 and 28). However, in recent years, studies from several laboratories used monoclonal Abs (MAbs) to demonstrate beneficial effects of Abs on various aspects of M. tuberculosis infection (11, 17, 30, 57, 76, 80).
ANTIBODIES TO GLYCOLIPIDS, LIPOGLYCANS, AND POLYSACCHARIDES CAN MODIFY THE OUTCOME OF MYCOBACTERIUM TUBERCULOSIS INFECTION
Some important examples of the role of Abs against M. tuberculosis include the experience with Abs to lipid- and polysaccharide-containing mycobacterial fractions. Costello and colleagues found that children with disseminated TB had lower immunoglobulin G (IgG) titers to the lipoglycan lipoarabinomannan (LAM) than controls (19). They also measured IgG Ab titers to mycobacterial antigens (Ags) in children from the United Kingdom who were not immunized with BCG and not infected with M. tuberculosis. They found that maternally transmitted IgG to mycobacterial antigens declined to a nadir at around 3 months of age and remained low until 2 years of age. The Ab levels then gradually increased, reflecting the subject's own production of Ab to mycobacterial antigens. Abs to LAM were particularly low during the 3-month to 2-year period, with little or no LAM-specific IgG detected (19). Interestingly, the peak incidence of disseminated tuberculosis occurs between the ages of 6 months and 2 years, when levels of antibody to LAM are minimal (19).
Khuller and colleagues demonstrated that the M. tuberculosis glycolipid phosphatidylinositol mannoside (PIM) could induce protective Ab responses in mice immunized with liposome-encapsulated or bovine serum albumin-conjugated vaccines (53, 71). Immunization with PIM decreased the bacterial burden in the lungs and prolonged survival. Passive immunization with sera from vaccinated mice also conferred protection in naive hosts challenged with live M. tuberculosis.
Seibert and colleagues tested Ab responses in rabbits after BCG immunization. They found that rabbits with resistance to TB produced high Ab titers to both mycobacterial proteins and polysaccharides, while rabbits that were susceptible to TB developed mostly protein-specific Abs, suggesting that polysaccharide-specific Abs confer protection (68). In a second study by the same group, production of antimycobacterial polysaccharide Ab following immunization with BCG and mycobacterial protein was inversely correlated with disease severity and survival time after challenge with a virulent strain of M. tuberculosis (69). The authors concluded that the production of Abs to mycobacterial polysaccharides in addition to antiprotein Abs was important for enhanced animal survival after exposure to M. tuberculosis (67). The investigators further hypothesized that free mycobacterial polysaccharide not encountered by Ab interfered with a protective immune response against M. tuberculosis (69).
Abs specific for lipid antigens may also play a role in host defense against M. tuberculosis. Trehalose 6,6′-dimycolate (cord factor)-specific Abs induced by immunization with cord factor complexed with methylated bovine serum albumin neutralized the toxic effects of mycolic acid, as manifested by prolonged survival and maintenance of body weight in mice receiving cord factor intraperitoneally (38).
The studies described above were performed with serum, which contains polyclonal antibodies. Recent studies with MAbs provide additional examples for the role of glycolipid-, lipoglycan-, and polysaccharide-specific Abs in host defense against M. tuberculosis. MAb 9d8, an IgG3 Ab directed to arabinomannan (AM), was demonstrated to prolong the survival of mice infected with a lethal dose of M. tuberculosis. Mice that received the M. tuberculosis Erdman strain coated with MAb 9d8 survived significantly longer than controls (76). Prolonged survival was seen in several mouse strains, including gamma interferon- and major histocompatibility complex (MHC) class II-deficient mice. The improved survival was associated with changes in the histological response to M. tuberculosis infection in the lungs of mice that were consistent with an enhanced granulomatous response (76).
Another study aimed to determine the effect of Ab on free mycobacterial polysaccharide, a concept that was originally introduced by Seibert and colleagues (68). In that study, the effect of MAb 5c11, a LAM-binding MAb, on the clearance and organ distribution of mannose-capped LAM (ManLAM) was examined in mice (30). In the absence of MAb 5c11, intravenously administered ManLAM was taken up by marginal-zone macrophages (Mφ) of the spleen and, to a lesser degree, by the liver. MAb 5c11 administered before the injection of ManLAM enhanced the clearance of ManLAM from the circulation and affected its organ distribution (30). In the presence of MAb 5c11, ManLAM was shunted towards the hepatobiliary system, where bile and bile salts caused a reduction in its immunoreactivity (30). Another study recently demonstrated that the LAM-binding MAb SMITHB14 prolonged survival, prevented weight loss, and reduced CFU in mice infected with M. tuberculosis when administered before or concurrently with mycobacterial challenge (31). It therefore appears that Abs reactive with glycolipid-, lipoglycan-, and polysaccharide-containing structures can confer protection against M. tuberculosis.
GLYCOLIPIDS AND LIPOGLYCANS ARE POTENT VIRULENCE FACTORS
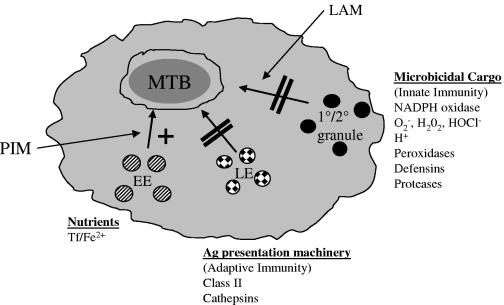
It is not known why Abs reactive to glycolipids, lipoglycans, and polysaccharides confer protection, but this could be related to findings that these molecules are potent virulence factors providing M. tuberculosis with several survival advantages. Ab-mediated neutralization of the effects of virulence factors could “swing the balance” in favor of the host. M. tuberculosis sheds cell wall glycolipid molecules, including LAM and its biosynthetic precursor PIM (7, 63), as well as the polysaccharide AM (29). LAM and PIM are major contributors to M. tuberculosis evasion and subversion of the host immune response (21, 37, 70, 78, 79). Several effects of LAM on the immune system have been described (13, 37, 59, 65, 70), but of great interest is the discovery that LAM inhibits functional maturation of the M. tuberculosis-containing Mφ phagosome (Fig. 1) (21, 78). Phagosome maturation is characterized by the signaling-regulated sequential delivery of endocytic vesicle cargo to the phagosome. The vesicle cargo includes a wide array of innate microbicidal agents, including proteolytic enzymes, and MHC class II, a trigger of adaptive immune responses (12, 26, 35). In contrast to LAM, PIM increases transport of early endosomes but not late endosomes or lysosomes to the M. tuberculosis phagosome (79). Early-endosome transport to phagosomes allows delivery of transferrin receptors, bearing transferrin/iron complexes (14, 79). Iron is critical for M. tuberculosis survival (reviewed in reference 15).
FIG. 1.
M. tuberculosis-derived glycolipids promote survival in the host Mφ and evasion of the host immune response. M. tuberculosis (MTB) resident in the host Mφ phagosome sheds PIM and LAM to exert effects on intracellular vesicle trafficking. LAM prevents trafficking of late endosomes (LE) to the phagosome, thus inhibiting delivery of Ag presentation machinery and inhibiting the adaptive immune response (21, 22). Although not directly proven, evasion of innate immune responses might be similarly mediated, via inhibition of primary and secondary granule trafficking. PIM enhances delivery of early and recycling endosomes (EE) to the phagosome and is suggested to increase levels of essential nutrients in the phagosome, thus contributing to M. tuberculosis survival (79). Tf, transferrin.
Thus, M. tuberculosis cell wall glycolipids are potent virulence factors that operate by enhancing nutrient delivery to the Mφ phagosome or contributing to evasion or subversion of Mφ-mediated innate and adaptive immune responses. Abs to LAM and PIM, for instance, could potentially interfere with inhibition of innate Mφ killing mechanisms and the initiation of adaptive immunity (Fig. 1). Little is known about how Abs confer protection against M. tuberculosis. Hamasur and colleagues demonstrated recently that F(ab′)2 fragments of anti-LAM Abs were equally protective to whole Ab, supporting a neutralization-based mechanism rather than Fc receptor-mediated effector functions for certain Abs (31). Therefore, it is essential that investigators determine the mechanism(s) by which Abs reactive with such virulence factors protect the host.
CD1-RESTRICTED T CELLS PROVIDE HELP FOR ANTIBODY PRODUCTION
CD1 represents a family of nonpolymorphic MHC class I molecules that present lipid-associated carbohydrate structures to CD1-restricted T cells (8, 58). Humans express five known CD1 isoforms (CD1a to -e) (8, 58). Mice express a single CD1 isoform that is highly homologous to human CD1d (58). The M. tuberculosis virulence factors LAM and PIM are glycolipid antigens that bind CD1b and CD1d, leading to activation of CD1b-restricted αβ Th cells and CD1d-restricted natural killer-like T (NKT) cells, respectively (20, 59). Other M. tuberculosis Ags, such as mycolic acid, also stimulate CD1b-restricted T cells (55). Of note, several galactosylceramide molecules from diverse bacterial species have been identified as CD1d Ags (39, 52), suggesting that observations related to M. tuberculosis Ags may be relevant to other bacterial species.
Expression of the single CD1d isoform in mice has resulted in more extensive study of the role of CD1d-restricted NKT cells in producing Abs reactive to CD1-binding Ags. The information obtained from such studies may guide research on CD1b- and CD1c-restricted T cells in Ab responses. All professional antigen-presenting cells (APCs), including B cells, present CD1d Ags (23, 24, 64, 72, 74). NKT cells stimulated by α-galactosylceramide (α-GalCer) produce interleukin-4 (IL-4), consistent with creating a cytokine environment necessary for Ab production (6, 9, 75). Kitamura and colleagues showed that α-GalCer stimulated NKT cells to activate B cells in vivo, resulting in increased expression of MHC class II, B7.2, and the activation marker CD69 (40). The effects of NKT cells on B cells were also shown to be IL-4 dependent (40). Thus far, published findings are consistent with CD1d/Ag expressed on B cells stimulating NKT cells to create a cytokine environment that in turn will activate B cells and promote Ab production.
Several laboratories have reported that NKT cells can provide help for Ab production in a CD1d-dependent manner (10, 25, 48, 66). The M. tuberculosis-derived CD1d-binding Ag PIM activates NKT cells (20), but it is not known whether a PIM-specific B-cell antigen receptor (BCR) can capture PIM to facilitate CD1d presentation to NKT cells and result in Ab production. PIM induces Ab production in the absence of a protein carrier, suggesting but not proving MHC class II-independent Ab production (71). Support for CD1-mediated Ab production is provided by the recent finding that production of Abs reactive with polysaccharide Ags from Streptococcus pneumoniae is CD1d dependent (42). In the S. pneumoniae study, CD1−/− mice immunized with pneumococcal polysaccharide Ags failed to elicit an IgG response (42). Importantly, this suggests that polysaccharide-derived Ag may also bind CD1d and activate NKT cells. These findings are very important to this discussion because they indicate that protective Abs specific for polysaccharides could also be produced in a CD1-dependent manner.
In a separate study, CD1−/− mice infected with the spirochete Borrelia hermsii demonstrated impaired production of specific Ab and had a higher pathogen burden than CD1d-expressing controls (5, 44). Schofield and colleagues showed that the glycosylphosphatidylinositol (GPI) moiety on the Plasmodium berghei-derived malarial circumsporozoite protein was required for production of Ab reactive to the protein component and that the response was abrogated in CD1−/− mice (66). This finding demonstrates that presentation of a CD1d-binding component (GPI) of a GPI-linked protein can stimulate help from CD1d-restricted T cells for production of protein-specific Ab, even though peptides derived from the protein are presented on MHC class II.
Galli and colleagues showed that human peripheral B cells cocultured with autologous NKT cells produced IgG in response to stimulation with α-GalCer (25). This suggests that human CD1d-restricted NKT cells contribute to Ab production. Campos et al. showed that production of IgM by B-1 B cells mediating contact sensitivity reactions was dependent on CD1d expression (10). Two separate studies also showed that sensitization of mice via subcutaneous OVA immunization, followed by intranasal OVA administration, induced airway hypersensitivity, eosinophilia, and anti-OVA IgE production in a CD1d/NKT-dependent manner (1, 48). Importantly, in both of these studies IgE production was not entirely dependent on CD1d-restricted T cells. This indicates that MHC class II- and CD1d-restricted T cells might both contribute to the Ab response, a finding that has been supported by studies showing that CD1d−/− and MHC class II−/− mice produce IgE, albeit at lower levels than in wild-type mice (82). Recently, α-GalCer was shown to have potential as an adjuvant that could be administered intranasally to induce protein Ag-specific IgA (41). This could have consequences for inducing antibody-mediated immunity to M. tuberculosis at mucosal surfaces.
Our recent research has addressed the mechanism for production of Abs reactive with CD1-binding Ags (45). We demonstrated in vitro that if CD1-binding glycolipid (α-GalCer) was targeted to the BCR (within anti-BCR Ab complexes), it resulted in a 100-fold-enhanced activation of CD1d-restricted NKT cells compared to nontargeted Ag. In the same study, we demonstrated that the BCR facilitated enhanced uptake of LAM or α-GalCer to CD1d-containing organelles and that colocalization of Ag with CD1d was essential for NKT activation. These findings support the hypothesis that CD1 Ag-specific BCR could capture Ag to facilitate intracellular acquisition by CD1d and subsequent presentation to NKT cells and in turn stimulate help for Ab production in vivo.
A substantial number of publications support the concept that CD1d-restricted T cells provide help for Ab production, but a few controversies remain. For example, in contrast to the findings of Schofield and colleagues (66), Procopio and colleagues showed that production of Abs reactive to GPI-anchored mucins of Trypanosoma cruzi was not CD1d dependent (60). This suggests that not all carbohydrate structures are presented by CD1d. Additionally, Molano and colleagues reported that production of Ab reactive to the GPI-linked circumsporozoite protein was not CD1 mediated (54). Differences in genetic background arising via different numbers of backcrosses of the CD1−/− mice may provide some explanation for the different results, but notably, when the Ab response was CD1d independent, a larger amount of irradiated sporozoites than in the experiments of Schofield et al. was used to infect the mice. Interestingly Schofield et al. reported that 1 ng of native GPI-anchored protein induced an Ab response comparable to that with 10 μg of recombinant (non-GPI linked) protein (66). In other words, CD1d-restricted T cells provided help in response to very small amounts of Ag. This suggests that the disparity in results between the two studies could have been caused by high Ag levels overcoming the requirement for CD1d-restricted T cells and stimulating MHC class II-restricted T cells to produce Ab. These studies suggest that CD1d- and MHC class II-restricted T cells may be operating at two different levels of sensitivity. Therefore, CD1d-restricted NKT cells may be capable of enhancing Ab production in response to very low levels of Ag early in infection.
One other area of interest pertaining to Ab production mediated by CD1-restricted T cells is the B-cell compartment(s) involved in such responses. Investigators have proposed that splenic marginal zone (MZ) B cells may produce Abs in a CD1-dependent manner. Murine MZ B cells express high levels of CD1 and produce Abs reactive to carbohydrate epitopes (reviewed in references 50 and 51). Direct evidence has been obtained by Belperron and colleagues, who demonstrated that MZ B cells produced Abs reactive with Borrelia hermsii carbohydrate Ags in a CD1d-dependent manner (5). This suggests the potential for MZ B-cell-dependent production of Abs reactive with M. tuberculosis-derived glycolipid, lipoglycan, or polysaccharide Ags.
A PROPOSED MECHANISM FOR CD1-MEDIATED ANTIBODY PRODUCTION
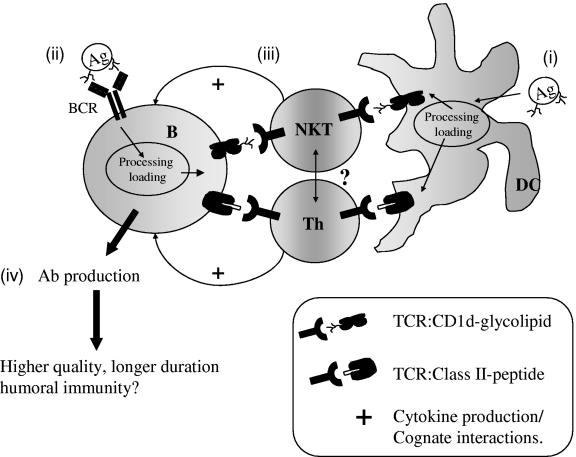
The evidence presented here supports the concept that CD1d-restricted T cells can provide help for Ab production in vivo. A model for this concept is shown in Fig. 2. We propose that the BCR specificity and Ag structure (and thus CD1-binding moieties) will influence the relative contribution of CD1-restricted T cells to Ab production. Vaccination with pure protein or CD1-binding Ags may skew the response towards MHC class II-restricted or CD1-restricted T cells, respectively (Fig. 2). A more likely scenario (during infection or following vaccination with carbohydrate-lipid-protein conjugates) is that due to uptake of antigenic complexes containing glycolipid and protein or polysaccharide and protein, MHC class II- and CD1-restricted T cells will provide help for Ab production. The BCR may be specific for a peptide or carbohydrate epitope (or a combination of both) and could capture protein, carbohydrate (including CD1-binding glycolipids/lipoglycans), or a structure consisting of all these moieties (either covalently bound or associated). Ag processing will then result in the generation of MHC class II-binding peptides as well as CD1-binding glycolipids/lipoglycans. Presentation on both MHC class II and CD1 will in turn elicit help from MHC class II- and CD1-restricted T cells. The relative contributions of MHC class II- and CD1-restricted T cells may also depend on the relative levels of MHC class II and CD1 Ag presentation.
FIG. 2.
Increased help for Ab production mediated by CD1-restricted Th or NKT cells. In this model, we propose the following. (i) DCs capture protein, glycolipids, or complexes and subsequently present Ag in the context of CD1d to CD1d-NKT cells or present MHC class II to MHC class II-restricted Th cells. DCs therefore prime NKT and Th cells for further activation by Ag-presenting B cells. (ii) B cells capture Ag via a specific BCR to facilitate presentation to DC-primed NKT and/or Th cells. (iii) The T cell is stimulated via cognate interactions to produce IL-4 and to increase receptor engagement between the T cell and the B cell. (iv) The cytokine environment combined with receptor-mediated help stimulates Ab production. In this model, the relative contribution of MHC class II- and CD1-restricted help may depend on the relative affinity of glycolipid and peptide Ag for the T-cell receptor (TCR). Whether CD1- and MHC class II-restricted T cells interact directly to affect Ab production is not known.
As shown in the model, other CD1-expressing professional APCs, particularly dendritic cells (DCs) and perhaps macrophages, will likely influence or even be required for the induction of CD1-mediated Ab responses. CD1 Ags such as α-GalCer (CD1d) and LAM (CD1b) are captured and presented by dendritic cells and Mφ (23). However, the exact effect (if any) of DCs and on CD1-dependent Ab production is at present unknown. DCs rather than B cells prime naive MHC class II-restricted Th cells to provide help for Ab production in vivo (36, 62). Whether B cells, DCs, or both prime CD1-restricted T cells and affect priming of MHC class II-restricted Th cells is not established. Furthermore, Ags with distinct structures may skew the in vivo Th1/Th2 balance via activation of APCs and lead to distinct immunological outcomes (cell-mediated versus humoral immunity) (56, 83). The contribution of other APCs to the Ab response may therefore be governed at least in part by the specific Ag.
IS THERE POTENTIAL FOR A NOVEL VACCINE STRATEGY AGAINST TB?
The polysaccharide vaccines are among the most successful to be used in recent years, and they include vaccines against Haemophilus influenzae type b, Streptococcus pneumoniae, Salmonella enterica serovar Typhi, and meningococcal serogroup C (49, 77). These are protein-conjugated polysaccharide vaccines that elicit effective T-cell-dependent Ab responses. The Salmonella enterica serovar Typhi conjugate vaccine serves as an example of a vaccine that provides protection against a facultative intracellular pathogen by eliciting a protective Ab response (47). To date, several polysaccharide conjugate vaccine candidates have been described, among them LAM-derived oligosaccharide conjugated to tetanus toxoid, cross-reactive mutant (CRM197) diphtheria toxoid (33), or antigen 85B or a 75-kDa protein of M. tuberculosis (32). A vaccine candidate consisting of AM conjugated to recombinant Pseudomonas aeruginosa exoprotein A has also been developed (29).
These vaccines were immunogenic and elicited IgG responses in immunized animals (29, 32). Vaccine candidates consisting of LAM-derived oligosaccharides conjugated to protein carriers prolonged survival and prevented weight loss in mice and guinea pigs infected with a virulent strain of M. tuberculosis (32). We demonstrated that immunization of mice with AM-recombinant P. aeruginosa exoprotein A conjugate vaccine resulted in a CFU reduction 7 days after infection with M. tuberculosis Erdman strain or Mycobacterium bovis BCG. It is worth highlighting that the effect on CFU was noted early after infection, prior to the development of effective cell-mediated immunity (29). As mentioned, PIM-containing liposomes or PIM-bovine serum albumin conjugates have been used as vaccines to stimulate Ab-mediated protection in mice challenged with M. tuberculosis (53, 71). These results provide a strong basis for continued investigation of polysaccharide- and glycolipid/lipoglycan-derived vaccines and new investigations into the mechanism(s) of protective Ab production.
Since it is possible that not all M. tuberculosis Ags induce protective Ab responses, the Ag (or mixture of Ags) chosen for vaccination is likely to be critical. In this regard, the difficulties encountered with the development of meningococcal polysaccharide vaccines (34, 73), especially to serogroup B (34), and the encouraging breakthrough made in recent years with the serogroup C meningococcal vaccine (73) highlight the importance of choosing a suitable Ag(s) for vaccine development. We hypothesize that there are several criteria that an Ag should fulfill to be useful as a vaccine component to enhance Ab-mediated protection against M. tuberculosis. The Ag should be critical to the growth and survival of M. tuberculosis and accessible to Ab. The Ag should be presented on MHC class II and/or CD1 to a T-cell receptor whereby the affinity of interaction stimulates an appropriate “T helper” response that favors Ab production. The CD1-restricted T cell should contribute to isotype switch and somatic hypermutation (at the immunoglobulin locus), thus generating Ab with sufficiently high affinity to confer protection against challenge with live M. tuberculosis. Additionally, the vaccine should trigger sufficiently long-term immunity, ideally stimulating lifelong protection. At present, we do not know what the relative contributions of CD1-restricted T cells to these intricate processes are. Careful experimentation using well defined CD1-binding Ags will be necessary to achieving the goal of understanding the contribution of CD1-restricted T cells to Ab production and how to stimulate protective Ab responses.
CONCLUDING REMARKS
Based on the studies reported thus far with MAbs specific for mycobacterial CD1-binding antigens, it appears that Abs can have beneficial effects on the course of M. tuberculosis infection if present at the time of host-pathogen engagement. Thus, we believe that Ab-inducing vaccine candidates should be developed with the intention of preventing infection rather than controlling M. tuberculosis replication after infection has already been established. The variables that are important for the successful development of such vaccines need to be extensively evaluated. Learning how to appropriately stimulate CD1-restricted T-cell responses may be critical in the successful development of such vaccines.
Acknowledgments
M.L.L is funded by NIH grant P20 RR16437 from the COBRE Program of the National Center for Research Resources. A.G.-F. has been funded by NIH grants AI001691 and AI053192 and by a pilot grant from the Center for AIDS Research at the Albert Einstein College of Medicine. A.G.-F. is a Center for AIDS Research investigator at the Albert Einstein College of Medicine.
We thank S. A. Porcelli and J. D. Nosanchuk (Albert Einstein Institute, Yeshiva University, Bronx, NY) for critical review of the manuscript and insightful discussions.
Editor: J. B. Kaper
REFERENCES
- 1.Akbari, O., P. Stock, E. Meyer, M. Kronenberg, S. Sidobre, T. Nakayama, M. Taniguchi, M. J. Grusby, R. H. DeKruyff, and D. T. Umetsu. 2003. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 9:582-588. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1996. The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. Morb. Mortal. Wkly. Rep. Recomm. Rep. 45:1-18. [PubMed] [Google Scholar]
- 3.Anonymous. 1952. Treatment of pulmonary tuberculosis with isoniazid; an interim report to the Medical Research Council by their Tuberculosis Chemotherapy Trials Committee. Br. Med. J. 2:735-746. [PMC free article] [PubMed] [Google Scholar]
- 4.Anonymous. 1948. Streptomycin treatment of pulmonary tuberculosis—a Medical Research Council investigation. Br. Med. J. 2:770. [PMC free article] [PubMed] [Google Scholar]
- 5.Belperron, A. A., C. M. Dailey, and L. K. Bockenstedt. 2005. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J. Immunol. 174:5681-5686. [DOI] [PubMed] [Google Scholar]
- 6.Bezbradica, J. S., A. K. Stanic, N. Matsuki, H. Bour-Jordan, J. A. Bluestone, J. W. Thomas, D. Unutmaz, L. Van Kaer, and S. Joyce. 2005. Distinct roles of dendritic cells and B cells in Va14Ja18 natural T cell activation in vivo. J. Immunol. 174:4696-4705. [DOI] [PubMed] [Google Scholar]
- 7.Brennan, P. J. 2003. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinburgh) 83:91-97. [DOI] [PubMed] [Google Scholar]
- 8.Brigl, M., and M. B. Brenner. 2004. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22:817-890. [DOI] [PubMed] [Google Scholar]
- 9.Burdin, N., L. Brossay, and M. Kronenberg. 1999. Immunization with alpha-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J. Immunol. 29:2014-2025. [DOI] [PubMed] [Google Scholar]
- 10.Campos, R. A., M. Szczepanik, A. Itakura, M. Akahira-Azuma, S. Sidobre, M. Kronenberg, and P. W. Askenase. 2003. Cutaneous immunization rapidly activates liver invariant Vα14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J. Exp. Med. 198:1785-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers, M. A., D. Gavier-Widen, and R. G. Hewinson. 2004. Antibody bound to the surface antigen MPB83 of Mycobacterium bovis enhances survival against high dose and low dose challenge. FEMS Immunol. Med. Microbiol. 41:93-100. [DOI] [PubMed] [Google Scholar]
- 12.Chua, J., I. Vergne, S. Master, and V. Deretic. 2004. A tale of two lipids: Mycobacterium tuberculosis phagosome maturation arrest. Curr. Opin. Microbiol. 7:71-77. [DOI] [PubMed] [Google Scholar]
- 13.Chujor, C. S., B. Kuhn, B. Schwerer, H. Bernheimer, W. R. Levis, and D. Bevec. 1992. Specific inhibition of mRNA accumulation for lymphokines in human T cell line Jurkat by mycobacterial lipoarabinomannan antigen. Clin. Exp. Immunol. 87:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clemens, D. L., and M. A. Horwitz. 1996. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J. Exp. Med. 184:1349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins, H. L. 2003. The role of iron in infections with intracellular bacteria. Immunol. Lett. 85:193-195. [DOI] [PubMed] [Google Scholar]
- 16.Collins, H. L., and S. H. Kaufmann. 2001. Prospects for better tuberculosis vaccines. Lancet Infect. Dis. 1:21-28. [DOI] [PubMed] [Google Scholar]
- 17.Conti, S., F. Fanti, W. Magliani, M. Gerloni, D. Bertolotti, A. Salati, A. Cassone, and L. Polonelli. 1998. Mycobactericidal activity of human natural, monoclonal, and recombinant yeast killer toxin-like antibodies. J. Infect. Dis. 177:807-811. [DOI] [PubMed] [Google Scholar]
- 18.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione, and C. Dye. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009-1021. [DOI] [PubMed] [Google Scholar]
- 19.Costello, A. M., A. Kumar, V. Narayan, M. S. Akbar, S. Ahmed, C. Abou-Zeid, G. A. Rook, J. Stanford, and C. Moreno. 1992. Does antibody to mycobacterial antigens, including lipoarabinomannan, limit dissemination in childhood tuberculosis? Trans. R. Soc. Trop. Med. Hyg. 86:686-692. [DOI] [PubMed] [Google Scholar]
- 20.Fischer, K., E. Scotet, M. Niemeyer, H. Koebernick, J. Zerrahn, S. Maillet, R. Hurwitz, M. Kursar, M. Bonneville, S. H. Kaufmann, and U. E. Schaible. 2004. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc. Natl. Acad. Sci. USA 101:10685-10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fratti, R. A., J. M. Backer, J. Gruenberg, S. Corvera, and V. Deretic. 2001. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 154:631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fratti, R. A., J. Chua, I. Vergne, and V. Deretic. 2003. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc. Natl. Acad. Sci. USA 100:5437-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii, S., K. Shimizu, M. Kronenberg, and R. M. Steinman. 2002. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat. Immunol. 3:867-874. [DOI] [PubMed] [Google Scholar]
- 24.Fujii, S., K. Shimizu, C. Smith, L. Bonifaz, and R. M. Steinman. 2003. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 198:267-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galli, G., S. Nuti, S. Tavarini, L. Galli-Stampino, C. De Lalla, G. Casorati, P. Dellabona, and S. Abrignani. 2003. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J. Exp. Med. 197:1051-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garin, J., R. Diez, S. Kieffer, J. F. Dermine, S. Duclos, E. Gagnon, R. Sadoul, C. Rondeau, and M. Desjardins. 2001. The phagosome proteome: insight into phagosome functions. J. Cell Biol. 152:165-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glatman-Freedman, A. 2003. Advances in antibody-mediated immunity against Mycobacterium tuberculosis: implications for a novel vaccine strategy. FEMS Immunol. Med. Microbiol. 39:9-16. [DOI] [PubMed] [Google Scholar]
- 28.Glatman-Freedman, A., and A. Casadevall. 1998. Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin. Microbiol. Rev. 11:514-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glatman-Freedman, A., A. Casadevall, Z. Dai, W. R. Jacobs, Jr., A. Li, S. L. Morris, J. A. Navoa, S. Piperdi, J. B. Robbins, R. Schneerson, J. R. Schwebach, and M. Shapiro. 2004. Antigenic evidence of prevalence and diversity of Mycobacterium tuberculosis arabinomannan. J. Clin. Microbiol. 42:3225-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glatman-Freedman, A., A. J. Mednick, N. Lendvai, and A. Casadevall. 2000. Clearance and organ distribution of Mycobacterium tuberculosis lipoarabinomannan (LAM) in the presence and absence of LAM-binding immunoglobulin M. Infect. Immun. 68:335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamasur, B., M. Haile, A. Pawlowski, U. Schroder, G. Kallenius, and S. B. Svenson. 2004. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab′) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin. Exp. Immunol. 138:30-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamasur, B., M. Haile, A. Pawlowski, U. Schroder, A. Williams, G. Hatch, G. Hall, P. Marsh, G. Kallenius, and S. B. Svenson. 2003. Mycobacterium tuberculosis arabinomannan-protein conjugates protect against tuberculosis. Vaccine 21:4081-4093. [DOI] [PubMed] [Google Scholar]
- 33.Hamasur, B., G. Kallenius, and S. B. Svenson. 1999. Synthesis and immunologic characterisation of Mycobacterium tuberculosis lipoarabinomannan specific oligosaccharide-protein conjugates. Vaccine 17:2853-2861. [DOI] [PubMed] [Google Scholar]
- 34.Healy, C. M., and C. J. Baker. 2005. The future of meningococcal vaccines. Pediatr. Infect. Dis. J. 24:175-176. [DOI] [PubMed] [Google Scholar]
- 35.Houde, M., S. Bertholet, E. Gagnon, S. Brunet, G. Goyette, A. Laplante, M. F. Princiotta, P. Thibault, D. Sacks, and M. Desjardins. 2003. Phagosomes are competent organelles for antigen cross-presentation. Nature 425:402-406. [DOI] [PubMed] [Google Scholar]
- 36.Inaba, K., J. P. Metlay, M. T. Crowley, and R. M. Steinman. 1990. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J. Exp. Med. 172:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplan, G., R. R. Gandhi, D. E. Weinstein, W. R. Levis, M. E. Patarroyo, P. J. Brennan, and Z. A. Cohn. 1987. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J. Immunol. 138:3028-3034. [PubMed] [Google Scholar]
- 38.Kato, M. 1972. Antibody formation to trehalose-6,6′-dimycolate (cord factor) of Mycobacterium tuberculosis. Infect. Immun. 5:203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinjo, Y., D. Wu, G. Kim, G. W. Xing, M. A. Poles, D. D. Ho, M. Tsuji, K. Kawahara, C. H. Wong, and M. Kronenberg. 2005. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434:520-525. [DOI] [PubMed] [Google Scholar]
- 40.Kitamura, H., A. Ohta, M. Sekimoto, M. Sato, K. Iwakabe, M. Nakui, T. Yahata, H. Meng, T. Koda, S. Nishimura, T. Kawano, M. Taniguchi, and T. Nishimura. 2000. Alpha-galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell Immunol. 199:37-42. [DOI] [PubMed] [Google Scholar]
- 41.Ko, S. Y., H. J. Ko, W. S. Chang, S. H. Park, M. N. Kweon, and C. Y. Kang. 2005. α-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J. Immunol. 175:3309-3317. [DOI] [PubMed] [Google Scholar]
- 42.Kobrynski, L. J., A. O. Sousa, A. J. Nahmias, and F. K. Lee. 2005. Cutting edge: antibody production to pneumococcal polysaccharides requires CD1 molecules and CD8+ T cells. J. Immunol. 174:1787-1790. [DOI] [PubMed] [Google Scholar]
- 43.Koch, R. 1932. Die aetiogie der tuberculose. Am. Rev. Tuberc. 25:285-323. [Google Scholar]
- 44.Kumar, H., A. Belperron, S. W. Barthold, and L. K. Bockenstedt. 2000. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J. Immunol. 165:4797-4801. [DOI] [PubMed] [Google Scholar]
- 45.Lang, G. A., P. A. Illarionov, A. Glatman-Freedman, G. S. Besra, and M. L. Lang. 2005. BCR targeting of biotin-α-galactosylceramide leads to enhanced presentation on CD1d and requires transport of BCR to CD1d-containing endocytic compartments. Int. Immunol. 17:899-908. [DOI] [PubMed] [Google Scholar]
- 46.Lehmann, J. 1946. Para-aminosalicylic acid in the treatment of tuberculosis. Lancet 250:15-16. [DOI] [PubMed] [Google Scholar]
- 47.Lin, F. Y., V. A. Ho, H. B. Khiem, D. D. Trach, P. V. Bay, T. C. Thanh, Z. Kossaczka, D. A. Bryla, J. Shiloach, J. B. Robbins, R. Schneerson, and S. C. Szu. 2001. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N. Engl. J. Med. 344:1263-1269. [DOI] [PubMed] [Google Scholar]
- 48.Lisbonne, M., S. Diem, A. de Castro Keller, J. Lefort, L. M. Araujo, P. Hachem, J. M. Fourneau, S. Sidobre, M. Kronenberg, M. Taniguchi, P. Van Endert, M. Dy, P. Askenase, M. Russo, B. B. Vargaftig, A. Herbelin, and M. C. Leite-de-Moraes. 2003. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 171:1637-1641. [DOI] [PubMed] [Google Scholar]
- 49.Marchant, C. D., Kumar, M. L. 2002. Immunizations, p. 232-262. In H. B. Jenson and R. S. Baltimore (ed.), Pediatric infectious diseases: principles and practices. W. B. Saunders, Philadelphia, Pa.
- 50.Martin, F., and J. F. Kearney. 2002. Marginal-zone B cells. Nat. Rev. Immunol. 2:323-335. [DOI] [PubMed] [Google Scholar]
- 51.Martin, F., A. M. Oliver, and J. F. Kearney. 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14:617-629. [DOI] [PubMed] [Google Scholar]
- 52.Mattner, J., K. L. Debord, N. Ismail, R. D. Goff, C. Cantu III, D. Zhou, P. Saint-Mezard, V. Wang, Y. Gao, N. Yin, K. Hoebe, O. Schneewind, D. Walker, B. Beutler, L. Teyton, P. B. Savage, and A. Bendelac. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434:525-529. [DOI] [PubMed] [Google Scholar]
- 53.Mehta, P. K., and G. K. Khuller. 1988. Protective immunity to experimental tuberculosis by mannophosphoinositides of mycobacteria. Med. Microbiol. Immunol. (Berlin) 177:265-284. [DOI] [PubMed] [Google Scholar]
- 54.Molano, A., S. H. Park, Y. H. Chiu, S. Nosseir, A. Bendelac, and M. Tsuji. 2000. Cutting edge: the IgG response to the circumsporozoite protein is MHC class II-dependent and CD1d-independent: exploring the role of GPIs in NK T cell activation and antimalarial responses. J. Immunol. 164:5005-5009. [DOI] [PubMed] [Google Scholar]
- 55.Moody, D. B., B. B. Reinhold, V. N. Reinhold, G. S. Besra, and S. A. Porcelli. 1999. Uptake and processing of glycosylated mycolates for presentation to CD1b-restricted T cells. Immunol. Lett. 65:85-91. [DOI] [PubMed] [Google Scholar]
- 56.Parekh, V. V., A. K. Singh, M. T. Wilson, D. Olivares-Villagomez, J. S. Bezbradica, H. Inazawa, H. Ehara, T. Sakai, I. Serizawa, L. Wu, C. R. Wang, S. Joyce, and L. Van Kaer. 2004. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct alpha- and beta-anomeric glycolipids. J. Immunol. 173:3693-3706. [DOI] [PubMed] [Google Scholar]
- 57.Pethe, K., S. Alonso, F. Biet, G. Delogu, M. J. Brennan, C. Locht, and F. D. Menozzi. 2001. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature 412:190-194. [DOI] [PubMed] [Google Scholar]
- 58.Porcelli, S. A., and R. L. Modlin. 1999. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 17:297-329. [DOI] [PubMed] [Google Scholar]
- 59.Prigozy, T. I., P. A. Sieling, D. Clemens, P. L. Stewart, S. M. Behar, S. A. Porcelli, M. B. Brenner, R. L. Modlin, and M. Kronenberg. 1997. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity 6:187-197. [DOI] [PubMed] [Google Scholar]
- 60.Procopio, D. O., I. C. Almeida, A. C. Torrecilhas, J. E. Cardoso, L. Teyton, L. R. Travassos, A. Bendelac, and R. T. Gazzinelli. 2002. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins from Trypanosoma cruzi bind to CD1d but do not elicit dominant innate or adaptive immune responses via the CD1d/NKT cell pathway. J. Immunol. 169:3926-3933. [DOI] [PubMed] [Google Scholar]
- 61.Robbins, J. B., R. Schneerson, and S. C. Szu. 1996. Hypothesis: how licensed vaccines confer protective immunity. Adv. Exp. Med. Biol. 397:169-182. [DOI] [PubMed] [Google Scholar]
- 62.Ronchese, F., and B. Hausmann. 1993. B lymphocytes in vivo fail to prime naive T cells but can stimulate antigen-experienced T lymphocytes. J. Exp. Med. 177:679-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russell, D. G., H. C. Mwandumba, and E. E. Rhoades. 2002. Mycobacterium and the coat of many lipids. J. Cell Biol. 158:421-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaible, U. E., F. Winau, P. A. Sieling, K. Fischer, H. L. Collins, K. Hagens, R. L. Modlin, V. Brinkmann, and S. H. Kaufmann. 2003. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat. Med. 9:1039-1046. [DOI] [PubMed] [Google Scholar]
- 65.Schlesinger, L. S., T. M. Kaufman, S. Iyer, S. R. Hull, and L. K. Marchiando. 1996. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J. Immunol. 157:4568-4575. [PubMed] [Google Scholar]
- 66.Schofield, L., M. J. McConville, D. Hansen, A. S. Campbell, B. Fraser-Reid, M. J. Grusby, and S. D. Tachado. 1999. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science 283:225-229. [DOI] [PubMed] [Google Scholar]
- 67.Seibert, F. B. 1958. The interplay of an immune substance with tuberculopolysaccharide and its antibody in tuberculosis. J. Infect. Dis. 103:52-60. [DOI] [PubMed] [Google Scholar]
- 68.Seibert, F. B. 1956. The significance of antigen-antibody reactions in tuberculosis. J. Infect. Dis. 99:76-83. [DOI] [PubMed] [Google Scholar]
- 69.Seibert, F. B., and M. V. Seibert. 1957. Relationship between immunity and circulating antibodies, complement and tuberculopoly-saccharide in tuberculosis. J. Infect. Dis. 101:109-118. [DOI] [PubMed] [Google Scholar]
- 70.Sibley, L. D., L. B. Adams, and J. L. Krahenbuhl. 1990. Inhibition of interferon-gamma-mediated activation in mouse macrophages treated with lipoarabinomannan. Clin. Exp. Immunol. 80:141-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh, A. P., and G. K. Khuller. 1993. Liposomes as a carrier for mannophosphoinositide antigens of mycobacteria. Indian J. Biochem. Biophys. 30:160-165. [PubMed] [Google Scholar]
- 72.Singh, N., S. Hong, D. C. Scherer, I. Serizawa, N. Burdin, M. Kronenberg, Y. Koezuka, and L. Van Kaer. 1999. Cutting edge: activation of NK T cells by CD1d and alpha-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 163:2373-2377. [PubMed] [Google Scholar]
- 73.Snape, M. D., and A. J. Pollard. 2005. Meningococcal polysaccharide-protein conjugate vaccines. Lancet Infect. Dis. 5:21-30. [DOI] [PubMed] [Google Scholar]
- 74.Spada, F. M., F. Borriello, M. Sugita, G. F. Watts, Y. Koezuka, and S. A. Porcelli. 2000. Low expression level but potent antigen presenting function of CD1d on monocyte lineage cells. Eur. J. Immunol. 30:3468-3477. [DOI] [PubMed] [Google Scholar]
- 75.Taniguchi, M., M. Harada, S. Kojo, T. Nakayama, and H. Wakao. 2003. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 21:483-513. [DOI] [PubMed] [Google Scholar]
- 76.Teitelbaum, R., A. Glatman-Freedman, B. Chen, J. B. Robbins, E. Unanue, A. Casadevall, and B. R. Bloom. 1998. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc. Natl. Acad. Sci. USA 95:15688-15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trotter, C. L., Ramsay, M. E., Kaczmarski, E. B. 2002. Meningococal serogroup C conjugate vaccination in England and Wales: coverage and initial impact of the campaign. Commun. Dis. Public Health 5:220-225. [PubMed] [Google Scholar]
- 78.Vergne, I., J. Chua, and V. Deretic. 2003. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J. Exp. Med. 198:653-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vergne, I., R. A. Fratti, P. J. Hill, J. Chua, J. Belisle, and V. Deretic. 2004. Mycobacterium tuberculosis phagosome maturation arrest: mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol. Biol. Cell 15:751-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams, A., R. Reljic, I. Naylor, S. O. Clark, G. Falero-Diaz, M. Singh, S. Challacombe, P. D. Marsh, and J. Ivanyi. 2004. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology 111:328-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.World Health Organization. 2004. Tuberculosis. Fact sheet 104. World Health Organization, Geneva, Switzerland.
- 82.Yoshimoto, T., B. Min, T. Sugimoto, N. Hayashi, Y. Ishikawa, Y. Sasaki, H. Hata, K. Takeda, K. Okumura, L. Van Kaer, W. E. Paul, and K. Nakanishi. 2003. Nonredundant roles for CD1d-restricted natural killer T cells and conventional CD4+ T cells in the induction of immunoglobulin E antibodies in response to interleukin 18 treatment of mice. J. Exp. Med. 197:997-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu, K. O., J. S. Im, A. Molano, Y. Dutronc, P. A. Illarionov, C. Forestier, N. Fujiwara, I. Arias, S. Miyake, T. Yamamura, Y. T. Chang, G. S. Besra, and S. A. Porcelli. 2005. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides. Proc. Natl. Acad. Sci. USA 102:3383-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]