Abstract
Toll-like receptor 9 (TLR-9) recognizes unmethylated CpG dinucleotides which are abundant in prokaryotic DNA and yet are rare in eukaryotic DNA. Little is known about the significance of TLR-9 in terms of recognition of different bacterial DNA species. In this study HEK293 cells stably transfected with human TLR-9 were used to analyze the immunostimulatory properties of 15 bacterial DNA preparations. In addition, bacterial genome data were analyzed for the frequency of unmethylated cytosine-guanosine ([CG]) dinucleotides. We observed that DNA samples of different bacteria showed considerable differences in their potential to stimulate TLR-9. This correlated with the frequency of [CG] dinucleotides. Based upon data from our experiments the estimate of immunostimulatory bacterial DNA concentrations translated to as high as 109 bacteria/ml. Application of the transfection reagent DOTAP resulted in a more efficient delivery of DNA into the cell, and this went along with increased TLR-9 activation. The data indicate that bacterial DNA preparations from different species differ in their capacity to activate TLR-9, which is dependent on the individual [CG] content. Moreover, increased intracellular delivery results in a marked enhancement of immunostimulation.
Innate immunity recognizes conserved microbial structures that are referred to as pathogen-associated molecular patterns (15). This is achieved by use of pattern recognition receptors, of which Toll-like receptors have been identified to play a crucial role. Prototypical substances constituting microbial patterns are components of the prokaryotic cell wall, e.g., lipopolysaccharide or lipoteichoic acid. However, it has been recognized early that also DNA possesses immunostimulatory potential when Tokunaga et al. identified DNA-containing fractions of mycobacteria to mediate immune modulation (23, 25). Later it was shown that this is due to the relative abundance of unmethylated cytosine-guanosine ([CG]) dinucleotides (11). Indeed, vertebrate DNA shows a suppression of [CG] dinucleotide frequency and, moreover, is characterized by an increased rate of C-5 methylation of cytosine residues (24). TLR-9 has been identified as the DNA-recognizing receptor (7).
It was noted early that stimulation by bacterial DNA could be mimicked by the use of [CG]-containing synthetic oligodesoxynucleotides (CpG-ODNs) (11). Since CpG-ODNs turned out to possess a considerable potential for immune modulation and since these compounds were easy to synthesize, subsequent work in the TLR-9 field has mainly focused on the use synthetic ODNs. It is intriguing that, despite the fact that basic principles in bacterial DNA/TLR-9 recognition have been known for years, information concerning the meaning of TLR-9 for infections or for the recognition of bacteria is still extremely limited.
Differences in the C/G composition (implying differences in [CG] content) are typical for different genera of bacteria. Since TLR-9 recognizes [CG]-containing DNA motifs, it can be speculated that DNA from different bacteria should vary in the TLR-9 activating capacity. In one study, DNA from four bacterial species had been analyzed, with Escherichia coli showing the highest activation and [CG] content (16). However, only a limited number of bacterial species have been tested, and the readout system was prone to activation by contaminants. Other studies analyzing side effects in plasmid vaccination showed that immunostimulation by plasmid DNA vectors correlated with [CG] content and that methylation (9) or elimination (27) of these motifs decreased immunostimulation. The data can be taken to hypothesize that the frequency of [CG] might be the basis for differences in immunostimulation by bacterial DNA. With the identification of more and more whole genomes of bacteria, it has become feasible to calculate for the real [CG] dinucleotide content in bacterial DNA.
Another question regarding the stimulation of TLR-9 has been whether sufficient concentrations of bacterial DNA can be reached to activate the receptor during infections. It is known that TLR-9 becomes activated within a lysosomal compartment and that DNA has to be taken up previously (1, 5, 13). As has been suggested for CpG-ODN/TLR-9 and RNA/TLR-7/-8, increasing the delivery efficiency seems to increase immunostimulation (6, 26). Whether this holds true for bacterial DNA from different species is not known.
In the present study we sought to analyze the effects of DNA preparations of 15 different bacteria in a TLR-9 specific reporter system.
MATERIALS AND METHODS
Reagents.
Phosphorothioate-modified CpG-ODN 2006 (TCGTCGTTTTGTCGTTTTGTCGTT) and control ODN 2006GC (TGCTGCTTTTGTGCTTTTGTGCTT), as well as phosphodiester CpG-ODN 1668 (TCCATGACGTTCCTGATG) were custom synthesized by TIB Molbiol (Berlin, Germany). Highly purified lipopolysaccharide was prepared from Salmonella enterica serovar Minnesota (HL63, smooth form) and was a gift from U. Seydel, Borstel, Germany. Transfection reagent N-[1-(2,3-dioleoyloxy)]-N,N,N-trimethylammonium propan methylsulfate (DOTAP) was from ROTH (Karlsruhe, Germany).
Cells and culture conditions.
RAW 264.7 cells, a murine macrophage cell line, were kindly provided by R. Schumann, Berlin, Germany. HEK293 cells were obtained from S. Bauer, Munich, Germany. Cells were cultured in Clicks/RPMI 1640 supplemented with either 5% fetal calf serum (FCS; for RAW264.7) or 10% FCS (HEK293 cells), 50 μM β-mercaptoethanol, and antibiotics (penicillin G and streptomycin). HEK-TLR9 cells were obtained by transfecting HEK293 cells with a human TLR-9-green fluorescent protein (GFP) expression plasmid (obtained from T. Espevik, Trondheim, Norway), and selection for stable expression in 0.8 mg of G418/ml. Cells were cloned and analyzed for CpG-DNA responsiveness.
Bacteria.
Bacteria were obtained from an in-house library. The following strains were used Campylobacter jejuni (patient isolate), Corynebacterium efficiens (DSM 44549), Enterococcus faecalis (ATCC 29212), Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 700603), Legionella pneumophila (wild-type corby strain, K Heuner, Wuerzburg, Germany), Mycobacterium tuberculosis (ATCC 27294), Neisseria meningitidis (patient isolate), Propionibacterium acnes (ATCC 6919), Pseudomonas aeruginosa (ATCC 27853), Salmonella enterica serovar Typhi (MCCM 01631), Staphylococcus aureus (ATCC 29213), Staphylococcus epidermidis (ATCC 12228), Streptococcus pneumoniae (patient isolate), and Yersinia enterocolitica (MCCM 01839).
DNA preparation.
Bacteria were grown in 30 ml of Luria-Bertani medium, in brain heart infusion, or on solid Schaedler plates and subsequently pelleted. Bacterial DNA was prepared suspending the bacteria in 20 mM Tris-HCl-0.2 mM EDTA (pH 8.0). Lysozyme (20 mg/ml) was added for 30 min at 37°C. Next, the samples were incubated in 100 mM NaCl-10 mM Tris-HCl-25 mM EDTA (pH 8.0) with proteinase K (0.2 mg/ml) and sodium dodecyl sulfate (0.5%) at 50°C overnight. DNA was purified from the lysate by repeated extraction with phenol-chloroform-isoamyl alcohol, precipitated with sodium acetate, and ethanol and then dissolved and stored at −20°C in aliquots. DNA content and purity were measured spectroscopically.
Western blotting.
A total of 5 × 106 cells were lysed for 30 min on ice in 250 μl of lysis buffer (50 mM Tris-HCl [pH 7.4]; 1% Igepal; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM EDTA; 1 mM phenylmethylsulfonyl fluoride; aprotinin, leupeptin, and pepstatin [1 μg/ml each]; 1 mM Na3VO4; 1 mM NaF). Lysates were cleared by centrifugation at 4°C for 10 min at 11,000 × g. Equal amounts of lysates were fractionated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and electrotransferred to polyvinylidene difluoride membranes. Membranes were stained with anti-GFP antibody (Santa Cruz Biotechnology) and visualized by enhanced chemiluminescence system (Amersham, Freiburg, Germany).
Flow cytometry.
TLR-9-GFP-transfected cells were analyzed for GFP expression on a PAS flow cytometer (Partec, Muenster, Germany).
Cell stimulation and cytokine measurement.
A total of 2 × 105 HEK-TLR9 or 1.5 × 105 RAW264.7 cells were stimulated in 96-well plates in duplicates overnight. Cell-free supernatants were analyzed for cytokine secretion by enzyme-linked immunosorbent assay (ELISA; OptEIA; BD Pharmingen, Heidelberg, Germany).
Quantitative PCR.
A total of 5 × 105 HEK-TLR9 cells were incubated with 10 μg of bacterial DNA/ml for 4 h. Cells were washed intensely four times and genomic DNA (host and bacteria) was prepared with Qiamp DNA kit (QIAGEN, Hilden, Germany). Quantitative PCR for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and eubacterial 16S rRNA was performed on three dilutions of the DNA samples as described previously (17). 16S rRNA amplicons were normalized to GAPDH.
Bioinformatics.
Genome data were analyzed with a software tool (GScan) developed by T. Mallée (Marburg, Germany) for this purpose. Sequence data were examined for the frequency of various nucleotide motifs by a string search.
RESULTS
Generation of a reporter system specific for CpG-DNA.
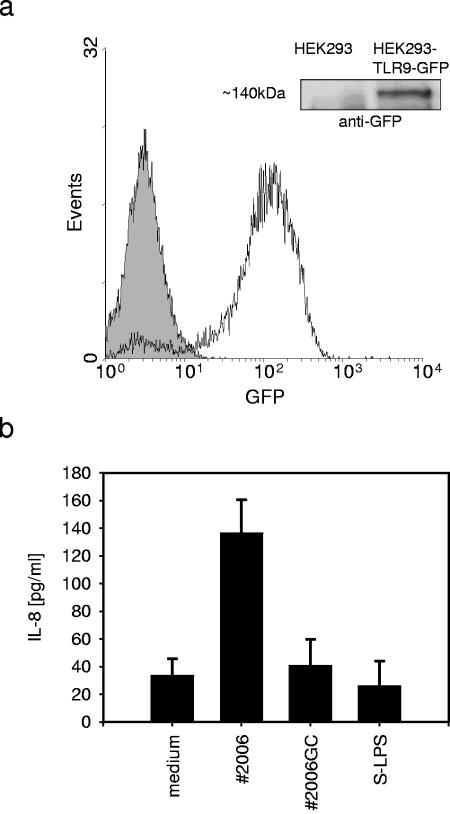
In order to evaluate differences in the immunostimulatory capacity of bacterial DNA samples, we decided to transfect CpG-DNA-unresponsive HEK293 cells with an expression plasmid for human TLR-9 fused to GFP. Initial experiments with transient transfections failed to give satisfactory results. Therefore, we generated six stably transfected cell clones of which one was used for further experiments. HEK-TLR9 cells expressed the transgene efficiently at the mRNA level (data not shown), as well as at the protein level as determined by flow cytometry (Fig. 1a). Moreover, the fusion protein had the expected size of ca. 140 kDa (Fig. 1a, inset). Flow cytometry using a TLR-9 specific antibody detected the same cells marked by GFP expression (data not shown). Functionally, HEK-TLR9 cells were sensitive to CpG-DNA stimulation using the synthetic ODN #2006 but did not respond to the control ODN #2006GC or to lipopolysaccharide (Fig. 1b). Also, ligands for TLR-1, -2, -3, and -6 did not activate the cells (data not shown). We observed that the experiments showed marked variance concerning quantitative interleukin-8 (IL-8) production between different experiments over time but showed no qualitative differences. To account for this, we used plateau concentrations of CpG-ODN #2006 as an internal standard in each experiment, which was set to 100% stimulation.
FIG. 1.
Characterization of the HEK-TLR9 cell line. (a) HEK293 cells stably transfected with a human TLR9-GFP expression plasmid were analyzed for GFP expression by flow cytometry (filled curve, wild type; solid line, HEK-TLR9 cells) and by Western blotting with anti-GFP (inset). (b) HEK-TLR9 cells were stimulated with 1 μM CpG-ODN 2006, the control ODN 2006GC, or with 100 ng of LPS/ml overnight, and supernatants were examined for IL-8 secretion by ELISA (means of duplicates plus the standard deviations [SD], one of three experiments).
Bacterial DNA activates TLR-9 in a concentration-dependent manner.
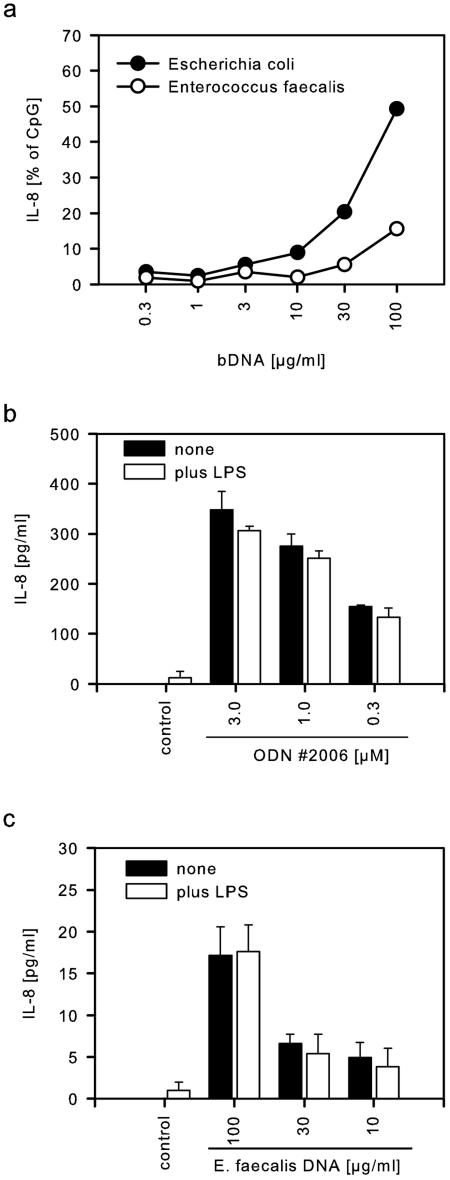
To determine the stimulatory range for bacterial DNA, HEK-TLR9 cells were incubated with DNA preparations of different bacterial species. DNA from E. coli induced significant release of IL-8 starting from 10 μg/ml and increasing up to 100 μg/ml (Fig. 2a). DNA from E. faecalis was effective at concentrations above 30 μg/ml but produced throughout less IL-8 than observed with DNA from E. coli. Although DNA was purified carefully, LPS contamination could not be excluded completely. To exclude that LPS, although itself nonactive (Fig. 1c), might exert costimulatory effects, we stimulated HEK-TLR9 cells with CpG-ODN #2006 (Fig. 2b) or DNA from E. faecalis (Fig. 2c) either in the absence or in the presence of 100 ng of LPS/ml. No differences could be observed; thus, LPS did not influence the reporter system used here.
FIG. 2.
Bacterial DNA stimulates TLR-9. (a) HEK-TLR9 cells were activated with different concentrations of bacterial DNA preparations from E. coli or E. faecalis. Normalized IL-8 secretion (mean of duplicates) of one of two experiments is shown. (b) HEK-TLR9 cells were stimulated with CpG-ODN 2006, and 100 ng of LPS/ml was added as indicated. (c) HEK-TLR9 cells were incubated with DNA from E. faecalis, and 100 ng of LPS/ml was added as indicated. IL-8 secretion was determined by ELISA (means of triplicates plus the SD, one of two experiments each).
Bacterial species differ in their potential to stimulate TLR-9 via DNA.
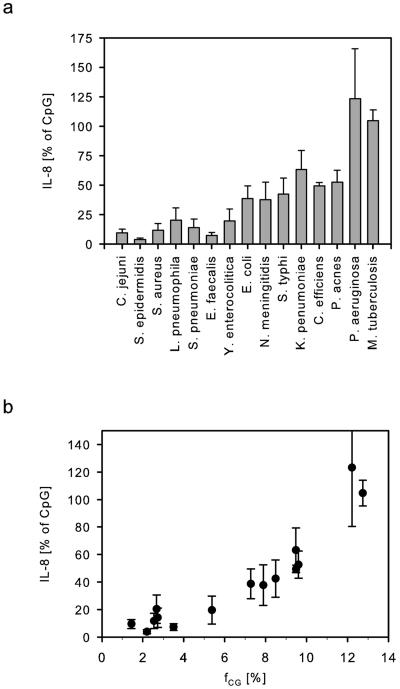
To account for differences in DNA preparation, as well as interassay variance, we next purified DNA from five independent cultures of 15 different bacterial species. Concentration and purity of the DNA samples was determined spectroscopically. The optical density ratio OD260/280 was in the range of 1.76 to 2.07. Genomic DNA was further analyzed quantitatively and qualitatively by gel electrophoresis, which showed only minor fragmentation due to the purification process (data not shown). The preparations were subsequently tested on HEK-TLR9 cells for IL-8 induction (Fig. 3a). We observed marked differences in the immunostimulation by DNA samples of the different species. Although Staphylococcus epidermidis only induced low amounts of IL-8 (3.8% of CpG-ODN), Mycobacterium tuberculosis (105%) and Pseudomonas aeruginosa (123%) were rather stimulatory.
FIG. 3.
Bacterial DNA activates TLR-9 depending on the individual [CG] dinucleotide content. (a) Five independent bacterial DNA preparations from each indicated species (100 μg/ml) were tested for their activity on HEK-TLR9 cells. IL-8 secretion normalized to 3 μM CpG-ODN 2006 is shown as the mean of n = 5 experiments plus the standard error of the mean. (b) Mean values (n = 5) of normalized IL-8 secretion of the different bacteria are plotted against the individual frequency of the dinucleotide [CG] (fCG).
[CG] dinucleotide content of bacterial DNA correlates with TLR-9 activity.
Bacteria differ in their frequency of cytosine and guanosine (fC+G); thus, differences in the frequency of [CG] dinucleotides (fCG) are also probable. We analyzed published whole-genome data of the tested bacteria for their nucleotide composition (Table 1). First, we observed that most often fCG correlated with fC+G, indicating that the dinucleotide [CG] showed a frequency as expected from the individual C+G content (this is indicated by a relative frequency of ∼1.0). However, there was an overrepresentation (pCG > 1.23) of [CG] in Salmonella enterica serovar Typhi and an underrepresentation (pCG < 0.78) in Streptococcus pneumoniae and Campylobacter jejuni. Also, the murine genome showed a severe underrepresentation of [CG]. Differences in fCG in general went along with differences in the frequency of [TCG], as well as the optimal murine CpG motive [Pu-Pu-C-G-Py-Py].
TABLE 1.
Microbial genome sequence analysis
| Genome (analyzed bp [Mb]) | fC + fGa (%) | fCG (%) | fTCG (%) | fPuPuCGPyPy (%) | pCGb |
|---|---|---|---|---|---|
| Pseudomonas aeruginosa (3.5) | 66.62 | 12.21 | 2.71 | 0.99 | 1.10 |
| Mycobacterium tuberculosis (4.4) | 65.61 | 12.74 | 2.81 | 0.84 | 1.18 |
| Corynebacterium efficiens (3.1) | 63.14 | 9.48 | 2.07 | 0.47 | 0.95 |
| Propionibacterium acnes (2.6) | 60.01 | 9.60 | 2.67 | 0.67 | 1.07 |
| Klebsiella pneumoniae (4.9) | 57.48 | 9.47 | 1.87 | 0.85 | 1.14 |
| Salmonella typhi (4.9) | 52.22 | 8.48 | 1.60 | 0.76 | 1.24 |
| Neisseria meningitidis (2.3) | 51.53 | 7.88 | 1.82 | 0.32 | 1.19 |
| Escherichia coli (5.5) | 50.58 | 7.27 | 1.47 | 0.58 | 1.14 |
| Yersinia enterocolitica (4.6) | 47.27 | 5.37 | 1.12 | 0.34 | 0.96 |
| Mus musculus (3.4) | 42.49 | 0.99 | 0.24 | 0.07 | 0.22 |
| Streptococcus pneumoniae (2.2) | 39.70 | 2.73 | 0.87 | 0.20 | 0.69 |
| Legionella pneumophila (3.4) | 38.24 | 2.67 | 0.78 | 0.20 | 0.73 |
| Enterococcus faecalis (3.2) | 37.53 | 3.50 | 1.00 | 0.30 | 0.99 |
| Staphylococcus aureus (2.8) | 32.84 | 2.54 | 0.81 | 0.22 | 0.94 |
| Staphylococcus epidermidis (2.5) | 32.10 | 2.20 | 0.72 | 0.19 | 0.85 |
| Campylobacter jejuni (1.6) | 30.55 | 1.44 | 0.42 | 0.11 | 0.62 |
Absolute frequency fx = number of base x/number of total nucleotides.
Relative frequency pxy = fxy/fxfy.
As we compared equal concentrations of the DNA samples in terms of immunostimulation, we next tested whether the absolute frequency of [CG] dinucleotides might correlate with the differences in TLR-9 activation (Fig. 3b). We observed that an increase in fCG went along with increased immunostimulation. M. tuberculosis and P. aeruginosa, which showed the best IL-8 inducing activity had also the highest frequency of [CG], while the low activating species were marked by a low fCG. Based upon the experimental data the nature of the correlation could not be deduced exactly (coefficient of determination R2 = 0.81). Qualitatively, the same results were obtained when fTCG (indicative of the activating core motif for human cells [TCG]) or fPuPuCGPyPy (exact murine CpG motif) was plotted against IL-8 secretion (data not shown, R2 = 0.78 and 0.75).
Intracellular delivery of bacterial DNA increases stimulation of TLR-9.
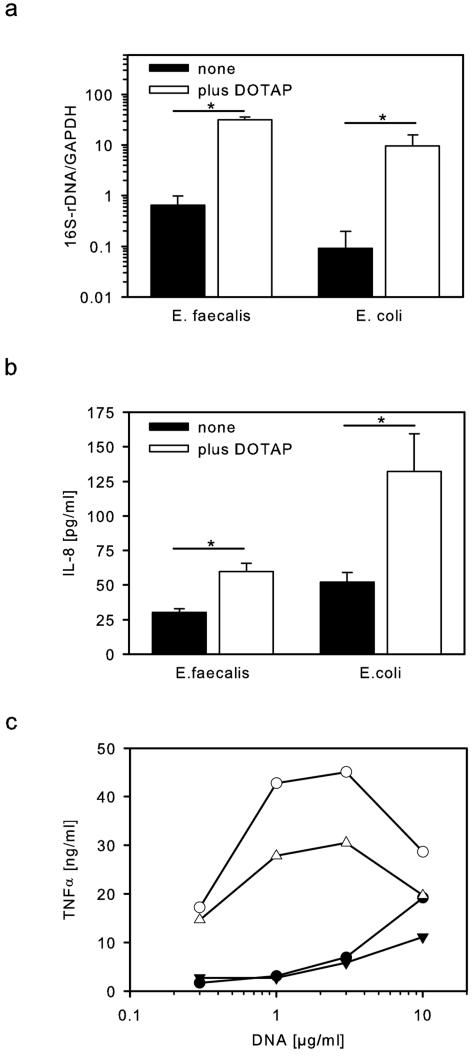
CpG-DNA has to be taken up in cells in order to stimulate intracellularly expressed TLR-9. We tested whether delivery of bacterial DNA by the transfection reagent DOTAP affects stimulation of TLR-9. Using eubacterial 16S rRNA PCR to measure uptake of bacterial DNA, we observed first that DNA samples from E. coli and E. faecalis indeed were taken up into HEK-TLR9 cells (Fig. 4a). Uptake of E. faecalis DNA seemed to be slightly more efficient than for E. coli, but differences were not significant. Moreover, analyzing uptake of DNA from all bacteria tested confirmed that only minor differences occurred which could not explain the differences in immunostimulation (data not shown). Using DOTAP packaged DNA there was a great increase in the uptake of bacterial DNA (20-100-fold) (Fig. 4a). In parallel stimulation of TLR-9 by low concentrations of DNA (10 μg/ml) was examined. Without DOTAP IL-8 secretion was only low, with E. coli being more active than E. faecalis (Fig. 4b). Upon delivery with DOTAP both DNA preparations increased in their capacity to activate TLR-9 (2- and 2.5-fold increase for E. faecalis and E. coli, respectively). Still, DNA from E. faecalis despite of similar uptake was less active than DNA from E. coli, indicating that differences in immunostimulation do not depend on different cellular delivery.
FIG. 4.
Increased intracellular delivery of bDNA improves stimulation of TLR-9. (a) HEK-TLR9 cells were stimulated with 10 μg of bacterial DNA/ml, which was delivered by the transfection reagent DOTAP as indicated. Cells were analyzed for uptake of bacterial DNA by means of quantitative eubacterial PCR. 16S rRNA gene amplicons were normalized to GAPDH (mean values plus the SD; controls, n = 6 experiments; DOTAP, n = 4). (b) Additionally, IL-8 was measured in the supernatant (mean values plus the SD, n = 3; medium, 15.1 ± 0.9 pg/ml). (c) RAW264.7 macrophages were stimulated with bacterial DNA from E. faecalis (triangles) or S. aureus (circles) either without (filled symbols) or with (open symbols) DOTAP. Tumor necrosis factor alpha (TNF-α) was measured in overnight supernatants by ELISA (means of duplicates of one of two experiments).
Based upon the genome size and composition of bacteria the average amount of DNA per bacterium can be calculated and is in the range of a few femtograms/germ. From this it becomes obvious that bacterial DNA concentrations of 10 to 100 μg/ml translate to 109 to 1010 bacteria/ml, which are rather high numbers. Since DOTAP increased delivery and immunostimulation of bacterial DNA in HEK-TLR9 cells (Fig. 4a), we examined whether this holds also true for macrophages. Indeed, DOTAP delivery of DNA from the gram-positive bacteria E. faecalis and S. aureus (to avoid influences from residual LPS) increased activation of RAW264.7 macrophages and shifted dose-response curves to lower DNA concentrations (Fig. 4c). DOTAP packaged DNA was effective at concentrations as low as 0.3 to 1 μg/ml, which would be equivalent to 107 to 108 bacteria/ml. At higher concentrations of DOTAP the activity dropped, which was due to the induction of cell death (data not shown). DOTAP also increased the stimulation by synthetic phosphodiester CpG-oligodesoxynucleotides (100 nM CpG-ODN 1668, 1.1 ± 0.3 ng of TNF-α/ml; plus DOTAP, 15.2 ± 2.2 ng/ml, n = 3).
DISCUSSION
In this study we have found evidence supporting the hypothesis that bacterial species differ in their DNA-dependent immunostimulatory capacity because of different [CG] frequencies.
The present study is based on the use of a TLR-9/DNA specific reporter system that exclusively measured activation by the DNA fraction. This is important since we and others have observed that rather high concentrations of DNA are necessary to stimulate TLR-9 (18, 19), and thus even minute amounts of contaminating TLR ligands might lead to wrong results if cells with a more complete set of TLRs are used. Therefore, we have adopted the well-known HEK-TLR complementation system for our purposes, as already reported by others for CpG-ODNs (2, 22). This system was exclusively responsive to CpG- but not GpC-ODNs. Among possible contaminants LPS is of special concern due to its high immunostimulatory capacity, and substimulatory doses have been shown to synergize powerfully with DNA (4). In our system neither LPS alone nor LPS in the coincubation setting with CpG-ODN or bacterial DNA influenced the experimental readout. Additionally, we analyzed the DNA preparations in HEK293 cells transfected with TLR-2, TLR-4, or NOD2 (data not shown). Samples from gram-negative bacteria showed residual TLR-4 activation indicative of minor LPS contamination, but all samples were inactive for TLR-2 or NOD2 stimulation. Of note, the HEK-TLR9 system did only work with stable transfectants but not with transient transfection. This might be due to an intrinsic stimulation during the transfection process since plasmid DNA has been reported to activate TLR-9 (20).
Upon analyzing TLR-9 activation by DNA from different species, we observed a wide range of stimulatory activity. Despite some variance the results could consistently be reproduced. In line with our results, a study from Neujahr et al. (using a different system and only four bacteria) also observed an improved activity of E. coli DNA over S. aureus DNA (16). In our study we for the first time were able not only to analyze TLR-9 activation but also to correlate this with genome sequence data. We analyzed genome data for frequencies of [CG] dinucleotides and were able to observe a positive correlation with immunostimulatory properties. High frequencies of [CG] could be observed for P. aeruginosa and M. tuberculosis and indeed the DNA fraction of M. tuberculosis had once been the starting point for identification of DNA immunostimulation (23). Other organisms, including E. coli and serovar Typhi, whose DNA preparations have commonly been used had intermediate [CG] frequencies. The results support the view of a [CG] content-dependent immunostimulation in bacterial DNA. In line with our results, others have shown that [CG] frequency in plasmids is correlated with immunostimulation in DNA vaccination (9, 27). It has also been suggested that some DNA sequences derived from adenoviruses (10) or composed of poly[G] (21) might inhibit TLR-9. However, we observed a clear positive correlation and thus were not able to confirm inhibitory sequences within the analyzed DNA samples.
Based upon these results the stimulatory potential of DNA is in the range of 1 to 100 μg/ml. One can calculate that this translates to rather high numbers of bacteria. Thus, stimulation of TLR-9 in real infections might be difficult to be achieved, yet synergism with other TLRs has been reported (3, 4, 28). Moreover, recently it was reported that increased delivery of RNA or CpG-ODNs with transfection reagents was able to enhance immunostimulation by TLR-7 and -9, respectively (6, 26). In line with this, we also observed that DOTAP delivered bacterial DNA efficiently into the cell, thereby increasing the local concentration. In parallel, this lowered the threshold for immunoactivation of TLR-9 by a factor of 10 to 100. The biological equivalent could be that TLR-9 activation by DNA is especially active if bacteria are enriched intracellularly. Indeed, TLR-9, but also TLR-7 and -8, is expressed intracellularly but not at the surface (1) and, accordingly, this whole group of nucleic acid-detecting receptors might serve a special function for only subgroups of microbes. Thus far, TLR-9-deficient mice have only been reported to be sensitive to herpes simplex virus and cytomegalovirus infection but not to bacteria (12, 14). Perhaps TLR-9-mediated activation through bacterial DNA is important only for few bacterial species. Thus, Propionibacterium acnes, which here showed rather high DNA-dependent immunostimulative activity, sensitizes mice for LPS by increasing gamma interferon, and this was dependent on TLR-9 (8).
Taken together, these findings show that activation of TLR-9 by bacterial DNA differs depending upon the bacterial species from which the DNA is prepared, and this correlates with the genomic frequencies of [CG] dinucleotides. Moreover, intracellular delivery of bacterial DNA increases the immunostimulatory potential.
Acknowledgments
This project was supported by the Deutsche Forschungsgemeinschaft Da 592/1, Da 592/2, and He 1452/4.
We thank Christine Barett, Adelina Dillmann, and Stefanie Penati for excellent technical support.
Editor: J. D. Clements
REFERENCES
- 1.Ahmad-Nejad, P., H. Hacker, M. Rutz, S. Bauer, R. M. Vabulas, and H. Wagner. 2002. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32:1958-1968. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, S., C. J. Kirschning, H. Hacker, V. Redecke, S. Hausmann, S. Akira, H. Wagner, and G. B. Lipford. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 98:9237-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalpke, A. H., M. Frey, S. Morath, T. Hartung, and K. Heeg. 2002. Interaction of lipoteichoic acid and CpG-DNA during activation of innate immune cells. Immunobiology 206:392-407. [DOI] [PubMed] [Google Scholar]
- 4.Gao, J. J., E. G. Zuvanich, Q. Xue, D. L. Horn, R. Silverstein, and D. C. Morrison. 1999. Cutting edge: bacterial DNA and LPS act in synergy in inducing nitric oxide production in RAW 264.7 macrophages. J. Immunol. 163:4095-4099. [PubMed] [Google Scholar]
- 5.Häcker, H., H. Mischak, T. Miethke, S. Liptay, R. Schmid, T. Sparwasser, K. Heeg, G. B. Lipford, and H. Wagner. 1998. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by nonspecific endocytosis and endosomal maturation. EMBO J. 17:6230-6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 7.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 8.Kalis, C., M. Gumenscheimer, N. Freudenberg, S. Tchaptchet, G. Fejer, A. Heit, S. Akira, C. Galanos, and M. A. Freudenberg. 2005. Requirement for TLR9 in the immunomodulatory activity of Propionibacterium acnes. J. Immunol. 174:4295-4300. [DOI] [PubMed] [Google Scholar]
- 9.Klinman, D. M., G. Yamshchikov, and Y. Ishigatsubo. 1997. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J. Immunol. 158:3635-3639. [PubMed] [Google Scholar]
- 10.Krieg, A. M., T. Wu, R. Weeranta, S. M. Efler, L. Love-Homan, L. Yang, A. K. Yi, Short, D., and H. L. Davis. 1998. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs. Proc. Natl. Acad. Sci. USA 95:12631-12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krieg, A. M., A. K. Yi, S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546-549. [DOI] [PubMed] [Google Scholar]
- 12.Krug, A., A. R. French, W. Barchet, J. A. A. Fischer, A. Dzionek, J. T. Pingel, M. M. Orihuela, S. Akira, W. M. Yokoyama, and M. Colonna. 2004. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21:107-119. [DOI] [PubMed] [Google Scholar]
- 13.Latz, E., A. Schoenemeyer, A. Visintin, K. A. Fitzgerald, B. G. Monks, C. F. Knetter, E. Lien, N. J. Nilsen, T. Espevik, and D. T. Golenbock. 2004. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5:190-198. [DOI] [PubMed] [Google Scholar]
- 14.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medzhitov, R., and C. A. Janeway. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91:295-298. [DOI] [PubMed] [Google Scholar]
- 16.Neujahr, D. C., C. F. Reich, and D. S. Pisetsky. 1999. Immunostimulatory properties of genomic DNA from different bacterial species. Immunobiology 200:106-119. [DOI] [PubMed] [Google Scholar]
- 17.Nonnenmacher, C., A. Dalpke, R. Mutters, and K. Heeg. 2004. Quantitative detection of periodontopathogens by real-time PCR. J. Microbiol. Methods 59:117-125. [DOI] [PubMed] [Google Scholar]
- 18.Nonnenmacher, C., A. Dalpke, S. Zimmermann, L. Flores-De-Jacoby, R. Mutters, and K. Heeg. 2003. DNA from periodontopathogenic bacteria is immunostimulatory for mouse and human immune cells. Infect. Immun. 71:850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparwasser, T., T. Miethke, G. B. Lipford, A. Erdmann, H. Häcker, K. Heeg, and H. Wagner. 1997. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-α-mediated shock. Eur. J. Immunol. 27:1671-1679. [DOI] [PubMed] [Google Scholar]
- 20.Spies, B., H. Hochrein, M. Vabulas, K. Huster, D. H. Busch, F. Schmitz, A. Heit, and H. Wagner. 2003. Vaccination with plasmid DNA activates dendritic cells via Toll-like receptor 9 (TLR9) but functions in TLR9-deficient mice. J. Immunol. 171:5908-5912. [DOI] [PubMed] [Google Scholar]
- 21.Stunz, L. L., P. Lenert, D. Peckham, A. K. Yi, S. Haxhinasto, M. Chang, A. M. Krieg, and R. F. Ashman. 2002. Inhibitory oligonucleotides specifically block effects of stimulatory CpG oligonucleotides in B cells. Eur. J. Immunol. 32:1212-1222. [DOI] [PubMed] [Google Scholar]
- 22.Takeshita, F., C. A. Leifer, I. Gursel, K. J. Ishii, S. Takeshita, M. Gursel, and D. M. Klinman. 2001. Cutting edge: role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J. Immunol. 167:3555-3558. [DOI] [PubMed] [Google Scholar]
- 23.Tokunaga, T., H. Yamamoto, S. Shimada, H. Abe, T. Fukuda, Y. Fujisawa, Y. Furutani, O. Yano, T. Kataoka, T. Sudo, N. Makiguchi, and T. Sugamura. 1984. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J. Natl. Cancer Inst. 72:955-962. [PubMed] [Google Scholar]
- 24.Wagner, H. 1999. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv. Immunol. 73:329-368. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto, S., T. Yamamoto, T. Kataoka, E. Kuramoto, O. Yano, and T. Tokunaga. 1992. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN and augment IFN-mediated natural killer activity. J. Immunol. 148:4072-4076. [PubMed] [Google Scholar]
- 26.Yasuda, K., P. Yu, C. J. Kirschning, B. Schlatter, F. Schmitz, A. Heit, S. Bauer, H. Hochrein, and H. Wagner. 2005. Endosomal translocation of vertebrate DNA activates dendritic cells via TLR9-dependent and -independent pathways. J. Immunol. 174:6129-6136. [DOI] [PubMed] [Google Scholar]
- 27.Yew, N. S., H. Zhao, I. H. Wu, A. Song, J. D. Tousignant, M. Przybylska, and S. H. Cheng. 2000. Reduced inflammatory response to plasmid DNA vectors by elimination and inhibition of immunostimulatory CpG motifs. Mol. Ther. 1:255-262. [DOI] [PubMed] [Google Scholar]
- 28.Yi, A. K., J. G. Yoon, S. C. Hong, T. W. Redford, and A. M. Krieg. 2001. Lipopolysaccharide and CpG DNA synergize for tumor necrosis factor-alpha production through activation of NF-κB. Int. Immunol. 13:1391-1404. [DOI] [PubMed] [Google Scholar]