Abstract
In an analysis of the molecular factors triggering amebiasis, we investigated the chemotaxis of Entamoeba histolytica toward tumor necrosis factor (TNF) in vitro, using quantitative imaging techniques. Our findings enabled us to propose a hitherto unknown role for TNF as a chemokinetic and chemoattractant agent for this parasite.
Entamoeba histolytica, an extracellular protozoan parasite, is the etiological agent of the human infectious disease amebiasis, with 50 million clinical cases and 100,000 deaths worldwide each year (17). Research into the inflammatory host response during amebiasis has shown that human intestinal epithelial cells produce interleukin-1β (IL-1β), IL-8, and cyclooxygenase-2, all compounds attracting neutrophils and macrophages to the site of invasion of the epithelial barrier by E. histolytica (11). Tumor necrosis factor (TNF) is secreted by both enterocytes and macrophages and is a major component involved in the amplification of this ameba-related inflammation (18). TNF was observed, by immunohistochemical localization, during development of hepatic abscesses (1). In vitro studies have shown that TNF-induced nitric oxide (NO) production by macrophages leads to cytotoxicity of E. histolytica. Gamma interferon-primed macrophages are enhanced for TNF and NO production in response to live amebae and amebic proteins (15). All these events highlight the major role of TNF in amebiasis. Cell motility is crucial for E. histolytica to invade host tissues (11, 12): in light of the parasite's ability to chemotax host molecules (notably zymosan-treated serum, intestine components, and the fibronectin moiety), it has been proposed that chemotaxis could play a role in the pathophysiology of amebiasis (4, 8, 13). During chemotaxis, cell locomotion changes from a nonbiased movement to a biased, directional movement. To investigate the cellular mechanisms that guide E. histolytica during the invasion of human tissue, we first sought to determine whether proinflammatory molecules could attract the parasite, as it is well known that chemokines regulate the navigation of immune cells. We screened over a dozen molecules implicated in inflammatory responses by using an under-agarose chemotaxis assay (13), and we found that recombinant human TNF attracted trophozoites. This result prompted us to examine, in more depth, E. histolytica chemotaxis toward human TNF.
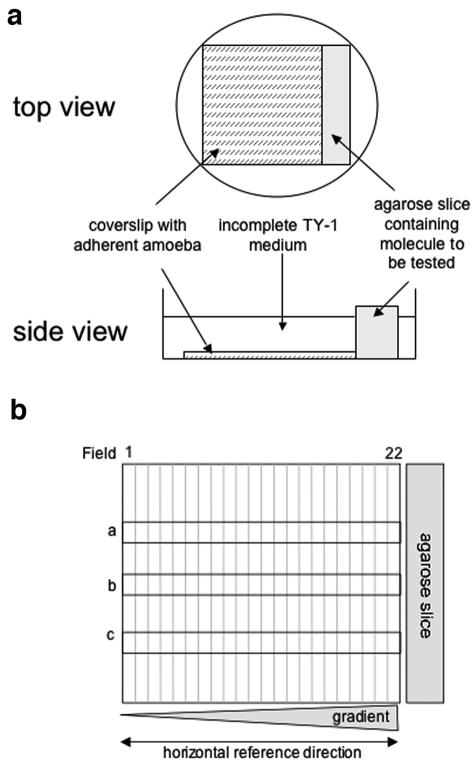
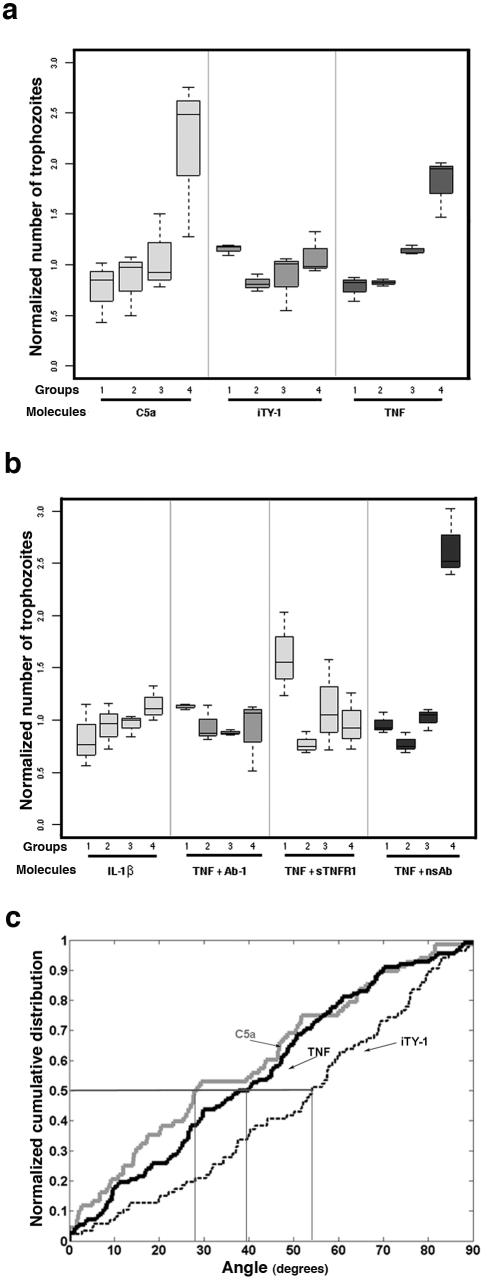
To determine the behavior of the trophozoites in a gradient of TNF, we set up a “chemotaxis-on-coverslip assay” that permitted us, by image analysis, to quantify the displacement of the E. histolytica population, with an accumulation of trophozoites near the molecule source indicating a chemotactic migration (Fig. 1a and b). Trophozoites were incubated for 2 h in the presence of agarose cubes injected with the molecule to be tested. Three independent experiments for each molecule were performed. The relative distribution of the E. histolytica population along the coverslip (pool of three horizontal rows a, b, and c) was separated into four groups (group 1, fields from 1 to 6; group 2, fields from 7 to 11; group 3, fields from 12 to 17; and group 4, fields from 18 to 22), as represented in the box plots (Fig. 2a and b). The data for each experiment were normalized by dividing the number of amebae in each group by the median for the four groups (median normalization). After verification of the homogeneity of variances, a one-way analysis of variance allowed us to compare the numbers of amebae in the different groups. We first analyzed the displacement of the population in the presence of a gradient of TNF (source, 50 nM) and C5a (source, 6 nM) (the component suggested to be chemoattractant in zymosan-treated serum [13]) and in the presence of incomplete TY-1 (iTY-1) (the culture medium TY-1-S-33 without serum). In presence of TNF and C5a, the distribution of the population along the coverslip accumulated toward the source (Fig. 2a). The analysis of variance allowed us to conclude that the difference between groups was significant for TNF (P = 1.6 × 10−4) and C5a (P = 8.7 × 10−3); in contrast, in iTY-1 the distribution was homogeneous (P = 0.8).
FIG. 1.
“Chemotaxis-on-coverslip assay” setup. (a) Experimental setup. One milliliter of incomplete TY-1 medium, without decomplemented serum, vitamins, or penicillin-streptomycin (iTY-1), containing 2.5 × 105 E. histolytica trophozoites previously treated with fluorescent CellTracker green (Molecular Probes) was deposited onto a coverslip placed in a 35-mm culture dish. The trophozoites were allowed to adhere for 10 min at 37°C, during which time the test molecules were injected into a slice of 1% agarose (20 mm by 5 mm by 5 mm). The excess medium was removed from the dishes, the agarose slice was placed next to the coverslip, and 1.5 ml of iTY-1 was then added to the dish. E. histolytica is an anaerobic organism, so the level of oxygen was minimized by placing the dishes in an anaerobic environment (GENbag anaer; Biomerieux). The cells were incubated at 37°C for 2 hours. The trophozoites were fixed with 3.7% paraformaldehyde for 20 min at room temperature. The coverslips were mounted onto glass slides so that the cell distribution could be examined. The progression of Ponceau red (5 μl of a 3.75% solution injected into the agarose slice) was monitored as a measure of the stability of the gradient. A stable, linear gradient was found at between 1 and 3 h. (b) To quantify the cell distribution, the trophozoites were imaged with a Zeiss inverted microscope in epifluorescence mode (fluorescein filters), running the “mosaic” option of the Simple PCI software. The coverslip's vertical axis was divided into 22 fields, with field 22 nearest the agarose slice. Three horizontal rows (a, b, and c) of 22 adjacent images were taken along the coverslip (covering 10% of the coverslip's area). The number of trophozoites was counted in each field for rows a, b, and c, so as to obtain the total number of amebae per field. The total population counted was obtained by summing the number of parasites in rows a, b, and c for the 22 fields.
FIG. 2.
TNF has a chemoattractant effect on E. histolytica. The displacement of the E. histolytica population was analyzed after 2 h of incubation in the presence of different molecules. The box plot shows the distribution of cells along the coverslip in groups 1 (fields 1 to 6), 2 (fields 7 to 11), 3 (fields 12 to 17), and 4 (fields 18 to 22). A one-way analysis of variance allowed us to compare the difference in amebae numbers between groups. (a) In the absence of a chemoattractant (agarose with iTY-1 [without serum]), the population was distributed homogeneously along the coverslip (P = 0.8). When the agarose slice was filled with C5a (6 nM) or TNF (50 nM), there was a displacement of the population up the gradient toward the test molecule source (C5a, P = 8.7 × 10−3; TNF, P = 1.6 × 10−4). In addition, a pairwise comparison using t tests between groups was applied. In the C5a gradient, the mean ameba number was significantly different between groups 1 and 4 (P = 0.008), groups 2 and 4 (P = 0.011), and groups 3 and 4 (P = 0.025), with an accumulation of amebae near the source of C5a. The mean per group increased along the gradient of TNF and was significantly different between groups 1 and 3 (P = 0.025), groups 1 and 4 (P = 0.015), groups 2 and 3 (P < 10−4), and groups 2 and 4 (P = 0.03). In contrast, for iTY-1 no significant difference between groups was found, in accord with an absence of chemotactic migration. (b) In the presence of a gradient of IL-1β (source, 50 nM) there was no displacement of the population (P = 0.08). When TNF (50 nM; 0.88 μg/ml) was incubated with a monoclonal anti-TNF antibody (500 μg/ml) (TNF + Ab-1) or with the soluble TNF receptor I (4 μg/ml) (TNF + sTNFRI) prior to injection into the agarose cube, chemotaxis was abrogated (P = 0.14 and P = 0.16, respectively). However, when TNF was incubated with a monoclonal antibody (500 μg/ml) not specific for human TNF (TNF + nsAb), chemotaxis toward the TNF source was not inhibited (P = 0.003). Total numbers of ameba analyzed: iTY-1, n = 3,303; IL-1β, n = 4,022; C5a, n = 8,385; TNF, n = 7,871; TNF + Ab-1, n = 7,527; TNF + sTNFRI, n = 1,677; TNF + nsAb, n = 1,927. (c) Normalized cumulative distribution of cell orientations. The distributions of orientations of cells moving toward C5a (6 nM) and TNF (50 nM) were significantly different from the distribution in iTY-1 (P < 0.0006 for C5a and P < 0.0025 for TNF). In absence of chemotaxis (iTY-1), the median angle value was 54.0°. In presence of a C5a or TNF gradient, the median angle values were 28.2° and 38.8°, respectively, showing that the majority of cells oriented preferentially along the gradient, as expected for chemotaxis. Total numbers of amebae analyzed: iTY-1, n = 86; C5a, n = 68; TNF, n = 112.
Since TNF is not known to be a chemoattractant for cells and since the TNF is purified from Escherichia coli, we used another cytokine also purified from E. coli, recombinant human IL-1β (R&D Systems) (50 nM), to ensure that no contaminating bacterial molecules such as lipopolysaccharide could be attracting the trophozoites. In parallel, we assayed the capacity of purified lipopolysaccharide from E. coli (O111B4; Sigma), at the maximum concentration (2 ng/ml) present in the 50 nM TNF solution, to attract the trophozoites. In both cases, there was no significant difference between the groups (P = 0.08 [Fig. 2b] and P = 0.27 [data not shown], respectively). To confirm that TNF was the only molecule responsible for chemotaxis, TNF (50 nM; 0.88 μg/ml) was incubated with (i) a monoclonal anti-TNF antibody, Ab-1 (clone J1D9; Neomarkers) (500 μg/ml), that is able to block TNF cytolytic activity and/or binding sites or (ii) the soluble TNF receptor I/Fc chimera (sTNFRI) (R&D Systems) (4 μg/ml) prior to injection into the agarose cube. When TNF was bound to Ab-1 or to sTNFRI, chemotactic migration was abrogated (Fig. 2b); the variance analysis of amebae showed that there was no significant difference between groups (P = 0.14 and P = 0.16, respectively). To verify whether nonspecific binding was involved in the inhibition of chemotactic migration, TNF was incubated with an isotypic monoclonal antibody (500 μg/ml) not specific for human TNF. As shown in Fig. 2b and confirmed by the variance analysis between the groups (P = 0.003), trophozoites in presence of a nonspecific antibody were still capable of chemotactic migration toward the source of TNF.
During chemotaxis, cell shape polarizes toward the chemoattractant source, and the cell's longitudinal axis tends to orient parallel to the chemotactic gradient (7). To examine the orientation of the cell axis near the chemoattractant source, the images (fields 20, 21, and 22) obtained with the mosaic option described above were used. Cell orientations were defined by manually drawing an axis of elongation (the longest axis) on each cell. The absolute value of the angle (ranging from 0 to 90 degrees) between the cell axis and the horizontal reference direction (Fig. 1b) was computed, and the cumulative distributions of the angles of the cell population in iTY-1, C5a, and TNF were generated (Fig. 2c). A two-sample, two-tailed Kolmogorov-Smirnov test was used to compare the distribution of angles between trophozoites in gradients of C5a or TNF and iTY-1. The angle distribution in a gradient of C5a (P = 0.0006) or TNF (P = 0.0025) was significantly different from the distribution in iTY-1. In addition, the parasites tended to reorient their cell axis parallel to the gradient of C5a and TNF, as shown by the calculated median angles (28.2° and 38.8°, respectively), which were smaller than the median angle of parasites in iTY-1 (54.0°) (Fig. 2c). These results confirmed that polarized E. histolytica cells were able to orient their migration up the TNF gradient.
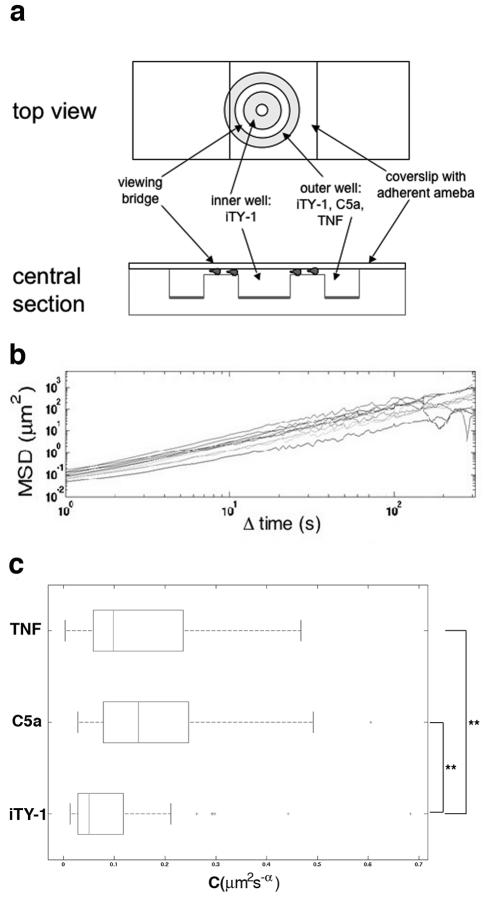
To study the dynamic parameters of cell migration, we performed a single-cell analysis of chemotaxis using a Dunn chemotaxis chamber (Weber, England) (2, 16, 20) (Fig. 3a). We checked that the gradient was formed and was linear after 25 min and was stable for over 3 h, using 10-kDa dextran Alexa Fluor 488 (Molecular Probes) (data not shown). Trophozoites were tracked in image sequences of 300 frames (one frame per second) using a computer program developed in house (21). The processed sequences corresponded to the period from 25 to 30 min after injection of the molecule into the Dunn chemotaxis chamber. Data were pooled from five experiments for each condition (iTY-1, C5a, and TNF). The recorded cell trajectories enabled us to compute the mean square displacements against time (Fig. 3b), from which we then extracted a motility rate for each cell, as previously described (6). For a given, fixed value of α, the coefficient C (μm2 s−α) can be regarded as a measure of the rate of movement. The values of rate of movement in a C5a (P = 2 × 10−6) or a TNF (P = 2 × 10−3) gradient were significantly different from the values in the control (iTY-1 alone), as confirmed by the Mann-Whitney test. The median motility rates for trophozoites in the presence of C5a (0.16 μm2 s−α) or TNF (0.10 μm2 s−α) gradients were 3 times and 2 times higher, respectively, than the median for iTY-1 (0.05 μm2 s−α) (Fig. 3c), indicating an enhanced rate of movement for the cells in presence of a C5a or TNF gradient.
FIG. 3.
TNF has a chemokinetic effect on E. histolytica. (a) Dunn chemotaxis chamber (20). The two circular wells were filled with iTY-1. A suspension of amebae was placed on a coverslip, and amebae were allowed to adhere for 10 min at 37°C. The coverslip was turned over and placed on the chamber so that the amebae bathed in the iTY-1. Once the chamber was set up, the outer well was emptied using a syringe and refilled with iTY-1 or the test molecule, C5a (6 nM) or TNF (30 nM). Phase-contrast images of moving cells were recorded by video microscopy at 1-second intervals for 45 min under a Zeiss inverted light microscope using a 10× objective in transmission mode. (b) Mean square displacements for 10 individual cells (different lines), calculated from the parasite trajectories obtained by computerized tracking (21) of the 300 images from 25 to 30 min. The approximate linearity of these curves on a logarithmic scale means that the mean square displacements fit well to power laws 〈(Δr2)〉 = C(Δt)α. The roughly constant exponents α ≈ 1.5 were equivalent to those found for E. histolytica in the absence of chemotactic gradients (6), thus indicating that E. histolytica chemotactic motility is superdiffusive. The constancy of the exponent enables us to use the coefficient C to measure each cell's rate of movement. (c) Rate of movement of E. histolytica during chemotaxis. Box plots of C for cells in iTY-1, C5a, and TNF (n = 58) are shown. E. histolytica trophozoites present in C5a and TNF gradients have a significantly higher rate of movement than trophozoites in iTY-1 (3 and 2 times, respectively). **, significantly different, with α < 0.01. Total numbers of ameba analyzed: iTY-1, n = 53; C5a, n = 43; TNF, n = 58.
The results of the present study demonstrate that TNF is a chemotactic and chemokinetic stimulus for E. histolytica. They also confirm the chemoattractant effect of C5a for E. histolytica and reveal its chemokinetic effect.
It is known that TNF can either promote or prevent immune cell migration, depending on its environmental context (3, 9, 14). TNF also stimulates the motility of human mammary epithelial cells (in part via the epidermal growth factor receptor) and induces autocrine signaling in this cell type (5). The mechanisms by which TNF induces E. histolytica chemotaxis and enhances trophozoite motility require further exploration. However, at least two hypotheses can be considered: TNF could bind an amebic surface component that either (i) activates (directly or through cross talk with a receptor) a signaling pathway involved in the regulation of motility or (ii) induces the release of extracellular factors promoting migration and/or facilitating passage through the extracellular matrix (ECM). TNF secreted from a site of injury can exist in a soluble form but can also interact with ECM components such as fibronectin and laminin (3). Interestingly, during amebiasis, macrophages and intestinal epithelial cells secrete TNF (18), suggesting that it may exist free in solution in the lumen and/or bound to ECM components.
Studies with severe combined immunodeficient mice with human intestinal xenografts (SCID-HU-INT mice) have shown the importance of TNF in the pathogenesis of amebiasis. First, the transcriptional analysis of human colonic xenografts after infection with E. histolytica showed increased expression of genes that are activated by TNF (such as IL-1β, IL-8, and IL-6) and also expressed in ulcerative colitis or Crohn's disease (19). Second, the involvement of TNF in the pathogenesis of amebiasis was studied in SCID-HU-INT mice by using chimerized monoclonal antibodies to TNF. The blockade of TNF by these antibodies not only reduced inflammation, with a significant decrease in IL-1β and IL-8 production, but also reduced intestinal damage and decreased neutrophil influx (18).
In conclusion, our results highlight a novel role for TNF: the molecule could generate critical chemotactic and chemokinetic signals which modulate the behavior of E. histolytica within the human colon by (i) stimulating the migration of commensal E. histolytica, (ii) delimiting the area of invasive lesions (microscopy studies show that trophozoites are usually clumped at the opening of an ulcer) (10), and (iii) facilitating the parasite's navigation through the ECM. TNF may have a dual role in amebiasis: orchestrating the host inflammatory response and attracting trophozoites. Potentially, chemotaxis toward TNF, during invasive amebiasis, could guide E. histolytica in the human tissue environment, leading to tissue destruction and dissemination of the parasite. This dual role now needs to be studied further in vitro and ex vivo by imaging the ameba's ability to penetrate human tissues. This should provide data on the role of TNF in the outcome of the disease. In addition our findings will open up new avenues for testing TNF antagonists (such as those already used in treatment of inflammatory disease) as inhibitors of parasite chemotaxis.
Acknowledgments
We thank S. Marion (Institut Pasteur) for manuscript advice and E. Perret (Dynamic Imaging Platform, Institut Pasteur) for help with microscopy experiments.
This study was supported by a “Programme Transversal de Recherche” grant from the Institut Pasteur (PTR 12).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Acevado, J. A., J. Pacheco-Yépez, J. Serrano-Luna, M. Espinosa-Cantallano, V. Tsutsumi, and M. Shibayama. 2000. Experimental amebic liver abscess: in vivo localization of TNF-α. Arch. Med. Res. 31:S98-S100. [DOI] [PubMed] [Google Scholar]
- 2.Allen, W. E., D. Zicha, A. J. Ridley, and G. E. Jones. 1998. A role for Cdc42 in macrophage chemotaxis. J. Cell Biol. 141:1147-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alon, R., L. Cahalon, R. Hershkoviz, D. Elbaz, B. Reizis, D. Wallach, S. K. Akiyama, K. M. Yamada, and O. Lider. 1994. TNF-alpha binds to the N-terminal domain of fibronectin and augments the beta 1-integrin-mediated adhesion of CD4+ T lymphocytes to the glycoprotein. J. Immunol. 152:1304-1313. [PubMed] [Google Scholar]
- 4.Bailey, G. B., G. J. Leitch, and D. B. Day. 1985. Chemotaxis by Entamoeba histolytica. J. Protozool. 32:341-346. [DOI] [PubMed] [Google Scholar]
- 5.Chen, W. N., R. L. Woodbury, L. E. Kathmann, L. K. Opresko, R. C. Zangar, H. S. Wiley, and B. D. Thrall. 2004. Induced autocrine signaling through the epidermal growth factor receptor contributes to the response of mammary epithelial cells to tumor necrosis factor alpha. J. Biol. Chem. 279:18488-18496. [DOI] [PubMed] [Google Scholar]
- 6.Coudrier, E., F. Amblard, C. Zimmer, P. Roux, J.-C. Olivo-Marin, M.-C. Rigothier, and N. Guillén. 2004. Myosin II and the Gal-GalNAc lectin play a crucial role in tissue invasion by Entamoeba histolytica. Cell Microbiol. 7:19-27. [DOI] [PubMed] [Google Scholar]
- 7.Eisenbach, M. 2004. Chemotaxis, p. 1-6. Imperial College Press, London, England.
- 8.Franco, E., J. Vazquez-Prado, and I. Meza. 1997. Fibronectin-derived fragments as inducers of adhesion and chemotaxis of Entamoeba histolytica trophozoites. J. Infect. Dis. 176:1597-1602. [DOI] [PubMed] [Google Scholar]
- 9.Issekutz, T. B. 1995. In vivo blood monocyte migration to acute inflammatory reactions, IL-1 alpha, TNF-alpha, IFN-gamma, and C5a utilizes LFA-1, Mac-1, and VLA-4. The relative importance of each integrin. J. Immunol. 154:6533-6540. [PubMed] [Google Scholar]
- 10.Martinez-Palomo, A., V. Tsutsumi, F. Anaya-Velazquez, and A. Gonzalez-Robles. 1989. Ultrastructure of experimental intestinal invasive amebiasis. Am. J. Trop. Med. Hyg. 41:273-279. [PubMed] [Google Scholar]
- 11.Stanley, S. L. 2001. Pathophysiology of amoebiasis. Trends Parasitol. 17:280-285. [DOI] [PubMed] [Google Scholar]
- 12.Stanley, S. L., Jr. 2003. Amoebiasis. Lancet 361:1025-1034. [DOI] [PubMed] [Google Scholar]
- 13.Urban, T., C. Jarstrand, and A. Aust-Kettis. 1983. Migration of Entamoeba histolytica under agarose. Am. J. Trop. Med. Hyg. 32:733-737. [DOI] [PubMed] [Google Scholar]
- 14.Vaday, G. G., R. Hershkoviz, M. A. Rahat, N. Lahat, L. Cahalon, and O. Lider. 2000. Fibronectin-bound TNF-alpha stimulates monocyte matrix metalloproteinase-9 expression and regulates chemotaxis. J. Leukoc. Biol. 68:737-747. [PubMed] [Google Scholar]
- 15.Wang, W., K. Keller, and K. Chadee. 1992. Modulation of tumor necrosis factor production by macrophages in Entamoeba histolytica infection. Infect. Immun. 60:3169-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb, S., J. Pollard, and G. Jones. 1996. Direct observation and quantification of macrophage chemoattraction to the growth factor CSF-1. J. Cell Sci. 109:793-803. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. 1997. WHO/PAHO/UNESCO report of a consultation of experts on amoebiasis. WHO Wkly. Epidemiol. Rec. 72:97-100. [Google Scholar]
- 18.Zhang, Z., S. Mahajan, X. Zhang, and S. L. Stanley, Jr. 2003. Tumor necrosis factor alpha is a key mediator of gut inflammation seen in amebic colitis in human intestine in the SCID mouse-human intestinal xenograft model of disease. Infect. Immun. 71:5355-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang, Z., and S. L. Stanley. 2004. Stereotypic and specific elements of the human colonic response to Entamoeba histolytica and Shigella flexneri. Cell. Microbiol. 6:535-554. [DOI] [PubMed] [Google Scholar]
- 20.Zicha, D., G. A. Dunn, and A. F. Brown. 1991. A new direct-viewing chemotaxis chamber. J. Cell Sci. 99:769-775. [DOI] [PubMed] [Google Scholar]
- 21.Zimmer, C., E. Labruyère, V. Meas-Yedid, N. Guillén, and J. C. Olivo-Marin. 2002. Segmentation and tracking of migrating cells in videomicroscopy with parametric active contours: a tool for cell-based drug testing. IEEE Trans. Med. Imaging 21:1210-1220. [DOI] [PubMed] [Google Scholar]