Abstract
Macrophages from inbred chickens that are resistant to salmonellosis show greater and more rapid expression of proinflammatory chemokines and cytokines, including the key Th1-inducing cytokine interleukin-18, upon Salmonella challenge than those from susceptible birds. This suggests the possibility that salmonellosis resistant-line macrophages signal more effectively and rapidly and are more able to induce protective Th1 adaptive responses.
Genetic resistance to systemic salmonellosis in the chicken is dependent on a number of factors, including slc11a1 (Nramp1), the major histocompatibility complex, Toll-like receptor 4, and a novel genetic locus termed SAL1 (7, 11, 13-15, 21). Previous studies of inbred White Leghorn chickens have shown that, of these factors, SAL1 plays the greatest role in experimental infection with Salmonella enterica serovar Gallinarum, the causative agent of fowl typhoid, and to a lesser extent, following infection with Salmonella enterica serovar Typhimurium (15, 21). In these studies, birds that were resistant to Salmonella showed decreased mortality and morbidity and on postmortem examination they showed small granuloma-like lesions in their livers relative to the large necrotic lesions shown in susceptible birds (15, 21). No difference in initial invasion or colonization of the gastrointestinal tract was found, suggesting minimal intestinal involvement, but bacterial numbers increased rapidly in the spleens and livers of susceptible birds, suggesting that differences in systemic innate immunity played a major role, and subsequent studies showed differences in in vitro biology of macrophages from Salmonella-resistant and -susceptible inbred chickens (21). While no difference in uptake was found between lines, resistant W1-line macrophages cleared bacteria within 24 to 48 h of infection, whereas Salmonella persisted in the susceptible 72-line cells (21). Macrophages from the resistant line produced a strong oxidative response to Salmonella, whereas little or no detectable response was found upon challenge in macrophages from the susceptible line, though macrophages from both lines responded equally well to nonspecific stimuli. These findings suggest that macrophages play a significant role in resistance to systemic salmonellosis in the chicken. The importance of the survival of Salmonella serovar Gallinarum within chicken macrophages is illustrated by the complete attenuation of strains with a mutation in the Salmonella pathogenicity island 2 type III secretion system and that survive poorly within chicken macrophages (8). The role of heterophils, avian polymorphonuclear cells, as mediators of genetic resistance has also been investigated in lines of broiler chickens, indicating a strong correlation between heterophil function and resistance to Salmonella enterica serovar Enteritidis infection (19).
The role of adaptive immunity in Salmonella resistance in chickens has only recently begun to be explored. A number of candidate genes, including T-cell markers, cytokines, and immunoglobulin genes, have shown linkage to resistance (5, 11, 12). Single-nucleotide polymorphisms have been identified in a number of genes, including CD28 and Tlr4 genes, that appear to be associated with resistance (13), but as yet, little immune function has been ascribed to Salmonella resistance. Signaling through cytokines and chemokines is likely to play a major role in both the activation of innate immunity and the subsequent development of the adaptive response. Differential expression of the cytokines interleukin-6 (IL-6) and IL-18 was described in inbred chicken lines that were resistant or susceptible to Marek's disease following infection with Marek's disease virus (10). Recently, differential expression of cytokines has been shown in Salmonella-resistant and -susceptible chicken line heterophils following Salmonella serovar Enteritidis challenge (20), with increased expression of the proinflammatory cytokines IL-6 and IL-8 and the Th1-associated cytokine IL-18 but significantly lower levels of the anti-inflammatory cytokine transforming growth factor β4 in cells from Salmonella-resistant birds in comparison to the susceptible-line cells. This suggested the possibility that resistant-line heterophils would be more effective in initiating both innate and Th1-mediated adaptive responses that appear to play a pivotal role in immunity to avian systemic salmonellosis (3, 23). Here we determine differences in the expression and kinetics of expression of a range of cytokines and chemokines by macrophages from Salmonella-resistant and -susceptible lines to in vitro challenge.
Primary macrophages were produced from monocytes isolated from heparinized blood taken from the wing vein of Salmonella-resistant or -susceptible chickens of 8 to 12 weeks of age. Specific-pathogen-free line W1 Salmonella-resistant and line 72 Salmonella-susceptible inbred White Leghorn chicks were obtained from the Poultry Production Unit, Institute for Animal Health, Compton, United Kingdom, and reared as described previously (21). To isolate peripheral blood monocytes, the blood was mixed with an equal volume of phosphate-buffered saline. Monocytes were isolated by centrifugation over Histopaque 1083 as previously described (21). Monocytes from each line, four birds for each experiment, were then pooled and cultured in supplemented RPMI 1640 for 48 h to obtain monocyte-derived macrophages (21). For both lines, cells were seeded to give a final concentration of 1 × 106 macrophages per ml in 24-well tissue culture plates, with each well containing 1 ml of cells. At this point, the culture medium was replaced by antibiotic-free medium and the cells were cultured for 4 h prior to challenge.
Spontaneous nalidixic acid-resistant mutants of the well-characterized strains Salmonella enterica serovar Gallinarum 9 and Salmonella enterica serovar Typhimurium F98 were used for macrophage challenge (2, 17, 18, 21, 25). Strains were maintained as glycerol stocks at −70°C and grown for 18 h in Luria-Bertani broth at 37°C in an orbital shaking incubator at 150 rpm. Macrophages were challenged with nonopsonized Salmonella serovar Gallinarum 9 or serovar Typhimurium F98 at a multiplicity of infection (MOI) of 10 Salmonella bacteria per macrophage as described previously (8, 22). The numbers of Salmonella bacteria that were taken up by or surviving within macrophages at 20 min and 1 and 4 h postchallenge were determined by a gentamicin protection assay as previously described (21). To obtain macrophage RNA, macrophages were challenged in parallel as described above. At 20 min, 1 h, and 4 h postinfection, supernatants were removed and then 350 μl of RLT lysis buffer from a QIAGEN RNeasy mini kit was added to each well and agitated to homogenize the cell sheet. The cell homogenates were removed and stored at −70°C prior to isolation of macrophage RNA. At each time point, cell homogenates were produced from unchallenged cells as controls. Each challenge experiment was performed in triplicate using different batches of isolated macrophages from different birds for three repeats for RNA expression. RNA was isolated from cell homogenates in RLT buffer by using RNeasy mini kits following the manufacturer's instructions. Isolated RNA was stored at −70°C until required. The expression levels of cytokine mRNA in control and Salmonella-challenged macrophages for the proinflammatory cytokines IL-1β and IL-6 (9), the Th1 cytokine IL-18 (10), the chemokine CXCLi1 (K60), and the MIP family CC chemokine CCLi2 (24, 25) were determined using previously described probes, primers, and conditions (9, 24, 25). Differences in RNA levels between samples were corrected against 28S rRNA levels as previously described (9). Results are expressed as differences (n-fold) between Salmonella-challenged samples and uninfected controls. Statistical analysis of mean values between groups was determined by analysis of variance using Microsoft Excel 2002 SP3. Significance was taken as P of <0.05.
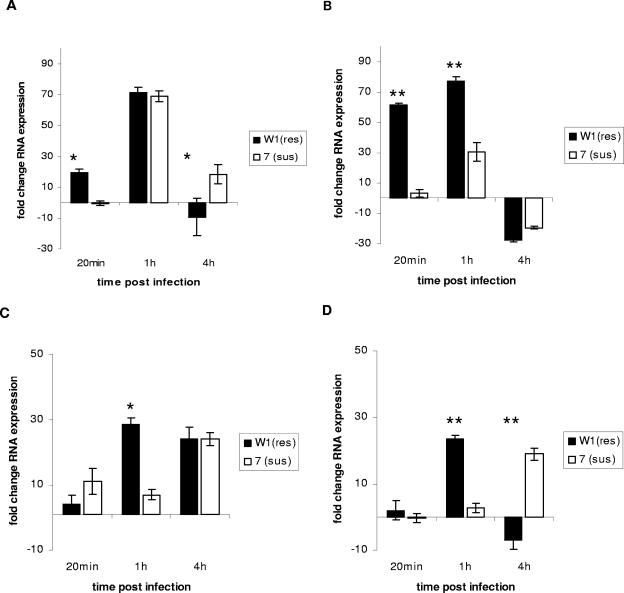
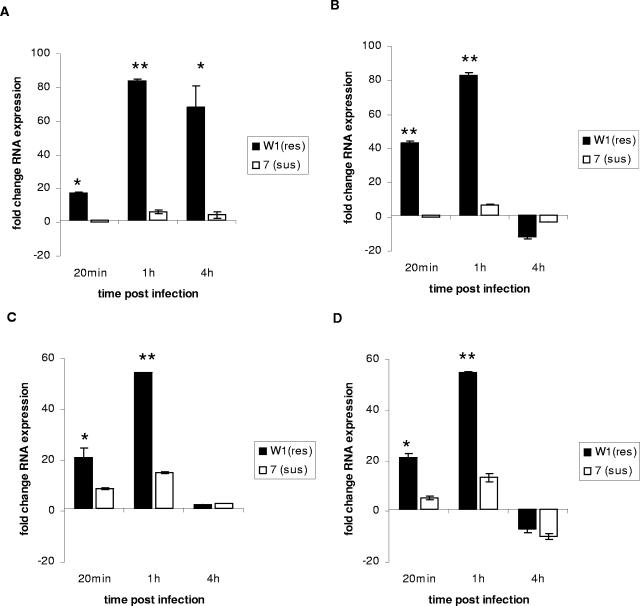
As described previously (21), Salmonella bacteria were taken up by both resistant- and susceptible-line macrophages in similar numbers, though significantly fewer Salmonella serovar Gallinarum bacteria were phagocytosed by cells from the susceptible 72-line relative to cells from the resistant line by 1 h after challenge (P = 0.02) (Table 1). Salmonella numbers declined significantly (P ≥ 0.05) in the resistant W1 macrophages between 1 and 4 h postinfection in resistant W1 cells, but macrophages from susceptible 72 line chickens remained at the same level or increased, which was consistent with previous studies (21). The expression levels of cytokines and chemokines were markedly different between chicken lines in both magnitude of expression and kinetics. Although macrophages from both resistant- and susceptible-line chickens showed similar levels of expression for IL-1β, with up to 70-fold increases in expression after 1 h (Fig. 1A) following Salmonella serovar Gallinarum challenge, expression following Salmonella serovar Typhimurium challenge was more rapid, with an increase of greater than 60-fold after 20 min and one higher still in macrophages from the Salmonella-resistant W1 line chickens after 1 h (Fig. 1B). The expression of IL-6 was significantly greater (P = 0.022) in macrophages from resistant-line W1 chickens at 1 h postchallenge with both serovars (Fig. 1C), though there appeared to be significant down-regulation of both IL-6 and IL-1 expression at 4 h postinfection following Salmonella serovar Typhimurium challenge in macrophages from the resistant line (Fig. 1B and D). Both CCLi2 and CXCLi1 chemokines were expressed at significantly higher levels by macrophages from the Salmonella-resistant line than by macrophages from the susceptible line following challenge by both serovars (P < 0.05). Expression of CXCLi1 mRNA was also quicker in macrophages from the Salmonella-resistant line (Fig. 2A and C), with significant expression detected at 20 min postchallenge with both serovars, while there was also more rapid expression of the MIP family CC chemokine CCLi2 by macrophages from the resistant line following Salmonella serovar Typhimurium challenge (Fig. 2B and D). The differences in these responses are consistent with the phenotype of increased resistance to experimental infection and more rapid killing of Salmonella by macrophages of the resistant W1 chicken line (21). Recently, differences in expression of cytokines in heterophils from resistant and susceptible broiler chickens following Salmonella challenge have been described (19). These studies indicated increased expression of the proinflammatory cytokines IL-6 and IL-8 and the Th1 cytokine IL-18 in Salmonella-resistant lines. In this study, we show increased expression of proinflammatory cytokines and chemokines in response to Salmonella challenge. This study also demonstrates that expression of proinflammatory signals is more rapid in macrophages from Salmonella-resistant chickens, with rapid expression of IL-1β, IL-6, and CXC found in the challenged line W1 cells. These findings suggest that, upon stimulation by Salmonella, macrophages from the resistant line are able to express proinflammatory cytokines more rapidly and at a greater level. In chickens, as in mammals, expression of these cytokines would lead to increased proinflammatory activity, including an increased influx of polymorphonuclear cells, increased macrophage activation, and in the case of IL-6, activation of lymphocytes. Such a response would be consistent with the pathology and cellular changes found following experimental infection of resistant-line chickens.
TABLE 1.
Salmonella uptake and survival in monocyte-derived macrophages from Salmonella-resistant and -susceptible inbred lines of chickensa
| Time postinfection | Mean log10 CFU (SEM) of Salmonella for:
|
|||
|---|---|---|---|---|
| W1 (resistant)
|
72 (susceptible)
|
|||
| Serovar Gallinarum 9 | Serovar Typhimurium F98 | Serovar Gallinarum 9 | Serovar Typhimurium F98 | |
| 20 min | 2.74 (0.17) | 4.71 (0.07) | 3.31 (0.33) | 4.69 (0.09) |
| 1 h | 4.28 (0.11) | 5.05 (0.29) | 3.42 (0.05) | 4.63 (0.08) |
| 4 h | 3.38 (0.21) | 4.13 (0.14) | 3.91 (0.23) | 4.64 (0.09) |
Results shown are as determined by a gentamicin protection assay (n = 6).
FIG. 1.
Expression of interleukin-1β (A and B) and interleukin-6 (C and D) by monocyte-derived macrophages from Salmonella-resistant (res) and-susceptible (sus) inbred chicken lines challenged with Salmonella serovar Gallinarum (A and C) or Salmonella serovar Typhimurium (B and D) at an MOI of 10. Expression was determined by quantitative reverse transcriptase (qRT)-PCR from RNA isolated in triplicate challenges from three repeats of macrophages pooled from four different birds for each repeat. Significant differences in expression between chicken lines at a particular time point (P ≤ 0.05) are indicated by an asterisk; highly significant differences (P ≤ 0.01) are indicated by a double asterisk (n = 9). Error bars indicate standard errors of the means.
FIG. 2.
Expression of the CXC chemokine CXCLi1 (K60) (A and B) and the MIP-family CC chemokine CCLi2 (C and D) by monocyte-derived macrophages from Salmonella-resistant and -susceptible inbred chicken lines challenged with Salmonella serovar Gallinarum (A and C) or Salmonella serovar Typhimurium (B and D) at an MOI of 10. Expression was determined by qRT-PCR from RNA isolated in triplicate challenges from three repeats of macrophages pooled from four different birds for each repeat. Significant differences in expression between chicken lines at a particular time point (P ≤ 0.05) are indicated by an asterisk; highly significant differences (P ≤ 0.01) are indicated by a double asterisk (n = 9). Error bars indicate standard errors of the means.
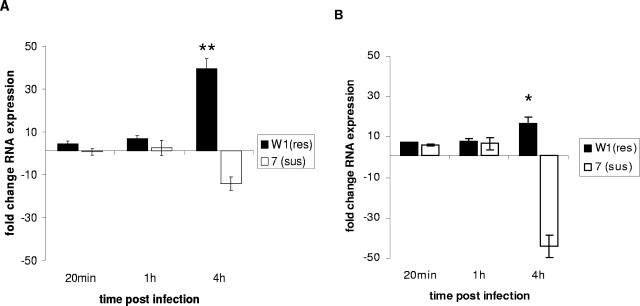
Limited expression of IL-18 was found in both lines at 1 h postchallenge, with higher levels of expression following Salmonella serovar Typhimurium challenge (Fig. 3) at 4 h postchallenge. Significantly higher expression was observed in macrophages from the resistant line W1 (P = 0.019). This was most pronounced in the macrophages challenged with Salmonella serovar Gallinarum. In contrast, a decrease in expression was observed in macrophages from the susceptible line 72. These differences in IL-18 (a cytokine expressed particularly by activated macrophages) expression is particularly interesting. The roles of IL-12 and IL-18 are pivotal in the immunity to primary Salmonella serovar Typhimurium infections of mice in the initiation of gamma interferon (IFN-γ) production by Th1 lymphocytes and NK cells (16). Initiation of such a response is crucial in the clearance of intracellular pathogens, including Salmonella, mycobacteria, and trypanosomes (6). Recently, the roles of T cells and IFN-γ in the clearance of primary Salmonella serovar Typhimurium systemic infections of chickens (3, 4, 24) and in the clearance of the live attenuated Salmonella serovar Gallinarum vaccine strain 9R (23) have been determined. The data here indicate that macrophages from the Salmonella-resistant line express significantly higher levels of IL-18 than do susceptible-line cells. As well as increased antimicrobial activity to Salmonella, macrophages from the resistant W1 line may be more efficient in initiating an adaptive response that leads to the eventual clearance of Salmonella from the spleen and liver. In general, the expression of cytokines and chemokines was more rapid in Salmonella serovar Typhimurium-challenged cells than in Salmonella serovar Gallinarum-challenged cells. Salmonella serovar Typhimurium was taken up by or invaded macrophages more rapidly than thenonmotile, nonflagellated Salmonella serovar Gallinarum (Table 1). Salmonella serovar Gallinarum is generally regarded as poorly invasive in host cells (1), primarily as a consequence of its poor motility. It appears that Salmonella serovar Typhimurium will invade cells more rapidly and efficiently in vitro than will Salmonella serovar Gallinarum. This may go some way to explaining the generally slower response to Salmonella serovar Gallinarum in vitro, though as Salmonella serovar Gallinarum is highly invasive to the spleen and liver in vivo, such differences may not occur during infection.
FIG. 3.
Expression of interleukin-18 by monocyte-derived macrophages from Salmonella-resistant and -susceptible inbred chicken lines challenged with Salmonella serovar Gallinarum (A) or Salmonella serovar Typhimurium (B) at an MOI of 10. Expression was determined by qRT-PCR from RNA isolated in triplicate challenges from three repeats of macrophages pooled from four different birds for each repeat. Significant differences in expression between chicken lines at a particular time point (P ≤ 0.05) are indicated by an asterisk; highly significant differences (P ≤ 0.01) are indicated by a double asterisk (n = 9). Error bars indicate standard errors of the means.
In this study, we have shown that macrophages from chickens that are genetically resistant or susceptible to systemic salmonellosis display differential expression of cytokines to Salmonella serovar Gallinarum and Salmonella serovar Typhimurium challenge in vitro. The findings are consistent with the infection biology of Salmonella in the lines used and with previous studies indicating that macrophages from resistant-line chickens are more efficient in killing Salmonella both in vitro and in vivo. The data presented here suggest that macrophages from resistant-line chickens are capable of rapid expression of proinflammatory cytokines and chemokines following challenge and that the macrophages become activated more quickly. In addition, the differences in expression of IL-18 are intriguing, suggesting that macrophages from resistant-line chickens are more efficient in the initiation of IFN-γ-dependent adaptive immune responses. This would suggest that resistant-line chickens not only have increased innate immunity to Salmonella infection but also are more capable of stimulating a protective adaptive immune response.
Acknowledgments
We thank the Biotechnology and Biological Sciences Research Council and the European Union for funding programs relating to this work.
Editor: F. C. Fang
Footnotes
Sadly, Nat Bumstead passed away during this study. We fondly dedicate this paper to his memory.
REFERENCES
- 1.Barrow, P. A., and M. A. Lovell. 1989. Invasion of Vero cells by Salmonella species. J. Med. Microbiol. 28:59-67. [DOI] [PubMed] [Google Scholar]
- 2.Barrow, P. A., J. F. Tucker, and J. M. Simpson. 1987. Inhibition of colonization of the chicken alimentary tract with Salmonella typhimurium gram-negative facultatively anaerobic bacteria. Epidemiol. Infect. 98:311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beal, R. K., C. Powers, P. Wigley, P. A. Barrow, and A. L. Smith. 2004. Temporal dynamics of the cellular, humoral and cytokine responses in chickens during primary and secondary infection with Salmonella enterica serovar Typhimurium. Avian Pathol. 33:25-33. [DOI] [PubMed] [Google Scholar]
- 4.Beal, R. K., P. Wigley, C. Powers, S. D. Hulme, P. A. Barrow, and A. L. Smith. 2004. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet. Immunol. Immunopathol. 100:151-164. [DOI] [PubMed] [Google Scholar]
- 5.Cheeseman, J. H., M. G. Kaiser, and S. J. Lamont. 2004. Genetic line effect on peripheral blood leukocyte cell surface marker expression in chickens. Poult. Sci. 83:911-916. [DOI] [PubMed] [Google Scholar]
- 6.Holscher, C. 2004. The power of combinatorial immunology: IL-12 and IL-12-related dimeric cytokines in infectious diseases. Med. Microbiol. Immunol. 193:1-17. [DOI] [PubMed] [Google Scholar]
- 7.Hu, J., N. Bumstead, P. Barrow, G. Sebastiani, L. Olien, K. Morgan, and D. Malo. 1997. Resistance to salmonellosis in the chicken is linked to NRAMP1 and TNC. Genome Res. 7:693-704. [DOI] [PubMed] [Google Scholar]
- 8.Jones, M. A., P. Wigley, K. L. Page, S. D. Hulme, and P. A. Barrow. 2001. Salmonella enterica serovar Gallinarum requires the Salmonella pathogenicity island 2 type III secretion system but not the Salmonella pathogenicity island 1 type III secretion system for virulence in chickens. Infect. Immun. 69:5471-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser, P., L. Rothwell, E. E. Galyov, P. A. Barrow, J. Burnside, and P. Wigley. 2000. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology 146:3217-3226. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser, P., G. Underwood, and F. Davison. 2003. Differential cytokine responses following Marek's disease virus infection of chickens differing in resistance to Marek's disease. J. Virol. 77:762-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer, J., M. Malek, and S. J. Lamont. 2003. Association of twelve candidate gene polymorphisms and response to challenge with Salmonella enteritidis in poultry. Anim. Genet. 34:339-348. [DOI] [PubMed] [Google Scholar]
- 12.Lamont, S. J., M. G. Kaiser, and W. Liu. 2002. Candidate genes for resistance to Salmonella enteritidis colonization in chickens as detected in a novel genetic cross. Vet. Immunol. Immunopathol. 87:423-428. [DOI] [PubMed] [Google Scholar]
- 13.Leveque, G., V. Forgetta, S. Morroll, A. L. Smith, N. Bumstead, P. Barrow, J. C. Loredo-Osti, K. Morgan, and D. Malo. 2003. Allelic variation in TLR4 is linked to susceptibility to Salmonella enterica serovar Typhimurium infection in chickens. Infect. Immun. 71:1116-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu, W., M. G. Kaiser, and S. J. Lamont. 2003. Natural resistance-associated macrophage protein 1 gene polymorphisms and response to vaccine against or challenge with Salmonella enteritidis in young chicks. Poult. Sci. 82:259-266. [DOI] [PubMed] [Google Scholar]
- 15.Mariani, P., P. A. Barrow, H. H. Cheng, M. M. Groenen, R. Negrini, and N. Bumstead. 2001. Localization to chicken chromosome 5 of a novel locus determining salmonellosis resistance. Immunogenetics 53:786-791. [DOI] [PubMed] [Google Scholar]
- 16.Mastroeni, P., and N. Menager. 2003. Development of acquired immunity to Salmonella. J. Med. Microbiol. 52:453-459. [DOI] [PubMed] [Google Scholar]
- 17.Smith, H. W. 1956. The use of live vaccines in experimental Salmonella gallinarum infection in chickens with observations on their interference effect. J. Hyg. 54:419-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith, H. W., and J. F. Tucker. 1975. The effect of antibiotic therapy on the faecal excretion of Salmonella typhimurium by experimentally infected chickens. J. Hyg. 75:275-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swaggerty, C. L., M. H. Kogut, P. J. Ferro, L. Rothwell, I. Y. Pevzner, and P. Kaiser. 2004. Differential cytokine mRNA expression in heterophils isolated from Salmonella-resistant and -susceptible chickens. Immunology 113:139-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swaggerty, C. L., I. Y. Pevzner, V. K. Lowry, M. B. Farnell, and M. H. Kogut. 2003. Functional comparison of heterophils isolated from commercial broiler chickens. Avian Pathol. 32:95-102. [DOI] [PubMed] [Google Scholar]
- 21.Wigley, P., S. D. Hulme, N. Bumstead, and P. A. Barrow. 2002. In vivo and in vitro studies of genetic resistance to systemic salmonellosis in the chicken encoded by the SAL1 locus. Microbes Infect. 4:1111-1120. [DOI] [PubMed] [Google Scholar]
- 22.Wigley, P., M. A. Jones, and P. A. Barrow. 2002. Salmonella enterica serovar Pullorum requires the Salmonella pathogenicity island 2 type III secretion system for virulence and carriage in the chicken. Avian Pathol. 31:501-506. [DOI] [PubMed] [Google Scholar]
- 23.Wigley, P., S. D. Hulme, C. Powers, R. Beal, A. Smith, and P. Barrow. 2005. Oral infection with the Salmonella enterica serovar Gallinarum 9R attenuated live vaccine as a model to characterise immunity to fowl typhoid in the chicken. BMC Vet. Res. 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Withanage G. S. K., P. Wigley, P. Kaiser, C. Powers, P. Mastroeni, H. Brooks, P. Barrow, A. Smith, D. Maskell, and I. McConnell. 2005. Cytokine and chemokine responses associated with clearance of a primary Salmonella enterica serovar Typhimurium infection in the chicken and in protective immunity to rechallenge. Infect. Immun. 73:5173-5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Withanage, G. S. K., P. Kaiser, P. Wigley, C. Powers, P. Mastroeni, H. Brooks, P. Barrow, A. Smith, D. Maskell, and I. McConnell. 2004. Rapid expression of chemokines and proinflammatory cytokines in newly hatched chickens infected with Salmonella enterica serovar Typhimurium. Infect. Immun. 72:2152-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]