Abstract
While coagulation often causes pathology during infectious disease, we recently demonstrated that fibrin, a product of the coagulation pathway, performs a critical protective function during acute toxoplasmosis (L. L. Johnson, K. N. Berggren, F. M. Szaba, W. Chen, and S. T. Smiley, J. Exp. Med. 197:801-806, 2003). Here, we investigate the mechanisms regulating the formation of this protective fibrin. Through comparisons of Toxoplasma-infected wild-type and cytokine-deficient mice we dissociate, for the first time, the relative fibrin-regulating capacities of pathogen products, host cytokines, and infection-stimulated hemorrhage. Remarkably, neither the pathogen burden nor hemorrhage is a primary regulator of fibrin levels. Rather, two type 1 cytokines exert dominant and counterregulatory roles: tumor necrosis factor alpha (TNF-α), acting via the type 1 TNF-α receptor, promotes fibrin deposition, while gamma interferon (IFN-γ), acting via STAT1 and IFN-γ receptors expressed on radioresistant cells, suppresses fibrin deposition. These findings have important clinical implications, as they establish that cytokines known to regulate pathological coagulation also dictate levels of protective fibrin deposition. We present a novel model depicting mechanisms by which the immune system can destroy infected tissue while independently restraining hemorrhage and promoting tissue repair through the deliberate deposition of protective fibrin.
Hemorrhage exposes plasma to extravascular tissue, thereby activating well-studied hemostatic coagulation pathways that culminate in the deposition of fibrin, an insoluble matrix that curtails blood loss and provides a scaffold for tissue repair (15). Coagulation and fibrin deposition also frequently accompany infection. In that setting, coagulation often functions pathologically: excessive and/or aberrant coagulation promotes abscess and adhesion formation during peritonitis (1, 31, 43, 44), prompts thrombotic microangiopathy during infection by enterohemorrhagic Escherichia coli (33), and contributes to multiorgan failure during sepsis (26). Deciphering the precise molecular mechanisms underlying infection-associated coagulation should advance efforts to generate effective therapeutics for coagulopathy. Plausible coagulation-promoting stimuli include hemorrhage and lesser vascular trauma as well as pathogens, their products, and host cytokines (48, 50, 54). Despite extensive research on their individual coagulation-promoting capacities, the relative contributions of these stimuli, which are often present simultaneously during infectious disease, have yet to be dissociated.
Coagulation can also function protectively during infection. Fibrin restricts the growth and dissemination of bacteria in vivo (1, 11-13, 35, 43, 58), and we recently demonstrated that fibrin performs a distinct, but equally important, protective function during acute toxoplasmosis. Mammals naturally acquire toxoplasmosis by ingesting encysted Toxoplasma gondii organisms (30). Following ingestion, these obligate intracellular protozoan parasites invade the intestinal wall and then disseminate throughout the tissues of the host. In the well-studied murine model, T. gondii infection elicits a robust type 1 immune response that abruptly restricts parasite replication but also causes significant collateral pathology (8, 23, 27, 28). Wild-type mice deposit fibrin within hepatic and gastrointestinal tissue during the acute phase of T. gondii infection and survive the collateral pathology (23). In contrast, fibrin-deficient mice exhibit profound hemorrhage and acutely succumb to the infection (23). Thus, fibrin prevents lethal hemorrhage during acute toxoplasmosis, thereby performing a critical protective function.
The mechanisms regulating fibrin deposition in mice during acute toxoplasmosis presumably reflect evolutionarily selected host-pathogen relationships: mice, like humans, are natural hosts for Toxoplasma (30), and coagulant processes function protectively and resolve naturally during T. gondii infection (23). We anticipate that the pathways that balance coagulation during toxoplasmosis become pathologically exacerbated during other infections, thereby causing coagulopathy. As such, we have begun to decipher the mechanisms regulating fibrin deposition during T. gondii infection. Given that hemorrhage accompanies acute toxoplasmosis (23), we initially anticipated that hemorrhage prompts fibrin deposition. However, fibrin is readily detectable by day 4 after the initiation of T. gondii infection, whereas hemorrhage is not evident until after day 6 (23). To investigate this paradox, here we compare the relative fibrin-promoting capacities of hemorrhage, pathogen burden, and host cytokines during toxoplasmosis. Remarkably, we demonstrate that immunity exerts powerful and primary control over fibrin deposition, apparently overriding the conventional hemostatic mechanisms activated by hemorrhage. We present a model that highlights the protective attributes of immune-associated coagulation and has important implications with regard to therapeutics aimed at suppressing coagulopathy.
MATERIALS AND METHODS
Mice.
Wild-type (WT) C57BL/6 mice were either purchased from The Jackson Laboratory (Bar Harbor, ME) or Taconic (Germantown, NY) or were bred at Trudeau Institute. C57BL/6-backcrossed gamma interferon (IFN-γ)-, IFN-γ receptor (IFN-γR)-, tumor necrosis factor alpha (TNF-α)-, TNF-α receptor 1 (TNFR1)-, TNFR2-, interleukin-12 (IL-12) p35-, and IL-12 p40-deficient mice were purchased from The Jackson Laboratory. Signal transducer and activator of transcription 1 (STAT1)-deficient and control 129S6/SvEv mice were purchased from Taconic. Chimeric mice were generated as described previously (41). In brief, 6-week-old recipients were exposed to 1,000 rads at 93 rads/min from a 137Cs source, administered as two doses separated by 4 h. Immediately after this split-dose irradiation procedure, the mice were reconstituted with 1 × 107 donor bone marrow cells. All animal experiments were reviewed and approved by the Trudeau Institute Animal Care and Use Committee.
Infections.
Mice were infected by peroral inoculation of 10 ME49 T. gondii cysts. Cysts were prepared from brains of chronically infected C57BL/6 mice as previously described (23). Sham-infected mice received equal volumes of brain suspension from uninfected C57BL/6 mice. Where indicated, mice were treated with monoclonal antibody (MAb) specific for IFN-γ (clone XMG1.2) or TNF-α (clone XT3.11) or with an isotype-matched control rat IgG1 MAb (RIgG; clone HRPN) (23). MAb were administered intraperitoneally as a 1-mg dose the day prior to infection, and cytokine depletion was assessed by performing enzyme-linked immunosorbent assay on plasma samples obtained at day 8 after the initiation of infection. All mice were infected when they were between 6 and 8 weeks old, except for chimeric mice, which were infected at 7 weeks after bone marrow reconstitution.
Measurement of fibrin deposition, hematocrits, fecal occult blood, parasite burden, and cytokine levels.
Fibrin levels were determined by quantitative Western blotting of tissue harvested from mice treated with heparin (500 U, intravenously) just prior to euthanasia by carbon dioxide narcosis (23). Hematocrits were determined by a Coulter Counter (23). Fecal occult blood, a sensitive indicator of gastrointestinal bleeding (42), was detected using the Hemoccult assay (Beckman Coulter) following the instructions provided by the manufacturer. Parasite burden was measured in liver tissue by determining levels of T. gondii p30 mRNA by real-time PCR and interpolating parasite numbers per milligram of tissue from a standard curve (23). Tissue levels of IFN-γ and TNF-α mRNA were measured in liver tissue by real-time PCR, normalized to levels of beta-2-microglobulin mRNA, and expressed as fold change relative to levels in uninfected WT mice (23).
Statistics.
Because some values were below the detection limits of our assays, data are routinely depicted as median and interquartile range and therefore were analyzed by nonparametric statistical methods. Specifically, statistical analyses were performed using the computer program Prism 4.0 (GraphPad Software, Inc.) using either the Mann Whitney test or the Kruskal-Wallis with Dunn's multiple comparison post test, as appropriate. Significant differences among frequencies of mice testing positive for occult blood were analyzed by the Chi-square test.
RESULTS
IFN-γ promotes hemorrhage but reduces pathogen burden and fibrin deposition during acute T. gondii infection.
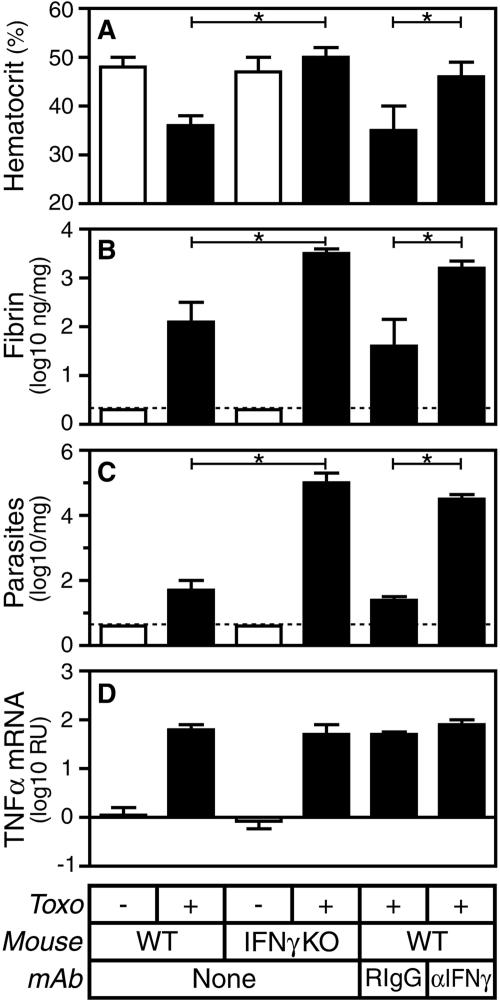
Fibrin-deficient C57BL/6 mice exhibit profound, IFN-γ-mediated anemia and overt hepatic hemorrhage on day 8 after oral infection with small doses of T. gondii cysts (23). Although less severely affected than fibrin-deficient mice, WT C57BL/6 mice also exhibit readily detectable, IFN-γ-dependent anemia and hemorrhage during acute toxoplasmosis. As shown in Fig. 1A, the hematocrit, a measure of circulating red cell numbers, is significantly reduced on day 8 after the initiation of T. gondii infection in WT mice but not in gene-targeted IFN-γ-deficient mice or WT mice treated with MAb specific for IFN-γ (P < 0.0005 for both). Likewise, 60% of WT mice produce stool samples that test positive for occult gastrointestinal blood on day 8 after the initiation of T. gondii infection, whereas 0% of IFN-γ-deficient mice produce positive samples (n = 15; P < 0.01). We conclude that overt hemorrhage accompanies toxoplasmosis in WT mice but not in IFN-γ-deficient mice.
FIG. 1.
IFN-γ deficiency suppresses hemorrhage but increases pathogen burden and fibrin deposition during acute T. gondii infection (Toxo). C57BL/6 WT or IFN-γ-deficient (IFN-γ knockout [KO]) mice were sham infected (open bars) or infected perorally with 10 ME49 T. gondii cysts (closed bars). Where indicated, WT mice were treated with IFN-γ-specific MAb (αIFN-γ) or isotype-matched rat IgG1 control MAb the day prior to infection. On day 8 after the initiation of infection, we measured hematocrits (A) and assayed levels of hepatic fibrin (B), parasite burden (C), and TNF-α mRNA (D). Data depict the median and interquartile range. Dashed lines depict limits of detection. Statistical comparisons were performed between T. gondii-infected WT and IFN-γ-deficient mice as well as between T. gondii-infected WT mice treated with IFN-γ-specific MAb or control rat IgG1 (RIgG; n = 9 to 11 mice per group; *, P < 0.0005). RU, relative units.
Hemorrhage exposes plasma to extravascular tissue, thereby activating hemostatic coagulant pathways that promote fibrin deposition (15). To evaluate the importance of hemorrhage as a fibrin-producing stimulus during toxoplasmosis, we compared levels of fibrin deposition in hemorrhage-prone WT mice and hemorrhage-resistant IFN-γ-deficient mice. Despite their resistance to T. gondii infection-stimulated hemorrhage, hepatic fibrin deposition actually increases more than 20-fold in both gene-targeted IFN-γ-deficient mice and WT mice treated with MAb specific for IFN-γ (Fig. 1B). As IFN-γ-deficient mice exhibit significantly less hemorrhage than WT mice (Fig. 1A) but significantly more fibrin deposition (Fig. 1B; P < 0.0005), we conclude that some stimulus other than hemorrhage must be a primary regulator of fibrin deposition during acute toxoplasmosis. In addition, we conclude that IFN-γ suppresses fibrin deposition during T. gondii infection.
In parallel with measurements of fibrin deposition, we also measured cytokine levels and the parasite burden within infected liver tissue. IFN-γ deficiency has little impact on levels of TNF-α mRNA, but consistent with prior reports (46), IFN-γ deficiency increases the parasite burden approximately 1,000-fold during T. gondii infection (Fig. 1C and 1D).
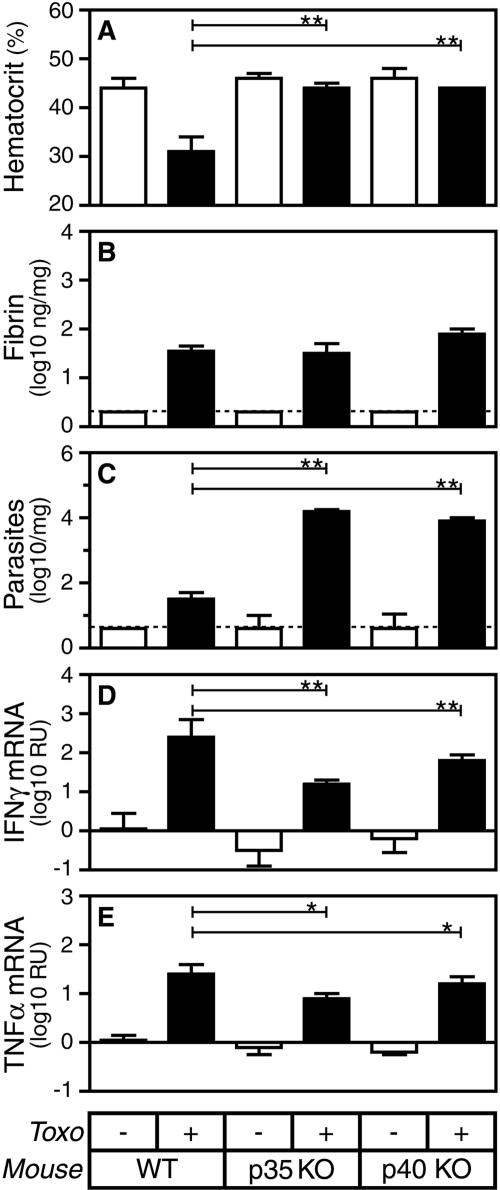
IL-12 promotes hemorrhage and reduces pathogen burden but does not significantly impact fibrin deposition during acute T. gondii infection.
IL-12 contributes to the production of IFN-γ during acute T. gondii infection (18). To evaluate whether IL-12 contributes to fibrin deposition during T. gondii infection, we measured levels of hepatic fibrin in gene-targeted mice lacking the capacity to produce the IL-12 p35 or p40 subunits. Like IFN-γ deficiency, IL-12 deficiency significantly reduces hemorrhage, as revealed by measurements of hematocrits (Fig. 2A; P < 0.001) and fecal occult blood (data not shown). Also like IFN-γ deficiency, and as reported previously (18), IL-12 deficiency increases the parasite burden (Fig. 2C; P < 0.001), presumably because IL-12-deficient mice produce reduced levels of IFN-γ and TNF-α (Fig. 2D and E; P < 0.001 and P < 0.05, respectively). However, in striking contrast to IFN-γ deficiency, IL-12 deficiency does not affect fibrin levels during acute toxoplasmosis (Fig. 2B). Thus, in comparison with WT mice, IFN-γ- and IL-12-deficient mice both display decreased hemorrhage and increased parasite burden, yet they differ dramatically from one another with respect to levels of fibrin deposition (Table 1). Together, these findings indicate that neither hemorrhage nor pathogen burden is a primary regulator of fibrin deposition during T. gondii infection.
FIG. 2.
IL-12 deficiency suppresses hemorrhage and increases pathogen burden but does not significantly impact fibrin deposition during acute T. gondii infection (Toxo). WT, IL-12 p35-deficient, and IL-12 p40-deficient mice were sham infected (open bars) or infected perorally with 10 ME49 T. gondii cysts (closed bars). On day 8 after the initiation of infection, we measured hematocrits (A) and assayed levels of hepatic fibrin (B), parasite burden (C), IFN-γ mRNA (D), and TNF-α mRNA (E). Data depict the median and interquartile range. Dashed lines depict limits of detection. Statistical comparisons were performed between T. gondii-infected WT and IL-12 p35-deficient mice as well as between T. gondii-infected WT and IL-12 p40-deficient mice (n = 5 to 10 mice per group; *, P < 0.05; **, P < 0.001). KO, knockout. RU, relative units.
TABLE 1.
Dissociation of roles for hemorrhage, pathogen burden, and cytokines in the regulation of fibrin deposition during acute T. gondii infection
| Mousea | Fibrin | Hemorrhageb | Parasites | IFN-γ | TNF-α |
|---|---|---|---|---|---|
| WT | ++ | ++ | ++ | ++ | ++ |
| IFN-γ deficient | ++++ | − | ++++ | − | ++ |
| IL-12 deficient | ++ | − | ++++ | + | + |
| TNF-α deficient | +/− | ++ | +++ | ++ | − |
| IFN-γ/TNF-α deficient | ++++c | − | ++++ | − | − |
Data are compiled from multiple experiments. Levels of hepatic fibrin deposition, parasite burden, IFN-γ, and TNF-α were quantified as in Fig. 1. A semiquantitative scoring system was applied: ++++, more than 10-fold greater than levels detected in WT mice; +++, 2- to 10-fold greater than levels detected in WT mice; ++, equivalent to levels detected in WT mice; +, below levels detected in WT mice but above the assay's detection limit; −, not detected.
Hemorrhage denotes evidence for both hematocrit reductions (i.e., anemia) and positive tests for fecal occult blood (i.e., bleeding).
As depicted in Fig. 5, fibrin levels in IFN-γ/TNF-α-deficient mice are greatly elevated in comparison with WT mice yet significantly lower than those observed in IFN-γ-deficient mice.
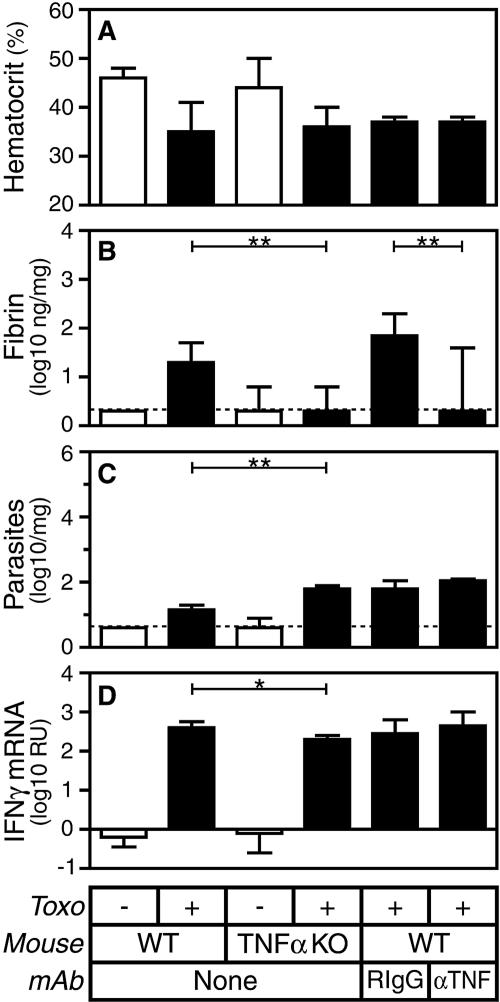
TNF-α does not contribute to hemorrhage and only modestly affects pathogen burden but promotes fibrin deposition during acute T. gondii infection.
As TNF-α promotes fibrin deposition in models of sepsis and peritonitis (12, 19, 29, 53), the disparate levels of fibrin deposition in T. gondii-infected IL-12- and IFN-γ-deficient mice could potentially reflect their disparate levels of TNF-α mRNA. Indeed, in comparison with WT control mice, both gene-targeted TNF-α-deficient mice and WT mice treated with TNF-α-specific MAb display greatly reduced fibrin deposition during T. gondii infection (Fig. 3B; P < 0.005 for both). However, TNF-α deficiency does not significantly impact levels of hemorrhage (Fig. 3A) and only modestly affects pathogen burden (Fig. 3C) and levels of IFN-γ mRNA (Fig. 3D). These findings further dissociate levels of fibrin deposition from hemorrhage and pathogen burden (Table 1). Moreover, they reveal that TNF-α promotes fibrin deposition during T. gondii infection.
FIG. 3.
TNF-α deficiency does not alter hemorrhage and only modestly impacts pathogen burden but reduces fibrin deposition during acute T. gondii infection (Toxo). WT or TNF-α-deficient mice were sham infected (open bars) or infected perorally with 10 ME49 T. gondii cysts (closed bars). Where indicated, WT mice were treated with TNF-α-specific MAb or isotype-matched rat IgG1 control MAb the day prior to infection. On day 8 after the initiation of infection, we measured hematocrits (A) and assayed levels of hepatic fibrin (B), parasite burden (C), and IFN-γ mRNA (D). Data depict the median and interquartile range. Dashed lines depict limits of detection. Statistical comparisons were performed between T. gondii-infected WT and TNF-α-deficient mice as well as between T. gondii-infected WT mice treated with TNF-α-specific MAb or control rat IgG1 (n = 8 to 13 mice per group; *, P < 0.05; **, P < 0.005). KO, knockout.
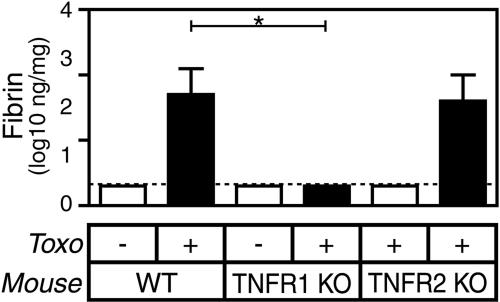
Two molecularly distinct receptors transmit TNF-α-mediated signals. As shown in Fig. 4, gene-targeted mice lacking TNFR1, like TNF-α-deficient animals, deposit significantly lower levels of fibrin during T. gondii infection (P < 0.0001). In contrast, gene-targeted mice lacking TNFR2 deposit WT levels of fibrin. We conclude that TNFR1 transmits the fibrin-promoting effects of TNF-α during T. gondii infection.
FIG. 4.
TNFR1 deficiency but not TNFR2 deficiency reduces fibrin deposition during acute T. gondii infection (Toxo). WT, TNFR1-deficient, and TNFR2-deficient mice were sham infected (open bars) or infected perorally with 10 ME49 T. gondii cysts (closed bars). Levels of hepatic fibrin were measured on day 8 after the initiation of infection. Data depict the median and interquartile range (n = 10 to 15 mice per group). The dashed line depicts the limit of detection. In comparison with T. gondii-infected WT mice, T. gondii-infected TNFR1-deficient mice produce significantly less fibrin (P < 0.0001) but TNFR2-deficient mice do not. KO, knockout.
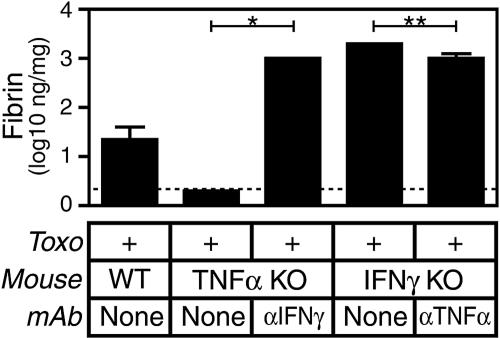
IFN-γ and TNF-α counterregulate fibrin deposition.
During T. gondii infection, mice lacking the capacity to produce either IFN-γ or TNF-α display increased or reduced levels of fibrin deposition, respectively (Fig. 1 and 3). To further assess the interplay between these cytokines and to assess whether TNF-α is a requisite stimulus for fibrin deposition during toxoplasmosis, we generated mice deficient in both TNF-α and IFN-γ by treating gene-targeted mice with neutralizing MAb. As depicted in Table 1, mice rendered deficient in both TNF-α and IFN-γ display suppressed hemorrhage and increased pathogen burden compared to those of WT mice. Mice rendered deficient in both TNF-α and IFN-γ also exhibit significantly elevated levels of infection-stimulated fibrin deposition compared to those of WT mice (Fig. 5, Table 1; P < 0.01). However, administration of MAb specific for TNF-α only moderately suppresses fibrin levels in IFN-γ-deficient mice (Fig. 5; P < 0.01), whereas administration of MAb specific for IFN-γ dramatically elevates fibrin levels in TNF-α-deficient mice (Fig. 5; P < 0.05). We conclude that TNF-α and IFN-γ counterregulate fibrin deposition during T. gondii infection and that the fibrin-promoting phenotype of IFN-γ deficiency dominates the fibrin-suppressing phenotype of TNF-α deficiency. In addition, we conclude that fibrin deposition does not absolutely require expression of TNF-α, as fibrin is readily apparent in gene-targeted TNF-α-deficient mice treated with IFN-γ-specific MAb.
FIG. 5.
IFN-γ and TNF-α counterregulate fibrin deposition during acute T. gondii infection (Toxo). WT, IFN-γ-deficient, or TNF-α-deficient mice were infected perorally with 10 ME49 T. gondii cysts. Where indicated, mice were treated with IFN-γ- or TNF-α-specific MAb the day prior to infection. On day 8 after the initiation of infection, we measured levels of hepatic fibrin deposition. Data depict the median and interquartile range. The dashed line depicts the limit of detection. In comparison with T. gondii-infected TNF-α-deficient mice, T. gondii-infected TNF-α-deficient mice treated with IFN-γ-specific MAb produce significantly greater levels of fibrin (n = 3 to 5 mice/group; *, P < 0.05). In comparison with T. gondii-infected IFN-γ-deficient mice, T. gondii-infected IFN-γ-deficient mice treated with TNF-α-specific MAb produce significantly less fibrin (n = 5 mice/group; **, P < 0.01). In comparison with WT animals, mice lacking both TNF-α and IFN-γ produce significantly elevated levels of fibrin (n = 8 to 10 mice per group; P < 0.01). KO, knockout.
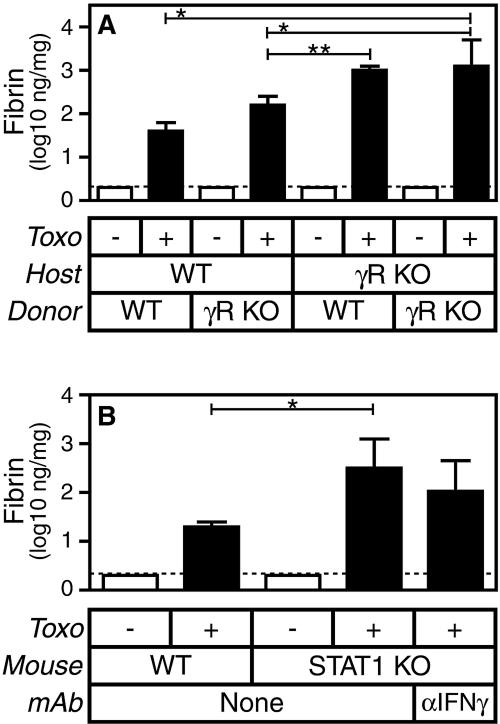
IFN-γ suppresses fibrin deposition via IFN-γ receptors expressed by radioresistant cells.
The IFN-γR is expressed by a wide variety of cell types, including both leukocytes and parenchymal cells. To begin characterizing the cell type that regulates levels of fibrin deposition in response to IFN-γ, we generated chimeric mice in which only certain cells could express the IFN-γR. Specifically, we lethally irradiated WT and IFN-γR-deficient “host” mice and then reconstituted their radiosensitive cells with “donor” bone marrow derived from either WT or IFN-γR-deficient mice. In so doing, we generated chimeric mice in which only radioresistant cells, only radiosensitive cells, both cell types, or neither cell type could express IFN-γR. We then infected these four types of chimeric mice with T. gondii and evaluated levels of infection-stimulated fibrin deposition.
Consistent with our analyses of IFN-γ-deficient mice (Fig. 1B), mice lacking expression of the IFN-γR on both host and donor cells deposit greatly increased levels of fibrin during T. gondii infection compared to mice expressing the IFN-γR on both host and donor cells (Fig. 6A; P < 0.001). Among the infected mixed chimeras, IFN-γR-deficient hosts reconstituted with WT bone marrow produce significantly more fibrin than WT hosts reconstituted with IFN-γR-deficient bone marrow (P < 0.01). We conclude that expression of the IFN-γR by radioresistant cells is most critical to suppressing fibrin deposition.
FIG. 6.
The suppression of fibrin deposition by IFN-γ during acute T. gondii infection (Toxo) is STAT1 dependent and primarily mediated by IFN-γR (γR) expressed by radioresistant cells. (A) Bone marrow chimeras were generated from WT and IFN-γR-deficient mice, as indicated. After reconstitution, mice were sham infected (open bars) or infected perorally with 10 ME49 T. gondii cysts (closed bars). Levels of hepatic fibrin were measured on day 8 after the initiation of infection. Data depict the median and interquartile range (n = 10 mice per group). The dashed line depicts the limit of detection. WT hosts reconstituted with either WT or IFN-γR-deficient bone marrow produce significantly less fibrin than IFN-γR-deficient hosts reconstituted with IFN-γR-deficient bone marrow (*, P < 0.001). By contrast, IFN-γR-deficient hosts produce similar levels of fibrin, regardless of whether they are reconstituted with WT or IFN-γR-deficient bone marrow. Among the mixed chimeras, WT hosts reconstituted with IFN-γR-deficient bone marrow produce significantly less fibrin than did IFN-γR-deficient hosts reconstituted with WT bone marrow (**, P < 0.01). (B) WT and STAT1-deficient mice were sham infected (open bars) or infected perorally with 10 ME49 T. gondii cysts (closed bars). Where indicated, STAT1-deficient mice were treated with IFN-γ-specific MAb 1 day prior to infection. Levels of hepatic fibrin were measured on day 8 after the initiation of infection. Data depict the median and interquartile range (n = 15 mice per group, except for n = 5 for mice treated with IFN-γ-specific MAb). The dashed line depicts the limit of detection. T. gondii-infected STAT1-deficient mice produce significantly more fibrin than WT mice (P < 0.0001) but do not further increase levels of fibrin upon treatment with IFN-γ-specific MAb. KO, knockout. RU, relative units.
STAT1 suppresses fibrin deposition during acute T. gondii infection.
The transcription factor STAT1 is essential for many IFN-γR-associated antimicrobial activities during acute toxoplasmosis (17). However, IFN-γ also mediates STAT1-independent responses (40). Figure 6B demonstrates that levels of infection-stimulated fibrin deposition are significantly elevated in STAT1-deficient mice compared with genetically matched 129/SvEv WT mice (P < 0.0001). We conclude that STAT1, like IFN-γ, suppresses fibrin deposition during acute toxoplasmosis. Moreover, antibody-based neutralization of IFN-γ fails to further increase fibrin levels in STAT1-deficient mice (Fig. 6B), suggesting that IFN-γ suppresses fibrin deposition via a STAT1-dependent pathway.
DISCUSSION
Upon infection with T. gondii, C57BL/6 mice mount robust type 1 immune responses that limit parasite replication but also elicit significant collateral pathology (8, 27, 28). Previously, we demonstrated that IFN-γ causes profound hemorrhage during T. gondii infection in fibrin-deficient C57BL/6 mice (23). Here, we demonstrated that IFN-γ also causes significant hemorrhage during acute toxoplasmosis in WT mice. Together, these findings indicate that fibrin restrains hemorrhage caused by IFN-γ during the acute phase of T. gondii infection.
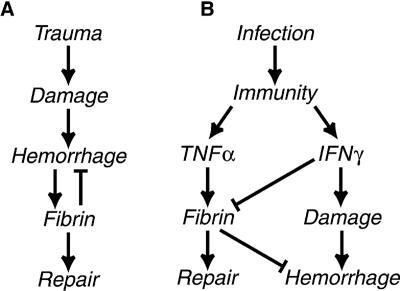
Hemorrhagic trauma activates conventional hemostatic pathways that culminate in the deposition of fibrin (Fig. 7A). Given that hemorrhage accompanies T. gondii infection, we anticipated that hemorrhage would likewise activate fibrin deposition during toxoplasmosis. However, kinetic studies revealed that fibrin deposition is readily detectable by day 4 after the initiation of T. gondii infection, whereas hemorrhage is not evident until after day 6 (23). To address this paradox, we investigated the relative fibrin-promoting capacities of hemorrhage, pathogen burden, and host cytokines. As presented collectively in Table 1, our analyses of mice lacking IFN-γ, IL-12, and/or TNF-α reveal that neither hemorrhage nor pathogen burden dictates fibrin levels during T. gondii infection. Rather, cytokines exert dominant regulatory roles.
FIG. 7.
Models depicting the disparate mechanisms that regulate fibrin deposition during trauma and infection. (A) The conventional trauma-activated hemostatic coagulation pathway. (B) Our proposed model of cytokine-regulated coagulation during infection: TNF-α promotes fibrin deposition while IFN-γ suppresses fibrin deposition and also promotes hemorrhage. Note that fibrin deposits in response to damage in the conventional hemostatic pathway, whereas fibrin can be deposited preemptively, thereby limiting hemorrhage and hastening tissue repair in the cytokine-regulated pathway.
As depicted in Table 1 and in the model in Fig. 7B, our data indicate that immunity provokes hemorrhage via IFN-γ while independently promoting the deposition of fibrin via TNF-α. IFN-γ-mediated hemorrhage presumably reflects either purposeful destruction of infected vascular cells or unintended collateral vascular damage. In either case, fibrin would limit the extent of immune-mediated hemorrhage, thereby functioning protectively. The key inference from our experimental observations is that immunity exerts deliberate and powerful control over the deposition of this protective fibrin. We propose that immunity does so in order to dictate the kinetics of this critical protective response. As such, immunity can preemptively deploy fibrin rather than waiting for tissue damage to secondarily activate fibrin deposition.
The model in Fig. 7B proposes that immunity not only segregates the tissue-damaging and tissue-protecting stimuli but also precisely regulates levels of fibrin deposition. Specifically, our data indicate that TNF-α promotes fibrin deposition while IFN-γ suppresses fibrin deposition. We suggest that this cytokine-mediated counterregulation of fibrin levels enables immunity to deliberately, powerfully, and precisely deploy protective fibrin without inciting coagulopathy. Notably, in the absence of IFN-γ, mice lacking TNF-α still produce fibrin (Fig. 5), indicating that additional stimuli can promote fibrin deposition under certain circumstances. Prior studies suggested that IL-1 and IL-6 can promote pathological coagulation (54). The murine toxoplasmosis model will permit detailed analyses of the relative contributions of these, and other, stimuli to the deposition of protective fibrin.
While we have thus far focused upon roles for fibrin in restraining hemorrhage, fibrin also has well-appreciated tissue repair functions that may play vital roles during infection (10). Indeed, histological examination of hepatic tissue during acute toxoplasmosis reveals large areas of coagulative necrosis in WT mice, which subsequently resolve as the infection is contained. In contrast, the infected hepatic tissue appears to disintegrate in the absence of fibrin, leaving hemorrhagic voids (23). It seems likely that this fibrin-dependent maintenance of tissue architecture must improve the rate of tissue repair during infection. Thus, by depositing fibrin while or even before inflicting damage, immunity almost certainly promotes tissue repair more effectively than could be accomplished if fibrin was only deposited in response to damage. In summary, we suggest that immunity must powerfully, deliberately, and precisely regulate fibrin deposition during infection in order to maintain tissue architecture, provide a scaffold for tissue repair, preemptively combat hemorrhage, and avoid coagulopathy.
We have begun to decipher the mechanisms by which TNF-α and IFN-γ counterregulate fibrin deposition during toxoplasmosis. We demonstrated an important role for TNF-α, acting via TNFR1, in promoting the deposition of protective fibrin (Fig. 3 and 4). Previous studies found that the administration of recombinant TNF-α promotes coagulation in humans via interactions with TNFR1, but not TNFR2 (2, 49, 51, 52, 55), and that TNF-α promotes the formation of pathological fibrin as well as fibrin-associated adhesions and abscesses in models of endotoxemia and peritonitis (12, 19, 29, 53). To our knowledge, the molecular pathways underlying TNF-α/TNFR1-stimulated coagulation have yet to be established in vivo. Prior observations suggest that TNF-α may promote fibrin deposition by stimulating vascular permeability (4, 5, 14), increasing expression of procoagulant molecules (3, 6, 37, 45), decreasing expression of anticoagulant molecules (37, 45), and/or suppressing fibrin degradation (9, 32, 56, 57). Investigating the TNFR1 dependency of these events in the toxoplasmosis model should help to establish their in vivo relevance with regard to infection-associated fibrin deposition.
Our finding that IFN-γ suppresses fibrin deposition during toxoplasmosis contrasts with prior reports that administration of recombinant IFN-γ reduces fibrinolytic activity, thereby potentially promoting fibrin deposition (20, 36). Moreover, cells exposed to IFN-γ in vitro upregulate fibrin-promoting activities (7, 34, 47). However, consistent with our observations, IFN-γ suppresses fibrin deposition during organ transplantation (21, 22), and Qiu et al. recently found that IFN-γ suppresses fibrin deposition and adhesion formation during bacterial peritonitis (38, 39). These findings do not dispute potential roles for IFN-γ as a fibrin-promoting cytokine but suggest that IFN-γ primarily antagonizes fibrin deposition in certain settings, including infection.
Therapeutic strategies aimed at manipulating IFN-γ must consider this cytokine's multiple roles during infection: while mediating protective immunity and promoting hemorrhage, it also suppresses fibrin deposition. In order to selectively manipulate these activities, IFN-γ-based therapeutics will need to specifically target the relevant underlying mechanisms. We anticipate that these mechanisms can be deciphered using the well-characterized murine toxoplasmosis model, where the multiple roles of IFN-γ, both protective and pathological, are readily apparent. Here, we have made several novel observations regarding the molecular pathways by which IFN-γ suppresses fibrin deposition (Fig. 6): (i) we found that radioresistant cells, rather than radiosensitive leukocytes, are primary mediators of the fibrin-suppressing effects of IFN-γ, (ii) we demonstrated an important role for STAT1 in the suppression of infection-stimulated fibrin deposition, and (iii) we found that treatment of STAT1-deficient mice with MAb specific for IFN-γ does not further increase fibrin deposition. Together, these observations suggest that IFN-γ suppresses fibrin deposition via a STAT1-dependent pathway operating in radioresistant cells, such as endothelial cells. Notably, expression of plasminogen activator inhibitor 1 (PAI-1) is STAT1 regulated (24), and IFN-γ suppresses expression of PAI-1 in endothelial cells (16). As PAI-1 antagonizes fibrin degradation, thereby promoting fibrin deposition, STAT1-mediated suppression of PAI-1 expression in endothelial cells may well account for the effects of IFN-γ on fibrin deposition during infection. Our laboratory is currently investigating this possibility through the application of sensitive assays that enable quantitative measurements of fibrin-promoting, fibrin-stabilizing, and fibrin-degrading activities in situ.
Finally, our findings have important implications for the treatment of infection-stimulated coagulopathy. First, our data strongly suggest that it is the immune response to the pathogen, not the pathogen itself, that primarily regulates coagulation during infection. This observation, together with clinical data indicating that diverse infectious agents elicit pathological coagulation (25), should bolster efforts to treat coagulopathy by focusing on the host immune response to infection rather than specific pathogen components. Second, our prior studies (23, 35), and those of others (1, 11-13, 43, 58), firmly establish that coagulation leading to fibrin deposition performs multiple protective functions during infection: fibrin restrains bacterial growth and dissemination, protects against hemorrhagic pathology, and presumably promotes tissue repair. As such, it stands to reason that therapeutics targeting coagulopathy should strive to limit, but not fully antagonize, coagulation-regulating cytokines and their downstream mediators. Lastly, in conjunction with prior studies (21, 22, 38, 39, 48, 50), our data indicate that TNF-α and IFN-γ counterregulate both protective and pathological coagulation during infection. Thus, further deciphering the pathways that regulate fibrin deposition in the well-characterized and readily manipulated murine toxoplasmosis model should speed efforts to develop effective therapies for coagulopathy.
Acknowledgments
This work was supported by National Institutes of Health grants HL72937 (S.T.S.), AI49823 (I.K.M.), and AI61587 (L.L.J.) and funds from Trudeau Institute.
We thank Andrea Cooper and David Woodland for critical reading of our manuscript and the employees of the Trudeau Institute Animal Breeding and Maintenance Facilities for dedicated care of the mice used in these studies.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Ahrenholz, D. H., and R. L. Simmons. 1980. Fibrin in peritonitis. I. Beneficial and adverse effects of fibrin in experimental E. coli peritonitis. Surgery 88:41-47. [PubMed] [Google Scholar]
- 2.Bauer, K. A., H. ten Cate, S. Barzegar, D. R. Spriggs, M. L. Sherman, and R. D. Rosenberg. 1989. Tumor necrosis factor infusions have a procoagulant effect on the hemostatic mechanism of humans. Blood 74:165-172. [PubMed] [Google Scholar]
- 3.Bevilacqua, M. P., J. S. Pober, G. R. Majeau, W. Fiers, R. S. Cotran, and M. A. Gimbrone, Jr. 1986. Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc. Natl. Acad. Sci. USA 83:4533-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brett, J., H. Gerlach, P. Nawroth, S. Steinberg, G. Godman, and D. Stern. 1989. Tumor necrosis factor/cachectin increases permeability of endothelial cell monolayers by a mechanism involving regulatory G proteins. J. Exp. Med. 169:1977-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clauss, M., C. Sunderkotter, B. Sveinbjornsson, S. Hippenstiel, A. Willuweit, M. Marino, E. Haas, R. Seljelid, P. Scheurich, N. Suttorp, M. Grell, and W. Risau. 2001. A permissive role for tumor necrosis factor in vascular endothelial growth factor-induced vascular permeability. Blood 97:1321-1329. [DOI] [PubMed] [Google Scholar]
- 6.Conway, E. M., R. Bach, R. D. Rosenberg, and W. H. Konigsberg. 1989. Tumor necrosis factor enhances expression of tissue factor mRNA in endothelial cells. Thromb. Res. 53:231-241. [DOI] [PubMed] [Google Scholar]
- 7.Del Prete, G., M. De Carli, R. M. Lammel, M. M. D'Elios, K. C. Daniel, B. Giusti, R. Abbate, and S. Romagnani. 1995. Th1 and Th2 T-helper cells exert opposite regulatory effects on procoagulant activity and tissue factor production by human monocytes. Blood 86:250-257. [PubMed] [Google Scholar]
- 8.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dosne, A. M., F. Dubor, F. Lutcher, M. Parant, and L. Chedid. 1988. Tumor necrosis factor (TNF) stimulates plasminogen activator inhibitor (PAI) production by endothelial cells and decreases blood fibrinolytic activity in the rat. Thromb. Res. (Suppl.) 8:115-122. [DOI] [PubMed] [Google Scholar]
- 10.Drew, A. F., H. Liu, J. M. Davidson, C. C. Daugherty, and J. L. Degen. 2001. Wound-healing defects in mice lacking fibrinogen. Blood 97:3691-3698. [DOI] [PubMed] [Google Scholar]
- 11.Dunn, D. L., and R. L. Simmons. 1982. Fibrin in peritonitis. III. The mechanism of bacterial trapping by polymerizing fibrin. Surgery 92:513-519. [PubMed] [Google Scholar]
- 12.Echtenacher, B., K. Weigl, N. Lehn, and D. N. Mannel. 2001. Tumor necrosis factor-dependent adhesions as a major protective mechanism early in septic peritonitis in mice. Infect. Immun. 69:3550-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flick, M. J., X. Du, D. P. Witte, M. Jirouskova, D. A. Soloviev, S. J. Busuttil, E. F. Plow, and J. L. Degen. 2004. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J. Clin. Investig. 113:1596-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedl, J., M. Puhlmann, D. L. Bartlett, S. K. Libutti, E. N. Turner, M. F. Gnant, and H. R. Alexander. 2002. Induction of permeability across endothelial cell monolayers by tumor necrosis factor (TNF) occurs via a tissue factor-dependent mechanism: relationship between the procoagulant and permeability effects of TNF. Blood 100:1334-1339. [PubMed] [Google Scholar]
- 15.Furie, B., and B. C. Furie. 1988. The molecular basis of blood coagulation. Cell 53:505-518. [DOI] [PubMed] [Google Scholar]
- 16.Gallicchio, M., P. Hufnagl, J. Wojta, and P. Tipping. 1996. IFN-gamma inhibits thrombin- and endotoxin-induced plasminogen activator inhibitor type 1 in human endothelial cells. J. Immunol. 157:2610-2617. [PubMed] [Google Scholar]
- 17.Gavrilescu, L. C., B. A. Butcher, L. Del Rio, G. A. Taylor, and E. Y. Denkers. 2004. STAT1 is essential for antimicrobial effector function but dispensable for gamma interferon production during Toxoplasma gondii infection. Infect. Immun. 72:1257-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gazzinelli, R. T., M. Wysocka, S. Hayashi, E. Y. Denkers, S. Hieny, P. Caspar, G. Trinchieri, and A. Sher. 1994. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 153:2533-2543. [PubMed] [Google Scholar]
- 19.Gibson, F. C., III, A. B. Onderdonk, D. L. Kasper, and A. O. Tzianabos. 1998. Cellular mechanism of intraabdominal abscess formation by Bacteroides fragilis. J. Immunol. 160:5000-5006. [PubMed] [Google Scholar]
- 20.Gluszko, P., A. Undas, S. Amenta, A. Szczeklik, and A. H. Schmaier. 1994. Administration of gamma interferon in human subjects decreases plasminogen activation and fibrinolysis without influencing C1 inhibitor. J. Lab. Clin. Med. 123:232-240. [PubMed] [Google Scholar]
- 21.Halloran, P. F., M. Afrouzian, V. Ramassar, J. Urmson, L. F. Zhu, L. M. Helms, K. Solez, and N. M. Kneteman. 2001. Interferon-gamma acts directly on rejecting renal allografts to prevent graft necrosis. Am. J. Pathol. 158:215-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halloran, P. F., L. W. Miller, J. Urmson, V. Ramassar, L. F. Zhu, N. M. Kneteman, K. Solez, and M. Afrouzian. 2001. IFN-gamma alters the pathology of graft rejection: protection from early necrosis. J. Immunol. 166:7072-7081. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, L. L., K. N. Berggren, F. M. Szaba, W. Chen, and S. T. Smiley. 2003. Fibrin-mediated protection against infection-stimulated immunopathology. J. Exp. Med. 197:801-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasza, A., D. L. Kiss, S. Gopalan, W. Xu, R. E. Rydel, A. Koj, and T. Kordula. 2002. Mechanism of plasminogen activator inhibitor-1 regulation by oncostatin M and interleukin-1 in human astrocytes. J. Neurochem. 83:696-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinasewitz, G. T., S. B. Yan, B. Basson, P. Comp, J. A. Russell, A. Cariou, S. L. Um, B. Utterback, P. F. Laterre, and J. F. Dhainaut. 2004. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism. Crit. Care 8:R82-R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levi, M., E. de Jonge, and T. van der Poll. 2004. New treatment strategies for disseminated intravascular coagulation based on current understanding of the pathophysiology. Ann. Med. 36:41-49. [DOI] [PubMed] [Google Scholar]
- 27.Liesenfeld, O., H. Kang, D. Park, T. A. Nguyen, C. V. Parkhe, H. Watanabe, T. Abo, A. Sher, J. S. Remington, and Y. Suzuki. 1999. TNF-alpha, nitric oxide and IFN-gamma are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 21:365-376. [DOI] [PubMed] [Google Scholar]
- 28.Liesenfeld, O., J. Kosek, J. S. Remington, and Y. Suzuki. 1996. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J. Exp. Med. 184:597-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathison, J. C., E. Wolfson, and R. J. Ulevitch. 1988. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J. Clin. Investig. 81:1925-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCabe, R. E., and J. S. Remington. 1990. Toxoplasma gondii, p. 2090-2103. In G. L. Mandell, R. G. Douglas, and J. E. Bennett (ed.), Principles and practices of infectious diseases, 3rd ed. Churchill Livingstone, Inc., New York, N. Y.
- 31.McRitchie, D. I., M. J. Girotti, M. F. Glynn, J. M. Goldberg, and O. D. Rotstein. 1991. Effect of systemic fibrinogen depletion on intraabdominal abscess formation. J. Lab. Clin. Med. 118:48-55. [PubMed] [Google Scholar]
- 32.Medcalf, R. L., E. K. Kruithof, and W. D. Schleuning. 1988. Plasminogen activator inhibitor 1 and 2 are tumor necrosis factor/cachectin-responsive genes. J. Exp. Med. 168:751-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moake, J. L. 2002. Thrombotic microangiopathies. N. Engl. J. Med. 347:589-600. [DOI] [PubMed] [Google Scholar]
- 34.Moon, D. K., and C. L. Geczy. 1988. Recombinant IFN-gamma synergizes with lipopolysaccharide to induce macrophage membrane procoagulants. J. Immunol. 141:1536-1542. [PubMed] [Google Scholar]
- 35.Mullarky, I. K., F. M. Szaba, K. N. Berggren, M. A. Parent, L. W. Kummer, W. Chen, L. L. Johnson, and S. T. Smiley. 2005. Infection-stimulated fibrin deposition controls hemorrhage and limits hepatic bacterial growth during listeriosis. Infect. Immun. 73:3888-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musial, J., P. Gluszko, A. Undas, F. Mahdi, S. Kang, A. Szczeklik, and A. H. Schmaier. 1998. Gamma interferon administration to patients with atopic dermatitis inhibits fibrinolysis and elevates C1 inhibitor. Thromb. Res. 89:253-261. [DOI] [PubMed] [Google Scholar]
- 37.Nawroth, P. P., and D. M. Stern. 1986. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J. Exp. Med. 163:740-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu, G., E. Gribbin, K. Harrison, N. Sinha, and K. Yin. 2003. Inhibition of gamma interferon decreases bacterial load in peritonitis by accelerating peritoneal fibrin deposition and tissue repair. Infect. Immun. 71:2766-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu, G., C. Wang, R. Smith, K. Harrison, and K. Yin. 2001. Role of IFN-gamma in bacterial containment in a model of intra-abdominal sepsis. Shock 16:425-429. [DOI] [PubMed] [Google Scholar]
- 40.Ramana, C. V., M. P. Gil, R. D. Schreiber, and G. R. Stark. 2002. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 23:96-101. [DOI] [PubMed] [Google Scholar]
- 41.Randall, T. D., F. E. Lund, M. C. Howard, and I. L. Weissman. 1996. Expression of murine CD38 defines a population of long-term reconstituting hematopoietic stem cells. Blood 87:4057-4067. [PubMed] [Google Scholar]
- 42.Rockey, D. C. 1999. Occult gastrointestinal bleeding. N. Engl. J. Med. 341:38-46. [DOI] [PubMed] [Google Scholar]
- 43.Rotstein, O. D. 1992. Role of fibrin deposition in the pathogenesis of intraabdominal infection. Eur. J. Clin. Microbiol. Infect. Dis. 11:1064-1068. [DOI] [PubMed] [Google Scholar]
- 44.Rotstein, O. D., and J. Kao. 1988. Prevention of intra-abdominal abscesses by fibrinolysis using recombinant tissue plasminogen activator. J. Infect. Dis. 158:766-772. [DOI] [PubMed] [Google Scholar]
- 45.Scarpati, E. M., and J. E. Sadler. 1989. Regulation of endothelial cell coagulant properties. Modulation of tissue factor, plasminogen activator inhibitors, and thrombomodulin by phorbol 12-myristate 13-acetate and tumor necrosis factor. J. Biol. Chem. 264:20705-20713. [PubMed] [Google Scholar]
- 46.Scharton-Kersten, T. M., T. A. Wynn, E. Y. Denkers, S. Bala, E. Grunvald, S. Hieny, R. T. Gazzinelli, and A. Sher. 1996. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 157:4045-4054. [PubMed] [Google Scholar]
- 47.Schwager, I., and T. W. Jungi. 1994. Effect of human recombinant cytokines on the induction of macrophage procoagulant activity. Blood 83:152-160. [PubMed] [Google Scholar]
- 48.Tapper, H., and H. Herwald. 2000. Modulation of hemostatic mechanisms in bacterial infectious diseases. Blood 96:2329-2337. [PubMed] [Google Scholar]
- 49.van der Poll, T., H. R. Buller, H. ten Cate, C. H. Wortel, K. A. Bauer, S. J. van Deventer, C. E. Hack, H. P. Sauerwein, R. D. Rosenberg, and J. W. ten Cate. 1990. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N. Engl. J. Med. 322:1622-1627. [DOI] [PubMed] [Google Scholar]
- 50.van der Poll, T., E. de Jonge, and M. Levi. 2001. Regulatory role of cytokines in disseminated intravascular coagulation. Semin. Thromb. Hemost. 27:639-651. [DOI] [PubMed] [Google Scholar]
- 51.van der Poll, T., P. M. Jansen, K. J. Van Zee, M. B. Welborn III, I. de Jong, C. E. Hack, H. Loetscher, W. Lesslauer, S. F. Lowry, and L. L. Moldawer. 1996. Tumor necrosis factor-alpha induces activation of coagulation and fibrinolysis in baboons through an exclusive effect on the p55 receptor. Blood 88:922-927. [PubMed] [Google Scholar]
- 52.van der Poll, T., M. Levi, H. R. Buller, S. J. van Deventer, J. P. de Boer, C. E. Hack, and J. W. ten Cate. 1991. Fibrinolytic response to tumor necrosis factor in healthy subjects. J. Exp. Med. 174:729-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Poll, T., M. Levi, S. J. van Deventer, H. ten Cate, B. L. Haagmans, B. J. Biemond, H. R. Buller, C. E. Hack, and J. W. ten Cate. 1994. Differential effects of anti-tumor necrosis factor monoclonal antibodies on systemic inflammatory responses in experimental endotoxemia in chimpanzees. Blood 83:446-451. [PubMed] [Google Scholar]
- 54.van Gorp, E. C., C. Suharti, H. ten Cate, W. M. Dolmans, J. W. van der Meer, J. W. ten Cate, and D. P. Brandjes. 1999. Review: infectious diseases and coagulation disorders. J. Infect. Dis. 180:176-186. [DOI] [PubMed] [Google Scholar]
- 55.van Hinsbergh, V. W., K. A. Bauer, T. Kooistra, C. Kluft, G. Dooijewaard, M. L. Sherman, and W. Nieuwenhuizen. 1990. Progress of fibrinolysis during tumor necrosis factor infusions in humans. Concomitant increase in tissue-type plasminogen activator, plasminogen activator inhibitor type-1, and fibrin(ogen) degradation products. Blood 76:2284-2289. [PubMed] [Google Scholar]
- 56.van Hinsbergh, V. W., T. Kooistra, M. A. Scheffer, J. Hajo van Bockel, and G. N. van Muijen. 1990. Characterization and fibrinolytic properties of human omental tissue mesothelial cells. Comparison with endothelial cells. Blood 75:1490-1497. [PubMed] [Google Scholar]
- 57.van Hinsbergh, V. W., T. Kooistra, E. A. van den Berg, H. M. Princen, W. Fiers, and J. J. Emeis. 1988. Tumor necrosis factor increases the production of plasminogen activator inhibitor in human endothelial cells in vitro and in rats in vivo. Blood 72:1467-1473. [PubMed] [Google Scholar]
- 58.Zinsser, H. H., and A. W. Pryde. 1952. Experimental study of physical factors, including fibrin formation, influencing the spread of fluids and small particles within and from the peritoneal cavity of the dog. Ann. Surg. 136:818-827. [DOI] [PMC free article] [PubMed] [Google Scholar]