Abstract
The capacity of the immune system in infants to develop stable T-cell memory in response to vaccination is attenuated, and the mechanism(s) underlying this developmental deficiency in humans is poorly understood. The present study focuses on the capacity for expression of in vitro recall responses to tetanus and diphtheria antigens in lymphocytes from 12-month-old infants vaccinated during the first 6 months of life. We demonstrate that supplementation of infant lymphocytes with “matured” dendritic cells (DC) cultured from autologous CD14+ precursors unmasks previously covert cellular immunity in the form of Th2-skewed cytokine production. Supplementation of adult lymphocytes with comparable prematured autologous DC also boosted vaccine-specific T-cell memory expression, but in contrast to the case for the infants, these cytokine responses were heavily Th1 skewed. Compared to adults, infants had significantly fewer circulating myeloid DC (P < 0.0001) and plasmacytoid DC (P < 0.0001) as a proportion of peripheral blood mononuclear cells. These findings suggest that deficiencies in the numbers of antigen-presenting cells and their functional competence at 12 months of age limit the capacity to express effector memory responses and are potentially a key factor in reduced vaccine responsiveness in infants.
Morbidity and mortality resulting from infectious diseases are inversely related to age, and the development of more effective vaccines for use in the highest-risk age group (infants) represents a major unmet need. Current practices associated with immunological evaluation of potential vaccines prior to their widespread release rely principally on assessment of vaccine-specific immunological memory development within the humoral compartment, restricted in the main to immunoglobulin G (IgG) antibody titers. However, an increasing body of evidence suggests that humoral immunity following vaccination does not necessarily equate directly with protection, with a notable example being resistance to pertussis (3, 8). A variety of animal (21, 24) and human (3) studies have highlighted the potentially important role of cellular immunity, in the form of cytokine production by vaccine-primed CD4+ T-memory cells, in vaccine-induced host resistance. However, assessment of the efficacy of vaccines in inducing cellular immune memory is not widely utilized in vaccine evaluation.
A complicating factor in this equation is the generalized functional immaturity of the immune system in human neonates and its relatively slow and highly variable kinetics of maturation over the first few years of life (13, 26, 35). This functional attenuation in early life is manifest particularly in the Th1 arm of the adaptive immune response, with genes such as that for gamma interferon (IFN-γ) displaying highly restricted patterns of expression in response to a variety of stimuli (1, 4, 27, 37), and in some situations this skewing may compromise the capacity to develop stable protective immunity following vaccination and/or to resist primary infections (5, 14, 26).
The mechanism(s) underlying the diminished capacity of the infant immune system to develop efficient sterilizing immunity is only partially understood. The available evidence suggests that the maturational defect is multifactorial and involves mechanisms intrinsic to the T-cell system and also within antigen-presenting cells (APC) of the immune system (6, 9, 10, 30, 33). With respect to the T-cell compartment, recent studies implicate hypermethylation of CpG motifs in the proximal promoter of the IFN-γ gene as a major factor limiting expression of Th1 immunity (39), especially in the early postnatal period (15, 36). However, information relating to the nature of the functional deficiency in APC during early life is more limited and is generally restricted to studies of cord blood APC (6, 9, 10, 30, 33). Much less is known concerning APC function during the postnatal period, an issue that may become especially relevant as maternal antibodies disappear. In particular, there is no direct information on the relationship between the functional status of APC populations in humans and the capacity to express cellular immunity following vaccination in the first few years of life.
Detailed mechanistic studies examining this issue are difficult to perform because only small volumes of blood can be obtained from young children. In the current prospective study, we addressed whether the capacity to develop in vitro recall responses of peripheral blood mononuclear cells (PBMC) to diphtheria toxoid or tetanus toxoid in 12-month-old infants vaccinated at 2, 4, and 6 months is limited by circulating APC. Having samples of cryobanked cord blood cells from these children made it possible to supplement PBMC cultures with autologous, “matured” dendritic cells (DC), leading to a marked increase in cytokine responses and revealing the presence of covert T-cell reactivity that was not detectable in parallel PBMC cultures without additional DC. The numbers of peripheral blood plasmacytoid DC and myeloid DC were reduced at 12 months of age relative to those in adults, suggesting that deficits in the number and function of circulating APC are likely to be key factors in reduced vaccine responsiveness in infants.
MATERIALS AND METHODS
Subjects.
Healthy infants (n = 48) were recruited into a prospective study on development of immune function in early life. All children were born at term (>37 weeks of gestation) following a normal pregnancy. Blood samples were collected from the umbilical cord at birth and from a peripheral vein at 12 months of age. Adult blood samples were obtained from healthy laboratory volunteers (n = 32) who had all received booster vaccinations against diphtheria and tetanus during adolescence or early adult life. This study was carried out with the approval of the Princess Margaret Hospital Research and Ethics Committee. Informed consent was obtained from all adult subjects and from the parents or guardians of the children.
Sample collection and cell preparation.
Blood samples were collected into an equal volume of RPMI 1640 (Invitrogen-Life Technologies, Melbourne, Australia) containing preservative-free heparin (20 U/ml). PBMC were isolated by density gradient centrifugation and cryopreserved in liquid nitrogen, as previously described (18). Cryobanked cord blood samples were necessary for the critical experiments employing PBMC cocultured with autologous DC (as described below), so all experiments were therefore performed using cryopreserved cells. Previous studies from our laboratory and elsewhere have demonstrated that this procedure does not distort cellular immune responses (3, 12, 18), nor does it influence the generation of DC from neonatal and adult monocytes cultured with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) (35). For functional studies, PBMC that had been cryopreserved were thawed and resuspended at 106 viable cells/ml in RPMI 1640 supplemented with l-glutamine, 5% pooled human AB serum (BioWhittaker Inc., Maryland), 2-mercaptoethanol (4 × 10−5 M; Sigma-Aldrich Co., Sydney, Australia), and gentamicin (Pharmacia Australia Pty. Ltd., Sydney, Australia), hereafter referred to as complete medium.
Antigen-specific T-cell responses.
PBMC (2 × 105 cells) were cultured in round-bottom 96-well plates (Nunc) in 200 μl of complete medium. Cells were cultured alone or with optimal stimulatory concentrations of diphtheria toxoid (0.5 Limes flocculation [Lf]/ml) or tetanus toxoid (0.5 Lf/ml). Both antigens were obtained from CSL Ltd., Melbourne, Australia, and dialyzed against phosphate-buffered saline to remove preservatives. Levels of cytokine protein in culture supernatant were measured at 48 h after the initiation of cultures, and all data were expressed as “delta” values, designating levels of cytokine synthesis above those in unstimulated control cultures.
In some experiments, PBMC were cultured in the presence of autologous monocyte-derived DC (2 × 104 cells per well, i.e., a PBMC/DC ratio of 10:1). Initial experiments with adults compared DC cultured with either unseparated PBMC or purified T cells; no significant difference in antigen-specific lymphoproliferation or cytokine synthesis was observed. Accordingly, experiments thereafter employed PBMC without further T-cell enrichment.
Generation of relatively mature DC from peripheral blood and cord blood.
Adult monocyte-derived DC were prepared from peripheral blood monocytes by using methods described previously (35). Because only small volumes of blood (typically 1 to 2 ml) were available from 12-month-old children, insufficient cells with which to generate DC directly were available, so DC were derived instead from cryobanked, autologous cord blood monocytes (n = 9). Briefly, monocytes (>90% CD14+) were isolated by negative selection using anti-CD2, -CD7, -CD16, -CD56, -CD19, and -glycophorin A antibodies, followed by anti-mouse immunomagnetic beads (Dynal Biotech Australia, Melbourne, Australia), and cultured for 7 days in the presence of 50 ng/ml GM-CSF (Leukomax; Schering-Plough, Sydney, Australia) and 20 ng/ml IL-4 (Chemicon Australia Pty. Ltd., Melbourne, Australia). Every 2 or 3 days, fresh medium, GM-CSF, and IL-4 were added. After 7 days of culture, nonadherent cells corresponding to the DC-enriched fraction were harvested, washed, and used for subsequent experiments. These cells contained over 90% DC as determined by morphology, the presence of HLA-DR, and the absence of CD14 expression. These DC expressed intermediate levels of HLA-DR and CD86 and were therefore more mature than circulating DC, but they still had the capacity to further increase their expression of HLA-DR and CD86 in response to lipopolysaccharide (LPS) in vitro (data not shown). However, for experiments examining T-cell responses to antigens, monocyte-derived DC were not stimulated with LPS.
Flow cytometric analysis.
For immunophenotyping, cells were washed in PBS supplemented with 0.1% sodium azide and 1% normal human serum and incubated for 30 min on ice with one of the following monoclonal antibodies (MAbs) (all obtained from BD Biosciences, Sydney, Australia): fluorescein isothiocyanate (FITC)-conjugated CD14, FITC-conjugated annexin V, propidium iodide, peridinin chlorophyll protein-conjugated HLA-DR, and allophycocyanin-conjugated CD4. Corresponding isotype-matched control MAbs were also used. Cells were analyzed immediately using a FACSCalibur flow cytometer with CellQuest software (Becton Dickinson).
For enumeration of DC subsets, cells were stained with a commercial four-color antibody kit (BD Biosciences) containing lineage-specific antibodies conjugated to FITC (a cocktail of MAbs specific for B cells, T cells, monocytes, and NK cells), CD123-phycoerythrin, HLA-DR-PerCp, and CD11c-allophycocyanin. Circulating DC were defined as “lineage-negative” cells expressing HLA-DR and further divided into plasmacytoid and myeloid subsets based on differential expression of CD123 (plasmacytoid DC) and CD11c (myeloid DC). At least 150,000 mononuclear cells were acquired on the flow cytometer in order to obtain sufficient numbers of DC subsets for accurate enumeration, and the results are expressed as percentages of mononuclear cells.
Detection of IL-5, IL-13, and IFN-γ.
The levels of cytokine protein in culture supernatants were determined by time-resolved fluorometry, using antibody pairs obtained from BD Biosciences, as described in detail elsewhere (28). Standard curves were generated using serial dilutions of recombinant IL-5, IL-13, and IFN-γ (BD Biosciences, Sydney, Australia). For detection, europium-labeled streptavidin (Delfia Wallac, Finland) was added, followed by enhancement solution (Delfia Wallac). The detection limits of the assays were 10 pg/ml for IL-5, 3 pg/ml for IL-13, and 20 pg/ml for IFN-γ.
Statistical analyses.
Group data were expressed as means and 95% confidence intervals. Data concerning numbers of DC subsets were normally distributed, so comparisons between infants and adults were analyzed with the unpaired t test. All other comparisons between groups were analyzed with the Wilcoxon signed rank test for paired data and the Mann-Whitney U test for unpaired data. All analyses were performed with SPSS version 11 for Macintosh (SPSS Inc., Chicago, Illinois).
RESULTS
Vaccine-specific cytokine responses.
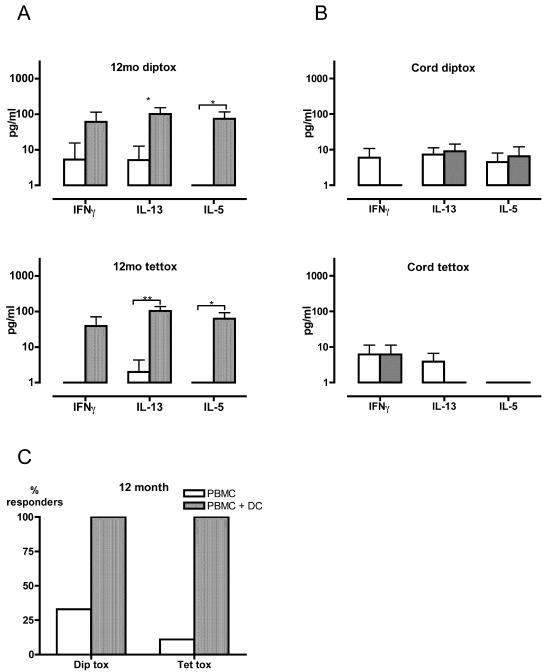
We first examined the expression of vaccine-specific T-cell memory in nine healthy 12-month-old children from whom sufficient numbers of cells were available both at birth and at 12 months of age. All the children in the study had received cellular diphtheria-tentanus-pertussis (whole-cell pertussis) vaccine immunization with alum at 2, 4, and 6 months of age, and sera from all subjects contained significant titers of vaccine-specific IgG against both diphtheria and tetanus antigens (data not shown). However, T-cell memory (detectable as cytokine production by PBMC exposed to tetanus toxoid and diphtheria toxoid) was small (Fig. 1A), with positive cytokine responses detectable in only a minority of individuals (Fig. 1C). Supplementation of vaccine antigen-stimulated PBMC cultures with autologous “matured” DC unmasked previously covert IFN-γ, IL-5, and IL-13 responses to both tetanus toxoid and diphtheria toxoid in all nine subjects (Fig. 1A). In contrast, cord PBMC did not respond to these vaccine antigens, even when cocultured with autologous “matured” DC (Fig. 1B). Given that in utero exposure to tetanus toxoid and diphtheria toxoid is extremely unlikely, this lack of response in the cord PBMC/DC cocultures suggests that the responses seen at 12 months were not due to nonspecific stimulation by DC but rather reflect antigen-specific recall.
FIG. 1.
Effects of DC supplementation on vaccine-specific cytokine production in infants and newborns. (A and B) PBMC from 12-month-old children (A) and newborns (cord blood) (B) were cultured for 48 h with diphtheria toxoid (diptox) or tetanus toxoid (tettox) in the presence or absence of additional autologous DC. Data are expressed as Δ values, designating levels of cytokine production above those in unstimulated control cultures. Error bars indicate 95% confidence intervals. (C) Percentages of subjects with positive cytokine responses to vaccine antigens. The data shown in panel A were analyzed in order to show the percentage of 12-month-old children manifesting at least one detectable cytokine response (whether IFN-γ, IL-5, or IL-13) following in vitro stimulation with diphtheria toxoid or tetanus toxoid. *, P < 0.05; **, P < 0.005 (Wilcoxon signed rank test).
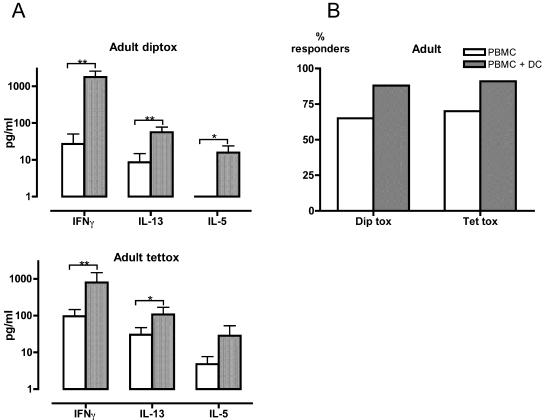
Vaccine-specific T-cell memory was also examined in healthy adult laboratory volunteers (n = 23). PBMC responses to tetanus toxoid and diphtheria toxoid were detectable in the majority of adults, and the magnitude of individual cytokine responses was much higher than that in the 12-month-old children (Fig. 2A and B). The addition of autologous DC further increased the magnitude of the adult PBMC responses (Fig. 2A) but had only a minor (statistically nonsignificant) effect on the proportion of adults in whom a positive cytokine response could be detected (Fig. 2B).
FIG. 2.
Effects of DC supplementation on vaccine-specific cytokine production in adults. PBMC from adults (n = 23) were cultured for 48 h with diphtheria toxoid (diptox) or tetanus toxoid (tettox) in the presence or absence of additional autologous DC. (A) Data are expressed as Δ values, designating levels of cytokine production above those in unstimulated control cultures. Error bars indicate 95% confidence intervals. (B) Percentages of subjects with positive cytokine responses to vaccine antigens. The data shown in panel A were analyzed in order to show the percentage of subjects manifesting at least one detectable cytokine response following in vitro stimulation with diphtheria toxoid or tetanus toxoid. *, P < 0.05; **, P < 0.005 (Wilcoxon signed rank test).
While DC supplementation augmented PBMC responses to vaccine antigens in adults, important quantitative and qualitative differences were seen compared to the effects of DC supplementation in 12-month-old children. In DC-supplemented cultures, IFN-γ production was considerably higher than IL-13 or IL-5 production in adults (Fig. 2A), whereas levels of IFN-γ, IL-13, and IL-5 production were similar in 12-month-old children (Fig. 1A). The major difference between DC-supplemented cultures from adults and from 12-month-old children was the markedly higher IFN-γ production seen in adults. Similar levels of the Th2 cytokines IL-13 and IL-5 were observed in both adults and children. As shown in Table 1, IFN-γ/IL-5 and IFN-γ/IL-13 ratios were high in adults but low in 12-month-old children. Thus, cytokine response patterns in DC-supplemented cultures were strongly Th1 skewed in adults but were unpolarized or even Th2 skewed in 12-month-old children.
TABLE 1.
Vaccine antigen-specific Th1/Th2 ratios in PBMC-DC cocultures from 12-month-old children and adults
| Ratio | Median (interquartile range)a
|
|||
|---|---|---|---|---|
| 12-mo-old children
|
Adults
|
|||
| Diphtheria toxoid | Tetanus toxoid | Diphtheria toxoid | Tetanus toxoid | |
| IFN-γ/IL-5 | 0.2 (0.1-1.0) | 0.4 (0.1-1.6) | 55.2b (3.6-196.5) | 25.8b (2.3-40.3) |
| IFN-γ/IL-13 | 0.4 (0.1-1.7) | 0.1 (0.0-1.2) | 19.9c (1.5-32.5) | 6.8b (1.7-18.1) |
The IFN-γ/IL-5 and IFN-γ/IL-13 ratios were calculated for each individual. The statistical significance of differences between 12-month-old children and adults was assessed using the Mann-Whitney U test.
P = 0.001 compared to value for 12-month-old children.
P = 0.004 compared to value for 12-month-old children.
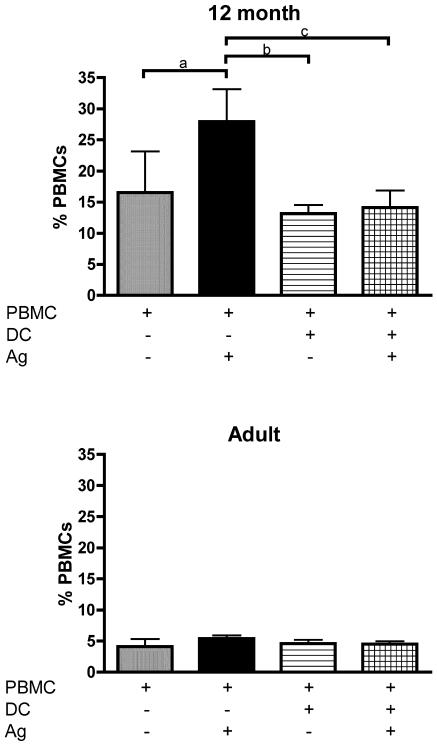
Further examination of PBMC cultures showed that reduced cell survival was a prominent feature in 12-month-old children but not in adults, as shown in Fig. 3. Antigen stimulation of PBMC from the infants led to a significant increase in the number of annexin-V-positive apoptotic CD4+ T cells (Fig. 3, top), whereas antigen stimulation did not significantly affect CD8+ T-cell apoptosis (data not shown). Similar results were obtained with diphtheria toxoid (data not shown). Addition of autologous matured DC reduced apoptosis in PBMC from 12-month-old children by approximately 50% (Fig. 3, top), but had no effect on the low levels of apoptosis seen in adult cultures.
FIG. 3.
Effects of DC supplementation on CD4+ T-cell apoptosis by tetanus toxoid-stimulated PBMC from 12-month-old children (n = 7) and adults (n = 7). Using flow cytometry, apoptotic CD4+ T cells were identified as annexin V-positive, propidium iodide-positive cells and the data expressed as a percentage of total PBMC. Ag, antigen. Error bars indicate 95% confidence intervals. a, P = 0.009; b, P = 0.02; c, P = 0.012 (Wilcoxon signed rank test).
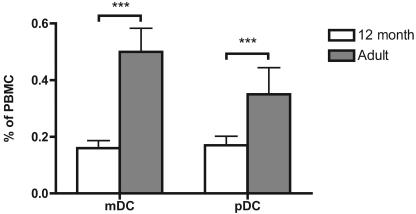
These findings suggested that there might be quantitative or qualitative deficiencies in circulating APC in 12-month-old children compared to those in adults. Myeloid DC and plasmacytoid DC are thought to be the principal APC in the peripheral blood, and as shown in Fig. 4, both these subsets were significantly less numerous at 12 months than in adult life (P < 0.0001 for both DC subsets). The expression of HLA-DR on these DC subsets at 12 months was less intense than that on adult DC subsets (data not shown). Numbers of monocytes were similar in 12-month-old children and adults (data not shown).
FIG. 4.
Enumeration of DC subsets in 12-month-old children (n = 48) and adults (n = 32). Using flow cytometry, myeloid DC (mDC) and plasmacytoid DC (pDC) were enumerated and expressed as a percentage of PBMC. Error bars indicate 95% confidence intervals. ***, P < 0.0001 (12-month-old children versus adults, unpaired t test).
DISCUSSION
The risk of serious infection is particularly high during infancy, and there is a need to better understand the determinants of host protection at this age, especially the mechanisms regulating development of stable immune memory directed against pathogenic microbes. Increasing interest in the determinants of cellular immunity directed against vaccine antigens led us to reexamine the role of APC maturity in this context. Previous research in this area has highlighted the potential importance of APC maturation as a determinant of the efficiency of the initial priming process in T-cell memory development. We demonstrate here for the first time in humans that, independent of steps during priming, the numbers of circulating APC and their functional competence may also determine the capacity to express effector memory responses (notably cellular immunity in the form of cytokine production) after the priming process is complete.
Our laboratory has previously shown for a prospective cohort of healthy children that the capacity to mount Th1 cytokine responses to vaccine antigens often remains low until beyond the age of 12 months (26). Similar findings are evident in the present study, in which we demonstrated that when PBMC from fully vaccinated 12-month-old children were cultured with diphtheria and tetanus antigens, cytokine responses were often small or below the threshold of detection. This finding is difficult to reconcile with the fact that 100% of cellular diphtheria-tetanus-pertussis (whole-cell pertussis) vaccine-primed 12-month-old infants in this study demonstrated vaccine-specific IgG antibody in peripheral blood, production of which is dependent on T-helper cell activity. Accordingly, either relevant T-helper memory cells do not recirculate in these infants or the culture system used to reactivate them (viz., whole PBMC plus vaccine antigen) is in some way deficient for this purpose, and the results of this study are compatible with the latter explanation. Thus, when PBMC cultures were supplemented with autologous cord blood monocytes that had been differentiated into DC by using GM-CSF and IL-4, a different picture of T-cell memory emerged. Supplementation with these relatively mature, antigen-presenting DC markedly increased cytokine responses to both diphtheria toxoid and tetanus toxoid and, in many cases, revealed the presence of covert T-cell reactivity that was undetectable in parallel PBMC cultures. These findings suggest that the minimal T-cell reactivity in PBMC cultures derived from vaccine-primed infants at 12 months is not entirely due to the low frequency of vaccine-specific memory T cells in the peripheral circulation but rather that the capacity to reactivate specific T cells is also constrained by endogenous, circulating APC populations.
As shown in Fig. 4, the numbers of peripheral blood myeloid DC and plasmacytoid DC relative to PBMC were significantly reduced in 12-month-old children compared to adults. Because of the nature of the specimen collection in this prospective study, it was not possible to measure absolute numbers of DC subsets per microliter of blood. However, we did not observe any differences in the numbers of other cell populations between adults and 12-month-old children, making it likely that these changes in differential cell counts reflect changes in absolute cell numbers. This is supported by reports showing differences in the absolute numbers of blood DC subsets in young children compared to older children and adults (11, 31). Similar reductions in DC numbers within mucosal tissue in early life have also been reported (23, 34).
Over and above the low numbers of blood DC at 12 months, it is likely that there are additional deficits in APC function at this age. Blood DC are functionally immature at birth relative to their adult counterparts (29) and continue to express a less differentiated surface phenotype during most of early childhood (11), while the capacity to synthesize IL-12, a key APC-derived cytokine, matures quite slowly during childhood (35). Even though the small volumes of blood available at 12 months precluded a detailed assessment of the function of purified DC subsets, the reduced expression of HLA-DR on myeloid and plasmacytoid DC is consistent with an immature phenotype, making it likely that both deficiencies of APC function and reduction in numbers of DC subsets combine to retard the expression of T-cell memory at this age.
It is noteworthy that DC supplementation boosted vaccine-specific T-cell reactivity in both adults and 12-month-old children, suggesting that the APC populations present in the peripheral circulation at all ages do not express full functional capacity. This is not unexpected, given the comprehensive literature indicating that in healthy animals the only sites at which fully mature APC (in particular DC) are present constitutively are primary lymphoid organs or sites of chronic immunoinflammatory lesions. The system employed to generate DC produces cells with APC activity that is comparable to that in the latter case, and our findings may thus reflect potential for in vivo responsiveness to vaccine antigens more accurately than the picture seen with conventional PBMC culture systems.
Despite the similarities in overall DC-mediated augmentation of vaccine-specific recall responses, the ensuing patterns of cytokine production varied markedly with age. Whereas IL-13 and IL-5 responses were similar in infants and adults, IFN-γ responses in the infants remained considerably below those observed in adults, a finding that is in broad agreement with the large body of murine studies showing that relative Th1/Th2 response patterns vary significantly with age. In particular, while infection or immunization during the neonatal period triggers transient Th1 and Th2 cytokine production, subsequent T-cell memory is largely Th2 polarized, whereas infection or immunization in adult animals induces Th1-polarized immunity (1, 5). Of particular note, we have demonstrated previously that Th1 memory responses to acellular diphtheria-tetanus-pertussis (acellular pertussis) vaccine are unstable during late infancy relative to the Th2 component, with the former requiring maturation of as-yet-undefined elements of systemic immune function to enable stable expression of T-cell memory that was primed during the first 6 months of life (26, 27). The relative inability to develop stable Th1 memory in early childhood has been variously attributed to intrinsic properties of T cells (2) or to deficiencies in APC function (25, 33), especially their capacity to secrete polarizing cytokines such as IL-12 (9, 10, 16, 35).
Our findings suggest that even in the presence of apparently mature antigen-presenting DC, there remains a relative deficiency in Th1 memory expression in childhood (Fig. 1 and 2 and Table 1). This may be due in part to the predilection of immature T cells to undergo apoptotic death following activation, as shown in Fig. 3, confirming previous work from our laboratory and others (17, 32). It is noteworthy that while introduction of mature autologous DC into these cultures markedly reduced T-cell attrition, the levels of overall cell loss in the infant cultures still remained approximately threefold higher than those observed in adults. Th1 effector cells appear to be more susceptible than Th2 effector cells to apoptosis following antigen stimulation in adult mice (38, 40), a situation that may be exaggerated during infancy (17), thereby contributing to the relative inability to develop stable Th1 memory, as discussed above. We have shown previously that antigen-induced apoptosis of cord blood T cells can be inhibited by IL-2, IL-4, and IL-7 (32), although whether these cytokines mediate the effects shown in Fig. 3 or whether additional mechanisms specific to DC are involved was not examined in the current study. It will be important for future research to examine the relative importance of T-cell apoptosis as a factor limiting vaccine responsiveness in infancy, using caspase inhibitors, for example.
It is clear that T-cell responses in infancy are both attenuated and intrinsically Th2 skewed relative to those in adults. However, while mature DC were able to augment the expression of vaccine-specific T-cell memory seen in infant PBMC cultures, mature DC were unable to overcome the Th2 skewing of infant responses and unleash the high-level IFN-γ responses seen in the adult cultures. Goriely and coworkers have previously shown that neonatal monocyte-derived DC exhibit impaired IL-12 p35 transcription in response to LPS compared with adult DC (9, 10), although addition of IFN-γ allows neonatal DC to secrete adult-equivalent amounts of bioactive IL-12 p70 (9, 10, 35). Neonatal CD4 cells also appear to be intrinsically deficient in their capacity to express Th1 effector function (2), a characteristic that can be partially attributed to hypermethylation within and adjacent to the IFN-γ promoter (36).
The clinical implication of these observations is that optimal induction of vaccine-specific T-cell memory in early childhood may require the use of strategies designed specifically to compensate for deficiencies in the number and/or function of APC. Antigen challenge together with adjuvants that up-regulate APC function allows neonatal animals to mount relatively mature Th1 responses (7, 20, 22), while mycobacterial vaccines with intrinsic adjuvant properties can induce vigorous IFN-γ responses in human infants (19). Accordingly, it appears that the deficiencies of APC function in young children are not immutable, as APC can be induced to mature via the provision of appropriate microenvironmental signals, whether these are adjuvants or cytokines with the capacity to induce DC maturation in vivo. More-detailed studies are required to elucidate the relationship between postnatal maturation of DC function and variations in the capacity to induce protective immunity, as a foundation on which to develop new vaccines strategies for use during infancy.
Acknowledgments
This work was funded by grants from the National Health and Medical Research Council of Australia (no. 211912) and the Faculty of Medicine and Dentistry, University of Western Australia.
The authors do not have a commercial or other association that might pose a conflict of interest.
Editor: J. T. Barbieri
REFERENCES
- 1.Adkins, B., Y. Bu, and P. Guevara. 2001. The generation of Th memory in neonates versus adults: prolonged primary Th2 effector function and impaired development of Th1 memory effector function in murine neonates. J. Immunol. 166:918-925. [DOI] [PubMed] [Google Scholar]
- 2.Adkins, B., Y. Bu, and P. Guevara. 2002. Murine neonatal CD4(+) lymph node cells are highly deficient in the development of antigen-specific Th1 function in adoptive adult hosts. J. Immunol. 169:4998-5004. [DOI] [PubMed] [Google Scholar]
- 3.Ausiello, C. M., R. Lande, F. Urbani, A. la Sala, P. Stefanelli, S. Salmaso, P. Mastrantonio, and A. Cassone. 1999. Cell-mediated immune responses in four-year-old children after primary immunization with acellular pertussis vaccines. Infect. Immun. 67:4064-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrios, C., P. Brawand, M. Berney, C. Brandt, P. H. Lambert, and C. A. Siegrist. 1996. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur. J. Immunol. 26:1489-1496. [DOI] [PubMed] [Google Scholar]
- 5.Culley, F. J., J. Pollott, and P. J. Openshaw. 2002. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J. Exp. Med. 196:1381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delespesse, G., L. P. Yang, Y. Ohshima, C. Demeure, U. Shu, D. G. Byun, and M. Sarfati. 1998. Maturation of human neonatal CD4+ and CD8+ T lymphocytes into Th1/Th2 effectors. Vaccine 16:1415-1419. [DOI] [PubMed] [Google Scholar]
- 7.Forsthuber, T., H. C. Yip, and P. V. Lehmann. 1996. Induction of TH1 and TH2 immunity in neonatal mice. Science 271:1728-1730. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano, M., P. Mastrantonio, A. Giammanco, A. Piscitelli, S. Salmaso, and S. G. Wassilak. 1998. Antibody responses and persistence in the two years after immunization with two acellular vaccines and one whole-cell vaccine against pertussis. J. Pediatr. 132:983-988. [DOI] [PubMed] [Google Scholar]
- 9.Goriely, S., C. Van Lint, R. Dadkhah, M. Libin, D. De Wit, D. Demonte, F. Willems, and M. Goldman. 2004. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J. Exp. Med. 199:1011-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goriely, S., B. Vincart, P. Stordeur, J. Vekemans, F. Willems, M. Goldman, and D. De Wit. 2001. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 166:2141-2146. [DOI] [PubMed] [Google Scholar]
- 11.Hagendorens, M. M., D. G. Ebo, A. J. Schuerwegh, A. Huybrechs, H. P. Van Bever, C. H. Bridts, L. S. De Clerck, and W. J. Stevens. 2003. Differences in circulating dendritic cell subtypes in cord blood and peripheral blood of healthy and allergic children. Clin. Exp. Allergy 33:633-639. [DOI] [PubMed] [Google Scholar]
- 12.Heaton, T., J. Rowe, S. Turner, R. C. Aalberse, N. de Klerk, D. Suriyaarachchi, M. Serralha, B. J. Holt, E. Hollams, S. Yerkovich, K. Holt, P. D. Sly, J. Goldblatt, P. Le Souef, and P. G. Holt. 2005. An immunoepidemiological approach to asthma: identification of in-vitro T-cell response patterns associated with different wheezing phenotypes in children. Lancet 365:142-149. [DOI] [PubMed] [Google Scholar]
- 13.Holt, P. G., J. Rowe, R. Loh, and P. D. Sly. 2003. Developmental factors associated with risk for atopic disease: implications for vaccine strategies in early childhood. Vaccine 21:3432-3435. [DOI] [PubMed] [Google Scholar]
- 14.Holt, P. G., and P. D. Sly. 2002. Interactions between RSV infection, asthma, and atopy: unraveling the complexities. J. Exp. Med. 196:1271-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katamura, K., T. Fukui, T. Kiyomasu, J. Iio, G. Tai, H. Ueno, T. Heike, M. Mayumi, and K. Furusho. 1998. IL-4 and prostaglandin E2 inhibit hypomethylation of the 5′ regulatory region of IFN-gamma gene during differentiation of naive CD4+ T cells. Mol. Immunol. 35:39-45. [DOI] [PubMed] [Google Scholar]
- 16.Lee, S. M., Y. Suen, L. Chang, V. Bruner, J. Qian, J. Indes, E. Knoppel, C. van de Ven, and M. S. Cairo. 1996. Decreased interleukin-12 (IL-12) from activated cord versus adult peripheral blood mononuclear cells and upregulation of interferon-gamma, natural killer, and lymphokine-activated killer activity by IL-12 in cord blood mononuclear cells. Blood 88:945-954. [PubMed] [Google Scholar]
- 17.Li, L., H. H. Lee, J. J. Bell, R. K. Gregg, J. S. Ellis, A. Gessner, and H. Zaghouani. 2004. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity 20:429-440. [DOI] [PubMed] [Google Scholar]
- 18.Macaubas, C., P. D. Sly, P. Burton, K. Tiller, A. Yabuhara, B. J. Holt, T. B. Smallacombe, G. Kendall, M. C. Jenmalm, and P. G. Holt. 1999. Regulation of T-helper cell responses to inhalant allergen during early childhood. Clin. Exp. Allergy 29:1223-1231. [DOI] [PubMed] [Google Scholar]
- 19.Marchant, A., T. Goetghebuer, M. O. Ota, I. Wolfe, S. J. Ceesay, D. De Groote, T. Corrah, S. Bennett, J. Wheeler, K. Huygen, P. Aaby, K. P. McAdam, and M. J. Newport. 1999. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J. Immunol. 163:2249-2255. [PubMed] [Google Scholar]
- 20.Martinez, X., C. Brandt, F. Saddallah, C. Tougne, C. Barrios, F. Wild, G. Dougan, P. H. Lambert, and C. A. Siegrist. 1997. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte responses in neonates and during early life. Proc. Natl. Acad. Sci. USA 94:8726-8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills, K. H., A. Barnard, J. Watkins, and K. Redhead. 1993. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect. Immun. 61:399-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min, B., K. L. Legge, J. C. Caprio, L. Li, R. Gregg, and H. Zaghouani. 2000. Differential control of neonatal tolerance by antigen dose versus extended exposure and adjuvant. Cell Immunol. 200:45-55. [DOI] [PubMed] [Google Scholar]
- 23.Nelson, D. J., and P. G. Holt. 1995. Defective regional immunity in the respiratory tract of neonates is attributable to hyporesponsiveness of local dendritic cells to activation signals. J. Immunol. 155:3517-3524. [PubMed] [Google Scholar]
- 24.Redhead, K., J. Watkins, A. Barnard, and K. H. Mills. 1993. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect. Immun. 61:3190-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridge, J. P., E. J. Fuchs, and P. Matzinger. 1996. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science 271:1723-1726. [DOI] [PubMed] [Google Scholar]
- 26.Rowe, J., C. Macaubas, T. Monger, B. J. Holt, J. Harvey, J. T. Poolman, R. Loh, P. D. Sly, and P. G. Holt. 2001. Heterogeneity in diphtheria-tetanus-acellular pertussis vaccine-specific cellular immunity during infancy: relationship to variations in the kinetics of postnatal maturation of systemic Th1 function. J. Infect. Dis. 184:80-88. [DOI] [PubMed] [Google Scholar]
- 27.Rowe, J., C. Macaubas, T. M. Monger, B. J. Holt, J. Harvey, J. T. Poolman, P. D. Sly, and P. G. Holt. 2000. Antigen-specific responses to diphtheria-tetanus-acellular pertussis vaccine in human infants are initially Th2 polarized. Infect. Immun. 68:3873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharp, M. J., J. Rowe, M. Kusel, P. D. Sly, and P. G. Holt. 2003. Specific patterns of responsiveness to microbial antigens staphylococcal enterotoxin B and purified protein derivative by cord blood mononuclear cells are predictive of risk for development of atopic dermatitis. Clin. Exp. Allergy 33:435-441. [DOI] [PubMed] [Google Scholar]
- 29.Sorg, R. V., G. Kogler, and P. Wernet. 1999. Identification of cord blood dendritic cells as an immature CD11c− population. Blood 93:2302-2307. [PubMed] [Google Scholar]
- 30.Taylor, S., and Y. J. Bryson. 1985. Impaired production of gamma-interferon by newborn cells in vitro is due to a functionally immature macrophage. J. Immunol. 134:1493-1497. [PubMed] [Google Scholar]
- 31.Teig, N., D. Moses, S. Gieseler, and U. Schauer. 2002. Age-related changes in human blood dendritic cell subpopulations. Scand. J. Immunol. 55:453-457. [DOI] [PubMed] [Google Scholar]
- 32.Thornton, C. A., J. W. Upham, M. E. Wikstrom, B. J. Holt, G. P. White, M. J. Sharp, P. D. Sly, and P. G. Holt. 2004. Functional maturation of CD4+CD25+CTLA4+CD45RA+ T regulatory cells in human neonatal T cell responses to environmental antigens/allergens. J. Immunol. 173:3084-3092. [DOI] [PubMed] [Google Scholar]
- 33.Trivedi, H. N., K. T. HayGlass, V. Gangur, J. G. Allardice, J. E. Embree, and F. A. Plummer. 1997. Analysis of neonatal T cell and antigen presenting cell functions. Hum. Immunol. 57:69-79. [DOI] [PubMed] [Google Scholar]
- 34.Tschernig, T., A. S. Debertin, F. Paulsen, W. J. Kleemann, and R. Pabst. 2001. Dendritic cells in the mucosa of the human trachea are not regularly found in the first year of life. Thorax 56:427-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Upham, J. W., P. T. Lee, B. J. Holt, T. Heaton, S. L. Prescott, M. J. Sharp, P. D. Sly, and P. G. Holt. 2002. Development of interleukin-12-producing capacity throughout childhood. Infect. Immun. 70:6583-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White, G. P., P. M. Watt, B. J. Holt, and P. G. Holt. 2002. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO− T cells. J. Immunol. 168:2820-2827. [DOI] [PubMed] [Google Scholar]
- 37.Wilson, C. B., J. Westall, L. Johnston, D. B. Lewis, S. K. Dower, and A. R. Alpert. 1986. Decreased production of interferon-gamma by human neonatal cells. Intrinsic and regulatory deficiencies. J. Clin. Investig. 77:860-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, C. Y., J. R. Kirman, M. J. Rotte, D. F. Davey, S. P. Perfetto, E. G. Rhee, B. L. Freidag, B. J. Hill, D. C. Douek, and R. A. Seder. 2002. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat. Immunol. 3:852-858. [DOI] [PubMed] [Google Scholar]
- 39.Young, H. A., P. Ghosh, J. Ye, J. Lederer, A. Lichtman, J. R. Gerard, L. Penix, C. B. Wilson, A. J. Melvin, M. E. McGurn, et al. 1994. Differentiation of the T helper phenotypes by analysis of the methylation state of the IFN-gamma gene. J. Immunol. 153:3603-3610. [PubMed] [Google Scholar]
- 40.Zhang, X., T. Brunner, L. Carter, R. W. Dutton, P. Rogers, L. Bradley, T. Sato, J. C. Reed, D. Green, and S. L. Swain. 1997. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J. Exp. Med. 185:1837-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]