Abstract
Infective larvae (L3) of nematodes secrete macromolecules that are critical to infection and establishment of the parasite in the host. The dog hookworm Ancylostoma caninum secretes an astacin-like metalloprotease, Ac-MTP-1, upon activation in vitro with host serum. Recombinant Ac-MTP-1 was expressed in the baculovirus/insect cell system as a secreted protein and was purified from culture medium by two separate methods, cation-exchange fast-performance liquid chromatography and gelatin-affinity chromatography. Recombinant MTP-1 was catalytically active and digested a range of native and denatured connective tissue substrates, including gelatin, collagen, laminin, and fibronectin. A dog was immunized with recombinant Ac-MTP-1 formulated with AS03 adjuvant, and the antiserum was used to immunolocalize the anatomic sites of expression within A. caninum L3 to secretory granules in the glandular esophagus and the channels that connect the esophagus to the L3 surface and to the cuticle. Antiserum inhibited the ability of recombinant MTP-1 to digest collagen by 85% and inhibited larval migration through tissue in vitro by 70 to 75%, in contrast to just 5 to 10% inhibition obtained with preimmunization serum. The metalloprotease inhibitors EDTA and 1,10-phenanthroline also reduced the penetration of L3 through skin in vitro by 43 to 61%. The data strongly suggest that Ac-MTP-1 is critical for the invasion process of hookworm larvae, and moreover, that antibodies against the enzyme can neutralize its function and inhibit migration.
Hookworms are blood-feeding nematodes that infect 740 million people in developing countries (4). Antihelminthics are the current method of control, but increasing drug resistance in nematodes of livestock and rapid reinfection following treatment of infected people have facilitated the need for recombinant vaccines (14, 17).
Most hookworms infect a host by penetrating the skin, although some species are orally infective. Third-stage infective larvae (L3) of the dog hookworm, Ancylostoma caninum, and the major human hookworm, Necator americanus, are developmentally arrested and wait in the soil or on blades of grass to come into contact with a mammalian host. They attach to the host upon skin contact and penetrate via hair follicles, eventually entering blood or lymphatic capillaries. Feeding recommences upon exposure to serum components (8), and the worms resume development. They are passively carried to the pulmonary microcirculation, where they undergo tracheal migration by penetrating into the alveoli, to be swept in mucus up the airways and then down into the gut.
Hookworm proteins involved in the tissue invasion process are particularly good candidate antigens for the development of vaccines and drugs. Proteases are central to tissue penetration by parasitic helminths, and the enzyme activities associated with this process have been characterized from crude protein extracts from numerous parasites, including filariae (7) and strongyle nematodes of livestock (5, 15) and humans (19). However, studies that have unequivocally attributed tissue penetration and migration to specific gene products are far less common; to our knowledge, the only gene product (confirmed with a recombinant protein) from a parasitic helminth with a proven role in skin penetration is the serine elastase secreted by Schistosoma mansoni cercariae (22, 24).
A. caninum L3 secrete a metalloprotease, Ac-MTP-1, in response to host serum (28). Ac-MTP-1 has sequence identity to members of a family of zinc metalloproteases called the astacins (2), named after a digestive protease from the crayfish Astacus astacus. MEROPS classification of Ac-MTP-1 places it in clan MA, family M12 as peptidase M12.310 (http://merops.sanger.ac.uk/index.htm). Members of this family are characterized by a short N-terminal signal peptide that targets them for secretion, followed by a short propeptide and a catalytic domain containing the characteristic zinc-binding region and “Met turn” (2). Members of the M12 peptidase family have a wide range of functions and varied specificities, including degradation of tissue matrix proteins. For example, procollagen C-peptidase cleaves the C-terminal propeptide during the maturation of type 1 procollagen, making it a drug target for fibrosis (23). Ac-MTP-1 also contains a C-terminal epidermal growth factor-like domain and a CUB (complement subcomponent C1r/C1s/embryonic sea urchin protein Uegf/bone morphogenetic protein)-like domain. It shares its highest identity with peptidases from nematodes, both free-living (Caenorhabditis elegans) and parasitic, including strongylastacin, a secreted protein from Strongyloides stercoralis L3 that is thought to be involved in the invasive process (6). Like strongylastacin, Ac-MTP-1 is expressed exclusively by the L3 stage of A. caninum and is actively secreted into the culture medium in vitro (28), supporting a role in tissue invasion.
A role for secreted metalloproteases in tissue invasion by nematode larvae has been inferred (3, 5, 11) but never proven with the use of highly purified native proteases or recombinant enzymes. Here we show that the native Ac-MTP-1 enzyme is produced in the secretory granules of the glandular esophagus of L3, transported to the cuticle via the body channels, and then potentially released from the cuticle. Recombinant Ac-MTP-1 digested connective tissue macromolecules, and antiserum to the recombinant enzyme inhibited the digestion of substrates by the recombinant protease and the tissue migration of hookworm L3 in vitro.
MATERIALS AND METHODS
Parasites.
Third-stage infective A. caninum larvae were collected from charcoal coprocultures at George Washington University and stored in BU buffer (50 mM Na2HPO4, 22 mM KH2PO4, 70 mM NaCl, pH 6.8) at 22°C until use.
Expression of recombinant Ac-MTP-1.
In a previous study (28), Ac-MTP-1 was expressed in Escherichia coli, but the recombinant protein was insoluble. We therefore decided to express Ac-MTP-1 as a secreted protein in a baculovirus system to promote the solubility and catalytic activity of the recombinant enzyme. The region encoding the prodomain to the C terminus in the Ac-MTP-1 open reading frame (ORF) (Gly-17 to Arg-547) was amplified by PCR from a pBluescript template and cloned into the shuttle vector pMelBac (Invitrogen, Carlsbad, CA) by incorporating NcoI and SacI restriction sites such that the N terminus fused to the melittin signal peptide encoded by the vector. The 3′ oligonucleotide carried the last 18 nucleotides of the ORF (excluding the stop codon), followed by codons for a six-His tag and a stop codon (CATCATCACCATCACCATTGA). The recombinant virus was generated by cotransfection, aided by Cellfectin (Invitrogen), of Spodoptera frugiperda Sf9 cells (derived from the pupal ovarian tissue of the fall army worm, Spodoptera frugiperda) with Bac-N-Blue viral DNA (Invitrogen) and pMelBac containing the Ac-MTP-1 construct. The recombinant virus was located by Western blotting using a mouse antiserum raised against Ac-MTP-1 expressed as an insoluble fusion protein in E. coli (28). The recombinant virus was isolated and amplified, and the resulting high-titer viral stock was stored at 4°C as recommended by the manufacturer (Invitrogen). Adherent Sf9 cell cultures were used for initial plaque purification of the recombinant virus and for small-scale amplifications. High Five cells (derived from ovarian cells of the cabbage looper, Trichoplusia ni) were used for large-scale cultures, in which the transfected cells were cultured in suspension flasks at 27°C in protein-free medium (JRH Biosciences, Lenexa, KS). The supernatant containing secreted Ac-MTP-1 was harvested 3 days later.
Purification of recombinant Ac-MTP-1.
Recombinant Ac-MTP-1 did not bind to nickel agarose under native conditions, despite the presence of a six-His tag, as confirmed by sequence analysis of the recombinant plasmid (not shown) and Western blotting of the purified protein using a monoclonal anti-six-His antibody (Invitrogen; see Fig. 1C). Ac-MTP-1 was therefore purified on gelatin Sepharose as described for the hemoglobin-degrading metalloprotease from adult-stage A. caninum, Ac-MEP-1 (27). Ac-MTP-1 was also purified from the culture medium by ion-exchange chromatography. Two liters of culture supernatant was concentrated by diafiltration using a 10-kDa-cutoff membrane as described elsewhere (25) and then desalted, and the buffer was exchanged with 50 mM sodium acetate (pH 4.8) using a Sephadex G-25 fine desalting column (Amersham Biosciences, Bucks, United Kingdom). One hundred ninety milliliters of desalted sample was loaded onto a HiPrep 16/10 SP FF ion-exchange column (Amersham Biosciences) that was connected to a high-performance liquid chromatography system (Waters, Milford, MA) and had been washed with 100 ml of 50 mM sodium acetate-0.5 M NaCl (pH 4.8). The column was then equilibrated with 100 ml of 50 mM sodium acetate buffer. The 190 ml of desalted supernatant was loaded onto the column at room temperature at a flow rate of 2 ml min−1. Bound protein on the column was eluted with 120 ml of 50 mM sodium acetate-0.5 M NaCl (pH 4.8) at room temperature with a flow rate of 2 ml min−1, using a linear gradient of 0 to 100% buffer, over a period of 60 min. The column eluate was collected in fractions of 4.0 ml and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 4 to 20% Tris-glycine gels (Invitrogen) stained with Coomassie brilliant blue R-250.
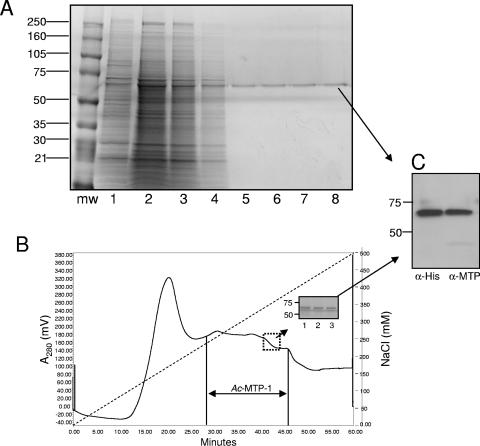
FIG. 1.
Purification of Ac-MTP-1 using either gelatin-affinity (A) or cation-exchange (B) chromatography. (A) Lanes: mw, molecular size markers (listed in kDa at the side); 1, recombinant MTP-1-containing baculovirus culture supernatant; 2, concentrated supernatant in binding buffer; 3, column flowthrough; 4, binding buffer wash; 5 to 8, successive 1.0-ml eluate fractions. (B) Chromatogram showing elution profile of recombinant Ac-MTP-1 from an SP FF cation-exchange column eluting with a linear gradient of 0 to 0.5 M NaCl. The inset shows the protein profiles of three successive 4.0-ml eluate fractions corresponding to the boxed region. (C) Western blot showing recognition of the eluted protein shown in the inset of panel B with a monoclonal antibody raised to the six-His tag on the recombinant protein (anti-His) or a polyclonal rabbit serum raised to recombinant Ac-MTP-1 expressed in E. coli (28). The purified protein shown in panel A was recognized in the same manner (not shown).
Assessment of enzymatic activity and substrate preferences.
The hydrolysis of various substrates was determined at neutral pH to replicate the pH of the skin surface during the hookworm invasion process. The incubation buffer was Tris-buffered saline (pH 7.5)-100 mM ZnCl2, with or without the metalloprotease inhibitor 1,10-phenanthroline (Sigma, St. Louis, MO) at a final concentration of 10 μM. Ten micrograms of purified recombinant Ac-MTP-1 was incubated for 24 h with 50 μg of various substrates found in connective tissues, including elastin, laminin, and fibronectin (Sigma). The cleavage of denatured and native collagen was assessed by using 10% gelatin gels (Sigma) as described elsewhere (27) and 0.5% Azocoll (Sigma) following the manufacturer's instructions. Briefly, 0.5% Azocoll in 100 mM potassium phosphate, pH 7.0, was incubated with recombinant Ac-MTP-1 at a range of final concentrations (1 to 50 μg ml−1) for 2 h at 37°C. The solution was then centrifuged for 5 min at 13,000 × g, the supernatant was removed, and the optical density (OD) was determined spectrophotometrically at 520 nm.
Animal husbandry and immunization.
A purpose-bred, parasite-naïve male beagle aged 8 weeks was purchased from Marshall Farms (North Rose, NY) and maintained in the George Washington University Animal Research Facility as previously described (12). The experiments were conducted through an approved protocol by the George Washington University Animal Care and Use Committee. Before the first vaccination and after each subsequent one, a serum sample was obtained. Ac-MTP-1 (100 μg) was formulated with the adjuvant AS03 as described elsewhere (16), and the dog was immunized intramuscularly three times beginning at age 62 days with Ac-MTP-1 and adjuvant as previously described (12). The dog was given boosters at 21-day intervals. Blood was drawn once every 21 days, and serum was separated from cells by centrifugation. Enzyme-linked immunosorbent assays were performed as previously described (12). Recombinant Ac-MTP-1 was used to coat microtiter plates at a concentration of 5.0 μg ml−1. Dog serum was titrated between 1:100 and 1:2 × 106 to determine the end-point titer (the final dilution where the mean OD of serum from the vaccinated dog was three times or more the mean OD of the control serum). Anti-canine immunoglobulin G1 (IgG1), IgG2, and IgE antibodies conjugated to horseradish peroxidase (Bethyl Laboratories, Montgomery, TX) were used at a dilution of 1:1,000.
Effect of anti-Ac-MTP-1 IgG on proteolytic activity.
Canine IgG was purified from the serum of the vaccinated dog and pooled sera from five control dogs (12), using protein A-agarose (Amersham Biosciences) as previously described (25). Purified IgG (0.5 μg) was incubated with 1.0 μg of recombinant Ac-MTP-1 for 45 min prior to assessment of the degradation of Azocoll as previously described (16). 1,10-Phenanthroline (final concentration, 10 μM) was used as a control for chemical inhibition of metalloprotease activity. The percent reduction in MTP-1-mediated cleavage of Azocoll was calculated, using Azocoll alone to determine the baseline value. Statistical assessment of the results was conducted using a one-tailed Student t test in Microsoft Excel.
Effect of anti-Ac-MTP-1 serum and metalloprotease inhibitors on larval skin penetration.
A. caninum L3 were incubated in undiluted serum from the vaccinated dog or pooled sera from control dogs and then placed on freshly removed dog skin for 30 min to determine the effect of anti-Ac-MTP-1 serum on larval skin penetration, as described elsewhere (1). The effects of metalloprotease inhibitors (10 mM EDTA and 10 or 100 μM 1,10-phenanthroline) on L3 migration through hamster skin were also assessed as described above. 1,10-Phenanthroline was solubilized in distilled H2O at 42°C to avoid adding dimethyl sulfoxide to L3. The numbers of larvae remaining on the skin (i.e., those that had not penetrated) were counted. Each trial was conducted in duplicate (those involving sera) or triplicate (those involving chemical inhibitors). Statistical assessment of the results was conducted using a one-tailed Student t test in Microsoft Excel.
Immunolocalization.
Exsheathed A. caninum L3 were fixed for 60 min at room temperature in 0.25% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, containing 1% sucrose and processed for immunoelectron microscopy as described previously (18). Thin sections of embedded worms were probed with dog or rabbit antiserum (1:100 dilution) raised against recombinant Ac-MTP-1. Rabbit antiserum was raised to recombinant MTP-1 expressed in E. coli by Zhan et al. (28). Sections stained with rabbit serum were incubated with a 1:20 dilution of goat anti-rabbit IgG (heavy plus light chains) coupled with 15-nm gold particles (Amersham Biosciences). Sections stained with the dog antibody were first incubated with 50 μg ml−1 rabbit anti-dog IgG (EM Sciences, Hatfield, PA) and then with protein A conjugated to 15-nm gold particles (Amersham Biosciences). Preimmune serum was used as the control.
RESULTS
Ac-MTP-1 is secreted in a catalytically active form and can be purified by ion-exchange or gelatin-affinity chromatography.
Ac-MTP-1 was expressed in baculovirus and purified at a yield of 6 mg liter−1 culture medium. The recombinant protein did not bind to nickel agarose, despite the presence of a six-His tag. We experienced a similar problem with recombinant Ac-MEP-1 (27), a neprilysin-like metalloprotease, and attributed the lack of binding to nickel to spatial inaccessibility of the charged six-His tag to the immobilized nickel. While the enzyme was purified to homogeneity (single band on sodium dodecyl sulfate-polyacrylamide gels stained with Coomassie brilliant blue) using gelatin Sepharose, the majority of the recombinant protein did not bind to the resin (Fig. 1A). The recombinant protein was instead purified by binding to an SP-FF cation-exchange column, and protein was eluted from the column with 235 to 375 mM NaCl (Fig. 1B). This method produced routinely high yields of recovery of pure protein (Fig. 1B, inset). The purified protein migrated with an apparent molecular mass of 69 kDa and was recognized by Western blotting with antiserum raised against recombinant MTP-1 expressed in E. coli (28) as well as with a monoclonal anti-six-His antibody (Fig. 1C). The observed molecular mass of 69 kDa was slightly larger than the predicted size of the fusion protein (62 kDa); however, N-linked glycosylation at two predicted sites (28) probably accounted for the discrepancy between the predicted and observed molecular masses.
Recombinant Ac-MTP-1 degrades connective tissue macromolecules.
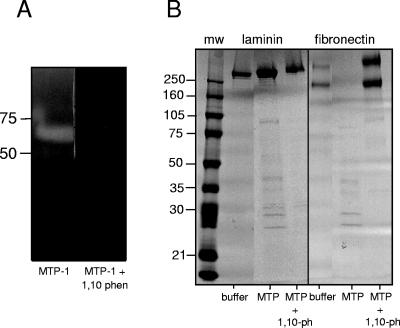
Ac-MTP-1 readily degraded gelatin, and its activity was completely inhibited by 1,10-phenanthroline (Fig. 2A), indicating that the secreted recombinant protein was catalytically active. Ac-MTP-1 digested proteins found in connective tissue, including laminin, fibronectin, and collagen, but not elastin. After 24 h at 37°C, MTP-1 completely digested fibronectin but only partially digested laminin, leaving the majority of the substrate protein intact (Fig. 2). MTP-1 also displayed collagenase activity when Azocoll was used as the substrate (Fig. 3). The digestion of all three substrates was completely inhibited in the presence of 10 μM 1,10-phenanthroline. Protein substrates that are not found in connective tissues but are cleaved by other hookworm proteases (hemoglobinases), such as hemoglobin and fibrinogen (26), were not degraded by MTP-1 (not shown).
FIG. 2.
(A) Hydrolysis of 10% gelatin in a zymogram gel by recombinant Ac-MTP-1 in the presence or absence of 10 μM 1,10-phenanthroline. Molecular mass markers (kDa) are listed on the side. (B) Recombinant Ac-MTP-1 completely degraded fibronectin and partially degraded laminin over a 24-h period, but not in the presence of 10 μM 1,10-phenanthroline. Molecular mass markers (in kDa) are listed on the side. The substrate used for each experiment is indicated at the top of the gel, and the presence or absence of a recombinant protease or protease inhibitor is indicated at the bottom of each lane.
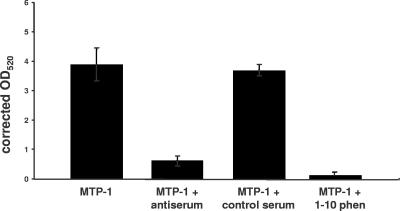
FIG. 3.
Digestion of Azocoll by recombinant Ac-MTP-1 and inhibition of proteolytic digestion by dog antiserum to Ac-MTP-1 and 1,10-phenanthroline but not by control dog serum. Values shown are the means of triplicate assays, and errors bars denote standard deviations.
Canine anti-MTP-1 IgG neutralizes proteolytic activity in vitro.
Fifty nanograms of purified IgG from a dog that was immunized with Ac-MTP-1 reduced MTP-1-mediated cleavage of Azocoll by 85% (P = 0.006; Fig. 3), in contrast with just a 5% reduction in Azocoll cleavage in the presence of normal dog IgG (P = 0.264). Preincubation of MTP-1 with 10 μM 1,10-phenanthroline resulted in a 98% reduction in Azocoll digestion (P = 0.002).
Anti-Ac-MTP-1 serum and chemical inhibitors of metalloproteases inhibit penetration of skin by L3.
Preincubation of A. caninum L3 with dog anti-MTP-1 serum inhibited 70 to 75% of L3 from penetrating canine skin in vitro (P = 0.024) in two separate trials, each consisting of three separate counts of L3 (Table 1). Serum taken from the same dog prior to immunization resulted in just a 5 to 10% reduction in larval migration. Preincubation of L3 with two different metalloprotease inhibitors, 10 mM EDTA and 1,10-phenanthroline (10 or 100 μM), also reduced the number of L3 that successfully penetrated skin by 51 (P = 0.006), 43 (P = 0.005), and 61% (P = 0.003), respectively (Table 1).
TABLE 1.
Inhibition of A. caninum L3 migration through dog skin in vitro by antiserum to recombinant Ac-MTP-1, but not by prevaccination serum, and inhibition of migration through hamster skin in vitro by the metalloprotease inhibitors EDTA and 1,10-phenanthroline (solubilized in distilled H2O)a
| L3 treatment | No. of L3 that did not penetrate skin/total no. of L3 (%)
|
P value | ||
|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | ||
| Prevaccination serum | 2/40 (5) | 4/40 (10) | ||
| Anti-MTP-1 serum | 30/40 (75) | 28/40 (70) | 0.024 | |
| Phosphate-buffered saline | 0/300 (0) | 20/300 (7) | 0/300 (0) | |
| EDTA (10 mM) | 180/300 (60) | 150/300 (50) | 130/300 (43) | 0.006 |
| 1,10-Phenanthroline (10 μM) | 130/300 (43) | 120/300 (40) | 140/300 (47) | 0.005 |
| 1,10-Phenanthroline (100 μM) | 170/300 (57) | 170/300 (57) | 200/300 (67) | 0.003 |
Each experiment was conducted two (sera) or three (chemical inhibitors) times, and the mean number of L3 that did not penetrate skin from three separate experiments per trial is presented.
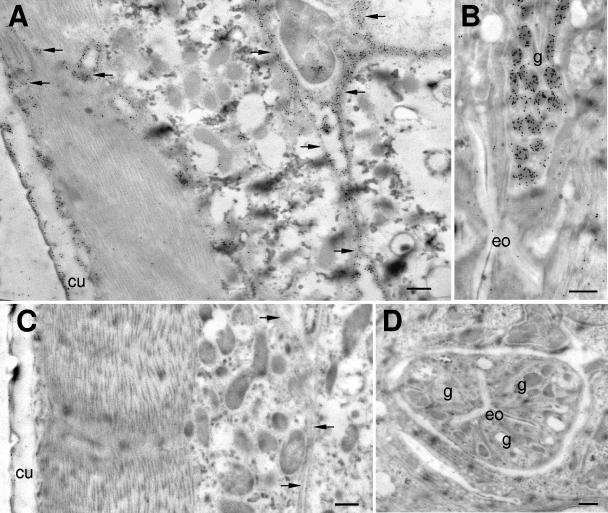
Localization of native Ac-MTP-1 enzyme in L3.
Ac-MTP-1 immunolocalized both to the secretory granules in the glandular esophagus and to the cuticles of exsheathed L3. In addition, the protein localized to the basal lamina of the body cavity or the channels that connect the glandular esophagus to the L3 surface (Fig. 4). However, it was not expressed in the esophageal lumen of A. caninum L3. There were some differences between the localization of the native protein by antibodies from a dog (Fig. 4A) and a rabbit (Fig. 4B), with the rabbit antibodies localizing the protein in both the glandular esophagus and the cuticle, whereas the dog antibody labeled primarily the surfaces and the basal layer of the L3 cuticles as well as the channels leading to the cuticle from the esophagus. No specific staining was observed with preimmune serum (Fig. 4C and D).
FIG. 4.
Localization of Ac-MTP-1 to body channels, cuticles (A), and secretory granules in the glandular esophagus (B) of serum-activated A. caninum L3, using antiserum against recombinant Ac-MTP-1 raised in a dog (A) and antiserum against recombinant Ac-MTP-1 raised in a rabbit (B). Control dog serum did not bind strongly to any structures (C and D). cu, cuticle; g, granules in the glandular esophagus; eo, esophagus. Arrows denote body channels. Bar, 500 nm.
DISCUSSION
The results of this study indicate that Ac-MTP-1 plays a critical role in skin penetration and the degradation of skin macromolecules. Enzymatically active Ac-MTP-1 readily hydrolyzed laminin, fibronectin, and collagen, supporting a role in host matrix degradation. Moreover, antiserum to MTP-1 inhibited proteolytic cleavage of collagen and greatly reduced larval penetration of skin in vitro, strongly suggesting that MTP-1 is critical for initial penetration of the epidermis.
Hotez et al. described an o-phenanthroline-sensitive metalloprotease activity secreted by live L3 of A. caninum and the human hookworm, Ancylostoma duodenale, that resolved into a major band of 68 kDa on substrate gels (11). It is highly likely that the activity described by Hotez et al. was attributable to Ac-MTP-1 for the following reasons (1). A. caninum L3 secrete a phenanthroline-sensitive protease that, like recombinant MTP-1, completely degrades fibronectin and partially degrades laminin but does not degrade elastin (2). The ∼68-kDa major protease that Hotez et al. described from substrate gels corresponds with the predicted molecular mass of the Ac-MTP-1 gene product (28) and the zone of hydrolysis that we observed in gelatin gels that had been digested with recombinant Ac-MTP-1 (Fig. 1).
Using immunoelectron microscopy, we localized the anatomic site of expression of MTP-1 to the granules of the glandular esophagus of A. caninum exsheathed L3. We did not, however, detect MTP-1 in the lumen of the esophagus, suggesting that MTP-1 does not exit L3 via the oral opening. Instead, MTP-1 was detected in the body channels that connect the esophagus to the cuticle, which are also intertwined between muscle blocks; the channels appear to exit onto the basal layer of the cuticle. The channels are believed to function as a network akin to vascular channels that are capable of efficiently transporting proteins to the cuticles of filarial nematodes (20). Since MTP-1 is also localized in the cortical layer of the cuticle and thus the surfaces of L3, it is potentially released into host tissues from the cuticle. Notably, MTP-1 is not found in the cuticles of developmentally arrested L3 (data not shown). Previously, it was also shown that MTP-1 is not secreted by developmentally arrested L3 in the soil but is rapidly released when worms are given a host-like stimulus (serum) (8, 13, 28). A family of pathogenesis-related proteins called the Ancylostoma secreted proteins (ASPs) are also secreted upon host-like activation of A. caninum larvae (9, 10). We recently localized the anatomic expression site of A. caninum ASP-2 (1) to the same structures as those reported here for MTP-1, implying that secreted proteins involved in the invasion process are packaged in esophageal secretory granules that are rapidly shuttled to the cuticle via a network of body channels. ASP-2, however, was also localized to the esophageal lumen of L3 and was suggested to also be secreted from the oral opening during the larval feeding process that resumes following entry into the host (1). The differences in anatomic localization with the dog and rabbit antibodies might be attributed to the presentation/recognition of different epitopes between dogs and rabbits. The expression hosts used to make the immunogens also differed—the rabbit antibody was raised to insoluble recombinant Ac-MTP-1 expressed in E. coli in an earlier study (28), while the dog antibody was raised to the catalytically active (and therefore properly folded) recombinant MTP-1 described herein.
Other parasitic nematodes secrete metalloproteases that are thought to be involved in host penetration, although catalytically active recombinant enzymes have not been reported for any of these parasites. Coincident with the third molt, larvae of the sheep barber's pole worm, Hemonchus contortus, begin to secrete protein into the culture medium, including a zinc metalloprotease (5). The native, purified 46-kDa protein digested several proteins of host origin, including fibrinogen and fibronectin, as well as gelatin; however, unlike the case with Ac-MTP-1, limited digestion of laminin and collagen was observed, suggesting that this enzyme might perform roles other than tissue migration, such as molting. Infective larvae of S. stercoralis penetrate the dermal matrix by using a secreted metalloprotease, termed Ss40 (3). The mRNA that is thought to encode Ss40, termed strongylastacin, was recently cloned, and the predicted protein, like Ac-MTP-1, was shown to belong to clan MA and be a multidomain protein, with astacin-, EGF-, and CUB-like domains, in that order (6).
In a previous study, the vaccination of dogs with recombinant Ac-MTP-1 expressed in E. coli in an insoluble form resulted in an inverse association between antibody titers and worm burdens after larval challenge (13). A similar inverse association was noted between IgG2 antibody titers and median quantitative egg counts (13). More recently, Ay-MTP-1, the orthologue of Ac-MTP-1 from the related hookworm Ancylostoma ceylanicum, was shown to provide significant reductions in worm burdens and egg counts when used to vaccinate hamsters in a challenge model of hookworm infection (21). Vaccination with a cocktail of Ay-MTP-1 and Ay-ASP-2 resulted in even greater protection levels than those obtained with each antigen alone when parasitological burdens and clinical outcomes (hemoglobin levels and body weights) were assessed (21).
Our findings presented here, particularly the ability of antiserum and metalloprotease inhibitors to inhibit protease function and larval skin penetration in vitro, suggest that MTP-1 is involved in skin penetration and migration through connective tissues, and MTP-1 appears to be a suitable candidate for the development of a vaccine that targets larval invasion of the host.
Acknowledgments
This work was supported by grants from The Bill and Melinda Gates Foundation, the Sabin Vaccine Institute, and National Institutes of Health (NIAID grant AI-32726). A.L. is the recipient of an R.D. Wright Career Development Award from the National Health and Medical Research Council of Australia.
We thank Yan Wang, Lilian Bueno, and Michael Smout for technical assistance.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Bethony, J. M., A. Loukas, M. J. Smout, S. Brooker, S. Mendez, J. Plieskatt, G. Goud, M. E. Bottazzi, B. Zhan, Y. Wang, A. L. Williamson, S. Lustigman, R. Corrêa-Oliveira, S. Xiao, and P. J. Hotez. 2005. Antibodies against a secreted protein from hookworm larvae reduce the intensity of hookworm infection in humans and vaccinated laboratory animals. FASEB J. 19:1743-1745. [DOI] [PubMed] [Google Scholar]
- 2.Bond, J. S., and R. J. Beynon. 1995. The astacin family of metalloendopeptidases. Protein Sci. 4:1247-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brindley, P. J., A. A. Gam, J. H. McKerrow, and F. A. Neva. 1995. Ss40: the zinc endopeptidase secreted by infective larvae of Strongyloides stercoralis. Exp. Parasitol. 80:1-7. [DOI] [PubMed] [Google Scholar]
- 4.de Silva, N. R., S. Brooker, P. J. Hotez, A. Montresor, D. Engels, and L. Savioli. 2003. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 19:547-551. [DOI] [PubMed] [Google Scholar]
- 5.Gamble, H. R., R. H. Fetterer, and L. S. Mansfield. 1996. Developmentally regulated zinc metalloproteinases from third- and fourth-stage larvae of the ovine nematode Haemonchus contortus. J. Parasitol. 82:197-202. [PubMed] [Google Scholar]
- 6.Gomez Gallego, S., A. Loukas, R. W. Slade, F. A. Neva, R. Varatharajalu, T. B. Nutman, and P. J. Brindley. 2005. Identification of an astacin-like metallo-proteinase transcript from the infective larvae of Strongyloides stercoralis. Parasitol. Int. 54:123-133. [DOI] [PubMed] [Google Scholar]
- 7.Haffner, A., A. Z. Guilavogui, F. W. Tischendorf, and N. W. Brattig. 1998. Onchocerca volvulus: microfilariae secrete elastinolytic and males nonelastinolytic matrix-degrading serine and metalloproteases. Exp. Parasitol. 90:26-33. [DOI] [PubMed] [Google Scholar]
- 8.Hawdon, J. M., and P. J. Hotez. 1996. Hookworm: developmental biology of the infectious process. Curr. Opin. Genet. Dev. 6:618-623. [DOI] [PubMed] [Google Scholar]
- 9.Hawdon, J. M., B. F. Jones, D. R. Hoffman, and P. J. Hotez. 1996. Cloning and characterization of Ancylostoma-secreted protein. A novel protein associated with the transition to parasitism by infective hookworm larvae. J. Biol. Chem. 271:6672-6678. [DOI] [PubMed] [Google Scholar]
- 10.Hawdon, J. M., S. Narasimhan, and P. J. Hotez. 1999. Ancylostoma secreted protein 2: cloning and characterization of a second member of a family of nematode secreted proteins from Ancylostoma caninum. Mol. Biochem. Parasitol. 99:149-165. [DOI] [PubMed] [Google Scholar]
- 11.Hotez, P., J. Haggerty, J. Hawdon, L. Milstone, H. R. Gamble, G. Schad, and F. Richards. 1990. Metalloproteases of infective Ancylostoma hookworm larvae and their possible functions in tissue invasion and ecdysis. Infect. Immun. 58:3883-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotez, P. J., J. Ashcom, Z. Bin, J. Bethony, A. Williamson, J. M. Hawdon, F. Jianjun, A. Dobardzic, I. Rizo, J. Bolden, Q. Jin, W. Yan, R. Dobardzic, S. Chung-Debose, M. Crowell, B. Datu, A. Delaney, D. Dragonovski, Y. Jiang, L. Yueyuan, K. Ghosh, A. Loukas, W. Brandt, P. K. Russell, and B. C. Zook. 2002. Effect of vaccinations with recombinant fusion proteins on Ancylostoma caninum habitat selection in the canine intestine. J. Parasitol. 88:684-690. [DOI] [PubMed] [Google Scholar]
- 13.Hotez, P. J., J. Ashcom, B. Zhan, J. Bethony, A. Loukas, J. Hawdon, Y. Wang, Q. Jin, K. C. Jones, A. Dobardzic, R. Dobardzic, J. Bolden, I. Essiet, W. Brandt, P. K. Russell, B. C. Zook, B. Howard, and M. Chacon. 2003. Effect of vaccination with a recombinant fusion protein encoding an astacin-like metalloprotease (MTP-1) secreted by host-stimulated Ancylostoma caninum third-stage infective larvae. J. Parasitol. 89:853-855. [DOI] [PubMed] [Google Scholar]
- 14.Hotez, P. J., B. Zhan, J. M. Bethony, A. Loukas, A. Williamson, et al. 2003. Progress in the development of a recombinant vaccine for human hookworm disease: the Human Hookworm Vaccine Initiative. Int. J. Parasitol. 33:1245-1258. [DOI] [PubMed] [Google Scholar]
- 15.Knox, D. P., and D. G. Jones. 1990. Studies on the presence and release of proteolytic enzymes (proteinases) in gastro-intestinal nematodes of ruminants. Int. J. Parasitol. 20:243-249. [DOI] [PubMed] [Google Scholar]
- 16.Loukas, A., J. M. Bethony, A. L. Williamson, G. N. Goud, S. Mendez, B. Zhan, J. M. Hawdon, M. Elena Bottazzi, P. J. Brindley, and P. J. Hotez. 2004. Vaccination of dogs with a recombinant cysteine protease from the intestine of canine hookworms diminishes the fecundity and growth of worms. J. Infect. Dis. 189:1952-1961. [DOI] [PubMed] [Google Scholar]
- 17.Loukas, A., S. L. Constant, and J. M. Bethony. 2005. Immunobiology of hookworm infection. FEMS Immunol. Med. Microbiol. 43:115-124. [DOI] [PubMed] [Google Scholar]
- 18.Lustigman, S., B. Brotman, T. Huima, A. M. Prince, and J. H. McKerrow. 1992. Molecular cloning and characterization of onchocystatin, a cysteine proteinase inhibitor of Onchocerca volvulus. J. Biol. Chem. 267:17339-17346. [PubMed] [Google Scholar]
- 19.McKerrow, J. H., P. J. Brindley, M. Brown, and A. A. Gam. 1990. Strongyloides stercoralis: identification of a protease that facilitates penetration of the skin by the infective larvae. Exp. Parasitol. 70:134-143. [DOI] [PubMed] [Google Scholar]
- 20.McKerrow, J. H., T. Huima, and S. Lustigman. 1999. Do filarid nematodes have a vascular system? Parasitol. Today 15:123. [DOI] [PubMed] [Google Scholar]
- 21.Mendez, S., B. Zhan, G. Goud, K. Ghosh, A. Dobardzic, W. Wu, S. Liu, V. Deumic, R. Dobardzic, Y. Liu, J. Bethony, and P. J. Hotez. 2005. Effect of combining the larval antigens Ancylostoma secreted protein 2 (ASP-2) and metalloprotease 1 (MTP-1) in protecting hamsters against hookworm infection and disease caused by Ancylostoma ceylanicum. Vaccine 23:3123-3130. [DOI] [PubMed] [Google Scholar]
- 22.Newport, G. R., J. H. McKerrow, R. Hedstrom, M. Petitt, L. McGarrigle, P. J. Barr, and N. Agabian. 1988. Cloning of the proteinase that facilitates infection by schistosome parasites. J. Biol. Chem. 263:13179-13184. [PubMed] [Google Scholar]
- 23.Robinson, L. A., D. M. Wilson, N. G. Delaet, E. K. Bradley, S. M. Dankwardt, J. A. Campbell, R. L. Martin, H. E. Van Wart, K. A. Walker, and R. W. Sullivan. 2003. Novel inhibitors of procollagen C-proteinase. Part 2. Glutamic acid hydroxamates. Bioorg. Med. Chem. Lett. 13:2381-2384. [DOI] [PubMed] [Google Scholar]
- 24.Salter, J. P., Y. Choe, H. Albrecht, C. Franklin, K. C. Lim, C. S. Craik, and J. H. McKerrow. 2002. Cercarial elastase is encoded by a functionally conserved gene family across multiple species of schistosomes. J. Biol. Chem. 277:24618-24624. [DOI] [PubMed] [Google Scholar]
- 25.Williamson, A. L., P. J. Brindley, G. Abbenante, P. Prociv, C. Berry, K. Girdwood, D. I. Pritchard, D. P. Fairlie, P. J. Hotez, J. P. Dalton, and A. Loukas. 2002. Cleavage of hemoglobin by hookworm cathepsin D aspartic proteases and its potential contribution to host specificity. FASEB J. 16:1458-1460. [DOI] [PubMed] [Google Scholar]
- 26.Williamson, A. L., P. J. Brindley, and A. Loukas. 2003. Hookworm cathepsin D aspartic proteases: contributing roles in the host-specific degradation of serum proteins and skin macromolecules. Parasitology 126:179-185. [DOI] [PubMed] [Google Scholar]
- 27.Williamson, A. L., P. Lecchi, B. E. Turk, Y. Choe, P. J. Hotez, J. H. McKerrow, L. C. Cantley, M. Sajid, C. S. Craik, and A. Loukas. 2004. A multi-enzyme cascade of hemoglobin proteolysis in the intestine of blood-feeding hookworms. J. Biol. Chem. 279:35950-35957. [DOI] [PubMed] [Google Scholar]
- 28.Zhan, B., P. J. Hotez, Y. Wang, and J. M. Hawdon. 2002. A developmentally regulated metalloprotease secreted by host-stimulated Ancylostoma caninum third-stage infective larvae is a member of the astacin family of proteases. Mol. Biochem. Parasitol. 120:291-296. [DOI] [PubMed] [Google Scholar]