Abstract
Postweaning multisystemic wasting syndrome (PMWS) is a disease of nursery and fattening pigs characterized by growth retardation, paleness of the skin, dyspnea, and increased mortality rates. Porcine circovirus 2 (PCV2) has been demonstrated to be the cause of PMWS. However, other factors are needed for full development of the syndrome, and porcine reproductive and respiratory syndrome virus (PRRSV) infection has been suggested to be one of them. Twenty-four conventional 5-week-old pigs were distributed in four groups: control (n = 5), PRRSV inoculated (n = 5), PCV2 inoculated (n = 7), and PRRSV and PCV2 inoculated (n = 7). The two groups inoculated with PRRSV showed growth retardation. Pigs inoculated with both PRRSV and PCV2 had increased rectal temperature. One of these pigs developed wasting, had severe respiratory distress, and died. The most important microscopic lesion in pigs inoculated with PCV2 was lymphocyte depletion with histiocytic infiltration of the lymphoid organs, more severe and in a wider range of tissues in doubly inoculated pigs. Interstitial pneumonia was observed in the three inoculated groups. PCV2 nucleic acid was found by in situ hybridization in larger amounts and in a wider range of lymphoid tissues in PRRSV- and PCV2-inoculated than in PCV2-inoculated pigs. TaqMan PCR was performed to quantify the PCV2 loads in serum during the experiment. PCV2 loads were higher in doubly inoculated pigs than in pigs inoculated with PCV2 alone. These findings indicate that severe disease can be reproduced in conventional 5-week-old pigs by inoculation of PRRSV and PCV2. Moreover, these results support the hypothesis that PRRSV infection enhances PCV2 replication.
Postweaning multisystemic wasting syndrome (PMWS) is a relatively new disease of swine associated with important mortality rates in nursery and fattening pigs (17). This disease was first described in Canada in 1991 (10) and now is considered to be widespread throughout the most important swine production areas of the world (2). Pigs affected with PMWS show growth retardation, dyspnea, paleness of the skin (occasionally icterus), and sometimes diarrhea (21). Characteristic macroscopic findings are enlargement of lymph nodes and noncollapsed lungs with tan mottling (7, 21). Microscopic lesions can be detected in a number of tissues, the most characteristic being those of lymphoid organs. These lesions consist of lymphocyte depletion with histiocytic and multinucleate giant cell infiltration in the lymphoid tissues. Characteristic intracytoplasmic viral inclusion bodies can also be found within the infiltrating histiocytes (21). Other common lesions described for PMWS include interstitial pneumonia, periportal to diffuse hepatitis, and interstitial nephritis (7, 21).
Porcine circovirus 2 (PCV2) is a member of the family Circoviridae that has been demonstrated to be the cause of PMWS (12, 14). Susceptible pigs inoculated with PCV2 develop the typical microscopic lesions of PMWS but only a mild form of the clinical disease (3, 4, 12, 13, 17). These results have suggested that other, concomitant factors may be needed for the development of clinical PMWS. Severe disease has been reproduced in a proportion of pigs coinfected with PCV2 and porcine parvovirus (PPV) (3, 8, 12, 13), but the mechanism of this synergy is not known yet. Since both viruses infect macrophages and their replication is dependent on cellular enzymes expressed during S phase of the cell cycle (29), it has been suggested that the previous activation of macrophages by PPV may promote the replication of PCV2 or, alternatively, that other, unknown factors may enhance the replication of both viruses (9). However, simultaneous PPV and PCV2 infections in the field are sporadic events (9) and may not explain most of the PMWS cases seen under field conditions. On the other hand, porcine reproductive and respiratory syndrome virus (PRRSV) infection is widespread in many parts of the world (5). Natural coinfection with PCV2 and PRRSV has been reported in proportions of pigs affected with PMWS ranging from 20% in western Canada (2) to 60% in the United States (27) and 48% in Spain (24a). Experimental studies of coinfection with PRRSV and PCV2 have also reproduced microscopic lesions of PMWS and/or PMWS (1, 11). Since PRRSV also replicates in macrophages, it has been suggested that this virus can produce an effect similar to that observed with PPV (1).
The objective of the present study was to reproduce PMWS in conventionally reared pigs by experimental inoculation with PRRSV and PCV2 Spanish isolates. Moreover, the proposed enhancement of PCV2 replication by PRRSV (1) was investigated by using quantitative methods.
MATERIALS AND METHODS
Animals.
Twenty-four conventional 31- to 40-day-old pigs from three different litters were used. The piglets were weaned at 2 weeks of age, bled, ear tagged, and kept in isolated experimental facilities. All pigs were found seronegative for PRRSV by an immunoperoxidase monolayer assay (IPMA) and were found to have slow titers of antibodies to PCV2 (1:20 to 1:320) by the same technique at the beginning of the experiment.
PRRSV inoculum.
A sixth passage of the Olot/91 PRRSV strain (18) was obtained with porcine alveolar macrophages, titrated (106.1 50% tissue culture infective doses [TCID50]/ml), divided into aliquots, and frozen at −80°C until used. PRRSV-inoculated pigs received 2.5 ml of the viral inoculum (total dose of 106.5 TCID50 per pig) by the intranasal route.
PCV2 inoculum.
A swine kidney (SK) cell line free of porcine circovirus 1 (PCV1) was inoculated with a tissue homogenate from a pig with PMWS to obtain a cell line persistently infected with PCV2. The homogenate was tested by PCR and/or IPMA with cell cultures to exclude the presence of PRRSV, PPV, pseudorabies virus, transmissible gastroenteritis virus, porcine respiratory coronavirus, swine influenza virus, and PCV1. The cells were passaged once, and the supernatant from the second passage was recovered, clarified by centrifugation at 650 × g, and further ultracentrifuged at 100,000 × g. The viral pellet was resuspended in Dulbecco modified Eagle medium, titrated with noninfected SK cells (104.8 TCID50/ml), divided into aliquots, and frozen at −80°C until used. PCV2-inoculated pigs received intranasally 2 ml of the viral inoculum (total dose of 105.1 TCID50 per pig).
Experimental design.
Pigs were randomly distributed into four groups kept in different isolation rooms. The first group consisted of five pigs kept as uninfected controls. The second group included five pigs inoculated with PRRSV on day 0 of the experiment. The third group consisted of seven pigs inoculated with PCV2 on day 7 of the experiment. The seven remaining pigs formed the fourth group and were inoculated with PRRSV on day 0 and PCV2 on day 7 of the experiment. Body weight was measured and blood samples were collected at 0, 7, 14, 21, 28, and 32 days of the experiment (DE). Blood samples were centrifuged, and serum was frozen at −80°C until used. Rectal temperatures were measured every second day during the entire experimental period. Pigs were euthanatized at 32 DE, and a complete necropsy was performed. Samples of lungs, inguinal and mesenteric lymph nodes, tonsils, thymus, spleen, liver, kidneys, ileum, and nasal turbinates were collected and fixed by immersion in 10% buffered formalin. Pigs were managed in accordance with European Guidelines for Animal Welfare.
Pathological analysis.
Tissue samples were dehydrated, embedded in paraffin wax, sectioned at 4 μm, and stained with hematoxylin and eosin. An immunohistochemical technique for PRRSV was performed by following previously published protocols (25). An in situ hybridization technique to detect PCV2 nucleic acid was carried out as previously described (21) by using a PCV2-specific 40-nucleotide probe (22). Evaluation of microscopic lesions, amount of PCV2 DNA, and amount of PRRSV antigen was done in a blind fashion. Interstitial pneumonia was considered mild, moderate, or severe based on the increased thickness of the alveolar walls due to the accumulation of mononuclear inflammatory cells, the presence of these cells in the lumen of alveoli, and the proportion of affected tissue. Lymphoid lesions were considered mild, moderate or severe based on lymphoid depletion, histiocytic infiltration, and presence of the intracytoplasmic inclusion bodies characteristic of PCV2 infection. Positive in situ hybridization results were scored as +, ++, or +++ based on the amount of labeled PCV2 DNA as previously described (21).
Serological analysis.
An IPMA technique to detect antibodies to PCV2 was performed (20) with PCV2-infected SK cells. Antibodies against PRRSV were detected by using a previously described IPMA technique (18).
PCV2 PCR.
PCV2 DNA was extracted from pig sera by using a QIAamp DNA blood minikit (Qiagen Inc., Valencia, Calif.) according to the manufacturer's instructions. A previously described PCR technique for the detection of PCV2 was performed (19).
PRRSV RT-PCR.
PRRSV RNA was extracted from 750 μl of pig sera by using Nucleospin RNA virus F (Macherey Nagel, Düren, Germany) according to the manufacturer's instructions. A single-step reverse transcription (RT)-PCR for the detection of PRRSV was performed. The primers were designed based on the sequence of the ORF7 Olot/91 PRRSV strain (GenBank accession number X92942). The primer sequence was kindly provided by Carmen Vela (Ingenasa, Madrid, Spain). The forward primer sequence was 5′-CCTCGTCAAGTATGGCCGGTA-3′, and the reverse primer sequence was 5′-GACTGTCAAATTAGCTTGCACCC-3′. These PRRSV-specific primers amplified a 400-bp DNA fragment. The RT-PCR mixture contained 10× reaction buffer (10 mM Tris [pH 8.3], 50 mM KCl), 200 μM each deoxynucleoside triphosphate, 2 mM MgCl2, 0.5 μM each primer, 20 U of RNase inhibitor, 20 U of Superscript II reverse transcriptase (Life Technologies, Paisley, United Kingdom), 1.25 U of AmpliTaq Gold polymerase (PE Applied Biosystems, Branchburg, N.J.), and 5 μl of RNA. The conditions for amplification were as follows: RT at 50°C (30 min); activation of TaqGold at 95°C (10 min); 35 cycles of denaturation at 95°C (1 min), annealing at 40°C (1 min), and extension at 72°C (1 min); and final extension at 72°C (5 min). The amplified products were run in a 2% agarose gel and visualized by staining with 0.5 μg of ethidium bromide/ml. The sensitivity of this RT-PCR technique was demonstrated to be 100 TCID50/ml.
TaqMan PCR for PCV2.
To quantify PCV2 DNA in serum samples at 14, 21, 28, and 32 DE, a real-time fluorescent-probe PCR was used. The TaqMan probe(Fam-ACGCTTGACAGTATATCCGAAGGTGCGG-Tamra) and primers(T004F, 5′-AATCTCAKCATGTCCACCGC; T004B, 5′-AAAAATGGCATCTTCAACACC) amplifying a 100-bp fragment from the cap gene of PCV2 were designed by using Primer Express Oligo Design software (Perkin-Elmer Biosystems, Weiterstadt, Germany). DNA was isolated from 200 μl of serum by using the QIAamp DNA blood minikit according to the manufacturer's instructions. TaqMan PCR was performed with a 50-μl volume and a 10-μl aliquot of a DNA preparation. Final concentrations were 250 nM each primer, 100 nM TaqMan probe, 50 ng of calf thymus DNA/μl, 6 mM MgCl2, and 1× PCR buffer II (0.9 μM roxithromycin, 200 μM each deoxynucleoside triphosphate, 1.25 U of AmpliTaq Gold polymerase; Perkin-Elmer). Cycling parameters were 95°C for 12 min and 45 cycles at 95°C for 20 s, 68°C for 20 s, and 72°C for 20 s; an ABI prism 5700 thermocycler with optical tubes was used. For a standard curve, serial dilutions of plasmid pIC2 (PCV2 genome cloned into pCR2.1) of 107 to 100 copies were used. Each assay was performed in duplicate.
Statistical methods.
Statistical analysis was performed by using the SAS system (SAS/STAT Users' Guide: Statistics, version 8 [SAS Institute Inc., Cary, N.C.]). The instruction LSMEANS of the general linear model procedure was used to analyze differences in average daily weight gain between groups. A mixed linear model (30) was designed by using the mixed procedure for the statistical analysis of the TaqMan PCR results.
RESULTS
Clinical evaluation.
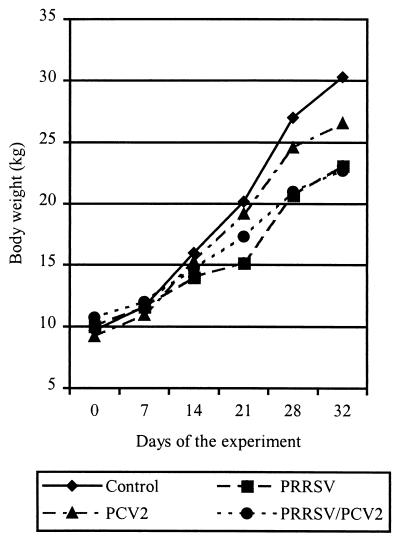
Only pigs inoculated with PRRSV and PCV2 (3 of 7) had increased rectal temperature (>40.5°C) from 22 to 29 DE. One of these pigs developed wasting (loss of 0.5 kg of body weight in 4 days), had severe respiratory distress, and died at 25 DE (18 days after PCV2 inoculation). One pig in the same group showed intense paleness of the skin from 20 DE to the end of the experiment. One pig inoculated with PCV2 alone died at 28 DE during blood extraction due to cardiorespiratory shock. The evolution of body weight during the experiment is shown in Fig. 1. Average daily weight gain was higher in control pigs (0.64 kg/day), followed in decreasing order by pigs inoculated with PCV2 (0.56 kg/day), PRRSV (0.41 kg/day), and both viruses (0.35 kg/day). Significant differences (P < 0.05) were found between groups of pigs inoculated with PRRSV (singly and doubly inoculated) and pigs in the other groups (control and PCV2 inoculated). No significant differences were found between pigs inoculated with PRRSV alone and pigs inoculated with PRRSV and PCV2. The difference between control pigs and pigs inoculated with PCV2 alone also was not significant.
FIG. 1.
Evolution of body weight during the experiment.
Gross findings.
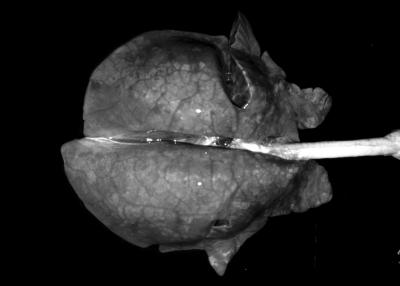
At necropsy, 5 of 5 PRRSV-inoculated, 3 of 7 PCV2-inoculated, and 7 of 7 PRRSV- and PCV2-inoculated pigs had a slight increase in the size of the lymph nodes. The pig that was inoculated with PRRSV and PCV2 and that died at 25 DE had noncollapsed lungs with interstitial edema and dark areas on the lung surface (Fig. 2); this animal also had serous liquid in the thoracic (180 ml) and abdominal (15 ml) cavities. No significant bacteria were isolated from the thoracic liquid and lungs of this pig (data not shown). Two more of the PRRSV- and PCV2-inoculated pigs and one PRRSV-inoculated pig had lungs with diffuse tan mottling. Slight hyperkeratosis of the gastric pars oesophagea was found in one of the PRRSV-inoculated pigs, three of the PCV2-inoculated pigs, and one of the PRRSV- and PCV2-inoculated pigs. Moreover, one PRRSV-inoculated pig had a slight laceration of the pars oesophagea of the stomach, and the dead pig (inoculated with both PRRSV and PCV2) had marked ulceration of the pars oesophagea. Control pigs did not have any macroscopic lesions.
FIG. 2.
Lung of the PRRSV- and PCV2-inoculated pig that died at 25 DE. Note the absence of pulmonary collapse and the interstitial edema. All images in this report were adjusted by using Adobe Photoshop 5.0 LE for Windows with an IBM-compatible PC.
Light microscopy examination.
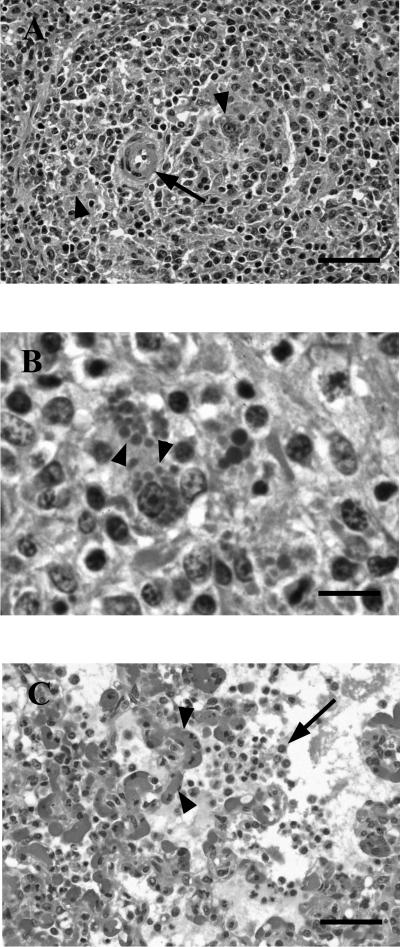
Microscopic lesions are summarized in Table 1. The pig that died at 25 DE (PRRSV and PCV2 inoculated) had moderate lymphocyte depletion with moderate histiocytic infiltration of the lymphoid organs examined (Fig. 3A). PCV2 intracytoplasmic inclusion bodies were seen within the infiltrating histiocytes in all studied lymphoid organs except the thymus (Fig. 3B). Moreover, this pig had moderate to severe diffuse interstitial pneumonia with intense interstitial edema, multifocal hemorrhages, and disseminated intravascular coagulation (Fig. 3C). Other tissues of this pig were processed for a larger histopathological study. Perivascular granulomatous inflammatory infiltrates were observed in the hypodermis, bone marrow, parotid gland, epididymis, and branches of the artery of the ductus deferens located between the testicle and the epididymis. No lesions were found in the colon, duodenum, stomach, urinary bladder, pancreas, adrenal gland, cardiac muscle, skeletal muscle, cerebral cortex, cerebellum, or testicles of this pig.
TABLE 1.
Microscopic lesions
| Group (n) | No. of pigs with the following lesions:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Interstitial pneumonia
|
Lymphoid lesions characteristic of PMWS
|
Mild multifocal lymphoplasmacytic hepatitis | Mild interstitial nephritis | Multifocal lymphoplasmacytic rhinitis | |||||
| Mild | Moderate | Severe | Mild | Moderate | Severe | ||||
| Control (5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| PRRSV (5) | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| PCV2 (7) | 3 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 5 |
| PRRSV and PCV2 (7) | 4 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 3 |
FIG. 3.
Microscopic findings. (A) Periarteriolar lymphoid sheet of the spleen of a PRRSV- and PCV2-inoculated pig. The arteriole (arrow) is surrounded by infiltrating histiocytes (arrowheads). Hematoxylin-eosin (HE) staining. Bar, 50 μm. (B) Inguinal lymph node of a PRRSV- and PCV2-inoculated pig. The node is infiltrated by histiocytes; note the characteristic PCV2 inclusion bodies within the cytoplasm of some of them (arrowheads). HE staining. Bar, 15 μm. (C) Lung of a PRRSV- and PCV2-inoculated pig. The lung shows interstitial pneumonia with mononuclear cells within the alveoli (arrow) and disseminated intravascular coagulation (arrowheads). HE staining. Bar, 50 μm.
PRRSV immunohistochemical analysis.
PRRSV antigen was found in the lungs of one pig inoculated with PRRSV and in the lungs of the doubly inoculated pig that died at 25 DE. The remainder of the examined tissues from pigs in all groups was negative.
PCV2 in situ hybridization.
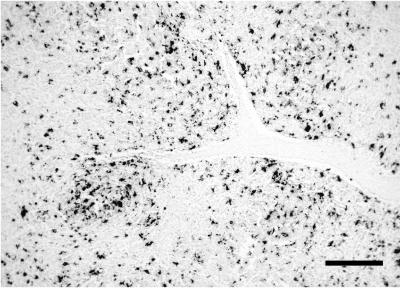
PCV2 in situ hybridization results are summarized in Table 2 and Fig. 4. Most of the additional tissues of the pig that died were positive: macrophage-like cells were labeled in the lamina propria of the stomach, duodenum, and colon; few positive nonglandular cells (endothelial and macrophage-like cells) were found in the pancreas and adrenal gland; large amounts of virus located in perivascular granulomatous infiltrates were detected in the dermis, bone marrow, parotid gland, epididymis, and branches of the artery of the ductus deferens; and fusiform cells resembling myoepithelial cells located around seminiferous tubules were labeled in the testicles. Tissues from the control and PRRSV-inoculated groups did not have PCV2 nucleic acid.
TABLE 2.
Results of the in situ hybridization technique to detect PCV2 nucleic acid
| Inoculum | Pig | Score for the following samplea:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lungs | Tonsils | Inguinal LN | Mesenteric LN | Spleen | Peyer's patches | Thymus | Liver | Kidneys | Nasal turbinates | ||
| PCV2 | 1 | − | + | + | − | + | + | − | − | − | − |
| 2 | − | − | + | − | − | − | − | − | − | − | |
| 3 | − | + | − | − | − | − | − | − | − | − | |
| 4 | − | − | ++ | + | − | − | − | − | − | − | |
| 5 | − | − | − | − | − | − | − | − | − | − | |
| 6 | − | + | + | − | − | − | − | − | − | − | |
| 7 | − | + | + | + | + | + | − | − | − | − | |
| Total | 0 | 4 | 5 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | |
| PRRSV and PCV2 | 8 | − | + | + | ++ | − | − | − | − | − | − |
| 9 | ++ | +++ | +++ | +++ | + | ++ | − | + | + | + | |
| 10 | − | − | + | + | − | − | − | − | − | − | |
| 11 | − | − | + | − | + | − | − | − | − | − | |
| 12 | − | − | + | + | + | − | − | − | + | − | |
| 13 | − | + | + | + | + | − | − | − | − | − | |
| 14 | ++ | +++ | +++ | +++ | +++ | +++ | +++ | + | + | − | |
| Total | 2 | 4 | 7 | 6 | 5 | 2 | 1 | 2 | 3 | 1 | |
LN, Lymph nodes; −, absence of PCV2 nucleic acid; +, slight presence of PCV2 nucleic acid; ++, moderate presence of PCV2 nucleic acid; +++, strong presence of PCV2 nucleic acid.
FIG. 4.
Detection of PCV2 DNA by in situ hybridization with fast green counterstain. A mesenteric lymph node of a PRRSV- and PCV2-inoculated pig shows numerous positive cells. Bar, 200 μm.
Serological analysis.
Antibodies to PRRSV were detected only in PRRSV-inoculated pigs; animals in the rest of the groups remained seronegative to PRRSV during the entire experiment. All PRRSV-inoculated pigs seroconverted at 14 DE. The pig that died at 25 DE had low PRRSV titers at 21 and 25 DE (1:320) compared to the titers in the other pigs (1:1,280 to 1: 5,120). Only pigs inoculated with PCV2 seroconverted to this virus; pigs in the rest of the groups remained seronegative to PCV2 during the experiment. Seroconversion was detected at 21 DE (14 days after PCV2 inoculation) for all but two pigs in the PRRSV- and PCV2-inoculated group. One of them, the pig that showed paleness of the skin from 20 to 32 DE, seroconverted at 28 DE with a low titer (1:320) compared to the titers in the other PCV2-inoculated pigs (1:1,280 to 1:20,480). The other one, the pig that died at 25 DE, seroconverted at 25 DE with a very low titer (1:80).
PCR.
PCR results for PCV2 and PRRSV are summarized in Table 3. PRRSV nucleic acid was detected only in pigs inoculated with PRRSV and with both PRRSV and PCV2. The PRRSV genome was detected from 7 to 21 DE in PRRSV-inoculated pigs and from 7 to 28 DE in PCV2- and PRRSV-inoculated pigs. One PRRSV-inoculated pig was found negative by PCR throughout the experiment but seroconverted to this virus. PCV2 DNA was present only in sera from pigs inoculated with PCV2 and with both PRRSV and PCV2. The PCV2 genome was detected from 14 DE (7 days after PCV2 inoculation) to the end of the experimental period (25 days after PCV2 inoculation). Stronger amplification signals were detected in pigs inoculated with both PRRSV and PCV2 than in pigs inoculated with PCV2 alone.
TABLE 3.
Results of the PCR and RT-PCR techniques to detect PCV2 and PRRSV, respectively, in serum samples
| Inoculum | No. of pigs in which the indicated virus was detected at the following DE/no. tested:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
7
|
14
|
21
|
28
|
32
|
|||||||
| PRRSV | PCV2 | PRRSV | PCV2 | PRRSV | PCV2 | PRRSV | PCV2 | PRRSV | PCV2 | PRRSV | PCV2 | |
| PRRSV | 0/5 | 0/5 | 3/5 | 0/5 | 2/5 | 0/5 | 2/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| PCV2 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 5/7 | 0/7 | 7/7 | 0/7 | 6/7 | 0/6 | 5/6 |
| PRRSV and PCV2 | 0/7 | 0/7 | 5/5a | 0/7 | 5/7 | 5/7 | 4/7 | 7/7 | 3/7b | 7/7b | 0/6 | 5/6 |
Two pigs were not tested.
The result for the sample taken at death from the pig that died at 25 DE is included.
TaqMan PCR for PCV2.
The PCV2 load was higher in pigs inoculated with both PRRSV and PCV2 than in pigs inoculated with PCV2 alone. The maximum loads for each pig ranged from 4.2 × 104 to 2.2 × 106 copies/ml in the PCV2-inoculated pigs and from 2.9 × 106 to 1.1 × 109 copies/ml in the PRRSV- and PCV2-inoculated pigs. Differences between both groups were significant (P < 0.05) at 14, 21, and 28 DE (7, 14, and 21 days after PCV2 inoculation, respectively). PCV2 load dynamics during the experiment are shown in Fig. 5. Maximum levels were found at 21 DE, except for the two pigs with the highest loads. One of them had the maximum amount of virus at 28 DE. The other one was the pig that died at 25 DE; this pig had the highest amounts of virus every sampling day compared to the other pigs and showed the highest load at 25 DE.
FIG. 5.
Evolution of PCV2 load in serum. The values represented are the mean of the results for the seven pigs in each group; error bars show standard deviations. The result for the sample taken at death from the pig that died at 25 DE is included in the 28 DE mean.
DISCUSSION
In the present work it was demonstrated that PMWS can be reproduced in conventional 5-week-old pigs by intranasal inoculation of PRRSV and PCV2. Only one out of seven doubly inoculated animals developed severe disease with clinical signs and lesions characteristic of both PMWS and PRRS. The other six PRRSV- and PCV2-inoculated pigs showed mild expression of clinical disease with growth retardation, mild fever, and various degrees of microscopic lesions. Also, it was possible to detect PCV2 within lymphoid tissues and sera by in situ hybridization or PCR and PRRSV in sera by PCR. All pigs in the PRRSV- and PCV2-inoculated group were infected with both viruses, as shown by different laboratory techniques; therefore, the levels of exposure to the infectious agents were theoretically identical for all animals. However, the small proportion of severely affected animals in this experiment is in agreement with what is seen on conventional farms with pigs affected with PMWS, where mortality is about 10 to 25% of pigs (16, 19). Several factors have been suggested to explain this epidemiological situation, including a litter effect, management, immunostimulation, and an individual effect (14, 16). The conditions of the present experiment cannot definitely disprove all of these effects. (i) Pigs were randomly distributed in the different groups, and clinical signs or lesions were not different among litters; however, the small number of litters studied (n = 3) does not allow us to disprove or confirm the litter effect. (ii) Management conditions were identical for all animals and therefore cannot be considered a variable factor among groups. (iii) A previous PRRSV infection can be considered systemic stimulation of the immune system (23), but all pigs in the doubly inoculated group were infected with this virus and only one developed severe disease. Therefore, differences in clinical and pathological outcomes may be associated with an individual effect (16) or other factors that have not been elucidated to date.
All pigs inoculated with PRRSV became infected, according to RT-PCR and serological results. As in other reports (25, 26), no respiratory clinical signs were observed in pigs inoculated with PRRSV alone. However, average daily weight gain was significantly (P < 0.05) lower for pigs in the PRRSV-inoculated groups than in the control group or the group inoculated with PCV2 alone. This result indicates that the growth retardation observed for pigs in this experiment was mainly an effect of PRRSV infection. Wasting and reduced feed conversion rates have also been described as features of PRRSV infection (5).
All pigs inoculated with PCV2 became infected, based on PCR, TaqMan PCR, serological, and in situ hybridization results. As in other PCV2 inoculation experiments published to date, pigs inoculated with this virus alone became infected but did not develop clinical PMWS (4, 8, 13, 17). However, other authors have reported reproduction of wasting disease in a proportion of pigs inoculated with PCV2 alone (3, 6, 11). These differences may be related to the use of different strains of PCV2, type of inoculum (homogenate versus virus isolated in cell culture), inoculation route, dose of virus, number of passages of virus in cell culture, source of pigs (conventional, gnotobiotic, colostrum-deprived [CD], or cesarean-derived, colostrum-deprived [CDCD] pigs), and age of inoculated pigs.
Two experiments besides the one presented here have been published on PRRSV and PCV2 interactions to date (1, 11). These experiments yielded very different results; while one of them (1) did not reproduce clinical signs compatible with PMWS, the other one (11) was able to reproduce a severe PMWS-like picture with high mortality in both PCV2-inoculated and PCV2- and PRRSV-inoculated pigs. In both experiments, microscopic lymphoid lesions characteristic of PMWS were reproduced, with higher severity in the one in which pigs died because of the wasting disease (11). In the present study, intermediate clinical and pathological results were achieved compared with the results of these previously published experiments. Again, these differences can be attributed to a number of factors, but major differences were associated with the source and age of the pigs, the PCV2 isolate, and the dose. The three experiments used conventional 5-week-old pigs (present study), CD 1- to 2-day-old piglets from a healthful farm (1), and CDCD 3-week-old pigs (11). Based on these and other published data (13, 14), it is difficult to conclude which is the most susceptible age of pig for experimentally reproducing clinical PMWS, but it seems that the most microbiologically naive pigs (gnotobiotic and CDCD pigs) are more prone to developing wasting disease under experimental conditions. Surprisingly, the experiment in which the PCV2 inoculum had the lowest titer (11) was able to fully reproduce the clinical outcome and lesions that are observed in naturally acquired PMWS (19). Finally, since the three experiments used isolates from field PMWS or PRRSV cases in different countries, it cannot be disproved that PCV2 strain variation may be the cause of the differences observed in the present and published studies.
In the present study, PRRSV was inoculated 1 week before challenge with PCV2 in 5-week-old nursery pigs. This experimental protocol was implemented based on the dynamics of natural PRRSV and PCV2 infections on conventional farms. On the one hand, PRRSV infection on farms on which PRRSV is enzootic usually occurs in nurseries soon after maternal immunity has declined (approximate duration, 3 weeks) (5). On the other hand, field cases of PMWS are usually seen at between 5 and 12 weeks of age (10, 21), also after the decline in the level of maternal antibodies.
The present study indicates that PRRSV can be a predisposing factor to the development of PMWS and/or PMWS lesions, as previously suggested (1, 11). A potentiation effect of PRRSV on PCV2 replication has been proposed (1), as suggested for PPV (3). In the present study, more severe microscopic lesions were found in pigs inoculated with both viruses than in pigs inoculated with PCV2 alone, and these lesions were detected in a wider range of tissues. In addition, the amount of PCV2 nucleic acid detected by in situ hybridization was higher in PRRSV- and PCV2-inoculated pigs than in PCV2-inoculated pigs, and it was located in a wider range of tissues. Moreover, previous infection with PRRSV in PCV2-inoculated pigs resulted in a higher PCV2 load in serum, as quantified by the TaqMan PCR technique. All of these results support the hypothesis that PRRSV infection promotes the replication of PCV2 (1). Although the nature of this interaction is not known, it can be hypothesized that PRRSV acts as a systemic immunostimulator (23), based on the pathogenic mechanism proposed by Krakowka et al. (14). Alternatively, PRRSV may interfere with PCV2 clearance by the reticuloendothelial system, as has been demonstrated for other blood-borne particles (28), favoring the persistence of the virus and subsequently allowing its replication. On the other hand, no quantification of PRRSV viremia was carried out in the present study; therefore, we do not know if PRRSV replication was also enhanced by PCV2 infection in doubly inoculated pigs. However, a longer duration of PRRSV viremia and a higher proportion of viremic pigs were observed for pigs inoculated with both PRRSV and PCV2, suggesting that PCV2 may also favor the replication of PRRSV.
A quantitative, competitive PCR method was previously used to detect PCV2 (15); in the present study, another quantitative PCR method, the TaqMan PCR method, was used for the first time to quantify PCV2 load in experimentally inoculated pigs. The results of this technique showed a strong correlation with the severity of the microscopic lesions, the amount of the PCV2 genome observed in tissues by in situ hybridization, and the development of clinical disease. It is noteworthy that the pig that developed severe wasting and respiratory signs and died 18 days after PCV2 inoculation had very high levels of PCV2 in serum compared with its penmates. These results are in agreement with those of a previously published work (15) which indicated that the development of PMWS may require a certain level of PCV2. On the other hand, it was demonstrated that clinically healthy pigs can also be infected with PCV2 (19); these animals had milder microscopic lymphoid lesions and lower levels of PCV2 nucleic acid, as detected by in situ hybridization, than pigs affected with PMWS. Therefore, quantitative PCR methods may be used in the future as a diagnostic tool for PMWS in living animals by use of blood or serum as a sample.
It is of interest that the dead pig with moderate to marked PMWS and PRRSV lesions apparently failed to mount a humoral immune response compared to its penmates (serologic titers to PCV2 and PRRSV for this particular pig were clearly below the mean for the group). Three hypotheses can be drawn to explain this fact. First, this pig might have been immunocompromised prior to the start of the experiment and would have developed PMWS after the double viral infection; however, this notion is in contrast to the recent demonstration that systemically immunostimulated pigs can develop clinical PMWS (14). Alternatively, the infection with PCV2 subsequent to the PRRSV infection might have caused immunosupression, as has been suggested for pigs naturally affected with PMWS (24). Finally, it cannot be disproved that complexing of antibodies by excess virus in the bloodstream led to the low titers of antibodies. Although these observations correspond to just one pig and cannot be generalized, they deserve further examination to test the effect of PCV2 in immunodepressed pigs and to evaluate the immune function of pigs affected with PMWS.
In summary, this experiment shows that severe wasting and respiratory disease can be reproduced in conventional pigs infected with both PRRSV and PCV2. Also, an enhancement of PCV2 load occurs in the serum of pigs inoculated with both viruses compared to pigs inoculated with PCV2 alone.
Acknowledgments
We thank S. Usero, B. Pérez, M. Pérez, P. Losada, J. Plana-Masllopart, and M. Aulinas for technical assistance and Llorenç Badiella, Joan Valls, and Edgar G. Manzanilla for statistical analysis.
This work was supported by project QLRT-PL-199900307 from Fifth Framework program 1998-2002 of the European Commission and project 2-FEDER-1997-1341 from the I+D National Plan (Spain).
REFERENCES
- 1.Allan, G. M., F. McNeilly, J. Ellis, S. Krakowka, B. Meehan, I. McNair, I. Walker, and S. Kennedy. 2000. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch. Virol. 145:2421-2429. [DOI] [PubMed]
- 2.Allan, G. M., and J. A. Ellis. 2000. Porcine circoviruses: a review. J. Vet. Diagn. Investig. 12:3-14. [DOI] [PubMed] [Google Scholar]
- 3.Allan, G. M., S. Kennedy, F. McNeilly, J. C. Foster, J. A. Ellis, S. J. Krakowka, B. M. Meehan, and B. M. Adair. 1999. Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J. Comp. Pathol. 121:1-11. [DOI] [PubMed] [Google Scholar]
- 4.Balasch, M., J. Segalés, C. Rosell, M. Domingo, A. Mankertz, A. Urniza, and J. Plana-Durán. 1999. Experimental inoculation of conventional pigs with tissue homogenates from pigs with post-weaning multisystemic wasting syndrome. J. Comp. Pathol. 121:139-148. [DOI] [PubMed] [Google Scholar]
- 5.Benfield, D. A., J. E. Collins, S. A. Dee, P. G. Halbur, H. S. Joo, K. M. Lager, W. L. Mengeling, M. P. Murtaugh, K. D. Rossow, G. W. Stevenson, and J. J. Zimmerman. 1999. Porcine reproductive and respiratory syndrome, p. 201-232. In B. E. Straw, S. D'Allaire, W. L. Mengeling, and D. J. Taylor (ed.), Diseases of swine, 8th ed. Iowa State University Press, Ames.
- 6.Bolin, S. R., W. C. Stoffregen, G. P. S. Nayar, and A. L. Hamel. 2001. Postweaning multisystemic wasting syndrome induced after experimental inoculation of cesarean-derived, colostrum-deprived piglets with type 2 porcine circovirus. J. Vet. Diagn. Investig. 13:185-194. [DOI] [PubMed] [Google Scholar]
- 7.Clark, E. G. 1997. Post-weaning multisystemic wasting syndrome. Proc. Am. Assoc. Swine Pract. 28:499-501. [Google Scholar]
- 8.Ellis, J., S. Krakowka, M. Lairmore, D. Haines, A. Bratanich, E. Clark, G. Allan, C. Konoby, L. Hassard, B. Meehan, K. Martin, J. Harding, S. Kennedy, and F. McNeilly. 1999. Reproduction of lesions of postweaning multisystemic wasting syndrome in gnotobiotic piglets. J. Vet. Diagn. Investig. 11:3-14. [DOI] [PubMed] [Google Scholar]
- 9.Ellis, J. A., A. Bratanich, E. G. Clark, G. Allan, B. Meehan, D. M. Haines, J. Harding, K. H. West, S. Krakowka, C. Konoby, L. Hassard, K. Martin, and F. McNeilly. 2000. Coinfection by porcine circoviruses and porcine parvovirus in naturally acquired postweaning multisystemic wasting syndrome. J. Vet. Diagn. Investig. 12:21-27. [DOI] [PubMed] [Google Scholar]
- 10.Harding, J. 1997. Post-weaning multisystemic wasting syndrome (PMWS): preliminary epidemiology and clinical presentation. Proc. Am. Assoc. Swine Pract. 28:503. [Google Scholar]
- 11.Harms, P. A., S. D. Sorden, G. P. Halbur, S. R. Bolin, K. M. Lager, I. Morozov, and P. S. Paul. 2001. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and PRRSV. Vet. Pathol. 38:528-539. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy, S., D. Moffett, F. McNeilly, B. Meehan, J. Ellis, S. Krakowka, and G. M. Allan. 2000. Reproduction of lesions of postweaning multisystemic wasting syndrome by infection of conventional pigs with porcine circovirus type 2 alone or in combination with porcine parvovirus. J. Comp. Pathol. 122:9-24. [DOI] [PubMed] [Google Scholar]
- 13.Krakowka, S., J. A. Ellis, B. Meehan, S. Kennedy, F. McNeilly, and G. Allan. 2000. Viral wasting syndrome of swine: experimental reproduction of postweaning multisystemic wasting syndrome in gnotobiotic swine by coinfection with porcine circovirus 2 and porcine parvovirus. Vet. Pathol. 37:254-263. [DOI] [PubMed] [Google Scholar]
- 14.Krakowka, S., J. A. Ellis, F. McNeilly, S. Ringler, D. M. Rings, and G. Allan. 2001. Activation of the immune system is the pivotal event in the production of wasting disease in pigs infected with the porcine circovirus-2 (PCV-2). Vet. Pathol. 38:31-42. [DOI] [PubMed] [Google Scholar]
- 15.Liu, Q., L. Wang, P. Willson, and L. A. Babiuk. 2000. Quantitative, competitive PCR analysis of porcine circovirus DNA in serum from pigs with postweaning multisystemic wasting syndrome. J. Clin. Microbiol. 38:3474-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madec, F., E. Eveno, P. Morvan, L. Hamon, P. Blanchard, R. Cariolet, N. Amenna, H. Morvan, C. Truong, D. Mahé, E. Albina, and A. Jestin. 2000. Post-weaning multisystemic wasting syndrome (PMWS) in pigs in France: clinical observations from follow-up studies on affected farms. Livest. Prod. Sci. 63:223-233. [Google Scholar]
- 17.Magar, R., R. Larochelle, S. Thibault, and L. Lamontagne. 2000. Experimental transmission of porcine circovirus type 2 (PCV2) in weaned pigs: a sequential study. J. Comp. Pathol. 123:258-269. [DOI] [PubMed] [Google Scholar]
- 18.Plana, J., M. Vayreda, J. Vilarrasa, M. Bastons, R. Rosell, M. Martínez, A. San Gabriel, J. Pujols, J. L. Badiola, J. A. Ramos, and M. Domingo. 1992. Porcine epidemic abortion and respiratory syndrome (mystery swine disease). Isolation in Spain of the causative agent and experimental reproduction of the disease. Vet. Microbiol. 33:203-211. [DOI] [PubMed] [Google Scholar]
- 19.Quintana, J., J. Segalés, C. Rosell, M. Calsamiglia, G. M. Rodríguez-Arrioja, F. Chianini, J. M. Folch, J. Maldonado, M. Canal, J. Plana-Durán, and M. Domingo. 2001. Clinical and pathological observations on pigs with postweaning multisystemic wasting syndrome. Vet. Rec. 149:357-361. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Arrioja, G. M., J. Segalés, M. Balasch, C. Rosell, J. Quintana, J. M. Folch, J. Plana-Durán, A. Mankertz, and M. Domingo. 2000. Serum antibodies to porcine circovirus type 1 and type 2 in pigs with and without PMWS. Vet. Rec. 146:762-764. [DOI] [PubMed] [Google Scholar]
- 21.Rosell, C., J. Segalés, J. Plana-Durán, M. Balasch, G. M. Rodríguez-Arrioja, S. Kennedy, G. M. Allan, F. McNeilly, K. S. Latimer, and M. Domingo. 1999. Pathological, immunohistochemical and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J. Comp. Pathol. 120:59-78. [DOI] [PubMed] [Google Scholar]
- 22.Rosell, C., J. Segalés, and M. Domingo. 2000. Hepatitis and staging of hepatic damage in pigs naturally infected with porcine circovirus type 2. Vet. Pathol. 37:687-692. [DOI] [PubMed] [Google Scholar]
- 23.Rossow, K. D., E. M. Bautista, S. M. Goyal, T. W. Molitor, M. P. Murtaugh, R. B. Morrison, D. A. Benfield, and J. E. Collins. 1994. Experimental porcine reproductive and respiratory syndrome virus infection in one-, four-, and ten-week-old pigs. J. Vet. Diagn. Investig. 6:3-12. [DOI] [PubMed] [Google Scholar]
- 24.Segalés, J., F. Alonso, C. Rosell, J. Pastor, F. Chianini, E. Campos, L. Lopez-Fuertes, J. Quintana, G. Rodríguez-Arrioja, M. Calsamiglia, J. Pujols, J. Domínguez, and M. Domingo. 2001. Changes in peripheral blood leukocyte populations in pigs with natural postweaning multisystemic wasting syndrome (PMWS). Vet. Immunol. Immonopathol. 81:37-44. [DOI] [PubMed] [Google Scholar]
- 24a.Segalés, J., M. Calsamiglia, C. Rosell, M. Soler, J. Maldonado, M. Martin, and M. Domingo. 2002. Porcine reproductive and respiratory syndrome virus (PRRSV) infection status in pigs naturally affected with post-weaning multisystemic wasting syndrome (PMWS) in Spain. Vet. Microbiol. 85:23-30. [DOI] [PubMed] [Google Scholar]
- 25.Segalés, J., M. Domingo, G. I. Solano, and C. Pijoan. 1999. Porcine reproductive and respiratory syndrome virus and Haemophilus parasuis antigen distribution in dually infected pigs. Vet. Microbiol. 64:287-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solano, G. I., J. Segalés, J. E. Collins, T. W. Molitor, and C. Pijoan. 1997. Porcine reproductive and respiratory syndrome virus (PRRSV) interaction with Haemophilus parasuis. Vet. Microbiol. 55:247-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorden, S. D., P. A. Harms, T. Sirinarumitr, I. Morozov, P. G. Halbur, K. J. Yoon, and P. S. Paul. 1998. Porcine circovirus and PRRSV co-infection in pigs with chronic bronchointerstitial pneumonia and lymphoid depletion: an emerging syndrome in midwestern swine, p. 75. In Proceedings of the Annual Meeting of the American Association of Veterinary Laboratory Diagnosticians. American Association of Veterinary Laboratory Diagnosticians, Minneapolis, Minn.
- 28.Thanawongnuwech, R., P. G. Halbur, M. R. Ackermann, E. L. Thacker, and R. L. Royer. 1998. Effects of low (modified-live virus vaccine)- and high (VR-2385)-virulence strains of porcine reproductive and respiratory syndrome virus on pulmonary clearance of copper particles in pigs. Vet. Pathol. 35:398-406. [DOI] [PubMed] [Google Scholar]
- 29.Tischer, I., D. Peters, R. Rasc, and S. Pocuili. 1987. Replication of porcine circovirus: induction by glucosamine and cell cycle dependence. Arch. Virol. 96:39-57. [DOI] [PubMed] [Google Scholar]
- 30.Verbeke, G., and G. Molenberghs. 2000. Linear mixed models for longitudinal data. Springer-Verlag, New York, N.Y.