Abstract
Recruitment of monocytes plays important roles during vegetation formation and endocardial inflammation in the pathogenesis of infective endocarditis (IE). Bacterial antigens or modulins can activate endothelial cells through the expression of cytokines or adhesion molecules and modulate the recruitment of leukocytes. We hypothesized that glucosyltransferases (GTFs), modulins of viridans group streptococci, may act directly to up-regulate the expression of adhesion molecules and also interleukin-6 (IL-6) to augment monocyte attachment to endothelial cells. Using primary cultured human umbilical vein endothelial cells (HUVECs) as an in vitro model, we demonstrated that GTFs (in the cell-bound or free form) could specifically modulate the expression of IL-6, and also adhesion molecules, in a dose- and time-dependent manner. Results of inhibition assays suggested that enhanced expression of adhesion molecules was dependent on the activation of nuclear factor κB (NF-κB) and extracellular signal-regulated kinase and that p38 mitogen-activated protein kinase pathways also contributed to the release of IL-6. Streptococcus-infected HUVECs or treatment with purified IL-6 plus soluble IL-6 receptor α enhanced the expression of ICAM-1 and the adherence of the monocytic cell line U937. These results suggest that streptococcal GTFs might play an important role in recruiting monocytic cells during inflammation in IE through induction of adhesion molecules and IL-6, a cytokine involved in transition from neutrophil to monocyte recruitment.
Bacterium-cell interactions through pathogen-associated molecular patterns (PAMPs) or modulins are important for triggering the innate immune response and inflammatory reactions at the site of entry. Infective endocarditis (IE) is a microbial infection initiated at the endothelial lining of the heart valves. IE is classified as “acute” or “subacute chronic” on the basis of different disease syndromes and progression (39). Staphylococci (S. aureus and S. epidermidis) are most frequently responsible for the acute type of infection, accompanied by rapid valve destruction, sepsis, and shock (32). Viridans group streptococci frequently cause subacute syndromes that have the protracted course of a chronic wasting disease. The most common streptococci isolated from patients with endocarditis are Streptococcus sanguinis, S. bovis, S. mutans, and S. mitis (39). These streptococci, which are closely related genetically, share some common molecular patterns or antigens that might trigger inflammatory responses to opportunistic systemic infections, such as IE and meningitis, or chronic inflammatory diseases, such as rheumatoid arthritis (19, 30).
The pathogenesis of IE is characterized by the formation of endocardial vegetations through an inflammatory reaction (36). These vegetations consist of a clot of fibrin and platelets mixed with leukocytes, in which the causative microorganisms are embedded and multiply. During the formation of vegetations, the coagulation system is activated through the extrinsic clotting pathway, which is factor VII and tissue factor dependent (9, 17). Monocytes could release tissue factor after interaction with either staphylococci or streptococci (7, 8), and such interactions are essential for vegetation formation in experimental endocarditis (52). Accompanied by vegetation formation, the pathogenesis of IE is characterized histopathologically by a chronic inflammatory reaction with infiltration of monocytes. Therefore, the recruitment and migration of monocytes play a critical role in both the coagulation and inflammation events orchestrated in IE.
Monocyte recruitment to the site of infection (e.g., damaged heart valves and endocardium in IE) requires the concerted interaction of cytokines, chemokines, and adhesion molecules expressed on the activated endothelial linings. The endothelium, a major responder for leukocyte recruitment, could be activated directly by interaction with bacteria or a PAMP, such as lipopolysaccharide (LPS), or indirectly through mediators of inflammation, such as tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) (41). After activation, the vascular endothelium recruits leukocytes via expression of adhesion molecules and chemokines (37): mainly IL-8 and monocyte chemoattractant protein 1 (MCP-1), key factors for the chemotaxis of neutrophils and monocytes, respectively (31). Limited information is available on endothelial cells and the activation or signaling induced by gram-positive bacteria. S. aureus, an IE-inducing pathogen, can induce the expression of IL-6 and IL-1β by cultured human umbilical vein endothelial cells (HUVECs) (58). Different species of viridans group streptococci can stimulate the production of IL-6 and IL-8 by human saphenous vein endothelial cells (HSVECs), and the protein I/II family of immunological and structurally related surface proteins, identified on viridans group streptococci, are potent modulins (53). Proteins I/IIf from S. mutans OMZ 175 could stimulate IL-8 production by HSVECs through interaction with α5β1 integrins and signaling pathways involving mitogen-activated protein kinases (MAPKs), phospholipase C-γ (PLC-γ), and protein kinase C (PKC) (2).
Glucosyltransferases (GTFs) are a group of cell-wall-associated or extracellular proteins that convert sucrose into exopolysaccharides (glucans), and isozymes have also been identified in several viridans group streptococci, such as S. mutans, S. sanguinis, S. gordonii, and S. salivarius. These GTFs, with molecular masses around 150 kDa, share conserved N- and C-terminal functional domains, which are coordinately responsible for sucrose splitting and glucan synthesis and binding (35). In addition to their essential role in dental biofilm formation, GTFs act as modulins on the circulating or spleen monocytes to produce preferentially various cytokines, IL-6 in particular, when added in vitro or challenged in vivo (11, 13). In the rat model of experimental endocarditis, we found that IL-6 was induced in situ earlier than TNF-α, that GTFs are the major modulins acting during the acute stage of inflammation on IL-6 release, and that the endothelial lining of the valvular regions might be the source of the IL-6 (46).
IL-6 combined with soluble IL-6 receptor α (sIL-6Rα) plays an important role in the transition from neutrophil to monocyte recruitment and the switch from acute to persistent chronic inflammation (29). Plasma concentrations of IL-6, but not TNF-α or IL-1, were found to be associated with inflammation in IE (3, 43). To explore the molecular mechanisms involved in monocyte recruitment by endothelial cells, triggered by common structures shared by IE-related streptococci, we hypothesized that GTFs may act directly to up-regulate the expression of adhesion molecules and also IL-6, which in combination with sIL-6Rα would further augment monocyte attachment to endothelial cells. Studies were performed with primary HUVECs as the targets to explore the interaction of GTFs and the signaling events induced. We found that GTF alone was capable of inducing the expression of adhesion molecules and, preferentially, the release of IL-6. Activation of HUVECs by GTFs might involve the activation of nuclear factor κB (NF-κB), a pivotal regulator of proinflammatory gene expression, including cytokines, chemokines, and adhesion molecules. Enhanced monocyte adherence was demonstrated on the GTF-activated HUVECs and could be augmented in the presence of IL-6.
MATERIALS AND METHODS
Bacterial strains and preparation of rGTFC.
Streptococcus mutans laboratory strain GS-5 and two clinical isolates, NTU-5526 and NTU-4312, were grown at 37°C and maintained in brain heart infusion (BHI) broth (Difco Laboratories Inc., Detroit, MI). Strain NTU-5526 was isolated from the peripheral blood of a patient suffering from infective endocarditis at the Department of Infectious Diseases, National Taiwan University Hospital (NTUH). Strain NTU-4312 is an oral isolate from a patient with rampant caries from the Department of Dentistry, NTUH. Three isogenic mutants derived from GS-5, GS-5DD (inactivated gtfD), NHR1DD (ΔgtfC, inactivated gtfD), and NHS1DD (ΔgtfB/C, inactivated gtfD) (50), which differ from the wild type in the expression of two or three gtf genes, were grown in BHI broth supplemented with both erythromycin (10 μg ml−1) and tetracycline (10 μg ml−1). The genetic stability or phenotypic characteristics were indistinguishable from those of their parental GS-5 strain, except in the expression of GTFs.
Escherichia coli DH5α and BL21 were used for cloning and protein expression. The full-length gtfC was digested with pshAI from pNH3 (50) and subcloned into the NheI- and HindIII-cleaved plasmid pRSETA (Invitrogen). The resulting plasmid, pRSET-gtfC, expresses recombinant GTFC (rGTFC) lacking the signal peptide (amino acid sequence 1 to 42) and contains an additional stretch of six histidine residues at the N terminus. His-tagged rGTFC proteins were purified using Ni-nitrilotriacetic acid-agarose resin (QIAGEN) as previously described (13). The purity of the rGTFC was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) after silver staining (Fig. 1B). The rGTFC exhibited two bands around 155 kDa reactive to anti-GTFB/C polyclonal antibody (10), and the identities of both bands were confirmed by the amino acid composition of tryptic digests on liquid chromatography-tandem mass spectrometry, as described previously (12). Any possible endotoxin content in the purified rGTFC was removed with polymyxin B-agarose beads (Sigma).
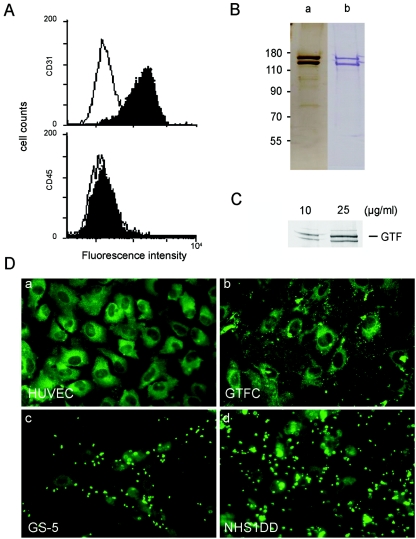
FIG. 1.
Adherence of S. mutans and purified rGTFC to HUVECs. (A) FACS analysis of trypsinized HUVECs revealed positive staining of CD31 without CD45-positive leukocytes. (B) SDS-PAGE analysis of purified rGTFC by silver staining (a) or Western blotting using rabbit antibodies specific for GTFB/C (b). (C) Detection of rGTFC bound to HUVECs by Western blotting. HUVECs were treated separately with the amounts of rGTFC indicated at 37°C for 1 h, and unbound protein was washed away. Total cell lysates were harvested and Western blotting of rGTFC was performed as described in Materials and Methods. (D) Detection of S. mutans or rGTFC binding to HUVECs by fluorescence. Confluent HUVECs, untreated (a) or stimulated with purified rGTFC (b), S. mutans GS-5 (c), or GTF-null mutant NHS1DD (d), were fixed and stained with an anti-S. mutans antibody for panels c and d or anti-GTFB/C antibody for panel b. The results were observed by fluorescence microscopy after reaction with a secondary FITC-conjugated anti-rabbit IgG antibody.
Preparation and characterization of cells.
HUVECs were isolated from human umbilical veins according to the method described previously (27). Briefly, cells obtained from type I collagenase digestion were washed, pooled, centrifuged for 7 min at 900 rpm, and resuspended in fresh M199 medium (Cambrex Bio Science) with heat-inactivated 20% fetal calf serum (FCS), 2 mM glutamine and antibiotics (penicillin, 100 U ml−1; streptomycin, 100 U ml−1; Cambrex Bio Science), sodium heparin (25 μg ml−1), and endothelial growth factor supplement (25 μg ml−1; Upstate). Before splitting, cells were growth for 3 to 4 days on 10-cm dish. HUVECs were harvested after trypsin-EDTA (0.05% trypsin, 0.02% EDTA; Cambrex Bio Science) digestion, seeded into 24-well plates, and grown in a humid atmosphere at 37°C with 5% CO2 until reaching confluence within 3 to 5 days. All experiments were performed on subcultures of confluent cells between the second and fifth passages. At this stage, the cells all expressed von Willebrand factor and retained their cobblestone appearance. The homogeneity of HUVECs was over 90%, as analyzed by flow cytometry using CD31 monoclonal antibody (MAb), and the cells were free from CD45-positive leukocytes (Fig. 1A). Cell culture media had an endotoxin content that never exceeded 0.04 ng ml−1, as tested by a chromogenic Limulus amoebocyte lysate assay purchased from Bio-Whittaker, Walkersville, MD.
U937, a human monocytic leukemia cell line, was cultured in RPMI 1640 medium (Cambrex Bio Science) supplemented with 10% heat-inactivated FCS, 100 U ml−1 of penicillin, and 100 μg ml−1 of streptomycin. U937 cells expressed CD45, CXCR1, and CXCR2 on their surface and were able to secrete IL-6, IL-8, and TNF-α after stimulation with phorbol myristate acetate.
Cell activation and bacterial adherence assay.
For infection studies, bacteria from the stationary phase were washed three times with phosphate-buffered saline (PBS) and adjusted to a density of 108 CFU ml−1 in M199 containing 10% heat-inactivated FCS without antibiotics. Bacteria were added to HUVECs grown to confluence in 24-well tissue culture plates at a multiplicity of infection (MOI) of 500:1 (5 × 107 bacteria at 1 × 105 cells per well). After treatment with bacteria or rGTFC, the culture supernatants and total cell lysates were collected at different time intervals for quantification of cytokines. To confirm that the observed effects were not caused by possible LPS contamination, the experiments were performed in the presence of polymyxin B (40 μg ml−1; Sigma). For the adherence assay, the HUVECs were infected with stationary-phase bacteria at an MOI of 500:1 in M199 culture medium for 2 h at 37°C in 5% CO2. The HUVECs were washed three times with PBS and subsequently lysed from the well by 0.5% Triton X-100 in PBS. The number of cell-adherent bacteria was determined by plating appropriate dilutions of the lysate onto BHI agar. The adherence of bacteria or rGTFC to HUVECs was also confirmed by immunofluorescence staining with anti-S. mutans antibody or specific anti-GTFC antibody and observation by fluorescein isothiocyanate (FITC)-conjugated secondary antibody.
For the inhibition assay, the confluent cells were preincubated with 300 μl of various inhibitors diluted in M199 for 1 h at 37°C and then stimulated with bacteria or purified rGTFC in M199 supplemented with 10% heat-inactivated FCS.
RNA isolation and RT-PCR.
Total RNA was isolated from confluent HUVECs by guanidine isothiocyanate-phenol-chloroform extraction as described previously (14). Reverse transcription (RT) of 2 μg of total RNA was conducted in a 50-μl reaction volume using 1 μg of oligo(dT18) and Moloney murine leukemia virus reverse transcriptase (Promega Corporation, Madison, Wis.) at 37°C for 1 h. A total of 2.5 μl of cDNA solution was used for RT-PCR in a total volume of 25 μl containing 0.04 μM of sense and antisense primers. The mRNA stimulated by S. mutans or rGTFC was initially screened with a multiplex PCR kit (Maxim Biotech) and confirmed subsequently by using specific primers. The specific primers for IL-1β, IL-6, IL-8, and GADPH were selected based on the published human cDNA sequences. The oligonucleotide primers used were as follows: (i) IL-1β, 5′-AAACAGATGAAGTGCTCCTTCCAGG-3′ and 5′-TGGAGAACACCACTTGTTGCTCCA-3′; (ii) IL-6, 5′-ATGAACTCCTTCTCCACAAGCGC-3′ and 5′-GAAGAGCCCTCAGGCTGGACTG; (iii); IL-8, 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ and 5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′; and (iv) GAPDH, 5′-GAAGGTGAAGGTCGGAGTC-3′ and 5′-GAAGATGGTGATGGGATTTG-3′. The PCR temperatures used were as follows: denaturing at 94°C for 30 s, annealing at 60°C for 30 s, and polymerization at 72°C for 1 min, followed by final extension for 7 min at 72°C. PCR products were separated by 2% agarose gel electrophoresis and visualized with ethidium bromide. The relative intensities of the bands were quantified by densitometric analysis using the Electrophoresis Documentation and Analysis System 120 (Scientific Imaging Systems, Eastman Kodak). The data were normalized as ratios to copies of an internal control gene coding for GAPDH in order to correct for any differences between samples in the efficiency of the RT and PCRs.
Detection of cytokine and chemokine by ELISA.
The IL-1β, IL-6, IL-8, TNF-α, or sIL-6Rα concentration in conditional medium or total cell lysates was quantified by sandwich immunoassays using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R and D Systems), according to the manufacturer's protocol. For inhibition assays, HUVECs were preincubated in culture medium containing specific inhibitors of PKC (Ro-31-8220, 1 or 10 μM; Sigma), phosphatidylinositol 3-kinase (PI3K; LY294002, 2 or 20 μM; Sigma), protein tyrosine kinases (PTKs [genistein], 25 or 50 μM; Sigma), ERK1/2 (PD98059, 25 or 50 μM; Cashmere Biotech), p38 MAPK (SB203580, 1 or 5 μM; Cashmere Biotech), JNK (SP600125, 1 or 5 μM; Cashmere Biotech), or NF-κB (pyrrolidine dithiocarbamate [PDTC], 25 or 50 μM; Sigma) for 1 h and then stimulated with bacteria or rGTFC as described above. After 24 h of incubation, the supernatants of cell cultures were collected for determination of cytokines by ELISA. Experiments were conducted in triplicate, and the results are shown as mean picograms per milliliter ± standard deviation. A two-sample t test was used to compare the mean levels of cytokine secretion following a particular treatment, and differences with P values of <0.05 were considered significant.
Cell lysate preparation and Western blotting.
rGTFC-treated or untreated cells were harvested at various time points, washed three times with ice-cold PBS, and lysed at 4°C with lysis buffer (150 mM NaCl, 0.5% NP-40, 50 mM Tris HCl, pH 7.4, 2 mM EDTA, 0.25% sodium deoxycholate, 10 mM Na3VO4) plus protease inhibitors (2 ng ml−1 leupeptin, 1 mM phenylmethylsulfonyl fluoride, 15 ng ml−1 aprotinin). Total protein in the cell extracts was determined by Bradford assay (Bio-Rad). Samples containing 20 μg of total protein were electrophoresed on 10% SDS-PAGE gels and then transferred to polyvinylidene difluoride membranes; afterwards, membranes were blocked with a 4% (wt/vol) solution of nonfat milk powder in Tris-buffered saline-Tween (TBST; 25 mM Tris, pH 7.4, 150 mM NaCl, 0.05% Tween 20) for 1 h at room temperature. Membranes were probed with polyclonal rabbit antibodies specific for GTFC at 4°C overnight. The membranes were then incubated with alkaline phosphatase-conjugated secondary antibodies (1:4,000 in 4% nonfat milk-TBST) for 2 h at room temperature. Following washing, immunoreactivity was detected using naphthol AS-MX phosphate and fast blue BB salt (Sigma).
Detection of NF-κB translocation by indirect immunofluorescence.
Confluent HUVECs on 12-mm glass coverslips, were treated with rGTF for 1 h at 37°C, washed carefully with ice-cold PBS, and then permeabilized with 1% Triton X-100 for 15 min at room temperature; for polyclonal rabbit antibodies specific for NF-κB p65 (clone c-20; Santa Cruz Biotechnology), permeabilization was performed at room temperature for 1 h. The cells were examined by fluorescence microscopy after incubation with FITC-conjugated secondary antibodies (Zymed Laboratories) at room temperature for 1 h. For inhibition assays, HUVECs were preincubated with culture medium containing a specific inhibitor of PKC (Ro-31-8220, 10 μM) or NF-κB (PDTC, 50 μM) for 1 h and then stimulated with rGTFC as described above.
Detection of adhesion molecule expression on HUVECs.
Cell surface expression of adhesion molecules was determined by indirect immunofluorescence followed by fluorescence-activated cell sorting (FACS; Becton Dickinson). Confluent HUVECs were stimulated with bacterial suspension (GS-5 or NHS1DD), rGTFC, purified IL-6 alone (1, 10, or 20 ng; PeproTech, UK) or IL-6 plus sIL-6Rα (20 ng; PeproTech, UK), or LPS (O111:B4; Sigma) for 6 h and then harvested by treating cells with trypsin-EDTA (0.005% trypsin, 0.002% EDTA) solution. Following fixation with 2% paraformaldehyde, trypsinized cells were resuspended in ice-cold PBS with a MAb (e-Bioscience) specific for CD54 (clone HA58), CD106 (clone STA), CD62E (clone CTB202), CD62P (clone AK-4), or CD45 (clone HI30) at 4°C for 1 h. The cells were stained with FITC-conjugated secondary antibodies at 4°C for 1 h. Washed cells were resuspended in PBS containing 1% paraformaldehyde and applied to a FACS analyzer. HUVECs treated with conjugated antibody alone served as controls for background fluorescence.
U937 adherence assay.
Monolayers of HUVECs were pretreated with a bacterial suspension (GS-5 or NHS1DD), rGTFC or IL-6 alone, or IL-6 plus sIL-6Rα for 6 h at 37°C. After treatment, the cells were washed twice with prewarmed M199 culture medium before adherence of U937 to stimulated HUVECs was assessed. Briefly, 5 × 105 U937 cells (3 to 5 monocytes per HUVEC) were added to the HUVEC monolayer and allowed to attach to HUVECs during 2 h of incubation at 37°C under static conditions. Loosely adherent cells were removed by being washed with prewarmed PBS three times. The cells were harvested, trypsinized followed by fixation in 2% paraformaldehyde, and then stained with MAb specific for CD45 and FITC-conjugated secondary antibodies. The numbers of HUVEC-bound monocytes were determined by FACS as described above. Data are expressed as the percentage of monocytes that bound to S. mutans-, rGTFC-, or IL-6-stimulated HUVECs.
RESULTS
GTFs bind directly to HUVECs but are not essential for S. mutans adherence.
Despite being implicated less frequently in the incidence of endocarditis than S. sanguinis or S. mitior (S. orlis and S. mitis), S. mutans is the best characterized of the viridans group streptococci in terms of its genomics (1), as well as the specific characteristics of its gtf genes. For these reasons, S. mutans was chosen as the representative species for further analysis in this study. Genetic (4, 24, 25) and genomic (1) analyses confirmed that S. mutans expresses three GTFs (GtfB, -C, and -D) with an amino acid sequence identity of over 50%. Interaction of S. mutans with HUVECs and the role of GTFs in mediating its attachment were investigated initially with GS-5, a laboratory strain, and a GTF-null mutant, NHS1DD. The homogeneity of HUVECs was over 90%, as analyzed by flow cytometry using the anti-CD31 MAb, and HUVECs were free from CD45-positive leukocytes (Fig. 1A). Stationary-phase GS-5 could bind directly to HUVECs at a density of 1.4 × 106 CFU/105 cells. Despite the deficiency of GTFs, NHS1DD could still bind to the HUVECs at a density of 6.7 × 106 CFU/105 cells, similar to the parental GS-5 (Fig. 1D, panels c and d). Previous results from another laboratory suggested that GtfG, a GTF of Streptococcus gordornii, could function as an adhesin and interact directly with endothelial cells in vitro (51). To investigate whether GTFs of S. mutans might play a similar role, binding directly to the HUVECs, His-tagged rGTFC was expressed in E. coli and purified (Fig. 1B). The rGTFC could bind directly to HUVECs (Fig. 1D, panel b), and dose-dependent binding of rGTFC to the cell membrane fractions was confirmed by Western blotting (Fig. 1C). Specific binding of rGTFC (at a concentration of 25 μg ml−1) to HUVECs could be inhibited by preincubation with the anti-GTFB/C rabbit immunoglobulin G (IgG) in a dose-dependent manner. These results indicated that rGTFC could bind directly to HUVECs and that bacterial cell-wall-associated components other than GTFs could mediate in vitro the binding of S. mutans to the HUVECs, because, the absence of GTFs, as in NHS1DD, did not affect the adherence of this microorganism to HUVECs.
GTFs modulate cytokine production in HUVECs.
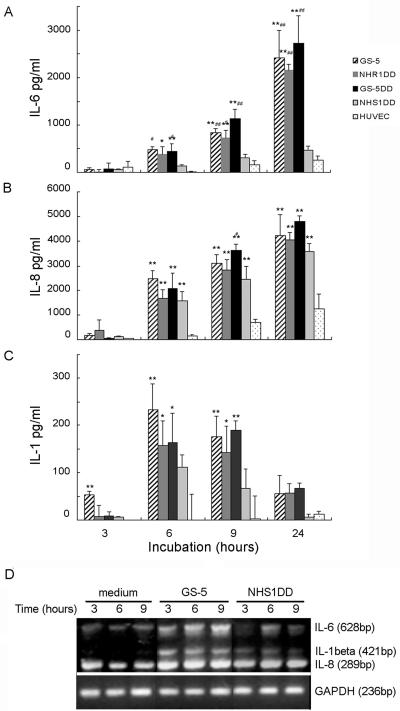
Human endothelial cells, such as HSVECs, respond to S. mutans cell surface protein I/II by producing the polymorphonuclear leukocyte chemokine IL-8 (53, 54). Previous results in vivo indicated that GTFs preferentially induced IL-6 from circulating monocytes (11) and the surrounding endocardium in situ (46). To delineate endothelial cell responses to GTFs, cytokine production by HUVECs was studied by comparing responses induced by wild-type S. mutans GS-5; a GTF-null mutant, NHS1DD; and two isogenic mutants, NHR1DD (which expresses GTFB only) and GS-5DD (which expresses GTFB and -C). Cytokine release into the culture supernatants was determined by cytokine-specific ELISA measurement of unstimulated HUVECs or cells stimulated with different strains at comparable infective doses. All four strains induced significant amounts of IL-8 from HUVECs, as found earlier for HSVECs (Fig. 2B), albeit a constitutively higher level of IL-8 (400 ± 56 pg ml−1) was found in HUVECs (26). The GTF-null or -defective mutants could still induce comparable levels of IL-8, because they express other proinflammatory agonists, such as protein I/II, and they express them at similar levels to parental GS-5, as seen on Western blots probed with a specific antiserum (data not shown). Unstimulated HUVECs released limited levels of IL-1β and TNF-α (<5 pg) and IL-6 (<200 pg). Addition of S. mutans GS-5, NHR1DD, or GS-5DD resulted in a marked increase (up to 10-fold) in the release of IL-6, but not TNF-α or IL-1β, in a time-dependent manner (Fig. 2A). However, the ability to induce IL-6 was completely abolished for the GTF-null mutant NHS1DD. The preferential induction of IL-6 by S. mutans was dose dependent and could be achieved by two other clinical isolates, NTU-5526 and NTU-4312, suggesting that the ability to induce IL-6 might be ubiquitous. Further analysis revealed that IL-β was found in the cell lysates, but not in the culture supernatant, after 3 h of stimulation with all strains tested. However, distinct from IL-6 or IL-8, the level of IL-1β decreased significantly thereafter (Fig. 2C). This inverse relationship between IL-6 and IL-1β, plus the notion that membrane-associated IL-1β was inactive (15), excluded the possibility that IL-6 production was secondary to IL-1β. However, a possible effect derived from IL-1α, which is active either intracellularly or pericellularly, was not excluded. These results confirmed our earlier findings in vivo and suggested that GTFs contribute significantly to IL-6 production and that endothelial cells could be targeted in addition to monocytes. The presence of only one cell-wall-associated GTF molecule, GTFB or GTFC, was significant for the observed stimulatory effect.
FIG. 2.
Induction of cytokines and chemokine by S. mutans wild type or mutants. The confluent monolayer of HUVECs was treated with wild-type strain GS-5 (hatched bars), GTFB-expressing strain NHR1DD (dark gray bars), GTFB/C-expressing strain GS-5DD (black bars), or the GTF-null mutant NHS1DD (light gray bars) and compared with the untreated control (spotted bars) for the time indicated. Culture supernatants or cell lysates were harvested for detection of IL-6, IL-8, and IL-1β levels by a sandwich ELISA. IL-6 (A) and IL-8 (B) were released by HUVECs, and IL-1β (C) was detected in the cell lysates but not in the supernatants. Data are expressed as means for triplicate experiments from three independent assays. Error bars indicate standard deviations. * and **, P ≤ 0.05 and P ≤ 0.01, respectively, relative to uninfected cells; # and ##, P ≤ 0.05 and P ≤ 0.01, respectively, relative to NHS1DD. (D) Upregulation of cytokine and chemokine mRNA expression. HUVECs (1 × 106 cells) were stimulated with 5 × 108 CFU of S. mutans or NHS1DD. Total cellular RNA was extracted, reverse transcribed, and analyzed by RT-PCR using synthetic oligonucleotides. PCR products were separated on 2% agarose gel and stained with ethidium bromide. Relative amounts of RNA were quantified by a densitometry analysis as described in Materials and Methods; GAPDH mRNA expression was used as an internal control.
Cell-free GTFC also induces expression of IL-6 and IL-8.
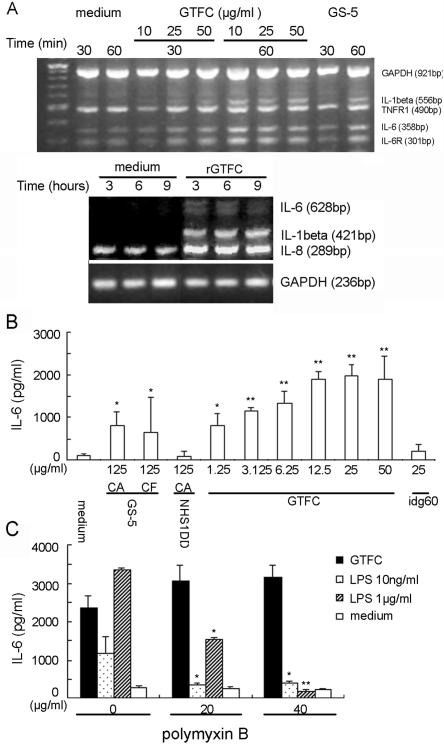
To confirm further the roles of GTFs in modulating cytokine expression, RT-PCR was performed with RNA isolated from untreated HUVECs or cells stimulated at various time intervals. Under our experimental conditions, untreated HUVECs incubated for 1 h with M199 culture medium did not contain any detectable IL-1β mRNA, but the basal levels of IL-6 and IL-8 gene expression were detectable (Fig. 3A). Treatment with live GS-5 upregulated the expression of the IL-6 gene significantly, up to a maximum after 9 h of exposure, whereas in GTF-null NHS1DD-treated cells, only a transient increase in IL-6 was observed after 6 h, followed by a decrease to almost basal levels (Fig. 2D). The IL-1β gene also was induced significantly by GS-5, reaching a plateau within 3 h and decreasing thereafter. Similar to the IL-6 gene, activation of IL-1β by NHS1DD was much lower in magnitude and declined faster than that with GS-5 (Fig. 2D). In contrast, expression of the IL-8 gene was increased to a similar level by either GS-5 or NHS1DD. These results are consistent with the earlier findings and confirmed that GTFs modulate cytokine expression, in particular that of IL-6, in HUVECs.
FIG. 3.
Dose-dependent induction of IL-6 release and up-regulation of cytokine mRNA in HUVECs induced by rGTFC. (A) Up-regulation of cytokine and chemokine mRNA expression in rGTFC-stimulated HUVECs. TNFR1, TNF receptor 1. (B) Dose-dependent induction of IL-6. A confluent monolayer of HUVECs (1 × 105) was stimulated by crude extracts of cell wall-associated (CA) or secreted (CF) proteins from wild-type GS-5 or from GTF-null mutant NHS1DD, purified rGTFC (1.56 to 50 μg ml−1), or recombinant immunodominant glycoprotein 60 (idg60; 25 μg ml−1) for 24 h. (C) Dose-dependent inhibition of LPS-stimulated IL-6 production in HUVECs stimulated by polymyxin B. rGTFC-induced IL-6 release was not affected by polymyxin B. HUVECs were stimulated in the presence or absence of 20 or 40 μg ml−1 polymyxin B for 24 h. IL-6 in supernatants was determined by sandwich ELISA. Data are expressed as means from triplicate experiments. Error bars indicated standard deviations. *, P ≤ 0.05; **, P ≤ 0.01.
To confirm that GTF in the cell-free form would exert effects on HUVECs similar to those observed when when present on the surface of the whole bacterium, rGTFC was tested with GS-5 whole bacterium as the positive control in parallel experiments. As shown in Fig. 3A, rGTFC up-regulated the expression of the IL-6 and IL-8 genes in a manner similar to that of the whole bacterium, detectable 30 min after interaction. Consistent with the mRNA levels, IL-6 release could be induced in a dose-dependent manner by rGTFC to a level similar to that of the whole bacterium GS-5 (Fig. 3B). In addition, the GTFs appeared to be the major components responsible for IL-6 induction in HUVECs, because the crude extracts of cell-wall-associated proteins from GS-5 induced the release of significantly higher levels of IL-6 than did extracts from NHS1DD (Fig. 3B). The preferential induction of IL-6 by GTFC was specific and not attributable to the His-tagged fusion, because another cell-wall-associated protein, IDG60 (12), carrying the identical fusion tag, exhibited negligible levels of released IL-6 (Fig. 3B). In addition, the induction of IL-6 by rGTFC was not affected in the presence of polymyxin B, which specifically represses the induction of IL-6 stimulated by LPS, a potent stimulator of the release of IL-6, IL-8, and IL-1β (Fig. 3C). Therefore, the polymyxin B inhibition assays excluded the possibility that the observed stimulatory effect on IL-6 production was attributed to minor contamination of LPS in the GTFC preparation. The stimulatory effect of rGTFC was abolished after heat inactivation, suggesting that the observed induction of cytokines was not attributed to the possible contamination of LPS-associated lipoprotein (22). However, the IL-6 induction by GTFC on HUVECs could be specifically inhibited by preincubation with anti-GTF rabbit IgG in a dose-dependent manner. Taken together, these results suggested that GTFs, such as GTFC, when released from or present on the entire bacterium (a situation that might occur in situ inside vegetations), are still capable of modulating and activating the surrounding endothelial cells through direct interaction.
GTFC enhances adhesion molecule expression on HUVECs.
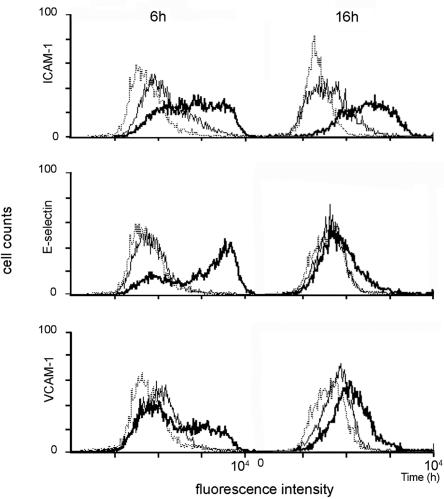
In addition to cytokine and chemokine production, activated endothelial cells exhibited up-regulated expression of several adhesion molecules, including E-selectin, ICAM-1, and VCAM-1. To test if GTFs could also exert similar effects, untreated HUVECs or HUVECs treated with rGTFC or LPS (positive control) at different time intervals were analyzed by FACS. Results from three independent assays are summarized in Table 1, and a representative FACS analysis is depicted in Fig. 4. The treatment with either S. mutans whole cells or rGTFC could significantly enhance the expression of all three tested adhesion molecules on the surface of HUVECs with distinct kinetics. E-selectin, the adhesion molecule involved in polymorphonuclear leukocyte trafficking, was induced rapidly to reach a plateau around 6 h and returned to a basal level comparable to that of the untreated controls (Fig. 4) after 16 h of stimulation with either whole bacterium or rGTFC. VCAM-1 followed similar kinetics to E-selectin, but the induction of ICAM-1, the adhesion molecule responsible mainly for monocyte recruitment, was persistently up-regulated after 16 h. These results indicated that GTFs in cell-free form could activate endothelial cells and result in up-regulation of IL-6 and adhesion molecules.
TABLE 1.
Surface expression of adhesion molecules in response to S. mutans, rGTFC, or LPS
| Stimulus | Expression of adhesion moleculea
|
|||||
|---|---|---|---|---|---|---|
| −NF-κB inhibitor (PDTC)
|
+NF-κB inhibitor (PDTC)
|
|||||
| ICAM-1 | E-selectin | VCAM-1 | ICAM-1 | E-selectin | VCAM-1 | |
| Medium | 6.4 ± 0.1 (1) | 2.7 ± 0.4 (1) | 1.7 ± 0.4 (1) | 1.7 ± 0.6 | 0.4 ± 0.04 | 0.5 ± 0.14 |
| GS-5 (108 CFU ml−1) | 51.8 ± 3.1* (8.1) | 52.6 ± 6.0* (19.9) | 20.9 ± 0.9* (12.4) | 0.7 ± 0.3 | 0.3 ± 0.04 | 0.4 ± 0.04 |
| NHS1DD (108 CFU ml−1) | 24.7 ± 3.7 (3.9) | 14.5 ± 1.6 (5.5) | 4.3 ± 0.2 (2.6) | 1.6 ± 0.2 | 0.3 ± 0.02 | 0.5 ± 0.13 |
| GTFC (25 μg ml−1) | 55.2 ± 1.9 (8.7) | 63.8 ± 6.8 (24) | 26.8 ± 0.5 (15.9) | 1.3 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.08 |
| LPS (1 μg ml−1) | 71.3 ± 0.2 (11.2) | 84.7 ± 0.4 (32.0) | 51.3 ± 0.5 (30) | 14.5 ± 2.1 | 1.1 ± 0.4 | 1.3 ± 0.48 |
The percentage of adhesion molecule expressed on the cells was determined by flow cytometry. Data represent the mean ± standard deviation of three independent experiments. *P ≤ 0.01 relative to NHS1DD-infected cells. For values in parentheses, baseline or constitutive expression was normalized and the increase (fold) in expression in response to bacteria was calculated.
FIG. 4.
Time-dependent expression of adhesion molecules on stimulated HUVECs. A confluent monolayer of HUVECs (1 × 105 cells) was treated with culture medium (solid line) or rGTFC (25 μg ml−1; heavy solid line) for 6 h or 16 h. The cells stained with secondary antibody served as a negative control (dot line). Cell surface expression of adhesion molecules was determined by indirect immunofluorescence and FACS analysis. Representative histograms are shown. The x axis indicates relative fluorescence intensity on a logarithmic scale; the y axis shows the number of cells on a linear scale. A total of 104 cells were analyzed for each histogram.
Enhanced adherence of monocytes to HUVECs activated by GTF or IL-6.
In the pathogenesis of IE, the adherence and recruitment of monocytes in situ play important roles in the persistent inflammation of the underlying endocardium. We hypothesized that S. mutans or GTF-activated HUVECs could enhance the adherence of monocytic cells and that IL-6, a cytokine involved in transition from neutrophil to monocyte recruitment (29), might contribute to this. To test this hypothesis, attachment of the monocytic cell line U937 to activated or untreated HUVECs was examined by FACS analysis. Untreated HUVECs arrested floating U937 cells (around 5.79% ± 1.44% of CD45-positive cells in the HUVEC-U937 mixture), probably through the constitutively expressed ICAM-1. Attachment of U937 increased significantly with stimulation by LPS (53.9% ± 3.01%), S. mutans GS-5 (42.3% ± 3.82%), or soluble rGTFC (44.1% ± 1.25%) and was also enhanced by NHS1DD (22.7% ± 1.08%), but to a lesser extent than by GS-5 or rGTFC. The increased level of U937 adherence correlated well with the enhanced expression of adhesion molecules ICAM-1, E-selectin, and VCAM-1, induced, respectively, by LPS, GS-5, or rGTFC, as shown in Table 1. In our experimental systems, HUVECs cultured in vitro expressed the IL-6 receptor gp130 on their surfaces, and after exposure to IL-6 for 6 h, ICAM-1 expression on HUVECs increased in a dose-dependent manner. Addition of sIL-6Rα in combination resulted in an additive effect from 1.63- to 2.9-fold. Enhanced expression of ICAM-1 on the cell surface was accompanied by a dose-dependent increased adhesion of the monocytic U937 cells to IL-6- or combined sIL-6Rα-activated HUVECs (Table 2). The fold increase in U937 adherence was similar to that observed earlier on GS-5-infected HUVECs. The supernatant from GS-5- or rGTFC-treated HUVECs contained undetectable sIL-6Rα and exhibited induced ICAM-1 at a much higher level than IL-6 alone, suggesting that GTF-induced ICAM-1 expression was not secondary to the effect of IL-6. Taken together, our results indicated that GTFs activate endothelial cells to recruit monocytes by up-regulation of adhesion molecules and IL-6, which in combination with sIL-6Rα could further augment the interactions per se.
TABLE 2.
Effect of IL-6 in combination with sIL-6Rα on cell surface expression of ICAM-1 and U937 adherence
| IL-6 concn (ng ml−1) | sIL-6Rα concn (ng ml−1) | % ICAM-1 expressiona | % U937 adherenceb |
|---|---|---|---|
| 0 | 0 | 8.9 ± 2.3 (1) | 7.6 ± 1.9 (1) |
| 1 | 0 | 11.4 ± 2.6 (1.28) | 10.1 ± 2.1 (1.33) |
| 10 | 0 | 14.2 ± 4.8 (1.6) | 12.1 ± 1.5 (1.59) |
| 20 | 0 | 14.5 ± 1.6 (1.63) | 13.9 ± 2.7 (1.83) |
| 0 | 20 | 17.7 ± 7.5 (1.99) | 15.5 ± 6.9 (1.99) |
| 1 | 20 | 20.4 ± 6.7 (2.29) | 18.4 ± 7.6 (2.42) |
| 10 | 20 | 22.3 ± 2.0 (2.51) | 20.0 ± 4.7 (2.63) |
| 20 | 20 | 25.8 ± 5.1 (2.9) | 21.1 ± 6.1 (2.9) |
The percentage of adhesion molecule expressed on the cells was determined by flow cytometry. The data represent the mean ± standard deviation of three independent experiments.
Baseline or constitutive expression was normalized, and the increase (fold) in the expression in response to bacteria was calculated.
Cell signaling events involved in GTFC activation.
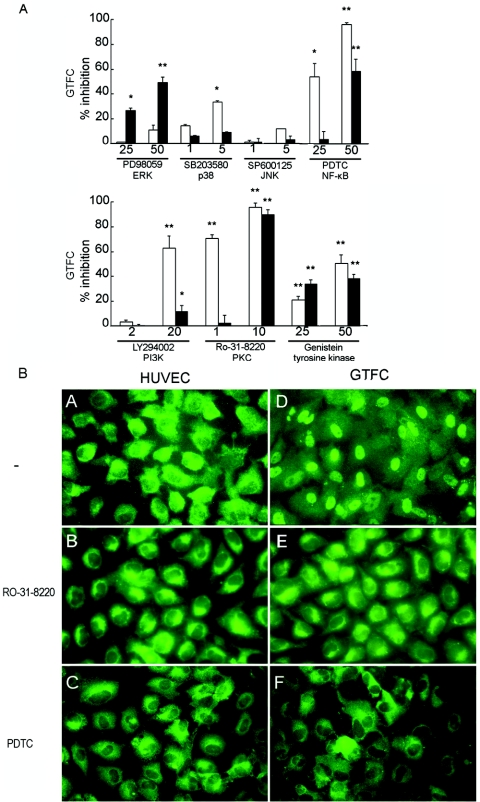
Although the cellular receptors or molecules on HUVECs that GTFs interact directly with are not clear at this moment, we attempted to explore the mechanism of activation triggered by GTFC and the intracellular signaling events that might be involved. In response to many types of PAMPs or modulins, MAPKs and/or NF-κB appears to be the pivotal regulator of the transcription of proinflammatory cytokines, chemokines, adhesion molecules, etc. (5, 48). To investigate whether MAPK signaling pathways might contribute to GTF-induced IL-6 or IL-8 production from HUVECs, selected inhibitors of p38 MAPK, ERK1/2, and JNK were prepared and added to the cell culture medium. As shown in Fig. 5A, rGTFC-induced IL-6 release by HUVECs could be inhibited dose dependently by both SB203580, a selective inhibitor of p38 MAPK at low concentrations, and PD98059, the specific MEK1 and MEK2 inhibitor that blocks the ERK1/2 signaling cascade. The maximal levels of inhibition of IL-6 release obtained with SB203580 or PD98059 were 33.6% ± 2.5% and 23% ± 1%, respectively. But only marginal inhibition of IL-6 production induced by rGTFC could be observed with the JNK inhibitor SP600125. Interestingly, the release of IL-8 was unaffected by p38 MAPK inhibitor, but it was significantly inhibited (up to 50%) when the ERK1/2 signaling cascade was blocked (Fig. 5A). The presence of dimethyl sulfoxide in the culture medium was found to have no effect on IL-6 or IL-8 release by infected HUVECs (data not shown). Activation of MAPKs could be mediated in concert through upstream activation of PI3K, PKC, and PTKs (18). In agreement with the concerted pathway, preincubation with respective antagonist LY294002 (PI3K) and inhibitor Ro-31-8220 (PKC) or genistein (PTKs), caused a significant reduction of rGTFC-induced IL-6 production by approximately 50% to 95% (Fig. 5B). These results suggested that p38 MAPK and ERK1/2, but not JNK, could be activated by rGTFC protein and contributed to the induction of IL-6 release from HUVECs.
FIG. 5.
Inhibitor-mediated inhibition of IL-6 or IL-8 and NF-κB translocation in rGTFC-stimulated HUVECs. (A) Inhibitor-mediated inhibition of IL-6 and IL-8 in stimulated HUVECs. HUVECs were pretreated with different inhibitors for 1 h and then stimulated with purified rGTFC (25 μg ml−1) for 24 h at 37°C. Levels of IL-6 (white bars) and IL-8 (dark bars) in the cell supernatants were determined by sandwich ELISA. The results are expressed as percentages of inhibition of IL-6 and IL-8 release and are means ± standard deviation for triplicate determinations from three independent assays. *, P ≤ 0.05; **, P ≤ 0.01. (B) Immunofluorescence detection of p65 translocation and PDTC-mediated inhibition in HUVECs. HUVECs were left unstimulated (A, B, and C) or were stimulated with purified rGTFC (D, E, and F) for 1 h, and then cells were fixed, permeabilized, and stained with an anti-NF-κB p65 antibody (visually by the FITC-labeled secondary antibody). For inhibition assays, HUVECs were pretreated with PKC inhibitor Ro-31028 (B and E) or with NF-κB inhibitor PDTC (C and F) and then stimulated with rGTFC. Original magnification, ×400.
The MAPK inhibitors did not abolish completely the production of IL-6 in rGTFC-treated HUVECs, suggesting that another signaling pathway(s) downstream of PTKs also might be involved. We considered NF-κB as a potential candidate in our experimental systems. Incubation of HUVECs with rGTFC provoked a marked nuclear translocation of NF-κB within 1 h in about 82% ± 10% (80 to 100 cells per field from 10 fields) of the cells (Fig. 5B). Activation of NF-κB by rGTFC was unaffected in the presence of polymyxin B, whereas the LPS-induced NF-κB activation in HUVECs was abolished by polymyxin B (data not shown). Preincubation of endothelial cells with the PKC inhibitor Ro-31-8220 or NF-κB inhibitor PDTC (Fig. 5B) markedly reduced (over 90%) the nuclear shift of NF-κB and the release of IL-6 (Fig. 5A) in response to rGTFC. An analogous inhibitory effect on IL-6 release was also observed when cells were pretreated with BAY 11-7082, an inhibitor of cytokine-induced IκB-α phosphorylation. The up-regulation of all three adhesion molecules at 6 h was abolished completely in the presence of PDTC, an inhibitor of NF-κB activation (Table 1). Taken together, these findings suggested that up-regulation of adhesion molecules on HUVECs induced by rGTFC was dependent on NF-κB activation that also contributed to the induction of IL-6.
DISCUSSION
This report demonstrates that GTFs, shared by different members of viridans group streptococci, can activate endothelial cells to produce cytokines, chemokines, and the expression of adhesion molecules following activation; such interactions provide a microenvironment facilitating the firm adhesion of monocytes, a critical step in the transmigration or recruitment of monocytes. Additionally, we provide evidence to support our previous findings in vivo that GTFs are the major modulins inducing IL-6 expression and confirm that the role of IL-6 is to augment the monocyte arrest on endothelial cells through an autocrine interaction. GTFs, either presented on the surface of bacteria or released, are efficient in activating endothelial cells. An interesting pathological finding derived from experimental endocarditis is that the infected microorganism is walled off inside the vegetations, surrounded by a platelet/fibrin layer, and bordered by an influx of immune cells (34, 38). Bacteria are rarely found in the surrounding valves or underlying endocardium, at which sites the persistent chronic inflammatory responses were most frequently observed. Modulins, such as GTFs, rather than the whole bacterium, might easily gain access to the surrounding endothelium or penetrate deep inside the underlying endocardium, where the interaction would take place directly with the endothelium or with the recruited monocytes. Therefore, bacterial modulins from these gram-positive microorganisms might play an important role in persistent endocardial inflammation during IE.
GTFs synthesize exopolysaccharide glucans using sucrose as a substrate, and both GTFs and glucans are important virulence factors for adherence and the formation of biofilm on the tooth surface in the oral cavity (49). S. mutans and other GTFs secreting viridans group streptococci can gain access readily to the blood circulation after routine dental procedures or traumatic injury (20). Distinct from the oral cavity, sucrose is found infrequently in human blood, and the results of previous and present studies demonstrated another role of GTFs during systemic infections. Complex roles of GTFs in pathogenesis might be attributed to the multiple domains that are well conserved in these molecules. GTFs of S. mutans consist of an amino-terminal enzymatic domain and a carboxyl-terminal glucan-binding domain (GBD) (45). The GBD of GTFs binds both dextran, rich in α-1,6 linkages, and mutan, which consists predominantly of α-1,3 linkages. The GBDs of three GTFs consist of repeating units of 21 amino acids, characterized by the consistent presence of one or more tyrosine residues near the beginning of the repeat and a highly conserved glycine in the middle of the repeat. Sequence comparison reveals that these repeats are homologous not only to each other within a protein but also to the repeats found in the GTFs from other oral streptococci, toxins A and B from Clostridium difficile, and the autolysins from Streptococcus pneumoniae and its bacteriophages (57). Similar to GTFs, toxin A exhibits two functional domains: the carboxyl-terminal portion of the toxins comprising the cellular binding domain and the amino-terminal portion containing the enzymatic activity (UDP-glucosyltransferase), which inactivates the intracellular GTP binding proteins RhoA and -B (28). Toxin A binds to a variety of cells from different lineages, and such broad binding specificity might be attributed to the interaction of C-terminal repeats with multiple cellular receptors of glycoproteins or glycolipid containing carbohydrate domains. Rabbit sucrase-isomaltase is a functional intestinal receptor for Clostridium difficile toxin A (42). Our current hypothesis is that GTFs, analogous to toxin A, might bind to multiple glycoproteins, such as integrins or proteoglycans, commonly identified on endothelial cells, and such binding is relevant to the signal transduction mechanisms that mediate in vitro and in vivo modulation activity.
In HUVECs, a well-characterized model of vascular endothelial cells, S. mutans or GTFs alone induce persistent expression of IL-6 and IL-8 and transient expression of IL-1β. An interesting finding was that the induction of IL-6, but not IL-8, was nearly abolished by NHSIDD, a GTF-null mutant, albeit the expression of other modulins, such as protein I/II, was unaffected on NHS1DD. These results are not considered to be contradictory to a previous report that proteins I and II were potent modulins of endothelial cells, inducing them to produce both IL-6 and IL-8 (53). The possibility was that different cell cultures and cell culture medium were tested. In the present study, HUVECs were the targeted cells, whereas the proteins I/II were tested primarily on HSVECs. The experiment to determine the effect of S. mutans whole cells or rGTFC on HUVECs was conducted in culture medium containing 10% heat-inactivated fetal calf serum in the present study, whereas the binding of proteins I and II to HSVECs and induced cytokine release could be inhibited by heat-inactivated normal human serum (2, 53). Whether a similar inhibitory effect on protein I/II by fetal calf serum might occur and such inhibition might be attributable to the defect in NHS1DD awaits further investigation. Tissue-specific phenotypical variations in terms of chemokine or chemokine receptor expression have been reported in endothelial cells of different origins (26). Common, as well as distinct, signaling pathways might be involved in the up-regulation of the IL-6 or IL-8 gene upon exposure of HUVECs to GTFs. The MAPK pathway is one of the major pathways for transmitting signals to immediate-early genes implicated in the regulation of cytokine responses. We demonstrated that inhibition of JNK with SP600125 resulted in no significant change in the total amounts of secreted IL-6 or IL-8. It has been proposed that JNK is not a major activator, but contributes to IL-8 gene expression (16). Using specific inhibitors of p38 MAPK and ERK1/2, we demonstrated that these MAPKs might contribute, but only partially, to the enhanced expression of IL-6 or IL-8. In addition, rGTFC-induced IL-8 release is weakly dependent on the p38 MAPK signaling pathway, because IL-8 release from activated HUVECs was unaffected in the presence of SB203580, while the release of IL-6 was inhibited by a total of 33% by a low concentration of SB203580 (5 μM). Differential regulation of IL-6 versus IL-8 expression via one or more MAPKs has also been demonstrated upon exposure of synoviocytes to S. mutans protein I/II or human brain microvascular endothelial cells to Neisseria menigitidis (40, 47). Interestingly, the IL-8 release from synoviocytes induced by S. mutans protein I/II also was weakly dependent on p38 MAPK, even though p38 MAPK stabilizes IL-8 mRNA and may contribute to IL-8 production by posttranscriptional mechanisms (21, 56).
NF-κB is a dimeric transcription factor formed by the hetero- or homodimerization of proteins in the Rel family, including p50 and p65. Activation of the NF-κB proteins plays a central role in inflammation through the regulation of genes encoding proinflammatory cytokines, chemokines, and adhesion molecules (6). Our results also demonstrated that GTFC-induced nuclear translocation of p65 containing NF-κB in HUVECs and a selective inhibitor of NF-κB, PDTC, inhibited the release of IL-6 to a much greater extent than IL-8 at both a low concentration (25 μM) and a higher concentration (50 μM). Such a discrepancy, as found in the MAPKs, suggested further the IL-6 and IL-8 might be differentially regulated in HUVECs upon stimulation with GTFs. Distinct pathways as well as the signaling events upstream await further investigation. In contrast to the activation of IL-6 or IL-8, induced expression of adhesion molecules E-selectin, VCAM-1, and ICAM-1 was totally dependent on the activation of NF-κB, because PDTC completely blocked the surface expression of these adhesion molecules. The promoters of the E-selectin, VCAM-l, and ICAM-1 genes contain recognition sequences for NF-κB. Direct binding of NF-κB to these sites in vitro has been demonstrated in HUVECs after TNF-α stimulation. The stimulatory effect achieved by rGTFC on the expression of adhesion molecules and adherence of U937 cells was comparable to that by the S. mutans whole cells, whereas the stimulation achieved by NHS1DD was reduced significantly. These results suggested that GTFs play a significant role in monocyte recruitment by up-regulation of adhesion molecules through the activation of NF-κB.
One important question in such a model is whether the induced adhesion of U937 by GTFC was direct or indirect through the combinatorial effects of IL-6 and sIL-6Rα. In our experimental model, gp130 protein expression could be detected in HUVECs but exogenous sIL-6Rα was undetectable. Nevertheless, IL-6 when added alone could enhance ICAM-I expression and U937 adherence. IL-6 has been reported to induce activation of HUVECs directly, as well as lymphocyte-endothelial cell adhesion (55), although HUVECs express the gp130 transducer but not IL-6Rα. In addition, IL-6 could exert its autocrine effect on HUVECs through an IL-6/IL-6Rα/gp130 complex to induce the release of MCP-1, a key chemokine for monocytes (33). Specifically, IL-6 is able to form a complex with sIL-6Rα, before forming a complex with gp130 homodimers, and activate HUVECs for chemokine production and adhesion molecule expression (44). Similar results were obtained in the present study: greater (fold) expression of ICAM-I and U937 adherence was achieved when exogenous sIL-6Rα was added in addition to IL-6. As well as MCP-1, IL-8 could also arrest rolling monocytes to adhere firmly onto HUVECs by inducing the expression of specific integrin adhesion receptor on the monocytes, probably through signaling pathways distinct from MCP-1 (23). Primary cultured HUVECs constitutively express IL-8, as demonstrated in this and another report (26), and might account for the basal level of U937 adherence detected in the untreated HUVECs. Addition of IL-6 and sIL-6Rα, within physiological concentrations (<500 ng ml−1), to thrombin-activated HUVECs induced the secretion of MCP-1 but not IL-8 (33). A similar observation also was made in the present study—that addition of IL-6 plus sIL-6Rα could induce the expression of MCP-1 but not IL-8 (data not shown).
In conclusion, we have demonstrated that streptococcal GTFs could activate endothelial cells to enhance the expression of IL-6 and adhesion molecules, which act in concert to arrest monocyte adherence to endothelial cells. Such an interaction might take place in vivo during IE, resulting in the chronic inflammation and damage to the underlying endocardium caused by persistent release of bacterial modulins, such as GTFs, instead of the whole bacterium.
Acknowledgments
We thank H. K. Kuramitsu for providing NHR1DD, GS-5DD, and NHS1DD strains. We appreciate Yang Yu-Shih, Department of Gynecology, National Taiwan University Hospital, for assistance with HUVEC preparation. We thank Tim J. Harrison, UCL, for kind review and help with the preparation of the manuscript. We thank Lien Huei-Ting for technical assistance with cell cultures and FACS.
This work was supported in part by the National Science Council (grants NSC-922320-B002-183, NSC-932320-B002-041, and NSC-942320-B-002-007) and National Health Research Institute grants (grant NHRI-EX91-9139SI, NHRI-EX92-9139SI, NHRI-EX93-9139SI, and NHRI-EX94-9432-SI).
Editor: V. J. DiRita
REFERENCES
- 1.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Okla, S., C. Chatenay-Rivauday, J. P. Klein, and D. Wachsmann. 1999. Involvement of alpha5beta1 integrins in interleukin 8 production induced by oral viridans streptococcal protein I/IIf in cultured endothelial cells. Cell Microbiol. 1:157-168. [DOI] [PubMed] [Google Scholar]
- 3.Alter, P., J. Hoeschen, M. Ritter, and B. Maisch. 2002. Usefulness of cytokines interleukin-6 and interleukin-2R concentrations in diagnosing active infective endocarditis involving native valves. Am. J. Cardiol. 89:1400-1404. [DOI] [PubMed] [Google Scholar]
- 4.Aoki, H., T. Shiroza, M. Hayakawa, S. Sato, and H. K. Kuramitsu. 1986. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect. Immun. 53:587-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldassare, J. J., Y. Bi, and C. J. Bellone. 1999. The role of p38 mitogen-activated protein kinase in IL-1 beta transcription. J. Immunol. 162:5367-5373. [PubMed] [Google Scholar]
- 6.Baldwin, A. S., Jr. 1996. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. [DOI] [PubMed] [Google Scholar]
- 7.Bancsi, M. J. L. M. F., M. H. A. M. Veltrop, R. M. Bertina, and J. Thompson. 1996. Influence of monocytes and antibiotic treatment on tissue factor activity of endocardial vegetations in rabbits infected with Streptococcus sanguis. Infect. Immun. 64:448-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bancsi, M. J. L. M. F., M. H. A. M. Veltrop, R. M. Bertina, and J. Thompson. 1998. Role of monocytes and bacteria in Staphylococcus epidermidis endocarditis. Infect. Immun. 66:448-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buiting, A. G., J. Thompson, D. van der Keur, W. C. Schmal-Bauer, and R. M. Bertina. 1989. Procoagulant activity of endocardial vegetations and blood monocytes in rabbits with Streptococcus sanguis endocarditis. Thromb. Haemostasis 62:1029-1033. [PubMed] [Google Scholar]
- 10.Chia, J.-S., R.-H. Lin, S.-W. Lin, J.-Y. Chen, and C.-S. Yang. 1993. Inhibition of glucosyltransferase activities of Streptococcus mutans by a monoclonal antibody to a subsequence peptide. Infect. Immun. 61:4689-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chia, J.-S., C.-M. You, C.-Y. Hu, B.-L. Chiang, and J.-Y. Chen. 2001. Human T-cell responses to the glucosyltransferases of Streptococcus mutans. Clin. Diagn. Lab. Immunol. 8:441-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chia, J.-S., L. Y. Chang, C.-T. Shun, Y.-Y. Chang, and J.-Y. Chen. 2001. A 60-kilodalton immunodominant glycoprotein is essential for cell wall integrity and the maintenance of cell shape in Streptococcus mutans. Infect. Immun. 69:6987-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chia, J.-S., H.-T. Lien, P.-R. Hsueh, P.-M. Chen, A. Sun, and J.-Y. Chen. 2002. Induction of cytokines by glucosyltransferases of Streptococcus mutans. Clin. Diagn. Lab. Immunol. 9:892-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 15.Dinarello, C. A. 1997. Interleukin-1. Cytokine Growth Factor Rev. 8:253-265. [DOI] [PubMed] [Google Scholar]
- 16.Doran, K. S., G. Y. Liu, and V. Nizet. 2003. Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J. Clin. Investig. 112:736-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drake, T. A., G. M. Rodgers, and M. A. Sande. 1984. Tissue factor is a major stimulus for vegetation formation in enterococcal endocarditis in rabbits. J. Clin. Investig. 73:1750-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eligini, S., B. S. Stella, V. Cavalca, M. Camera, M. Brambilla, M. De Franceschi, E. Tremoli, and S. Colli. 2005. Diversity and similarity in signaling events leading to rapid Cox-2 induction by tumor necrosis factor-alpha and phorbol ester in human endothelial cells. Cardiovasc. Res. 65:683-693. [DOI] [PubMed] [Google Scholar]
- 19.Enting, R. H., J. de Gans, J. P. Blankevoort, and L. Spanjaard. 1997. Meningitis due to viridans streptococci in adults. J. Neurol. 244:435-438. [DOI] [PubMed] [Google Scholar]
- 20.Fekete, T. 1990. Controversies in the prevention of infective endocarditis related to dental procedures. Dent. Clin. N. Am. 34:79-90. [PubMed] [Google Scholar]
- 21.Frevel, M. A., T. Bakheet, A. M. Silva, J. G. Hissong, K. S. A. Khabar, and B. R. G. Williams. 2003. p38 mitogen-activated protein kinase-dependent and -independent signaling of mRNA stability of AU-rich element-containing transcripts. Mol. Cell. Biol. 23:425-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao, B., and M. F. Tsan. 2003. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor alpha release by murine macrophages. J. Biol. Chem. 278:174-179. [DOI] [PubMed] [Google Scholar]
- 23.Gerszten, R. E., E. A. Garcia-Zepeda, Y. C. Lim, M. Yoshida, H. A. Ding, M. A. Gimbrone, Jr., A. D. Luster, F. W. Luscinskas, and A. Rosenzweig. 1999. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 398:718-723. [DOI] [PubMed] [Google Scholar]
- 24.Hanada, N., and H. K. Kuramitsu. 1988. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect. Immun. 56:1999-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanada, N., and H. K. Kuramitsu. 1989. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect. Immun. 57:2079-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hillyer, P., E. Mordelet, G. Flynn, and D. Male. 2003. Chemokines, chemokine receptors and adhesion molecules on different human endothelia: discriminating the tissue-specific functions that affect leucocyte migration. Clin. Exp. Immunol. 134:431-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaffe, E. A., R. L. Nachman, C. G. Becker, and C. R. Minick. 1973. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 52:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Just, I., J. Selzer, C. Eichel-Streiber, and K. Aktories. 1995. The low molecular mass GTP-binding protein Rho is affected by toxin A from Clostridium difficile. J. Clin. Investig. 95:1026-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplanski, G., V. Marin, F. Montero-Julian, A. Mantovani, and C. Farnarier. 2003. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 24:25-29. [DOI] [PubMed] [Google Scholar]
- 30.Kempsell, K. E., C. J. Cox, M. Hurle, A. Wong, S. Wilkie, E. D. Zanders, J. S. H. Gaston, and J. S. Crowe. 2000. Reverse transcriptase-PCR analysis of bacterial rRNA for detection and characterization of bacterial species in arthritis synovial tissue. Infect. Immun. 68:6012-6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnaswamy, G., J. Kelley, L. Yerra, J. K. Smith, and D. S. Chi. 1999. Human endothelium as a source of multifunctional cytokines: molecular regulation and possible role in human disease. J. Interferon Cytokine Res. 19:91-104. [DOI] [PubMed] [Google Scholar]
- 32.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 33.Marin, V., F. A. Montero-Julian, S. Gres, V. Boulay, P. Bongrand, C. Farnarier, and G. Kaplanski. 2001. The IL-6-soluble IL-6Ralpha autocrine loop of endothelial activation as an intermediate between acute and chronic inflammation: an experimental model involving thrombin. J. Immunol. 167:3435-3442. [DOI] [PubMed] [Google Scholar]
- 34.McCormick, J. K., H. Hirt, C. M. Waters, T. J. Tripp, G. M. Dunny, and P. M. Schlievert. 2001. Antibodies to a surface-exposed, N-terminal domain of aggregation substance are not protective in the rabbit model of Enterococcus faecalis infective endocarditis. Infect. Immun. 69:3305-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monchois, V., R. M. Willemot, and P. Monsan. 1999. Glucansucrases: mechanism of action and structure-function relationships. FEMS Microbiol. Rev. 23:131-151. [DOI] [PubMed] [Google Scholar]
- 36.Moreillon, P., Y. A. Que, and A. S. Bayer. 2002. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect. Dis. Clin. N. Am. 16:297-318. [DOI] [PubMed] [Google Scholar]
- 37.Muller, W. A. 2003. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 24:327-334. [DOI] [PubMed] [Google Scholar]
- 38.Munro, C. L., and F. L. Macrina. 1993. Sucrose-derived exopolysaccharides of Streptococcus mutans V403 contribute to infectivity in endocarditis. Mol. Microbiol. 8:133-142. [DOI] [PubMed] [Google Scholar]
- 39.Mylonakis, E., and S. B. Calderwood. 2001. Infective endocarditis in adults. N. Engl. J. Med. 345:1318-1330. [DOI] [PubMed] [Google Scholar]
- 40.Neff, L., M. Zeisel, J. Sibilia, M. Scholler-Guinard, J. P. Klein, and D. Wachsmann. 2001. NF-kappaB and the MAP kinases/AP-1 pathways are both involved in interleukin-6 and interleukin-8 expression in fibroblast-like synoviocytes stimulated by protein I/II, a modulin from oral streptococci. Cell Microbiol. 3:703-712. [DOI] [PubMed] [Google Scholar]
- 41.Peters, K., R. E. Unger, J. Brunner, and C. J. Kirkpatrick. 2003. Molecular basis of endothelial dysfunction in sepsis. Cardiovasc. Res. 60:49-57. [DOI] [PubMed] [Google Scholar]
- 42.Pothoulakis, C., R. J. Gilbert, C. Cladaras, I. Castagliuolo, G. Semenza, Y. Hitti, J. S. Montcrief, J. Linevsky, C. P. Kelly, S. Nikulasson, H. P. Desai, T. D. Wilkins, and J. T. LaMont. 1996. Rabbit sucrase-isomaltase contains a functional intestinal receptor for Clostridium difficile toxin A. J. Clin. Investig. 98:641-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rawczynska-Englert, I., T. Hryniewiecki, and D. Dzierzanowska. 2000. Evaluation of serum cytokine concentrations in patients with infective endocarditis. J. Heart Valve Dis. 9:705-709. [PubMed] [Google Scholar]
- 44.Romano, M., M. Sironi, C. Toniatti, N. Polentarutti, P. Fruscella, P. Ghezzi, R. Faggioni, W. Luini, V. van Hinsbergh, S. Sozzani, F. Bussolino, V. Poli, G. Ciliberto, and A. Mantovani. 1997. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6:315-325. [DOI] [PubMed] [Google Scholar]
- 45.Shimamura, A., Y. J. Nakano, H. Mukasa, and H. K. Kuramitsu. 1994. Identification of amino acid residues in Streptococcus mutans glucosyltransferases influencing the structure of the glucan product. J. Bacteriol. 176:4845-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shun, C.-T., S.-Y. Lu, C.-Y. Yeh, C.-P. Chiang, J.-S. Chia, and J.-Y. Chen. 2005. Glucosyltransferases of viridans streptococci are modulins of interleukin-6 induction in infective endocarditis. Infect. Immun. 73:3261-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sokolova, O., N. Heppel, R. Jagerhuber, K. S. Kim, M. Frosch, M. Eigenthaler, and A. Schubert-Unkmeir. 2004. Interaction of Neisseria meningitidis with human brain microvascular endothelial cells: role of MAP- and tyrosine kinases in invasion and inflammatory cytokine release. Cell Microbiol. 6:1153-1166. [DOI] [PubMed] [Google Scholar]
- 48.Tak, P. P., and G. S. Firestein. 2001. NF-kappaB: a key role in inflammatory diseases. J. Clin. Investig. 107:7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamesada, M., S. Kawabata, T. Fujiwara, and S. Hamada. 2004. Synergistic effects of streptococcal glucosyltransferases on adhesive biofilm formation. J. Dent. Res. 83:874-879. [DOI] [PubMed] [Google Scholar]
- 50.Tsumori, H., and H. Kuramitsu. 1997. The role of the Streptococcus mutans glucosyltransferases in the sucrose-dependent attachment to smooth surfaces: essential role of the GtfC enzyme. Oral Microbiol. Immunol. 12:274-280. [DOI] [PubMed] [Google Scholar]
- 51.Vacca-Smith, A. M., C. A. Jones, M. J. Levine, and M. W. Stinson. 1994. Glucosyltransferase mediates adhesion of Streptococcus gordonii to human endothelial cells in vitro. Infect. Immun. 62:2187-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veltrop, M. H. A. M., M. J. L. M. F. Bancsi, R. M. Bertina, and J. Thompson. 2000. Role of monocytes in experimental Staphylococcus aureus endocarditis. Infect. Immun. 68:4818-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vernier, A., M. Diab, M. Soell, G. Haan-Archipoff, A. Beretz, D. Wachsmann, and J.-P. Klein. 1996. Cytokine production by human epithelial and endothelial cells following exposure to oral viridans streptococci involves lectin interactions between bacteria and cell surface receptors. Infect. Immun. 64:3016-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vernier-Georgenthum, A., S. Al Okla, B. Gourieux, J. P. Klein, and D. Wachsmann. 1998. Protein I/II of oral viridans streptococci increases expression of adhesion molecules on endothelial cells and promotes transendothelial migration of neutrophils in vitro. Cell Immunol. 187:145-150. [DOI] [PubMed] [Google Scholar]
- 55.Watson, C., S. Whittaker, N. Smith, A. J. Vora, D. C. Dumonde, and K. A. Brown. 1996. IL-6 acts on endothelial cells to preferentially increase their adherence for lymphocytes. Clin. Exp. Immunol. 105:112-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winzen, R., M. Kracht, B. Ritter, A. Wilhelm, C. Y. Chen, A. B. Shyu, M. Muller, M. Gaestel, K. Resch, and H. Holtmann. 1999. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 18:4969-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wren, B. W. 1991. A family of clostridial and streptococcal ligand-binding proteins with conserved C-terminal repeat sequences. Mol. Microbiol. 5:797-803. [DOI] [PubMed] [Google Scholar]
- 58.Yao, L., V. Bengualid, F. D. Lowy, J. J. Gibbons, V. B. Hatcher, and J. W. Berman. 1995. Internalization of Staphylococcus aureus by endothelial cells induces cytokine gene expression. Infect. Immun. 63:1835-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]