Abstract
Gram-negative flagellin, a Toll-like receptor 5 (TLR5) agonist, is a potent inducer of innate immune effectors such as cytokines and nitric oxide. In the lung, flagellin induces a localized and transient innate immune response characterized by neutrophil infiltration and the production of cytokines and chemokines. In view of the extraordinary potency of flagellin as an inducer of innate immunity and the contribution of innate responses to the development of adaptive immunity, we evaluated the efficacy of recombinant Salmonella flagellin as an adjuvant in an acellular plague vaccine. Mice immunized intranasally or intratracheally with the F1 antigen of Yersinia pestis and flagellin exhibited dramatic increases in anti-F1 plasma immunoglobulin G (IgG) titers that remained stable over time. In contrast, control mice had low or undetectable antibody responses. The IgG1/IgG2a ratio of antibody titers against F1 in immunized mice is consistent with a Th2 bias. However, no significant antigen-specific IgE production was detected. Interferons, tumor necrosis factor alpha, and interleukin-6 were not essential for the adjuvant effects of flagellin. Preexisting antiflagellin antibodies had no significant effect on the adjuvant activity of flagellin. Importantly, intranasal immunization with flagellin and the F1 antigen was protective against intranasal challenge with virulent Y. pestis CO92, with 93 to 100% survival of immunized mice. Lastly, vaccination of cynomolgus monkeys with flagellin and a fusion of the F1 and V antigens of Y. pestis induced a robust antigen-specific IgG antibody response.
Yersinia pestis, the causative agent of plague, is a gram-negative organism responsible for approximately 200 million deaths during three major pandemics. In humans, plague has three forms designated by the nature of the infection: bubonic, pneumonic, and septicemic. Whereas bubonic plague is spread via bites from infected fleas, the pneumonic form may be transmitted person to person. Without medical treatment, pneumonic plague is a rapidly progressing disease with a mortality rate approaching 100% (28, 52). The use of whole-cell vaccines for plague has raised safety concerns. Although the shift in vaccine development from complete pathogens to individual antigens has led to safer vaccines, efficacy has been markedly reduced. Vaccine adjuvants are necessary to promote strong adaptive responses to soluble recombinant protein antigens. Immunization with the F1 antigen of Y. pestis and an appropriate adjuvant elicits a protective response that strongly correlates with the titer of anti-F1 immunoglobulin G1 (IgG1) antibodies (3, 50). A synergistic protective effect is obtained when animals are immunized with both F1 and V antigens or a recombinant F1/V fusion protein (13, 20, 29, 57). Although highly variable responses were observed, a phase 1 clinical trial demonstrated that intramuscular immunization with a vaccine containing F1 and V is immunogenic in humans (56).
Recognition of conserved microbial products by Toll-like receptors (TLRs) expressed on cells such as endothelial and epithelial cells, monocytes, macrophages, and immature dendritic cells stimulates the production of proinflammatory cytokines (24, 39) as well as the maturation and migration of dendritic cells to secondary lymphoid sites (1, 34). Eleven mammalian TLRs (59) have been identified and are characterized by extracellular leucine-rich repeat domains and an intracellular Toll/interleukin-1 (IL-1) receptor domain. Microbial ligands for TLRs include bacterial lipopolysaccharide (LPS), peptidoglycan and lipoproteins, yeast cell wall components, viral double-stranded RNA, and the unmethylated CpG motifs of bacterial DNA, as well as bacterial flagellin. As these components are essential for survival or pathogenicity, they are highly conserved and allow the limited number of TLRs to recognize a multitude of pathogenic organisms.
The proinflammatory effects of TLR agonists such as gram-negative LPS and bacterial CpG DNA have led to evaluation of their adjuvant properties and effects on dendritic cells (23, 26, 46). In this regard, Arnon and colleagues demonstrated that recombinant flagellin containing foreign epitopes elicited protective immune responses in the absence of any additional adjuvant (5, 25, 31). Effects of flagellin on CD4+ T-lymphocyte proliferative and cytokine responses have also been reported (9, 37). Most TLR agonists function as adjuvants by stimulating the production of cytokines and the maturation of dendritic cells, thereby linking innate and adaptive immunity. Flagellin from gram-negative organisms signals via TLR5 and has effects on both innate and adaptive immune responses (22) and induces dendritic cell maturation (8, 9, 53). We recently demonstrated the stimulatory effects of flagellin on innate immunity in the mouse lung (21). Recombinant Salmonella flagellin instilled intratracheally (i.t.) induced transient neutrophil infiltration of the lungs and the production of a subset of cytokines and chemokines. In view of the established role of the innate immune response in the development of adaptive immunity, the strong innate response to flagellin in the lung, and the adjuvant activity of flagellin, we explored the possibility that flagellin might be a highly efficacious adjuvant for protection from respiratory infection with Y. pestis.
MATERIALS AND METHODS
Plasmids and cell culture.
The coding sequence for the F1 antigen of Yersinia pestis, caf1 (plasmid containing the entire caf operon kindly provided by J. B. Bliska, State University of New York, Stony Brook), was subcloned into the NdeI and XhoI sites of the pET29a expression vector from Novagen (EMD Biosciences, Inc., Madison, WI). The recombinant F1/V fusion construct (20) (provided by G. Andrews and P. Worsham, USAMRIID) was sequenced and subcloned into pET16b. Sequencing revealed the absence of 21 amino acids corresponding to the signal sequence of F1.
Reagents and antibodies.
Purified, recombinant His-tagged flagellin from Salmonella enterica serovar Enteritidis was prepared as described previously (21, 36). The F1 and F1/V antigens of Y. pestis were purified in an identical manner, as well as the 229 mutant flagellin. This truncated form of flagellin expresses only amino acids 297 to 471 of the hypervariable region and is unable to signal through TLR5 (21, 36, 41). However, it is prepared in the same manner as bioactive flagellin and would therefore include any potential stimulatory contaminants. Endotoxin levels were ≤1 pg/μg as detected by the QCL-1000 chromogenic Limulus amebocyte lysate test kit from Cambrex Corporation (East Rutherford, NJ). Tumor necrosis factor alpha (TNF-α) was detected using the BD OptEIA enzyme-linked immunosorbent assay (ELISA) kit (mono/mono) per the manufacturer's instructions (BD Biosciences). An anti-F1 mouse monoclonal IgG1, clone YPF19 obtained from Research Diagnostics, Inc. (Flanders, NJ), was used as a control in the anti-F1 ELISA. Goat anti-mouse IgG-horseradish peroxidase and rat anti-mouse IgE were purchased from Southern Biotechnology (Birmingham, AL). Goat anti-mouse IgG1 and IgG2a were purchased from Roche Diagnostics (Indianapolis, IN). Goat anti-monkey IgG-horseradish peroxidase was purchased from Research Diagnostics, Inc. (Flanders, NJ).
Mice.
Female BALB/cAnNCr, BALB/cAnNCr-nu/nu, C3H/HeNCr, and C3H/HeJCr mice were purchased from the Frederick Cancer Research and Development Center (Frederick, MD). Female IL-6−/− mice (B6;129S2-Il6tm1Kopf/J), TNFR−/− mice (B6;129S-Tnfrsf1atm1Imx Tnfrsf1btm1Imx/J), gamma interferon−/− (IFN-γ−/−) mice (B6.129S7-Ifngtm1Ts/J), and control mice (C57BL/6J, B6;129SF2/J, and 129/SvJ) were purchased from The Jackson Laboratory (Bar Harbor, ME). Female IgH−/− [C.129(B6)-IgH-Jtm1DhuN?+2] mice on a BALB/c background were purchased from Taconic (Hudson, NY). These mice have a targeted deletion of the J gene segments of the immunoglobulin heavy chain, a mutation that prevents rearrangement and blocks B-cell development (6). IFN-α/βR−/− mice were provided by C. Schindler, Columbia University, New York, NY (44). Mice were maintained in a specific-pathogen-free facility, and all research complied with federal and institutional guidelines set forth by the Wake Forest University Animal Care and Use Committee.
Nonsurgical intratracheal and intranasal (i.n.) immunization of mice.
For intratracheal immunization, mice were anesthetized with Avertin (2,2,2-tribromoethanol [Sigma]; tert-amyl alcohol [Fisher]) by intraperitoneal injection and suspended from a length of wire by their front incisors. With the use of a sterile gel-loading tip inserted gently into the trachea (21), 10 μg F1 antigen and the indicated amount of flagellin or the flagellin mutant 229 were administered in a total of 50 μl pyrogen-free phosphate-buffered saline (PBS). For intranasal immunization, small volumes (9 to 12 μl total) containing antigen and adjuvant in PBS were administered to the nostrils of anesthetized mice. Preliminary experiments using patent blue dye revealed that intranasal immunization with larger volumes resulted in significant amounts of dye in the stomach due to swallowing. Mice were boosted at 4 weeks, and plasma was collected 2 to 3 weeks postboost for analysis of antibody titers.
Immunization of monkeys.
Fifteen healthy adult female cynomolgus monkeys (Macaca fascicularis) were maintained in accordance with federal and institutional guidelines set forth by the Wake Forest University Animal Care and Use Committee. Animals were anesthetized with 7 to 10 mg/kg of body weight ketamine intramuscularly (i.m.) for immunizations and blood collection. For intranasal immunization, 150 μg F1/V fusion protein and 50 μg flagellin were delivered dropwise (100 μl/nostril) to animals in a recumbent position. Intramuscular immunizations were administered into the quadriceps in a volume of 1 ml. Control animals received PBS intranasally and intramuscularly.
Analysis of plasma IgG titers by ELISA.
Heparinized tubes (StatSpin; Fisher Scientific) or BD Vacutainer PST tubes were used for plasma collection. Plasma was then aliquoted and frozen at −70°C until analysis. ELISA plates were coated with 100 μl of antigen (F1 or F1/V) at 10 μg/ml in sterile PBS overnight at 4°C and blocked with 10% fetal bovine serum in PBS. Duplicate or triplicate plasma dilutions were added, and the plate was incubated overnight at 4°C, followed by secondary anti-Ig antibodies for 2 h at room temperature. Peroxidase activity was detected with 3,3′,5,5′-tetramethylbenzidine (TMB) liquid substrate system (Sigma-Aldrich) and stopped with 2 N H2SO4. Endpoint dilution titers were defined as the inverse of the highest dilution that resulted in an absorbance value (optical density at 450 nm) of >0.1 over that of naive plasma.
Respiratory challenge with Yersinia pestis CO92.
The Centers for Disease Control (CDC) Division of Vector-Borne Infectious Diseases (Fort Collins, CO) provided a stock culture of Yersinia pestis CO92 biovar orientalis, a strain isolated from a fatal case of human primary pneumonic plague (10). Heart infusion broth was inoculated with a single colony from a subculture plate and grown at 28°C to an approximate density of 1 × 109 CFU/ml. Mice were challenged intranasally with 10 μl of culture diluted in PBS to ∼1.8 × 107 CFU/ml, a dose equivalent to 150 50% lethal doses (LD50s). Actual CFU/ml values were determined by plating serial dilutions onto tryptose blood agar plates. Mice were monitored twice daily for survival and were euthanized if showing obvious signs of illness, such as lethargy, dehydration, or abnormal posture. All experiments were conducted according to CDC-approved standard operating protocols for the biosafety level 3 and animal biosafety level 3 facility at the Infectious Disease Unit at Virginia Tech (CDC approval no. C20031120-0016).
Statistical analysis.
Data are represented as means with standard errors. SigmaStat3.1 (Systat Software, Inc., Richmond, CA) was used for statistical calculations. The F test for equality of variance and Student's one-sided t test were used to assign statistical significance at P < 0.05 or P < 0.01. LD50s were determined by nonlinear regression.
RESULTS
Immunization with flagellin promotes a potent adaptive response to the F1 antigen of Yersinia pestis.
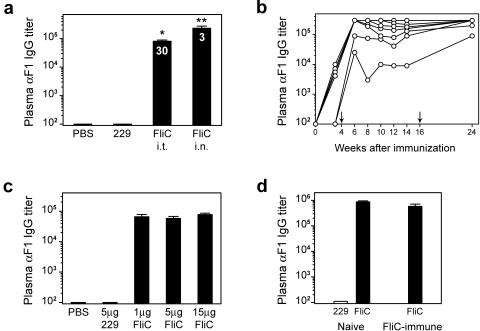
To evaluate the ability of flagellin to promote a humoral immune response against the F1 antigen of Y. pestis, BALB/c mice were immunized with 10 μg F1 antigen and 1 μg recombinant flagellin (FliC) i.t. or i.n. Control animals were immunized with F1 antigen in PBS or F1 and a mutant form of flagellin, designated 229. Four weeks later, mice were boosted in an identical manner, and plasma was collected for analysis of circulating antibody titers at various times postboost. No F1-specific IgG was detected in the groups of control mice. However, a vaccine containing F1 and flagellin stimulated a dramatic increase in total IgG titers (Fig. 1a), with high levels of F1-specific IgG1 and IgG2a. The mean ratio of IgG1 to IgG2a (indicated within the bars) ranged from 30 to 170 when flagellin and F1 were administered i.t. and was approximately 3 when they were given i.n. Although i.n. and i.t. immunization resulted in mixed Th responses, the bias towards an apparent Th2 response was more evident following intratracheal immunization. It is important that flagellin did not promote significant F1-specific IgE production. Mice immunized with F1 and flagellin exhibited sustained titers of anti-F1 IgG after two immunizations (Fig. 1b). A tertiary immunization at 16 weeks improved the antibody response in the two mice that had lower initial titers.
FIG. 1.
Immunization with flagellin and the F1 antigen of Yersinia pestis results in substantial anti-F1 antibody production. (a) Female BALB/c mice were immunized i.t. or i.n. with 10 μg F1 plus 1 μg flagellin (FliC). Control animals were immunized i.t. with 10 μg F1 alone or with 1 μg of the 229 mutant flagellin. Mice were boosted in an identical manner at 4 weeks, and plasma was collected 2 weeks later for analysis by ELISA. Numbers within the bars indicate ratio of IgG1/IgG2a isotypes. A single asterisk indicates statistical significance over controls, and a double asterisk indicates that i.n. titers are statistically greater than i.t. (P < 0.007). (b) Anti-F1 antibody titers from mice immunized i.n. with 10 μg F1 plus 1 μg flagellin. Each line represents one mouse, and arrows indicate booster immunizations. (c) Female BALB/c mice were immunized i.t. with 10 μg F1 and increasing amounts of FliC or 5 μg of 229 and boosted at 4 weeks. Plasma anti-F1 IgG titers were determined 2 weeks postboost. (d) A group of female BALB/c mice was immunized i.n. with 5 μg of flagellin alone and boosted in an identical manner at 4 weeks. Anti-FliC antibody titers were determined 2 weeks later (mean anti-FliC titer = 8.5 × 105), and flagellin-immune mice were then immunized and boosted with 10 μg F1 plus 1 μg FliC i.n. Two weeks postboost, anti-F1 titers were determined and compared to titers of flagellin-naive animals immunized with 10 μg F1 plus 1 μg FliC or 229. Bars represent mean antibody titers ± standard errors of the means. Seven female BALB/c mice were used per immunization group.
In a previous study, we demonstrated that the induction of TNF-α in response to i.t. instillation of flagellin in the lung was maximal with doses of flagellin in the range of 5 to 15 μg (21). To determine if there was a linear relationship between the magnitude of the cytokine response and the antibody response promoted by flagellin, BALB/c mice were immunized with F1 antigen and 1 μg, 5 μg, and 15 μg of flagellin (Fig. 1c). Anti-F1 IgG titers were not significantly different at these doses, indicating that the adjuvant effect is maximal at 1 μg flagellin. However, we noticed a trend towards a greater IgG1/IgG2a ratio with increasing doses of flagellin. Thus, it appears that a maximal adaptive response may not require a maximal innate response.
Preexisting immunity to flagellin is an obvious concern in considering its use as an adjuvant. Therefore, we evaluated the effectiveness of immunization with flagellin and F1 in the presence of high titers of antiflagellin antibodies. Female BALB/c mice were immunized and boosted with 5 μg flagellin i.n., and antiflagellin antibody titers were determined. Flagellin-specific IgG titers were below the level of detection prior to immunization and increased significantly with a mean anti-FliC IgG titer of 8.5 × 105. These mice were then immunized and boosted with 10 μg F1 and 1 μg FliC i.n. Two weeks postboost, anti-F1 IgG titers were similar between naive and FliC-immune mice (Fig. 1d), indicating that circulating antiflagellin antibodies did not positively or negatively alter the response to flagellin. Our results, in conjunction with those of Ben-Yedidia and Arnon (5), support the conclusion that flagellin is an effective adjuvant in the presence of prior immunity to flagellin.
The adjuvant effect of flagellin stimulates antigen-specific responses and is dependent on T lymphocytes.
The development of immunological memory is an essential feature of an effective vaccine, preparing the immune system to respond rapidly following subsequent exposure to the antigen during infection. To evaluate the requirement for flagellin stimulation in the secondary immunization, four groups of BALB/c mice were immunized with F1 antigen and flagellin and subsequently boosted with PBS, flagellin alone, F1 antigen alone, or flagellin and F1 (Fig. 2a). F1-specific IgG titers in mice boosted with PBS or flagellin alone remained between 500 and 1,100, values that are typical for postprimary responses. However, mice that received F1 antigen alone in the secondary immunization had dramatically increased anti-F1 IgG titers. Although flagellin was not required in the boost, there was a significant increase in anti-F1 antibody titers when flagellin was present. These findings are analogous to those reported by Pasare and Medzhitov (47). The authors suggested that, once CD4+ memory is established using LPS as an adjuvant, TLR stimulation is no longer required for activation of these lymphocytes. Although the memory response in our system remains to be fully characterized, the lack of an F1-specific IgG response in athymic nude mice (BALB/cAnNCr-nu/nu) immunized with flagellin and F1 (Fig. 2b) demonstrates a requirement for T cells in the humoral response to this vaccine.
FIG. 2.
Flagellin stimulates antigen-specific responses and requires T cells. (a) Groups of seven female BALB/c mice were immunized i.n. with 10 μg F1 antigen plus 1 μg flagellin (FliC) and boosted at 4 weeks with PBS, 1 μg FliC alone, 10 μg F1 alone, or 10 μg F1 plus 1 μg FliC. Plasma was collected 3 weeks after boosting for analysis by ELISA. A single asterisk indicates statistical significance over animals boosted with PBS or FliC alone, and a double asterisk indicates that boosting with F1 plus FliC results in antibody titers statistically greater than F1 antigen alone (P < 0.01). Bars represent mean antibody titers ± standard errors of the means. (b) A group of seven athymic nude mice (BALB/cAnNCr-nu/nu) was immunized and boosted i.n. with 10 μg F1 plus 1 μg FliC. Plasma was collected 2 weeks postboost for analysis of anti-F1 IgG titers by ELISA. A single asterisk indicates statistical significance compared to normal BALB/c mice immunized in an identical manner (P < 0.001).
TNF-α, IL-6, and interferons are not required for the adjuvant effects of flagellin.
Previously, we determined that flagellin induces high levels of TNF-α and IL-6 in the lung (21). In view of these results and the well-characterized role of these cytokines in the promotion of adaptive immunity, we evaluated the impact of these cytokines on the adjuvant activity of flagellin. TNF-α is a pleiotropic cytokine that promotes dendritic cell maturation (4), and the role of this cytokine in the adjuvant effects of flagellin was determined using mice lacking the ability to respond to TNF-α (TNFR−/−). As shown in Fig. 3a, the anti-F1 antibody response remains extremely high in these mice, indicating that TNF-α is not required for the adjuvant effect of flagellin. However, flagellin-stimulated TNF-α production appears to enhance the antibody response against F1 antigen, as the titers in TNFR−/− mice were reduced approximately twofold relative to wild-type B6;129 mice. The role of IL-6, a cytokine that promotes B-cell proliferation and differentiation (27), in the adjuvant activity of flagellin was evaluated using IL-6−/− mice. There was no defect in anti-F1 IgG production in these mice following immunization with F1 and FliC (Fig. 3b), indicating that this cytokine is also not essential for the adjuvant effect of flagellin.
FIG. 3.
Requirements for the adjuvant effects of flagellin. (a and b) TNFR−/− or IL-6−/− and wild-type B6;129 control mice were immunized i.t. with 10 μg F1 antigen plus 1 μg flagellin (FliC) or mutant flagellin (229). A single asterisk indicates that TNFR−/− titers are statistically less than those of B6;129 control (P < 0.001). (c) C3H/HeJ (Tlr4 P712H mutant) and wild-type C3H/HeN mice were immunized with 10 μg F1 plus 1 μg FliC or 229. (d and e) IFN-α/βR−/− and IFN-γ−/− mice and corresponding wild-type controls were immunized i.n. with 10 μg F1 plus 1 μg FliC or 229. Seven female mice were used in each immunization group. Mice were boosted in the same manner at 4 weeks, and plasma was collected 2 weeks later for analysis of anti-F1 IgG titers by ELISA. Bars represent mean antibody titers ± standard errors of the means.
In vitro, flagellin stimulates nitric oxide and beta interferon (IFN-β) production via signaling through functional TLR5/4 heteromeric complexes (40). C3H/HeJ mice, which possess a nonfunctional mutant TLR4 (48), provide a model to separate the effects of TLR5/4 heteromeric and TLR5/5 homomeric signaling in vivo. In the lung, flagellin-stimulated production of TNF-α, IL-6, granulocyte colony-stimulating factor, keratinocyte-derived chemokine, macrophage inflammatory protein 2, and macrophage inflammatory protein 1α was not disrupted in C3H/HeJ mice (21); however, interferon production was not evaluated. Type I IFNs are proposed to link innate and adaptive immune responses by stimulating the upregulation of major histocompatibility complex and costimulatory molecules on antigen-presenting cells (30). To determine the role of TLR5/4 signaling on the adjuvant effect of flagellin, anti-F1 antibody titers in C3H/HeJ mice were compared to their wild-type counterpart, C3H/HeN mice (Fig. 3c), following immunization with F1 antigen and flagellin. As there was no defect in antibody production, signaling via TLR5/4 complexes is not required for the adjuvant effects of flagellin. The role of interferons in the adjuvant activity of flagellin was evaluated directly by determining antibody responses in mice lacking either type I interferon signaling (IFN-α/βR−/−) or the ability to produce IFN-γ (IFN-γ−/−) (Fig. 3d and e). As both strains of mice responded in a manner similar to that of wild-type mice immunized with F1 antigen and flagellin, interferons are also not required for the adjuvant effect of flagellin.
Flagellin promotes a protective response for intranasal challenge with Yersinia pestis CO92.
The fundamental test of a vaccine is the ability to provide protection against challenge with a pathogen. As a model for respiratory infection, immunized and control mice were challenged intranasally with virulent Y. pestis CO92. To ensure that vaccination induced adequate protection, a challenge dose of ∼150 times the LD50 for i.n. infection with Y. pestis was selected on the basis of recommendations from a recent Plague Vaccine Workshop sponsored by the NIAID and the FDA (13 to 14 October 2004). BALB/c mice immunized and boosted i.n. with flagellin and F1 had a 93% survival rate, versus only 7% in the control group (Fig. 4a), following challenge with a dose equivalent to 100× the LD50. B-cell-deficient IgH−/− mice were used to evaluate the B-cell/antibody dependence of the protective response (Fig. 4b). All control and immunized mice succumbed to Y. pestis infection at a dose of approximately 150× the LD50, indicating that protection at this challenge dose is B cell mediated and thus presumably antibody mediated. Previously, Elvin and Williamson (12) examined the role of type 1 effector functions in protection from Y. pestis infection using Stat4−/− animals that have defective IL-12- and IFN-γ-mediated cellular immune responses. Whereas immunized Stat4−/− mice produced levels of anti-F1 and anti-V IgG similar to those of their wild-type counterpart, these animals were less protected from high-dose challenge. To address the role of IFN-γ-mediated protection following intranasal challenge in our system, groups of IFN-γ−/− and wild-type C57BL/6 mice were immunized and boosted with F1 antigen and flagellin prior to challenge with 150× the LD50 of Y. pestis. Wild-type C57BL/6 mice were completely protected by i.n. immunization with flagellin and F1, versus only 10% of controls (Fig. 4c). Immunized IFN-γ−/− mice had 80% survival following challenge (Fig. 4d), indicating that IFN-γ-mediated responses are not required for protection. As these animals had high titers of anti-F1 IgG, these results confirm the importance of F1-specific antibodies in protection from Y. pestis infection and support the utility of circulating IgG titers as a correlate of protective efficacy. The observation that two IFN-γ−/− mice succumbed to infection suggests that IFN-γ may augment antibody-mediated protection from Y. pestis, possibly through the promotion of the respiratory burst in phagocytes.
FIG. 4.
Flagellin promotes a protective response for intranasal infection with Yersinia pestis CO92. (a) Groups of 15 female BALB/c mice were immunized i.n. with 10 μg F1 antigen plus 1 μg flagellin (FliC) or PBS alone and boosted in an identical manner at 4 weeks. Plasma was collected 2 weeks postboost for analysis of antibody titers by ELISA (mean anti-F1 titer = 9.4 × 105). One week later mice were challenged i.n. with a dose of Y. pestis CO92 equivalent to 100× the LD50. Mice were monitored for 30 days postchallenge. (b) Groups of 10 antibody-deficient IgH−/− mice were immunized i.n. with 10 μg F1 plus 1 μg FliC or PBS alone and boosted in an identical manner at 4 weeks. Mice were challenged 2 weeks later i.n. with a dose of Y. pestis equivalent to 155× the LD50. (c and d) Groups of 10 female IFN-γ−/− mice and wild-type C57BL/6 mice were immunized and boosted i.n. with 10 μg F1 plus 1 μg FliC. Plasma was collected 2 weeks postboost for analysis of antibody titers by ELISA (anti-F1 titer, ≥1 × 106). One week later mice were challenged i.n. with a dose of Y. pestis equivalent to 150× the LD50 and monitored for 16 days postchallenge.
Flagellin is an effective adjuvant in nonhuman primates.
In view of the ability of flagellin to promote protective adaptive immune responses in murine models, we next evaluated the effectiveness of flagellin as an adjuvant in nonhuman primates. A recombinant fusion protein consisting of the F1 and V antigens of Y. pestis was used for immunization of female cynomolgus macaques. Groups of six monkeys were immunized with 150 μg F1/V fusion and 50 μg flagellin i.n. or i.m. Additional control animals (n = 3) received PBS by both routes. Prior to immunization, the monkeys exhibited antiflagellin antibody titers of approximately 9.8 × 104. Monkeys immunized with flagellin exhibited no change in body temperature or plasma TNF-α levels during the first 24 h following immunization, and no observable inflammation occurred at the site of injection. Animals were boosted in an identical manner at 4 weeks, and plasma anti-F1/V IgG titers were determined 2 weeks later (Fig. 5). Immunized monkeys exhibited a striking increase in F1/V-specific antibody titers. No antigen-specific IgE was detected. These results clearly establish that flagellin is an effective adjuvant for the development of an antibody response in nonhuman primates, even in the presence of circulating antiflagellin antibodies.
FIG. 5.
Flagellin is an effective adjuvant in nonhuman primates. Female cynomolgus monkeys (Macaca fascicularis) were immunized intranasally (n = 6) or intramuscularly (n = 6) with 150 μg F1/V fusion protein plus 50 μg flagellin. Control animals (n = 3) were immunized i.n. and i.m. with PBS alone. No significant change in body temperature occurred over 12 h, and TNF-α was not detected in plasma collected at 4 h, 12 h, and 24 h postimmunization. Animals were boosted in an identical manner at 4 weeks, and plasma was collected 2 weeks later for analysis by ELISA. Bars indicate mean anti-F1/V antibody titers ± standard errors of the means, and a single asterisk indicates statistical significance over intranasal immunization (P < 0.006).
DISCUSSION
Vaccination with whole-cell plague vaccines, using live attenuated or formalin-inactivated Y. pestis, has limited effectiveness for protection from virulent challenge and has been associated with significant side effects (49, 55, 58). To address these concerns, acellular vaccines composed of the F1 or V antigen of Y. pestis have been developed (2, 3, 43, 50). Vaccines combining F1 and V (13, 14, 15) or recombinant F1/V fusion proteins (18, 20, 29) have been used successfully when appropriate adjuvants are utilized. Passive immunization with polyclonal or monoclonal antibodies directed against these antigens confers protection (2, 43), and the level of circulating IgG1 in immunized animals correlates with protection (50, 58), with 90% protection at a serum titer of approximately 2 × 105 (57). Various routes of immunization have been investigated, including i.m., i.n., and subcutaneous vaccination, as well as numerous methods of antigen delivery, such as coencapsulation in poly-lactic acid microspheres (14, 15), expression by Salmonella (17, 29, 42), and DNA vaccination (19, 54). Adsorption of antigens to aluminum hydroxide has been the most common adjuvant in acellular plague vaccines, but cholera toxin (13, 14) as well as Ribi emulsion adjuvants (3, 11) has also been examined.
The results presented in this study demonstrate that flagellin is a potent adjuvant for the development of humoral immune responses to Y. pestis in both murine and nonhuman primate models. Although this study focused on the detection of circulating anti-F1 IgG, the titer of which closely correlates with protection from lethal challenge (50, 57, 58), future studies will investigate the levels of immunoglobulin present at mucosal surfaces. In addition to determining the antigen-specific IgA and IgG titers in nasal washes, immunization of IgA−/− mice with flagellin and F1 will clarify the role of IgA in protection from respiratory Y. pestis infection.
The titers of circulating IgG following immunization with flagellin and the F1 antigen were equivalent to or greater than those reported in previous studies that utilized a variety of adjuvants and routes of administration (3, 13, 14, 15, 57, 58). The F1-specific IgG response following i.t. and i.n. immunization was predominantly IgG1 in several mouse strains examined (Fig. 1 and data not shown). In spite of this apparent Th2 bias, no significant antigen-specific IgE production was detected in mice or monkeys following immunization including flagellin, suggesting that the development of hypersensitivity reactions is not a concern. In preliminary experiments, comparable levels of F1-specific IgG were induced following i.t. immunization with 10 μg F1 antigen and 10 μg CpG DNA, another TLR agonist with adjuvant properties (35, 46). These mice also had an IgG1/IgG2a ratio similar to those immunized with flagellin and F1.
Importantly, i.n. immunization with flagellin and the F1 antigen protected 93 to 100% of immunized BALB/c and C57BL/6 mice from respiratory challenge with virulent Y. pestis CO92. In preliminary experiments, we have found that passive transfer of plasma from immune monkeys completely protected naive BALB/c mice from challenge with 180× the LD50 of Y. pestis CO92.
Vaccine adjuvants function by stimulating innate immunity, thereby promoting the development of subsequent adaptive immune responses. Dendritic cells are critical for induction of an effective adaptive immune response, connecting the innate and adaptive immune systems by presenting antigens from the site of exposure to naive lymphocytes in secondary locations. Similar to other TLR agonists that have adjuvant activity, such as LPS and CpG oligonucleotides, flagellin promotes the maturation of dendritic cells as well as their migration to lymph nodes (9, 53). Type I IFNs and TNF-α have been demonstrated to promote the survival, maturation, and migration of dendritic cells to secondary lymphoid organs (4, 32, 33). However, mice with defective responses to these cytokines exhibited strong humoral responses to the F1 antigen following immunization with F1 and flagellin. These results indicate that individually these cytokines are not required intermediates for the adjuvant effect of flagellin. While it is possible that the redundancy of the immune system compensated for the loss of individual cytokines, we favor the hypothesis that flagellin is acting directly on dendritic cells to promote their maturation and thus drive the adaptive immune response. However, we also believe that the overall adjuvant effect of flagellin is enhanced by the induction of cytokines. This conclusion is consistent with the results of work with other TLR agonists. For example, LPS-induced maturation of dendritic cells without concomitant cytokine production is inadequate for the initiation of CD4+ T-lymphocyte responses (47), and indirect activation by LPS or CpG is not sufficient for the development of effector function by CD4+ T cells (51).
Although Means et al. (38) reported that flagellin induced the maturation of human, but not murine, dendritic cells, other investigators (8, 9, 53) have demonstrated upregulation of costimulatory molecules and cytokine production by murine dendritic cells in vitro. Variation in the responses of in vitro-cultured dendritic cells may be due to differing amounts of serum factors that modulate flagellin responsiveness. This hypothesis is supported by earlier findings that flagellin responsiveness may increase with differentiation of monocytes (7). Treatment of the human promonocytic line U38 with phorbol esters induced the cells to become more macrophage-like and significantly enhanced TNF-α production following flagellin stimulation. In vivo, cytokine production might prime immature dendritic cells to become flagellin responsive.
There are a number of advantages to the use of flagellin as an adjuvant. Intranasal vaccination is appealing as it does not require needles for administration. Although flagellin is an extraordinarily potent stimulator of immune responses, it appears to be a safe adjuvant. No local or systemic inflammation was observed following i.n. and i.m. immunization of cynomolgus monkeys, and i.t. instillation of flagellin to the lungs of mice induced only a limited and transient inflammatory response (21). Neurotoxicity is an additional concern for intranasal vaccines and adjuvants. For example, cholera toxin may accumulate in the olfactory nerves and olfactory bulbs (16). An association has been established between a nasal influenza virus vaccine containing heat-labile enterotoxin and Bell's palsy (45). Intranasal administration of flagellin did not induce any detectable inflammation in the central nervous system (A. N. Honko, N. D. Kock, and S. B. Mizel, unpublished observations), a finding that supports the safety of flagellin as an intranasal adjuvant.
In summary, the results of our study as well as those of other investigators (5, 25, 31) have demonstrated that flagellin is a highly effective adjuvant when administered i.n. Flagellin promotes a strong humoral immune response against Y. pestis antigens in both murine and nonhuman primate models. Furthermore, this response is protective for respiratory challenge with Y. pestis in mice. Our results support the conclusion that flagellin may be an efficacious adjuvant for use in humans.
Acknowledgments
This study was supported by NIH grant R01-AI051319 and program project grant P01-AI060642. A.N.H. was a predoctoral fellow on NIH training grant T32-AI007401.
We acknowledge Aaron H. Graff and Catherine Carlson for their expert technical assistance.
Editor: J. B. Bliska
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, G. W., Jr., P. L. Worsham, C. R. Bolt, G. P. Andrews, S. L. Welkos, A. M. Friedlander, and J. P. Burans. 1997. Protection of mice from fatal bubonic and pneumonic plague by passive immunization with monoclonal antibodies against the F1 protein of Yersinia pestis. Am. J. Trop. Med. Hyg. 56:571-573. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, G. P., D. G. Heath, G. W. Anderson, Jr., S. L. Welkos, and A. M. Friedlander. 1996. Fraction 1 capsular antigen (F1) purification from Yersinia pestis CO92 and from an Escherichia coli recombinant strain and efficacy against lethal plague challenge. Infect. Immun. 64:2180-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.-J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Yedidia, T., and R. Arnon. 1998. Effect of pre-existing carrier immunity on the efficacy of synthetic influenza vaccine. Immunol. Lett. 64:9-15. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J., M. Trounstine, F. W. Alt, F. Young, C. Kurahara, J. F. Loring, and D. Huszar. 1993. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int. Immunol. 5:647-656. [DOI] [PubMed] [Google Scholar]
- 7.Ciacci-Woolwine, F., P. F. McDermott, and S. B. Mizel. 1999. Induction of cytokine synthesis by flagella from gram-negative bacteria may be dependent on the activation or differentiation state of human monocytes. Infect. Immun. 67:5176-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datta, S. K., V. Redecke, K. R. Prilliman, K. Takabayashi, M. Corr, T. Tallant, J. DiDonato, R. Dziarski, S. Akira, S. P. Schoenberger, and E. Raz. 2003. A subset of Toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. J. Immunol. 170:4102-4110. [DOI] [PubMed] [Google Scholar]
- 9.Didierlaurent, A., I. Ferrero, L. A. Otten, B. Dubois, M. Reinhardt, H. Carlsen, R. Blomhoff, S. Akira, J.-P. Kraehenbuhl, and J.-C. Sirard. 2004. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J. Immunol. 172:6922-6930. [DOI] [PubMed] [Google Scholar]
- 10.Doll, J. M., P. S. Zeitz, P. Ettestad, A. L. Bucholtz, T. Davis, and K. Gage. 1994. Cat-transmitted fatal pneumonic plague in a person who traveled from Colorado to Arizona. Am. J. Trop. Med. Hyg. 51:109-114. [DOI] [PubMed] [Google Scholar]
- 11.Elvin, S. J., and E. D. Williamson. 2000. The F1 and V subunit vaccine protects against plague in the absence of IL-4 driven immune responses. Microb. Pathog. 29:223-230. [DOI] [PubMed] [Google Scholar]
- 12.Elvin, S. J., and E. D. Williamson. 2004. Stat 4 but not Stat 6 mediated immune mechanisms are essential in protection against plague. Microb. Pathog. 37:177-184. [DOI] [PubMed] [Google Scholar]
- 13.Eyles, J. E., S. J. Elvin, A. Westwood, C. S. LeButt, H. O. Alpar, S. Somavarapu, and E. D. Williamson. 2004. Immunisation against plague by transcutaneous and intradermal application of subunit antigens. Vaccine 22:4365-4373. [DOI] [PubMed] [Google Scholar]
- 14.Eyles, J. E., G. J. E. Sharp, E. D. Williamson, I. D. Spiers, and H. O. Alpar. 1998. Intra nasal administration of poly-lactic acid microsphere co-encapsulated Yersinia pestis subunits confers protection from pneumonic plague in the mouse. Vaccine 16:698-707. [DOI] [PubMed] [Google Scholar]
- 15.Eyles, J. E., I. D. Spiers, E. D. Williamson, and H. O. Alpar. 1998. Analysis of local and systemic immunological responses after intra-tracheal, intra-nasal and intra-muscular administration of microsphere co-encapsulated Yersinia pestis sub-unit vaccines. Vaccine 16:2000-2009. [DOI] [PubMed] [Google Scholar]
- 16.Fujihashi, K., T. Koga, F. W. van Ginkel, Y. Hagiwara, and J. R. McGhee. 2002. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine 20:2431-2438. [DOI] [PubMed] [Google Scholar]
- 17.Garmory, H. S., K. F. Griffin, K. A. Brown, and R. W. Titball. 2003. Oral immunisation with live aroA attenuated Salmonella enterica serovar Typhimurium expressing the Yersinia pestis V antigen protects mice against plague. Vaccine 21:3051-3057. [DOI] [PubMed] [Google Scholar]
- 18.Glynn, A., L. C. Freytag, and J. D. Clements. 2005. Effect of homologous and heterologous prime-boost on the immune response to recombinant plague antigens. Vaccine 23:1957-1965. [DOI] [PubMed] [Google Scholar]
- 19.Grosfeld, H., S. Cohen, T. Bino, Y. Flashner, R. Ber, E. Mamroud, C. Kronman, A. Shafferman, and B. Velan. 2003. Effective protective immunity to Yersinia pestis infection conferred by DNA vaccine coding for derivatives of the F1 capsular antigen. Infect. Immun. 71:374-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heath, D. G., G. W. Anderson, Jr., J. M. Mauro, S. L. Welkos, G. P. Andrews, J. Adamovicz, and A. M. Friedlander. 1998. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine 16:1131-1137. [DOI] [PubMed] [Google Scholar]
- 21.Honko, A. N., and S. B. Mizel. 2004. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect. Immun. 72:6676-6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honko, A. N., and S. B. Mizel. 2005. Effects of flagellin on innate and adaptive immunity. Immunol. Res. 33:83-102. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987-995. [DOI] [PubMed] [Google Scholar]
- 24.Janssens, S., and R. Beyaert. 2003. Role of Toll-like receptors in pathogen recognition. Clin. Microbiol. Rev. 16:637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon, S. H., T. Ben-Yedidia, and R. Arnon. 2002. Intranasal immunization with synthetic recombinant vaccine containing multiple epitopes of influenza virus. Vaccine 20:2772-2780. [DOI] [PubMed] [Google Scholar]
- 26.Kaisho, T., and S. Akira. 2002. Toll-like receptors as adjuvant receptors. Biochim. Biophys. Acta 1589:1-13. [DOI] [PubMed] [Google Scholar]
- 27.Kamimura, D., K. Ishihara, and T. Hirano. 2003. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev. Physiol. Biochem. Pharmacol. 149:1-38. [DOI] [PubMed] [Google Scholar]
- 28.Krishna, G., and R. K. Chitkara. 2003. Pneumonic plague. Semin. Respir. Infect. 18:159-167. [PubMed] [Google Scholar]
- 29.Leary, S. E. C., K. F. Griffin, H. S. Garmory, E. D. Williamson, and R. W. Titball. 1997. Expression of an F1/V fusion protein in attenuated Salmonella typhimurium and protection of mice against plague. Microb. Pathog. 23:167-179. [DOI] [PubMed] [Google Scholar]
- 30.Le Bon, A., and D. F. Tough. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432-436. [DOI] [PubMed] [Google Scholar]
- 31.Levi, R., and R. Arnon. 1996. Synthetic recombinant influenza vaccine induces efficient long-term immunity and cross-strain protection. Vaccine 14:85-92. [DOI] [PubMed] [Google Scholar]
- 32.Luft, T., K. C. Pang, E. Thomas, P. Hertzog, D. N. J. Hart, J. Trapani, and J. Cebon. 1998. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 161:1947-1953. [PubMed] [Google Scholar]
- 33.Lutz, M. B., N. Kukutsch, A. L. J. Ogilvie, S. Rößner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 34.Mazzoni, A., and D. M. Segal. 2004. Controlling the Toll road to dendritic cell polarization. J. Leukoc. Biol. 75:721-730. [DOI] [PubMed] [Google Scholar]
- 35.McCluskie, M. J., and H. L. Davis. 1999. CpG DNA as mucosal adjuvant. Vaccine 18:231-237. [DOI] [PubMed] [Google Scholar]
- 36.McDermott, P. F., F. Ciacci-Woolwine, J. A. Snipes, and S. B. Mizel. 2000. High-affinity interaction between gram-negative flagellin and a cell surface polypeptide results in human monocyte activation. Infect. Immun. 68:5525-5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McSorley, S. J., B. D. Ehst, Y. Yu, and A. T. Gewirtz. 2002. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J. Immunol. 169:3914-3919. [DOI] [PubMed] [Google Scholar]
- 38.Means, T. K., F. Hayashi, K. D. Smith, A. Aderem, and A. D. Luster. 2003. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J. Immunol. 170:5165-5175. [DOI] [PubMed] [Google Scholar]
- 39.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 40.Mizel, S. B., A. N. Honko, M. A. Moors, P. S. Smith, and A. P. West. 2003. Induction of macrophage nitric oxide production by Gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. J. Immunol. 170:6217-6223. [DOI] [PubMed] [Google Scholar]
- 41.Mizel, S. B., A. P. West, and R. R. Hantgan. 2003. Identification of a sequence in human Toll-like receptor 5 required for the binding of Gram-negative flagellin. J. Biol. Chem. 278:23624-23629. [DOI] [PubMed] [Google Scholar]
- 42.Morton, M., H. S. Garmory, S. D. Perkins, A. M. O'Dowd, K. F. Griffin, A. K. Turner, A. M. Bennett, and R. W. Titball. 2004. A Salmonella enterica serovar Typhi vaccine expressing Yersinia pestis F1 antigen on its surface provides protection against plague in mice. Vaccine 22:2524-2532. [DOI] [PubMed] [Google Scholar]
- 43.Motin, V. L., R. Nakajima, G. B. Smirnov, and R. R. Brubaker. 1994. Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect. Immun. 62:4192-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Müller, U., U. Steinhoff, L. F. L. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 45.Mutsch, M., W. Zhou, P. Rhodes, M. Bopp, R. T. Chen, T. Linder, C. Spyr, and R. Steffen. 2004. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 350:896-903. [DOI] [PubMed] [Google Scholar]
- 46.O'Hagan, D. T., M. L. MacKichan, and M. Singh. 2001. Recent developments in adjuvants for vaccines against infectious diseases. Biomol. Eng. 18:69-85. [DOI] [PubMed] [Google Scholar]
- 47.Pasare, C., and R. Medzhitov. 2004. Toll-dependent control mechanisms of CD4 T cell activation. Immunity 21:733-741. [DOI] [PubMed] [Google Scholar]
- 48.Poltorak, A., X. He, I. Smirnova, M.-Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 49.Russell, P., S. M. Eley, S. E. Hibbs, R. J. Manchee, A. J. Stagg, and R. W. Titball. 1995. A comparison of plague vaccine, USP and EV76 vaccine induced protection against Yersinia pestis in a murine model. Vaccine 13:1551-1556. [DOI] [PubMed] [Google Scholar]
- 50.Simpson, W. J., R. E. Thomas, and T. G. Schwan. 1990. Recombinant capsular antigen (fraction 1) from Yersinia pestis induces a protective antibody response in BALB/c mice. Am. J. Trop. Med. Hyg. 43:389-396. [DOI] [PubMed] [Google Scholar]
- 51.Spörri, R., and C. Reis e Sousa. 2005. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat. Immunol. 6:163-170. [DOI] [PubMed] [Google Scholar]
- 52.Titball, R. W., and E. D. Williamson. 2001. Vaccination against bubonic and pneumonic plague. Vaccine 19:4175-4184. [DOI] [PubMed] [Google Scholar]
- 53.Tsujimoto, H., T. Uchida, P. A. Efron, P. O. Scumpia, A. Verma, T. Matsumoto, S. K. Tschoeke, R. F. Ungaro, S. Ono, S. Seki, M. J. Clare-Salzler, H. V. Baker, H. Mochizuki, R. Ramphal, and L. L. Moldawer. 2005. Flagellin enhances NK cell proliferation and activation directly and through dendritic cell-NK cell interactions. J. Leukoc. Biol. 78:888-897. [DOI] [PubMed] [Google Scholar]
- 54.Wang, S., D. Heilman, F. Liu, T. Giehl, S. Joshi, X. Huang, T. Chou, J. Goguen, and S. Lu. 2004. A DNA vaccine producing LcrV antigen in oligomers is effective in protecting mice from lethal mucosal challenge of plague. Vaccine 22:3348-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williamson, E. D., S. M. Eley, A. J. Stagg, M. Green, P. Russell, and R. W. Titball. 1997. A sub-unit vaccine elicits IgG in serum, spleen cell cultures and bronchial washings and protects immunized animals against pneumonic plague. Vaccine 15:1079-1084. [DOI] [PubMed] [Google Scholar]
- 56.Williamson, E. D., H. C. Flick-Smith, C. LeButt, C. A. Rowland, S. M. Jones, E. L. Waters, R. J. Gwyther, J. Miller, P. J. Packer, and M. Irving. 2005. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect. Immun. 73:3598-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williamson, E. D., P. M. Vesey, K. J. Gillhespy, S. M. Eley, M. Green, and R. W. Titball. 1999. An IgG1 titre to the F1 and V antigens correlates with protection against plague in the mouse model. Clin. Exp. Immunol. 116:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williamson, E. D., S. M. Eley, A. J. Stagg, M. Green, P. Russell, and R. W. Titball. 2000. A single dose sub-unit vaccine protects against pneumonic plague. Vaccine 19:566-571. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, D., G. Zhang, M. S. Hayden, M. B. Greenblatt, C. Bussey, R. A. Flavell, and S. Ghosh. 2004. A Toll-like receptor that prevents infection by uropathogenic bacteria. Science 303:1522-1526. [DOI] [PubMed] [Google Scholar]