Abstract
The contribution of amebiasis to the burden of diarrheal disease in children and the degree to which immunity is acquired from natural infection were assessed in a 4-year prospective observational study of 289 preschool children in an urban slum in Dhaka, Bangladesh. Entamoeba histolytica infection was detected at least once in 80%, and repeat infection in 53%, of the children who completed 4 years of observation. Annually there were 0.09 episodes/child of E. histolytica-associated diarrhea and 0.03 episodes/child of E. histolytica-associated dysentery. Fecal immunoglobulin A (IgA) anti-parasite Gal/GalNAc lectin carbohydrate recognition domain (anti-CRD) was detected in 91% (183/202) of the children at least once and was associated with a lower incidence of infection and disease. We concluded that amebiasis was a substantial burden on the overall health of the cohort children. Protection from amebiasis was associated with a stool anti-CRD IgA response. The challenge of producing an effective vaccine will be to improve upon naturally acquired immunity, which does not provide absolute protection from reinfection.
Diarrhea is a major contributor to childhood mortality and morbidity in the developing world, causing an estimated 2.5 million deaths each year and long-term effects on growth and cognitive function (23, 27). One etiology of diarrheal disease is amebiasis, which is endemic in the developing world. Amebiasis is also a problem in the developed world in travelers, immigrants, and men who have sex with men (13, 14, 18, 20, 34, 36). Entamoeba histolytica usually causes asymptomatic infection but in a minority of cases causes symptoms ranging from a few loose stools to profuse bloody diarrhea (18). The recent identification of candidate vaccines and the classification of Entamoeba histolytica as a category B priority biodefense agent have heightened interest in preventing infection by the organism. However, the lack of prospective community-based observational studies leaves uncertain both the disease burden and the natural history of amebiasis. Such information has been an important aid in resource allocation and vaccine development against other intestinal pathogens, such as rotavirus and cholera (5, 24, 26, 31, 35). It is now possible to conduct observational studies of E. histolytica infection because of the availability of diagnostic tests that easily distinguish it from the morphologically identical but nonpathogenic parasites Entamoeba dispar and Entamoeba moshkovskii (10, 19).
We sought to quantify both the burden of amebiasis and the acquired immunity conferred by natural E. histolytica infection. This information could be used to provide an estimate of the benefit that could be expected from vaccination. We hypothesized that a mucosal immunoglobulin A (IgA) response against the parasite Gal/GalNAc lectin carbohydrate recognition domain (CRD) would provide immunity. The Gal/GalNAc lectin is an amebic surface molecule that mediates parasite adherence to the colon via its CRD (18, 34). Here we report the results at 4 years of a prospective observational study of a cohort of children living in the Mirpur district of Dhaka, Bangladesh. Interim analyses from this cohort demonstrated that immunity from colonization was associated with anti-CRD IgA, but there were too few cases of amebic diarrhea to determine if immunity extended to disease (12, 15, 16, 20).
MATERIALS AND METHODS
Study protocol.
Preschool children (2 to 5 years old) from Mirpur, an urban slum in Dhaka, Bangladesh, were enrolled starting in January 1999 as described previously (15). Briefly, we recruited 1,164 children 2 to 5 years of age by going door to door in the Mirpur community of Dhaka, Bangladesh. Overall, 15% (170/1,164) of the children screened for the study were anti-E. histolytica serum antibody positive (15). The entry ages of 2 to 5 years were selected for the study because amebiasis is reportedly rare in children younger than age 2 in Dhaka (14, 17). Since the original study was designed to test the protective role of anti-E. histolytica serum IgG, the sample was chosen to have equal proportions of children with and without these antibodies. Of the 170 anti-E. histolytica IgG positive children potentially eligible for the study, 145 (85%) gave consent to participate. Of the 994 anti-E. histolytica IgG negative children, we randomly selected 170 children who were generally matched for age, sex, and area of living, of whom 145 consented to participate, but 1 negative child dropped out after 3 weeks, leaving 144 IgG-negative children.
The inhabitants of Mirpur are of Bihari ethnic origin and settled there after the war of independence with Pakistan in 1971. The area is densely populated and has poor sanitary and hygienic conditions. Most residents use pit latrines that filter into open sewage that flows through the slum. The mean monthly family income is 3,820 ± 234 Taka (approximately $65 U.S.).
Children and their parents were visited and interviewed every other day over 4 years (until July 2003) by health care workers, and diarrheal stools were tested for E. histolytica. In addition, surveillance stool specimens were obtained every month for detection of E. histolytica infection. Stool samples were tested for anti-CRD IgA at 4-month intervals. Blood samples were drawn every 4 months for IgG antibody testing, and DNA was extracted for HLA typing to assess genetic susceptibility to infection. All enrolled children and their family members received free primary health care services, including medications, from the project office in Mirpur. Episodes of diarrhea were treated with oral rehydration and antibiotics or antiamebic medications as needed.
Informed consent was obtained from the parents or guardians, and the human experimentation guidelines of the U.S. Department of Health and Human Services were followed. The study was reviewed and approved by the Institutional Review Boards of the University of Virginia, Johns Hopkins University, and the Centre for Health and Population Research, International Centre for Diarrheal Disease Research, Dhaka, Bangladesh.
Diagnosis of E. histolytica infection.
E. histolytica infection was diagnosed by detection of amebic antigen in stool, using the E. histolytica II test, and E. dispar infection was determined by the Entamoeba stool antigen detection test (both produced by TechLab, Inc., Blacksburg, VA) (19). Stool samples were also cultured for E. histolytica and E. dispar in Robinson's medium within 6 h of collection to confirm the presence of living parasites in antigen-positive stool samples, and strain-specific PCR was conducted on a subset of the stool samples to distinguish new from relapsed infections, as described previously (16).
Antibody testing and HLA analysis.
Stool secretory and nonsecretory IgA against the Gal/GalNAc lectin CRD was determined by enzyme-linked immunosorbent assay as described previously (15). For HLA typing, DNA was purified from 200 μl of peripheral blood obtained from the children. Genomic DNA was isolated with QIAGEN spin columns after the use of QIAGEN protease and lysing buffer. The DNA was eluted from the membrane with an elution buffer, according to the manufacturer's instructions. HLA typing of DRB1 and DQB1 was performed by the use of the PCR and sequence-specific oligonucleotides at Dynal, Inc. (Bromborough, United Kingdom) (12).
Definitions.
Entamoeba histolytica infection was defined as a positive test for amebic antigen in stool. Infection could be asymptomatic or symptomatic. A “new episode” of E. histolytica infection during the period of observation was defined as a positive E. histolytica stool antigen and/or culture result preceded by >2 monthly surveillance stool samples with negative results. This definition was based on earlier studies with this cohort which demonstrated that the requirement for 2 months of preceding negative stool samples gave the highest level of separation between new and relapsed infections as judged by DNA fingerprinting of the parasite by serine-rich E. histolytica protein (SREHP) polymorphism (16).
“Person-days” was defined as the number of total days of observation contributed by the cohort study population.
E. histolytica-associated diarrhea was defined as three or more unformed stools in a 24-h period accompanied by a new episode of E. histolytica infection. This definition was validated previously in this cohort by demonstrating that diarrhea was approximately five times more common in the setting of a new infection (age-adjusted odds ratio (OR) for the association of new E. histolytica infection with diarrhea of 4.7; 95% confidence interval [CI], 2.9 to 7.6) (20). Amebic dysentery was defined as a diarrheal stool sample containing occult or gross blood that was positive for E. histolytica antigen (20).
Statistical analyses.
A comparison of means for different variables was done using the Student t test or by a nonparametric test for data that are not normally distributed. The χ2 and Fisher exact tests were used for categorical variables to compare proportions between two groups.
We used an extension of the Cox proportional hazards model, the Andersen-Gill model, that allows for repeat infections to determine the relative risk with robust variance estimates (1, 8, 9, 22, 29). The Anderson-Gill method addresses ordered events (first infection, second infection, third infection, etc.) using a counting process and assumes that all failure types are equal. This method was used to calculate the time to an infection from entry into the study until the event and then time from the event until the next event or the end of follow-up. The outcome was E. histolytica infection diagnosed by stool antigen or E. histolytica-associated diarrhea. The numbers of previous E. histolytica infections were treated as dummy variables, and a history of one previous infection defined the reference group. In a second model, we also stratified the number of previous infections by the presence of fecal anti-CRD IgA prior to the infection. Age, gender, baseline lectin IgG, and the area within Mirpur that the children lived were treated as potential confounding factors and were included in the proportional hazards model. The proportional hazard assumption was assessed using scaled Schoenfeld residuals against log of time and was found to be negligible in the global test.
The magnitude of the association between HLA markers and the production of fecal IgA against the lectin CRD was measured with an OR by use of univariate logistic regression (32). As a result of the high prevalence of E. histolytica infection, the ORs and relative hazards presented were not interpreted as risk ratios (39).
For statistical analyses, we used the cohort excluding siblings (n = 253), so only one individual per family is included, since these individuals would not be independent and may have both environmental and genetic clustering. The results were consistent, and we present the more conservative models excluding siblings. Siblings were excluded from all genetic analyses (HLA), since siblings share 50% of their alleles. We also present summary statistics including incidence of disease for all participants in the cohort (n = 289) and those who remained enrolled at the end of the study on 31 July 2003 (n = 202), as noted. A P value of ≤0.05 was considered to be statistically significant. All data were computer coded and analyzed by either SPSS version 10.0 or STATA version 8.0.
RESULTS
Overall characteristics of the study.
Of the 289 children originally enrolled in 1999, there were 202 children (70%) who completed a mean of 4.2 ± 0.01 years of follow-up with complete disease and immunity information. Of the 87 children who dropped out of the study, 90% were due to migration and 10% were not willing to continue in the study because of the rigorous protocol. No children were lost due to death or severe morbidity associated with diarrhea or infection (one child died from severe burns). The children who were lost to follow-up did not differ significantly in baseline characteristics from children who remained enrolled (Table 1).
TABLE 1.
Baseline characteristics of the present cohort and children lost to follow-up
| Parameter | Value for group
|
P value | |
|---|---|---|---|
| Present cohorta | Children lost to follow-upb | ||
| Sex (% male) | 48 | 57.5 | 0.14 |
| Mean age (mo) | 49.96 ± 0.81 | 48.15 ± 1.2 | 0.22 |
| Weight for age, Z score | −1.66 ± 0.07 | −1.89 ± 0.09 | 0.07 |
| Height for age, Z score | −1.27 ± 0.10 | −1.37 ± 0.15 | 0.56 |
| % Malnourished | 36.1 | 46.0 | 0.12 |
| % Stunted | 32.2 | 32.2 | 0.99 |
| % Lectin IgG positive | 54.5 | 43.7 | 0.09 |
| % Stool CRD IgA positive at baseline | 5.4 | 4.6 | 0.77 |
n = 202.
n = 87.
There was no significant difference in nutritional status, family size, or income between children with or without anti-E. histolytica IgG antibody, although the seropositive children had a mean age of 51.3 versus 47.5 months for the seronegative children (P = 0.005).
There were 1,712 episodes of diarrhea for the 289 children in this cohort (1,612 episodes of diarrhea for 202 children completing 4.2 years of observation). Seventy-seven percent of the episodes of diarrhea were ≤2 days in duration. Of the 1,712 episodes of diarrhea, 1,107 stool samples (64%) were available for analysis and 86 (7.8%) were positive for E. histolytica. Oral rehydration was given in 98% (1,085/1,107), antibiotics (trimethoprim-sulfamethoxazole or nalidixic acid) in 82% (914/1,107), and metronidazole in 1.5% (17/1,107) of these cases of diarrhea.
Incidence of E. histolytica infection.
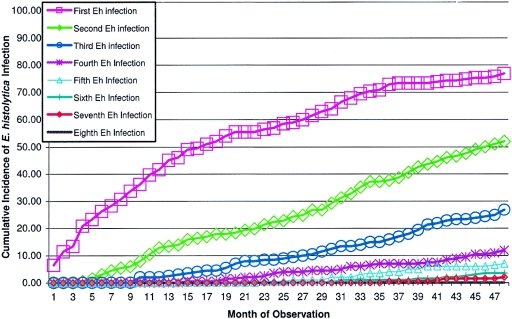
Among those children who completed 4.2 years of the study, 80% (162/202) were infected with E. histolytica at least once as determined by detection of parasite antigen in diarrhea and monthly surveillance stool samples (Fig. 1). Fecal IgA anti-CRD was detected in a higher proportion of children (see below), suggesting that some infections were missed by parasite antigen detection. Children with and without E. histolytica infection as determined by antigen detection did not differ in nutritional status, age, sex or duration of breast-feeding (Table 2). There was a total of 384 episodes of E. histolytica infection in the 202 children for an overall incidence of 0.45 infections per child per year. For all 289 children, the children who upon entry to the study were anti-E. histolytica IgG positive had a slightly higher infection rate with E. histolytica (79% for IgG-positive versus 69% for IgG-negative children; P = 0.05).
FIG. 1.
Cumulative incidences of first and subsequent Entamoeba histolytica infections in 202 children from Mirpur, Dhaka, Bangladesh, who completed 4 years of observation.
TABLE 2.
Baseline characteristics of children with and without E. histolytica infection
| Parameter | Value for group
|
P value | |
|---|---|---|---|
| Without infectiona | With infectionb | ||
| Age (mo) (mean ± SE) | 51.80 ± 1.67 | 49.51 ± 0.93 | 0.26 |
| Duration of breast feeding (mo) (mean ± SE) | 26.50 ± 1.68 | 22.75 ± 0.83 | 0.11 |
| Weight for age Z score (mean ± SE) | −4.66 ± 0.15 | −1.67 ± 0.08 | 0.96 |
| Height for age Z score (mean ± SE) | −1.10 ± 0.25 | −1.32 ± 0.11 | 0.41 |
| No. (%) male | 22 (55) | 75 (46) | 0.32 |
| No. (%) malnourished | 14 (35) | 59 (36) | 0.86 |
| No. (%) stunted | 10 (25) | 55 (34) | 0.27 |
n = 40.
n = 162.
Of the children with E. histolytica infection, 75% (122/162) had asymptomatic infections. The median duration of asymptomatic infection with E. histolytica was 2 months. 25% (40/162) of the children with E. histolytica developed diarrhea, and 15% (24/162) developed dysentery at least once during the study. The duration of diarrhea was 3.0 ± 0.36 days (mean ± standard error). There were 73 episodes of E. histolytica-associated diarrhea (0.09 episodes/child/year) and 26 episodes of dysentery (0.03 episodes/child/year) during the study. Coinfection with Shigella or Campylobacter in children with E. histolytica-associated diarrhea was observed for 9% (6/69) of the episodes for which a complete microbiologic workup was performed (data not shown). Treatment for amebiasis with metronidazole was given in 19% (14/73) of episodes of E. histolytica-associated diarrhea. Diarrhea had resolved before treatment for amebiasis could be provided in the other 81% of cases.
SREHP gene polymorphisms identified 70 different E. histolytica strains among the 151 tested. Nonpathogenic E. dispar was detected in 98% of the children, with 698 total E. dispar infections during the study from 202 children.
Repeat infections with E. histolytica.
E. histolytica reinfections occurred in 53% (107/202) of the children (Fig. 1). The incidence of initial infection versus one to three reinfections was consistent (0.10 to 0.13 infections per 100-child days); however, after three reinfections, the likelihood of another infection increased (0.26 infections per 100 child days; P < 0.0001) (Table 3). Diarrhea and dysentery were not less common with repeat infections: the percentage of infections associated with diarrhea was 8.9% of first infections and 14%, 15%, 15%, and 16% for episodes two to five.
TABLE 3.
Risk of reinfection in children with E. histolytica infectionsa
| No. of previous infections | Person-time (days)b | No. of infections | Incidencec | Adjusted relative hazard | 95% CI | P value |
|---|---|---|---|---|---|---|
| 0 | 154,946 | 180 | 0.12 | |||
| 1 | 99,342 | 100 | 0.10 | 0.97 | 0.76, 1.25 | 0.85 |
| 2 | 43,095 | 50 | 0.12 | 1.15 | 0.80, 1.65 | 0.45 |
| 3 | 14,681 | 19 | 0.13 | 1.28 | 0.74, 2.22 | 0.37 |
| ≥4 | 10,985 | 29 | 0.26 | 2.54 | 1.69, 3.81 | <0.0001 |
The incidence of Entamoeba histolytica infection and reinfection and the adjusted hazard ratio for repeat infections were calculated using the Andersen-Gill proportional hazards method adjusted for age in months, gender, baseline serum anti-E. histolytica IgG, and area of residence within Mirpur. Siblings (n = 36) were excluded from this analysis.
No. of total days of observation contributed by cohort study population.
No. of infections per 100 child-days.
Stool IgA anti-CRD-associated protection.
Fecal IgA anti-CRD was detected in 91% (183/202) of the children at least once during the study by monitoring the surveillance stool samples every 4 months. The presence of IgA specific for the CRD domain of lectin was associated with a significantly lower risk of repeat infections compared to those with one infection and negative for fecal IgA anti-CRD. The strong protective effect of anti-CRD IgA against reinfection was present for individuals in all categories with one or more previous E. histolytica infections (Table 4). The average time interval between each infection was 899 ± 210 days. For stool IgA anti-CRD-positive and -negative children, the intervals were 1,336 ± 164 and 462 ± 55 days, respectively. So the average duration of protection afforded by anti-CRD IgA was 437 days (95% CI, 346 to 528 days). IgA anti-CRD was also associated with a significantly lower risk of E. histolytica-associated diarrhea (Table 5). HLA DQB1 and DRB1 alleles were not significantly different between children with or without fecal anti-CRD IgA (Table 6). The lack of association of IgA anti-CRD with class II alleles suggests that the previously observed protection from infection associated with the class II DQB1*601/DRB1*1501 haplotype is not due to the production of anti-CRD IgA (12).
TABLE 4.
Incidence of Entamoeba histolytica reinfection in children with and without prior stool anti-CRD IgAa
| No. of previous infections | Previous stool anti-CRD IgA | Person-time (days) | No. of infections | Incidenceb | Adjusted relative hazard | 95% CI | P value |
|---|---|---|---|---|---|---|---|
| 1 | Negative | 26,192 | 53 | 0.20 | |||
| 1 | Positive | 73,150 | 47 | 0.06 | 0.28 | 0.18, 0.44 | <0.0001 |
| 2 | Negative | 17,236 | 32 | 0.18 | 0.85 | 0.53, 1.36 | 0.50 |
| 2 | Positive | 25,859 | 18 | 0.07 | 0.30 | 0.17, 0.50 | <0.0001 |
| ≥3 | Negative | 12,465 | 35 | 0.28 | 1.20 | 0.74, 1.95 | 0.45 |
| ≥3 | Positive | 13,201 | 13 | 0.10 | 0.40 | 0.20, 0.79 | 0.008 |
Incidence of Entamoeba histolytica reinfection and adjusted relative risk for repeat infections were calculated using the Andersen-Gill proportional hazards method adjusted for age in months, gender, baseline serum anti-E. histolytica IgG, and area of residence within Mirpur. Siblings (n = 36) were excluded from this analysis. See Table 3 for definition of person-time.
No. of infections per 100 child-days.
TABLE 5.
Incidence of Entamoeba histolytica diarrheal episodes in children with and without prior stool anti-CRD IgAa
| No. of previous diarrheal ep.b | Previous stool anti-CRD IgA | Person-time (days) | No. of diarrheal episodes | Incidencec | Adjusted relative hazard | 95% CI | P value |
|---|---|---|---|---|---|---|---|
| 0 | Negative | 46,214 | 22 | 0.05 | |||
| 0 | Positive | 242,231 | 17 | 0.007 | 0.16 | 0.08, 0.30 | <0.0001 |
| 1 | Negative | 6,522 | 12 | 0.18 | 3.66 | 1.62, 8.24 | 0.002 |
| 1 | Positive | 14,840 | 3 | 0.02 | 0.48 | 0.14, 1.66 | 0.25 |
| ≥2 | Negative | 5,886 | 17 | 0.29 | 7.07 | 3.50, 14.29 | <0.0001 |
| ≥2 | Positive | 5,938 | 2 | 0.03 | 0.75 | 0.20, 2.83 | 0.68 |
Incidence of Entamoeba histolytica-associated diarrheal episodes and adjusted relative risk for repeat diarrheal episodes were calculated using the Andersen-Gill proportional hazards method adjusted for age in months, gender, baseline IgG, and area of residence within Mirpur. Siblings (n = 36) were excluded from this analysis. See Table 3 for definition of person-time.
ep., episodes.
No. of diarrheal episodes per 100 child-days.
TABLE 6.
Unadjusted odds ratio for stool anti-CRD IgA-positive children versus stool anti-CRD IgA-negative children after 4.2 years by major histocompatibility complex class II DQB1 or DRB1 allelea
| Class II allele | Odds ratio | 95% CI | P value |
|---|---|---|---|
| DQB1*0202 | 2.73 | 0.60, 12.4 | 0.19 |
| DQB1*0301 | 0.70 | 0.23, 2.12 | 0.53 |
| DQB1*0503 | 1.65 | 0.36, 7.58 | 0.52 |
| DQB1*0601 | 0.73 | 0.27, 2.00 | 0.55 |
| DRB1*0701 | 0.96 | 0.34, 2.72 | 0.93 |
| DRB1*1501 | 0.77 | 0.28, 2.11 | 0.61 |
Univariate logistic regression analysis of ever being IgA positive after 4.2 years of follow-up. Only those MHC class II alleles with a frequency of ≥10% were assessed in the regression models.
DISCUSSION
Amebiasis resulted in a substantial burden of illness. One-fifth of the children suffered from E. histolytica-associated diarrhea or dysentery. This high incidence contradicts conventional wisdom that amebiasis is “a very infrequent cause of childhood diarrhea or dysentery in developing countries” (6). Both infection and diarrhea associated with E. histolytica were predominantly self-limited, with only 19% of episodes of E. histolytica-associated diarrhea requiring metronidazole therapy.
Contributing to the burden of amebic infection in these children were reinfections, similar to other enteric infections (4, 38). The 4.2-year period of every-other-day observation and the use of highly specific diagnostic tests for amebiasis demonstrated the existence of partial immunity to disease and infection. Immunity was associated with an intestinal IgA response specific for the active site (CRD) of the major parasite adhesin.
There are several possible limitations of this study. The morbidity of amebiasis was likely underestimated because of interventions that included every-other-day family visits by health assistants, oral rehydration, and provision of antiamebic and antibiotic therapy, all at no cost to the children and their families. Immunity associated with anti-CRD IgA may also have been underestimated because of the 4-month sampling interval used to detect IgA in the study. Additionally, the incidence of amebiasis may have been underestimated, since stool specimens were not always available to be tested for E. histolytica. Although this natural history cohort has extensive and comprehensive follow-up, this was an observational study, and there is the possibility of residual confounding from unmeasured or unknown factors. There is also the potential for selection bias, since only 289 children out of 1,164 were selected for inclusion in the study. However, we believe the children included in this study are representative of an urban slum population, for except for the entry criterion that 50% of the children be anti-E. histolytica IgG positive, no enrollment limitations were made. To avoid potential bias, we included baseline anti-E. histolytica IgG status as a covariate (where appropriate) in our analyses.
The ratio of symptomatic to asymptomatic amebiasis was low, as has been previously observed with children and adults (7, 13). Parenthetically, no hepatic abscesses were detected during the prospective study, which was expected, since amebic liver abscess is predominantly a disease of adult males (18, 34). We do not know the relative contributions of acquired immune responses versus differences between E. histolytica strains to the low rate of invasive infections. Enrollment of a birth cohort in this prospective study is planned to address the former. However, we doubt that invasive disease is more common in children under the age of 2, since it is rare for this age group to be admitted to the International Centre for Diarrheal Disease Hospital of Dhaka with amebiasis (14).
Reinfection with E. histolytica was common in this cohort and has also been observed in adults in Vietnam (7). In the case of rotavirus, where reinfection is also common, the lack of complete cross-protection between circulating rotavirus strains is a contributing factor. Extensive genetic diversity exists between E. histolytica isolates in Mirpur, Vietnam, and South Africa (2, 7, 16, 30). Intraspecies diversity, combined with the transient acquired immunity observed here, likely contributes to the high incidence of reinfection.
The poor sanitary conditions in Mirpur undoubtedly contributed to the high incidence of infection and reinfection. Mirpur is unfortunately not atypical of the conditions in the developing world: the World Health Organization estimates that 2.4 billion people worldwide are without access to improved sanitation and 1.1 billion lack access to improved water. Universal access to water and sanitation would likely result in dramatic reductions in morbidity and mortality not only from amebiasis but from most enteric infections. Enormous strides have been made in the last decade, but these efforts are keeping only slightly ahead of population growth (37).
This study extends the observation that a mucosal anti-CRD IgA response is associated with resolution of existing infection and in a delay of repeat infection (15, 16). The 4-year length of the study allowed the discovery that immunity associated with fecal anti-CRD IgA was short-lived but importantly was associated with protection not only from infection but from disease. Also encouraging for the potential application of CRD in a subunit vaccine was the lack of any HLA class II association with the ability of children to mount an anti-CRD IgA response observed here, as well as previous observations of its sequence conservation between isolates of E. histolytica and utility as a subunit vaccine in animal models of amebiasis (3, 11, 21, 25, 28, 33, 40).
It is remarkable that during the 4 years of the study, some of the children were never detected to have E. histolytica infection. This contrasts not only with the high rate of reinfection with E. histolytica in the infected children but also with the 98% infection rate of the same children with the nonpathogen E. dispar. Genetic differences between children may be playing an important role in influencing susceptibility to amebiasis, if the environmental factors that contribute to infection with E. histolytica and E. dispar are similar. The finding that children with three observed infections were at greater risk for additional new infections also suggests that there are host factors that influence susceptibility to amebiasis. Supporting a contribution of host genetic factors to susceptibility is the previous finding of an association of the HLA haplotype DQB1*0601/DRB1*1501 with a delay in infection onset (12). Future discovery of other genes influencing susceptibility to amebiasis will be enlightening as to the immune responses that provide protection.
In summary, the major conclusions of our study are that amebiasis is common in these urban slum children and that immunity to amebic diarrhea and colitis exists. The substantial burden of disease due to E. histolytica suggests that an effective vaccine would result in a measurable improvement in child health.
Acknowledgments
This study was conducted at the ICDDR, B Centre for Health and Population Research, with the support of a grant (AI-43596) from the NIH. This research was supported in part by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health. R.H. is a Howard Hughes Medical Institute International Research Scholar.
W.A.P. received research support from TechLab, Inc., and royalties from a patent license agreement with TechLab for a diagnostic test for amebiasis. These royalties accrue to the American Society of Tropical Medicine and Hygiene without benefit to W.A.P.
We thank the parents and children of Mirpur for their participation.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Andersen, P. K., and R. D. Gill. 1982. Cox's regression model for counting process: a large sample study. Ann. Stat. 10:1100-1120. [Google Scholar]
- 2.Ayeh-Kumi, P., I. K. M. Ali, L. Lockhart, et al. 2001. Entamoeba histolytica: genetic diversity of clinical isolates from Bangladesh as demonstrated by polymorphisms in the serine-rich gene. Exp. Parasitol. 99:80-88. [DOI] [PubMed] [Google Scholar]
- 3.Beck, D. L., M. Tanyuksel, A. Mackey, et al. 2002. Sequence conservation of the Gal/GalNAc lectin from clinical isolates. Exp. Parasitol. 101:157-163. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein, D. I., M. M. McNeal, G. M. Schiff, and R. L. Ward. 1989. Induction and persistence of local rotavirus antibodies in relation to serum antibodies. J. Med. Virol. 28:90-95. [DOI] [PubMed] [Google Scholar]
- 5.Bhan, M. K., J. F. Lew, S. Sazawal, B. K. Das, J. R. Gentsch, and R. I. Glass. 1993. Protection conferred by neonatal rotavirus infection against subsequent rotavirus diarrhea. J. Infect. Dis. 168:282-287. [DOI] [PubMed] [Google Scholar]
- 6.Black, R. E., and C. F. Lanata. 2002. Epidemiology of diarrheal diseases in developing countries, p. 11-29. In M. J. Blaser, P. D. Smith, J. I. Ravdin, H. B. Greenberg, and R. L. Guerrant (ed.), Infections of the gastrointestinal tract, 2nd ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 7.Blessman, J., I. K. M. Ali, P. A. Ton Nu, et al. 2003. Longitudinal study of intestinal Entamoeba histolytica infections in asymptomatic adult carriers. J. Clin. Microbiol. 41:4745-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleves, M. 1999. Analysis of multiple failure-time data with Stata. Stata Tech. Bull. 9:38-49. [Google Scholar]
- 9.Cox, D. R. 1972. Regression models and life tables. J. R. Stat. Soc. B 34:187-220. [Google Scholar]
- 10.Diamond, L. S., and C. G. Clark. 1993. A redescription of Entamoeba histolytica Shaudinn 1903 (amended Walker 1911) separating it from Entamoeba dispar (Brumpt 1925). J. Eukaryot. Microbiol. 40:340-344. [DOI] [PubMed] [Google Scholar]
- 11.Dodson, J. M., P. W. Lenkowski, Jr., A. C. Eubanks, et al. 1999. Role of the Entamoeba histolytica adhesin carbohydrate recognition domain in infection and immunity. J. Infect. Dis. 179:460-466. [DOI] [PubMed] [Google Scholar]
- 12.Duggal, P., R. Haque, S. Roy, et al. 2004. Influence of human leukocyte antigen class II alleles on susceptibility to Entamoeba histolytica infection in Bangladeshi children. J. Infect. Dis. 189:520-526. [DOI] [PubMed] [Google Scholar]
- 13.Gathiram, V., and T. F. H. G. Jackson. 1987. A longitudinal study of asymptomatic carriers of pathogenic zymodemes of E. histolytica. S. Afr. J. Med. Sci. 72:669-672. [PubMed] [Google Scholar]
- 14.Haque, R., I. K. M. Ali, and W. A. Petri, Jr. 1999. Prevalence and immune response to Entamoeba histolytica infection in preschool children in Bangladesh. Am. J. Trop. Med. Hyg. 60:1031-1034. [DOI] [PubMed] [Google Scholar]
- 15.Haque, R., I. K. M. Ali, R. B. Sack, et al. 2001. Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J. Infect. Dis. 183:1787-1793. [DOI] [PubMed] [Google Scholar]
- 16.Haque, R., P. Duggal, I. K. M. Ali, et al. 2002. Innate and acquired resistance to amebiasis in Bangladeshi children. J. Infect. Dis. 186:547-552. [DOI] [PubMed] [Google Scholar]
- 17.Haque, R., A. S. G. Faruque, P. Hahn, et al. 1997. Entamoeba histolytica and Entamoeba dispar infection in children in Bangladesh. J. Infect. Dis. 175:734-736. [DOI] [PubMed] [Google Scholar]
- 18.Haque, R., C. D. Huston, M. Hughes, E. Houpt, and W. A. Petri, Jr. 2003. Current concepts: amebiasis. N. Engl. J. Med. 348:1565-1573. [DOI] [PubMed] [Google Scholar]
- 19.Haque, R., N. U. Mollah, I. K. M. Ali, et al. 2000. Diagnosis of amebic liver abscess and intestinal infection with the Techlab Entamoeba histolytica II antigen detection and antibody tests. J. Clin. Microbiol. 38:3235-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haque, R., D. Mondal, B. D. Kirkpatrick, S. Akther, B. M. Farr, R. B. Sack, and W. A. Petri, Jr. 2003. Epidemiologic and clinical characteristics of acute diarrhea with emphasis on E. histolytica infections in preschool children in urban slum of Dhaka, Bangladesh. Am. J. Trop. Med. Hyg. 69:398-405. [PubMed] [Google Scholar]
- 21.Houpt, E., L. Barroso, L. Lockhart, et al. 2004. Prevention of intestinal amebiasis by vaccination with the Entamoeba histolytica Gal/GalNac lectin. Vaccine 22:612-618. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan, E. L., and P. Meier. 1958. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53:457-481. [Google Scholar]
- 23.Kosek, M., C. Bern, and R. L. Guerrant. 2003. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. W. H. O. 81:197-204. [PMC free article] [PubMed] [Google Scholar]
- 24.Levine, M. M., R. E. Black, M. L. Clemens, et al. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143:818-821. [DOI] [PubMed] [Google Scholar]
- 25.Lotter, H., T. Zhang, K. B. Seydel, et al. 1997. Identification of an epitope on the Entamoeba histolytica 170 kDa lectin conferring antibody-mediated protection against invasive amebiasis. J. Exp. Med. 185:1793-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moulton, L. H., M. A. Staat, M. Santosham, and R. L. Ward. 1998. The protective effectiveness of natural rotavirus infection in an American Indian population. J. Infect. Dis. 178:1562-1566. [DOI] [PubMed] [Google Scholar]
- 27.Murray, C. J. L., and A. D. Lopez. 1997. Alternative projections of mortality and disability by cause 1990-2020: global burden of disease study. Lancet 349:1498-1504. [DOI] [PubMed] [Google Scholar]
- 28.Petri, W. A., Jr., and J. I. Ravdin. 1991. Protection of gerbils from amebic liver abscess by immunization with the galactose-specific adherence lectin of Entamoeba histolytica. Infect. Immun. 59:97-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prentice, R. L., B. J. Williams, and A. V. Peterson. 1981. On the regression analysis of multivariate failure time data. Biometrika 68:373-379. [Google Scholar]
- 30.Ravdin, J. I., M. D. Abd-Alla, S. L. Welles, et al. 2003. Intestinal anti-lectin immunoglobulin A antibody response and immunity to Entamoeba dispar infection following cure of amebic liver abscess. Infect. Immun. 71:6899-6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reves, R. R., M. M. Hossain, K. Midthun, et al. 1989. An observational study of naturally acquired immunity to rotaviral diarrhea in a cohort of 363 Egyptian children: calculation of risk for second episodes using age-specific person-years of observation. Am. J. Epidemiol. 130:981-988. [DOI] [PubMed] [Google Scholar]
- 32.Schlesselman, J. J. 1982. Case-control studies: design, conduct and analysis. Oxford University Press, New York, N.Y.
- 33.Soong, C. J. G., K. C. Kain, M. D. Abd-Alla, et al. 1995. A recombinant cysteine-rich section of the Entamoeba histolytica galactose inhibitable lectin is efficacious as a subunit vaccine in the gerbil model of amebic liver abscess. J. Infect. Dis. 171:645-651. [DOI] [PubMed] [Google Scholar]
- 34.Stanley, S. L., Jr. 2003. Amoebiasis. Lancet 361:1025-1034. [DOI] [PubMed] [Google Scholar]
- 35.Velazquez, F. R., D. O. Matson, J. J. Calva, et al. 1996. Rotavirus infections in infants as protection against subsequent infections. N. Engl. J. Med. 335:1022-1028. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. 1997. Amoebiasis. Wkly. Epidemiol. Rec. 72:97-99.9100475 [Google Scholar]
- 37.World Health Organization. 2005. Global water supply and sanitation assessment 2000 report. World Health Organization, Geneva, Switzerland.
- 38.Yuan, L., A. Geyer, and L. J. Saif. 2001. Short-term immunoglobulin A B-cell memory resides in intestinal lymphoid tissues but not in bone marrow of gnotobiotic pigs inoculated with Wa human rotavirus. Immunology 103:188-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, J., and K. F. Yu. 1998. What's a relative risk? a method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280:1690-1691. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, T., and S. L. Stanley, Jr. 1994. Protection of gerbils from amebic liver abscess by immunization with a recombinant protein derived from the 170-kilodalton surface adhesin of Entamoeba histolytica. Infect. Immun. 62:2605-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]