Abstract
Staphylococcal protein A (SpA) is representative of a new class of antigens, the B-cell superantigens (SAgs). These antigens bind to the Fab regions of immunoglobulin molecules outside their complementarity-determining regions. SpA, the best-studied B-cell SAg, reacts with the Fabs of most VH3+ immunoglobulins, which are expressed on 30 to 60% of human peripheral B cells. Therefore, B-cell SAgs like SpA have great potential to elicit inflammatory responses in vivo. We previously reported that the interaction of SpA with VH3+ immunoglobulin molecules leads to activation of the complement cascade and produces a histologic pattern of inflammation in the skin of a rabbit indicative of immune complex injury. To elucidate the cellular and molecular events contributing to this type of unconventional immune complex-mediated inflammation, we established a mouse peritoneal Arthus reaction model. Mice treated intravenously with human polyclonal immunoglobulin G (IgG), followed by intraperitoneal injection of SpA, showed neutrophil influx into the peritoneal cavity with peak numbers appearing at 8 h. This inflammatory reaction was dependent on the interaction of SpA with VH3+ IgG. Mast cells, FcγRIII, complement components, and tumor necrosis factor alpha play obligatory roles, and the reaction is associated with the local release of the CXC chemokines macrophage inflammatory protein 2 and KC. The data provide further compelling evidence for the induction of immune complex-mediated injury by a B-cell SAg and highlight important factors contributing to the pathogenesis of this novel type of inflammatory reaction.
B-cell superantigens (SAgs), unlike conventional antigens, bind to the Fab regions of immunoglobulin (Ig) molecules outside their complementarity-determining regions (CDRs) (reviewed in references 20 and 38). These unconventional antigens can react with a substantial amount of a host's peripheral B-cell repertoire and serum Igs by virtue of their ability to interact with many members of an entire variable region heavy (VH) or variable region light (VL) gene family (reviewed in reference 21). Staphylococcal protein A (SpA) is the most-studied B-cell SAg. Although it had long been known that this microbial product binds to the Fc region of IgG, it became clear that SpA also binds, via an alternative site, to determinants outside the CDRs in the Fab region of Igs. SpA reacts with the Fabs of most VH3+ Igs, which are expressed on 30 to 60% of human peripheral B cells. Other proteins defined as B-cell SAgs include human immunodeficiency virus (HIV) gp120, protein Fv (a human liver sialoprotein), protein L (a coat protein of Peptostreptococcus magnus), and staphylococcal enterotoxin D (5, 6, 7, 39).
The ability of B-cell SAgs to bind to a large amount of serum Ig molecules endows them with an array of potentially dangerous biologic properties. A B-cell SAg could inflict tissue damage by a number of inflammatory mechanisms. Its interaction with cytophilic IgG, IgE, or IgA molecules could lead to cross-linking of the respective Ig Fc receptors on inflammatory cells, thereby resulting in the release of proinflammatory mediators. Indeed, SpA, protein L, protein Fv, and HIV gp120 induce the release of proinflammatory mediators from human basophils and/or mast cells by interacting with the Fab region of IgE molecules bound to high-affinity Fcɛ receptors on these cells (10, 22, 27, 28, 29).
A B-cell SAg could also have deleterious effects on the host by virtue of its ability to form relatively large amounts of immune complexes. Unlike a conventional antigen, prior encounter of the B-cell SAg is not a prerequisite for this reaction to occur since the reactive VH or VL antibodies are found in the host's standing pool of serum Igs. We have been particularly interested in the capacity of these unconventional antigens to induce immune complex-mediated inflammation. With SpA as a model B-cell SAg, we provided evidence that its unconventional binding to reactive Igs leads to activation of the classical complement pathway (19). Activation of this pathway has long been considered to be an integral step in the development of inflammation caused by conventional antigen-antibody complexes (8). We subsequently reported (18) that such unconventional immune complexes formed in rabbits elicited the characteristic histologic features of the Arthus reaction, the classical model of tissue inflammation mediated by conventional antigen-antibody complexes.
Because it is difficult to study the pathogenesis of this novel type of immune complex inflammation in the rabbit, we developed a mouse model that enables us to begin to elucidate the cellular and molecular bases of B-cell SAg-induced immune complex inflammation. We adapted a murine model of peritonitis commonly used to investigate the pathogenesis of immune complex-mediated injury caused by conventional antigens (11, 15, 31). We now demonstrate that the SpA-IgG-induced Arthus reaction is likely caused by the superantigenic binding properties of this B-cell SAg. The reaction is characterized by the recruitment of neutrophils into the peritoneal cavity. The influx of neutrophils is associated with the release of the CXC chemokines KC and macrophage inflammatory protein 2 (MIP-2) into the peritoneal cavity and is dependent on the availability of mast cells, FcγRIIIs, C3 and C5, and tumor necrosis factor alpha (TNF-α).
MATERIALS AND METHODS
Animals.
Female BALB/c mice were purchased from Taconic Farms, Germantown, NY. WBB6F1-W/Wv, WBB6F1-+/+, C5-deficient, TNF-α knockout (TNF−/−), and FcγRIII knockout mice and their respective wild-type controls were purchased from The Jackson Laboratory (Bar Harbor, ME). Female C3-deficient mice, generated as previously described (40), and their wild-type controls were maintained at the University of Pennsylvania. All mouse strains except WBB6F1-W/Wv and WBB6F1-+/+ were used at 5 to 7 weeks of age. The WBB6F1-W/Wv and WBB6F1-+/+ mice were studied at 3 to 4 months of age. The mice were maintained under specific-pathogen-free conditions. The experimental protocols were approved by the Philadelphia Veterans' Administration Medical Center and the University of Pennsylvania institutional animal care and use committees.
Administered reagents.
Lyophilized, pooled normal human IgG (hIgG; Baxter Healthcare Co., Glendale, CA) was reconstituted to 60 mg/ml with sterile 0.9% saline. Recombinant SpA (Repligen, Waltham, MA) was diluted in phosphate-buffered saline (PBS). The C5aR antagonist peptide Ac-Phe-(Orn-Pro-dCha-Trp-Arg) (23) was synthesized and diluted in PBS. The above reagents were sterile filtered through 0.2-μm filters (Millipore, Bedford, MA).
Preparation of hyperiodinated SpA.
SpA was hyperiodinated to abrogate its IgG Fc-binding activity as previously described (18).
Conjugation of hyperiodinated SpA to Seph.
We suspended 300 μg of freeze-dried, CNBr-activated Sepharose 4B (Seph; Sigma, St. Louis, MO) in 12.5 ml of 1 mM HCl; the Seph was subsequently rotated for 15 min at room temperature to swell the beads (300 μg of Seph yields 1 ml of gel.) The beads were then centrifuged at 2,500 rpm for 2 min at room temperature. The supernatant was aspirated, and the beads were resuspended in 10 ml of 1 mM HCl and centrifuged as described above. Following the repetition of these steps, the beads were washed in coupling buffer (0.1 M NaHCO3, 0.5 M NaCl [pH 8.3]). The supernatant was aspirated, and 4 mg of SpA plus coupling buffer or coupling buffer alone (for a total volume of 1.5 ml) was added to 1 ml of swelled Seph. Following rotation for 2 h, the beads were centrifuged as described above and washed once in coupling buffer. Next, 2 ml of 0.2 M glycine was added to 1 ml of swelled Seph and rotated for 1 h at room temperature. The Seph beads were centrifuged, washed with an acetate buffer (0.1 M sodium acetate, 0.5 M NaCl [pH 4.0]), and then washed with a Tris buffer (0.1 M Tris-HCl, 0.5 M NaCl [pH 8.0]). This procedure was repeated two more times. The hyperiodinated-SpA-conjugated and unconjugated Seph beads were stored in 0.1% bovine serum albumin-PBS with 0.02% sodium azide at 4°C until needed. At the time of use, the beads were washed extensively with the appropriate buffer.
Depletion of VH3+ IgG from pooled hIgG.
The pooled normal hIgG was passed over either a hyperiodinated-SpA-conjugated Seph column or an unconjugated Seph column (sham-depleted hIgG). Polyprep chromatography columns (Bio-Rad, Hercules, CA) were packed with 10 ml of either hyperiodinated-SpA-Seph or Seph alone and washed with 100 ml of 1× running buffer (1:5 dilution of 5× running buffer [pH 7.0] containing 0.079 M KH2PO4, 0.48 M Na2HPO4, and 0.77 M NaCl). Next, 1 ml of pooled IgG (60 mg/ml) diluted with 250 μl of 5× running buffer was added to each column and allowed to pass into the Seph. The column was then closed, and the IgG was incubated in the column for 5 min. This procedure was repeated twice for each column. Afterward, 10 ml of 1× running buffer was added and 1-ml fractions were collected. This process was repeated until the A280 of the fractions was <0.100. The column was subsequently flushed with 50 ml of 1× running buffer. The IgG concentrations of the collected fractions were determined by the following method: A280/extinction coefficient for IgG (1.43). Effluent fractions with a concentration of >1 mg/ml were pooled and dialyzed two times against PBS at 4°C. The samples were concentrated in an Ultrafree-50 centrifugal filter (Millipore), and IgG concentrations were determined again as described above. The effluent fractions were then passed over their respective columns a second time, and the dialysis and concentration procedures were repeated.
Preparation of mast cells.
Bone marrow cells from femurs of WBB6F1-+/+ mice were cultured for 4 weeks in WEHI-3 cell conditioned medium as previously described (31). Mast cells harvested from cultures (>98% purity) were administered intraperitoneally (i.p.) to WBB6F1-W/Wv mice (107 mast cells per mouse). The bone marrow-derived mast cells initially resemble mucosal mast cells (34) and will differentiate into connective tissue mast cells in the peritoneal cavity (26, 30).
Peritoneal Arthus reaction.
Nine milligrams of polyclonal hIgG was injected intravenously (i.v.), followed 5 min later by i.p. injection of SpA (100 μg). Two control groups were examined: (i) mice injected i.v. with saline rather than hIgG, followed by i.p. injection of SpA, and (ii) mice injected i.v. with hIgG, followed by i.p injection of saline. In some experiments, BALB/c mice were treated with hIgG depleted of VH3+ Ig molecules or sham-depleted hIgG as described above. The mice were sacrificed at various time points after SpA injection, and their peritoneal cavities were subjected to lavage with 2 ml of ice-cold PBS. Total cell counts were performed on lavage fluid with a hemocytometer. Differential cell counts were performed on cytospin slides prepared from lavage fluids that were stained with Hema-3 stain. A minimum of 500 cells were counted per slide.
Quantitation of proinflammatory molecules.
Immunoreactive MIP-2 and KC levels in peritoneal lavage fluids were quantitated by the proinflammatory molecule Searchlight Multiplex assay according to the manufacturer's protocol (Pierce Biotechnology, Needham, MA).
RESULTS
Recruitment of neutrophils in B-cell SAg-immune complex-mediated peritoneal Arthus reaction.
We adapted a model of the peritoneal Arthus reaction induced by conventional antigen-IgG immune complexes to begin to study mechanisms involved in the elicitation of B-cell SAg-induced immune complex-mediated tissue injury (11, 15, 31). This adaptation was a natural extension of our earlier studies of B-cell SAg-induced cutaneous Arthus reactions in the rabbit (18). We focused on the number of neutrophils measured in the lavage fluid since selective recruitment of these cells has long served as the hallmark of the Arthus reaction induced by conventional antigen-IgG immune complexes (3) and this parameter had proved a useful marker of the B-cell SAg-induced cutaneous Arthus reaction observed in our rabbit model (18). In the peritoneal Arthus reaction induced by conventional antigen, neutrophils account for approximately 40 to 60% of the inflammatory infiltrate observed at 6 to 8 h after elicitation (11).
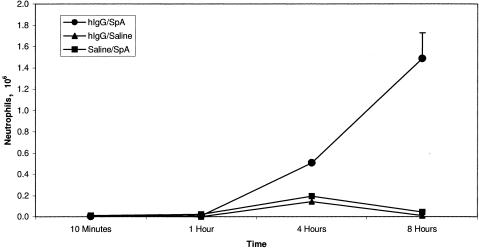
We observed the gradual appearance of neutrophils in the peritoneal cavities of BALB/c mice treated with hIgG and SpA. Accumulation of neutrophils was not apparent until 4 h, with numbers peaking at 8 h (Fig. 1). Their accumulation required the presence of hIgG since neutrophils failed to accumulate in the peritoneal cavities of mice injected i.p. with SpA following i.v. injection of saline in place of hIgG. Similarly, neutrophil recruitment into the peritoneal cavities of mice treated with hIgG i.v. and saline i.p. was not observed. These results indicate that the interaction of endogenous mouse Igs with either the Fab-binding site or the Fcγ-binding site of SpA is not sufficient to elicit this inflammatory reaction.
FIG. 1.
Kinetics of neutrophil infiltration in the peritoneal Arthus reaction. Inset indicates treatment conditions. BALB/c mice were treated i.v. with human polyclonal IgG and i.p. with SpA. At various points thereafter, their peritoneal cavities were subjected to lavage and the total number of neutrophils was determined. The number of neutrophils was highest at 8 h (n = 7 for each time point, mean ± standard deviation, P < 0.005).
Dependence of the peritoneal Arthus reaction on the superantigenic binding properties of SpA.
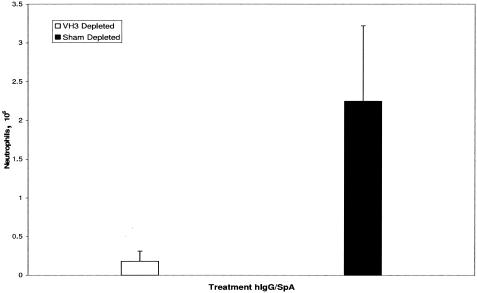
To further explore the dependence of this peritoneal Arthus reaction on the superantigenic binding properties of SpA, we took advantage of the knowledge that this B-cell SAg's superantigenic Fab-binding activity is restricted to VH3+ Igs (35, 36). Accordingly, the hIgG preparation was passed over a hyperiodinated-SpA-Seph column to deplete it of most VH3+ molecules as previously reported (18). Hyperiodination abrogates the Fcγ-binding activity of SpA, leaving the VH3 Fab-binding activity intact. Sham-depleted hIgG was prepared by passing the IgG reagent over a Seph column. As previously reported (18), the VH3-depleted IgG preparation had a 100-fold reduction in its binding to hyperiodinated SpA compared to the sham-depleted hIgG that had been passed over a Seph column (data not shown). The neutrophil infiltrate observed at 8 h in the peritoneal cavities of mice treated with sham-depleted hIgG was abolished in mice treated with VH3-depleted hIgG (Fig. 2).
FIG. 2.
Requirement of VH3+ hIgGs for SpA-induced neutrophil influx. SpA-induced inflammatory reactions were markedly reduced at 8 h in mice that had received hIgG depleted of molecules bearing VH3 heavy chains (n = 6, mean ± standard deviation, P < 0.0004).
Accumulation of proinflammatory molecules in the peritoneal cavity during the course of SpA-induced peritoneal Arthus reactions.
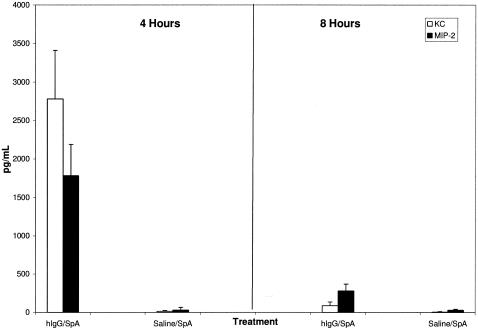
To further dissect the mechanisms responsible for the SpA-induced peritoneal Arthus reaction, we analyzed peritoneal lavage specimens for MIP-2 and KC, two chemokines that are considered to play key roles in the recruitment of neutrophils into inflamed tissue compartments (11). Both have been detected in the peritoneal cavities of mice undergoing conventional antigen-IgG peritoneal Arthus reactions (11). In our model, we detected levels of KC and MIP-2 in the peritoneal cavity that were higher at 4 h than at 8 h (Fig. 3).
FIG. 3.
Accumulation of KC and MIP-2 in SpA-hIgG-induced peritoneal Arthus reactions. Levels of the CXC chemokines KC and MIP-2 in the peritoneal cavity were determined with a Searchlight Multiplex assay after induction of the SpA-hIgG peritoneal Arthus reaction. Higher levels of both chemokines were seen at 4 h than at 8 h (n = 5 for each time point).
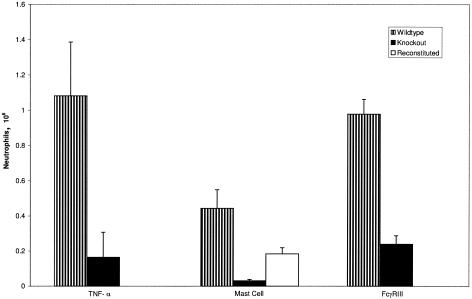
TNF-α is also released into the peritoneal cavities of mice undergoing conventional antigen-IgG immune complex Arthus reactions (11, 15, 31). Its role in the recruitment of neutrophils in the conventional antigen-induced peritoneal Arthus reaction remains unclear since functional inhibition of TNF-α had no effect on peritoneal neutrophil influx (15). To address this issue in the B-cell SAg-induced peritoneal model, we conducted experiments with mice homozygous for a TNF-α-targeted mutation that renders them TNF-α deficient. SpA-IgG-induced neutrophil recruitment to the peritoneal cavity was abolished in TNF−/− mice, underscoring an important role for this cytokine (Fig. 4).
FIG. 4.
Neutrophil recruitment into the peritoneal cavities of TNF-α knockout, mast cell-deficient, and FcγRIII knockout mice 8 h after injection of hIgG and SpA. The number of neutrophils was markedly reduced in TNF-α knockout versus wild-type mice (n = 6 in each group, mean ± standard deviation, P < 0.0008), mast cell-deficient versus wild-type mice (n = 9 in each group, mean ± standard deviation, P < 0.0007) and mast cell-deficient versus mast cell-reconstituted mice (n = 9, mean ± standard deviation, P < 0.0001), and in FcγRIII knockout mice versus wild-type mice (n = 10 in each group, mean ± standard deviation, P < 0.007).
Dependence of SpA-IgG immune complex peritoneal Arthus reaction on resident mast cells.
Mast cells have been demonstrated to play a key role in the pathogenesis of conventional antigen-IgG immune complex-induced peritonitis (15, 31, 32, 42). Therefore, we sought to determine if this resident peritoneal cell type also contributed to the B-cell SAg-induced inflammatory reactions observed in these studies. We used WBB6F1-W/Wv mice to address this issue. Mice belonging to this strain are mast cell deficient due to a mutation in Kit, a tyrosine kinase essential for the growth and differentiation of these cells. Controls were provided by mast cell-sufficient WBB6F1-+/+ mice. Neutrophil infiltration at 8 h was abolished in mice lacking mast cells compared to their mast cell-sufficient counterparts (Fig. 4). The essential contribution of mast cells was further underscored by the accumulation of neutrophils in the peritoneal cavities of WBB6F1-W/Wv mice reconstituted with mast cells (Fig. 4).
Dependence of SpA-IgG immune complex peritoneal Arthus reaction on FcγRIII.
We used FcγRIII knockout mice to examine the involvement of this Fcγ receptor in the development of SpA-induced immune complex peritonitis. These receptors were previously shown to play important roles in the inflammation induced by conventional antigen-antibody complexes in several tissue compartments (17, 33). As shown in Fig. 4, neutrophil recruitment was abrogated in the knockout mice versus their wild-type controls at 8 h, the peak time of appearance of these cells.
Dependence of SpA-IgG immune complex peritoneal Arthus reaction on complement activation.
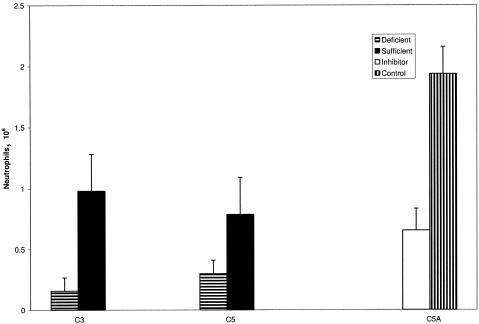
Next, we conducted a series of experiments to determine the importance of complement components in the development of these unconventional immune complex-mediated inflammatory reactions. Initial studies were carried out with C3-deficient mice and C5-deficient mice. As shown in Fig. 5, neutrophilic recruitment was reduced, on average, by 60% in the C5-deficient mice and 90% in the C3-deficient mice versus their respective wild-type controls.
FIG. 5.
Dependence of SpA-hIgG-induced peritoneal Arthus reaction on complement. The 8-h SpA-induced peritoneal Arthus reaction was reduced 90% in C3-deficient versus wild-type mice (n = 8 in each group, mean ± standard deviation, P < 0.04) and 60% in C5-deficient versus wild-type mice (n = 4 in each group, mean ± standard deviation, P < 0.004). BALB/c mice treated with a C5aR inhibitor (50 μg) demonstrated a 64% reduction in the number of neutrophils in the peritoneal cavity 8 h after injection of hIgG and SpA compared to those treated with saline (n = 10 in each group, mean ± standard deviation, P < 0.001).
To further explore the role of C5a, we attempted to block its activity with a potent C5aR antagonistic peptide [Ac-Phe-(Orn-Pro-dCha-Trp-Arg)] (23). In animals treated with 50 μg, neutrophil recruitment at 8 h was inhibited by a mean of 64%, reproducing the results observed in the C5-deficient mice (Fig. 5). Taken together, the results indicate that complement activation is intimately involved in the neutrophil influx elicited by SpA-IgG. The greater inhibition observed in C3-deficient mice than in C5-deficient mice and mice treated with the C5aR inhibitor suggests that C3 exerts effects independently of its obligatory role in the downstream activation of C5.
DISCUSSION
Given its ability to react with large amounts of serum Igs, a B-cell SAg has great potential to inflict tissue injury. Indeed, we have previously reported that SpA induces a cutaneous Arthus reaction-like reaction in rabbits (18). This inflammatory reaction was attributed to the superantigenic binding property of SpA since it was observed only in animals treated with polyclonal hIgG containing VH3+ molecules. The use of the rabbit in these experiments made it very difficult to conduct mechanistic studies. Therefore, we established an animal model that provided the opportunity to elucidate the cellular and molecular events occurring in this novel type of immune complex-elicited response.
We adapted a mouse peritonitis model previously used to delineate the pathogenesis of the tissue injury caused by conventional antigen-IgG immune complexes (11, 15, 31). Those studies used a reverse passive Arthus reaction. Mice were injected i.v. with ovalbumin immediately prior to i.p injection of IgG antiovalbumin antibodies. The ensuing inflammatory reaction was characterized by a peak recruitment of neutrophils into the peritoneal cavity at 6 to 8 h, along with the appearance of a number of biologic mediators (11, 15, 32, 42).
We assumed that the BALB/c mouse would have sufficient amounts of SpA-binding VH3-like IgG molecules in its standing pool of serum Igs to promote SpA-IgG immune complex-mediated inflammation. This assumption was based on the finding that VH3-like genes are expressed on BALB/c peripheral B cells (37). Indeed, recent reports have described in vivo functional consequences of the interaction of B-cell SAgs, including SpA, with the membrane-bound form of IgM on peripheral B cells, i.e., depletion of reactive B1 and marginal-zone B cells (12, 13, 41). However, in our experiments, BALB/c mice did not develop signs of inflammation, reminiscent of our early experience with our rabbit model. This surprising finding suggests that the endogenous BALB/c serum IgGs lack the qualitative and/or quantitative properties needed to give rise to immune complex-mediated inflammation. Therefore, following the lead of the experimental design used in the rabbit studies, we injected the mice i.v. with polyclonal hIgG immediately prior to i.p. injection of SpA. This ensured a rich source of VH3+ IgG molecules and indeed elicited the recruitment of neutrophils into the peritoneal cavity. Their accumulation was not apparent until 4 h, with higher numbers observed at 8 h. This kinetic pattern replicates that observed in the peritoneal Arthus reaction induced by conventional antigen-antibody complexes (11).
We believe that this reaction was caused by the superantigenic binding activity of SpA since it did not occur in animals treated with hIgG-depleted SpA-binding VH3+ IgG molecules. As previously reported (18), the VH3-depleted hIgG fraction proved no longer to bind hyperiodinated SpA but retained its Fcγ-binding activity in relevant enzyme-linked immunosorbent assays. The fact that the VH3-depleted hIgG and sham-depleted IgG fractions had equivalent amounts of SpA-binding activity (data not shown) indicates that the Fc IgG functional activity of the VH3-depleted IgG fraction was not compromised. Thus, the reaction requires the Fab-binding (superantigenic) properties of SpA. We cannot exclude the possibility that the Fc-binding region of SpA also contributes to the observed inflammatory reaction. Such binding could lead to the formation of large and possibly very stable immune complexes. The presence of mouse IgG in these complexes could enhance their binding to both mouse FcγR and mouse C1q. We attempted to investigate whether the Fc-binding site of SpA contributes to the observed inflammatory reaction by using hyperiodinated SpA rather than unmodified SpA. To control for the hyperiodination reaction, we carried out parallel experiments with hyperiodinated bovine serum albumin. Unfortunately, this approach proved untenable since both types of hyperiodinated proteins elicited a nonspecific inflammatory peritoneal inflammatory reaction even when not accompanied by i.v. administration of hIgG. Further experiments are necessary to resolve this issue.
We also cannot exclude the possibility that the reactions were caused, in part, by immune complexes containing SpA and hIgG anti-SpA antibodies. Such antibodies, which bind SpA via their CDRs, cannot be easily distinguished from those that bind via their VH framework regions. To our knowledge, such conventional anti-SpA antibodies have not been unequivocally demonstrated and thus are of only theoretical concern. To rule out this possibility, we are conducting studies with IgG1 xenomice (kindly provided by Geoff Davis, Abgenix, Fremont, CA) in which the endogenous mouse Ig VH and VL repertoires have been replaced with human VH and VL genes (9). In preliminary experiments, we observed the development of a typical localized Arthus reaction following the i.p. injection of these mice with SpA. Given the fact that such mice are maintained in a specific-pathogen-free facility and have not been intentionally immunized with SpA, we believe that deposition of immune complexes consisting of VH3+ IgG and SpA is the most likely cause of the Arthus reactions observed in the experiments reported herein.
Role of proinflammatory mediators.
In these studies, we focused on three proinflammatory mediators that have been identified as factors released downstream of conventional antigen-antibody complex deposition and that potentially contribute to the induced inflammatory response (11, 15, 17). Several groups have reported high levels of TNF-α not only in the peritoneal washings but also in the bronchial lavage fluids of mice undergoing lung-targeted Arthus reactions (11, 15, 17). However, the role played by this cytokine in the recruitment of neutrophils into the peritoneal cavity has remained ambiguous since functional inhibition of TNF-α with anti-TNF-α antibody had little effect on this response (23). Because of this uncertainty, we asked whether TNF-α plays a permissive role in the peritoneal neutrophilic inflammatory response elicited by SpA-hIgG immune complexes. To obtain an unambiguous answer, we conducted experiments with TNF−/− mice. We found that recruitment of neutrophils into the peritoneal cavities of these mice was abolished. This finding underscores the primacy of TNF-α in the events responsible for this B-cell SpA-IgG immune complex-mediated inflammatory response, in contrast to the nonessential role of this cytokine in the conventional antigen-induced peritoneal Arthus reaction. We presume that TNF-α mediates its effect by promoting transmigration of neutrophils across vascular endothelium following its upregulation of adhesion molecules on these cell populations.
We also observed accumulation of the CXC chemokines MIP-2 and KC in our model. Their levels were higher at 4 h than at 8 h after the administration of hIgG and SpA. Both of these chemokines are considered to play proinflammatory roles in conventional antigen-antibody-induced inflammatory reactions in the mouse peritoneal cavity, where they were found to reach peak levels at 2.5 h after elicitation of the Arthus reaction (11). This time period was not sampled in our studies. Thus, it is difficult to say whether the kinetics of chemokine accumulation are different in these two types of peritoneal Arthus reactions. Nevertheless, our data are consistent with the involvement of these chemokines in the accumulation of neutrophils in the peritoneal cavities of SpA-hIgG-challenged mice. Further studies are necessary to substantiate this supposition.
Role of mast cells in SpA-IgG-induced peritonitis.
The experiments with WBB6F1-W/Wv mice indicate that mast cells are required for generation of the peritoneal inflammatory response. First, the response was abolished in this strain, which lacks mast cells in its tissues. Second, the SpA-induced inflammatory response was enabled in these animals following their reconstitution with WBB6F1-+/+ mast cells. This finding conforms to the important role of mast cells in the murine peritoneal Arthus reaction induced by conventional antigen-IgG immune complexes (11, 15, 31). In that model, peritoneal mast cells are rich sources of mediators that promote the recruitment of neutrophils including leukotrienes, TNF-α, MIP-2, and KC (11, 32, 42). In those studies, activation of the mast cells was found to be effected via C5a and Fcγ receptors (11, 15).
Involvement of FcγRIII and complement.
Recent studies have highlighted the important roles of both activating FcγRs and C5aR in the pathogenesis of Arthus reactions elicited by immune complexes containing conventional antigens and specific IgG antibodies (11, 15, 17, 33). This codominance reflects the interplay between these two receptor systems with evidence for the upregulation of activating and downregulation of inhibitory FcγRs by C5a (11, 33). Dominance of one system versus the other appears to be dictated by the tissue compartment affected and the genetic background of the mouse under study (4, 15).
However, the results reported herein highlight key differences between the importance of these receptor systems in conventional antigen- and B-cell SAg-mediated peritoneal Arthus reactions. FcγRIIIs are an obligatory component of B-cell SAg-induced immune complex peritoneal inflammation since neutrophilic influx into the peritoneal cavity was abrogated in FcγRIII knockout mice. By contrast, FcγRIIIs had only a minor effect on neutrophil migration induced by the deposition of conventional antigen-containing immune complexes in the peritoneal cavity (11). Second, C3 and C5 were both identified as major contributors to the SpA-induced peritoneal Arthus reaction. Neutrophilic influx was essentially abolished in the C3 knockout mice compared to the 60% inhibition observed in both the C5-deficient mice and mice treated with a C5aR inhibitor. By contrast, blockade of the C5aR pathway markedly diminished neutrophil migration in the conventional antigen-induced peritoneal Arthus reaction, whereas blocking the C3aR pathway had no effect (11). Indeed, C3-deficient mice showed only little attenuation of the inflammatory response accompanying the Arthus reaction induced by conventional antigens in other tissue compartments.
The explanation for these differences in the relative importance of FcγRIII and complement components observed in conventional antigen and our B-cell SAg Arthus reaction model is not obvious. However, they may be due to qualitative differences in (i) the ability of Fc fragments of complexed mouse and hIgG to bind mouse FcγRs or to activate mouse complement and/or (ii) the physical properties of the complexes formed by the conventional antigen, chicken ovalbumin, versus the B-cell SAg SpA. At the very least, the greater inhibition of neutrophil influx observed in C3-deficient mice than in C5-deficient mice and mice treated with the C5aR inhibitor suggests that C3 exerts effects in the B-cell SAg-induced Arthus reaction independently of its role in the downstream activation of C5. Studies are under way to determine whether this can be explained by the action of C3a.
In summary, the SpA-IgG-induced peritoneal Arthus reaction has an absolute dependence on mast cells, FcγRIII, TNF-α, and C3, with a lesser, albeit substantial, requirement for C5. These findings are consistent with a model wherein mast cells are activated via FCγRIII and complement receptors. Importantly, although there may be interaction between these receptor systems, they do not appear to subserve redundant functions in this B-cell SAg-peritoneal Arthus reaction model. It is likely that the mast cells release TNF-α, which, along with the CXC cytokines MIP-2 and KC, C5a, and perhaps leukotrienes (42), contributes to the recruitment of neutrophils. The results of these experiments provide further evidence for the induction of immune complex-mediated injury by a B-cell SAg and highlight both similarities and differences in the importance of the factors involved in the pathogenesis of immune complex-mediated inflammation elicited by a B-cell SAg and conventional antigens.
Such an in vivo response to a B-cell SAg could have profound clinical significance. For example, SpA immunoadsorption has been used as a novel therapy in a variety of diseases that are mediated by pathogenic autoantibodies (1, 14, 16). In addition, the therapy is being investigated as a treatment for cancer, thrombotic thrombocytopenic purpura, hemolytic-uremic syndrome, myasthenia gravis, and HIV infection (2, 24, 25). However, a significant number of the patients treated have developed severe adverse side effects, most notably leukocytoclastic vasculitis (2, 24). Histologically, the dermal lesions are characterized by superficial and deep perivascular infiltrates containing mostly neutrophils (24). In addition, diapedesis of erythrocytes was observed. These effects could be due to an interaction between SpA-VH3+ Igs and the formation of immune complexes that bind to and activate FcγR-bearing cells and complement. Further studies are necessary to determine whether this type of inflammatory reaction is associated with the in vivo response to other B-cell SAgs.
Acknowledgments
The studies described in this report were supported by a Merit Review grant from the Veterans' Administration.
Editor: F. C. Fang
REFERENCES
- 1.Ainsworth, S. K., P. A. Pilla, S. H. Pepkowitz, and P. O'Brien. 1988. Toxicity following protein A treatment of metastatic breast adenocarcinoma. Cancer 61:1495-1500. [DOI] [PubMed] [Google Scholar]
- 2.Arbiser, J. L., J. S. Dzieczkowski, J. V. Harmon, and L. M. Duncan. 1995. Leukocytoclastic vasculitis following staphylococcal protein A column immunoadsorption therapy: two cases and a review of the literature. Arch. Dermatol. 131:707-709. [PubMed] [Google Scholar]
- 3.Arthus, M. 1903. Injections répetéés de sérum de cheval chez le lapin. C. R. Soc. Biol. 55:817-820. [Google Scholar]
- 4.Baumann, U. N., B. Couchakova, B. Gewecke, J. Kohl, M. C. Carroll, R. E. Schmidt, and J. E. Gessner. 2001. Distinct tissue site-specific requirements of mast cells and complement components C3/5a receptor in IgG immune complex-induced injury of skin and lung. J. Immunol. 167:1022-1027. [DOI] [PubMed] [Google Scholar]
- 5.Berberian, L., L. Goodglick, T. J. Kipps, and J. Braun. 1993. Immunoglobulin VH3 gene products: natural ligands for HIV gp120. Science 261:1588-1591. [DOI] [PubMed] [Google Scholar]
- 6.Björck, L. 1988. Protein L: a novel bacterial cell wall protein with affinity for Ig L chains. J. Immunol. 140:1194-1197. [PubMed] [Google Scholar]
- 7.Domiati-Saad, R., J. F. Attrep, H. P. Brezinschek, A. H. Cherrie, D. R. Karp, and P. E. Lipsky. 1996. Staphylococcal enterotoxin D functions as a human B cell superantigen by rescuing VH4 expressing B cells from apoptosis. J. Immunol. 156:3608-3620. [PubMed] [Google Scholar]
- 8.Fearon, D. T., and W. W. Wong. 1983. Complement ligand-receptor interactions that mediate biological responses. Annu. Rev. Immunol. 1:243-271. [DOI] [PubMed] [Google Scholar]
- 9.Gallo, M. L., E. V. Jakobovits, and G. C. Davis. 2000. The human immunoglobulin loci introduced into mice: V (D) and J gene segment usage similar to that of adult humans. Eur. J. Immunol. 30:534-540. [DOI] [PubMed] [Google Scholar]
- 10.Genovese, A., J. P. Bouvet, G. Florio, B. Lamparter-Schummert, L. Bjorck, and G. Marone. 2000. Bacterial immunoglobulin superantigen proteins A and L activate human heart mast cells by interacting with immunoglobulin E. Infect. Immun. 68:5517-5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godau, J., T. Heller, H. Hawlisch, M. Trappe, E. Howells, J. Best, J. Zwirner, S. Verbeek, P. M. Hogarth, C. Gerard, N. van Rooijen, A. Klos, J. Gessner, and J. Kohn. 2004. C5a initiates the inflammatory cascade in immune complex peritonitis. J. Immunol. 173:3437-3445. [DOI] [PubMed] [Google Scholar]
- 12.Goodyear, C. S., M. Narita, and G. J. Silverman. 2004. In vivo VL-targeted activation-induced apoptotic supraclonal deletion by a microbial B cell toxin. J. Immunol. 1:2870-2877. [DOI] [PubMed] [Google Scholar]
- 13.Goodyear, C. S., and G. J. Silverman. 2004. Staphylococcal toxin induced preferential and prolonged in vivo deletion of innate-like B lymphocytes. Proc. Natl. Acad. Sci. USA 10:11392-11397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guthrie, T. H., Jr., and A. Oral. 1989. Immune thrombocytopenia purpura: a pilot study of staphylococcal protein A immunomodulation in refractory patients. Semin. Hematol. 26:3-9. [PubMed] [Google Scholar]
- 15.Heller, T., J. E. Gessner, R. E. Schmidt, A. Klos, W. Bautsch, and J. Köhl. 1999. Cutting edge: Fc receptor type I for IgG on macrophages and complement mediate the inflammatory response in immune complex peritonitis. J. Immunol. 162:5657-5661. [PubMed] [Google Scholar]
- 16.Jones, F. R., J. P. Balint, Jr., and H. W. Snyder, Jr. 1986. Selective extracorporeal removal of immunoglobulin G and circulating immune complexes: a review. Plasma Ther. Transfus. Technol. 7:333-349. [Google Scholar]
- 17.Kohl, J., and J. E. Gessner. 1999. On the role of complement and Fcγ receptors in the Arthus reaction. Mol. Immunol. 36:893-903. [DOI] [PubMed] [Google Scholar]
- 18.Kozlowski, L. M., W. Li., M. Goldschmidt, and A. I. Levinson. 1998. In vivo inflammation induced by a prototypic B cell superantigen. Elicitation of an Arthus reaction by staphylococcal protein A requires its immunoglobulin VH binding site. J. Immunol. 160:5246-5252. [PubMed] [Google Scholar]
- 19.Kozlowski, L. M., G. Silverman, J. D. Lambris, and A. I. Levinson. 1996. Complement activation by a B cell superantigen. J. Immunol. 157:1200-1206. [PubMed] [Google Scholar]
- 20.Levinson, A. I., L. Kozlowski, Y. Zheng, and L. M. Wheatley. 1995. B cell superantigens: definition and potential impact on the immune response. J. Clin. Immunol. 15:26S-36S. [DOI] [PubMed] [Google Scholar]
- 21.Levinson, A. I., and L. Kozlowski. 1996. Staphylococcal protein A: functional properties of a model B-cell superantigen, p. 99-106. In M. Zouali (ed.), Human B-cell superantigens. Landes Bioscience Publishers, Austin, Tex.
- 22.Marone, G., M. Tamburini, M. G. Giudizi, R. Biagiotti, F. Almerigogna, and S. Romagnani. 1987. Mechanism of activation of human basophils by Staphylococcus aureus Cowan I. Infect. Immun. 55:803-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mastellos, D., J. C. Papadimitriou, S. Franchini, P. A. Tsonis, and J. D. Lambris. 2001. A novel role of complement: mice deficient in the fifth component of complement exhibit impaired liver regeneration. J. Immunol. 166:2479-2486. [DOI] [PubMed] [Google Scholar]
- 24.Messerschmidt, G. L., D. H. Henry, H. W. Snyder, Jr., J. Bertram, A. Mittelman, S. Ainsworth, J. Fiore, M. V. Viola, J. Louie, E. Ambinder, F. R. MacKintosh, D. J. Higby, P. O'Brien, D. Kiprov, M. Hamberger, Jr., J. P. Balint, L. D. Fisher, W. Perkins, C. M. Pinsky, and F. R. Jones. 1989. Protein A immunotherapy in the treatment of cancer: an update. Semin. Hematol. 26:19-24. [PubMed] [Google Scholar]
- 25.Mittelman, A., J. Bertram, D. H. Henry, H. W. Snyder, Jr., G. L. Messerschmidt, D. Ciavarella, S. Ainsworth, D. Kiprov, and Z. Arlin. 1989. Treatment of patients with HIV thrombocytopenia and hemolytic uremic syndrome with protein A (Prosorba column) immunoadsorption. Semin. Hematol. 26:15-18. [PubMed] [Google Scholar]
- 26.Nakano, T., T. Sonoda, C. Hayashi, A. Yamatodani, Y. Kanayama, T. Yamamura, H. Asai, T. Yonezawa, Y. Kitamura, and S. J. Galli. 1985. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer to genetically mast cell-deficient W/Wv mice. J. Exp. Med. 162:1025-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patella, V., J.-P. Bouvet, and G. Marone. 1993. Protein Fv produced during viral hepatitis is a novel activator of human basophils and mast cells. J. Immunol. 151:5685-5698. [PubMed] [Google Scholar]
- 28.Patella, V., V. Casolaro, L. Björck, and G. Marone. 1990. Protein L: a bacterial Ig-binding protein that activates human basophils and mast cells. J. Immunol. 145:3054-3061. [PubMed] [Google Scholar]
- 29.Patella, V., G. Florio, A. Petraroli, and G. Marone. 2000. HIV-1 gp120 induces IL-4 and IL-13 release from human FcγRI+ cells through interaction with the VH3 region of IgE. J. Immunol. 164:589-595. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi, R., and B. A. Jakschik. 1988. The role of mast cells in thioglycollate-induced inflammation. J. Immunol. 141:2090-2096. [PubMed] [Google Scholar]
- 31.Ramos, B. F., R. Qureshi, K. M. Olsen, and B. A. Jakschik. 1990. The importance of mast cells for the neutrophil influx in immune complex-induced peritonitis in mice. J. Immunol. 145:1868-1873. [PubMed] [Google Scholar]
- 32.Ramos, B. F., Y. Zhang, R. Qureshi, and B. A. Jakschik. 1991. Mast cells are critical for the production of leukotrienes responsible for neutrophil recruitment in immune complex-induced peritonitis in mice. J. Immunol. 147:1636-1641. [PubMed] [Google Scholar]
- 33.Ravetch, J. V. 2002. A full complement of receptors in immune complex diseases. J. Clin. Investig. 110:1759-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Razin, E., R. L. Stevens, F. Akiyama, K. Schmid, and K. F. Austen. 1982. Culture from mouse bone marrow of a subclass of mast cells possessing a distinct chondroitin sulfate proteoglycan with glycosaminoglycans rich in N-acetylgalactosamine-4,6-disulfate. J. Biol. Chem. 257:7229-7236. [PubMed] [Google Scholar]
- 35.Sasso, E. H., G. J. Silverman, and M. Mannik. 1989. IgM molecules that bind staphylococcal protein A contain VHIII H chains. J. Immunol. 142:2778-2783. [PubMed] [Google Scholar]
- 36.Sasso, E. H., G. J. Silverman, and M. Mannik. 1991. Human IgA and IgG (F(ab′)2) that bind to staphylococcal protein A belong to the VHIII subgroup. J. Immunol. 147:1877-1883. [PubMed] [Google Scholar]
- 37.Seppala, I., M. Kaartinen, S. Ibrahim, and O. Makela. 1990. Mouse Ig coded by VH families S107 and J606 bind to protein A. J. Immunol. 145:2989-2993. [PubMed] [Google Scholar]
- 38.Silverman, G. J. 1997. B cell superantigens. Immunol. Today 18:379-386. [DOI] [PubMed] [Google Scholar]
- 39.Silverman, G. J., P. Roben, J.-P. Bouvet, and M. Sasano. 1995. Superantigen properties of a human sialoprotein involved in gut-associated immunity. J. Clin. Investig. 96:417-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strey, C. W., M. Markiewski, D. Mastellos, R. Tudoran, L. Greenbaum, and J. D. Lambris. 2003. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J. Exp. Med. 198:913-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viau, M., N. S. Longo, P. E. Lipsky, L. Bjorck, and M. Zouali. 2004. Specific in vivo deletion of B-cell subpopulations expressing human immunoglobulins by the B-cell superantigen protein L. Infect. Immun. 72:3515-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang, Y., B. F. Ramos, and B. A. Jakschik. 1992. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science 258:1957-1959. [DOI] [PubMed] [Google Scholar]