Abstract
Pseudomonas aeruginosa, a gram-negative, facultative pathogen, causes severe and often even lethal infections in immunocompromised patients, as well as cystic fibrosis patients. We show here that a variety of P. aeruginosa strains activate phospholipase A2 (PLA2), cultured epithelial cells, and fibroblasts, resulting in increased intracellular and extracellular arachidonic acid release. The use of different PLA2 inhibitors revealed that P. aeruginosa-induced arachidonic acid release is mediated by activation of cytosolic PLA2 (cPLA2), whereas iPLA2 or sPLA2 do not seem to be involved in the response to P. aeruginosa. Likewise, the cPLA2-specific inhibitors MAFP and AACOCF3 prevented apoptosis of cultured epithelial cells upon P. aeruginosa infection, whereas inhibitors specific for iPLA2 or sPLA2 were without effect. The physiological significance of these findings is indicated by an inhibition of apoptosis in tracheal epithelial cells upon in vivo infection with P. aeruginosa. The data indicate that arachidonic acid generation by activation of cPLA2 during P. aeruginosa infection plays an important role in the induction of host cell death.
Pseudomonas aeruginosa infections are a severe clinical problem, in particular in immunocompromised individuals and in patients with cystic fibrosis (CF) (53). CF patients are highly susceptible to P. aeruginosa bacteria, leading to chronic lung infection in the majority of these patients. This lung disease is characterized by persistent pulmonary inflammation that finally results in fibrosis and destruction of lung tissue and respiratory failure. At present, P. aeruginosa is the leading cause of mortality in CF patients. Infection of mammalian cells with P. aeruginosa results in the release of cytokines (11, 23, 45), internalization of the bacteria (20, 40, 41), and induction of apoptosis of the infected host cells (5, 19-21, 42). We sought here to identify novel mechanisms of P. aeruginosa-induced apoptosis. It was previously shown that mice unable to respond with host cell apoptosis to pulmonary infection with P. aeruginosa are highly sensitive to the infection, develop sepsis, and die (19). Further, it was demonstrated that P. aeruginosa-induced apoptosis is delayed in cells lacking functional cystic fibrosis conductance regulator (5), whose mutations cause cystic fibrosis. Grassmé et al. (19), Jendrossek et al. (25), and Cannon et al. (5) demonstrated an upregulation of the CD95-CD95-ligand system on the surface of infected cells that resulted in apoptosis of P. aeruginosa-infected cells by ligation of the endogenous CD95 receptor. Genetic deficiency of CD95 prevented P. aeruginosa-induced apoptosis (19, 25), suggesting a central role of the CD95-CD95-ligand system for P. aeruginosa-initiated cell death. Further, an involvement of mitochondria (in particular mitochondrial depolarization, synthesis of reactive oxygen intermediates, and release of cytochrome c), an activation of Jun N-terminal kinases (JNK), and a stimulation of caspases were demonstrated to be involved in the induction of apoptosis in mammalian cells upon infection with P. aeruginosa (19, 25, 26).
In the present study we tested a potential role of phospholipases A2 (PLA2) in P. aeruginosa-triggered cell death. PLA2 are lipolytic enzymes that hydrolyze membrane phospholipids and thereby release fatty acids, particularly arachidonic acid from the sn-2 position of glycerophospholipids (13). PLA2 plays an important role in signal transduction, in particular by generation of proinflammatory mediators as prostaglandins and leukotrienes and by membrane remodeling. Several subtypes of mammalian PLA2 have been described that are divided in four main groups according to their function, localization, and calcium dependency. Secretory PLA2 (low molecular mass enzymes belonging to groups I, II, III, V, and X) are cysteine-rich, secreted proteins that require millimolar concentrations of Ca2+ for activity without a preference for a specific fatty acid in the sn-2 position of the phospholipid substrate (13). The second class of PLA2 includes specific acetylhydrolases such as platelet-activating factor. A third class is composed of Ca2+-independent PLA2, e.g., iPLA2 isolated from myocardium (55), CHO cells, and macrophages (1, 28). The group IV cytosolic PLA2 (cPLA2) includes three PLA2 named α, β, and γ. cPLA2α, an 85-kDa protein, requires micromolar Ca2+ concentrations for activity and has a preference for arachidonic acid (8). The recently described cPLA2β, a 110-kDa protein, shows 30% sequence identity with cPLA2α and also depends on Ca2+ but is less selective for cleavage at the sn-2 position than cPLA2α (39, 49). cPLA2γ with a molecular mass of 61 kDa and 29% sequence identity with cPLA2α is Ca2+ independent but distinguishable from iPLA2 by its preference for arachidonic acid at the sn-2 position (3). Previous studies implied PLA2 in the host response to such diverse pathogens as Staphylococcus aureus, Escherichia coli, Aeromonas hydrophila, Pasteurella haemolytica, and Bacillus anthracis (6, 9, 16, 18, 24, 44, 54). PLA2 seem to have a dual function in the infection of mammalian cells by pathogens, since many bacterial toxins seem to kill mammalian cells via PLA2 (4, 12, 16, 18, 27), whereas expression of PLA2 was shown to protect mice from acute infections at least with S. aureus, P. aeruginosa, and E. coli (15, 33). Thus, the exact function of PLA2 in infectious processes requires definition.
In the present study, we investigated the role of PLA2 for the in vitro and in vivo infection of cultured epithelial cells or fibroblasts, respectively, as well as tracheal epithelial cells with P. aeruginosa. We demonstrate an activation of PLA2 by a variety of P. aeruginosa strains and reveal by the use of a panel of PLA2 inhibitors a significant role of cPLA2 for the induction of host cell apoptosis by P. aeruginosa both in vitro and in vivo.
MATERIALS AND METHODS
Materials and cell culture.
The human conjunctiva epithelial cell line Chang (ATCC CCL 20.2) was cultured in RPMI 1640 (Gibco-BRL/Life Technologies) supplemented with 5% fetal calf serum (FCS) at 37°C as monolayers in tissue culture flasks in 5% CO2 atmosphere. The human lung fibroblast cell line WI-38 was maintained in minimal essential medium supplemented with 10% FCS, 2 mM glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 1% nonessential amino acids, and 1% penicillin-streptomycin. Infections were performed in RPMI 1640 supplemented with 10 mM HEPES (pH 7.2) to avoid interactions of serum proteins with the bacteria. Prior to infections, FCS and antibiotics were omitted from the culture media. The PLA2 inhibitors MAFP, BEL, 12-Episcalaradial, and AACOCF3 were purchased from Biomol.
Bacterial strains.
Three clinical isolates and two laboratory strains of P. aeruginosa were used. The isolate 762 was originally obtained from an urinary tract infection, strain 696 was isolated from the sputum of a hospitalized patient, and strain 769 was from a patient with urosepsis (18). The laboratory strains used were P. aeruginosa ATCC 27853 and PAO-1. Furthermore, we infected cells with a previously described (26) P. aeruginosa strain deficient for the type III secretion system (P. aeruginosa PAK pcrD::Smr, kindly provided by J. Heesemann), S. aureus strain ATCC 8325, and a nonpathogenic E. coli isolate.
Infection experiments.
Bacteria originating from glycerol stock cultures were plated overnight on tryptic soy agar plates at 37°C, resuspended in tryptic soy broth (TSB) at an optical density at 550 nm of 0.25, shaken at 120 rpm for 1 h at 37°C, and harvested during logarithmic growth phase by pelleting and resuspension in fresh TSB. Prior to infection, cells were washed twice in RPMI 1640 (Chang cells) or minimal essential medium (WI-38 cells) and maintained in the same medium during infection. Infection was performed by inoculating subconfluent cell layers at a host cell/bacterium ratio of 1:1,000, 1:100, 1:50, or 1:10. Synchronous infection conditions and an enhanced bacterium-host cell interaction were achieved by a 2-min centrifugation (35 × g) of the bacteria onto the cells. The end of the centrifugation step was defined as the starting point of all infections.
Arachidonic acid release assays.
Chang cells (0.4 × 105 per well) or WI-38 cells (0.3 × 105 per well) were labeled for 18 to 20 h with 0.05 μCi of [3H]arachidonic acid [5,6,8,9,11,12,14,15-3H(N)] (0.1 mCi/ml stock; New England Nuclear)/ml. Prior to infection, cells ware washed three times with a buffer containing 132 mM NaCl, 20 mM HEPES, 5 mM KCl, 1 mM CaCl2, 0.7 mM MgCl2, and 0.8 mM MgSO4 (H/S) supplemented with 1% bovine serum albumin and 10 mM glucose and incubated in the same buffer during infection. Infection was performed as described above at the indicated multiplicity of infection (MOI).
Cells were preincubated with the PLA2 inhibitors MAFP, 12-episcalaradial, and bromoenol lactone (BEL) at the indicated concentrations for 15 min prior to infection. The slow-acting inhibitor AACOCF3 was added to the cells 3 h prior to infection.
Extracellular arachidonic acid release.
To determine the release of arachidonic acid into the extracellular space, supernatants were collected after the indicated time of infection and centrifuged at 2,000 rpm for 5 min in a microcentrifuge to remove any cells that may have detached. The supernatants were quantified by liquid scintillation counting. The remaining cell-bound radioactivity was determined by adding 0.5 ml of 0.1% Triton X-100 in H2O to the cells, and lysed cells were removed from the plates and subjected to liquid scintillation counting. Extracellular arachidonic acid release was calculated as the percentage of radioactivity released into the supernatant of the total radioactivity (the latter being the sum of released and cell-bound radioactivity). Since only free arachidonic acid is released into the supernatant, the measurement of radioactivity in the supernatant is an accurate measurement for the formation of extracellular arachidonic acid.
Intracellular arachidonic acid release.
Chang cells were seeded at a density of 3 × 105 cells/well in six-well plates. Labeling with [3H]arachidonic acid, pretreatment with PLA2 inhibitors, and infection were performed as described above. Infections were terminated by removal of the supernatants, washing, and the addition of 1 ml of methanol-15% acetic acid to each well. Cells were scraped off the plates, and wells were washed with 0.5 ml of methanol. Chloroform (0.75 ml) was added, and samples were extracted by incubation for 30 min at room temperature. Next, 0.75 ml each of chloroform and H2O were added, and the phases were separated by low-speed centrifugation for 10 min. The lower phase containing the fatty acids was removed, dried, solubilized in 30 μl of petrol ether-diethyl ether (96:1), spotted onto a silica gel 60 plate, and separated by thin-layer chromatography (TLC) in a solvent system consisting of ethyl acetate-glacial acetic acid-2,2,4-trimethyl pentane-H2O (45:10:25:50, by volume). An arachidonic acid standard was run on each plate to confirm migration of free arachidonic acid. After separation, TLC plates were air dried and a Biomax MS film was exposed at −80°C for several days. To quantify free arachidonic acid, spots representing arachidonic acid were scraped off the plates and subjected to liquid scintillation counting. The TLC analysis permits separation of free arachidonic acid from arachidonic acid incorporated into lipids and, therefore, the analysis of free intracellular arachidonic acid.
Detection and quantification of apoptosis.
Apoptosis was quantified by fluorescein isothiocyanate (FITC)-annexin V staining and confirmed by trypan blue staining. To this end, cells were infected as described above, treated with trypsin, washed twice in 10 mM HEPES (pH 7.4), 140 mM NaCl2, and 5 mM CaCl2 and resuspended in the same buffer supplemented with FITC-annexin V (dilution 1:50; Roche Biochemicals, Mannheim, Germany). After incubation for 15 min cells were subjected to fluorescence-activated cell sorter (FACS) analysis using a FACSCalibur flow cytometer using the CELLQuest software (Becton Dickinson, Mountain View, Calif.).
In vivo infections.
To perform infection of tracheal epithelial cells in vivo, C57BL/6 wild-type mice were anesthetized by intraperitoneal injection of avertin, the trachea was carefully isolated, and the distal part was catheterized and ligated immediately proximal of the catheter. We then injected MAFP, AACOCF3, or buffer into the proximal part of the trachea. After an incubation period of 15 min for MAFP, we injected 108 CFU of P. aeruginosa 762 into the proximal trachea. Mice were killed after 45 min, and the tracheas were rapidly excised and fixed for 30 min in 2% paraformaldehyde (PFA) in phosphate-buffered saline (PBS; pH 7.2). Epithelial cells were isolated by disintegration of the trachea by scratching with a 27-gauge needle and a 5-min digestion in trypsin. Cells were washed and stained with FITC-annexin V to detect apoptosis. Apoptosis of epithelial cells in the trachea was also determined by TUNEL staining. To this end, the trachea was excised and fixed in 4% PFA in PBS (pH 7.2) for 36 h. The tissues were embedded in paraffin, and sections were deparaffinized and treated for 5 min in 0.1 M citrate (pH 6.0) with 350-W microwave irradiation for 5 min. The sections were then stained for 30 min at 37°C with FITC-coupled dUTP in the presence of terminal deoxynucleotidyl transferase. The samples were washed, incubated at 70°C for 10 min to reduce unspecific binding, and stained with alkaline phosphatase-coupled anti-FITC-antibodies. The slides were finally developed using Fast Red Tablets (Roche) as a substrate resulting in the formation of a red dye.
RESULTS
P. aeruginosa induces extra- and intracellular arachidonic acid release upon infection of epithelial cells.
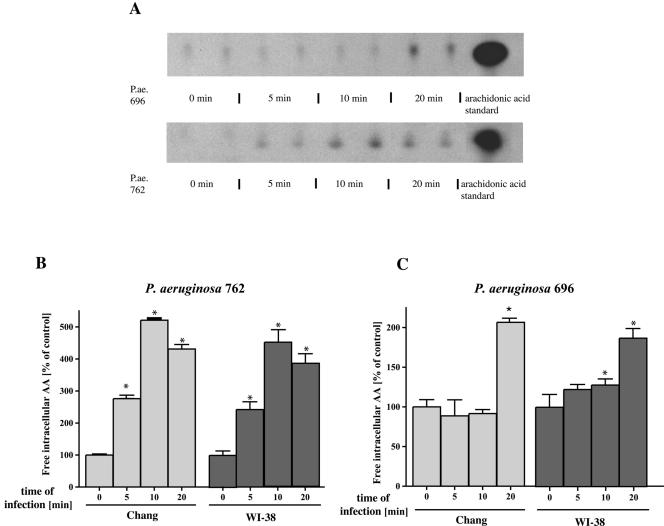
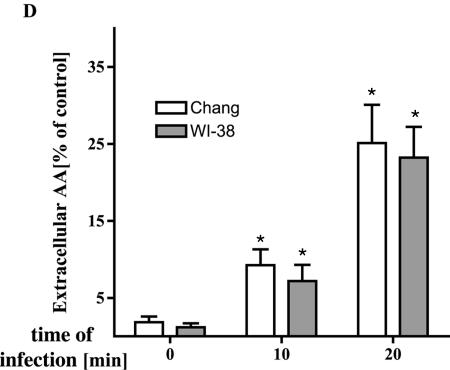
To gain insight into the interactions of P. aeruginosa with epithelial cells, we investigated the role of PLA2 upon bacterial infection. To this end, we measured P. aeruginosa-induced PLA2 activity by quantification of the extracellular and intracellular arachidonic acid release from two different cell lines, i.e., Chang conjunctiva epithelial cells and WI-38, a lung fibroblast cell line. Intracellular arachidonic acid release was determined by prelabeling cells with [3H]arachidonic acid, extraction of fatty acids, and subsequent separation by TLC. Extracellular arachidonic acid release was determined by labeling cells with [3H]arachidonic acid and quantification of released radioactivity in the supernatant by liquid scintillation counting. The latter data were related to the total radioactivity recovered from the samples. The results revealed a marked increase of free, intra- and extracellular arachidonic acid after bacterial infection with the P. aeruginosa strains 696, 762, 769, ATCC 27853, and PAO-1 (Fig. 1 and Fig. 2).
FIG. 1.
P. aeruginosa induces intra- and extracellular arachidonic acid release. (A) P. aeruginosa (P.ae.) 762 or 696 infection induces rapid intracellular arachidonic acid (AA) release as shown by extraction of free fatty acids, followed by TLC separation of [3H]arachidonic acid-labeled Chang conjunctiva epithelial cells. Cells were infected 5, 10, and 20 min with the bacteria or left uninfected and then harvested, and intracellular arachidonic acid was quantified as described above. P. aeruginosa induced a rapid increase in intracellular arachidonic acid release with a maximum at 10 min for P. aeruginosa strain 762 and at 20 min for P. aeruginosa strain 696. The data were determined in duplicate and are representative for three independent experiments. (B and C) Quantification of intracellular arachidonic acid release was performed by removing the arachidonic acid spots from the TLC plates, followed by liquid scintillation counting. The data refer to the percentages of untreated control and represent means ± the standard deviations (SD) of three independent experiments. (D) Release of extracellular arachidonic acid release 0, 10, and 20 min after P. aeruginosa 762 infection of Chang epithelial cells and WI-38 fibroblasts. Cells were labeled with [3H]arachidonic acid overnight and infected, and the radioactivity released into the supernatant was quantified by liquid scintillation counting. The data are normalized to total incorporated arachidonic acid and represent means ± the SD of at least four independent experiments. ✽, Significant differences compared to the control (P < 0.05, t test for unpaired samples).
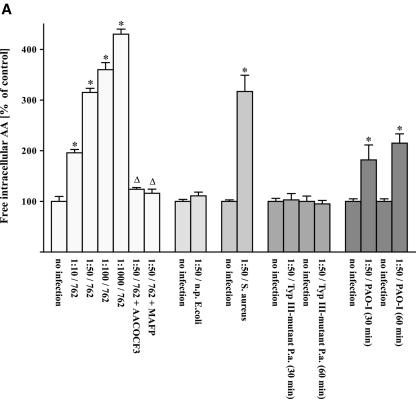
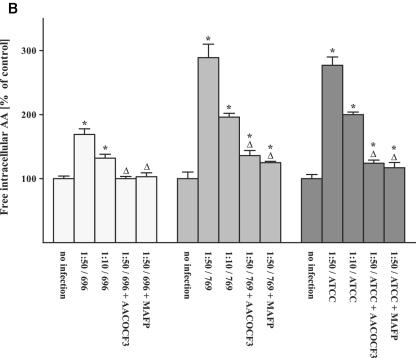
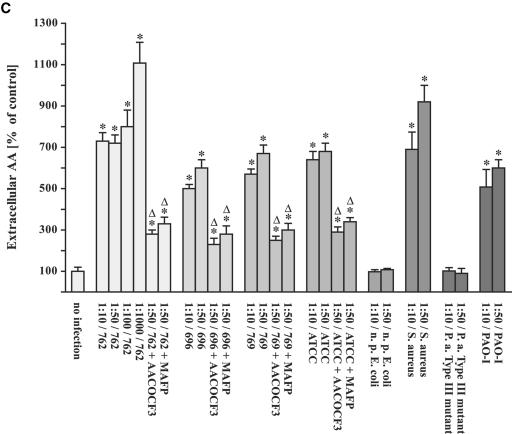
FIG. 2.
Infection of human cells with different P. aeruginosa strains results in arachidonic release prevented by cPLA2 inhibitors. Chang cells were prelabeled with [3H]arachidonic acid and infected with P. aeruginosa 762 for 20 min, P. aeruginosa 769 or ATCC 27853 for 45 min, and P. aeruginosa 696 or PAO-1 or a type III-deficient P. aeruginosa mutant for 60 min, respectively. In addition, the cells were infected with a nonpathogenic E. coli strain for 120 min or S. aureus strain 8325 for 60 min. Cells were infected at the indicated MOIs, and the release of intracellular (A and B) and extracellular (C) arachidonic acid was determined. The effect of the cPLA2 inhibitors MAFP or AACOCF3 was determined for each P. aeruginosa strain at an MOI of one cell to 50 bacteria. Displayed are the means ± the SD of each of three independent experiments. ✽, Significant differences between infected samples and noninfected controls and significant differences between infected cells treated with inhibitor or left untreated, respectively, are labeled with a delta (P < 0.05, t test for unpaired samples).
The release of arachidonic acid occurred very rapidly after infection with P. aeruginosa (Fig. 1) and was dependent on the MOI used in the infection experiments (Fig. 2). However, even at the lowest MOI of 10 bacteria/cell, we were still able to detect some release of arachidonic acid (Fig. 2). A type III mutant of P. aeruginosa did not induce a release of intra- or extracellular arachidonic acid (Fig. 2A).
The release of arachidonic acid was not restricted to P. aeruginosa and was also observed upon infection with a different pathogen, i.e., S. aureus (Fig. 2A). In contrast, nonpathogenic E. coli were unable to trigger a significant release of arachidonic acid (Fig. 2A).
Arachidonic acid release induced by P. aeruginosa infection is diminished by cPLA2 inhibitors but not by sPLA2 or iPLA2 inhibitors.
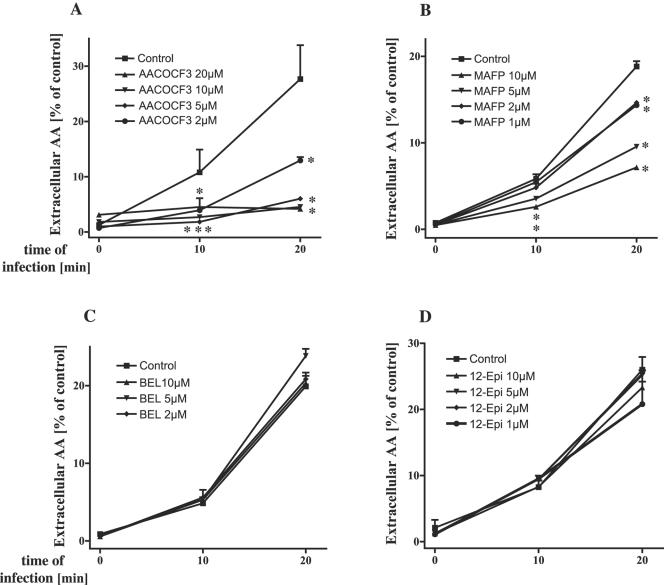
Next, we aimed to further characterize the PLA2 subclass involved in P. aeruginosa-induced arachidonic acid release. To this end, we investigated the influence of various PLA2 inhibitors on arachidonic acid release into the supernatant and on cell-bound arachidonic acid release. Two inhibitors (MAFP, AACOCF3) specific for both cPLA2 and iPLA2 were used, as well as the sPLA2-specific inhibitor 12-Episcalaradial and the iPLA2-specific inhibitor BEL. The results reveal that MAFP and AACOCF3 significantly reduced intra- and extracellular arachidonic acid release upon infection with P. aeruginosa strains 696, 762, 769, and ATCC 27853 (Fig. 2). A dose-response analysis revealed that doses of 1 μM MAFP or 2 μM AACOCF3, respectively, were already sufficient to reduce arachidonic acid release (Fig. 3A and B). In contrast, neither the iPLA2 inhibitor BEL nor the sPLA2 inhibitor 12-episcalaradial affected P. aeruginosa-induced arachidonic acid release upon infection of WI-38 or Chang cells (Fig. 3C and D).
FIG. 3.
Influence of various PLA2 inhibitors on P. aeruginosa-induced arachidonic acid release. Preincubation of WI-38 lung fibroblasts with cPLA2 inhibitors AACOCF3 (A) and MAFP (B) prevents the release of extracellular arachidonic acid in a dose-dependent way, whereas the iPLA2 inhibitor BEL (C) or sPLA2 inhibitor 12-Episcalaradial (D) was without effect. The data are means ± the SD of duplicate samples and representative of three experiments. Significant differences between inhibitor-treated and -untreated values are indicated by asterisks (P < 0.05, t test for unpaired samples).
Since MAFP specifically blocks cPLA2 and iPLA2, whereas BEL specifically targets iPLA2 and 12-Episcalaradial inhibits type II sPLA2, the data suggest that cPLA2 is activated by P. aeruginosa.
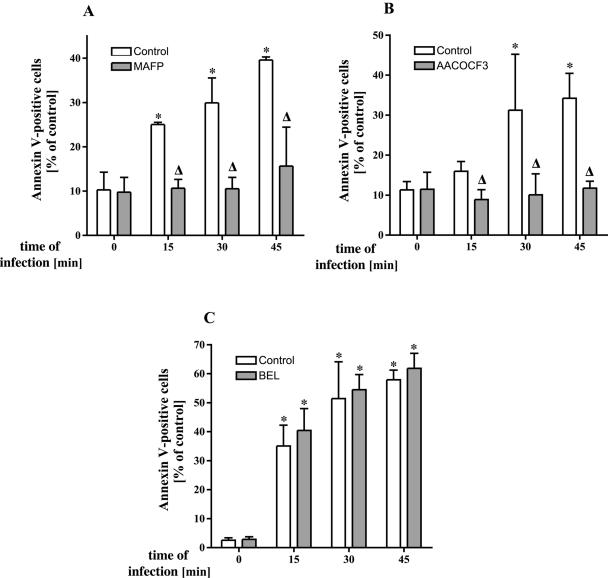
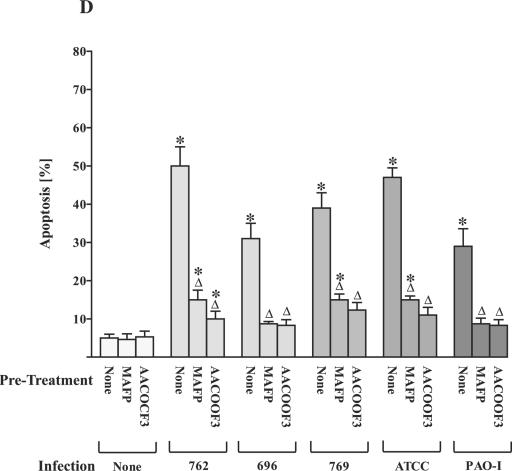
The cPLA2 inhibitors MAFP and AACOCF3 reduce P. aeruginosa-induced apoptosis.
To gain insight into the functional significance of arachidonic acid release, we investigated the role of PLA2 for the induction of apoptosis during P. aeruginosa infection. P. aeruginosa-induced apoptosis of epithelial cells has been previously demonstrated (5, 19, 20) and seems to be critical for the defense of the host against acute infections with these bacteria (19). First signs of apoptosis as determined by breakdown of phosphatidylserine asymmetry in the cell membrane were detected within 15 to 30 min after infection with P. aeruginosa 762. MAFP and AACOCF3 dose dependently inhibited P. aeruginosa-induced apoptosis (Fig. 4A and B), whereas pretreatment with 12-Episcalaradial (sPLA2 inhibitor) (Fig. 4C) was without effect on apoptosis induction. Inhibition of apoptosis by MAFP and AACOCF3, respectively, was not restricted to P. aeruginosa strain 762 and also observed for the P. aeruginosa strains 696, 769, ATCC 27853, and PAO-1 (Fig. 4D), as well as the S. aureus strain ATCC 8325 (not shown).
FIG. 4.
cPLA2 and iPLA2 inhibitors prevent P. aeruginosa-induced apoptosis. P. aeruingosa strain 762-induced apoptosis is abrogated by preincubation of Chang epithelial cells with the cPLA2 inhibitors MAFP (A) or AACOCF3 (B) as determined by FITC-annexin staining, but not with the sPLA2-specific inhibitor 12-Episcalaradial (C). Cells were infected with P. aeruginosa 762 for the indicated times, stained with FITC-labeled annexin V, and subjected to flow cytometry analysis. (D) Inhibition of PLA2 with MAFP or AACOCF3 also prevents apoptosis triggered by other P. aeruginosa strains 696, 769, ATCC 27853, and PAO-1. Cells were infected at an MOI of 1:100 with the strains 696 and PAO-1 for 6 h, with 769 and ATCC 27853 for 90 min. Apoptosis was quantified by FITC-annexin V binding and FACS analysis. Displayed are means ± the SD of two to four independent experiments. Significant differences between infected samples and noninfected controls are indicated by asterisks, and significant differences between infected cells treated with inhibitor or left untreated are indicated by a delta (P < 0.05, t test for unpaired samples).
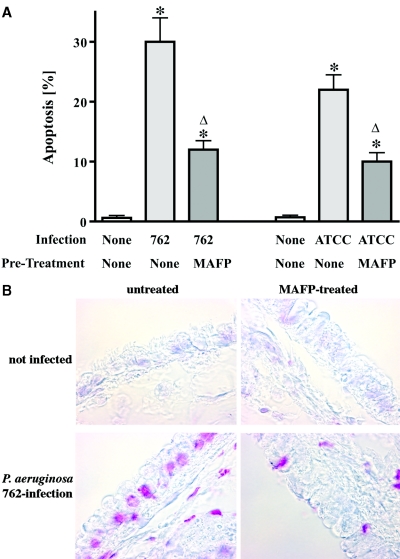
To test the in vivo role of PLA2 activation for P. aeruginosa-induced apoptosis, we used an in vivo infection model. To this end, we applied the PLA2 inhibitor MAFP into the trachea in vivo, followed by local infection with P. aeruginosa strains 762 or ATCC 27853. The results reveal an induction of apoptosis in respiratory epithelial cells in vivo upon infection with P. aeruginosa, which was prevented by inhibition of cPLA2 (Fig. 5A and B).
FIG. 5.
Inhibition of PLA2 in vivo prevents apoptosis of tracheal epithelial cells after P. aeruginosa infection. The PLA2 inhibitor MAFP was locally applied into the trachea in vivo. The trachea was infected with P. aeruginosa strain 762 or ATCC 27853, respectively, 15 min after application of the inhibitor. Apoptosis was determined by TUNEL and quantified by counting TUNEL-positive cells. Apoptosis was confirmed by staining with FITC-annexin V after disintegration of the trachea. (A and B) Shown are the means ± the SD of three independent experiments (A) and a typical result of the TUNEL staining (B). Asterisks indicate significant differences between control and infected tracheae, and significant differences between infected samples treated with MAFP or left untreated are indicated by a delta (P < 0.05, t test for unpaired samples).
DISCUSSION
In the present study we investigated the role of PLA2 in P. aeruginosa infection and its significance in P. aeruginosa-induced epithelial cell death. We show that several P. aeruginosa strains, i.e., the clinical isolates P. aeruginosa 762, 696, and 769, as well as the laboratory strains P. aeruginosa ATCC 27853 and PAO-1, activate cPLA2, release arachidonic acid, and employ this pathway to induce apoptosis of host cells. The use of various subtype-specific inhibitors indicates an activation of cPLA2 by P. aeruginosa-mediating the release of arachidonic acid. Our data further show that cPLA2 activation plays a significant role in P. aeruginosa apoptosis in vitro as well as in vivo, since cPLA2 inhibitors reduce P. aeruginosa-induced cell death. A P. aeruginosa mutant that lacked the type III secretion system failed to stimulate PLA2 and to induce apoptosis. This is consistent with previous findings that demonstrated the failure of a P. aeruginosa type III mutant to induce significant apoptosis of epithelial cells (26).
At present the signals mediating the activation of cPLA2 upon infection with P. aeruginosa are unknown. We have previously shown that P. aeruginosa triggers an activation of the acid sphingomyelinase and the formation of ceramide (19). Ceramide forms membrane platforms that serve to reorganize receptor molecules involved in internalization of the bacteria, as well as the induction of apoptosis (19). However, it is possible that ceramide does not only triggers the reorganization of receptor molecules in ceramide-enriched membrane domains upon infection but also mediates a stimulation of PLA2 (22, 31). In this context it is interesting that ceramide has been shown to bind to the CaLB domain of PLA2 and facilitates membrane docking of and lipid hydrolysis by PLA2 (22, 31). Thus, ceramide might be involved in the spatial reorganization of PLA2 required for the function of this enzyme. In addition, a recent study demonstrated an involvement of ceramide-kinase in the activation of PLA2 by ceramide (36, 37). Since ceramide-1-phosphate was shown to be a direct activator of PLA2 (36, 37), the metabolism of ceramide to ceramide-1-phosphate after infection with P. aeruginosa may also contribute to PLA2 activation. Further, we have previously shown that infection of human and murine epithelial cells and fibroblasts with P. aeruginosa results in the induction of apoptosis by upregulation of the endogenous CD95 receptor/CD95 ligand system (5, 19). The critical role of the CD95/CD95 ligand system for induction of apoptosis was demonstrated by the use of CD95- and CD95-ligand-deficient cells. These cells were resistant to P. aeruginosa-induced cell death (19, 25). Recent studies from Cannon et al. confirmed the CD95-dependent induction of apoptosis in mammalian cells by P. aeruginosa (5). CD95 might also mediate the activation of PLA2 upon infection with P. aeruginosa, although the activation of PLA2 by CD95 and the role of this enzyme for CD95-triggered death seem to be controversial. For instance, studies of Ulisse et al. (51) and Cifone et al. (7) demonstrated an activation of PLA2 by CD95 in Sertoli and HuT78 cells that was mediated by a signaling pathway involving extracellular regulated kinases. Activation of PLA2 activity was required, at least in part, for the induction of CD95-induced cell death in these cells. On the other hand, it was shown by Luschen et al. (32), Enari et al. (17), de Valck et al. (14), and Atsumi et al. (2) that an activation of PLA2 is not required for CD95-triggered apoptosis and cPLA2 seems to be even cleaved by proteolysis after CD95 stimulation.
Next, it is also possible that P. aeruginosa triggers an activation of PLA2 independent of the acid sphingomyelinase, ceramide, and the CD95 receptor/CD95 ligand system. In this respect, it is interesting that activation of PLA2 by P. aeruginosa required a functional type III secretion system. Although the exact nature of proapoptotic P. aeruginosa factors that are delivered into infected cells and mediate apoptosis is unknown, it might be possible that bacterial factors directly or indirectly activate PLA2.
Finally, P. aeruginosa expresses enzymes that display PLA2 activity and the observed PLA2 activity could be caused by an activation of a bacterial PLA2 (43, 44). In particular, it was shown that P. aeruginosa exotoxin U exhibits a PLA2 activity that is activated by still unknown mammalian cell factors (38, 49). Moreover, the PLA2 activity of P. aeruginosa exotoxin U was inhibited by the cPLA2 inhibitor MAFP and, accordingly, MAFP also prevented exotoxin U-induced cell death (38). However, we used doses of MAFP of 10 μM, while inhibition of P. aeruginosa exotoxin U by MAFP required concentrations of the inhibitor up to 1.35 mM and prolonged incubation of the enzyme with the inhibitor up to 16 h (38). Therefore, it seems to be unlikely that the observed effects of MAFP are due to an inhibition of the bacterial protein, and our data strongly suggest that a cellular PLA2 is activated by P. aeruginosa to mediate the release of arachidonic acid.
P. aeruginosa-induced PLA2 might be involved in apoptosis by the activation of several proapoptotic pathways. It was demonstrated that arachidonic acid, but not products of the lipoxygenase and the cyclooxygenase pathway, induces a depolarization of mitochondria, a typical event of mitochondrial alterations during apoptosis (29, 30, 34, 35, 46, 50, 52). Furthermore, it was suggested that PLA2 induces the formation of proapoptotic reactive oxygen intermediates and an activation of sphingomyelinases with a concomitant release of ceramide and induction of apoptosis (10, 56). Finally, a recent study demonstrated a change of the nuclear architecture by PLA2 in the course of apoptosis (47). However, the molecular details of arachidonic acid-induced apoptosis are still unknown.
The present data suggest PLA2 and arachidonic acid as novel components of the death machinery in the host cell and the pulmonary host response upon P. aeruginosa infection. Our studies show that P. aeruginosa infection activates cPLA2, resulting in arachidonic acid release. Pharmacological inhibition of PLA2 prevents P. aeruginosa-induced apoptosis, suggesting an important role of PLA2 in the biological response of the lung to infections with P. aeruginosa.
Acknowledgments
The study was supported by DFG grant Gu 335-10/3 and MO 435/17-3, a BMBF program, and the MANAD program to E.G.
Editor: V. J. DiRita
REFERENCES
- 1.Ackermann, E. J., E. S. Kempner, and E. A. Dennis. 1994. Ca2+-independent cytosolic phospholipase A from macrophage-like P388D1 cells: isolation and characterization. J. Biol. Chem. 269:9227-9233. [PubMed] [Google Scholar]
- 2.Atsumi, G., M. Tajima, A. Hadano, Y. Nakatani, M. Murakami, and I. Kudo. 1998. Fas-induced arachidonic acid release is mediated by Ca2+-independent phospholipase A2 but not cytosolic phospholipase A2, which undergoes proteolytic inactivation. J. Biol. Chem. 273:13870-13877. [DOI] [PubMed] [Google Scholar]
- 3.Balsinde, J. M., P. Balboa, A. Insel, and E. A. Dennis. 1999. Regulation and inhibition of phospholipase A2. Annu. Rev. Pharmacol. Toxicol. 39:175-189. [DOI] [PubMed] [Google Scholar]
- 4.Bernheimer, A. W., and B. Rudy. 1986. Interactions between membranes and cytolytic peptides. Biochim. Biophys. Acta 864:123-141. [DOI] [PubMed] [Google Scholar]
- 5.Cannon, C. L., M. P. Kowalski, K. S. Stopak, and G. B. Pier. 2003. Pseudomonas aeruginosa-induced apoptosis is defective in respiratory epithelial cells expressing mutant cystic fibrosis transmembrane conductance regulator. Am. J. Respir. Cell Mol. Biol. 29:188-197. [DOI] [PubMed] [Google Scholar]
- 6.Chopra, A. K., X. Xu, D. Ribardo, M. Gonzalez, K. Kuhl, J. W. Peterson, and C. W. Houston. 2000. The cytotoxic enterotoxin of Aeromonas hydrophila induces proinflammatory cytokine production and activates arachidonic acid metabolism in macrophages. Infect. Immun. 68:2808-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cifone, M. G., P. Roncaioli, R. De Maria, G. Camarda, A. Santoni, G. Ruberti, and R. Testi. 1995. Multiple pathways originate at the Fas/APO-1 (CD95) receptor: sequential involvement of phosphatidylcholine-specific phospholipase C and acidic sphingomyelinase in the propagation of the apoptotic signal. EMBO J. 14:5859-5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark, J. D., L.-L. Lin, R. W. Kriz, C. S. Ramesha, L. A. Sultzman, A. Y. Lin, N. Milona, and J. L. Knopf. 1991. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell 65:1043-1051. [DOI] [PubMed] [Google Scholar]
- 9.Conde, G., P. Garcia-Barreno, A. M. Muicico, and A. Suarez. 1981. In vitro and in vivo effect of Escherichia coli endotoxin on mitochondrial phospholipase A2 activity. FEBS Lett. 127:115-120. [DOI] [PubMed] [Google Scholar]
- 10.Cummings, B. S., J. Mchowat, and R. G. Schnellmann. 2000. Phospholipase A2s in cell injury and death. J. Pharmacol. Exp. Ther. 294:793-799. [PubMed] [Google Scholar]
- 11.Cusumano, V., M. A. Tufano, G. Mancuso, M. Carbone, F. Rossano, M. T. Fera, F. A. Ciliberti, E. Ruocco, R. A. Merendino, and G. Teti. 1997. Porins of Pseudomonas aeruginosa induce release of tumor necrosis factor alpha and interleukin-6 by human leukocytes. Infect. Immun. 65:1683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel, L. W., L. King, and M. Kennedy. 1988. Phospholipase activity of bacterial toxins. Methods Enzymol. 165:298-301. [DOI] [PubMed] [Google Scholar]
- 13.Dennis, E. A. 1997. The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem. Sci. 22:1-2. [DOI] [PubMed] [Google Scholar]
- 14.De Valck, D., D. Vercammen, W. Fiers, and R. Beyaert. 1998. Differential activation of phospholipases during necrosis or apoptosis: a comparative study using tumor necrosis factor and anti-Fas antibodies. J. Cell. Biochem. 71:392-399. [PubMed] [Google Scholar]
- 15.Dubouix, A., C. Campanac, J. Fauvel, M.-F. Simon, J.-P. Salles, C. Roques, H. Chap, and N. Marty. 2003. Bactericidal properties of group IIa secreted phospholipase A2 against Pseudomonas aeruginosa clinical isolates. J. Med. Microbiol. 52:1039-1045. [DOI] [PubMed] [Google Scholar]
- 16.Durkin, J. P., and W. T. Shier. 1981. Staphylococcal delta toxin stimulates endogenous phospholipase A2 activity and prostaglandin synthesis in fibroblasts. Biochim. Biophys. Acta 663:467-479. [DOI] [PubMed] [Google Scholar]
- 17.Enari, M., H. Hug, M. Hayakawa, F. Ito, Y. Nishimura, and S. Nagata. 1996. Different apoptotic pathways mediated by Fas and the tumor-necrosis-factor receptor: cytosolic phospholipase A2 is not involved in Fas-mediated apoptosis. Eur. J. Biochem. 236:533-538. [DOI] [PubMed] [Google Scholar]
- 18.Fink, D., M. L. Contreras, P. I. Lelkes, and P. Lazarovici. 1989. Staphylococcal aureus alpha-toxin activates phospholipases and indices a Ca2+ influx in PC12 cells. Cell. Signal. 1:387-393. [DOI] [PubMed] [Google Scholar]
- 19.Grassmé, H., S. Kirschnek, J. Riethmueller, A. Riehle, G. von Kuerthy, F. Lang, M. Weller, and E. Gulbins. 2000. Host defense to Pseudomonas aeruginosa requires CD95/CD95 ligand interactions on epithelial cells. Science 290:527-530. [DOI] [PubMed] [Google Scholar]
- 20.Grassmé, H., V. Jendrossek, A. Riehle, G. von Kürthy, J. Berger, H. Schwarz, M. Weller, R. Kolesnick, and E. Gulbins. 2003. Host defense against P. aeruginosa requires ceramide-rich membrane rafts. Nat. Med. 9:322-330. [DOI] [PubMed] [Google Scholar]
- 21.Hauser, A. R., and L. N. Engel. 1999. Pseudomonas aeruginosa induces type-III-secretion-mediated apoptosis of macrophages and epithelial cells. Infect. Immun. 67:5530-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huwiler, A., B. Johansen, A. Skarstad, and J. Pfeilschifter. 2001. Ceramide binds to the CaLB domain of cytosolic phospholipase A2 and facilitates its membrane docking and arachidonic acid release. FASEB J. 15:7-9. [DOI] [PubMed] [Google Scholar]
- 23.Inoue, H., P. P. Massion, I. F. Ueki, K. M. Grattan, M. Hara, A. F. Dohrman, B. Chan, J. A. Lausier, J. A. Golden, and J. A. Nadel. 1994. Pseudomonas stimulates interleukin-8 mRNA expression selectively in airway epithelium, in gland ducts, and in recruited neutrophils. Am. J. Respir. Cell. Mol. Biol. 11:651-663. [DOI] [PubMed] [Google Scholar]
- 24.Jan, J., B. Chen, S. Ma, C. Liu, H. Tsai, H. Wu, S. Jiang, K. Yang, and M. Shaio. 2000. Potential Dengue virus-triggered apoptotic pathway in human neuroblastoma cells: arachidonic acid, superoxide anion, and NF-κB are sequentially involved. J. Virol. 74:8680-8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jendrossek, V., H. Grassme, I. Mueller, F. Lang, and E. Gulbins. 2001. Pseudomonas aeruginosa-induced apoptosis involves mitochondria and stress-activated protein kinases. Infect. Immun. 69:2675-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jendrossek, V., S. Fillon, C. Belka, I. Muller, B. Puttkammer, and F. Lang. 2003. Apoptotic response of Chang cells to infection with Pseudomonas aeruginosa strains PAK and PAO-I: molecular ordering of the apoptosis signaling cascade and role of type IV pili. Infect. Immun. 71:2665-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeyaseelan, S., M. S. Kannan, S. L. Hsuan, A. K. Singh, T. F. Walseth, and S. K. Maheswaran. 2001. Pasteurella (Mannheimia) haemolytica leukotoxin-induced cytolysis of bovine leukocytes: role of arachidonic acid and its regulation. Microb. Pathog. 30:59-69. [DOI] [PubMed] [Google Scholar]
- 28.Jones, S. S., J. Tang, R. Kriz, M. Shaffer, J. Knopf, and J. Seehra. 1996. Isolation, molecular cloning and expression of a novel calcium-independent phospholipase A2. FASEB J. 10:A977. [Google Scholar]
- 29.Katz, E., M. R. Deehan, S. Seatter, C. Lord, R. D. Sturrock, and M. M. Harnett. 2001. B-cell receptor-stimulated mitochondrial phospholipase A2 activation and resultant disruption of mitochondrial membrane potential correlate with the induction of apoptosis in WEHI-231 B cells. J. Immunol. 166:137-147. [DOI] [PubMed] [Google Scholar]
- 30.Katz, E., C. Lord, C. A. Ford, S. B. Gauld, N. A. Carter, and M. M. Harnett. 2004. Bcl-(xL) antagonism of BCR-coupled mitochondrial phospholipase A2 signaling correlates with protection from apoptosis in WEHI-231 B cells. Blood 103:168-176. [DOI] [PubMed] [Google Scholar]
- 31.Koumanov, K. S., A. B. Momchilova, P. J. Quinn, and C. Wolf. 2002. Ceramides increase the activity of the secretory phospholipase A2 and alter its fatty acid specificity. Biochem. J. 363:45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luschen, S., S. Ussat, M. Kronke, and S. Adam-Klages. 1998. Cleavage of human cytosolic phospholipase A2 by caspase-1 (ICE) and caspase-8 (FLICE). Biochem. Biophys. Res. Commun. 253:92-98. [DOI] [PubMed] [Google Scholar]
- 33.Laine, V. J. O., D. S. Grass, and T. J. Nevalainen. 2000. Resistance of transgenic mice expressing human group II phospholipase A2 to Escherichia coli infection. Infect. Immun. 68:87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishi, E., and Y. Watanabe. 1997. The regulation of mitogenesis and apoptosis in response to the persistent stimulation of alpha1-adrenoceptors: a possible role of 15-lipoxygenase. Br. J. Pharmacol. 122:1516-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penzo, D., V. Petronilli, A. Angelin, C. Cusan, R. Colonna, L. Scorrano, F. Pagano, M. Prato, F. Di Lisa, and P. Bernardi. 2004. Arachidonic acid released by phospholipase A2 activation triggers Ca2+-dependent apoptosis through the mitochondrial pathway. J. Biol. Chem. 279:25219-25225. [DOI] [PubMed] [Google Scholar]
- 36.Pettus, B. J., A. Bielawska, S. Spiegel, P. Roddy, Y. A. Hannun, and C. E. Chalfant. 2003. Ceramide kinase mediates cytokine- and calcium ionophore-induced arachidonic acid release. J. Biol. Chem. 278:38206-38213. [DOI] [PubMed] [Google Scholar]
- 37.Pettus, B. J., A. Bielawska, P. Subramanian, D. S. Wijesinghe, M. Maceyka, C. C. Leslie, J. H. Evans, J. Freiberg, P. Roddy, Y. A. Hannun, and C. E. Chalfant. 2004. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J. Biol. Chem. 279:11320-11326. [DOI] [PubMed] [Google Scholar]
- 38.Phillips, R. M., D. A. Six, E. A. Dennis, and P. Ghosh. 2003. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J. Biol. Chem. 278:41326-41332. [DOI] [PubMed] [Google Scholar]
- 39.Pickard, R. T., B. A. Strifler, R. M. Kramer, and J. D. Sharp. 1999. Molecular cloning of two new human paralogs of 85-kDa cytosolic phospholipase A2. J. Biol. Chem. 274:8823-8831. [DOI] [PubMed] [Google Scholar]
- 40.Pier, G. B., M. Grout, T. S. Zaidi, J. C. Olsen, L. G. Johnson, J. R. Yankaskas, and J. B. Goldberg. 1996. Role of mutant CFTR in hypersensitivity of cystic fibrosis patients to lung infections. Science 271:64-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plotkowski, M. C., A. M. Saliba, S. H. Pereira, M. P. Cervante, and O. Bajolet-Laudinat. 1994. Pseudomonas aeruginosa selective adherence to and entry into human endothelial cells. Infect. Immun. 62:5456-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajan. S., G. Cacalano, R. Bryan, A. J. Ratner, C. U. Sontich, A. van Heerckeren, P. Davis, and A. Prince.2000. Pseudomonas aeruginosa induction of apoptosis in respiratory epithelial cells. Am. J. Respir. Cell Mol. Biol. 23:304-312. [DOI] [PubMed] [Google Scholar]
- 43.Sato, H., and D. W. Frank. 2004. ExoU is a potent intracellular phospholipase. Mol. Microbiol. 53:1279-1290. [DOI] [PubMed] [Google Scholar]
- 44.Sato, H., D. W. Frank, C. J. Hillard, J. B. Feix, R. R. Pankhaniya, K. Moriyama, V. Finck-Barbancon, A. Buchaklian, M. Lei, R. M. Long, J. Wiener-Kronish, and T. Sawa. 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 22:2959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultz, M. J., A. W. Rijneveld, S. Florquin, S. Speelman, S. J. Van Deventer, and T. van der Poll. 2002. Role of interleukin-1 in the pulmonary immune response during Pseudomonas aeruginosa pneumonia. Am. J. Physiol. Lung Cell Mol. Physiol. 282:L285-L290. [DOI] [PubMed] [Google Scholar]
- 46.Scorrano, L., D. Penzo, V. Petronilli, F. Pagano, and P. Bernardi. 2001. Arachidonic acid causes cell death through the mitochondrial permeability transition. Implications for tumor necrosis factor-alpha apoptotic signaling. J. Biol. Chem. 276:12035-12040. [DOI] [PubMed] [Google Scholar]
- 47.Shinzawa, K., and Y. Tsujimoto. 2003. PLA2 activity is required for nuclear shrinkage in caspase-independent cell death. J. Cell Biol. 163:1219-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song, C., X. J. Chang, K. M. Bean, M. S. Proia, J. L. Knopf, and R. W. Kriz. 1999. Molecular characterization of cytosolic phospholipase A2-β. J. Biol. Chem. 274:17063-17067. [DOI] [PubMed] [Google Scholar]
- 49.Steinbrueckner, B. E., J. Aufenanger, A. Hartinger, N. E. Saris, P. Kinnunen, and G. E. Hoffmann. 1995. Phospholipase A activity in Pseudomonas aeruginosa. Zentbl. Bakteriol. 282:54-66. [PubMed] [Google Scholar]
- 50.Taketo, M. M., and M. Sonoshita. 2002. Phospholipase A2 and apoptosis. Biochim. Biophys. Acta 1585:72-76. [DOI] [PubMed] [Google Scholar]
- 51.Ulisse, S., B. Cinque, G. Silvano, N. Rucci, L. Biordi, M. G. Cifone, and M. D'Armiento. 2000. Erk-dependent cytosolic phospholipase A2 activity is induced by CD95 ligand cross-linking in the mouse derived Sertoli cell line TM4 and is required to trigger apoptosis in CD95 bearing cells. Cell Death Differ. 7:916-924. [DOI] [PubMed] [Google Scholar]
- 52.Vance, R. E., S. Hong, K. Gronert, C. N. Serhan, and J. J. Mekalanos. 2004. The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc. Natl. Acad. Sci. USA 101:2135-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vidal, F., J. Mensa, M. Almela, J. A. Martinez, F. Marco, C. Casals, J. M. Gatell, E. Soriano, and M. T. Jimenez de Anta. 1996. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch. Intern. Med. 156:2121-2126. [PubMed] [Google Scholar]
- 54.Wang, Z., C. Clarke, and K. Clinkenbeard. 1998. Pasteurella haemolytica leukotoxin-induced increase in phospholipase A2 activity in bovine neutrophils. Infect. Immun. 66:1885-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolf, R. A., and R. W. Gross. 1985. Identification of neutral active phospholipase C which hydrolyzes choline glycerophospholipids and plasmalogen selective phospholipase A2 in canine myocardium. J. Biol. Chem. 260:7295-72303. [PubMed] [Google Scholar]
- 56.Zhao, S., X. Y. Du, M. Q. Chai, J. S. Chen, Y. C. Zhou, and J. G. Song. 2002. Secretory phospholipase A2 induces apoptosis via a mechanism involving ceramide generation. Biochim. Biophys. Acta 1581:75-88. [DOI] [PubMed] [Google Scholar]