Most wounds, of whatever aetiology, heal without difficulty. Some wounds, however, are subject to factors that impede healing, although these do not prevent healing if the wounds are managed appropriately. A minority of wounds will become chronic and non-healing. In these cases the ultimate goal is to control the symptoms and prevent complications, rather than healing the wound.
Figure 1.
Wounds are not just skin deep, and accurate assessment is an essential part of treatment
Table 1.
Causes of ulceration
| • Vascular (venous, arterial, lymphatic, vasculitis) |
| • Neuropathic (for example, diabetes, spina bifida, leprosy) |
| • Metabolic (for example, diabetes, gout) |
| • Connective tissue disease (for example, rheumatoid arthritis, scleroderma, systemic lupus erythematosus) |
| • Pyoderma gangrenosum (often reflection of systemic disorder) |
| • Haematological disease (red blood cell disorders (for example, sickle cell disease); white blood cell disorders (for example, leukaemia); platelet disorders (for example, thrombocytosis)) |
| • Dysproteinaemias (for example, cryoglobulinaemia, amyloidosis) |
| • Immunodeficiency (for example, HIV, immunosuppressive therapy) |
| • Neoplastic (for example, basal cell carcinoma, squamous cell carcinoma, metastatic disease) |
| • Infectious (bacterial, fungal, viral) |
| • Panniculitis (for example, necrobiosis lipoidica) |
| • Traumatic (for example, pressure ulcer, radiation damage) |
| • Iatrogenic (for example, drugs) |
| • Factitious (self harm, “dermatitis artefacta”) |
| • Others (for example, sarcoidosis) |
It is important that the normal processes of developing a diagnostic hypothesis are followed before trying to treat the wound. A detailed clinical history should include information on the duration of ulcer, previous ulceration, history of trauma, family history of ulceration, ulcer characteristics (site, pain, odour, and exudate or discharge), limb temperature, underlying medical conditions (for example, diabetes mellitus, peripheral vascular disease, ischaemic heart disease, cerebrovascular accident, neuropathy, connective tissue diseases (such as rheumatoid arthritis), varicose veins, deep venous thrombosis), previous venous or arterial surgery, smoking, medications, and allergies to drugs and dressings. Appropriate investigations should be carried out.
Table 2.
Some complications of chronic wounds
| • Sinus formation |
| • Fistula |
| • Unrecognised malignancy |
| • Malignant transformation in the ulcer bed (Marjolin's ulcer) |
| • Osteomyelitis |
| • Contractures and deformity in surrounding joints |
| • Systemic amyloidosis |
| • Heterotopic calcification |
| • Colonisation by multiple drug resistant pathogens, leading to antibiotic resistance |
| • Anaemia |
| • Septicaemia |
Table 3.
Local and systemic factors that impede wound healing
| Local factors | Systemic factors |
| • Inadequate blood supply | • Advancing age and general immobility |
| • Obesity | |
| • Increased skin tension | • Smoking |
| • Poor surgical apposition | • Malnutrition |
| • Deficiency of vitamins and trace elements | |
| • Wound dehiscence | • Systemic malignancy and terminal illness |
| • Poor venous drainage | • Shock of any cause |
| • Presence of foreign body and foreign body reactions | • Chemotheraphy and radiotherapy |
| • Immunosuppressant drugs, corticosteroids, anticoagulants | |
| • Continued presence of microorganisms | • Inherited neutrophil disorders, such as leucocyte adhesion deficiency |
| • Infection | • Impaired macrophage activity (malacoplakia) |
| • Excess local mobility, such as over a joint |
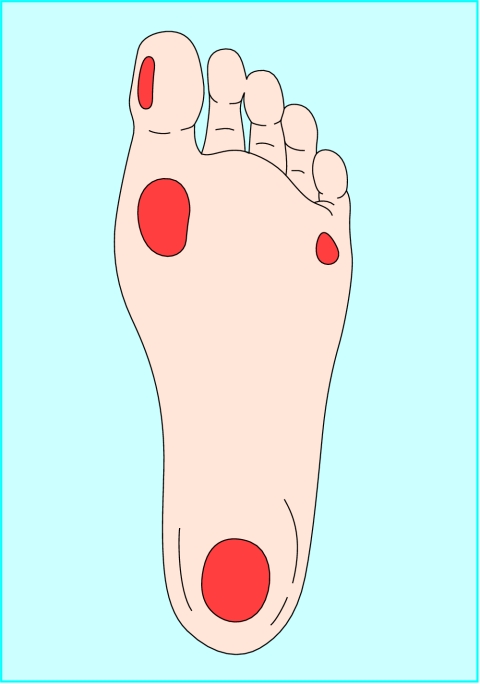
Figure 2.
Areas of abnormal pressure distribution in the diabetic foot. Plantar ulcers are most commonly seen under the hallux, on the first and fifth metatarsal heads, and under the heel
This is the first in a series of 12 articles
Assessing wounds
Size of wound
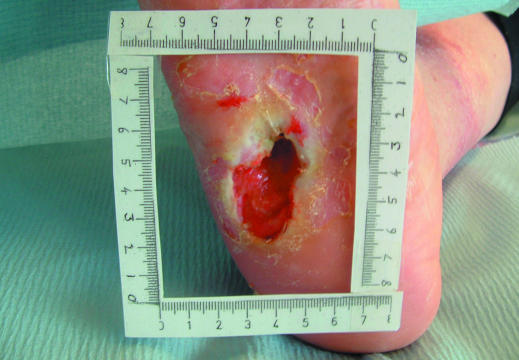
The size of the wound should be assessed at first presentation and regularly thereafter. The outline of the wound margin should be traced on to transparent acetate sheets and the surface area estimated: in wounds that are approximately circular, multiply the longest diameter in one plane by the longest diameter in the plane at right angles; in irregularly shaped wounds, add up the number of squares contained within the margin of the outline of the wound from an acetate grid tracing. These methods are the simplest, but it should be recognised that they are not precise. However, they do provide a means by which progress over time to wound closure can be identified. Patient positioning, body curvature, or tapering of the limbs will affect the accuracy of these techniques.
Figure 3.
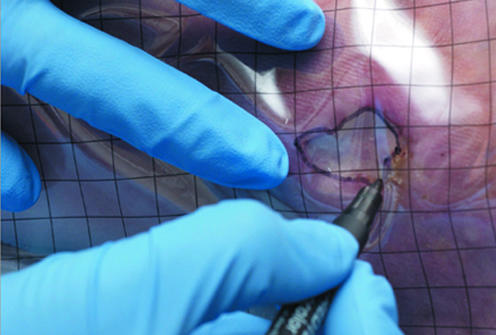
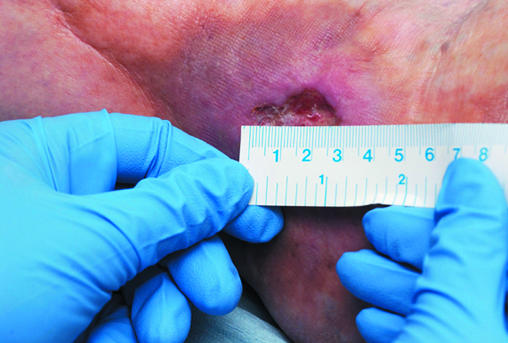
Tracing a wound for measurement and measuring a wound
Table 5.
Laboratory investigations before treating a wound
| Investigation | Rationale |
|---|---|
| Haemoglobin | Anaemia may delay healing |
| White cell count | Infection |
| Platelet count | Thrombocytopenia |
| Erythrocyte sedimentation rate; C reactive protein | Non-specific markers of infection and inflammation; useful in diagnosis and monitoring treatment of infectious or inflammatory ulceration |
| Urea and creatinine | High urea impairs wound healing. Renal function important when using antibiotics |
| Albumin | Protein loss delays healing |
| Glucose, haemoglobin A1C | Diabetes mellitus |
| Markers of autoimmune disease (such as rheumatoid factor, antinuclear antibodies, anticardiolipin antibodies, lupus anticoagulant) | Indicative of rheumatoid disease, systemic lupus erythematosus, and other connective tissue disorders |
| Cryoglobulins, cryofibrinogens, prothrombin time, partial thromboplastin time | Haematological disease |
| Deficiency or defect of antithrombin III, protein C, protein S, factor V Leiden | Vascular thrombosis |
| Haemoglobinopathy screen | Sickle cell anaemia, thalassaemia |
| HIV status | Kaposi's sarcoma |
| Serum protein electrophoresis; Bence-Jones proteins | Myeloma |
| Urine analysis | Useful in connective tissue disease |
| Wound swab | Not routine; all ulcers colonised (not the same as infection); swab only when clinical signs of infection |
Edge of wound
Although not diagnostic, examination of the edge of the wound may help to identify its aetiology in the context of the history of the wound. For example, venous leg ulcers generally have gently sloping edges, arterial ulcers often appear well demarcated and “punched out,” and rolled or everted edges should raise the suspicion of malignancy. A biopsy should be taken of any suspicious wound.
Figure 4.
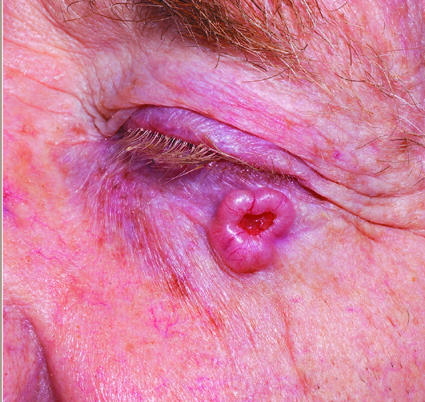
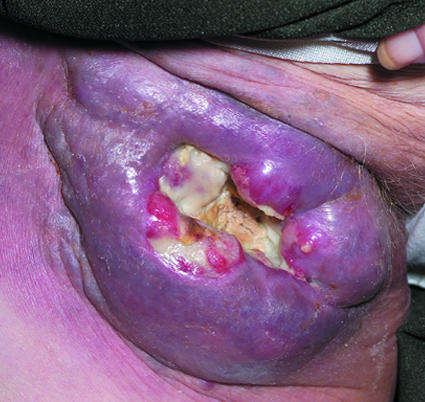
Left: Basal cell carcinoma with rolled edges. Right: Lymphoma presenting as groin ulceration
Table 4.
Would edge characteristics
| Edges | Type of ulcer |
|---|---|
| Sloping | Venous ulcer |
| Punched out | Arterial or vasculitic ulcer |
| Rolled | Basal cell carcinoma |
| Everted | Squamous cell carcinoma |
| Undermining | Tuberculosis, syphilis |
| Purple | Vasculitic (such as pyoderma gangrenosum) |
Site of wound
The site of the wound may aid diagnosis; diabetic foot ulcers often arise in areas of abnormal pressure distribution arising from disordered foot architecture. Venous ulceration occurs mostly in the gaiter area of the leg (see next article in this series). Non-healing ulcers, sometimes in unusual sites, should prompt consideration of malignancy.
Figure 5.
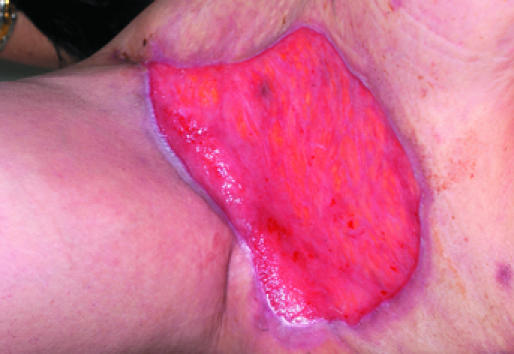
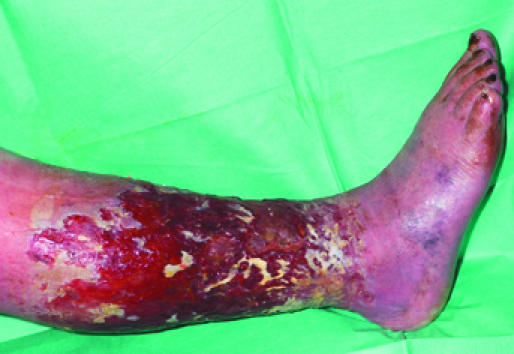
Left: Healthy granulation tissue in a hidradenitis suppurativa excision wound. Right: Unhealthy granulation tissue in a venous leg ulcer
Table 6.
Site of wound and type of ulcer
| Site | Type of ulcer |
|---|---|
| Gaiter area of the leg | Venous ulcer |
| Sacrum, greater trochanter, heel | Pressure ulcer |
| Dorsum of the foot | Arterial or vasculitic ulcer |
| Shin | Necrobiosis lipoidica |
| Lateral malleolus | Venous, arterial, or pressure ulcer or hydroxyurea induced ulceration |
| Plantar and lateral aspect of foot and toes | Diabetic ulcer |
| Sun exposed areas | Basal cell carcinoma; squamous cell carcinoma |
Wound bed
Healthy granulation tissue is pink in colour and is an indicator of healing. Unhealthy granulation is dark red in colour, often bleeds on contact, and may indicate the presence of wound infection. Such wounds should be cultured and treated in the light of microbiological results. Excess granulation or overgranulation may also be associated with infection or non-healing wounds. These often respond to simple cautery with silver nitrate or with topically applied steroid preparations. Chronic wounds may be covered by white or yellow shiny fibrinous tissue (see next article in this series). This tissue is avascular, and healing will proceed only when it is removed. This can be done with a scalpel at the bedside.
The type of tissue at the base of the wound will provide useful information relating to expectation of total healing time and the risk of complications—for example, bone at the base may suggest osteomyelitis and delayed or non-healing.
Figure 6.
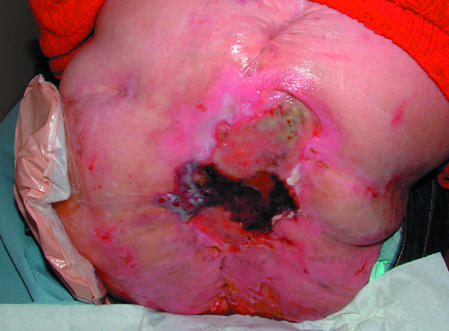
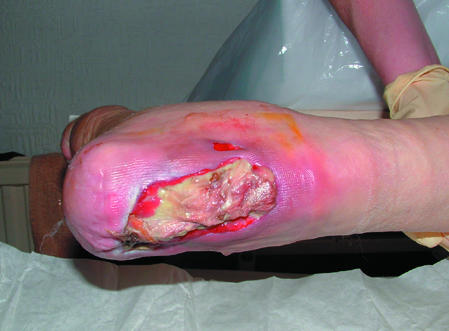
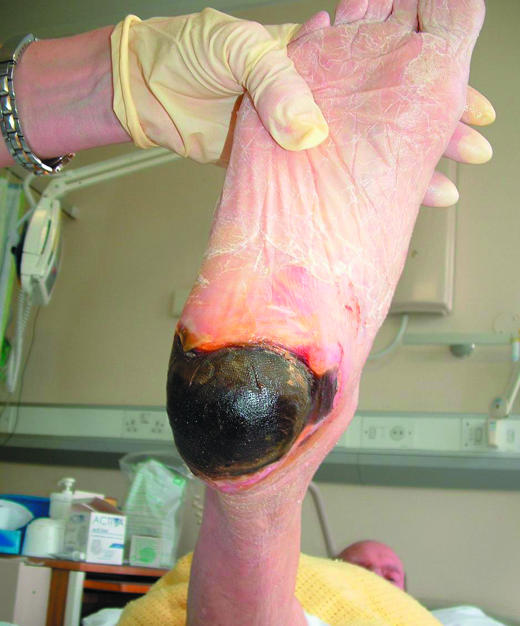
Top: Necrotic tissue (black areas) in a pressure ulcer. Bottom: Slough at the base of a pressure ulcer. Right: Eschar covering a heel pressure ulcer
Necrotic tissue, slough, and eschar
The wound bed may be covered with necrotic tissue (non-viable tissue due to reduced blood supply), slough (dead tissue, usually cream or yellow in colour), or eschar (dry, black, hard necrotic tissue). Such tissue impedes healing. Necrotic tissue and slough may be quantified as excessive (+++), moderate (++), minimal (+), or absent (-).
Since necrotic tissue can also harbour pathogenic organisms, removal of such tissue helps to prevent wound infection. Necrotic tissue and slough should be debrided with a scalpel so that the wound bed can be accurately assessed and facilitate healing. Eschar may be adherent to the wound bed, making debridement with a scalpel difficult. Further debridement, as part of wound management, may be required using other techniques.
Figure 7.
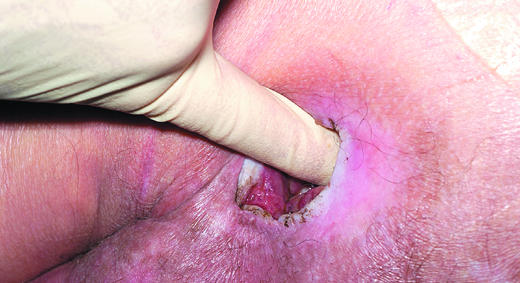

Left: Digital examination of a wound. Right: Examining a wound with a probe
Table 7.
Types of debridement
| Sharp—At the bedside (using scalpel or curette) |
| Surgical—In the operating theatre |
| Autolytic—Facilitation of the body's own mechanism of debridement with appropriate dressings |
| Biological—Larval (maggot) therapy |
| Enzymatic—Not widely used; pawpaw (papaya) or banana skin used in developing countries |
| Mechanical—Wet-to-dry dressings (not widely used in the UK) |
Depth
Accurate methods for measuring wound depth are not practical or available in routine clinical practice. However, approximate measurements of greatest depth should be taken to assess wound progress. Undermining of the edge of the wound must be identified by digital examination or use of a probe. The depth and extent of sinuses and fistulas should be identified. Undermining areas and sinuses should be packed with an appropriate dressing to facilitate healing. Undermining wounds and sinuses with narrow necks that are difficult to dress may be amenable to be laid open at the bedside to facilitate drainage and dressing. Wounds associated with multiple sinuses or fistulas should be referred for specialist surgical intervention.
Figure 8.
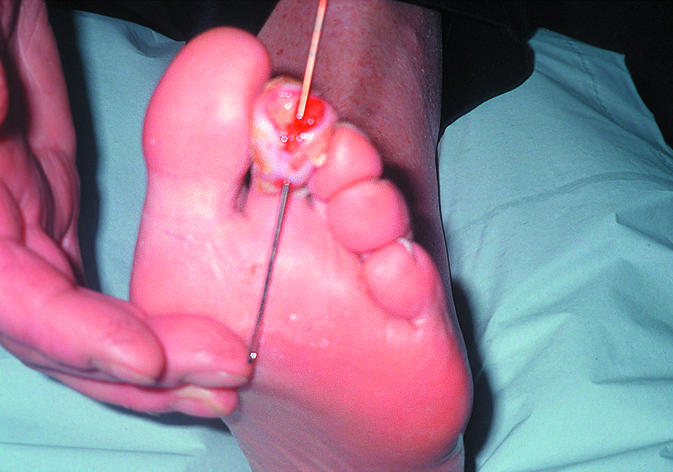
Fistula in a diabetic foot ulcer
Surrounding skin
Cellulitis associated with wounds should be treated with systemic antibiotics. Eczematous changes may need treatment with potent topical steroid preparations. Maceration of the surrounding skin is often a sign of inability of the dressing to control the wound exudate, which may respond to more frequent dressing changes or change in dressing type. Callus surrounding and sometimes covering neuropathic foot ulcers (for example, in diabetic patients) must be debrided to (a) visualise the wound, (b) eliminate potential source of infection, and (c) remove areas close to the wound subject to abnormal pressure that would otherwise cause enlargement of the wound. This can be done at the bedside.
Figure 9.
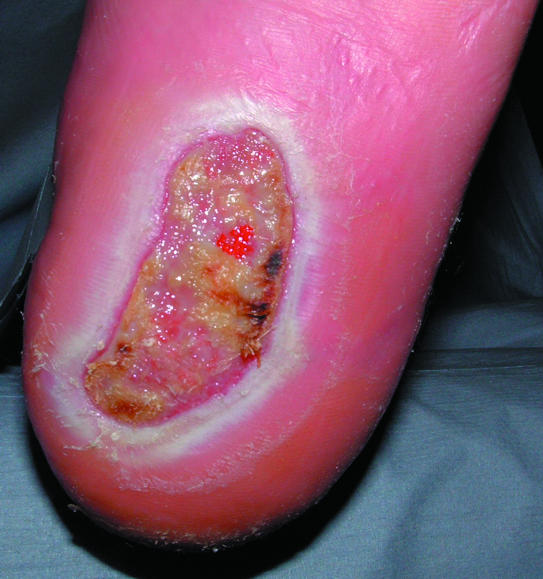
Maceration of the skin surrounding a diabetic foot ulcer
Infection
All open wounds are colonised. Bacteriological culture is indicated only if clinical signs of infection are present or if infection control issues (such as methicillin resistant staphylococcus aureus (MRSA)) need to be considered. The classic signs of infection are heat, redness, swelling, and pain. Additional signs of wound infection include increased exudate, delayed healing, contact bleeding, odour, and abnormal granulation tissue. Treatment with antimicrobials should be guided by microbiological results and local resistance patterns.
Table 8.
Wound exudate
| • Wound exudate may be serous, serosanguinous, or sanguinous |
| • The quantity of exudate is usually classified as heavy (+++ (dressing soaked)), medium (++ (dressing wet)), or minimal (+ (dressing dry)) |
| • Excessive exudate may be due to wound infection or gross oedema in the wound area and may complicate wound healing |
| • The exudate should be controlled with the use of dressings appropriate for the level of exudate and any infection treated |
| • Barrier films applied to the surrounding skin help to prevent further maceration (see the ninth article in the series) |
| • The oedematous leg should be raised when the patient is seated |
Bone at the base of a wound may suggest a protracted healing time and the possibility of underlying osteomyelitis
Pain
Pain is a characteristic feature of many healing and non-healing wounds. Pain can be caused by both nociceptive and neuropathic stimuli. Intermittent pain is often related to dressing removal or recent application of new dressings and may necessitate the use of analgesia before the dressing is changed. Constant pain may arise as a result of the underlying condition, such as ischaemia, neuropathy, tissue oedema, chronic tissue damage (for example, lipodermatosclerosis), infection, or scarring (for example, atrophie blanche). The nature and type of pain should be identified and treated appropriately. Pain assessment tools can help to assess the nature and severity of pain. With recalcitrant pain, or pain that is difficult to control, consider referral to a local pain team.
Table 9.
Clinical features of non-healing wounds
| • Absence of healthy granulation tissue | • Lack of adequate blood supply |
| • Failure of re-epithelialisation | |
| • Presence of necrotic and unhealthy tissue in the wound bed | • Cyclical or persistent pain |
| • Recurrent breakdown of wound | |
| • Clinical or subclinical infection | |
| • Excess exudate and slough |
Non-healing wounds
Non-healing wounds have traditionally been defined as those that fail to progress through an orderly sequence of repair in a timely fashion. Such wounds are sometimes thought of as being caused by neglect, incompetence, misdiagnosis, or inappropriate treatment strategies. However, some wounds are resistant to all efforts of treatment aimed at healing, and alternative end points should be considered; measures aimed at improving the quality of life will be paramount in these instances.
Figure 10.
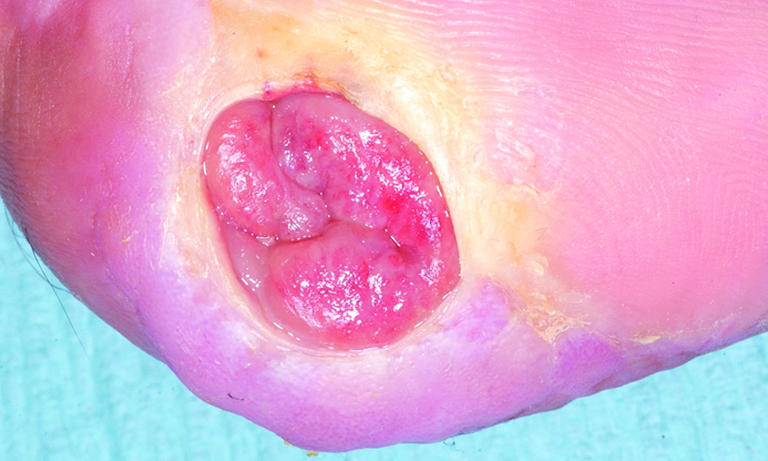
Overgranulation may be a sign of infection or non-healing
Quality of life
Several studies have shown that patients with non-healing wounds have a decreased quality of life. Reasons for this include the frequency and regularity of dressing changes, which affect daily routine; a feeling of continued fatigue due to lack of sleep; restricted mobility; pain; odour; wound infection; and the physical and psychological effects of polypharmacy. The loss of independence associated with functional decline can lead to changes, sometimes subtle, in overall health and wellbeing. These changes include altered eating habits, depression, social isolation, and a gradual reduction in activity levels. Many patients with non-healing wounds complain of difficulties with emotions, finances, physical health, daily activities, friendships, and leisure pursuits.
Quality of life is not always related to healing of the wound. It may be clear from the outset that wounds in some patients will be unlikely to heal. In such patients control of symptoms and signs outlined above—particularly odour, exudate, and pain—may improve the individual's quality of life. Additionally, optimal chronic wound management will lead to a reduction in the frequency of dressing changes, further enhancing quality of life. In a minority of instances, seemingly drastic measures—such as amputation in a person with chronic leg ulceration—may need to be considered when the quality of life is severely affected by the non-healing wound and its complications.
The causes of malodorous wounds include infection and the presence of necrotic tissue. Infection should be treated with antibiotics. Odour associated with necrotic tissue may be reduced by removal of the necrotic tissue or use of agents impregnated with antiseptics or charcoal. Treatment with topical metronidazole and use of odour absorbing dressings may help to reduce odour from fungating malignant wounds. Larval therapy may also be helpful in the debridement of malodorous tissue
The drawing on page 285 is adapted from one provided by Wendy Tyrrell, School of Health and Social Sciences, University of Wales Institute, Cardiff.
The ABC of wound healing is edited by Joseph E Grey (joseph.grey@cardiffandvale.wales.nhs.uk), consultant physician, University Hospital of Wales, Cardiff and Vale NHS Trust, Cardiff, and honorary consultant in wound healing at the Wound Healing Research Unit, Cardiff University, and by Keith G Harding, director of the Wound Healing Research Unit, Cardiff University, and professor of rehabilitation medicine (wound healing) at Cardiff and Vale NHS Trust. The series will be published as a book in summer 2006.
Competing interests: KGH's unit receives income from many commercial companies for research and education, and for advice. It does not support one company's products over another.
References
- • Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, Rodeheaver G, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol 1994;130: 489-93. [PubMed] [Google Scholar]
- • Izadi K, Ganchi P. Chronic wounds. Clin Plast Surg 2005;32: 209-22. [DOI] [PubMed] [Google Scholar]
- • Falanga V, Phillips TJ, Harding KG, Moy RL, Peerson LJ, eds. Text atlas of wound management. London: Martin Dunitz, 2000.