Abstract
The cerebral complement system is hypothesized to contribute to neurodegeneration in the pathogenesis of AIDS-associated neurological disorders. Our former results have shown that the human immunodeficiency virus (HIV) strongly induces the synthesis of complement factor C3 in astrocytes. This upregulation explains in vivo data showing elevated complement levels in the cerebrospinal fluid of patients with AIDS-associated neurological symptoms. Since inhibition of complement synthesis and activation in the brain may represent a putative therapeutic goal to prevent virus-induced damage, we analyzed in detail the mechanisms of HIV-induced modulation of C3 expression. HIV-1 increased the C3 levels in astrocyte culture supernatants from 30 to up to 400 ng/ml; signal transduction studies revealed that adenylate cyclase activation with upregulation of cyclic AMP is the central signaling pathway to mediate that increase. Furthermore, activity of protein kinase C is necessary for HIV induction of C3, since inhibition of protein kinase C by prolonged exposure to the phorbol ester tetradecanoyl phorbol acetate partly abolished the HIV effect. The cytokines tumor necrosis factor alpha and gamma interferon were not involved in mediating the HIV-induced C3 upregulation, since neutralizing antibodies had no effect. Besides whole HIV virions, the purified viral proteins Nef and gp41 are biologically active in upregulating C3, whereas Tat, gp120, and gp160 were not able to modulate C3 synthesis. Further experiments revealed that neurons were also able to respond on incubation with HIV with increased C3 synthesis, although the precise pattern was slightly different from that in astrocytes. This strengthens the hypothesis that HIV-induced complement synthesis represents an important mechanism for the pathogenesis of AIDS in the brain.
Infection of the brain by human immunodeficiency virus type 1 (HIV-1) is a frequent finding in patients with AIDS (14, 23, 43) and results in neurological manifestations in 20 to 30% of HIV-1-infected individuals. The AIDS dementia complex is the most prominent of these neurological complications, with cognitive, motor and behavioral dysfunctions. Classical hallmarks of AIDS dementia complex are neuron loss, reactive astrocytosis, microgliosis, and myelin pallor (11). The pathogenesis of AIDS dementia complex is unknown, since only a limited number of brain cells are infected by HIV. Current hypotheses indicate that virus-induced mediators are involved in inducing the neurological lesions.
Complement is an important antimicrobial defense mechanism of innate immunity. It recognizes a large variety of pathogens and targets them for destruction either directly by formation of a lytic pore or by opsonization and recruitment of phagocytes. The complement system is of special importance in the brain because the elements of adaptive immunity have only limited access due to the blood-brain barrier. Furthermore, astrocytes induce a deactivation of penetrating monocytes-macrophages and T cells (16, 51), thus enhancing the importance of the autonomous complement cascade system in the central nervous system. Therefore, complement activation during HIV infection of the brain might represent a protective defense mechanism by limiting virus spread within the brain and decreasing the viral burden, either directly by viral lysis or indirectly by activation of microglial immune cells by complement activation products like C3a and C5a (33).
However, there is also some evidence from other neurological diseases like Alzheimer's disease and multiple sclerosis that chronic complement activation is associated with brain inflammation and neurodegeneration (10, 22, 31, 39, 49, 52; reviewed in reference 46). Since HIV and HIV-infected cells activate the complement cascade by all three pathways (reviewed in reference 45) and complement activation products harbor a variety of biological functions toward brain cells, it is intriguing to hypothesize that chronic complement activation in the HIV-infected brain may represent an important mediator of virus-induced brain damage.
The complement factor C3 is a central protein of the cascade, and its fragments (C3b, iC3b, C3d, and C3a) affect many cellular processes in the brain, such as activation of signaling pathways (30, 35, 36) and modulation of cytokine synthesis (17, 41). In general, all complement proteins can be synthesized by various brain cells, including astrocytes, neurons, microglia, and oligodendrocytes, with astrocytes being the most potent complement producers (13, 32). Although normal synthesis in the brain is low, with C3 concentrations being 300 times lower in the cerebrospinal fluid than in the blood (24), inflammatory cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) considerably increase complement production, especially of complement factor C3 (4, 15, 40). Furthermore, the mRNA level of C3 was markedly upregulated in affected lesion areas of brains from Alzheimer's patients (52). Viral infection with Norwalk virus, a neurotropic paramyxovirus, also induces an increase in C3 production in astrocytes (40). In addition, increased levels of C3 and C4 were found in the cerebrospinal fluid of HIV-infected patients with neurological symptoms and signs of central nervous system dysfunction (24), supporting the hypothesis of an association between complement and HIV-induced neurodegeneration.
Previous studies have shown that an HIV-induced upregulation of C3 expression in astrocytes may be an important reason for increased complement levels in the cerebrospinal fluid of HIV-infected patients (48). Since inhibition of complement synthesis and activation may be an interesting therapeutic approach to prevent neurological damage in the virus-infected brain, we studied in detail the mechanism of how HIV upregulates complement synthesis in astrocytic cells. Signal transduction studies revealed that increase in cyclic AMP (cAMP) and activation of protein kinase C participated in the regulation of C3 expression by HIV. Purified viral proteins like Nef and gp41 were also able to modulate C3 expression, whereas proteins like Tat, Rev, and gp120 showed no effect. Furthermore, we could show that neuronal cells, similar to astrocytes, react on incubation with HIV with increased complement synthesis, strengthening the role of complement in the pathogenesis in HIV infection of the brain.
MATERIALS AND METHODS
Cell lines and culture.
The human glioblastoma/astrocytoma cell line U373MG was purchased from the Medical Research Council (MRC) Centralised Facility for AIDS Reagents, Hertfordshire, United Kingdom, and cultured in either Ham's F12 medium (Life Technologies, Vienna, Austria) supplemented with 10% fetal calf serum (FCS; BioWhittaker, Verviers, Belgium), penicillin-streptomycin (100 U/ml and 100 μg/ml, respectively), 0.1 mM nonessential amino acids, and 2 mM l-glutamine (Life Technologies). The neuroblastoma cell line SK-N-SH was from the American Type Culture Collection (Rockville, Md.; HTB-11) and grown in Dulbecco's modified Eagle's medium (DMEM) (Life Technologies) with the same supplements.
The T-lymphoblastoid cell line M8166 used for virus propagation was cultivated in RPMI 1640 medium (Life Technologies) supplemented with 10% FCS, penicillin-streptomycin, and 2 mM l-glutamine.
Virus propagation and purification.
The HIV-1 IIIB strain was from the MRC (7, 37) and grown for propagation in cell line M8166. Virus-containing culture supernatants were harvested after 3 days and frozen at −70°C. For mock treatment of cells, culture supernatant of noninfected M8166 was collected at the same time points. For some experiments, virus was purified by ultracentrifugation at 20,000 × g for 60 min. The pellet was resuspended in phosphate-buffered saline (PBS) and stored at −70°C.
Virus yield was determined by p24 antigen enzyme-linked immunosorbent assay (ELISA) developed at the Institute of Applied Microbiology (Vienna, Austria). For that assay, monoclonal antibodies 37G12 and Mo1 were used, which were a kind gift from H. Katinger (Institute for Applied Microbiology). Briefly, microplates were coated overnight with the first monoclonal antibody and washed three times with PBS-0.1% Tween 20 (Serva, Heidelberg, Germany). Dilution series of M8166 culture supernatants or purified virus stocks were applied to the plate, together with the second antibody conjugated with biotin, for 1 h. The amount of bound p24 was quantified by adding resorufin-β-d-galactopyranoside substrate solution and measuring the optical density at 550 nm using an ELISA reader. The p24 assay was calibrated using baculovirus-derived recombinant p24 as a standard.
Antibodies, inhibitors, and purified proteins.
C3 sandwich ELISA was established using the monoclonal C3d antibody BB5 (W. Prodinger, Innsbruck, Austria) as the catching antibody and monoclonal C3c antibody (Dako, Glostrup, Denmark) as the detection antibody.
The inhibitors rapamycin, wortmannin, SQ22536 [9-(tetrahydro-2′-furyl)adenine] and PD98059 were from Calbiochem (San Diego, Calif.). Dibutyryl-cAMP (dibutyryl-adenosine 3′,5′-cyclic monophosphate, N6,O2′) and tetradecanoyl acetate (TPA) were also purchased from Calbiochem.
For some experiments, the cells were incubated with neutralizing antibody against the cytokines TNF-α and IFN-γ. The monoclonal antibody against TNF-α was clone TA-31 (Sigma, St. Louis, Mo.) and was described to neutralize the biological activity of TNF-α in cell culture supernatants. The monoclonal anti-human IFN-γ antibody clone 25718.111 was purchased from R&D Systems (Minneapolis Minn.) and had been selected for its ability to neutralize the bioactivity of human IFN-γ.
Purified viral proteins NefIIIB (deposit by V. Erfle), TatIIIB (J. Raina), Rev (J. Karn), gp120SF2 (J. Steiner), gp120W61D (C. Bruck), and gp160IIIB (J. Raina) were purchased from the MRC (Blanche Lane, United Kingdom).
Since gp41 is rather hydrophobic, a recombinant fusion protein between maltose-binding protein (MBP) and an 82-amino-acid-long extracellular region (amino acids 565 to 647) of gp41 was used for the experiments (Intracel, Cambridge, Mass.). To rule out possible side effects of MBP, we used recombinant MBP (New England Biolabs, Beverly, Mass.) as a negative control.
RNA preparation, reverse transcription, and PCR.
At different time points of incubation, cells were lysed and RNA was isolated using the RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. For quantification, 2 μg of total RNA was reverse transcribed into cDNA using oligo(dT) as a primer and Moloney murine leukemia virus reverse transcriptase (Life Technologies) in a final volume of 25 μl. PCRs to analyze the expression of different complement factors were performed as previously described (48). Half of the PCR mixture was applied on an agarose gel and transferred to a nylon membrane (Bio-Rad, Munich, Germany) by alkaline capillary blotting. Filters were cross-linked by UV and hybridized with 32P-labeled probes under stringent conditions according to the method of Church and Gilbert (8). The radioactive signal obtained after hybridization was quantified using a Phosphoimager (Fuji) and expressed as photosensitive luminescence.
Experimental settings and measurement of C3 by ELISA.
Astrocytes were seeded in 96-well plates at a concentration of 1.5 × 104 cells/ml and incubated with HIV either directly as virus-containing M8166 culture supernatants or purified by ultracentrifugation. Control cells were incubated with culture supernatant from uninfected M8166 or PBS. Culture supernatants were harvested at the indicated time points.
To quantify C3 in cell culture supernatants, ELISA microplates were coated overnight at 4°C with the monoclonal C3d antibody in 0.1 M NaHCO3, pH 9.6. Unspecific binding was inhibited by saturation with 1% bovine serum albumin (BSA) (Sigma) in PBS. Cell culture supernatants were added to the coated wells and incubated for 1 h at room temperature. To calculate the exact amount of C3 in the supernatants, purified C3 protein (Sigma) was used as a standard. After the washing procedure, bound C3 was detected with monoclonal C3c antibody and a second peroxidase-labeled anti-mouse immunoglobulin G antibody (Dako).
Statistical analysis.
Statistical analysis (Student's t test) was performed using the Origin 4.1 software (serial no. 6014906).
RESULTS
Adenylate cyclase and protein kinase C participate in the signal transduction pathway for HIV-induced C3 expression.
U373 astrocytes were incubated with HIV-1 strain IIIB, and the production of C3 was quantified by ELISA using purified C3 protein as the standard. After incubation with HIV, the amount of C3 in the cell culture supernatants reached up to 350 ng/ml after 3 days, whereas the culture supernatants of the control cells contained 31 ng/ml (Fig. 1). The continuous presence of HIV is necessary for stimulation of C3 synthesis to that extent; a minimal incubation time of 24 h with HIV sufficed to obtain a significant difference in the control cells (data not shown).
FIG. 1.
Signal transduction studies of C3 protein synthesis after incubation of U373 astrocytes with HIV IIIB. U373 astrocytes were either mock treated (−) or incubated with HIV IIIB p24 (20 ng/ml, +). In addition, SQ22536 (200 μM), dibutyrl-cAMP (100 μM), forskolin or 1,9D-forskolin (10 μM), pertussis toxin (50 ng/ml), PD98059 (50 μM), rapamycin (50 nM), or wortmannin (500 nM) was added to the cells. Cell culture supernatants were harvested after 3 days. The amount of C3 protein was quantified by a specific ELISA. Results are the mean ± standard deviation (SD) of triplicate determinations. Statistical significance of HIV-induced C3 production with inhibitors versus HIV-induced C3 levels without inhibitors was evaluated by Student's t test. ∗∗, P < 0.01; ∗∗∗, P < 0.005.
To analyze the signal transduction pathway that is activated by HIV-1 and results in enhanced C3 expression, cells were stimulated with HIV in the presence or absence of specific inhibitors. Several chemical compounds which affect the activity of the cAMP-producing enzyme adenylate cyclase or the cAMP level directly in the cell were tested for their influence on the stimulation of C3 synthesis by HIV-1. Incubation of cells with SQ22536, a cell-permeating adenylate cyclase inhibitor, almost completely abolished the induction of C3 expression by HIV (Fig. 1). Dibutyryl-cAMP, a stable cAMP analogue that preferentially activates cAMP-dependent protein kinases, even enhanced the effect of HIV on C3 synthesis, having no effect when applied without virus. Furthermore, forskolin, which rapidly and reversibly activates adenylate cyclase, also increased the HIV-induced stimulation of C3 synthesis, whereas the biologically inactive analogue 1,9-dideoxy-forskolin had no effect. These substances also had no effect in the absence of HIV-1. Pertussis toxin, an inhibitor of Gi proteins and thus an activator of adenylate cyclase, also amplified the HIV effect.
Inhibitors of other signal transduction pathways like wortmannin, a specific inhibitor of phosphatidylinositol 3 kinase, and PD98059, which inhibits the activity of the mitogen-activated protein kinase, did not modulate the induction of C3 synthesis by HIV-1. Similarly, blocking of the p70S6 kinase, a mitogen-activated protein kinase that plays a central role in the control of mRNA, by the highly specific inhibitor rapamycin had no influence on C3 synthesis. (Fig. 1)
Since C3 was described to be regulated on a transcriptional level by a protein kinase C-dependent pathway, we also studied the effect of protein kinase C blocking on the HIV-induced synthesis of C3 in U373 astrocytes. Protein kinase C activity was downmodulated by exposure of the cells to TPA for 24 h. After that incubation, the cell culture medium was exchanged and the cells were incubated with fresh TPA either with or without HIV-1. Control cells were incubated with HIV-1 without prior protein kinase C downmodulation. The amount of C3 in the supernatants was quantified after 3 and 5 days.
HIV upregulated the amount of C3 in the supernatant from 30 ng/ml in the uninfected control cells to 410 ng/ml after 3 days and 570 ng/ml after 5 days (Fig. 2). Cells which had been incubated with TPA to downmodulate protein kinase C activity secreted much lower amounts of C3 in the supernatants, with 100 ng/ml after 3 days and 280 ng/ml after 5 days. Incubation with TPA alone had no effect on the C3 production of U373 cells.
FIG. 2.
Role of protein kinase C in HIV-induced C3 protein synthesis in U373 astrocytes. U373 astrocytes were either mock treated with medium or incubated with 50 nM TPA to exhaust protein kinase C activity. After 24 h the medium was changed, and HIV IIIB p24 (20 ng/ml) was added to the cells; fresh TPA was given to the cells pretreated with TPA. Cell culture supernatants were harvested 3 and 5 days after the medium change. The amount of C3 protein therein was quantified by a specific ELISA. Results are the mean ± SD of triplicate determinations. Statistical significance of HIV-induced C3 production with TPA versus HIV-induced C3 levels without TPA was evaluated by Student's t test. ∗∗∗, P < 0.005.
These data suggest that activation of adenylate cyclase and protein kinase C is the main signal transduction pathway for the induction of C3 synthesis by HIV-1.
Role of cytokines in the modulation of C3 production by HIV.
Since several cytokines have been described to activate the production of complement factor C3, we analyzed whether the effect of HIV might be mediated by cytokines. Neutralizing antibodies were added to the cells, with subsequent stimulation with HIV or medium as a control. Control cells were incubated with an isotype antibody.
The neutralizing antibodies against the cytokines IFN-γ and TNF-α had no effect on the C3 synthesis of the astrocytes, nor did they inhibit the stimulation of C3 synthesis by the virus (Fig. 3). The amount of C3 in the culture supernatants corresponded to that in those without antibody, indicating that the stimulatory activity of HIV is likely not the result of one of these cytokines.
FIG. 3.
Antibodies neutralizing IFN-γ and TNF-α show no effect on HIV-induced C3 synthesis in astrocytes. U373 astrocytes were either mock treated or incubated with 20 ng of HIV IIIB p24 per ml. To some wells, neutralizing antibodies against IFN-γ (A) (1 μg/ml) or against TNF-α (5 μg/ml) were added. Culture supernatants were harvested at the indicated time points, and the C3 level was determined by ELISA. Results are the mean ± SD of triplicate determinations.
Modulation of complement synthesis by purified viral proteins.
We sought to evaluate whether not only complete HIV virions but also isolated viral proteins have the capacity to influence the cellular synthesis of complement factor C3. Both regulatory proteins like Nef, Tat, and Rev and structural proteins (gp120, gp41, and gp160) were tested. An incubation with HIV-1 was performed in parallel to guarantee the principal responsiveness of the cells to external stimulation.
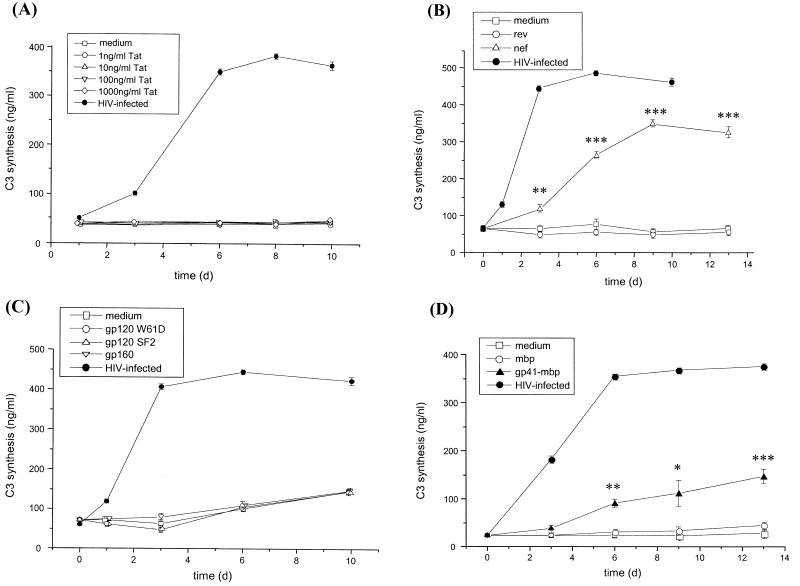
Since Tat is a very potent transactivator of cellular genes, we incubated the U373 cells with different concentrations of purified Tat protein (Fig. 4A). Even a Tat protein concentration of 1,000 ng/ml did not increase the C3 production of the cells, whereas HIV-1 was highly active in inducing the synthesis of C3. In contrast, the viral Nef protein was able to mimic the upregulation of C3, whereas Rev had no effect (Fig. 4B). A significant difference in the C3 synthesis in Nef-treated cells was visible already after 3 days compared to the Rev-treated cells. A maximal effect was reached after 9 days.
FIG. 4.
Induction of C3 synthesis by purified HIV proteins. U373 astrocytes were incubated with either Tat protein at increasing concentrations (between 1 and 1,000 ng/ml) (A); with Nef (500 ng/ml) or Rev (500 ng/ml) (B); with gp120 of virus strains SF2 and W61D (500 ng/ml) or gp160 (500 ng/ml) (C); or with gp41-MBP (500 ng/ml) or MBP (500 ng/ml) (D). As a positive control for C3 induction, some wells were incubated with HIV IIIB p24 (20 ng/ml). Cell culture supernatants were harvested after different time points, and the C3 levels were quantified by ELISA. Results are the mean ± SD of triplicate determinations. Statistical significance of protein-induced C3 production in Nef-treated and gp41-MBP-treated cells versus C3 levels of cells incubated with Rev or MBP was evaluated by Student's t test. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.005.
Similar to Tat and Rev, incubation of cells with the structural protein gp120 derived from two different virus strains (W61D and SF2) or with the precursor protein gp160 did not modulate the synthesis of C3 in astrocytes (Fig. 4C). In contrast, the viral protein gp41, applied as a fusion construct with MBP, did increase the production of C3 after 3 days, compared to the MBP-treated control cells (Fig. 4D). The difference was statistically significant after 6 days.
To compare the activity of C3 induction by gp41 and Nef, we tested these proteins in parallel at different concentrations. As negative controls, C3 was quantified in the supernatant of cells treated with either Rev or MBP. Nef was shown to be slightly more active than gp41, with concentrations up to 100 ng/ml significantly increasing the astrocytic synthesis of C3 protein (Fig. 5A). A maximal effect was reached with 1 μg/ml, and a higher concentration did not induce higher C3 production. The effect of gp41-MBP was significant at a concentration of 500 ng/ml and reached a maximum at 2 μg/ml (Fig. 5B), as higher concentrations did not further increase C3 production (data not shown).
FIG. 5.
Effect on C3 synthesis of purified Rev, Nef, gp41-MBP, and MBP. U373 astrocytes were incubated with either Rev or Nef protein at increasing concentrations between 20 ng/ml and 2 μg/ml (A) or with gp41-MBP or MBP at increasing concentrations (B). Cell culture supernatants were harvested after 5 days, and the C3 levels were quantified by ELISA. Results are the mean ± SD of triplicate determinations. Statistical significance of protein-induced C3 production versus C3 levels in mock-treated cells was evaluated by Student's t test. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.005.
Induction of C3 synthesis by HIV in neurons.
Further experiments aimed to test the role of HIV as an inducer of C3 synthesis in neurons. To that purpose, the neuronal cell line SK-N-SH was incubated with HIV, and the synthesis of different complement factors was quantified by semiquantitative PCR on an mRNA level. The mRNA levels of most complement factors (C1q, C2, C4, C5, C7, and C9) did not change during the incubation period of 7 days (Fig. 6A). The mRNA for C9 was not present in neurons, although it was clearly detected in astrocytes. However, a strong signal of C3 mRNA appeared already at day 1 after addition of the virus to the cells and increased over the incubation time. A quantification of the signal for C3 mRNA by radioactive hybridization revealed a more than 20-fold increase after incubation with HIV (Fig. 6B). Infection of the neurons was proven by detection of nef mRNA in the cells (Fig. 6A).
FIG. 6.
Expression of mRNA of different complement factors by HIV-infected and mock-treated SK-N-SH neurons. Cells were incubated with HIV IIIB p24 (20 ng/ml, +) or mock treated with medium (−). RNA was isolated at different time points, and expression levels were quantified by RT-PCR. (A) PCR amplification products were resolved on a 1.5% agarose gel and detected by hybridization with specific 32P-labeled probes. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (B) Densitometric quantification of the PCR signals for C3 using a phosphoimager after hybridization with a 32P-labeled probe. The signals are calculated as photosensitive luminescence units.
The increase in C3 induction was also measured on the protein level. Cell culture supernatants of SK-N-SH neurons either mock treated or incubated with HIV were harvested at different time points, and the amount of C3 was quantified by ELISA. For comparison, the astrocytic cell line U373 was treated in parallel. The kinetics of C3 increase in the supernatant of SK-N-SH neurons are rather similar to those of U373 astrocytes, with a prolonged lag phase (Fig. 7). After 4 days (U373, 3 days), a significant increase in C3 protein was measured, with 40-fold higher levels of C3 in the medium after 11 days (U373, 45-fold).
FIG. 7.
Comparison of HIV-induced C3 production in astrocytes and neuronal cells. U373 astrocytes and SK-N-SH neurons were either mock treated or incubated with HIV IIIB p24 (20 ng/ml). C3 levels in the supernatants were quantified by ELISA. The results are the mean ± SD of triplicate determinations.
These results imply that induction of complement by HIV is not restricted to astrocytes, since neurons, although to a lesser extent, show the same reaction. However, differences from astrocytes were visible, since the level of C2 mRNA did not change in neurons, whereas an increase had been observed in astrocytes (47).
Purified Nef protein can modulate C3 production in neurons.
To further compare the complement induction by HIV in neurons and astrocytes, we incubated SK-N-SH neurons with the purified proteins Tat, gp41-MBP, and gp120. None of these proteins was able to increase C3 synthesis in SK-N-SH cells (Fig. 8A).
FIG. 8.
Effect of purified viral proteins on C3 synthesis in SK-N-SH neuronal cells. (A) SK-N-SH neurons were mock treated with medium or incubated with either HIV IIIB p24 (20 ng/ml) or purified Tat, gp41-MBP, or gp120 protein (500 ng/ml). (B) SK-N-SH neurons and U373 astrocytes were either mock treated with medium or incubated with purified Nef or Rev protein (50 ng/ml). Cell culture supernatants were harvested at the indicated time points, and the C3 levels were quantified by ELISA. Results are the mean ± SD of triplicate determinations.
The activity of Nef protein toward C3 induction was compared between SK-N-SH neurons and U373 astrocytes with mock-treated control cells and cells incubated with purified Rev protein. In U373 astrocytes, 50 ng of Nef per ml induced a significant rise in C3 synthesis in comparison to Rev-treated cells and buffer-treated cells, and a 20-fold higher amount was measured after 9 days (Fig. 8B). Similarly, the same concentration of Nef induced C3 synthesis in SK-N-SH, but to a much lower extent and with a longer lag phase. A slight increase was visible after 6 days, and a significant threefold upregulation was visible after 9 days.
DISCUSSION
Complement as a central element of innate immunity is supposed to be of special importance in the brain, and its activation during HIV infection might have both positive (protective) and negative (degenerative) aspects. Normal complement synthesis in the brain is low, but previous studies have shown that C3 production in astrocytes is upregulated by infection with HIV-1 (48). This upregulation is likely to be responsible for the increased C3 levels found in the cerebrospinal fluid of patients with HIV infection and signs of central nervous system dysfunction (24). Since this correlation implies a role of complement in the pathogenesis of HIV-induced neurological dysfunction, the signal transduction pathway of C3 induction was studied in detail.
By signal transduction studies with various inhibitory and stimulatory substances, we revealed that activation of adenylate cyclase and subsequent production of cAMP is the relevant signaling pathway by which enhancement of C3 synthesis is induced. Inhibitors of adenylate cyclase completely abolished the HIV effect on C3 synthesis, whereas substances which increase the cAMP level in the cells further increased the activation of C3 production by HIV. These results fit well with previous reports showing that HIV upregulates intracellular cAMP concentrations in vitro and that HIV-positive individuals show significant increases in cAMP levels (19, 20, 34). Furthermore, the viral protein gp41, which can also enhance the synthesis of C3, was also described to increase the activity of adenylate cyclase and the cAMP levels in various cell types (3, 47). In addition, dibutyryl-cAMP, a stable homologue of cAMP, induces the expression of C3 mRNA and protein in Schwann cells (9), thus correlating the regulation of C3 expression with the second-messenger cAMP.
Adenylate cyclase and cAMP are not likely to be the only signal transduction pathway which translates the signal of HIV infection into increased C3 expression. Protein kinase C activity is also a prerequisite for C3 upregulation by HIV-1, since downmodulation by the phorbol ester TPA also abolished the activity of HIV toward C3 expression.
The cytokines TNF-α and IFN-γ were shown to differentially regulate the expression of complement factor C3 (4, 15, 42) in astrocytes. However, experiments with neutralizing antibodies against these cytokines indicated that the effect of HIV on C3 synthesis is not indirect, excluding the induction of TNF-α and IFN-γ as an intermediate step. These results strengthen the hypothesis that HIV-1 exerts its effect presumably by direct modulation of either C3 promoter activity or the stability of C3 mRNA. The results fit well with the result that an increase in the amount of C3 mRNA is visible already after 5 h, leaving nearly no time for an indirect mechanism.
In addition to complete HIV virions, the purified viral proteins gp41 and Nef also harbor C3-inducing activity. To our surprise, the viral regulator protein Tat, which is known to modulate the expression of numerous cellular proteins, had no effect on C3 synthesis, even at very high concentrations of 1,000 ng/ml.
Nef is the predominant viral protein produced in HIV-infected astrocytes. It can modulate the intracellular signaling of astrocytes, reversibly increases the potassium ion current in neurons, and can induce apoptosis (reviewed in reference 5). Furthermore, Nef protein stimulates a productive HIV-1 infection from latency (12). The close relation of Nef protein to cellular signaling pathways is due to the Src homology 3-like (SH3) domain of Nef that is homologous to cellular SH3 domains found in tyrosine kinases, transcription factors, and adapter molecules (reviewed in reference 18). Our results show that Nef can also stimulate the expression of C3 in astrocytes. The Nef protein is released extracellularly in vivo (6) and is thus likely to be able to also affect complement synthesis in noninfected distant brain regions. The induction of complement synthesis by Nef strengthens the hypothesis of complement as a mediator of HIV neuropathogenesis, since Nef expression in astrocytes in vivo is associated with dementia (38). Further experiments will show which cellular protein interacts with Nef, presumably via the SH3 domain, to activate C3 synthesis.
The transmembrane glycoprotein gp41 was described to play an important role in HIV pathogenesis and exerts several biological and immunological functions both in the blood and in the central nervous system. gp41 is readily detected in the brains of HIV-infected adult and pediatric patients (1, 28, 29, 44). Its function in the HIV-infected brain includes induction of nitric oxide synthesis, inhibition of excitatory amino acid transport, and upregulation of the cytokines TNF-α, interleukin-1 (IL-1), and IL-10 (2, 21, 26, 27, 47). The cell signaling mechanism activated by gp41 is known only for its induction of IL-10. gp41 induces cAMP accumulation in monocytes, as shown by direct measurement of cAMP levels in the cells and by use of a specific adenylate cyclase inhibitor (3). Furthermore, a similar pattern was shown for IL-10 induction in astrocytes (47). These results fit well with our experiments that the C3 increase caused by HIV is associated with adenylate cyclase activation and cAMP levels in the cells. However, the p70S6 kinase, whose activity is a prerequisite for the induction of IL-10, plays no role in the modulation of C3 expression, as shown by use of the specific inhibitor rapamycin, indicating that the signaling cascades for the two molecules are not identical.
The finding that HIV upregulates C3 synthesis not only in astrocytes but also in neurons emphasizes the possible role of complement in the pathogenesis of HIV in the brain. Neurons respond on incubation with HIV with enhanced levels of C3 in the culture supernatant. The increase is particularly marked because spontaneous C3 mRNA expression in unstimulated cells is very low. Interestingly, neurons express both chemokine receptors and predominant HIV coreceptors CXCR4 and CCR5 (25, 50), which were shown to be important for the HIV effect in astrocytes, since blocking of the two receptors significantly suppressed the upregulation of C3 expression (47). The enhanced C3 synthesis in infected neurons might also contribute to the increased C3 levels found in the cerebrospinal fluid of patients with HIV infection and signs of central nervous system dysfunction (24). The increased complement synthesis in two important cell types of the central nervous system further supports the hypothesis that complement might play a pivotal role in AIDS-associated pathogenesis of the brain.
Although the increase in C3 expression in neurons is similar to that in astrocytes, some differences between these two cell types are evident. The expression of C3 by neurons is lower than that of astrocytes, with a longer lag phase after addition of the virus. Whereas HIV-infected astrocytes upregulate the expression of both C2 and C3 (48), neurons responded only with enhanced levels of C3, leaving the amount of C2 mRNA unaltered. Furthermore, gp41 is inactive in neurons, whereas it can induce C3 synthesis in astrocytes. These data indicate a cell specificity for complement induction by HIV. The lack of gp41 responsiveness of the neurons might be due to the lack of gp41 receptors on the cell surface.
In conclusion, our experiments elucidate the signaling pathway of HIV-induced complement expression in astrocytes and assign to neurons a novel role in complement synthesis in the HIV-infected brain. Further experiments will focus on the role of HIV-induced complement synthesis by astrocytes and neurons in the antiviral defense mechanisms of the brain and in the development of neurodegenerative AIDS dementia complex.
Acknowledgments
This study was supported by the Ludwig Boltzmann Society, the FWF (project P15375), the BMSG, and the State of Tyrol.
We gratefully acknowledge the generous gift of monoclonal anti-C3 antibody BB5 from W. Prodinger (Institute of Hygiene and Social Medicine, Innsbruck, Austria). We are grateful to the MRC for providing various reagents. We thank H. Katinger of the Institute of Applied Microbiology (Vienna, Austria) for the p24 antibodies. The excellent technical assistance of M. Hagleitner is acknowledged.
REFERENCES
- 1.Achim, C. L., R. Wang, D. K. Miners, and C. A. Wiley. 1994. Brain viral burden in HIV infection. J. Neuropathol. Exp. Neurol. 53:284-294. [DOI] [PubMed] [Google Scholar]
- 2.Adamson, D. C., B. Wildemann, M. Sasaki, J. D. Glass, J. C. McArthur, V. I. Christov, T. M. Dawson, and V. L. Dawson. 1996. Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science 274:1917-1921. [DOI] [PubMed] [Google Scholar]
- 3.Barcova, M., C. Speth, L. Kacani, F. ℘berall, H. Stoiber, and M. P. Dierich. 1999. Involvement of adenylate cyclase and p70S6-kinase activation in IL-10 upregulation in human monocytes by gp41 envelope protein of human immunodeficiency virus type 1. Eur. J. Physiol. 437:538-546. [DOI] [PubMed] [Google Scholar]
- 4.Barnum, S. R., and J. L. Jones. 1995. Differential regulation of C3 gene expression in human astroglioma cells by interferon-γ and interleukin-1β. Neurosci. Lett. 197:121-124. [DOI] [PubMed] [Google Scholar]
- 5.Brack-Werner, R. 1999. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 13:1-22. [DOI] [PubMed] [Google Scholar]
- 6.Cheingsong-Popov, R., C. Panagiotidi, M. Ali, S. Bowcock, P. Watkins, A. Aronstam, M. Wassef, and J. Weber. 1990. Antibodies to HIV-1 nef (p27): prevalence, significance and relationship to seroconversion. AIDS Res. Hum. Retroviruses 9:1099-1105. [DOI] [PubMed] [Google Scholar]
- 7.Cheng-Mayer, C., and J. A. Levy. 1988. Distinct biological and serological properties of human immunodeficiency viruses from the brain. Ann. Neurol. 23:58-61. [DOI] [PubMed] [Google Scholar]
- 8.Church, G. M., and W. Gilbert. 1984. Genomic sequencing. Proc. Natl. Acad. Sci. USA 81:1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dashiell, S. M., P. Vanguri, and C. L. Koski. 1997. Dibutyryl cyclic AMP and inflammatory cytokines mediate C3 expression in Schwann cells. Glia 20:308-321. [PubMed] [Google Scholar]
- 10.Eikelbloom, P., C. E. Hack, J. M. Rozemuller, and F. C. Stam. 1989. Complement activation in amyloid plaques in Alzheimer's dementia. Arch. Cell. Pathol. 56:259-262. [DOI] [PubMed] [Google Scholar]
- 11.Epstein, E. G., and H. E. Gendelman. 1993. Human immunodeficiency virus type 1 infection of the nervous system: pathogenetic mechanisms. Ann. Neurol. 33:429-436. [DOI] [PubMed] [Google Scholar]
- 12.Fujinaga, K., Q. Zhong, T. Nakaya, M. Kameoka, T. Meguro, K. Yamada, and K. Ikuta. 1995. Extracellular Nef protein regulates productive HIV-1 infection from latency. J. Immunol. 155:5289-5298. [PubMed] [Google Scholar]
- 13.Gasque, P., Y. D. Dean, E. P. McGreal, J. VanBeek, and B. P. Morgan. 2000. Complement components in the innate immune system in health and disease in the central nervous system. Immunopharmacology 49:171-186. [DOI] [PubMed] [Google Scholar]
- 14.Gray, F., F. Scaravilli, I. Everall, F. Chretien, S. An, D. Boche, H. Adle-Biassette, L. Wingertsmann, M. Durigon, B. Hurtrel, F. Chiodi, J. Bell, and P. Lantos. 1996. Neuropathology of early HIV-1 infection. Brain Pathol. 6:1-15. [DOI] [PubMed] [Google Scholar]
- 15.Haga, S., T. Aizawa, T. Ishii, and K. Ikeda. 1996. Complement gene expression in mouse microglial and astrocytes in culture: comparison with mouse peritoneal macrophages. Neurosci Lett. 216:191-194. [DOI] [PubMed] [Google Scholar]
- 16.Hailer, N. P., F. L. Heppner, D. Haas, and R. Nitsch. 1998. Astrocytic factors deactivate antigen presenting cells that invade the central nervous system. Brain Pathol. 8:459-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heese, K., C. Hock, and U. Otten. 1998. Inflammatory signals induce neurotrophin expression in human microglial cells. J. Neurochem. 70:699-707. [DOI] [PubMed] [Google Scholar]
- 18.Herna Remenka, G., and K. Saksela. 2000. Interactions of HIV-1 Nef with cellular signal transducing proteins. Front. Biosci. 5:D268-D283. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann, B., P. Nishanian, T. Nguyen, M. Liu, and J. L. Fahey. 1993. Restoration of T-cell function in HIV infection by reduction of intracellular cAMP levels with adenosine analogues. AIDS 7:659-664. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann, B., P. Nishanian, T. Nguyen, P. Insixiengmay, and J. L. Fahey. 1993. Hum. immunodeficiency virus proteins induce the inhibitory cAMP/protein kinase A pathway in normal lymphocytes. Proc. Natl. Acad. Sci. USA 90:6676-6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hori, K., P. R. Burd, K. Furuke, J. Kutza, K. A. Weih, and K. A. Clouse. 1999. Human immunodeficiency virus-1-infected macrophages induce inducible nitric oxide synthase and nitric oxide (NO) production in astrocytes: astrocytic NO as a possible mediator of neural damage in acquired immunodeficiency syndrome. Blood 93:1843-1850. [PubMed] [Google Scholar]
- 22.Huang, J., L. J. Kim, R. Mealey, H. C. Marsh, Y. Zhang, A. J. Tenner, E. S. Connolly, and D. J. Pinsky. 1999. Neuronal protection in stroke by an sLEx-glycosylated complement inhibitory protein. Science 285:595-599. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, R. T., J. D. Glass, J. C. McArthur, and B. W. Chesebro. 1996. Quantitation of human immunodeficiency virus in brain of demented and nondemented patients with acquired immunodeficiency syndrome. Ann. Neurol. 39:392-395. [DOI] [PubMed] [Google Scholar]
- 24.Jongen, P. J. H., W. H. Doesburg, J. L. M. Ibrahim-Stappers, W. A. J. G. Lennens, O. R. Hommes, and K. J. B. Lamers. 2000. Cerebrospinal fluid C3 and C4 indexes in immunological disorders of the central nervous system. Acta Neurol. Scand. 101:116-121. [DOI] [PubMed] [Google Scholar]
- 25.Klein, R. S., K. C. Williams, X. Alvarez-Hernandez, S. Westmoreland, T. Force, A. A. Lackner, and A. D. Luster. 1999. Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J. Immunol. 163:1636-1646. [PubMed] [Google Scholar]
- 26.Koka, P., K. He, J. A. Zack, S. Kitchen, W. Peacock, I. Fried, T. Tran, S. S. Yashar, and J. E. Merrill. 1995. Human immunodeficiency virus 1 envelope proteins induce interleukin 1, tumor necrosis factor alpha and nitric oxide in glial cultures derived from fetal, neonatal, and adult human brain. J. Exp. Med. 182:941-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kort, J. J. 1998. Impairment of excitatory amino acid transport in astroglial cells infected with the human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 14:1329-1339. [DOI] [PubMed] [Google Scholar]
- 28.Kure, K., J. F. Llena, W. D. Lyman, R. Soeiro, K. M. Weidenheim, A. Hirano, and D. W. Dickson. 1991. Human immunodeficiency virus-1 infection of the nervous system: an autopsy study of 268 adult, pediatric and fetal brains. Hum. Pathol. 22:700-710. [DOI] [PubMed] [Google Scholar]
- 29.Kure, K., K. M. Weidenheim, W. D. Lyman, and D. W. Dickson. 1990. Morphology and distribution of HIV-1 gp41-positive microglia in subacute AIDS encephalitis. Pattern of involvement resembling a multisystem degeneration. Acta Neuropathol. 80:393-400. [DOI] [PubMed] [Google Scholar]
- 30.Moller, T., C. Nolte, R. Burger, A. Verkhratsky, and H. Kettenmann. 1997. Mechanisms of C5a and C3a complement fragment-induced [Ca2+]I signalling in mouse microglia. J. Neurosci. 17:615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan, B. P. 1993. Role of complement in the brain, p. 353-375. In K. Whaley, M. Loos, and J. M. Weiler (ed.), Complement in health and disease, 2nd ed. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 32.Morgan, B. P., and P. Gasque. 1996. Expression of complement in the brain: role in health and disease. Immunol. Today 17:461-466. [DOI] [PubMed] [Google Scholar]
- 33.Nataf, S., P. F. Stahel, N. Davoust, and S. R. Barnum. 1999. Complement anaphylatoxin receptors on neurons: new tricks for old receptors? Trends Neurosci. 22:397-402. [DOI] [PubMed] [Google Scholar]
- 34.Nokta, M., and R. Pollard. 1991. Human immunodeficiency virus infection: association with altered intracellular lebels of cAMP and cGMP in MT-4 cells. Virology 181:211-217. [DOI] [PubMed] [Google Scholar]
- 35.Osaka, H., A. McGinty, U. E. Hoepken, B. Lu, C. Gerard, and G. M. Pasinetti. 1999. Expression of C5a receptor in mouse brain: role in signal transduction and neurodegeneration. Neuroscience 88:1073-1082. [DOI] [PubMed] [Google Scholar]
- 36.Osaka, H., P. Mukherjee, P. S. Aisen, and G. M. Pasinetti. 1999. Complement-derived anaphylatoxin C5a protects against glutamate-mediated neurotoxicity. J. Cell. Biochem. 73:303-311. [PubMed] [Google Scholar]
- 37.Popovic, M., M. G. Sarngadharan, E. Read, and R. C. Gallo. 1984. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497-500. [DOI] [PubMed] [Google Scholar]
- 38.Ranki, A., M. Nyberg, V. Ovod, M. Haltia, I. Elovaara, R. Raininko, H. Haapasalo, and K. Krohn. 1995. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS 9:1001-1008. [DOI] [PubMed] [Google Scholar]
- 39.Rogers, J., N. R. Cooper, S. Webster, J. Schultz, P. L. McGeer, S. Styren, W. H. Civin, L. Brachova, B. Bradt, P. Ward, and I. Lieberburg. 1992. Complement activation by β-amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. USA 89:10016-10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rus, H. G., L. M. Kim, F. I. Niculescu, and M. L. Shin. 1992. Induction of C3 expression in astrocytes is regulated by cytokines and Newcastle disease virus. J. Immunol. 148:928-933. [PubMed] [Google Scholar]
- 41.Sayah, S., A. M. Ischenko, A. Zhakhov, A. S. Bonnard, and M. Fontaine. 1999. Expression of cytokines by human astrocytomas following stimulation by C3a and C5a anaphylatoxins: specific increase in interleukin-6 mRNA expression. J. Neurochem. 72:2426-2436. [DOI] [PubMed] [Google Scholar]
- 42.Sheerin, N. S., W. Zhou, S. Adler, and S. H. Sacks. 1996. TNF-α regulation of C3 gene expression and protein biosynthesis in rat glomerular endothelial cells. Kidney Int. 51:703-710. [DOI] [PubMed] [Google Scholar]
- 43.Sinclair, E., F. Gray, A. Ciardi, and F. Scaravilli. 1994. Immunohistochemical changes and PCR detection of HIV provirus DNA in brains of asymptomatic HIV-positive patients. J. Neuropathol. Exp. Neurol. 53:43-50. [DOI] [PubMed] [Google Scholar]
- 44.Soontornniyomkij, V., J. A. Nieto-Rodriguez, A. J. Martinez, L. A. Kingsley, C. L. Achim, and C. A. Wiley. 1998. Brain HIV burden and length of survival after AIDS diagnosis. Clin. Neuropathol. 17:95-99. [PubMed] [Google Scholar]
- 45.Speth, C., L. Kacani, and M. P. Dierich. 1997. Complement receptors in HIV infection. Immunol. Rev. 159:49-67. [DOI] [PubMed] [Google Scholar]
- 46.Speth, C., R. Würzner, H. Stoiber, and M. P. Dierich. 1999. The complement system: pathophysiology and clinical relevance. Wien. Klin. Wochenschr. 111/110:378-391. [PubMed] [Google Scholar]
- 47.Speth, C., B. Joebstl, M. Barcova, and M. P. Dierich. 2000. HIV-1 envelope protein gp41 modulates expression of interleukin-10 and chemokine receptors on monocytes, astrocytes and neurons. AIDS 14:629-636. [DOI] [PubMed] [Google Scholar]
- 48.Speth, C., G. Stöckl, I. Mohsenipour, R. Würzner, H. Stoiber, C. Lass-Flörl, and M. P. Dierich. 2001. Human immunodeficiency virus type 1 induced expression of complement factors in human astrocytes. J. Virol. 75:2604-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veerhuis, R., I. Janssen, C. E. Hack, and P. Eikelenboom. 1996. Early complement components in Alzheimer's disease brains. Acta Neuropathol 91:53-60. [DOI] [PubMed] [Google Scholar]
- 50.Westmoreland, S. V., J. B. Rottman, K. C. Williams, A. A. Lackner, and V. G. Sassevill. 1998. Chemokine receptor expression on resident and inflammatory cells in the brain of macaques with simian immunodeficiency virus encephalitis. Am. J. Pathol. 152:659-665. [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao, B. G., A. Diab, J. Zhu, P. van der Meide, and H. Link. 1998. Astrocytes induce hyporesponses of myelin basic protein-reactive T and B cell function. J. Neuroimmunol. 89:113-121. [DOI] [PubMed] [Google Scholar]
- 52.Yasojima, K., C. Schwab, E. G. McGeer, and P. L. McGeer. 1999. Up-regulated production and activation of the complement system in Alzheimer's disease brain. Am. J. Pathol. 154:927-936. [DOI] [PMC free article] [PubMed] [Google Scholar]