Abstract
Human T-lymphotropic virus type 1 (HTLV-1) is the agent of an aggressive malignancy of CD4+ T lymphocytes, called adult T-cell lymphoma/leukemia, and is associated with numerous immune-mediated diseases. To establish infection, HTLV-1 must activate targeted T cells during early stages of infection. We recently demonstrated that the HTLV-1 accessory protein p12I is critical for persistent infection in vivo and for viral infectivity in quiescent primary lymphocytes, suggesting a role for p12I in lymphocyte activation. To test whether p12I modulates signaling pathways required for T-lymphocyte activation, we examined AP-1-, NF-κB-, and nuclear factor of activated T cells (NFAT)-driven reporter gene activity in p12I-expressing Jurkat T cells compared to vector-transfected control cells. HTLV-1 p12I specifically induced NFAT-mediated transcription approximately 20-fold in synergy with the Ras/mitogen-activated protein kinase pathway, but did not influence AP-1- or NF-κB-dependent gene expression. Inhibition of calcium-dependent signals by cyclosporin A, BAPTA-AM [glycine, N,N′-1,2-ethanediylbis(oxy-2,1-phenylene)-bis-N-2-(acetyloxy)methoxy-2-oxoethyl]-[bis(acetyloxy)methyl ester], and a dominant negative mutant of NFAT2 abolished the p12I-mediated activation of NFAT-dependent transcription. In contrast, inhibition of phospholipase C-γ and LAT (linker for activation of T cells) did not affect p12I-induced NFAT activity. Importantly, p12I functionally substituted for thapsigargin, which selectively depletes intracellular calcium stores. Our data are the first to demonstrate a role for HTLV-1 p12I in calcium-dependent activation of NFAT-mediated transcription in lymphoid cells. We propose a novel mechanism by which HTLV-1, a virus associated with lymphoproliferative disease, dysregulates common T-cell activation pathways critical for the virus to establish persistent infection.
Human T-lymphotropic virus type 1 (HTLV-1) is the etiologic agent of adult T-cell lymphoma-leukemia (ATL), an aggressive non-Hodgkin's lymphoma-leukemia that is composed of mature T cells of CD4+ CD25+ cell surface phenotype (53, 58). HTLV-1 infection is further associated with tropical spastic paraparesis/HTLV-1-associated myelopathy and a variety of other immune-mediated disorders (4, 27, 60). While the diseases linked to the viral infection have been extensively characterized, the early molecular mechanisms leading to persistent infection of lymphocytes by HTLV-1 are poorly defined.
As a complex retrovirus, HTLV-1 encodes several regulatory and accessory proteins in four open reading frames (pX ORF I to IV) in the 3′ pX region of its genome. pX ORF III and IV encode the well-characterized posttranscriptional regulator Rex and the viral transactivator Tax, respectively (6, 47). Although the expression levels of both Tax and Rex during the natural infection in vivo are controversial, the requirement for the two proteins in viral replication is well appreciated. In addition, pX ORF I and II encode four accessory proteins, p12I, p27I, p13II, and p30II (5, 35). Messenger RNA species specific for these proteins can be detected in HTLV-1-transformed cell lines and in peripheral blood mononuclear cells (PBMC) of diseased human patients and asymptomatic carriers (5, 9, 12, 35). Similar to Tax and Rex, antibodies (11, 18) as well as cytotoxic T lymphocytes (52) directed against the four proteins have been detected in patients with and without disease. Thus, p12I, p27I, p13II, and p30II are chronically produced during the viral infection at levels sufficient to elicit specific antibody, as well as cell-mediated immune responses.
Despite this evidence, the functional role of the accessory protein p12I in the establishment of persistent infection characteristic of HTLV-1 has only recently been investigated. We have demonstrated that p12I is critical for establishment of persistent infection in vivo by comparing the infectivity of a wild-type infectious clone of HTLV-1, ACH (15, 31), and its corresponding pX ORF I deletion mutant, ACH.p12, in a rabbit model of infection (14). Furthermore, we reported that p12I is necessary for viral infectivity in quiescent primary lymphocytes in vitro (2). Taken together, our findings indicated an involvement of p12I in activation of T lymphocytes, enhancing early stages of HTLV-1 infection. This is significant, as the vast majority of circulating and tissue-associated T cells in individuals exposed to HTLV-1 are quiescent and must be activated to allow establishment of the persistent infection (27).
Biochemical evidence also suggests that HTLV-1 p12I is a candidate protein to mediate virus-induced T-cell activation. The 99-amino-acid protein contains two putative transmembrane domains and four proline-rich SH3-binding motifs (22). In addition, p12I associates with the H+ vacuolar ATPase, as well as the interleukin-2 (IL-2) receptor β- and γ-chain (23, 36, 45). Although it does not appear to dysregulate IL-2 receptor signaling pathways in HTLV-1-immortalized cell lines (13), the transduction of primary lymphocytes with a retrovirus vector expressing p12I causes a modest increase in Stat5 phosphorylation and may reduce IL-2 requirements for T-cell proliferation (48).
We have recently demonstrated that HTLV-1 p12I localizes to the endoplasmic reticulum (ER) and cis-Golgi compartments and associates with two ER-resident calcium-binding proteins, calreticulin and calnexin (19), the former of which has been linked to activation of nuclear factor of activated T cells (NFAT) (29). Taken together, these findings suggest a role for p12I in dysregulation of calcium-dependent signaling pathways in lymphocytes during early stages of HTLV-1 infection.
A potential target for p12I-mediated T-cell activation is the T-cell receptor (TCR) signaling pathway, which leads to induction of NF-κB-, AP-1-, and NFAT-mediated transcription via protein kinase C, the Ras/mitogen-activated protein kinase (MAPK) pathway, and calcium-mediated signaling events, respectively (17, 57, 61). NFAT-mediated transcription is induced by the cooperative DNA binding of any one of four cytosolically activated NFAT isoforms (NFAT1 to -4) and a constellation of nuclear transcription factors, for example, AP-1 (16, 40). Among the four cytosolic isoforms of NFAT, NFAT2 is the predominantly induced isoform in activated T cells (55). Furthermore, NFAT2 is highly sensitive to activation by calcium (50) and crucial for proliferation of mature lymphocytes (63).
To elucidate the function of HTLV-1 p12I during early lymphocyte activation, we examined levels of AP-1-, NF-κB-, and NFAT-mediated transcription in p12I-expressing Jurkat T cells. We demonstrate a robust (∼20-fold) and specific induction of NFAT activity in Jurkat T cells expressing p12I compared to vector-transfected control cells in the presence of phorbol myristate acetate (PMA). This effect was sensitive to treatment with cyclosporin A, BAPTA-AM [glycine-N,N′-1,2-ethanediylbis(oxy-2,1-phenylene)-bis-N-2-(acetyloxy)methoxy-2-oxoethyl]-[bis(acetyloxy)methyl ester], and a dominant negative mutant of NFAT2, as well as the MEK-1 inhibitor U0126 and dominant negative AP-1, indicating a synergistic effect between HTLV-1 p12I and the RAS/MAPK pathway. Importantly, p12I functionally substituted for thapsigargin, which selectively depletes intracellular calcium stores. Moreover, we demonstrate colocalization of p12I with the ER-resident protein calreticulin in Jurkat T cells, further suggesting a direct involvement of p12I in dysregulation of calcium-mediated signaling in infected T lymphocytes. Thus, p12I provides a crucial step in the establishment of persistent infection by HTLV-1 through activation of newly targeted lymphocytes. Based on these findings, we propose a novel mechanism by which HTLV-1 modulates its cellular milieu during the early stages of infection to promote replication and ultimately disease.
MATERIALS AND METHODS
Cells.
Jurkat T cells (clone E6-1; American Type Culture Collection catalog number TIB-152) and the LAT-deficient Jurkat T-cell line J.Cam2.5 (provided by A. Weiss, University of California-San Francisco) (59) were maintained in RPMI 1640 (BRL Life Sciences, Rockville, Md.) supplemented with 15% fetal bovine serum (FBS), 100 μg of streptomycin-penicillin per ml, 2 mM l-glutamine, and 10 mM HEPES (Life Technologies, Rockville, Md.) (complete RPMI [cRPMI]).
Plasmids and antibodies.
pME-18S (pME) and pME-p12, which expresses a fusion of HTLV-1 p12I with the influenza virus hemagglutinin (HA1) tag, were provided by G. Franchini (National Cancer Institute, National Institutes of Health) (34). The AP-1- and NF-κB-luciferase reporter plasmids were purchased from Stratagene (La Jolla, Calif.). The NFAT-luciferase construct pNFAT-luc contains a trimerized human distal IL-2 NFAT site inserted into the minimal IL-2 promoter (50) and was a generous gift from G. Crabtree (Stanford University).
pSH160c and pSH102CΔ418 (49), encoding wild-type and dominant negative NFAT2, respectively, were also provided by G. Crabtree. CMV500 and CMV500-A-Fos, which encode a dominant negative mutant of the transcription factor AP-1 (51), were obtained from C. Vinson (National Institutes of Health). The β-galactosidase-encoding reporter gene plasmid pCMV.SPORT-β-gal was purchased from Life Technologies. pc-AUPLC-Li, encoding an AU1-tagged lipase-inactive, dominant-negative mutant of phospholipase Cγ (PLCγ), and the empty vector pcDNA (30) were kindly provided by R. Abraham (Duke University).
Monoclonal antibodies directed against the AU1 (clone AU1), HA1 (clone 16B12), and Flag tag (clone M2) were purchased from Covance Research Products (Richmond, Calif.) and Sigma Chemical Corporation (St. Louis, Mo.), respectively. Monoclonal antibodies specific for phospho-ERK1/2 (clone E10) and nonphosphorylated ERK2 (clone 33) were obtained from Cell Signaling Technologies (Berkeley, Mass.) and BD Transduction Laboratories (San Diego, Calif.), respectively. The polyclonal anti-Erk1/2 antibody K-23 and the polyclonal anti-p300 antibody N-15 were purchased from Santa Cruz (Santa Cruz, Calif.).
Polyclonal antibodies directed against calreticulin were purchased from Affinity Bioreagents (Golden, Colo.). The NFAT2 monoclonal antibody (clone 7A6) was from BD Pharmingen (San Diego, Calif.). The Alexa 488-conjugated anti-rabbit immunoglobulin (Ig) and indocarbocyanine-3-conjugated anti-mouse Ig antibodies used for immunofluorescence staining were purchased from Molecular Probes (Eugene, Oreg.) and Jackson ImmunoResearch (West Grove, Pa.), respectively. Anti-CD3 (clone HIT3a) and CD28 (clone CD28.2) monoclonal antibodies were from BD Pharmingen.
Transfections and measurement of luciferase activity.
For analysis of AP-1, NF-κB, and NFAT transcriptional activity in pME- and pME-p12-transfected Jurkat T cells, 107 cells were electroporated in cRPMI (350 V, 975 μF; Bio-Rad Gene Pulser II) with 30 μg of expression vector, 10 μg of reporter plasmid (AP-1-luc, NF-κB-luc, or NFAT-luc) and 1 μg of pCMV.SPORT-β-gal to normalize for transfection efficiency. Vectors encoding dominant-negative mutants were cotransfected at concentrations that gave maximum inhibition of reporter gene activity, determined in functional titration assays. Corresponding empty control vectors were included at identical concentrations to equilibrate the total amount of DNA transfected. After transfection, cells were seeded in six-well plates at a density of 5 × 105/ml. Cells were either left untreated or stimulated with PMA (20 ng/ml), ionomycin (2 μM) (Sigma), or both, as well as thapsigargin (Sigma) at various concentrations 6 h posttransfection, followed by incubation for 18 h prior to lysis for analysis of luciferase activity.
Stimulations with anti-CD3 and/or anti-CD28 antibodies (each at 3 μg/ml) were carried out 18 h posttransfection for a period of 8 h. For investigation of the effect of pharmacological inhibitors, cyclosporin A (Sigma) (200 nM), BAPTA-AM [glycine, N,N′-1,2-ethanediylbis(oxy-2,1-phenylene)-bis-N-2-(acetyloxy)methoxy-2-oxoethyl]-[bis(acetyloxy)methyl ester] (Molecular Probes) (10 μM), the MEK-1 inhibitor U0126 [1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene] (Promega, Madison, Wis.) (10 μM), or wortmannin (Sigma) (100 nM) was added to transfected cells 6 h posttransfection for 30 min at 37°C, followed by stimulation with either PMA or ionomycin for 18 h.
For measurement of luciferase activity, cell lysates were prepared with cell culture lysis reagent (Promega). Luciferase activity was measured using the Promega luciferase assay reagent (Promega). Values were normalized for transfection efficiency based on β-galactosidase activity, which was determined using Lumigal (Lumigen, Southfield, Mich.). Data are depicted as graphs indicating the fold induction of NFAT-luciferase activity in cells expressing p12I (pME-p12) over those transfected with empty vector (pME). Data points were expressed as the mean of at least two independent experiments conducted in duplicate. Statistical analysis was performed by Student's t test.
Immunoprecipitation and immunoblotting.
For analysis of p12I expression in transfected Jurkat and J.CaM2.5 T cells, 107 cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (10) 24 h posttransfection. Lysate supernatants were precleared with 30 μl of a 50% slurry of protein A-Sepharose beads (Sigma) for 1 h at 4°C. Precleared supernatants were subsequently incubated with 4 μg of polyclonal anti-HA1 antibody (Babco) per ml and 30 μl of a 50% bead slurry for 4 h at 4°C. Beads were washed three times with RIPA buffer and resuspended in sodium dodecyl sulfate (SDS) buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with a monoclonal anti-HA1 antibody (1:1,000). Coexpression of HTLV-1 p12I and dominant negative signaling proteins and levels of ERK1/2 were analyzed by immunoblot using 10 or 15% polyacrylamide gels (2). Inhibitors and stimulants were added and cells were lysed as described above. For all immunoblot assays, protein concentrations were determined by the BCA assay (Micro BCA Protein Assay; Pierce, Rockford, Ill.).
Indirect immunofluorescence assays.
For visualization of p12I intracellular localization, transfected Jurkat T cells (4 × 105) were seeded onto two-well Labtek chamber slides (Nalgene Nunc International, Rochester, N.Y.) coated with CellTak (BD Biosciences, San Diego, Calif.) and fixed for 15 min with 4% paraformaldehyde. Cells were washed twice with phosphate-buffered saline (PBS) and incubated with primary antibody in antibody dilution buffer (ADB) (10 mM NaPO4, 0.5 M NaCl, 0.5% Triton X-100, 2% bovine serum albumin, 5% normal goat serum) for 1 h. After one additional wash with PBS, cells were incubated with appropriate fluorescein isothiocyanate (FITC)- or indocarbocyanine-3 (Cy3-)-labeled secondary antibody in ADB for another 45 min before final washing and mounting in glycerol-PBS. Cell nuclei were stained using bisbenzimide H33258 (Hoechst 33258; Calbiochem, San Diego, Calif.). Fluorescence microscopy and image collection were performed using a Bio-Rad MRC1024 microscope with Bio-Rad Lasersharp software.
RESULTS
Activation of NFAT-mediated transcription by HTLV-1 p12I.
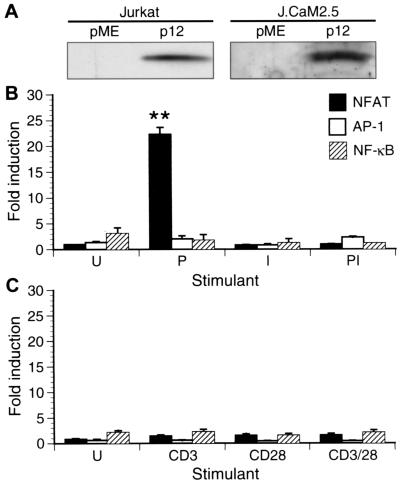
To obtain a model system that would test the function of p12I in the preferred target cell of HTLV-1, the CD4+ T lymphocyte, we optimized the expression of p12I in Jurkat T cells (E6-1 clone), as well as in the LAT-deficient Jurkat T-cell line J.CaM 2.5 (Fig. 1A). To test whether p12I could complement specific and commonly used stimulators of T-cell signaling pathways, we examined the activity of NF-κB, AP-1, and NFAT in p12I-transfected Jurkat T cells during stimulation with PMA or ionomycin. While PMA specifically increases NF-κB- and AP-1-mediated transcription (3) via either protein kinase C or RasGRP (20, 38, 54), ionomycin activates calcium-mediated signals (8).
FIG. 1.
Specific activation of NFAT transcriptional activity in lymphoid cells by HTLV-1 p12I and the Ras/MAPK pathway. (A) Expression of HA1-tagged HTLV-1 p12I in Jurkat T cells and the LAT-deficient Jurkat T-cell line J.Cam2.5, as analyzed by immunoprecipitation and immunoblot. (B) Jurkat T cells transfected with pME or pME-p12 and corresponding reporter plasmids were either left untreated (U) or treated with PMA (P), ionomycin (I), or a combination of PMA and ionomycin (PI). Graphs depict the fold induction of NFAT-luciferase activity in cells expressing p12I (pME-p12) over those transfected with empty vector (pME). Data points are the means of triplicate samples and at least four independent experiments. Statistical significance of NFAT-luciferase induction was analyzed by Student's t test. (C) Transfected Jurkat T cells were treated with anti-CD3 or anti-CD28 or both antibodies. Data points are the mean of triplicate samples and two independent experiments.
We observed an approximately 20-fold induction of NFAT-luciferase activity in HTLV-1 p12I-transfected cells over vector-transfected control cells in the presence of PMA (Fig. 1B). This indicated that HTLV-1 p12I effectively substituted for ionomycin-induced, calcium-mediated signals leading to activation of NFAT. PMA provides a stimulatory signal for activation of AP-1, which then synergizes with NFAT to induce transcription from the NFAT:AP-1 compound promoter element driving the NFAT reporter construct (50). While Jurkat T cells were highly responsive to treatment with PMA or ionomycin, no significant increase in NFAT-dependent transcription in p12I-expressing cells over vector control cells was detected in the absence of stimulation or in the presence of ionomycin or both ionomycin and PMA (Fig. 1B). While the addition of ionomycin masks the stimulatory effect of p12I by potently inducing calcium-mediated signals itself, unstimulated cells expressing p12I are lacking the costimulatory signal provided by PMA. Furthermore, the stimulatory effects of p12I specifically involved NFAT-dependent transcription, as the activity of NF-κB or AP-1 remained unchanged regardless of the stimulation conditions (Fig. 1B). Taken together, these results indicate that HTLV-1 p12I effectively substitutes for the calcium ionophore.
Furthermore, p12I did not increase NF-κB-, AP-1-, or NFAT-dependent gene expression during broad stimulation of Jurkat T cells with anti-CD3 and anti-CD28 antibodies (Fig. 1C). These findings are also expected, because CD3 and CD28 ligation either induces calcium-mediated signals, masking the requirement for p12I expression, or induces antiapoptotic signals that do not synergize with p12I-activated NFAT (8, 43). Our results were not related to insufficient stimulation of transfected cells by the antibodies used, as treatment of Jurkat T cells with a combination of anti-CD3 and CD28 antibodies resulted in significantly increased absolute values of NFAT-luciferase activity (377 ± 36 [standard error of the mean (SEM)] untreated to 684 ± 72 arbitrary light units [ALU] in antibody-treated cells). Furthermore, in accordance with previous reports (43, 55), absolute NFAT activity was also increased by anti-CD3 but not anti-CD28 (769 ± 98 ALU for anti-CD3- and 424 ± 52 ALU for anti-CD28-treated cells).
Genetic inhibition of NFAT2 abrogates p12I-mediated NFAT activation.
The results presented in Fig. 1 strongly suggested that HTLV-1 p12I induces transcription from the NFAT:AP-1 compound promoter by activation of the NFAT transcription factor family, but not AP-1, since no induction of AP-1-luciferase activity was observed in the presence of p12I compared to vector-transfected control cells. To confirm this hypothesis, we tested whether a dominant negative mutant of NFAT2 could inhibit p12I-mediated activation of NFAT-dependent gene expression by performing NFAT-driven reporter gene assays in the presence or absence of a dominant negative mutant of NFAT2 (dn-NFAT2) (49).
Among the four NFAT isoforms, NFAT2 is the most prominently upregulated NFAT isoform in activated T cells and is highly sensitive to induction by calcium (49, 55). The dn-NFAT2 mutant, which lacks the DNA-binding and C-terminal transactivation domain but retains its ability to interact with calcineurin, inhibited PMA- and ionomycin-induced NFAT-driven luciferase activity in a concentration-dependent manner (Fig. 2A). As expected, cotransfection of dn-NFAT2 with p12I completely abrogated the p12I-induced activation of NFAT-luciferase activity (Fig. 2B), further demonstrating that HTLV-1 p12I specifically activates the NFAT transcription factor family.
FIG. 2.
HTLV-1 p12I-induced NFAT-dependent gene expression is inhibited by dominant-negative NFAT2. (A) A dominant negative NFAT2 (dn-NFAT2) inhibits NFAT-dependent gene expression induced in Jurkat T cells by PMA-ionomycin costimulation. Values represent the means of triplicate samples and two independent experiments. Cells were transfected with NFAT-luciferase reporter but no dominant-negative NFAT2 and left unstimulated to obtain a value for basal reporter gene activity (0/−). An additional control included mock-transfected cells without the luciferase reporter and left unstimulated (m). (B) Expression of dn-NFAT2 in HTLV-1 p12I-transfected Jurkat T cells (+ column as shown above) results in complete abrogation of p12I-mediated NFAT activation. Only values for PMA-treated cells are shown. dn-NFAT2 or empty vector (20 μg) was cotransfected with pME-p12. Data points are the means of quadruplicate samples and two independent experiments. Statistical significance was analyzed by Student's t test. Inset shows detection of dn-NFAT2 by immunoprecipitation with monoclonal antibody 7A6, followed by immunoblotting with an anti-HA1 monoclonal antibody.
To further confirm the involvement of NFAT in the observed increase in reporter gene activity, we tested whether transfection of a wild-type NFAT2 (wt-NFAT2) construct could mimic the increase in NFAT-mediated transcription observed in the presence of p12I and PMA. Upon transfection into Jurkat T cells and stimulation with PMA, wt-NFAT2 induced NFAT-luciferase activity in a concentration-dependent manner analogous to that observed in HTLV-1 p12I-expressing and PMA-treated cells (data not shown).
Inhibition of calcium-dependent signals inhibits p12I-mediated NFAT activation.
Cytosolically retained NFAT is activated by the phosphatase calcineurin, which itself is activated upon calcium release from intracellular stores, mainly the ER (65). To test whether inhibition of calcineurin could abrogate the HTLV-1 p12I-mediated increase in NFAT activity, we performed NFAT reporter gene assays in the presence and absence of cyclosporin A and BAPTA-AM. While cyclosporin A blocks the ability of calcineurin to bind cytosolic calcium directly, BAPTA-AM sequesters intracellular calcium, removing the stimulatory signal for calcineurin. Both cyclosporin A and BAPTA-AM completely abolished the increase in NFAT activity observed in p12I-transfected Jurkat T cells (Fig. 3A and B), indicating that p12I requires calcium-dependent signaling events to increase NFAT-mediated transcription. Both inhibitors specifically blocked calcineurin activity, as neither cyclosporin A nor BAPTA-AM nor their solvent, dimethyl sulfoxide, affected levels of phospho-ERK1/2 or AP-1 and NF-κB reporter gene activity (data not shown).
FIG. 3.
HTLV-1 p12I-mediated increase in NFAT activity is sensitive to inhibition of calcineurin by the immunosuppressant cyclosporin A (CsA) (A) and the reduction of intracellular calcium concentration by BAPTA-AM (B). Only values for PMA-treated cells are shown. Values are the means of quadruplicate samples and three independent experiments. Statistical significance was analyzed by Student's t test.
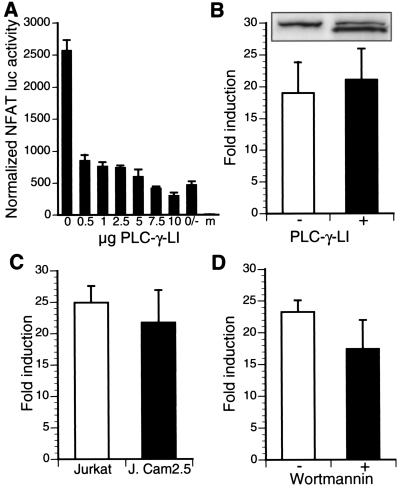
Inhibition of PLC-γ, LAT, or PI3K does not abolish p12I-mediated NFAT activation.
Upon ligation of the TCR, PLC-γ is recruited to the plasma membrane by the adapter LAT (64) and hydrolyzes phosphatidylinositol-4,5-bisphosphate (PI-[4,5]P2) into diacylglycerol (DAG) and inositol triphosphate (IP3). IP3 induces calcium release from the ER by binding to its corresponding receptor on the ER membrane (8). To test whether a dominant-negative mutant of PLC-γ (PLC-γ-LI) could inhibit the p12I-mediated increase in NFAT activity, we analyzed NFAT-luciferase activity in p12I-transfected Jurkat T cells in the presence or absence of PLC-γ-LI. PLC-γ-LI is a lipase-inactive mutant of PLC-γ and potently inhibits CD3- and CD28-stimulated NFAT activity (Fig. 4A). However, when cotransfected with p12I, PLC-γ-LI could not abrogate the p12I-induced activation of NFAT (Fig. 4B). Furthermore, the LAT-deficient Jurkat T-cell clone J.Cam2.5 displayed a p12I-induced increase in NFAT activity identical to that observed in the wild-type Jurkat T cell clone E6-1 (Fig. 4C). Taken together, these results indicate that HTLV-1 p12I acted on the calcium signaling pathway at the level of the ER.
FIG. 4.
Inhibition of PLC-γ1 and LAT or PI3K does not affect p12I-mediated induction of NFAT activity. (A) A lipase-inactive mutant of PLC-γ1 (PLC-γ-LI) potently inhibits CD3- and CD28-mediated T-cell stimulation. Control (0/−), 0 μg of PLC-γ-LI, no stimulation; m, mock transfection. (B) PLC-γ-LI (10 μg of pc-AUPLC-LI) does not inhibit HTLV-1 p12I-mediated activation of NFAT. Only values for PMA-treated cells are shown. Values represent the means of quadruplicate samples and two independent experiments. Statistical significance was analyzed by Student's t test. Expression of PLC-γ-LI was determined by AU1-specific immunoblot and is shown in the graph inset (lower band). (C) HTLV-1 p12I activates NFAT-dependent gene expression in the LAT-deficient Jurkat line J.CaM2.5 to levels comparable to those in wild-type Jurkat T cells. Values represent the means of quadruplicate samples and two independent experiments. Statistical significance was analyzed by Student's t test. (D) Inhibition of PI3K does not abolish p12I-mediated NFAT activation. Jurkat T cells transfected with pME or pME-p12 and pNFAT-luc were treated with wortmannin as described. Values represent the means of quadruplicate samples and two independent experiments
Phosphatidylinositol 3-kinase (PI3K) produces the membrane phospholipid phosphatidyl-3, 4,5-triphosphate (PIP3), which recruits a variety of pleckstrin homology domain (PH)-containing proteins to the plasma membrane, including Akt/protein kinase B and the Tec family of kinases. While Akt mainly activates antiapoptotic signaling pathways via Bcl-2 and NF-κB via MAPKKK (37), Tec kinases activate PLC-γ, enhancing calcium-dependent signals (56). To analyze the effect of PI3K on p12I-induced NFAT activation, we performed NFAT-driven reporter gene assays in the presence or absence of the PI3K inhibitor wortmannin. Wortmannin treatment did not affect the activity of either AP-1 or NF-κB or the phosphorylation status of ERK1/2 (data not shown), nor did it block the p12I-mediated increase in NFAT activity (Fig. 4D), suggesting that p12I acted independently of PI3K on NFAT.
Localization of HTLV-1 p12I in Jurkat T cells.
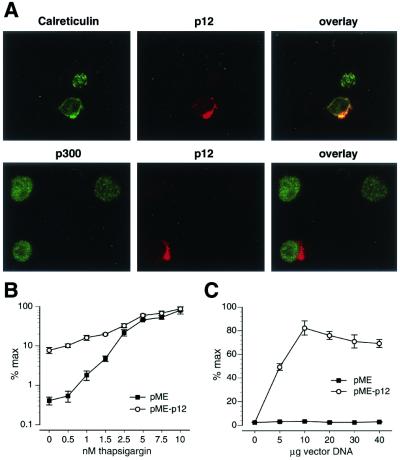
Our laboratory (19) and others (28) have reported the localization of HTLV-1 p12I to endomembranes, including the ER of transiently transfected fibroblast cell lines. Furthermore, we have demonstrated the association of p12I with the ER-resident, calcium-regulating proteins calreticulin and calnexin (19), suggesting a possible influence of p12I on calcium homeostasis. To test whether HTLV-1 p12I localizes to cellular compartments consistent with the ER in T cells, we performed immunofluorescence staining of p12I-transfected Jurkat T cells. HTLV-1 p12I localized to structures in the perinuclear region resembling the ER, which was confirmed by double staining with anticalreticulin antibodies (Fig. 5A, upper panel). The perinuclear localization was further confirmed by nuclear staining using either a polyclonal anti-p300 antibody (Fig. 5A, lower panel) or Hoechst 33258 (data not shown).
FIG. 5.
HTLV-1 p12I localizes to the ER in lymphoid cells and functionally substitutes for thapsiargin. (A) Jurkat T cells transfected with pME (not shown) or pME-p12 were fixed and stained for p12I, endogenously expressed calreticulin, or the nuclear transcriptional activator p300. HTLV-1 p12I colocalizes with the ER-resident protein calreticulin (upper panel), while no colocalization was observed with p300 (lower panel). (B) Jurkat T cells transfected with pME or pME-p12 were stimulated with PMA (20 ng/ml) and the indicated amounts of thapsigargin. NFAT-luciferase activity was measured as described and plotted on a logarithmic scale as a percentage of maximal activation induced by the addition of thapsigargin. (C) To confirm that the increased NFAT-luc activity in B was due to the expression of HTLV-1p12I, increasing amounts of pME and pME-p12I were transfected and cells were stimulated with 0.5 nM thapsigargin and PMA (20 ng/ml). The highest luciferase value obtained with 10 μg of pME-p12I was set at 100%.
Substitution of thapsigargin-modified calcium fluxes to the ER by HTLV-1 p12I.
To confirm that the ER localization of p12I indeed correlated with the ability of p12I to release calcium from that store, we tested whether p12I could functionally substitute for thapsigargin. Unlike ionomycin, which acts at both the ER and the cell membrane, thapsigargin blocks capacitative calcium influx into the ER and thereby selectively depletes intracellular calcium stores in the ER. Jurkat T cells were transfected with empty vector (pME) or p12I-encoding vector (pME-p12) and treated with increasing amounts of thapsigargin. Release of calcium from the ER was measured by NFAT-luciferase activity (25).
Jurkat T cells transfected with pME responded to thapsigargin in a sigmoidal response curve, with saturation occurring at approximately 5 nM (Fig. 5B, open circles). In sharp contrast, Jurkat T cells expressing HTLV-1 p12I had a significant (20-fold) activation of NFAT-driven transcription in the absence of thapsigargin, but also a marked enhancement of NFAT activity at low concentrations of thapsigargin (Fig. 5B, solid squares). To confirm that the increase in NFAT-luciferase activity was due to the presence of HTLV-1 p12I, increasing amounts of pME and pME-p12 were transfected into Jurkat T cells, and the cells were stimulated with 0.5 nM thapsigargin and 20 ng of PMA per ml. A marked increase in NFAT-luc activity was observed in the p12I-expressing cells even at lower concentrations of pME-p12 DNA (5 μg), and increases in NFAT-luc activity depended on the amount of transfected pME-p12 DNA, with saturation at 10 μg (Fig. 5C). These data demonstrate that HTLV-1 p12I can functionally substitute for thapsigargin in activating NFAT reporter gene activity in synergy with PMA.
Synergistic activation of NFAT by HTLV-1 p12I and the Ras/MAPK pathway.
The requirement for PMA for the p12I-induced activation of NFAT-mediated transcription suggested an involvement of the Ras/MAPK pathway. This pathway contributes to NFAT-dependent gene expression by providing the nuclear component of NFAT, designated NFATn, to represent the whole constellation of nuclear transcription factors that can serve this function. The identity of this component has been controversial (24) and appears to be dependent on the promoter providing the specific NFAT response element (39). However, recent reports provide compelling evidence that the transcription factor AP-1 synergizes with NFAT at the NFAT response element of the interleukin-2 promoter (39).
To test whether genetic and pharmacological inhibition of the Ras/MAPK pathway could abolish the p12I-mediated NFAT activation, we first inhibited the activity of the MAPKK MEK-1 by treatment of transfected cells with the MEK-1 inhibitor U0126. Addition of U0126 to p12I-expressing Jurkat T cells resulted in complete loss of p12I-induced NFAT activation (Fig. 6A). This was due to direct inhibition of MEK-1, as we observed an absence of phosphorylated ERK1/2 in U0126-treated Jurkat T cells, but no effect of U0126 on NF-κB transcriptional activity (data not shown).
FIG. 6.
HTLV-1 p12I-mediated activation of NFAT is sensitive to inhibition of the Ras/MAPK pathway. (A) Treatment of pME- and pME-12-transfected Jurkat T cells with U0126 results in complete abrogation of p12I-mediated NFAT activity. Only values for PMA-treated cells are shown. Values represent the means of quadruplicate samples and two independent experiments. Statistical significance was analyzed by Student's t test. (B) The expression of a dominant negative mutant of AP-1 (A-Fos) (+ column) completely abrogates the p12I-mediated induction of NFAT activity. Jurkat T cells were cotransfected with pME or pME-p12 and 10 μg of pCMV500-A-Fos or the corresponding empty vector pCMV500. Only values for PMA-treated cells are shown. Values represent the means of quadruplicate samples and two independent experiments. Statistical significance was analyzed by Student's t test. Expression of A-Fos was examined by immunoblot (inset) using a monoclonal antibody directed against the A-Fos N-terminal Flag tag. (C) A-Fos potently inhibits PMA- and ionomycin-stimulated NFAT activity. Jurkat T cells were cotransfected with pNFAT-luc and the indicated amounts of pCMV500-A-Fos and treated with PMA plus ionomycin.
To test specifically whether AP-1 synergized with NFAT to increase NFAT-mediated transcription, we performed cotransfection studies using a dominant negative form of AP-1, A-Fos (51). This mutant completely abrogated the p12I-induced NFAT activation in the presence of PMA (Fig. 6B). Importantly, A-Fos was confirmed to be expressed in transfected cells (Fig. 6B, insert) and to inhibit PMA- and ionomycin-stimulated transcription from the NFAT-luciferase reporter plasmid (Fig. 6C). These data demonstrate that AP-1 synergizes with NFAT to initiate transcription from the IL-2 promoter-derived NFAT response element and further support an important role for AP-1 in NFAT-mediated gene expression.
DISCUSSION
With the exception of the human and simian immunodeficiency virus (HIV and SIV) lentiviruses, retroviruses, including HTLV-1, require actively dividing cells for integration of the proviral DNA and subsequent viral gene expression. Herein, we provide data that support a novel mechanism by which the accessory protein p12I of HTLV-1 synergistically with the Ras/MAPK pathway promotes NFAT2 activation and thus may facilitate host cell activation and establishment of persistent HTLV-1 infection. Importantly, NFAT is critical for proliferation of peripheral lymphocytes (63), the primary target cells for HTLV-1 infection. In particular, expression of HTLV-1 p12I in Jurkat T cells leads to an approximately 20-fold induction of NFAT-dependent gene expression that was dependent on cytosolic calcium. The inhibitory effects of a dominant-negative mutant of NFAT2 further confirmed the specific activation of the NFAT transcription factor family by p12I.
Membrane-proximal signaling molecules were dispensable for p12I-mediated NFAT activation, as p12I also caused a significant activation of NFAT in LAT-deficient Jurkat T cells (J.CaM2.5) and in the presence of a dominant negative mutant of PLC-γ. HTLV-1 p12I colocalized with the ER-resident calcium-binding protein calreticulin in Jurkat T cells, supporting our previous finding that p12I directly binds both calreticulin and calnexin (19). Taken together, these results suggest a direct effect of HTLV-1 p12I on calcium homeostasis in the ER, possibly through a functional interaction with calreticulin and calnexin that modulates the regulation of intracellular calcium levels and NFAT activation in T lymphocytes.
To initiate transcription, NFAT complexes with a variety of nuclear transcription factors at most of its respective promoter response elements, for example, AP-1 at the NFAT response element in the IL-2 promoter (39). As expected, the induction of NFAT reporter gene activity by HTLV-1 p12I required activation of the Ras/MAPK pathway, as shown by the inhibitory effect of U0126 and a dominant negative AP-1. This indicates that p12I effectively substitutes for calcium ionophore and that the Ras/MAPK pathway, in particular AP-1, synergizes with NFAT, as reported previously (50). Moreover, the 20-fold induction of NFAT activity in cells expressing p12I over vector-transfected control cells is only observed in the presence of PMA and not upon stimulation with ionomycin or both PMA and ionomycin. As reported by others (54), we used PMA to activate PKC and thereby promote the nuclear component (e.g., AP-1) necessary for complete NFAT activation (20, 38). Since ionomycin itself potently raises cytosolic calcium, it would be predicted to override the requirement for p12I-mediated calcium release from the ER. Furthermore, unstimulated cells lack the positive effects of HTLV-1 p12I on NFAT-dependent gene expression, since they lack the PMA-induced AP-1 coactivation.
Importantly, we show that HTLV-1 p12I can functionally substitute for thapsigargin in inducing NFAT-driven transcription. Thapsigargin blocks the capacitative calcium influx in the ER and thus selectively depletes intracellular calcium stores. Together, our data support the tenet that HTLV-1 p12I causes an increase in calcium release from the ER to activate NFAT. Interestingly, the cellular protein CAML (Ca2+-modulating cyclophilin ligand) induces calcium release from the ER in a fashion proposed for HTLV-1 p12I (25). Like HTLV-1 p12I, CAML contains two putative transmembrane domains, colocalizes with calreticulin in the ER (26), and leads to NFAT activation (7). Thus, the accessory protein p12I of HTLV-1 appears to mimic the function of a host cell protein to increase cytosolic calcium and facilitate pathological T-cell activation and eventually viral infection and replication.
Our data presented here and in our other published reports (2, 14, 19) support a novel role for p12I in HTLV-1-mediated host cell activation (Fig. 7). In this model, p12I expression would result in the release of calcium from the ER by modulating the function of the calcium-binding proteins, such as calreticulin and calnexin. This could lead to an initial spike in cytosolic calcium, as suggested by the capacity of p12I to substitute for thapsigargin (21, 46). Indeed, our current studies indicate that p12I-expressing Jurkat cells contain elevated cytoplasmic calcium levels compared to vector-transfected control cells (W. Ding, personal communications).
FIG. 7.
Model for HTLV-1 p12I-mediated activation of NFAT in infected T lymphocytes. HTLV-1 p12I causes an increase in the release of calcium from the ER, possibly through interaction with calcium-binding proteins such as calreticulin or calnexin. Increased levels of cytosolic calcium lead to activation of the phosphatase calcineurin (CN) and to dephosphorylation of cytosolically retained NFAT. Upon dephosphorylation, NFAT translocates to the nucleus and synergizes with AP-1 at NFAT:AP1 composite promoter elements to activate NFAT-dependent gene expression. Boxes indicate membrane-localized and membrane-activated proteins. NF-κB and NF-AT response elements (re) are indicated.
In support of our model, Mesaeli et al. (44) demonstrated impaired nuclear translocation of NFAT3 in calreticulin-deficient (crt−/−) mice, which can be rescued by transfection of crt−/−-deficient fibroblasts with a calreticulin expression vector. Their data provide a functional link between calreticulin and NFAT activation, suggesting that similar to its importance in cardiac development, the calreticulin/Ca2+/calcineurin/NFAT regulatory pathway could play an essential role during T-cell activation.
Intriguingly, calcium-dependent activation of NFAT is essential for efficient infectivity of HIV in primary lymphocytes (32, 33). Primary CD4+ T cells stably expressing NFAT2 become highly susceptible to infection by HIV, while cells transduced with empty vector do not become infected. Consistent with our hypothesis of a functional role for p12I during early stages of the infection, Kinoshita et al. (32) demonstrate that NFAT facilitates early stages during HIV replication, specifically completion of reverse transcription. Interestingly, the inhibitory effect of cyclsporin A on HIV infectivity is strongly dependent on the presence of a functional nef gene (1).
Two recent reports have delineated the induction of NFAT activity in Nef-expressing Jurkat T cells (41, 42). Similar to HTLV-1 p12I, this induction was dependent on calcium-mediated signals and required the synergistic action of the Ras/MAPK pathway. In sharp contrast to HTLV-1 p12I, however, Nef-mediated NFAT activation is dependent on plasma membrane localization of the viral protein and appears to require association with PAK2, suggesting an involvement of the PAK2/Vav/CASK pathway. This indicates that HIV Nef induces NFAT activity via a molecular mechanism that is distinct from that used by HTLV-1 p12I.
However, with respect to viral replication, both Nef and p12I may serve very similar functions. We have previously demonstrated that HTLV-p12I enhances viral infectivity by promoting activation of quiescent primary T lymphocytes (2). This would lead to disruption of the nuclear envelope and increased accessibility of the host chromatin for viral integration. Intriguingly, Wu et al. recently demonstrated in quiescent T cells the selective transcription of HIV Nef and Tat prior to viral integration (62). While the de novo expression of Nef may not necessarily induce T-cell proliferation, it clearly leads to T-cell activation, as demonstrated by increased IL-2 production by infected cells upon stimulation via CD3 or CD28. Such a scenario could well be envisioned for HTLV-1 p12I and would support the data presented here. In fact, Franchini and coworkers recently demonstrated that p12I-transduced human PBMC have a slight proliferative advantage over nontransduced control cells upon suboptimal CD3 or CD28 stimulation (48). While the authors propose that this is due to a positive effect of p12I on STAT5 signaling, their data do not exclude the possibility that their observation results from an effect secondary to p12I-induced T-cell activation. This, as is the case for Nef, could then lead to increased IL-2 production by the p12I-expressing cells themselves.
In summary, ours is the first study to describe a mechanism for HTLV-1 p12I-mediated activation of NFAT-dependent gene expression. Importantly, the results presented here are consistent with our previous findings of the importance of p12I expression in the in vivo rabbit model of HTLV-1 infection (14) and in quiescent primary cells in vitro (2). Taken together, our findings have significant implications for a better understanding of HTLV-1 virus-host interactions and establishment of persistent infection by human retroviruses in general. In addition, our data suggest that HTLV-1 p12I is a potential target for antiviral therapy or vaccine development and provide an attractive model system for further studies of T lymphocyte activation.
Acknowledgments
This work was supported by National Institutes of Health grants RR-14324 from the National Center for Research Resources and CA-70529 from the National Cancer Institute, awarded through the Ohio State University Comprehensive Cancer Center. M. Lairmore is supported by an Independent Scientist Career Award from the National Institutes of Health (K02 AI01474). B. Albrecht is supported by a Boehringer Ingelheim Fonds predoctoral fellowship. S. Tridandapani is a Leukemia and Lymphoma Society Fellow.
We thank G. Franchini, G. Crabtree, C. Vinson, R. Abraham, and A. Weiss for generously providing plasmid vectors and cell lines. We are indebted to J. Nisbet for technical assistance, T. Vojt for preparation of figures, and P. Green for helpful discussion and critical review of the manuscript.
REFERENCES
- 1.Aiken, C. 1998. Mechanistic independence of Nef and cyclophilin A enhancement of human immunodeficiency virus type 1 infectivity. Virology 248:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht, B., N. D. Collins, M. T. Burniston, J. W. Nisbet, L. Ratner, P. L. Green, and M. D. Lairmore. 2000. Human T-lymphotropic virus type 1 open reading frame I p12I is required for efficient viral infectivity in primary lymphocytes. J. Virol. 74:9828-9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altman, A., N. Isakov, and G. Baier. 2000. Protein kinase C theta: a new essential superstar on the T-cell stage. Immunol. Today 21:567-573. [DOI] [PubMed] [Google Scholar]
- 4.Bangham, C. R. 2000. HTLV-1 infections. J. Clin. Pathol. 53:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berneman, Z. N., R. B. Gartenhaus, M. S. Reitz, W. A. Blattner, A. Manns, B. Hanchard, O. Ikehara, R. C. Gallo, and M. E. Klotman. 1992. Expression of alternatively spliced human T-lymphotropic virus type 1 pX mRNA in infected cell lines and in primary uncultured cells from patients with adult T-cell leukemia/lymphoma and healthy carriers. Proc. Natl. Acad. Sci. USA 89:3005-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bex, F., and R. B. Gaynor. 1998. Regulation of gene expression by HTLV-I Tax protein. Methods 16:83-94. [DOI] [PubMed] [Google Scholar]
- 7.Bram, R. J., and G. R. Crabtree. 1994. Calcium signalling in T cells stimulated by a cyclophilin B-binding protein. Nature 371:355-358. [DOI] [PubMed] [Google Scholar]
- 8.Cantrell, D. 1996. T cell antigen receptor signal transduction pathways. Annu. Rev. Immunol. 14:259-274. [DOI] [PubMed] [Google Scholar]
- 9.Cereseto, A., Z. Berneman, I. Koralnik, J. Vaughn, G. Franchini, and M. E. Klotman. 1997. Differential expression of alternatively spliced pX mRNAs in HTLV-I-infected cell lines. Leukemia 11:866-870. [DOI] [PubMed] [Google Scholar]
- 10.Chacko, G. W., J. T. Brandt, K. M. Coggeshall, and C. L. Anderson. 1996. Phosphoinositide 3-kinase and p72syk noncovalently associate with the low affinity Fc gamma receptor on human platelets through an immunoreceptor tyrosine-based activation motif. Reconstitution with synthetic phosphopeptides. J. Biol. Chem. 271:10775-10781. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Y. A., S. Chen, C. Fu, J. Chen, and M. Osame. 1997. Antibody reactivities to tumor-suppressor protein p53 and HTLV-I TOF, REX, and TAX in HTLV-I-infected people with differing clinical status. Int. J. Cancer 71:196-202. [DOI] [PubMed] [Google Scholar]
- 12.Ciminale, V., G. N. Pavlakis, D. Derse, C. P. Cunningham, and B. K. Felber. 1992. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV type I. J. Virol. 66:1737-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins, N. D., C. D'Souza, B. Albrecht, M. D. Robek, L. Ratner, W. Ding, P. L. Green, and M. D. Lairmore. 1999. Proliferation response to interleukin-2 and Jak/Stat activation of T cells immortalized by human T-cell lymphotropic virus type 1 is independent of open reading frame I expression. J. Virol. 73:9642-9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins, N. D., G. C. Newbound, B. Albrecht, J. L. Beard, L. Ratner, and M. D. Lairmore. 1998. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood 91:4701-4707. [PubMed] [Google Scholar]
- 15.Collins, N. D., G. C. Newbound, L. Ratner, and M. D. Lairmore. 1996. In vitro CD4+ lymphocyte transformation and infection in a rabbit model with a molecular clone of human T-cell lymphotropic virus type 1. J. Virol. 70:7241-7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabtree, G. R. 1999. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell 96:611-614. [DOI] [PubMed] [Google Scholar]
- 17.Crabtree, G. R. 2001. Calcium, calcineurin, and the control of transcription. J. Biol. Chem. 276:2313-2316. [DOI] [PubMed] [Google Scholar]
- 18.Dekaban, G. A., A. A. Peters, J. C. Mulloy, J. M. Johnson, R. Trovato, E. Rivadeneira, and G. Franchini. 2000. The HTLV-I orfI protein is recognized by serum antibodies from naturally infected humans and experimentally infected rabbits. Virology 274:86-93. [DOI] [PubMed] [Google Scholar]
- 19.Ding, W., B. Albrecht, R. Luo, W. Zhang, J. R. Stanley, G. C. Newbound, and M. D. Lairmore. 2001. Endoplasmic reticulum and cis-Golgi localization of human T-lymphotropic virus type 1 p12I: association with calreticulin and calnexin. J. Virol. 75:7672-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebinu, J. O., S. L. Stang, C. Teixeira, D. A. Bottorff, J. Hooton, P. M. Blumberg, M. Barry, R. C. Bleakley, H. L. Ostergaard, and J. C. Stone. 2000. RasGRP links T-cell receptor signaling to Ras. Blood 95:3199-3203. [PubMed] [Google Scholar]
- 21.Fanger, C. M., M. Hoth, G. R. Crabtree, and R. S. Lewis. 1995. Characterization of T cell mutants with defects in capacitative calcium entry: genetic evidence for the physiological roles of CRAC channels. J. Cell Biol. 131:655-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franchini, G. 1995. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood 86:3619-3639. [PubMed] [Google Scholar]
- 23.Franchini, G., J. C. Mulloy, I. J. Koralnik, A. Lo Monico, J. J. Sparkowski, T. Andresson, D. J. Goldstein, and R. Schlegel. 1993. The human T-cell leukemia/lymphotropic virus type I p12I protein cooperates with the E5 oncoprotein of bovine papillomavirus in cell transformation and binds the 16-kilodalton subunit of the vacuolar H+ ATPase. J. Virol. 67:7701-7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genot, E., S. Cleverley, S. Henning, and D. Cantrell. 1996. Multiple p21ras effector pathways regulate nuclear factor of activated T cells. EMBO J. 15:3923-3933. [PMC free article] [PubMed] [Google Scholar]
- 25.Holloway, M. P., and R. J. Bram. 1996. A hydrophobic domain of Ca2+-modulating cyclophilin ligand modulates calcium influx signaling in T lymphocytes. J. Biol. Chem. 271:8549-8552. [DOI] [PubMed] [Google Scholar]
- 26.Holloway, M. P., and R. J. Bram. 1998. Colocalization of calcium-modulating cyclophilin ligand with intracellular calcium pools. J. Biol. Chem. 273:16346-16350. [DOI] [PubMed] [Google Scholar]
- 27.Hollsberg, P. 1999. Mechanisms of T-cell activation by human T-cell lymphotropic virus type I. Microbiol. Mol. Biol. Rev. 63:308-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson, J. M., C. Nicot, J. Fullen, V. Ciminale, L. Casareto, J. C. Mulloy, S. Jacobson, and G. Franchini. 2001. Free major histocompatibility complex class I heavy chain is preferentially targeted for degradation by human T-cell leukemia/lymphotropic virus type 1 p12I protein. J. Virol. 75:6086-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kane, L. P., J. Lin, and A. Weiss. 2000. Signal transduction by the TCR for antigen. Curr. Opin. Immunol. 12:242-249. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy, A. P., A. Sekulic, B. J. Irvin, A. E. Nilson, S. M. Dilworth, and R. T. Abraham. 1998. Polyomavirus middle T antigen as a probe for T cell antigen receptor-coupled signaling pathways. J. Biol. Chem. 273:11505-11513. [DOI] [PubMed] [Google Scholar]
- 31.Kimata, J. T., F. Wong, J. Wang, and L. Ratner. 1994. Construction and characterization of infectious human T-cell leukemia virus type 1 molecular clones. Virology 204:656-664. [DOI] [PubMed] [Google Scholar]
- 32.Kinoshita, S., B. K. Chen, H. Kaneshima, and G. P. Nolan. 1998. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell 95:595-604. [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita, S., L. Su, M. Amano, L. A. Timmerman, H. Kaneshima, and G. P. Nolan. 1997. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity 6:235-244. [DOI] [PubMed] [Google Scholar]
- 34.Koralnik, I. J., J. Fullen, and G. Franchini. 1993. The p12, p13 and p30 proteins encoded by human T-cell leukemia/lymphotropic virus type-1 open reading frames I and II are localized in three different cellular compartments. J. Virol. 67:2360-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koralnik, I. J., A. Gessain, M. E. Klotman, A. Lo Monico, Z. N. Berneman, and G. Franchini. 1992. Protein isoforms encoded by the pX region of human T-cell leukemia/lymphotropic virus type 1. Proc. Natl. Acad. Sci. USA 89:8813-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koralnik, I. J., J. C. Mulloy, T. Andresson, J. Fullen, and G. Franchini. 1995. Mapping of the intermolecular association of the human T cell leukaemia/lymphotropic virus type I p12I and the vacuolar H+-ATPase 16 kDa subunit protein. J. Gen. Virol. 76:1909-1916. [DOI] [PubMed] [Google Scholar]
- 37.Lin, J., and A. Weiss. 2001. T cell receptor signalling. J. Cell Sci. 114:243-244. [DOI] [PubMed] [Google Scholar]
- 38.Lorenzo, P. S., M. Beheshti, G. R. Pettit, J. C. Stone, and P. M. Blumberg. 2000. The guanine nucleotide exchange factor RasGRP is a high-affinity target for diacylglycerol and phorbol esters. Mol. Pharmacol. 57:840-846. [PubMed] [Google Scholar]
- 39.Macian, F., C. Garcia-Rodriguez, and A. Rao. 2000. Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. EMBO J. 19:4783-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macian, F., C. Lopez-Rodriguez, and A. Rao. 2001. Partners in transcription: NFAT and AP-1. Oncogene 20:2476-2489. [DOI] [PubMed] [Google Scholar]
- 41.Manninen, A., R. G. Herma, and K. Saksela. 2000. Synergistic activation of NFAT by HIV-1 nef and the Ras/MAPK pathway. J. Biol. Chem. 275:16513-16517. [DOI] [PubMed] [Google Scholar]
- 42.Manninen, A., P. Huotari, M. Hiipakka, G. H. Renkema, and K. Saksela. 2001. Activation of NFAT-dependent gene expression by Nef: conservation among divergent nef alleles, dependence on SH3 binding and membrane association, and cooperation with protein kinase C-theta. J. Virol. 75:3034-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masuda, E. S., R. Imamura, Y. Amasaki, K. Arai, and N. Arai. 1998. Signalling into the T-cell nucleus: NFAT regulation. Cell Signal. 10:599-611. [DOI] [PubMed] [Google Scholar]
- 44.Mesaeli, N., K. Nakamura, E. Zvaritch, P. Dickie, E. Dziak, K. H. Krause, M. Opas, D. H. MacLennan, and M. Michalak. 1999. Calreticulin is essential for cardiac development. J. Cell Biol. 144:857-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulloy, J. C., R. W. Crowley, J. Fullen, W. J. Leonard, and G. Franchini. 1996. The human T-cell leukemia/lymphotropic virus type 1 p12I protein binds the interleukin-2 receptor β and γc chains and affects their expression on the cell surface. J. Virol. 70:3599-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negulescu, P. A., N. Shastri, and M. D. Cahalan. 1994. Intracellular calcium dependence of gene expression in single T lymphocytes. Proc. Natl. Acad. Sci. USA 91:2873-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neuveut, C., and K. T. Jeang. 2000. HTLV-I Tax and cell cycle progression. Prog. Cell Cycle Res. 4:157-162. [DOI] [PubMed] [Google Scholar]
- 48.Nicot, C., J. C. Mulloy, M. G. Ferrari, J. M. Johnson, K. Fu, R. Fukumoto, R. Trovato, J. Fullen, W. J. Leonard, and G. Franchini. 2001. HTLV-1 p12I protein enhances STAT5 activation and decreases the interleukin-2 requirement for proliferation of primary human peripheral blood mononuclear cells. Blood 98:823-829. [DOI] [PubMed] [Google Scholar]
- 49.Northrop, J. P., S. N. Ho, L. Chen, D. J. Thomas, L. A. Timmerman, G. P. Nolan, A. Admon, and G. R. Crabtree. 1994. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature 369:497-502. [DOI] [PubMed] [Google Scholar]
- 50.Northrop, J. P., K. S. Ullman, and G. R. Crabtree. 1993. Characterization of the nuclear and cytoplasmic components of the lymphoid-specific nuclear factor of activated T cells (NF-AT) complex. J. Biol. Chem. 268:2917-2923. [PubMed] [Google Scholar]
- 51.Olive, M., D. Krylov, D. R. Echlin, K. Gardner, E. Taparowsky, and C. Vinson. 1997. A dominant negative to activation protein-1 (AP1) that abolishes DNA binding and inhibits oncogenesis. J. Biol. Chem. 272:18586-18594. [DOI] [PubMed] [Google Scholar]
- 52.Pique, C., and M. C. Dokhelar. 2000. In vivo production of rof and tof proteins of HTLV type 1: evidence from cytotoxic T lymphocytes. AIDS Res. Hum. Retrovir. 16:1783-1786. [DOI] [PubMed] [Google Scholar]
- 53.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puente, L. G., J. C. Stone, and H. L. Ostergaard. 2000. Evidence for protein kinase C-dependent and -independent activation of mitogen-activated protein kinase in T cells: potential role of additional diacylglycerol binding proteins. J. Immunol. 165:6865-6871. [DOI] [PubMed] [Google Scholar]
- 55.Rao, A., C. Luo, and P. G. Hogan. 1997. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15:707-747. [DOI] [PubMed] [Google Scholar]
- 56.Schaeffer, E. M., and P. L. Schwartzberg. 2000. Tec family kinases in lymphocyte signaling and function. Curr. Opin. Immunol. 12:282-288. [DOI] [PubMed] [Google Scholar]
- 57.Stewart, S., and G. R. Crabtree. 2000. Transcription. Regulation of the regulators. Nature 408:46-47. [DOI] [PubMed] [Google Scholar]
- 58.Takatsuki, K., T. Uchiyama, K. Sagawa, and J. Yodoi. 1977. Adult T-cell leukemia in Japan, p. 73-77. In S. Seno, F. Takaku, and S. Irino (ed.), Topics in hematology. Excerpta Medica, Amsterdam, The Netherlands.
- 59.Taylor, N., K. B. Bacon, S. Smith, T. Jahn, T. A. Kadlecek, L. Uribe, D. B. Kohn, E. W. Gelfand, A. Weiss, and K. Weinberg. 1996. Reconstitution of T cell receptor signaling in ZAP-70-deficient cells by retroviral transduction of the ZAP-70 gene. J. Exp. Med. 184:2031-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uchiyama, T. 1997. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu. Rev. Immunol. 15:15-37. [DOI] [PubMed] [Google Scholar]
- 61.Vanoers, N. S. 1999. T cell receptor-mediated signs and signals governing T cell development. Semin. Immunol. 11:227-237. [DOI] [PubMed] [Google Scholar]
- 62.Wu, Y., and J. W. Marsh. 2001. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science 293:1503-1506. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida, H., H. Nishina, H. Takimoto, L. E. Marengere, A. C. Wakeham, D. Bouchard, Y. Y. Kong, T. Ohteki, A. Shahinian, M. Bachmann, P. S. Ohashi, J. M. Penninger, G. R. Crabtree, and T. W. Mak. 1998. The transcription factor NF-ATc1 regulates lymphocyte proliferation and Th2 cytokine production. Immunity 8:115-124. [DOI] [PubMed] [Google Scholar]
- 64.Zhang, W. G., J. Sloanlancaster, J. Kitchen, R. P. Trible, and L. E. Samelson. 1998. LAT: The ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92:83-92. [DOI] [PubMed] [Google Scholar]
- 65.Zhu, J., and F. Mckeon. 2000. Nucleocytoplasmic shuttling and the control of NF-AT signaling. Cell. Mol. Life Sci. 57:411-420. [DOI] [PMC free article] [PubMed] [Google Scholar]