Abstract
Background: Dry eye syndrome (DES) is a prevalent ocular condition, but information on risk or protective factors is lacking.
Objective: We aimed to determine the association between dietary intake of n-3 and n-6 fatty acids and their ratio and the presence of DES.
Design: Of the 39,876 female health professionals in the Women's Health Study (WHS), we studied cross-sectionally 32,470 women aged 45 to 84 years who provided information on diet and DES. We assessed intake of fatty acids by a validated food frequency questionnaire, and DES using self-reports of clinically diagnosed cases. Of the sample, 1546 (4.7%) subjects reported a clinical diagnosis of DES. We used logistic regression models to estimate the odds ratios (OR) and 95% confidence intervals (CI) to describe the relationships of fatty acid intake with DES. We analyzed the association between consumption of fish and DES in a similar way. Results: After adjusting for demographic factors, hormone therapy, and total fat intake, the OR (CI) for the highest versus lowest fifth of n-3 fatty acids was 0.83 (0.70-0.98), P[trend]=0.05. A higher ratio of n-6/n-3 fatty acid consumption was associated with significantly increased risk of DES, OR (CI) =2.51 (1.13-5.58) for >15/1 versus <4/1 (P[trend]=0.01). In addition, tuna consumption was inversely associated with DES (OR=0.81, CI=0.66-0.99 for 2-4 113 g (4 oz) servings/week, and OR=0.32, CI=0.13-0.79 for 5-6 servings/week versus ≤1 servings/week; P[trend]=0.005).
Conclusion: These results suggest that a higher dietary intake of n-3 fatty acids is associated with a decreased presence of DES in women. These findings are consistent with anecdotal clinical observations and postulated biological mechanisms.
Keywords: Epidemiology, dry eye syndrome, diet, n-3 fatty acids, n-6 fatty acids, risk factors
INTRODUCTION
Dry eye syndrome (DES) is one of the most prevalent ocular conditions in the US and a frequent reason for seeking eye care (1). Ocular discomfort is the most prominent patient complaint (2). In addition, DES commonly leads to decreased functional visual acuity (3), and problems reading, using a computer, driving at night and carrying out professional work (4, 5).
Despite progress in determining the etiology and pathogenesis of DES, current knowledge remains inadequate and no preventive strategies have been found. Moreover, the most common therapy for DES, artificial tears, provides only temporary and incomplete symptomatic relief. Therefore, identification of modifiable risk factors for DES may suggest avenues for investigation of novel preventive and treatment measures.
Lacrimal gland, meibomian gland, and ocular surface inflammation play a significant role in DES (6, 7). Patients with DES have an increased concentration of inflammatory cytokines, such as interleukin-1, interleukin-6, and tumor necrosis factor alpha, in the tear film (8). Research has shown that dietary intake of n-3 fatty acids (n-3 FA) and the ratio of their consumption in relation to n-6 (n-6 FA) intake affects the overall level of inflammatory activity in the body (9, 10). Anecdotal evidence has suggested a possible protective role of n-3 FA supplementation in treatment of DES (11, 12) but this has never been established in a systematic study. Both n-3 and n-6 FA are essential for human health and must be consumed directly in the diet. Therefore, we investigated the relationship of dietary intake of n-3 and its ratio to n-6 FA with DES in a large well-characterized population of women participating in the Women's Health Study (WHS).
SUBJECTS AND METHODS
Study population
The WHS is a randomized, double-blind, placebo-controlled trial among 39,876 female health professionals to assess the benefits and risks of low-dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer (13). At baseline, all the participants, aged between 39 and 90 years, were free of cancer (except non-melanoma skin cancer), myocardial infarction, stroke, transient cerebral ischemia, liver disease, renal disease, peptic ulcer, or gout. Women using corticosteroids, anticoagulants or supplements of vitamin A and E were excluded. For the purpose of the present analysis we excluded WHS participants who did not provide information on diet or DES, leaving 32,470 women.
Ascertainment of Diet
A semiquantitative food frequency questionnaire (SFFQ) was administered at baseline and captured information on 134 commonly consumed food items. For each item, a portion size was specified and each woman was asked, how often, on average, during the past year, she had consumed that amount. Nine responses were possible, ranging from ‘never or less than once a month’ to ‘six per day.’ A detailed description of the SFFQ and the procedures used for calculating nutrient intake, as well as data on reproducibility and validity have been published previously (14). We computed nutrient scores by multiplying the frequency of consumption of each unit of food from the SFFQ by the nutrient content of that specific portion-size of the food according to food composition tables from the US Department of Agriculture (15) and other sources (14, 16).
We obtained information on n-6 FA consumption, mostly linoleic acid (18:2 n-6, LA), primarily via questions on the consumption of margarine, butter, mayonnaise or other creamy salad dressing, peanuts, and other nuts; as well as the type of cooking oil used at home in the preparation of foods, and kind of fat used for frying, sautéing, and baking. In the typical American diet, n-3 FA are primarily derived from seafood sources, which contain the long-chain n-3 FA eicosapentanoic acid (20:5 n-3, EPA) and docosahexanoic acid (22:5, n-3 DHA). The SFFQ included questions on intake of 1) canned tuna fish (85-113 g portion size); 2) other dark meat fish such as mackerel, salmon, sardines, bluefish, swordfish (85-142 g portion size); 3) light flesh fish (85-142 g portion size); and 4) shrimp, lobster and/or scallops (as a main dish). We calculated the intake of EPA and DHA by assigning grams per serving as follows: 1.51 g for dark-meat fish, 0.42 g for canned tuna fish, 0.48 g for light flesh fish, and 0.32 g for shrimp, lobster, or scallops. These n-3 FA values were derived by weighting the mean values of n-3 FA for the most common types of fish based on US landings in 1984 (US Department of Commerce), described elsewhere (17). Intake of the n-3 FA alpha-linolenic acid (18:3, n-3 ALA), obtained primarily from plant sources, and other n-3 FA were also estimated and used to calculate total n-3 FA intake.
Dry Eye Ascertainment
We assessed history of DES on the four-year follow up questionnaire by asking participants “Have you ever been diagnosed, by a clinician, with dry eye syndrome?” and if so requesting the date of diagnosis. For the present study, we considered a woman to have DES if she reported a clinical diagnosis of DES. We used clinically diagnosed cases because in a validation study in which we examined 53 subjects, this endpoint was a sensitive and specific predictor of the presence of a clinical finding of DES (18). For example, the sensitivity of this question was 77% with a specificity of 83% for clinical DES as defined by a Schirmer test score of ≤ 10 mm in at least one eye or tear break-up time of <10 seconds in at least one eye, two commonly used clinical tests for diagnosis of DES. A total of 1,546 (4.7%) of the 32,470 women in the present study reported having clinically diagnosed DES.
Statistical Analysis
We categorized energy-adjusted total n-3 FA, EPA, DHA and total n-6 FA consumption using quintile cut-points of intake based on the distribution of these FA among all subjects. We categorized the ratio of n-6/n-3 FA into 4 categories of <4/1, ≥4/1 to <10/1, ≥10/1 to <15/1, and ≥15/1; ranges which approximately correspond to a theoretically ideal intake, the World Health Organization's recommended intake, an approximate average intake in a typical Western diet, and a high but still prevalent ratio in a typical American diet, respectively (9).
We initially examined the distribution of potential confounders according to the dietary intake of n-3 and n-6 FA, and the n-6/n-3 ratio. We then used logistic regression models to estimate the odds ratios (OR) and 95% confidence intervals (CI), for the relationships of n-3 FA, EPA, DHA as well as n-6 FA and the n-6/n-3 ratio with DES. In an initial set of models, we controlled for age, randomized aspirin and vitamin E assignments, hormone therapy, race, education, household income level, frequency of eye examination, US census region, and total fat intake. In a second set of models we additionally controlled for history of diabetes mellitus, hypertension and any connective tissue diseases (lupus, rheumatoid arthritis, etc.), as these variables are potential risk factors for DES and may be related to diet.
Since fish and seafood intake accounts for such a large proportion of the total n-3 FA intake in a typical American diet, we also specifically examined the association of fish and seafood intake with DES. In this analysis, we categorized average daily tuna fish consumption into 3 categories: ≤1 serving/week, 2 to 4 servings/week; and ≥5 to 6 servings/week. Intakes of the less commonly consumed other dark flesh fish, light flesh fish, and seafood were each divided into 2 categories: ≤1 serving/week, or ≥ 2-4 servings/week. We used logistic regression models to assess the relationships of fish and seafood consumption with DES and obtain OR and CI estimates for these associations.
RESULTS
The mean daily intake of n-3 FA was 1.40 g (roughly equal to eating three 113 g [4 oz] portions of canned tuna per week), ranging from 0.27 to 4.63 g. Mean daily intake of n-6 FA was 10.82 g, (roughly equal to eating just under 2 T of mayonnaise per day), ranging from 2.04 to 36.80 g. The mean ratio of n-6/n-3 FA was 7.97 with a range from 1.01 to 32.93. Baseline characteristics of the study participants distributed by quintiles of intake of n-3 and n-6 FA and the n-6/n-3 ratio are presented in Table 1. Older women were more likely to have a higher intake of n-3 and n-6 FA, and a lower n-6/n-3 ratio. In addition, there was a direct relationship of total fat with n-3 and n-6 FA intake and with the n-6/n-3 ratio. Women with history of diabetes mellitus had higher intake of n-3 and n-6 FA; and there was also a slightly higher intake of both n-3 and n-6 FA among women with hypertension and those with higher body mass index. Neither n-3 nor n-6 FA intake was different among categories of hormone therapy users.
Table 1.
Association of baseline characteristics with dietary intake of n-3 fatty acids
| Fifth of n-3 Fatty Acid Intake | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P-value for Trend | |
| Range of n-3 (g) | (0.27-1.07) | (1.08-1.25) | (1.26-1.43) | (1.44-1.67) | (1.68-4.63) | |
| Total Subjects in Quintile Group | n=6473 | n=6396 | n=6606 | n=6572 | n=6423 | |
| n-3 Intake in Quintile Group (g)* | 0.92 (0.12) | 1.17 (0.05) | 1.34 (0.05) | 1.55 (0.07) | 1.99 (0.33) | <0.0001 |
| n-6 Intake in Quintile Group in (g) | 8.38 (2.11) | 9.69 (1.95) | 10.62 (2.07) | 11.66 (2.19) | 13.72 (3.31) | <0.0001 |
| n-6/n-3 Ratio in Quintile Group | 9.2 | 8.3 | 7.9 | 7.5 | 6.9 | <0.0001 |
| Age (y) | 57.35 (6.84) | 57.51 (6.85) | 57.80 (6.90) | 58.17 (7.04) | 58.63 (7.09) | <0.0001 |
| BMI (kg/m2) | 26.29 (5.16) | 26.58 (5.22) | 26.55 (5.07) | 26.77 (5.29) | 26.83 (5.32) | <0.0001 |
| Total Fat Intake (g) | 54.33 (12.7) | 56.29 (11.35) | 57.44 (11.01) | 58.82 (10.43) | 61.71 (11.71) | <0.0001 |
| Postmenopausal Hormone Therapy (%) | ||||||
| None | 20.62 | 19.84 | 20.11 | 19.79 | 19.65 | |
| Estrogen | 18.78 | 19.57 | 20.58 | 20.73 | 20.33 | |
| Estrogen + Progesterone | 20.07 | 19.62 | 20.46 | 20.42 | 19.43 | 0.25 |
| Diabetes Mellitus (%) | ||||||
| Absent | 20.15 | 19.73 | 20.41 | 20.14 | 19.57 | |
| Present | 15.36 | 18.92 | 18.99 | 22.42 | 24.30 | <0.0001 |
| Hypertension (%) | ||||||
| Absent | 20.49 | 19.90 | 20.74 | 19.77 | 19.12 | |
| Present | 19.05 | 19.38 | 19.72 | 21.00 | 20.85 | <0.0001 |
| Any Connective Tissue Disease (%) | ||||||
| Absent | 19.93 | 19.71 | 20.39 | 20.22 | 19.75 | |
| Present | 20.27 | 19.13 | 18.79 | 20.96 | 20.84 | 0.56 |
| Fifth of n-6 Fatty Acid Intake | ||||||
| 1 | 2 | 3 | 4 | 5 | P-value for Trend | |
| Range of n-6 (g) | (2.04-8.36) | (8.37-9.81) | (9.82-11.19) | (11.20-12.99) | (13.00-36.80) | |
| Total Subjects in Quintile Group | n=6447 | n=6498 | n=6512 | n=6516 | n=6497 | |
| n-3 Intake in Quintile Group (g) | 1.09 (0.3) | 1.24 (0.27) | 1.36 (0.28) | 1.49 (0.28) | 1.78 (0.43) | <0.0001 |
| n-6 Intake in Quintile Group (g) | 7.15 (0.97) | 9.12 (0.42) | 10.49 (0.40) | 12.03 (0.52) | 15.25 (2.33) | <0.0001 |
| n-6/n-3 Ratio in Quintile Group | 6.9 | 7.6 | 8.0 | 8.3 | 9.0 | <0.0001 |
| Age (y) | 57.69 (6.88) | 57.82 (6.95) | 57.63 (6.83) | 57.98 (6.98) | 58.33 (7.13) | <0.0001 |
| BMI (kg/m2) | 26.09 (4.95) | 26.39 (5.13) | 26.75 (5.17) | 26.86 (5.33) | 26.93 (5.44) | <0.0001 |
| Total Fat Intake (g) | 47.48 (10.90) | 54.43 (9.56) | 58.06 (9.07) | 61.35 (9.04) | 67.17 (9.78) | <0.0001 |
| Postmenopausal Hormone Therapy (%) | ||||||
| None | 20.35 | 19.96 | 19.93 | 19.85 | 19.91 | |
| Estrogen | 19.22 | 19.74 | 20.15 | 19.98 | 20.91 | |
| Estrogen + Progesterone | 19.76 | 20.36 | 20.15 | 20.47 | 19.26 | 0.73 |
| Diabetes Mellitus (%) | ||||||
| Absent | 20.01 | 20.02 | 20.09 | 19.99 | 19.88 | |
| Present | 16.41 | 19.76 | 19.20 | 21.79 | 22.84 | <0.0001 |
| Hypertension (%) | ||||||
| Absent | 20.37 | 20.29 | 19.93 | 19.65 | 19.77 | |
| Present | 19.03 | 19.57 | 20.26 | 20.75 | 20.39 | 0.0004 |
| Any Connective Tissue Disease (%) | ||||||
| Absent | 19.84 | 20.03 | 20.03 | 20.09 | 20.02 | |
| Present | 20.50 | 19.48 | 20.96 | 19.36 | 19.70 | 0.66 |
| Fifth of n-6/n-3 Ratio | ||||||
| 1 | 2 | 3 | 4 | 5 | P-value for Trend | |
| Range of n-6/n-3 Ratio | (1.01-6.59) | (6.60-7.45) | (7.46-8.20) | (8.21-9.17) | (9.18-32.93) | |
| Total Subjects in Quintile Group | n=6528 | n=6457 | n=6472 | n=6503 | n=6510 | |
| n-3 Intake in Quintile Group (g) | 1.6 (0.45) | 1.48 (0.37) | 1.42 (0.38) | 1.32 (0.32) | 1.16 (0.29) | <0.0001 |
| n-6 Intake in Quintile Group (g) | 9.02 (2.45) | 10.41 (2.65) | 11.08 (2.93) | 11.37 (2.77) | 12.19 (3.08) | <0.0001 |
| n-6/n-3 Ratio in Quintile Group | 5.7 | 7.0 | 7.8 | 8.6 | 10.6 | <0.0001 |
| Age (y) | 58.56 (7.07) | 58.02 (6.97) | 57.82 (6.93) | 57.44 (6.73) | 57.61 (7.03) | <0.0001 |
| BMI (kg/m2) | 26.21 (5.01) | 26.58 (5.11) | 26.63 (5.12) | 26.87 (5.34) | 26.74 (5.46) | <0.0001 |
| Total Fat Intake (g) | 49.29 (10.77) | 55.39 (10.32) | 58.58 (10.42) | 61.02 (10.38) | 64.32 (10.79) | <0.0001 |
| Postmenopausal Hormone Therapy (%) | ||||||
| None | 19.87 | 19.74 | 20.15 | 19.66 | 20.57 | |
| Estrogen | 20.11 | 20.16 | 19.70 | 20.27 | 19.75 | |
| Estrogen + Progesterone | 20.44 | 19.82 | 19.84 | 20.33 | 19.56 | 0.16 |
| Diabetes Mellitus (%) | ||||||
| Absent | 20.02 | 19.89 | 19.92 | 20.08 | 20.09 | |
| Present | 22.00 | 19.76 | 20.18 | 18.99 | 19.06 | 0.07 |
| Hypertension (%) | ||||||
| Absent | 19.90 | 19.93 | 20.08 | 19.96 | 20.14 | |
| Present | 20.44 | 19.82 | 19.69 | 20.13 | 19.91 | 0.43 |
| Any Connective Tissue Disease (%) | ||||||
| Absent | 20.07 | 19.89 | 19.89 | 20.08 | 20.07 | |
| Present | 21.41 | 19.70 | 21.41 | 18.22 | 19.25 | 0.21 |
Numbers provided are mean (standard deviation) for continuous variables, and percent in each category for categorical variables.
In the initial set of logistic regression models adjusting for age, randomized aspirin and vitamin E assignments, demographic factors, post-menopausal hormone therapy, and total fat intake, women with a higher intake of n-3 FA tended to have a lower risk of DES (Table 2). For the highest versus the lowest fifth of n-3 FA intake the OR was 0.83 with a CI= 0.70-0.98. In a second set of models, additional control for connective tissue diseases, diabetes mellitus, and hypertension, did not appreciably modify these associations (Table 2). Similarly, women with the highest intake of DHA had a significantly lower risk of DES (OR= 0.88, CI = 0.74-1.04 for the highest versus lowest quintile, P for trend =0.01). Although results for EPA were not statistically significant (P trend = 0.08), the estimated magnitude of the association was not different to that for DHA (OR= 0.87, CI= 0.73-1.03 for highest versus lowest quintile) (data for other quintiles of DHA and EPA not shown).
Table 2.
Relative risks and 95% confidence intervals of dry eye syndrome among 32,470 participants in the Women's Health Study, according to dietary intake of n-3 and n-6 fatty acids
| Fifth of Dietary Intake | n | n with | Model 1* | Model 2† |
|---|---|---|---|---|
| (Mean Intake in Group) | DES | OR (95% CI) | OR (95% CI) | |
| n-3 Fatty Acids | ||||
| Quintile Group 1 (0.92 g) | 6473 | 329 | 1.0 | 1.0 |
| Quintile Group 2 (1.17 g) | 6396 | 296 | 0.89 (0.76-1.05) | 0.89 (0.76-1.05) |
| Quintile Group 3 (1.34 g) | 6606 | 318 | 0.92 (0.78-1.08) | 0.92 (0.78-1.08) |
| Quintile Group 4 (1.55 g) | 6572 | 314 | 0.90 (0.76-1.06) | 0.90 (0.76-1.05) |
| Quintile Group 5 (1.99 g) | 6423 | 289 | 0.83 (0.70-0.98) | 0.83 (0.70-0.98) |
| P for trend | 0.05 | 0.04 | ||
| n-6 Fatty Acids | ||||
| Quintile Group 1 (7.15 g) | 6447 | 329 | 1.0 | 1.0 |
| Quintile Group 2 (9.12 g) | 6498 | 304 | 0.92 (0.78-1.09) | 0.93 (0.79-1.09) |
| Quintile Group 3 (10.50 g) | 6512 | 306 | 0.95 (0.80-1.12) | 0.94 (0.80-1.12) |
| Quintile Group 4 (12.03 g) | 6516 | 307 | 0.94 (0.78-1.12) | 0.94 (0.79-1.12) |
| Quintile Group 5 (15.25 g) | 6497 | 300 | 0.91 (0.75-1.11) | 0.92 (0.76-1.12) |
| P for trend | 0.72 | 0.74 |
Odds ratios (OR) & 95% confidence intervals (CI) are from logistic regression models (separate models for n- 3 and n-6 fatty acids) controlling for age (5–year categories), randomized assignments to aspirin, beta-carotene and Vitamin E (each versus placebo), postmenopausal hormone therapy (none, estrogen only, estrogen plus progesterone/progestins), race (white, black, Hispanic, Asian/Pacific Islanders, Native Americans, other), education (licensed practical or visiting nurse training; 2-year associate's degree for registered nurse [RN]; 3-year RN diploma program; bachelor's, master's, or doctoral degree), household income level (<$10,000, $10,000-19,999, $ 20,000-29,999, $30,000-39,999, $ 40,000-49,999, $ 50,000- 99,999, ≥100,000), history of an eye examination in the past 2 years, US census region (West, Midwest, Northeast, South, outside of the United States [Puerto Rico, Guam, and other US territories], and total fat intake (g).
In addition to the variables in Model 1, we controlled for diagnoses of diabetes mellitus, hypertension, and any connective tissue diseases.
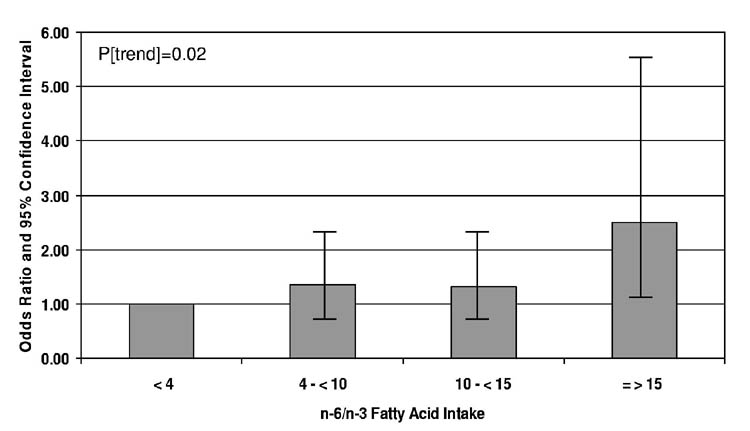
We observed no significant relationship between n-6 FA intake and DES (Table 2). On the other hand, a higher n-6/n-3 FA ratio was associated with a significantly increased risk of DES. The OR was 2.51, with a CI =1.13-5.58 for comparison of extreme categories (≥15/1 vs <4/1) (Figure). Additional control for connective tissue diseases, diabetes mellitus, and hypertension had no important impact on these estimates.
Figure.
Relative Risk of Dry Eye Syndrome According to Dietary n-6/n-3 Fatty Acid Ratio. Depicted are the odds ratios (OR) together with their 95% confidence intervals (CI) for the risk of dry eye syndrome according to categories of the ratio of dietary intake of n-6 to n-3 fatty acids. OR and CI are from a logistic regression model controlling for age, randomized assignments to aspirin and Vitamin E, post-menopausal hormone therapy, race, education, household income level, history of an eye examination in the past 2 years, US census region, and total fat intake.
In analyses examining the frequency of fish consumption in relation to DES, we observed a significant inverse association between tuna fish consumption and DES (OR=0.81, CI=0.66-0.99) for 2-4 servings/week, and OR=0.32, (0.13-0.79) for ≥5-6 servings/week versus ≤1 servings/week. None of the other specific types of fish reached statistical significance. The OR (CI) for DES with seafood intake of ≥2-4 servings/week compared to ≤ 1 serving/week was 0.58, (0.27-1.24). Consumption of dark fish other than tuna, or light-flesh fish were also not significantly related to risk of DES (OR [CI]= 0.70 [0.42-1.17], and 0.84 [0.66-1.07] for ≥2-4 servings/week compared to ≤ 1 serving/week of dark fish, or light flesh fish, respectively). Results for the relations of fish consumption with DES were not affected by additional control for diabetes, hypertension, and connective tissue diseases (Table 3).
Table 3.
Relative risks of dry eye syndrome among 32,470 Women's Health Study participants, according to dietary intake of fish and seafood
| Diet Variable* | n | n with Dry | Model 1† | Model 2‡ |
|---|---|---|---|---|
| Eye Syndrome | OR (95% CI) | OR (95% CI) | ||
| Tuna fish | ||||
| ≤1 serving/week | 29,424 | 1431 | 1.0 | 1.0 |
| 2-4 servings/week | 2,728 | 110 | 0.81 (0.66-0.99) | 0.80 (0.65-0.98) |
| ≥5 servings/week | 318 | 5 | 0.32 (0.13-0.79) | 0.32 (0.13-0.77) |
| P for trend | 0.005 | 0.003 | ||
| Other dark fish | ||||
| ≤1 serving/week | 32,047 | 1530 | 1.0 | 1.0 |
| ≥2-4 servings/week | 423 | 16 | 0.70 (0.42-1.17) | 0.70 (0.42-1.16) |
| Light flesh fish | ||||
| ≤1 serving/week | 30,816 | 1475 | 1.0 | 1.0 |
| ≥2-4 servings/week | 1654 | 71 | 0.84 (0.66-1.07) | 0.83 (0.65-1.06) |
| Seafood | ||||
| ≤ serving/week | 32,226 | 1539 | 1.0 | 1.0 |
| ≥2-4 servings/week | 244 | 7 | 0.58 (0.27-1.24) | 0.58 (0.27-1.23) |
Serving sizes were 85-113g for tuna, 85-142g for other dark fish and light flesh fish, and “as a main dish” for seafood.
Odds ratios (OR) & 95% confidence intervals (CI) are from logistic regression models (separate models for each type of fish) controlling for age (5–year categories), randomized assignments to aspirin, beta-carotene and Vitamin E (each versus placebo), post-menopausal hormone therapy (none, estrogen only, estrogen plus progesterone/progestins), race (white, black, Hispanic, Asian/Pacific Islanders, Native Americans, other), education (licensed practical or visiting nurse training; 2-year associate's degree for registered nurse [RN]; 3-year RN diploma program; bachelor's, master's, or doctoral degree), household income level (<$10,000, $10,000-19,999, $ 20,000-29,999, $30,000-39,999, $ 40,000-49,999, $ 50,000-99,999, ≥100,000), history of an eye examination in the past 2 years, US census region (West, Midwest, Northeast, South, outside of the United States [Puerto Rico, Guam, and other US territories], and total fat intake (g).
In addition to the variables in Model 1, we controlled for diagnoses of diabetes mellitus, hypertension, and any connective tissue disease
DISCUSSION
DES is a significant public health problem affecting over 10 million Americans (19, 20). However, few risk or protective factors for DES have been identified and none relate thus far to diet. The present study indicates that women with a higher dietary intake of n-3 FA have a lower prevalence of DES, including a 68% reduction in women who consumed ≥5-6 113 g (4-ounce) servings per week compared to ≤1 servings per week of tuna fish, one of the largest contributors of n-3 FA in the typical American diet. In contrast, we did not observe any independent relationship of n-6 FA intake with DES; however, a high ratio of n-6/n-3 FA (>15:1) was associated with a greater than two-fold higher prevalence of DES.
The central role of inflammation in the development of DES (21), and the known anti-inflammatory potential of n-3 FA are consistent with the correlations observed in the present study. Essential fatty acids are natural modulators of inflammatory activity via their metabolism to eicosanoids, locally acting hormone-like lipids involved in the control of inflammatory and immune responses. Eicosanoids are derived from three fatty acid precursors, dihomogammalinoleic acid (20:3, n-6 DGLA), arachidonic acid (20:4, n-6 AA), and EPA. The modulation of inflammatory activity is based on the balance of these precursors. One of the possible ways in which n-3 FA can reduce inflammatory activity is through their ability to suppress the biosynthesis of AA-derived eicosanoids. Since the balance of n-3 and n-6 FA in cellular membranes is largely dependent on dietary intake (22); high intakes of n-3 FA result in replacement of the usually more abundant AA with EPA and DHA. Eicosanoids derived from AA such as prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) are vigorously pro-inflammatory, whereas the 3-series prostaglandins and 5-series leukotrienes from EPA are 10 to 100-fold less biologically active (23). A higher intake of n-3 FA also reduces the desaturation and elongation of LA to AA (24, 25). Further, n-3 FA suppress COX-2 and have greater affinity resulting in higher formation of EPA-derived as compared to AA-derived eicosanoids (26-28). When the ratio of n-6/n-3 FA is approximately 4/1 or lower, there is also competitive inhibition of the conversion of DGLA to AA (22); resulting in enhanced metabolism of DGLA to the 1-series prostaglandins including PGE1, which has a number of anti-inflammatory actions (29).
Apart from the importance of FA in modulating inflammatory response, their eicosanoid metabolites have a variety of other actions. Particularly salient to DES, PGE1 appears to be an important stimulator of aqueous tear secretion (30). Early studies hypothesized that aqueous tear deficiency in Sjogren's-related DES was the result of PGE1 precursor deficiency due to impaired delta-6 desaturase activity and a resultant reduction of the metabolism of LA to gamma-linoleic acid (18:3, n-6 GLA) (31). GLA is elongated to DGLA, which forms PGE1. Investigators hypothesized that supplementation with GLA directly could correct this deficiency (31). However, a recent randomized trial of GLA versus placebo among 90 Sjogren's syndrome patients showed no significant difference between the active treatment and placebo groups in signs and symptoms of DES (32). In contrast, another randomized trial of 28.5mg LA plus 15mg GLA twice a day versus placebo among 26 patients with aqueous deficient DES resulted in reported reductions in DES symptoms, lissamine green staining, and ocular surface inflammation (33). An additional trial among 60 subjects undergoing photorefractive keratectomy, reported significant beneficial effects of a once daily dose of 28.5 mg LA plus 15.1 mg GLA on tear function tests and ocular symptoms (34). Observational studies have also suggested a link between n-3 FA and DES in Sjogren's syndrome. In a cross-sectional study of 41 patients with primary Sjogren's syndrome (35), fatty acid levels within erythrocyte phospholipids, plasma phospholipids, plasma triglycerides and plasma cholesterol esters were investigated for associations with immunopathological and clinical disease parameters. In this study, DHA was inversely correlated with the clinical DES status, a finding that is in general agreement with those of the present study. In a separate study, 68 women with Sjogren's syndrome were found to have a lower dietary intake of n-3 FA compared to age-matched controls (36).
N-3 FA may also have a direct effect on the polar portion of the lipid layer of tear film by increasing the amount of n-3 FA present or by affecting the ratio of n-6/n-3 FA (37). Finally, n-3 FA intake may decrease endogenous estrogen production (38), which may impact risk of DES (39).
One of the main limitations of our study lies in our questionnaire-based assessment of DES. Additionally, we could not differentiate between evaporative versus aqueous deficient subtypes of DES. However, previous studies have suggested the validity of the type of assessment we used (19, 39, 40) and our own validation study among 53 patients demonstrated good sensitivity and specificity versus commonly used clinical tests for DES (18). Although our classification of DES was certainly not perfect, misclassification would tend to bias estimates toward the null, unless it was associated with the exposure of interest. Although it is theoretically possible that women who consume higher amounts of n-3 FA are less likely to receive a diagnosis of DES, this seems particularly unlikely since increased consumption was correlated with factors such as older age and diabetes, which are associated with an increased risk of DES. Moreover, control for frequency of eye examinations (i.e. opportunity for diagnosis) did not eliminate the association between n-3 FA and DES. Confounding by unmeasured factors, such as medication use, is another concern. Although we could not address this directly as information on medication use was not available, control for major diseases such diabetes mellitus, hypertension and connective tissue diseases did not alter the observed associations. Confounding due to differential use of contact lenses or artificial tears across levels of FA intake is also unlikely given that neither of these factors was related to FA intake in a subgroup of 341 women for whom we had this information (each P for trend > 0.6; data not shown). Nonetheless, as in any epidemiological study, it remains possible that the relationships we observed could be explained by other differences between the women who consumed greater versus lesser amounts of n-3 FA.
Our study has several significant advantages including a large sample size, nationwide sampling of the study participants, control of most known or potential confounders, and use of a well-validated means of assessing dietary intake of essential fatty acids. Studies have shown that estimates of nutrient intake derived from the SFFQ are reflective of long-term dietary intake (14). Additionally, a positive association between the n-6/n-3 ratio and DES in the setting of a healthy population such as the WHS, where 99% of participants had a n-6/n-3 ratio below the mean for a typical Western diet (41), and 90% of them had a ratio below current recommendations (42), may point to an even greater influence of FA imbalances on DES in the general population.
To our knowledge, this is the first study of dietary intake of n-3 and/or n-6 FA, as they may relate to prevention of DES. Historically, there were appreciable amounts of n-3 FA in the diet provided by wild plants and wild game, and humans are thought to have evolved eating a ratio of n-6 FA to n-3 FA of close to 1:1. Many natural sources of n-3 FA have now been depleted from the diet, coupled with an oversupply of n-6 FA, particularly in last half of century, which has resulted in distortion of n-6/n-3 ratio to current levels, typically in the range of 12-16:1. Given the biology and importance of these fatty acids, and their opposing biological effects, it seems quite likely that such an imbalance would be related to some pathology (22).
In the present study, women with a higher intake of n-3 FA appear to have a lower risk of DES. Further, a high ratio of n-6/n-3 FA is associated with increased risk of DES. This is the first report of such an association. In light of the plausibility of hypothesized biological mechanisms, these findings suggest that increasing dietary intake of n-3 FA may reduce the risk of DES, an important and prevalent cause of ocular complaints.
ACKNOWLEDGEMENT
Each author contributed to the work described in this manuscript, including conception and study design (DAS), collection of data (DAS, JEB), analysis of data (DAS, BM, KAT), writing the manuscript (DAS, BM, KAT, JPG), and providing significant advice or consultation (JEB, MRD, JPG). Except for JPG, who is the Founder & CEO of Advanced Vision Research, Inc., the other authors do not have any conflicts of interest as pertains to information provided in this manuscript.
Footnotes
From the Division of Preventive Medicine (BM, JEB, DAS), Brigham and Women's Hospital and the Schepens Eye Research Institute (KAT, MRD, JPG, DAS) and the Massachusetts Eye and Ear Infirmary (MRD, JPG, DAS), Department of Ophthalmology, Harvard Medical School, Boston; the Department of Ambulatory Care and Prevention (JEB), Harvard Medical School, Boston and the Department of Epidemiology (JEB, DAS), Harvard School of Public Health, Boston.
Supported in part by NIH grants CA47988 & HL43851, and a grant from Advanced Vision Research, Inc., Woburn, MA.
Reprints not available. Address correspondence to: Debra A Schaumberg, Division of Preventive Medicine, 900 Commonwealth Avenue East, Boston, MA 02215, E-mail: dschaumberg@rics.bwh.harvard.edu
REFERENCES
- 1.Lemp MA. Epidemiology and classification of dry eye. Adv Exp Med Biol. 1998;438:791–803. doi: 10.1007/978-1-4615-5359-5_111. [DOI] [PubMed] [Google Scholar]
- 2.Lubniewski AJ. Diagnosis and management of dry eye and ocular surface disorders. Ophthalmol Clin North Am. 1990;3:575–594. [Google Scholar]
- 3.Goto E, Yagi Y, Matsumoto Y, Tsubota K. Impaired functional visual acuity of dry eye patients. Am J Ophthalmol. 2002;133:181–6. doi: 10.1016/s0002-9394(01)01365-4. [DOI] [PubMed] [Google Scholar]
- 4.Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Association for Research in Vision and Ophthalmology (ARVO) ARVO 2004; Fort Laudrerdale, Florida: 2004. Impact Of Dry Eye Syndrome On Vision-related Quality Of Life Among Women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson JD, Helms H, Fiscella R, Southwell Y, Hirsch JD. A new look at dry eye disease and its treatment. Adv Ther. 2000;17:84–93. doi: 10.1007/BF02854841. [DOI] [PubMed] [Google Scholar]
- 6.Kunert KS, Tisdale AS, Stern ME, Smith JA, Gipson IK. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000;118:1489–96. doi: 10.1001/archopht.118.11.1489. [DOI] [PubMed] [Google Scholar]
- 7.Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren syndrome. Ophthalmology. 1999;106:811–6. doi: 10.1016/S0161-6420(99)90171-9. [DOI] [PubMed] [Google Scholar]
- 8.Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–92. [PubMed] [Google Scholar]
- 9.Simopoulos AP. Human requirement for N-3 polyunsaturated fatty acids. Poult Sci. 2000;79:961–70. doi: 10.1093/ps/79.7.961. [DOI] [PubMed] [Google Scholar]
- 10.Simopoulos AP,MD, Robinson Jo. The Omega Diet. Harper Collins Publishers Inc; New York: 1999. [Google Scholar]
- 11.Ambrosio RJ, Stelzner SK. Nutrition and Dry Eye the Role of Lipids. Review of Refractive Surgery 2002 August. 2002:29–32. [Google Scholar]
- 12.Boerner Dry eye successfully teated with oral flaxseed oil. Ocular surgery news. 2000:147. [Google Scholar]
- 13.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women's Health Study. J Womens Health Gend Based Med. 2000;9:19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 14.Willett W. Nutritional epidemiology. 2nd Oxford University Press; New York: 1998. [Google Scholar]
- 15.Agriculture UDo . Composition of foods- raw, processed and prepared, 1963-1988. US Government Printing Office; Washington, DC: 1989. [Google Scholar]
- 16.Holland GW, Unwin AA, Buss ID, Paul DH, Dat AA. Royal Society of Chemistry and Ministry of Agriculture, Fisheries and Food; Cambridge, UK: 1991. The Composition of Foods. [Google Scholar]
- 17.Iso H, Rexrode KM, Stampfer MJ, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. Jama. 2001;285:304–12. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- 18.Gulati DAS DA, Sullivan DA, Dana R. Association for Research in Vision and Ophthalmology (ARVO) ARVO 2004; Fort Lauderdale, Florida: 2004. Clinical Validation of a Short Dry Eye Questionnaire. [Google Scholar]
- 19.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118:1264–8. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 20.Schein OD, Hochberg MC, Munoz B, et al. Dry eye and dry mouth in the elderly: a population-based assessment. Arch Intern Med. 1999;159:1359–63. doi: 10.1001/archinte.159.12.1359. [DOI] [PubMed] [Google Scholar]
- 21.Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78:409–16. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–79. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 23.Alexander JW. Immunonutrition: the role of omega-3 fatty acids. Nutrition. 1998;14:627–33. doi: 10.1016/s0899-9007(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 24.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 25.Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 1999;83:217–44. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 26.Ringbom T, Huss U, Stenholm A, et al. Cox-2 inhibitory effects of naturally occurring and modified fatty acids. J Nat Prod. 2001;64:745–9. doi: 10.1021/np000620d. [DOI] [PubMed] [Google Scholar]
- 27.Culp BR, Titus BG, Lands WE. Inhibition of prostaglandin biosynthesis by eicosapentaenoic acid. Prostaglandins Med. 1979;3:269–78. doi: 10.1016/0161-4630(79)90068-5. [DOI] [PubMed] [Google Scholar]
- 28.Grimm H, Mayer K, Mayser P, Eigenbrodt E. Regulatory potential of n-3 fatty acids in immunological and inflammatory processes. Br J Nutr. 2002;87(Suppl 1):S59–67. doi: 10.1079/bjn2001457. [DOI] [PubMed] [Google Scholar]
- 29.Calder PC, Zurier RB. Polyunsaturated fatty acids and rheumatoid arthritis. Curr Opin Clin Nutr Metab Care. 2001;4:115–21. doi: 10.1097/00075197-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Pholpramool C. Secretory effect of prostaglandins on the rabbit lacrimal gland in vivo. Prostaglandins Med. 1979;3:185–92. doi: 10.1016/0161-4630(79)90102-2. [DOI] [PubMed] [Google Scholar]
- 31.Horrobin DF, Campbell A. Sjogren's syndrome and the sicca syndrome: the role of prostaglandin E1 deficiency. Treatment with essential fatty acids and vitamin C. Med Hypotheses. 1980;6:225–32. doi: 10.1016/0306-9877(80)90120-6. [DOI] [PubMed] [Google Scholar]
- 32.Theander E, Horrobin DF, Jacobsson LT, Manthorpe R. Gammalinolenic acid treatment of fatigue associated with primary Sjogren's syndrome. Scand J Rheumatol. 2002;31:72–9. doi: 10.1080/03009740252937577. [DOI] [PubMed] [Google Scholar]
- 33.Barabino S, Rolando M, Camicione P, et al. Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea. 2003;22:97–101. doi: 10.1097/00003226-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Macri A, Giuffrida S, Amico V, Iester M, Traverso CE. Effect of linoleic acid and gamma-linolenic acid on tear production, tear clearance and on the ocular surface after photorefractive keratectomy. Graefes Arch Clin Exp Ophthalmol. 2003;241:561–6. doi: 10.1007/s00417-003-0685-x. [DOI] [PubMed] [Google Scholar]
- 35.Oxholm P, Asmussen K, Wiik A, Horrobin DF. Essential fatty acid status in cell membranes and plasma of patients with primary Sjogren's syndrome. Correlations to clinical and immunologic variables using a new model for classification and assessment of disease manifestations. Prostaglandins Leukot Essent Fatty Acids. 1998;59:239–45. doi: 10.1016/s0952-3278(98)90136-3. [DOI] [PubMed] [Google Scholar]
- 36.Cermak JM, Papas AS, Sullivan RM, Dana MR, Sullivan DA. Nutrient intake in women with primary and secondary Sjogren's syndrome. Eur J Clin Nutr. 2003;57:328–34. doi: 10.1038/sj.ejcn.1601543. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan BD, Cermak JM, Sullivan RM, et al. Correlations between nutrient intake and the polar lipid profiles of meibomian gland secretions in women with Sjogren's syndrome. Adv Exp Med Biol. 2002;506:441–7. doi: 10.1007/978-1-4615-0717-8_62. [DOI] [PubMed] [Google Scholar]
- 38.Noble LS, Takayama K, Zeitoun KM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600–6. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- 39.Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. Jama. 2001;286:2114–9. doi: 10.1001/jama.286.17.2114. [DOI] [PubMed] [Google Scholar]
- 40.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–26. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 41.Simopoulos AP. n-3 fatty acids and human health: defining strategies for public policy. Lipids. 2001;36(Suppl):S83–9. doi: 10.1007/s11745-001-0687-7. [DOI] [PubMed] [Google Scholar]
- 42.Medicine Io . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. National Academies Press website; 2002. [DOI] [PubMed] [Google Scholar]