Abstract
Since ancient times, mulberry leaves (Morus spp.) have been used to rear the silkworm Bombyx mori. Because the silkworm grows well on mulberry leaves, the toxicities and defensive activities of these leaves against herbivorous insects have been overlooked. Here we show that mulberry leaves are highly toxic to caterpillars other than the silkworm B. mori, because of the ingredients of the latex, a milky sap exuded from mulberry leaf veins. The toxicity of mulberry leaves was lost when the latex was eliminated from the leaves, and artificial diets containing latex showed toxicity. Mulberry latex contained very high concentrations of alkaloidal sugar-mimic glycosidase inhibitors reported to have antidiabetic activities, such as 1,4-dideoxy-1,4-imino-d-arabinitol, 1-deoxynojirimycin, and 1,4-dideoxy-1,4-imino-d-ribitol. The overall concentrations of these inhibitors in latex reached 1.5-2.5% (8-18% dry weight) in several mulberry varieties, which were ≈100 times the concentrations previously reported from whole mulberry leaves. These sugar-mimic alkaloids were toxic to caterpillars but not to the silkworm B. mori, indicating that the silkworm can circumvent the mulberry tree's defense. Our results suggest that latex ingredients play key roles in defense of this tree and of other plants against insect herbivory, and they imply that plant latexes are treasuries of bioactive substances useful as medicines and pesticides.
Keywords: plant defense, plant-insect interactions, Morus spp., Eri silkworm, Bombyx mori
Latex is widely found among plant species; 12,000-35,000 species have been reported to exude it (1-3). Plant latex has been suggested to play an important role in plant defense against insect herbivory (1-6) for two reasons: first, latex often contains toxic compounds such as morphine in poppy and cardenolide in milkweeds (2-4), and, second, many herbivorous insects specialized to feed on latex-containing plants have developed behaviors to avoid consuming latex and to inactivate laticifers by cutting leaf veins or making trenches (1, 4-6). However, neither toxins nor toxicities have been reported in a majority of latex-producing plants. In these cases, the stickiness of latex that would trap the mouth parts of herbivorous insects has been suggested to be involved in defense against insect herbivory. Recently, several latex-producing plants with no reported toxicities have been found to be strongly toxic to insects because of the ingredients of latex (7). For example, papaya (Carica papaya, Moraceae) and fig (Ficus virgata, Moraceae) leaves are strongly toxic to insects because of the cysteine proteases in their latex (7). These recent findings indicated a need to investigate latex ingredients and their biological functions.
Mulberry trees (Morus spp. Moraceae) grow in Asia, and their leaves have been used for rearing an economically very important insect, the silkworm Bombyx mori, for thousands of years. Moraceae plants are characterized by the presence of latex, and mulberry trees also exude latex when their leaves are damaged by caterpillars (Fig. 1 A-D). However, there have been no detailed studies about the defense activities of mulberry trees against insect herbivory or the involvement of mulberry latex in plant defense. In this study, we addressed these subjects, and we found that surprisingly high concentrations of alkaloidal sugar-mimic glycosidase inhibitors, which are reported to have antidiabetic activities (8), exist in mulberry latex and that these compounds play key roles in defense of mulberry trees.
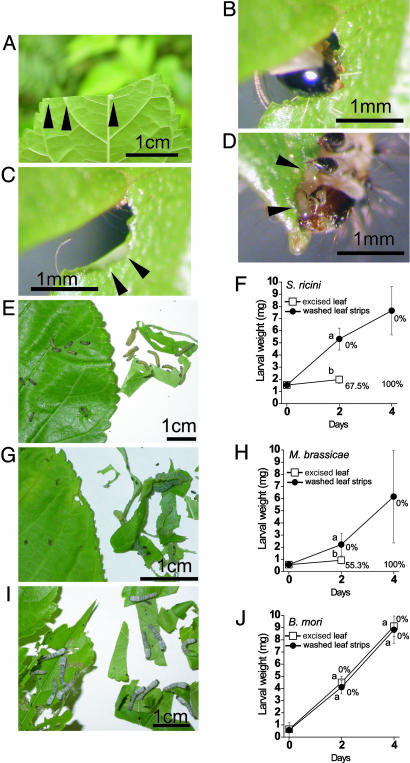
Fig. 1.
Mulberry latex and its involvement in mulberry defense against insect herbivory. (A) When mulberry leaf is damaged, latex (arrowheads) is exuded from veins. (B) A newly hatched larva of S. ricini, tiny in size, biting into a mulberry leaf. (C) A photo taken immediately after B was taken. Latex is exuded at the point of insect attack (arrowheads). (D) When eating a mulberry leaf, the caterpillar has to drink a very large amount of latex (arrowheads) relative to its small body size. (E-J) Comparison of caterpillar growth on excised mulberry leaves with latex and on narrow leaf strips that have been washed with water to remove the latex. Leaves of wild M. australis are fed to larvae of three caterpillar species. (E and F) S. ricini. (G and H) M. brassicae. (I and J) B. mori.(E, G, and I) Photos taken on day 4. Shown are excised leaves that have been clipped at the petioles (left) and washed leaf strips (right). (F, H, and J) Larval weight and mortality. Error bars indicate SD. Values shown with different letters are significantly different (P < 0.01, t test; n = 20 for J, and n = 40 for F and H).
Results
Mulberry leaves exude latex when damaged by caterpillars (Fig. 1 A-D). To examine whether mulberry latex is involved in mulberry defense against insect herbivory, we made bioassays using larvae of two polyphagous lepidopteran species, the Eri silkmoth (Samia ricini, Saturniidae), which is successfully used to detect a number of plant defenses (7, 9), and the cabbage moth (Mamestra brassicae, Noctuidae), a notorious pest species. In natural conditions, mulberry trees are not the host plants for either of these caterpillar species. When the excised leaves of wild mulberry, Morus australis (which is native to Japan), were fed, larvae of both species bit into the leaves (Fig. 1 B-D), neither species of caterpillar grew well, and the mortality of each was very high (Fig. 1 E-H). All of the larvae died before day 4. In contrast, larvae of both species fed the washed mulberry leaf strips grew very well without dying (Fig. 1 E-H). In contrast with these two nonmulberry specialist caterpillars, the silkworm B. mori grew very well without dying regardless of whether they ate excised leaves or washed leaf strips, with no significant difference in the results between these treatments (Fig. 1 I and J). These results suggested that mulberry leaves are highly toxic to caterpillars other than the silkworm B. mori and that mulberry latex is involved in the mulberry defense against insect herbivory.
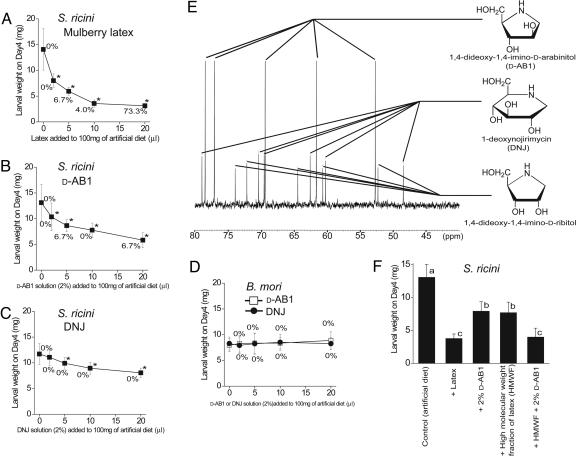
To examine the defensive effect of latex and the ingredients in latex responsible for the defensive activity, we chemically analyzed latex from M. australis (wild Ishigaki population) accompanied by bioassays using the Eri silkworm, S. ricini. When newly hatched larvae were fed an artificial diet containing 2-5% mulberry latex, growth was inhibited significantly, and when they were fed a diet containing 20% latex, high mortality was observed on day 4 (Fig. 2A). These results show that mulberry latex retains toxicity against caterpillars. The observation that mulberry latex has a unique bittersweet taste indicated that it is rich in chemicals. We therefore analyzed mulberry latex directly using NMR spectra, and we detected strong signals characteristic of three alkaloidal sugar-mimic glycosidase inhibitors; 1,4-dideoxy-1,4-imino-d-arabinitol (d-AB1), 1-deoxynojirimycin (DNJ), and 1,4-dideoxy-1,4-imino-d-ribitol (Fig. 2E). A basic fraction of mulberry latex, purified by using cation and anion exchange columns, showed only 16 clear 13C NMR peak signals, all of which were assigned to those three inhibitors with 16 carbon atoms altogether (Fig. 2E), which means that these sugar-mimic glycosidase inhibitors, which are reported to have antidiabetic activities (8), could be purified easily by using this method. The concentrations of sugar-mimic glycosidase inhibitors were surprisingly high, and those of d-AB1, DNJ, and 1,4-dideoxy-1,4-imino-d-ribitol (calculated from peak intensities of 13C-NMR signals of whole mulberry latex) reached 1.63%, 0.36%, and 0.48% of the latex collected from M. australis (wild population on Ishigaki Island, Okinawa, Japan) (Table 1). These three compounds together make up 2.46% (180 mM) of latex (wet weight) and 18.2% (>1 M) of dried latex. These results corresponded well with the fact that we purified 41.6 mg as a basic fraction (a pure mixture of the three compounds) out of 1.928 ml of latex, indicating a 2.16% (wet weight) total concentration of the tree compounds. The total concentration of sugar-mimic alkaloids in dried mulberry latex (18.2%) is ≈100 times more than those reported by other authors analyzing dried materials from other mulberry tissues, such as root bark (0.18%), fruits (0.11%), and leaves (0.14%) of mulberry in previous studies (10). In fact, it may be the highest concentration in the plant kingdom ever reported. The concentration of sugar-mimic alkaloids differed remarkably among mulberry populations and cultivars. A wild mulberry population in Tsukuba, Japan [M. australis (or Morus bombycis)] and two cultivars, Yukishirazu and Ichibei, both of which were derived from this population, showed patterns similar to that of wild M. australis on Ishigaki island, with d-AB1 being the most abundant, except that 1,4-dideoxy-1,4-imino-d-ribitol was totally absent (Table 1). In contrast, from the latex Shinichinose, a cultivar that derived from Morus alba, a species that originated in China, only DNJ (0.32% of wet latex) was detected, without a trace of D-AB1 or 1,4-dideoxy-1,4-imino-d-ribitol (Table 1).
Fig. 2.
Sugar-mimic alkaloids in mulberry latex and their toxic effects on caterpillars. (A) Effects of mulberry latex on S. ricini. (B) Effects of d-AB1 on S. ricini. (C) Effects of DNJ on S. ricini.(D) Effects of d-AB1 and DNJ on B. mori. (A-D) Larval weights and mortality on day 4 are measured. Error bars indicate SD. Values followed by * are significantly smaller than controls fed an artificial diet only (P < 0.01, t test; n = 15-30). (E) The 13C NMR spectrum of a basic fraction (acidified) of mulberry latex. All 16 peak signals are assigned to three sugar mimic alkaloids, d-AB1, DNJ, and 1,4-dideoxy-1,4-imino-d-ribitol, which altogether have 16 carbon molecules. (F) Evaluating the contribution of d-AB1 and the HMWF (molecular weight > 3,000) of mulberry latex to the toxicity of mulberry latex. Ten microliters of mulberry latex or a 10 μl equivalent of d-AB1, HMWF, or d-AB1 plus HMWF was added to artificial diets and fed to S. ricini larvae. Larval weights were measured on day 4. Error bars indicate SD. Values shown with different letters are significantly different (P < 0.01, Tukey's test for multiple comparison; n = 10-20). Mulberry latex used in A, E, and F was collected from a wild population of M. australis on Ishigaki Island.
Table 1. Concentrations of sugar-mimic alkaloids in mulberry latex.
| Concentrations in latex, %
|
|||
|---|---|---|---|
| Species, populations, and cultivars | d-AB1 | DNJ | 1,4-Dideoxy-1,4-imino-d-ribitol |
| M. australis (or M. bombycis) | |||
| Wild, Ishigaki Island | 1.63 ± 0.21 | 0.36 ± 0.09 | 0.48 ± 0.03 |
| Wild, Tsukuba | 1.28 ± 0.46 | 0.45 ± 0.23 | 0 |
| Cultivar Yukishirazu | 1.41 ± 0.26 | 0.35 ± 0.04 | 0 |
| Cultivar Ichibei | 1.04 ± 0.02 | 0.47 ± 0.07 | 0 |
| M. alba | |||
| Cultivar Shinichinose | 0 | 0.32 ± 0.00 | 0 |
Values indicate means ± SD (n = 4–5 for wild populations, and n = 2–3 for cultivars).
To examine the defensive role of sugar-mimic alkaloids in mulberry latex, the effects of authentic sugar-mimic alkaloids on the Eri silkworm, S. ricini, were analyzed. When Eri silkworms were fed 100 mg of an artificial diet containing more than 2-5 μl of 2% d-AB1 or DNJ, the growth of the larvae was significantly retarded, and the growth inhibition by d-AB1 was slightly stronger than that by DNJ (Fig. 2 B and C). These results show that the sugar-mimic alkaloids have significant adverse effects against the growth of the Eri silkworm at a very low concentration (i.e., 0.1% per diet). The results also indicate that the high concentrations of sugar-mimic alkaloids in mulberry latex contribute to the mulberry's latex-borne defense against insect herbivory. In contrast, the silkworm B. mori showed no significant growth retardation even when they were fed 100 mg of an artificial diet containing 20 μl of 2% d-AB1 or DNJ (Fig. 2D), suggesting that B. mori, a mulberry specialist, is well adapted to mulberry and its defensive chemicals. Because 2% is the approximate concentration of sugar-mimic alkaloids in mulberry latex, we compared the adverse effects of mulberry whole latex and 2% of d-AB1 or DNJ on S. ricini. The comparison showed, however, that the adverse effects of mulberry latex are stronger than 2% of either d-AB1 or DNJ and that not all of the adverse effects of mulberry latex can be explained by these sugar-mimic alkaloids. We found that a high-molecular-weight fraction (HMWF) of mulberry latex (molecular weight > 3,000) retains growth-inhibitory activity that is as strong as that of d-AB1, and, when 2% of d-AB1 is added to the HMWF, this mixture has a growth-inhibitory activity just as strong as that of mulberry latex (Fig. 2F). These results indicated that the sugar-mimic alkaloids, together with unidentified high-molecular-weight factor(s), can completely account for the toxicity of mulberry latex.
Discussion
We found in the present study that mulberry leaves are highly toxic to caterpillars other than the silkworm B. mori and that this toxicity is contained in mulberry latex, which has surprisingly high concentrations of sugar-mimic alkaloids. Because sugar-mimic alkaloids are potent inhibitors of glycosidases and sugar-metabolizing enzymes, they seem to exert adverse effects on insects by interfering with sugar metabolism in ways that are similar to their antidiabetic activities (8, 10-13). Because the silkworm B. mori has been a very important insect economically, there have been a number of studies on silkworm-mulberry interactions. For example, in a classic study published several decades ago in Nature (14), Hamamura et al. reported that B. mori feeds on the mulberry tree because biting factors and swallowing factors coexist in mulberry leaves. However, without knowing the defense factors in mulberry latex, our understanding of mulberry-silkworm interactions is incomplete. The more fundamental view suggested from our study is that mulberry has a latex-borne defense with sugar-mimic alkaloids and that B. mori can feed on it because it has developed some adaptive mechanism. Several studies have discussed the defensive roles of sugar-mimic alkaloid in several plants (12, 13), and it was found, interestingly, that B. mori glycosidases are less affected by sugar-mimic alkaloids than those of other organisms (10). However, there has been no direct experimental proof that sugar-mimic alkaloids serve as an effective defense of mulberry against insect herbivory in natural conditions. Probably, the defense effects of sugar-mimic alkaloids have been elusive because the involvement of latex very rich in these compounds has not been considered, because the average concentration of these compounds in mulberry leaves as a whole, which is observed by using traditional extraction methods with solvents, is too low to show defense activities against caterpillars (10). It is difficult to estimate how much latex caterpillars consume while eating mulberry leaves, but young caterpillars tiny in size likely ingest relatively very large amounts of latex rich in noxious substances, as suggested by the photos in Fig. 1 B-D. It is very interesting that many other plants reported to contain sugar-mimic alkaloids belong to families rich in latex-exuding species, such as Omphalea diandra (Euphorbiaceae) (8, 12) and Ipomoea carnea (Convolvulaceae) (8), and it is possible that sugar-mimic alkaloids in latex in concentrations similar to those found in mulberry might be observed in these plants.
A number of substances, proteins, peptides, and chemicals with no known biological functions have been found in plant latex. Papain, a cysteine protease in papaya latex, had been an example of such a substance until our recent studies (7) proved that it is indispensable in the defense of papaya against insect herbivory. It has been suggested that the stickiness of latex is important in latex-borne defense of many latex-exuding plants; however, the defensive roles of latex ingredients are not well investigated except for a few cases, such as milkweed latex rich in cardenolides and poppy latex rich in alkaloids (1-4). However, our results from papaya latex (7) and from mulberry latex in the present study suggest that ingredients of latex toxic to insects are also very important in many cases. The concept that latex and its ingredients toxic to insects (besides its stickiness) are very important in plant defense will help us understand the mechanisms of plant-insect interactions, as well as the roles and functions of various chemicals and proteins found in plant latex with no known biological functions. Furthermore, we found that surprisingly high concentrations (1.5-2.5% wet weight, 8-18% dry weight) of medically applicable sugar-mimic alkaloids exist in mulberry latex and that it is easy to purify them in a simple method (Fig. 2E). These results indicate that plant latexes could be both good targets to discover novel medicines and good materials to start purifications. Although latex may be a tiny sap, it plays very important roles in plant defense systems, and it could be a potential treasury of bioactive substances useful as medicines and pesticides.
Materials and Methods
Insects. Newly hatched larvae of a line of the Eri silkmoth (S. ricini, Saturniidae) maintained at our institute as an experimental insect were used to assay the toxic effects of mulberry leaves and latex on nonmulberry-specialist caterpillars. These larvae have been successfully used in bioassays to evaluate plant defense levels against herbivorous insects (7, 9). Newly molted second-instar larvae of M. brassicae (kindly provided by Hokko Chemical Industry, Tokyo, and then maintained at our institute) were also used for bioassays. Newly hatched larvae of Shinasagiri, a line of the silkworm B. mori, were used to assay the effects of mulberry leaves and latex on mulberry specialists.
Mulberry Latex. Mulberry latex was collected from a wild population of M. australis that grows on Ishigaki Island (24°N, 124°E); from a wild population of M. australis that grows in Tsukuba, Honshu, Japan (36°N, 140°E) (the Honshu population is also referred to as M. bombycis); from cultivars Yukishirazu and Ichibei, which originated from a Honshu population of M. australis; and from cultivar Shinichinose, which originated from M. alba in China. All of the cultivars were used to rear the silkworm B. mori and were grown at our institute. Latex from the wild Ishigaki population was used for all of the bioassays. Latex was collected by cutting the petioles. Latex exuded from the cut petioles was collected in ice-cooled test tubes, lyophilized, and kept at -20°C. Lyophilized latex powder was dissolved in water in the original concentration, and this solution was the latex used in bioassays.
Bioassays. From wild mulberry tree M. australis, young leaves that had reached approximately half their mature size and were still soft were collected by cutting the petioles and were used for the bioassays. The collected leaves were fed directly to the larvae of S. ricini (first instar), M. brassicae (second instar), and B. mori (first instar) to examine the toxicities of mulberry leaves. To examine the involvement of mulberry latex in leaf toxicity, we attempted to wash latex from the leaves before feeding the leaves to larvae. For this purpose, leaves (1-3 g) were cut into narrow strips (2-5 mm in width) with scissors, and these strips were washed twice in 500 ml of water. The leaves were patted dry with paper towels and then given to larvae. To examine the toxic effects of mulberry latex, 30 μl of an aqueous solution containing an indicated amount of latex was added to 100 mg of artificial diet [L4M diet for polyphagous insects, based on soybean powder (Nihon Nosan Kogyo, Yokohama, Japan)], and then fed to the larvae (to five individuals). To examine the toxic effect of sugar-mimic alkaloids, 30 μl of aqueous solution containing the indicated amount of 2% aqueous solution of authentic d-AB1 (Wako Pure Chemical Industries) or DNJ (Sigma) was added to 100 mg of L4M diet and fed to the larvae. The HMWF of mulberry latex was prepared by using Microcon YM-3 tubes (molecular-size-filtration centrifugal tubes using cellulose filters, molecular weight limit of 3,000, Millipore). Mulberry latex (300 μl) was applied to a YM-3 tube and then centrifuged (10,000 × g for 1.5 h). Then 400 μl of water was added to the upper part of the tube, which was again centrifuged (10,000 × g for 1.5 h). The small amount of solution remaining in the upper part was recovered, dissolved in 300 μl of water, and used as HMFW. In the bioassays, HMFW was added to the L4M diet alone or with d-AB1. Larval weight and mortalities were observed 2 and 4 days after the onset of the experiments. The leaves or artificial diets were exchanged with fresh ones every other day.
Chemical Analyses. A basic fraction of latex was prepared by applying 2 ml of mulberry latex, from which high-molecular-weight substances such as proteins had been removed by ethanol precipitation (70% ethanol), to a column with 10 ml of Amberlite IR-120 (plus) ion-exchanging resin (Sigma-Aldrich). After washing the column with 50 ml of distilled water to remove the neutral and acidic compounds, basic and amphoteric fractions were eluted with 100 ml of 0.5 N NH4OH solution. Eluted solution was evaporated, dissolved in 2 ml of water, and then applied to 25 g of the OH form of Dowex 1 × 2 column (200-400 mesh, Supelco). The basic fraction that was not absorbed was eluted with 100 ml of water. The evaporation and lyophilization of the fraction gave 41.6 mg of a sticky substance. The basic fraction and lyophilized latex powder derived from 100 μl of latex were dissolved in 100 μl of D2O. A floating creamy oil was discarded by centrifugation, if present, and the transparent solution was acidified to pH 2 with HCl to protonate sugar-mimic alkaloids. Then the solutions were lyophilized and again dissolved in 600 μl of D2O. The solution was centrifuged again, insoluble precipitate was discarded, and then NMR spectra were measured with a Bruker AVANCE 500 spectrometer equipped with a highly sensitive 13C-{1H} CryoProbe at 303 K in the presence of 15.0 μmol of 2-methyl-2-propanol as an internal standard (d13C 31.3 ppm). All 13C NMR spectra were obtained by inverse gated decoupling pulse sequence (15), with a relaxation delay of 1 min to minimize the change in sensitivity of each signal because of both nuclear Overhauser effect and incomplete relaxation, and were obtained with 1,024 scans. In this condition, primary and secondary carbon atoms give signal intensities proportional to the numbers of atoms present in a sample irrespective of the positions of carbon atoms in molecules, and the effects of the solvent (D2O) could also be neglected; therefore, we used this method for the quantification of chemicals as well as the qualification of chemicals. The 13C NMR spectrum of the basic fraction showed only 16 clear peaks, all of which were assigned to protonated forms of three sugar-mimic alkaloids. d-AB1 protonated form: 13C NMR (125 MHz, d2O) δ [ppm] 52.7, 61.7, 69.4, 77.0, 78.4. 1,4-dideoxy-1,4-imino-d-ribitol protonated form: 13C NMR (125 MHz, D2O) δ [ppm] 52.3, 60.7, 64.5, 72.1, 73.9. DNJ protonated form: 13C NMR (125 MHz, D2O) δ [ppm] 48.5, 60.3, 62.6, 69.6, 70.4, 78.9. The spectra assigned to the former two compounds were in good agreement with those reported in previous studies (11), and those assigned to the last compound corresponded exactly to the spectra that we obtained from the protonated form of authentic DNJ. The 13C NMR spectra of latex that was not acidified and was directly measured also had good correspondence to spectra of protonated forms of the three sugar-mimic alkaloids but not to the spectra of deprotonated forms. This finding indicated that the sugar-mimic alkaloids exist in mulberry latex as protonated forms in natural conditions. The sugar-mimic alkaloids were quantified by comparing the average signal intensities of five or six (in the case of DNJ) carbons in each compound, with the signal intensity of the methyl carbons in 2-methyl-2-propanol added as an internal control. The concentrations in latex were expressed in wt/vol percentage calculated as deprotonated forms of sugar-mimic alkaloids.
Acknowledgments
We thank M. Hazeyama, H. Oguchi, and H. Niki for help with the insect rearing and plant growing and Hokko Chemical Industry for the insects. This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and by a Research Grant Project from the Ministry of Agriculture, Forestry and Fishery of Japan.
Author contributions: K. Konno and H.O. designed research; K. Konno and H.O. performed research; K. Konno, H.O., M.N., K.T., C.H., Y.T., M.H., A.K., and K. Kohno contributed new reagents/analytic tools; K. Konno and H.O. analyzed data; and K. Konno wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: d-AB1, 1,4-dideoxy-1,4-imino-d-arabinitol; DNJ, 1-deoxynojirimycin; HMWF, high-molecular-weight fraction.
References
- 1.Dussourd, D. E. & Denno, R. F. (1991) Ecology 72, 1383-1396. [Google Scholar]
- 2.Farrell, B. D., Dussourd, D. E. & Mitter, C. (1991) Am. Nat. 138, 881-900. [Google Scholar]
- 3.Harborne, J. B. (1993) in Introduction to Ecological Biochemistry (Academic, London), 4th Ed., pp. 186-210.
- 4.Dussourd, D. E. (1993) in Caterpillars: Ecological and Evolutionary Constraints on Foraging, eds. Stamp, N. E. & Casey, T. M. (Chapman & Hall, New York), pp. 92-131.
- 5.Dussourd, D. E. & Eisner, T. (1987) Science 237, 898-901. [DOI] [PubMed] [Google Scholar]
- 6.Dussourd, D. E. & Denno, R. F. (1994) Ecology 75, 69-78. [Google Scholar]
- 7.Konno, K., Hirayama, C., Nakamura, M., Tateishi, K., Tamura, Y., Hattori, M. & Kohno, K. (2004) Plant J. 37, 370-378. [DOI] [PubMed] [Google Scholar]
- 8.Asano, N., Nash, R. J., Molyneux, R. J. & Fleet, W. J. (2000) Tetrahedron Asymmetry 11, 1645-1680. [Google Scholar]
- 9.Fukui, A., Murakami, M., Konno, K., Nakamura, M. & Ohgushi, T. (2002) Entomol. Sci. 5, 263-266. [Google Scholar]
- 10.Asano, N., Yamashita, T., Yasuda K., Ikeda, K., Kizu, H., Kameda, Y., Kato, A., Nash, R. J., Lee, H. S. & Ryu, K. S. (2001) J. Agric. Food Chem. 49, 4208-4213. [DOI] [PubMed] [Google Scholar]
- 11.Asano, N., Oseki, K. & Matsui, K. (1994) J. Med. Chem. 37, 3701-3706. [DOI] [PubMed] [Google Scholar]
- 12.Kite, G. C., Scofield, A. M., Lees, D. C., Hughes, M. & Smith, N. G. (1997) J. Chem. Ecol. 23, 119-135. [Google Scholar]
- 13.Campbell, B. C., Molyneux, R. J. & Jones, K. C. (1987) J. Chem. Ecol. 13, 1759-1770. [DOI] [PubMed] [Google Scholar]
- 14.Hamamura, Y., Hayashiya, K., Naito, K.-I., Matsuura, K. & Nishida, J. (1962) Nature 194, 754-755. [Google Scholar]
- 15.Freeman, R., Hill, H. D. W. & Kaptein, R. (1972) J. Magn. Reson. 7, 327-329. [Google Scholar]