Abstract
Varicella-zoster virus (VZV) encodes six genes that do not have homologs in herpes simplex virus. One of these genes, VZV open reading frame 2 (ORF2), was expressed as a 31-kDa phosphoprotein in the membranes of infected cells. Unlike equine and bovine herpesvirus type 1 ORF2 homologs that are associated with virions, VZV virions contained no detectable ORF2 protein. The ORF2 deletion mutant established a latent infection in cotton rats at a frequency and with a number of VZV genomes similar to that of the parental virus. ORF63 transcripts, a hallmark of latent infection, were present in ganglia latently infected with both the ORF2 deletion mutant and parental VZV. Thus, ORF2 is the first VZV gene shown to be dispensable for establishment of latent infection in an animal model.
Varicella-zoster virus (VZV) is a member of the alphaherpesvirus subfamily. This subfamily includes the genera Simplexvirus, such as herpes simplex virus (HSV), and Varicellovirus. The varicelloviruses include VZV, equine herpesvirus type 1 (EHV-1), bovine herpesvirus type 1 (BHV-1), and simian varicella virus (SVV). VZV encodes at least 70 unique genes, most of which are conserved in HSV. However, open reading frames (ORFs) 1, 2, 13, 32, 57, and S/L do not have homologs in HSV (6). Five of these genes, ORFs 1, 13, 32, 57, and S/L have been shown to be dispensable for replication of VZV in vitro (4, 5, 9, 17, 26).
VZV ORF2 is predicted to encode a 26-kDa protein containing 238 amino acids (10). Although VZV ORF2 does not have an HSV homolog, it does share positional and limited sequence homology with several animal alphaherpesvirus genes. These genes include the BHV-1 circ gene, EHV-1 gene 3 (UL1), EHV-4 gene 3, and Marek's disease virus ORF71 (12, 16, 29, 30). Interestingly, ORF2 is the only gene in VZV that does not have a homolog in SVV (13).
VZV and HSV establish lifelong latent infections in sensory ganglia in humans. Unlike latency in HSV infection, in which only the latency-associated transcript RNA is expressed, multiple viral transcripts have been detected during VZV latency (7, 8, 18, 20, 24). While many studies have evaluated which HSV genes are dispensable for latency, it is unknown whether specific VZV genes are required for the virus to establish a latent infection. Here we show that VZV ORF2 is dispensable for viral replication in cell culture and for establishment of latent infection in an animal model.
VZV ORF2 encodes a 31-kDa phosphoprotein.
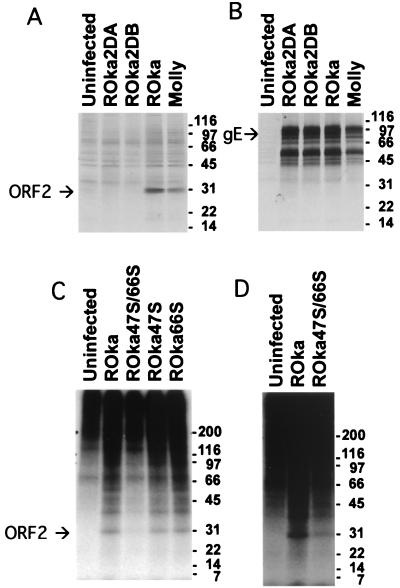
RNA prepared from VZV-infected cells revealed a 900-base transcript in infected cells using a probe complementary to ORF2 (data not shown). To verify that the ORF2 protein is expressed in VZV-infected cells, rabbit antibody to a maltose binding protein-ORF2 fusion protein containing amino acids 28 to 238 of ORF2 was produced. Immunoprecipitation of [35S]methionine-labeled cells using antiserum to ORF2 showed a 31-kDa protein in VZV-infected cells (Fig. 1A).
FIG. 1.
Immunoprecipitation of ORF2 protein from VZV-infected cells. (A) Antiserum to ORF2 immunoprecipitates a 31-kDa protein from cells infected with ROka VZV and a low-passage clinical isolate (Molly strain) but not from uninfected cells or cells infected with ORF2 deletion mutants of VZV (ROka2DA or ROka2DB). (B) Cells infected with VZV ROka and ORF2 deletion mutants express 44- to 100-kDa proteins that immunoprecipitate with monoclonal antibody to VZV gE (Chemicon, Temucula, Calif.). (C and D) ORF2 encodes a phosphoprotein. Cells infected with parental VZV or with ORF47 or ORF66 mutant viruses were radiolabeled with [32P]orthophosphoric acid, and ORF2 protein was immunoprecipitated. ORF2 protein is phosphorylated in cells infected with all of the VZV mutants. Panel D is a darker exposure of panel C showing the ORF2 phosphoprotein in cells infected with ROka47S/66S. The numbers to the right of the blots indicate molecular masses (in kilodaltons).
While ORF2 is predicted to encode a 26-kDa protein on the basis of its amino acid sequence, the larger size of the protein in virus-infected cells suggested that the protein may undergo posttranslational modification(s). Treatment of VZV-infected cells with tunicamycin or with neuraminidase and O-glycosidase did not result in a change in the size of ORF2 protein (data not shown). Thus, ORF2 is not N linked or O linked glycosylated. To determine if ORF2 protein is phosphorylated, VZV-infected cells were radiolabeled with [32P]orthophosphoric acid, and ORF2 protein and gE were immunoprecipitated. ORF2 protein was radiolabeled in cells infected with recombinant Oka (ROka) or VZV mutants with stop codons in the ORF47 and ORF66 protein kinases (ROka47S/66S, ROka47S, and ROka66S [14, 15]) but not in uninfected cells (Fig. 1C and D). Thus, ORF2 protein is phosphorylated in the absence of the VZV protein kinases.
VZV ORF2 is located in the membrane fraction of infected cells.
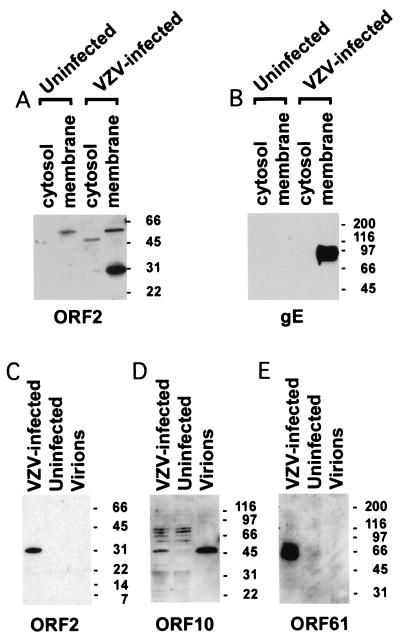
Cytosolic and membrane fractions were prepared (5) from virus-infected cells to localize the ORF2 protein. Immunoblotting using antiserum to ORF2 protein showed that the protein was located in the membrane fraction but not in the cytosol of VZV-infected cells (Fig. 2A). To determine whether ORF2 protein is located in virions, cells were infected with a low-passage-number clinical isolate of VZV and virions were isolated (22) and immunoblotted with antiserum to ORF2 protein. ORF2 protein was detected in lysates from VZV-infected cells but not in purified virions (Fig. 2C). The purity of the virion preparation was verified by the presence of the ORF10 tegument protein and the absence of the ORF61 nonstructural protein (21) in virions (Fig. 2D and E).
FIG. 2.
ORF2 protein is present in the membrane fraction of VZV-infected cells but not in virions. Membrane and cytosolic fractions from VZV-infected cells were prepared, and immunoblotting shows a band of 31-kDa corresponding to the ORF2 protein in the membrane fraction of VZV-infected cells (A). Immunoblotting with antibody to VZV gE shows gE also in the membrane fraction of infected cells (B). Cell lysates from VZV-infected cells, uninfected cells, or purified VZV virions were boiled in sample buffer, and immunoblotting was performed with antibodies (21, 25) to VZV ORF2 protein (C), ORF10 protein (D), and ORF61 protein (E). The numbers to the right of the blots indicate molecular masses (in kilodaltons).
VZV unable to express ORF2 protein is not impaired for replication in cell culture.
A VZV ORF2 deletion mutant was constructed to determine whether ORF2 is necessary for growth of VZV. ORF2 is encoded by VZV nucleotides 1,134 to 1,847 (10). The RecA-assisted restriction endonuclease cleavage technique (11) was used to produce VZV with a deletion in ORF2. A 60-base oligonucleotide, 5′-GATCCCCCCGTCAACAGCCGGTCTGTCACCGGGTTAGATTTGCCGAACCTACGGAGGGCG-3′, centered around the HpaII site at VZV nucleotide 1,808 in ORF2 was incubated with plasmid NS (5) in the presence of RecA protein. The unblocked HpaII sites in plasmid NS were methylated using HpaII methylase. After heat inactivation and dissociation of the DNA-RecA complex, the plasmid was digested with PmeI and HpaII. The large DNA fragment was blunt ended and ligated to itself to generate plasmid NS2D. The NotI-SacI fragment of plasmid NS2D was inserted in place of the wild-type NotI-SacI sequence in cosmid VZV NotI A to create cosmid VZV NotI A-2D. Sequence analysis of two clones showed that cosmid NotI A-2DA had nucleotides 1,214 to 1,808 (codons 27 to 226 of the 238-amino-acid ORF2 protein) deleted, whereas cosmid NotI A-2DB had nucleotides 1,213 to 1,808 deleted.
Melanoma cells were transfected with cosmids NotI BD, MstII A, MstII B, and NotI A-2DA or NotI A-2DB as described previously (4). Two viruses, ROka2DA and ROka2DB, were obtained, and Southern blots confirmed that the genomes had the expected configuration (data not shown). Immunoprecipitation of ORF2 from cells infected with VZV ROka or ORF2 deletion mutants showed that the ORF2 protein was absent in cells infected with the mutants (Fig. 1).
SVV is the herpesvirus most closely related to VZV. The sequence of SVV indicates that it has a homolog for each of the VZV genes, except for ORF2 (13). While VZV replicates more efficiently in human cells than in simian cells, the converse is true for SVV. Thus, ORF2 might provide a growth advantage for VZV in human cells compared to simian cells. Several human (melanoma, fibroblast, and schwannoma), and simian (Vero and BSC-1) cell lines were infected with parental VZV (ROka) and the ORF2 deletion mutants, and plaque sizes were similar for each virus. In addition, infection of melanoma cells with the parental and mutant viruses showed that all grew to similar titers (data not shown).
VZV ORF2 is dispensable for establishment of latency.
Guinea pigs and rats inoculated with VZV develop latent infection with viral DNA present in trigeminal (2, 23) or dorsal root (1, 27) ganglia. Since cotton rats are more permissive than other rodents for certain virus infections (3), we tested the ability of cotton rat cells to support the growth of VZV. While VZV growth in cell culture was thought to be limited to human, simian, and guinea pig cells, we found that VZV grew in cotton rat fibroblasts in vitro (data not shown).
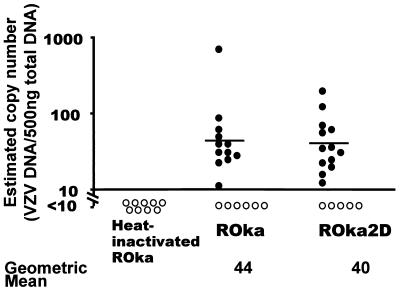
To study VZV latency, 7-week-old cotton rats were injected intramuscularly along both sides of the thoracic and lumbar spine with melanoma cells containing 3 × 105 PFU of VZV ROka or ROka2DA. None of the animals developed signs of varicella. One month after inoculation, the animals were sacrificed and DNA was isolated from pooled thoracic and lumbar dorsal root ganglia. VZV DNA was amplified by PCR using primers for VZV ORF21 (2), and Southern blots were performed with an ORF21 probe. The radioactive signal on the blot was quantified using a phosphorimager. A standard curve was constructed on the basis of the amount of PCR product obtained from serial dilutions of cosmid NotI A in 500 ng of DNA from ganglia of uninfected animals. The assay could reliably detect 10 copies of VZV cosmid DNA mixed with 500 ng of DNA from uninfected dorsal root ganglia.
Positive signals (≥10 copies/500 ng of DNA) were detected in ganglia from 12 of 18 VZV ROka-infected and 13 of 18 ROka2DA-infected animals. Two controls were performed to show specificity. First, DNA extracted from ganglia of animals inoculated with uninfected cells showed no viral DNA. Second, DNA from ganglia of animals inoculated with cells containing VZV ROka that had been heat inactivated (95°C for 10 min) contained no viral DNA. The amount of virion DNA in the inoculum after heat inactivation was similar to that without heat treatment, as assessed by Southern blotting (data not shown). Thus, infectious virus is necessary for establishment of latency. To estimate the copy number of VZV DNA in ganglia from latently infected animals, densitometry was performed using autoradiograms from Southern blots of PCR products. The geometric means of VZV genome copies from PCR-positive ganglia per 500 ng of ganglia DNA for animals infected with VZV ROka (44 copies) and ROka2DA (40 copies) were similar (Fig. 3).
FIG. 3.
Infection with parental VZV and the ORF2 deletion mutant results in similar VZV DNA copy numbers in dorsal root ganglia. Copy number was estimated by densitometry. The VZV genome copy numbers for animals that were considered positive for VZV (filled circles) and for animals that had genome copy numbers below the threshold for reliable detection of DNA (<10 copies of VZV DNA per 500 ng of ganglia DNA) (open circles) are shown. The geometric mean copy numbers of VZV genomes per 500 ng of ganglia DNA in PCR-positive animals are shown below the graphs.
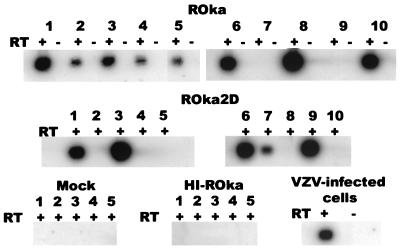
To verify that animals were latently infected with VZV, RNA was isolated from dorsal root ganglia, treated with DNase, and heated to inactivate DNase. cDNA, obtained by reverse transcription, was amplified by PCR using ORF63 (TTATCTAATGGGTCGCACC and GTATATTCCGCGGTTTCTGC) or ORF40 (2) primers. Southern blotting with a probe for ORF63 showed that ORF63 transcripts were present in ganglia from 13 of 18 VZV ROka-infected and 12 of 18 ROka2DA-infected animals (Fig. 4 and data not shown). In contrast, ORF40 transcripts were present in ganglia from none of 18 VZV ROka-infected and 1 of 18 ROka2DA-infected cotton rats (data not shown). ORF63 RNA has been detected in ganglia from rats inoculated with VZV and humans (8, 18, 27), while ORF40 transcripts have rarely been associated with latent infection (7, 18, 19). Thus, the pattern of latent infection in cotton rats is similar to that reported previously.
FIG. 4.
ORF63 transcripts are detected in ganglia from latently infected animals. RNA was prepared from animals inoculated with uninfected cells or cells containing VZV ROka, ROka2D, or heat-inactivated ROka (HI-ROka). RNA was also isolated from VZV-infected melanoma cells. cDNA was prepared from the RNA, and PCR was performed using ORF63 primers followed by Southern blotting with an ORF63 probe. ORF63 RNA was detected in VZV-infected melanoma cells and in ganglia from animals 1, 2, 3, 4, 5, 6, 8, and 10 infected with VZV ROka and in animals 1, 3, 6, 7, and 9 infected with ROka2D. Samples were incubated in the presence (+) or absence (−) of reverse transcriptase (RT) during preparation of cDNA.
ORF2 is the first VZV gene shown to be dispensable for establishing a latent infection in an animal model. ORF2 may still play a role in latency such as in reactivation from latency. While reactivation of VZV is generally limited to a single dermatome in humans, reactivation of SVV in monkeys is rarely evident, but when detected, it presents as a widely disseminated rash (28). Thus, ORF2, which is the only VZV gene that is not conserved in SVV, may help to regulate reactivation of VZV from latency.
Acknowledgments
We thank Paula Annunziato for advice on animal infections, Paul Kinchington for advice on virion purification and providing antibody to ORF61 protein, Claire Hallahan for advice on statistical analysis, and Stephen Straus for reviewing the manuscript.
Hitoshi Sato is the recipient of a Japan Society for the Promotion of Science (JSPS) fellowship in biomedical and behavioral research at the National Institutes of Health.
REFERENCES
- 1.Annunziato, P., P. LaRussa, P. Lee, S. Steinberg, O. Lungu, A. A. Gershon, and S. Silverstein. 1998. Evidence of latent varicella-zoster virus in rat dorsal root ganglia. J. Infect. Dis. 178(Suppl. 1):S48-S51. [DOI] [PubMed] [Google Scholar]
- 2.Brunell, P. A., L. C. Ren, J. I. Cohen, and S. E. Straus. 1999. Viral gene expression in rat trigeminal ganglia following neonatal infection with varicella-zoster virus. J. Med. Virol. 58:286-290. [DOI] [PubMed] [Google Scholar]
- 3.Byrd, L. G., and G. A. Prince. 1997. Animal models of respiratory syncytial virus infection. Clin. Infect. Dis. 25:1363-1368. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, J. I., and K. E. Seidel. 1993. Generation of varicella-zoster virus (VZV) and viral mutants from cosmid DNAs: VZV thymidylate synthetase is not essential for replication in vitro. Proc. Natl. Acad. Sci. USA 90:7376-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, J. I., and K. E. Seidel. 1995. Varicella-zoster virus open reading frame 1 encodes a membrane protein that is dispensable for growth of VZV in vitro. Virology 206:835-842. [DOI] [PubMed] [Google Scholar]
- 6.Cohen, J. I., and S. E. Straus. 2001. Varicella-zoster virus and its replication, p. 2702-2730. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott, Williams, & Wilkins, Philadelphia, Pa.
- 7.Cohrs, R. J., M. Barbour, and D. H. Gilden. 1996. Varicella-zoster virus (VZV) transcription during latency in human ganglia: detection of transcripts mapping to genes 21, 29, 62, and 63 in a cDNA library enriched for VZV RNA. J. Virol. 70:2789-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohrs, R. J., J. Randall, J. Smith, D. H. Gilden, C. Dabrowski, H. van Der Keyl, and R. Tal-Singer. 2000. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J. Virol. 74:11464-11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox, E., S. Reddy, I. Iofin, and J. I. Cohen. 1998. Varicella-zoster virus ORF57, unlike its pseudorabies virus UL3.5 homolog, is dispensable for viral replication in cell culture. Virology 250:205-209. [DOI] [PubMed] [Google Scholar]
- 10.Davison, A. J., and J. E. Scott. 1986. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 67:1759-1816. [DOI] [PubMed] [Google Scholar]
- 11.Ferrin, L. J., and R. D. Camerini-Otero. 1991. Selective cleavage of human DNA: RecA-assisted restriction endonuclease (RARE) cleavage. Science 254:1494-1497. [DOI] [PubMed] [Google Scholar]
- 12.Fraefel, C., U. V. Wirth, B. Vogt, and M. Schwyzer. 1993. Immediate-early transcription over covalently joined genome ends of bovine herpesvirus 1: the circ gene. J. Virol. 67:1328-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray, W. L., B. Starnes, M. W. White, and R. Mahalingam. 2001. The DNA sequence of the simian varicella virus genome. Virology 284:123-130. [DOI] [PubMed] [Google Scholar]
- 14.Heineman, T. C., and J. I. Cohen. 1995. The varicella-zoster virus (VZV) open reading frame 47 (ORF47) protein kinase is dispensable for viral replication and is not required for phosphorylation of ORF63 protein, the VZV homolog of herpes simplex virus ICP22. J. Virol. 69:7367-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heineman, T. C., K. Seidel, and J. I. Cohen. 1996. The varicella-zoster virus ORF66 protein induces kinase activity and is dispensable for viral replication. J. Virol. 70:7312-7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izumiya, Y., H. K. Jang, M. Ono, and T. Mikami. 2001. A complete genomic DNA sequence of Marek's disease virus type 2, strain HPRS24. Curr. Top. Microbiol. Immunol. 255:191-221. [DOI] [PubMed] [Google Scholar]
- 17.Kemble, G. W., P. Annunziato, O. Lungu, R. E. Winter, T. A. Cha, S. J. Silverstein, and R. R. Spaete. 2000. Open reading frame S/L of varicella-zoster virus encodes a cytoplasmic protein expressed in infected cells. J. Virol. 74:11311-11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy, P. G., E. Grinfeld, and J. E. Bell. 2000. Varicella-zoster virus gene expression in latently infected and explanted human ganglia. J. Virol. 74:11893-11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy, P. G., E. Grinfeld, S. Bontems, and C. Sadzot-Delvaux. 2001. Varicella-zoster virus gene expression in latently infected rat dorsal root ganglia. Virology 289:218-223. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy, P. G., E. Grinfeld, and J. W. Gow. 1999. Latent varicella-zoster virus in human dorsal root ganglia. Virology 258:451-454. [DOI] [PubMed] [Google Scholar]
- 21.Kinchington, P. R., D. Bookey, and S. E. Turse. 1995. The transcriptional regulatory proteins encoded by varicella-zoster virus open reading frames (ORFs) 4 and 63, but not ORF 61, are associated with purified virus particles. J. Virol. 69:4274-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinchington, P. R., J. K. Hougland, A. M. Arvin, W. T. Ruyechan, and J. Hay. 1992. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J. Virol. 66:359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowry, P. W., C. Sabella, C. M. Koropchak, B. N. Watson, H. M. Thackray, G. M. Abbruzzi, and A. M. Arvin. 1993. Investigation of the pathogenesis of varicella-zoster virus infection in guinea pigs by using polymerase chain reaction. J. Infect. Dis. 167:78-83. [DOI] [PubMed] [Google Scholar]
- 24.Meier, J. L., R. P. Holman, K. D. Croen, J. E. Smialek, and S. E. Straus. 1993. Varicella-zoster virus transcription in human trigeminal ganglia. Virology 193:193-200. [DOI] [PubMed] [Google Scholar]
- 25.Moriuchi, H., M. Moriuchi, R. Pichyangkura, S. J. Triezenberg, S. E. Straus, and J. I. Cohen. 1995. Hydrophobic cluster analysis predicts an amino-terminal domain of varicella-zoster virus open reading frame 10 required for transcriptional activation. Proc. Natl. Acad. Sci. USA 92:9333-9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy, S. M., E. Cox, I. Iofin, W. Soong, and J. I. Cohen. 1998. Varicella-zoster virus (VZV) ORF32 encodes a phosphoprotein that is posttranslationally modified by the VZV ORF47 protein kinase. J. Virol. 72:8083-8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadzot-Delvaux, C., S. Debrus, A. Nikkels, J. Piette, and B. Rentier. 1995. Varicella-zoster virus latency in the adult rat is a useful model for human latent infection. Neurology 45:S18-S20. [DOI] [PubMed] [Google Scholar]
- 28.Soike, K. F., S. R. Rangan, and P. J. Gerone. 1984. Viral disease models in primates. Adv. Vet. Sci. Comp. Med. 28:151-199. [DOI] [PubMed] [Google Scholar]
- 29.Telford, E. A., M. S. Watson, K. McBride, and A. J. Davison. 1992. The DNA sequence of equine herpesvirus-1. Virology 189:304-316. [DOI] [PubMed] [Google Scholar]
- 30.Telford, E. A., M. S. Watson, J. Perry, A. A. Cullinane, and A. J. Davison. 1998. The DNA sequence of equine herpesvirus-4. J. Gen. Virol. 79:1197-1203. [DOI] [PubMed] [Google Scholar]