Abstract
The association of the histone deacetylase (HDAC) inhibitor valproate (VPA) with atypical antipsychotics has become a frequent treatment strategy for schizophrenia and bipolar disorder. Because the VPA doses administered are elevated, one cannot assume that the benefits of the VPA plus antipsychotic treatment are exclusively related to the covalent modifications of nucleosomal histone tails. We compared the actions of N-(2-aminophenyl)-4-[N-(pyridin-3-yl-methoxycarbonyl)aminomethyl]benzamide derivative (MS-275), which is a potent HDAC inhibitor in vitro, with the actions of VPA for their ability to (i) increase the acetylated status of brain nucleosomal histone tail domains and (ii) to regulate brain histone-RELN and histone-GAD67 promoter interactions. MS-275 increases the content of acetylhistone 3 (Ac-H3) in the frontal cortex. Whereas this response peaks after a s.c. injection of 15 μmol/kg, the increase in Ac-H3 content in the hippocampus becomes significant only after an injection of 60 μmol/kg, suggesting that MS-275 is 30- to 100-fold more potent than VPA in increasing Ac-H3 in these brain regions. In contrast to VPA, MS-275, in doses up to 120 μmol/kg, fails to increase Ac-H3 content in the striatum. Chromatin immunoprecipitation shows that MS-275 increases Ac-H3-RELN and Ac-H3-GAD67 promoter interaction in the frontal cortex. These results suggest that MS-275 is a potent brain region-selective HDAC inhibitor. It is likely that, in addition to MS-275, other benzamide derivatives, such as sulpiride, are brain-region selective inhibitors of HDACs. Hence, some benzamide derivatives may express a greater efficacy than VPA as an adjunctive to antipsychotics in the treatment of epigentically induced psychiatric disorders.
Keywords: bipolar disorder, reelin, schizophrenia, histone code, chromatin remodeling
Aprefrontal cortex GABAergic neuron dysfunction, which is characterized by a reduction of the 67-kDa form of glutamic acid decarboxylase (GAD67) and reelin expression, is one of the most consistent neuropathological findings in postmortem brain studies of schizophrenia (SZ) and bipolar (BP) disorder (1-9). These expression deficits cannot be explained by reelin or GAD67 gene haploinsufficiency (10, 11). Converging epidemiological (12), histological, and biochemical (10, 13-17) evidence suggests that the pathogenesis of this dysfunction may be related to a disruption of epigenetic signaling, resulting in the selective hypermethylation of several GABAergic gene promoters that characterize SZ as a selective defect of gene transcription in GABAergic cortical neurons (13, 14). Such hypermethylation is very likely mediated by the overexpression of DNA methyltransferase 1 (DNMT1) (15, 18), which has been found to be operative in cortical and subcortical GABAergic interneurons of SZ and BP patients.
The long-term objective of this line of research is to identify drugs that selectively correct a basic defect of SZ, which is an epigenetic GABAergic neuron dysfunction, by directly or indirectly reducing the RELN and GAD67 promoter hypermethylation that is likely caused by a pathological increase of GABAergic DNMT1 expression (15, 18).
A logical strategy for the treatment of the GABAergic dysfunction expressed in SZ would be to normalize the SZ-related increase of DNMT1 GABAergic expression, reducing the hypermethylation of RELN and GAD67 promoters with the use of inhibitors of DNMT1 catalytic activity. However, the most potent DNMT1 inhibitors available today (5-azacytidine and zebularine) fail to readily cross the blood-brain barrier when administered systemically and are active only in the S phase of the cell cycle (19). Hence, a new approach to the treatment of SZ may result from a search for drugs that act on nondividing cells and display DNMT1-inhibitory activity in differentiated neurons.
In the nucleosome, histones package DNA and a posttranslational modification of these histones can regulate the access of DNMT1 or putative DNA-demethylases to DNA. For example, by hyperacetylating nucleosomal histone tails with histone deacetylase (HDAC) inhibitors, such as valproate (VPA) and trichostatin A (TSA), one could (i) prevent DNMT1 from accessing methylation sites expressed in promoter DNA segments (20) and/or (ii) induce the expression of DNA demethylase activity (21). Either of these activities could result in demethylation of the gene promoters that are hypermethylated by the increase of DNMT1 expression associated with SZ and BP disorders. These events could facilitate long-term changes in neuronal function (22, 23) and target acetylated nucleosomal histone tails to RELN and GAD67 promoters (24, 25).
Thus, a pharmacological strategy with great potential to normalize the reduced amount of reelin, GAD67, or other protein expression in cortical GABAergic neurons of SZ or BP patients is to use drugs that, by inhibiting HDACs, can reduce pathological RELN, GAD67, or other promoter hypermethylation. The efficacy of such a strategy is supported by the report that the weak HDAC inhibitor VPA can act as an adjunct to increase the efficacy of antipsychotics in the treatment of psychosis (26, 27). VPA, injected into mice in doses that hyperacetylate brain histone 3 (H3) tails, increases RELN, GAD67, and other promoter interactions with nucleosomal acetylated-H3 (Ac-H3) and up-regulates reelin and GAD67 expression (28).
Because VPA is an inhibitor of HDACs with millimolar affinity, it is possible that its actions on RELN, GAD67, or other gene transcriptions and its beneficial effects on the behavioral deficits observed in psychiatric disorders are mediated by mechanisms that are independent of the inhibition of nucleosomal histone core acetylation. Hence, as a proof of concept, experiments with more potent and specific HDAC inhibitors should help to clarify whether the molecular mechanisms underlying the beneficial action of VPA on the down-regulation of GAD67 and reelin expression in the frontal cortex GABAergic interneurons of SZ and BP patients is due to the blockade of selected HDAC molecular forms.
We report here on the identification of N-(2-aminophenyl)-4-[N-(pyridin-3-yl-methoxycarbonyl)aminomethyl]benzamide (MS-275) as a potent HDAC inhibitor, which, when administered to mice in low micromolar concentrations, increases Ac-H3 in a brain region-selective manner. MS-275 is a potent HDAC inhibitor with micromolar affinity for class I HDACs and with selectivity for HDAC1 over HDAC3 and HDAC8 (29-31). As a control drug to exclude pharmacological actions unrelated to the HDAC inhibitory activity of MS-275, we used equimolar doses of the 3′-aminophenylbenzamide isomer of MS-275 (3′-NH2-MS), which is inactive on HDACs (29). MS-275 is chemically unrelated to VPA and, unlike other potent HDAC inhibitors used in cancer therapy (i.e., suberoylanilide hydroxamic acid), has a long half-life (29), and, when injected s.c., can enter into the brain, where it is effective in increasing brain Ac-H3 levels in low μmol/kg doses. Thus, MS-275 appears to be an interesting prototypic drug to be used as a model in the synthesis of novel benzamide derivatives with potent and brain-region specific HDAC inhibitory activity. Such drugs will help develop a better understanding of the mechanisms operative in the beneficial actions of the VPA/antipsychotic association.
Results
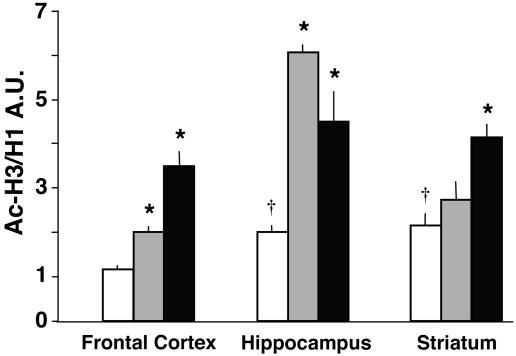
The MS-275-Induced Acetylation of H3 Tails Is Brain-Region Selective. MS-275, injected into mice in doses ranging from 1 to 120 μmol/kg s.c., increases Ac-H3 content in the frontal cortex (Fig. 1). In agreement with the long half-life of MS-275 established in pharmacokinetic studies (29, 32), the increase of Ac-H3 content (≈100%) elicited by 120 μmol/kg is maximal after 2 h and persists unabated up to 8 h after s.c. injection. The increase of Ac-H3 in the frontal cortex is maximal with a dose of 15 μmol/kg, whereas, in the hippocampus, a significant increase is seen only at 60 μmol/kg (Fig. 1). Importantly, in the striatum, MS-275 up to a dose of 120 μmol/kg fails to increase Ac-H3 content (Fig. 1).
Fig. 1.
MS-275 elicits an increase in Ac-H3 content in the frontal cortex and hippocampus but not in the striatum. Each value is the mean ±SE of three mice; *, P < 0.05 (one-way ANOVA followed by Bonferroni multiple comparison). The Ac-H3/H1 ratio is expressed in arbitrary immunoreactivity units (A.U.). Values are normalized to vehicle (VEH)-treated mice (see Materials and Methods). Mice were treated with MS-275 2 h before measurements.
Fig. 2 compares Ac-H3 levels in the frontal cortex, hippocampus, and striatum of mice treated with maximally effective doses of MS-275 (120 μmol/kg s.c.), or VPA (2 mmol/kg s.c.) (33). Whereas MS-275 is most effective in increasing Ac-H3 histones in the frontal cortex and hippocampus, it is virtually ineffective in the striatum. In contrast, VPA is almost equally effective in all three brain areas studied (Fig. 2). In view of the MS-275 brain-region selectivity in increasing Ac-H3, it is interesting to note that in vehicle (VEH)-treated mice, the content of Ac-H3 in the striatum and hippocampus is approximately twice as high as that in the frontal cortex (Fig. 2).
Fig. 2.
Comparison of the effects of MS-275 and VPA on Ac-H3 levels in different brain areas. Open bars, VEH; gray bars, MS-275, 120 μmol/kg; black bars, VPA, 2 mmol/kg. Animals were injected with drugs s.c. 2 h before measurements. *, P < 0.05 (ANOVA followed by Student-Newman-Keuls multiple comparison) when Ac-H3/H1 ratios of MS-275- and VPA-treated mice are compared with the corresponding values of VEH-treated mice. †, P < 0.05 when VEH Ac-H3/H1 ratios from different brain areas are compared with frontal cortex values.
When the increase in Ac-H3 frontal cortex content elicited by MS-275 is compared with that elicited by VPA, MS-275 appears to be 50- to 100-fold more potent than VPA. In the frontal cortex, the increase of Ac-H3 content expressed as a percent over the control value (VEH) is 20 ± 4.1 for VPA 0.5 mmol/kg, 102* ± 18 for VPA 1.0 mmol/kg, and 107* ± 15 for MS-275 15 μmol/kg (*, P < 0.01, when comparing VPA 1 mmol/kg or MS-275 15 μmol/kg vs. control or vs. VPA 0.5 mmol/kg treated groups; n = 3).
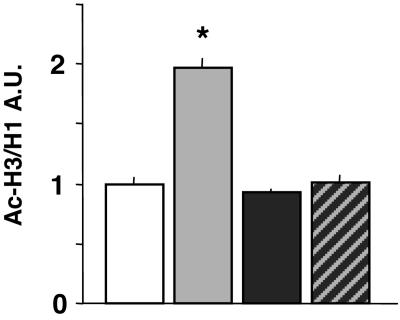
Fig. 3 shows that in the same mouse in which MS-275 (120 μmol/kg s.c.) elicits a clear increase of frontal cortex Ac-H3 content, the levels of frontal cortex Ac-H4, phospho-Ac-H3, and dimethyl-H3, measured with specific antibodies, remain virtually unchanged.
Fig. 3.
Covalently modified histones in the frontal cortex of MS-275- and 3′-NH2-MS-treated mice. Gray bars, MS-275, 120 μmol/kg; black bars, 3′-NH2-MS, 120 μmol/kg. Mice were injected s.c. 2 h before measurements. *, P < 0.05; **, P < 0.01 when compared with VEH (control)-treated mice. Data are expressed as the mean ± SE of five animals. P-Ac-H3, phosphorylated Ac-H3; Ac-H4, acetylated histone 4; Dimethyl-H3, dimethylhistone 3. For details on the antibodies see Materials and Methods.
The MS-275 isomer 3′-NH2-MS, which fails to inhibit HDACs in tumor cell lines in vitro (29), also fails to increase Ac-H3 or Ac-H4 content in the frontal cortex (Fig. 3). At a dose of 120 μmol/kg, 3′-NH2-MS prevents the increase of Ac-H3 elicited by an equimolar dose of MS-275 (Fig. 4). This finding suggests that the 2′-amino group of MS-275 plays an important and specific role in mediating the MS-275-induced inhibition of brain HDAC activity. Unexpectedly, 3′-NH2-MS given alone increases dimethyl-H3 content in the frontal cortex (Fig. 3). We did not investigate the mechanism operative in this increase.
Fig. 4.
3′-NH2-MS blocks the increase of Ac-H3 in the frontal cortex of mice treated with MS-275. Open bar, VEH; gray bar, MS-275; black bar, 3′-NH2-MS; hatched bar, 3′-NH2-MS + MS-275. 3′-NH2-MS (120 μmol/kg) was injected s.c. 30 min before MS-275 (120 μmol/kg) administration. Ac-H3 was measured 2 h after MS-275 injection. Each value is the mean ± SE of three mice. *, P < 0.05 when the MS-275-treated group was compared with the other groups (one-way ANOVA followed by Student-Newman-Keuls multiple comparison method).
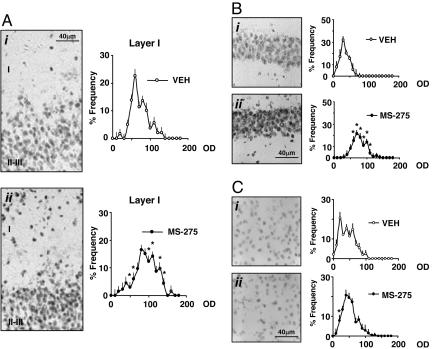
Immunohistochemical Detection of Ac-H3. Mice treated with MS-275 (15 μmol/kg) show an increase in the number of high-density Ac-H3 immunoreactive cells in layers I and II of the frontal cortex compared with mice injected with VEH (Fig. 5A). Confocal immunofluorescence images with Neu-N antibodies labeling neuronal nuclei and Ac-H3 antibodies labeling acetylated histones demonstrate that >95% of Ac-H3 immunoreactivity is colocalized with anti-Neu-N antibodies (data not shown).
Fig. 5.
MS-275 elicits an increase of neuronal Ac-H3 immunoreactivity in the piriform cortex and hippocampus but not in the striatum. (A Left) Typical Ac-H3 immunostaining in piriform cortex neurons (layers I and II-III) of mice treated with VEH (i) or MS-275 (15 μmol/kg) (ii). (Right) Densitometric distribution profiles of Ac-H3-immunopositive neurons in layer I: VEH-(open circles) and MS-275-treated mice (solid circles). (B Left) Typical immunostaining in CA1 hippocampal neurons of mice injected with VEH (i) or MS-275 (120 μmol/kg) (ii). (Right) Densitometric distribution profiles of Ac-H3-immunopositive neurons: VEH-(open circles) and MS-275-(solid circles) treated mice. (C Left) Typical Ac-H3 immunostaining in the striatal neurons of mice injected with VEH (i) or MS-275 (120 μmol/kg) (ii). (Right) Densitometric distribution profiles of Ac-H3-immunopositive neurons: VEH-(open circles) and MS-275-(solid circles) treated mice. OD, optical density of Ac-H3 immunostaining in each cell with a 256 gray scale used as a reference. For the densitometric distribution profiles, each point is the mean ± SE of three mice. Mice were injected s.c. 2 h before perfusion. * denotes statistically significant differences between VEH- and MS-275-treated mice (P < 0.05) when data were analyzed by two-way repeated measures ANOVA followed by Student-Newman-Keuls multiple comparison.
In the hippocampus of the same animals, we also observed an increase of Ac-H3 immunopositive neurons in CA1 (Fig. 5B) and in the dentate gyrus (data not shown), but this increase becomes consistent only after doses of MS-275 of 60 μmol/kg or higher (Fig. 5B). In the striatum, MS-275 failed to change the expression level of Ac-H3 in neuronal cells even at the highest dose of 120 μmol/kg of MS-275 (Fig. 5C).
Optical density (OD) analyses of Ac-H3-positive neurons support the qualitative observations reported in Figs. 5 A-C. In layers I and II of the frontal cortex and in the hippocampus of MS-275-treated mice, there is a significant shift to the right in the population of neurons with relatively higher OD compared with neurons of VEH-treated mice. In contrast, in the striatum, the relative intensity of Ac-H3-positive neurons was not shifted to the right after MS-275 treatment.
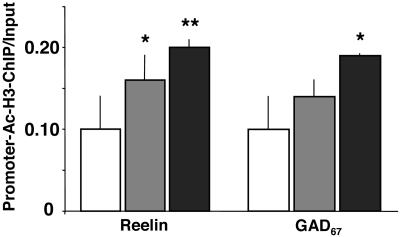
MS-275 Enhances the Expression of Frontal Cortex RELN and GAD67 Promoters That Are Immunoprecipitated with Ac-H3 Antibodies. The chromatin immunoprecipitation technique was used to study whether the increase of nucleosomal histone tail acetylation elicited by MS-275 treatment up-regulates the recruitment of the underlying RELN and GAD67 gene promoters. Fig. 6 shows that, in the frontal cortex, the ratio between reelin promoter immunoprecipitated with Ac-H3 antibodies and the amount of RELN promoter expressed in the initial (nonimmunoprecipitated) extract is significantly increased in a dose-related fashion in mice receiving MS-275. Similarly, the ratio between GAD67 promoter immunoprecipitated with Ac-H3 antibodies and the amount of GAD67 promoter in the initial nonimmunoprecipitated extracts is increased in MS-275-treated mice.
Fig. 6.
MS-275 dose dependently enhances the amount of RELN and GAD67 promoters immunoprecipitated with anti-Ac-H3 antibody. Open bars, VEH; gray bars, MS-275, 15 μmol/kg; black bars, MS-275, 30 μmol/kg. Depicted are the ratios between the amount of RELN (Left) and GAD67 (Right) promoters immunoprecipitated with Ac-H3 antibody and the amount of RELN and GAD67 promoter fragments in the initial nonimmunoprecipitated extract (input). Mice were injected s.c. with VEH or MS-275 (15 or 30 μmol/kg) 2 h before chromatin immunoprecipitated (ChIP) measurements. *, P < 0.05, Student's t test.
Discussion
In the present study, we demonstrate that the 2′-aminophenylbenzamide derivative MS-275, a potent inhibitor of HDACs in vitro (29) in low μmol/kg doses increases Ac-H3 expression in the mouse brain. Furthermore, we demonstrated that the increase in Ac-H3 is found in the histones associated with RELN and GAD67 gene promoters. The effect of MS-275 on brain HDACs appears specific because 3′-NH2-MS, an isomeric analogue of MS-275, is devoid of HDAC inhibitory action.
MS-275 is ≈100-fold more potent than VPA in increasing Ac-H3 expression in the frontal cortex, and its action is longer lasting than that of VPA. For example, 8 h after a single s.c. injection of MS-275, the levels of Ac-H3 in the frontal cortex are still as high as at 2 h after the injection, whereas the level of Ac-H3 in the frontal cortex of VPA-treated mice reaches a peak at 2 h but then declines toward control levels at 8 h (33). This long-lasting action of MS-275 on Ac-H3 agrees with pharmacodynamic studies in animals and humans (29, 32) and suggests that this compound may have a slow rate of disappearance from the brain.
The most important feature that differentiates MS-275 from VPA is that the increased expression of Ac-H3 elicited by MS-275 is brain-region selective. As shown in Figs. 1, 2, 3, 4 and 5, the dose of MS-275 that maximally increases Ac-H3 in the frontal cortex is 15 μmol/kg, whereas to act in the hippocampus, MS-275 requires a dose of 60 μmol/kg or higher. In the striatum, a MS-275 dose of 120 μmol/kg, which is ≈10-fold higher than the maximally effective dose in the cortex, fails to change the striatal Ac-H3 content.
Moreover, MS-275 appears to be specific for Ac-H3 because Ac-H4 and other covalent histone modifications are not affected by this compound (Fig. 3). Although chromatin immunoprecipitation studies reported in Fig. 6 indicate that nucleosomal H3 acetylation is a signal necessary to regulate the interaction of RELN and GAD67 promoters with nucleosomal histones, the synergistic or antagonistic role played by nucleosomal amino-terminal covalent modification of the H2 histones has not yet been studied and cannot be excluded at this time.
To interpret the data on MS-275 specificity for Ac-H3 and its brain region selectivity, we must consider that the steady-state level of histone acetylation in the brain is dynamically maintained by the opposing actions of histone acetyltransferases (HATs) and HDACs (34). There are two main classes of HAT: type A is nuclear, and type B is cytosolic. Three families of type A (GNAT, MYST, and p300/CPB) have been studied in vitro. They all acetylate histones, but their specificity is further defined by the formation of complexes with other enzymes acting on chromatin remodeling (35). So far, 17 genes that encode different forms of HDAC have been identified; they fall into three main classes (HDAC I, II, and III) (36, 37). However, the histone tail substrate specificity for such enzymes is just beginning to be characterized (38). Class I HDACs (HDAC 1, 2, 3, and 8) are located in the nucleus and their catalytic activities have been characterized as the key constituents of transcriptional repressor complexes (Sin3-HDAC and NuRD-Mi2-NDR) (37, 39). In contrast, class II HDACs can shuttle between the cytoplasm and the nucleus. The activity of class I and II HDACs and their participation in the formation of multiprotein complexes is also regulated by posttranslational modifications (i.e., phosphorylation). Class III HDACs form a distinct class of NAD-dependent enzymes that are similar to yeast SIR2 proteins (37).
Although the distribution of the majority of the HATs and HDACs in different tissues has recently been described (37), information regarding their distribution and abundance in various neuronal subtypes expressed in different brain regions is lacking. The observation reported here, that Ac-H3 is constitutively higher in the striatum than in the frontal cortex and hippocampus, suggests that histone acetylation mechanisms may be brain-area selective and that future studies should be aimed at defining the brain region and cell distribution of HATs and HDACs.
Given the complexity of histone acetylation mechanisms, the lack of precise information on (i) the contributions of different forms of HATs and HDACs to the acetylation steady state of brain histones, and (ii) the lack of information on the distribution of MS-275 in different brain regions, it is difficult to correctly interpret the mechanisms whereby MS-275 elicits an increase of Ac-H3 expressed in the frontal cortex and hippocampus but not in the striatum. In support of the possibility that MS-275 may selectively enhance cortical levels of Ac-H3 by blocking specific HDACs, we must consider that (i) among class I HDACs, HDAC1 is the most sensitive to MS-275, whereas HDAC3 and -8 have significantly lower sensitivity (30, 31), and (ii) 3′-NH2-MS, which is a structurally isomeric analogue of MS-275 but lacks HDAC inhibitor activity, also fails to increase acetylation of H3 in the same brain areas where MS-275 is active. Thus, the development of new HDAC inhibitors targeted to specific class I HDACs, together with information on the brain region distribution of HDAC subtypes, may make it possible to tailor the use of HDAC inhibitors in neurobiology.
Conclusions
Central to our thinking is that, in SZ and BP patients, epigenetic modifications of chromatin remodeling complexes play an important regulatory role in the down-regulation of specific genes (RELN and GAD67) in telencephalic GABAergic neurons. It is now clear that posttranslational nucleosomal histone amino-terminal covalent modifications, which act as docking sites for chromatin remodeling complexes, contribute to the changes in the epigenetic status of individual genes.
The large array of posttranslational modifications that decorate histone tails is the basis of the “Histone Code” operative as a differential regulatory mechanism of nucleosome histone/DNA chromatin interactions (40).
In our study, we have shown that covalent hyperacetylation of lysine residues in H3 tails, which has been induced in the mouse cortex by MS-275, is a putative histone code signal allowing access of the underlying RELN and GAD67 promoters to nucleosome histone tails (Fig. 6), thereby modulating the rates of their translation processes and increasing their expression: reelin/β-actin OD ratio = 0.45 ± 0.03 in frontal cortex of VEH-treated mice and 0.64 ± 0.05 in MS-275-treated mice (30 μmol/kg s.c., once a day for 7 days); GAD67/GAD65 OD ratio = 0.71 ± 0.02 in VEH-treated mice and 0.81 ± 0.06 in MS-275-treated mice; n = 3.
MS-275 has been used in clinical phase I trials in patients with a variety of solid tumors or lymphoma (32); a dose of 2 mg/m2 increases H3 acetylation in peripheral blood mononuclear cells. At this dose, MS-275 is reasonably well tolerated without producing any measurable behavioral side effect after 3 months of treatment.
Relevant for a rational approach to the treatment of SZ morbidity is to determine whether the telencephalic hypermethylation of RELN and GAD67 promoters, which has been elicited by methionine in an epigenetic mouse model of SZ (28, 41), may be impaired by MS-275 treatment, either alone or in combination with typical or atypical antipsychotics. These considerations also suggest an investigation of whether antipsychotics may influence neuronal gene expression by inducing posttranslational histone tail modifications. In preliminary studies, we have observed that sulpiride and amisulpride (two benzamide derivatives with antipsychotic properties) increase brain Ac-H3 content and also enhance the binding of RELN and GAD67 promoters to nucleosomal hyperacetylated histones when administered to mice in doses of 30-60 μmol/kg. Hence, the use of corticoselective HDAC inhibitors may allow the identification of specific histone code modifications in cortical GABAergic neurons that attract particular types of chromatin remodeling complexes. These research trends may also open new avenues for a pharmacological intervention targeted to influence telencephalic epigenetic chromatin remodeling disorders in SZ and BP patients.
Materials and Methods
Animals and Drug Treatments. Adult male Swiss albino mice (Harlan Breeders, Indianapolis), 20-22 g of body weight, were used for all experiments. Animals were injected s.c. with VEH (10 ml/kg) or MS-275 (1-120 μmol/kg) (dissolved with a drop of glacial acetic acid brought to pH 6 with the addition of NaOH), 3′-NH2-MS (120 μmol/kg), and VPA (0.5-2 mmol/kg). All animal procedures were approved by the University of Illinois at Chicago Animal Care Committee.
Western Blotting. All of the extraction procedures were carried out at 4°C in the presence of a mixture of protease inhibitors (Sigma). Samples were homogenized in 0.25 M sucrose/3.3 mM calcium acetate/1 mM PMSF and washed twice with PBS and 1 mM PMSF. Nuclear histones were extracted in 0.2 M H2SO4/1 mM PMSF. The extracted histones were precipitated with trichloroacetic acid (final concentration 33%), washed in 100% acetone, 0.05 M HCl, and 100% acetone, and then resuspended in H2O. To ensure similar protein loading, the total protein concentration of these extracts was determined by using the Bradford method with BSA as the standard. Samples were eluted in Laemmli buffer, denatured at 100°C, run on 10-20% Tris-glycine gradient gel, and blotted on nitrocellulose membranes (Invitrogen). Membrane preparations were incubated overnight at 4°C with anti-histone antibodies diluted in 3% dry milk/1.5% Tween 20 in PBS, washed, and further processed with horse-radish peroxidase-conjugated secondary antibodies (Amersham Pharmacia Biosciences). Immunocomplexes were revealed by enhanced chemiluminescence with an ECL Plus Chemiluminescence Western Blotting kit (Amersham Pharmacia Biosciences) by using the Blue Fluorescence/Chemiluminescence Storm System (Molecular Dynamics) and analyzed with imagequant 5.0 software. To profile the pattern of histone tail modifications, the following antibodies were used: polyclonal anti-dimethyl-Lys-9-histone H3 (dimethyl-H3) (1:500); polyclonal anti-acetyl-Lys-9,14-histone H3 (Ac-H3) (1:3,000); polyclonal anti-phospho-Ser-10-acetyl-Lys-14-histone H3, P-Ac-H3 (1:1,500); polyclonal anti-acetyl-Lys-5,8,12,16-histone H4 (Ac-H4) (1:1,000); and monoclonal (clone AE-4) anti-histone H1 (H1) (1:1,500) (Up-state, Charlottesville, VA). The antibodies were highly specific; in fact, after immunoblotting, only one major reactive band was recognized, and its molecular weight corresponded to that of the expected modified histone. It has also been reported that preadsorbing some of the Abs with the corresponding synthetic peptides resulted in a complete loss of signal (42). In addition, the intensity of histone H1 immunofluorescence was determined on the same blots with an anti-H1 antibody (clone AE-4, Upstate) and used to monitor the amount of histones applied to the gels. In all blots, the OD of the Ac-H3, Ac-H4, dimethyl-H3, and P-Ac-H3 bands was quantified and normalized relative to the OD of the correspondent H1 band. To determine the increase in Ac-H3 after treatment with HDAC inhibitors, the normalized Ac-H3 values for VEH-treated samples were adjusted to 1. Each sample was run in triplicate by using serial dilutions to obtain images in the linear exposure range (33). Samples from VEH- and drug-treated animals were analyzed in the same immunoblot, and values were calculated relative to the VEH-treated mice.
Immunohistochemistry. Ac-H3 neuronal staining. Tissues were fixed with 4% paraformaldehyde in PBS. Floating sections (30 μm) were immunostained for Ac-H3 as follows: Tissue slides were rinsed with PBS, then blocked for 30 min with 3% normal goat serum (NGS) in PBS. Sections were incubated overnight at 4°C with anti-Ac-H3 antibody (Upstate) diluted (1:8,000) in PBS containing 1% NGS. Afterward, sections were washed in PBS and incubated with a biotinylated-secondary antibody for 1 h at room temperature. Slices were then reacted with diaminobenzidine-nickel ammonium sulfate.
OD frequency analysis of Ac-H3-positive neurons. Immunolabeled sections were visualized by using a ×40 objective lens. All analyses were carried out in comparable areas under the same optical and light conditions. Black and white images were digitized and viewed on a computer using axio vision software (Zeiss). Staining intensity inside each cell body perimeter was measured by using scion image software (Scion, Frederick, MD); the OD measurement for each cell is the mean gray of the pixels inside the perimeter of the cell body, with a 256 gray scale as a reference. The OD background of each section was subtracted from the OD measurement of each cell. To graphically represent the cell immunostaining intensity distribution in each section, we divided the OD values included between 0 and 256 gray scale in decimal unit categories. Then, for each brain region studied, we plotted these OD categories against the percentage of cells (frequency) that fell into a specific category.
Chromatin Immunoprecipitation (ChIP). About 10 mg of frontal cortex tissue was used for the assay. Formaldehyde was added to homogenized tissues to a final concentration of 1% in PBS (containing 1 mM PMSF, 1 μg/ml aprotinin, and 1 μg/ml pepstatin) at 37°C for 15 min to crosslink histones to DNA. After washing with cold PBS, pellets were resuspended in lysis buffer (ChIP Assay Kit, Upstate Cell Signaling Solutions, Charlottesville, VA) and sonicated by Bioruptor UCD-200 (Diagenode, New York) with 10 bursts of 30 sec each. This sonication setting allowed us to obtain 250-500-bp genomic DNA fragments (28). An aliquot of the sonicated lysate was used as the reference for the total amount of DNA (input) in each sample. To quantify the Ac-H3/DNA crosslinked chromatin complex, chromatin fragments were incubated overnight with an Ac-H3-specific antibody (Upstate). The immunoprecipitated DNA was recovered with phenol/chloroform extraction and ethanol precipitation. RELN and GAD67 promoters were quantified by competitive PCR amplification using a homologous DNA internal standard as described in detail by Dong et al. (28).
Statistical Analysis. Results are expressed as the mean ± SE. Student's t test, one-way ANOVA followed by the Student-Newman-Keuls multiple comparison test, or two-way repeated-measures ANOVA followed by multiple comparison was used. The criterion for significance was P < 0.05.
Acknowledgments
We thank Dr. S. Akbarian (Department of Psychiatry, University of Massachusetts Medical School, Worchester), Dr. N. H. Neff (Departments of Pharmacology and Psychiatry, Ohio State University College of Medicine, Columbus), and Dr. D. R. Weinberger (Genes, Cognition, and Psychosis Program, National Institute of Mental Health, Bethesda) for constructive criticisms and suggestions in the preparation of the manuscript. This work was supported in part by National Institute of Mental Health Grants MH 071667 (to E.C.) and MH 070855 (to A.G.) and by Merck Grant 03-4-699 (to E.C.).
Author contributions: M.V.S., L.M.C., E.C., and A.G. designed research; M.V.S., E.D., E.M., and M.V. performed research; M.L.C. contributed new reagents/analytic tools; M.V.S., E.D., E.M., M.V., E.C., and A.G. analyzed data; and M.V.S., E.C., and A.G. wrote the paper.
Conflict of interest statement: L.M.C. is a stockholder in Merck Sharp & Dohme.
Abbreviations: Ac-H3, acetylated histone 3; BP, bipolar; DNMT1, DNA methyltransferase 1; GAD67, 67-kDa glutamic acid decarboxylase; H3, histone 3; HAT, histone acetyltransferase; HDAC, histone deacetylases; MS-275, N-(2-aminophenyl)-4-[N-(pyridin-3-yl-methoxycarbonyl)aminomethyl]benzamide; 3′-NH2-MS, 3′-aminophenylbenzamide isomer of MS-275; VEH, vehicle; VPA, valproate; SZ, schizophrenia.
References
- 1.Akbarian, S., Kim, J. J., Potkin, S. G., Hagman, J. O., Tafazzoli, A., Bunney, W. E., Jr., & Jones, E. G. (1995) Arch. Gen. Psychiatry 52, 258-266. [DOI] [PubMed] [Google Scholar]
- 2.Benes, F. M. & Berretta, S. (2001) Neuropsychopharmacology 25, 1-27. [DOI] [PubMed] [Google Scholar]
- 3.Eastwood, S. L. & Harrison, P. J. (2003) Mol. Psychiatry 8, 821-831. [DOI] [PubMed] [Google Scholar]
- 4.Fatemi, S. H., Earle, J. A. & McMenomy, T. (2000) Mol. Psychiatry 5, 654-663. [DOI] [PubMed] [Google Scholar]
- 5.Guidotti, A., Auta, J., Davis, J. M., DiGiorgi-Gerevini, V., Dwivedi, Y., Grayson, D. R., Impagnatiello, F., Pandey, G., Pesold, C., Sharma, R., et al. (2000) Arch. Gen. Psychiatry 57, 1061-1069. [DOI] [PubMed] [Google Scholar]
- 6.Impagnatiello, F., Guidotti, A., Pesold, C., Dwivedi, Y., Caruncho, H., Pisu, M., Uzunov, D., Smalheiser, N., Davis, J., Pandey, G., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 15718-15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knable, M. B., Barci, B. M., Webster, M. J., Meador-Woodruff, J. & Torrey, E. F. (2004) Mol. Psychiatry 9, 609-620. [DOI] [PubMed] [Google Scholar]
- 8.Volk, D. W., Austin, M. C., Pierri, J. N., Sampson, A. R. & Lewis, D. A. (2000) Arch. Gen. Psychiatry 57, 237-245. [DOI] [PubMed] [Google Scholar]
- 9.Woo, T. U., Walsh, J. P. & Benes, F. M. (2004) Arch. Gen. Psychiatry 61, 649-657. [DOI] [PubMed] [Google Scholar]
- 10.Costa, E., Chen, Y., Davis, J., Dong, E., Noh, J. S., Tremolizzo, L., Veldic, M., Grayson, D. R. & Guidotti, A. (2002) Mol. Interv. 2, 47-57. [DOI] [PubMed] [Google Scholar]
- 11.Goldberger, C., Gourion, D., Leroy, S., Schurhoff, F., Bourdel, M. C., Leboyer, M. & Krebs, M. O. (2005) Am. J. Med. Genet. B Neuropsychiatr. Genet. 137, 51-55. [DOI] [PubMed] [Google Scholar]
- 12.Petronis, A. (2004) Biol. Psychiatry 55, 965-970. [DOI] [PubMed] [Google Scholar]
- 13.Abdolmaleky, H. M., Cheng, K. H., Russo, A., Smith, C. L., Faraone, S. V., Wilcox, M., Shafa, R., Glatt, S. J., Nguyen, G., Ponte, J. F., et al. (2005) Am. J. Med. Genet. B Neuropsychiatr. Genet. 134, 60-66. [DOI] [PubMed] [Google Scholar]
- 14.Grayson, D. R., Jia, X., Chen, Y., Sharma, R., Mitchell, C., Guidotti, A. & Costa, E. (2005) Proc. Natl. Acad. Sci. USA 102, 9341-9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veldic, M., Guidotti, A., Maloku, E., Davis, J. M. & Costa, E. (2005) Proc. Natl. Acad. Sci. USA 102, 2152-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guidotti, A., Auta, J., Davis, J. M., Dong, E., Grayson, D. R., Veldic, M., Zhang, X. & Costa, E. (2005) Psychopharmacology 180, 191-205. [DOI] [PubMed] [Google Scholar]
- 17.Lewis, D. A., Hashimoto, T. & Volk, D. W. (2005) Nat. Rev. Neurosci. 6, 312-324. [DOI] [PubMed] [Google Scholar]
- 18.Veldic, M., Caruncho, H. M., Liu, W. S., Davis, J., Satta, R., Grayson, D. R., Guidotti, A. & Costa, E. (2004) Proc. Natl. Acad. Sci. USA 101, 348-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo, C. B., Cheng, J. C. & Jones, P. A. (2004) Biochem. Soc. Trans. 32, 910-912. [DOI] [PubMed] [Google Scholar]
- 20.Robertson, A. K., Geiman, T. M., Sankpal, U. T., Hager, G. L. & Robertson K. D. (2004) Biochem. Biophys. Res. Commun. 322, 110-118. [DOI] [PubMed] [Google Scholar]
- 21.Detich, N., Bovenzi, V. & Szyf, M. (2003) J. Biol. Chem. 278, 27586-27592. [DOI] [PubMed] [Google Scholar]
- 22.Levenson, J. M., O'Riordan, K. J., Brown, K. D., Trinh, M. A., Molfese, D. L. & Sweatt, J. D. (2004) J. Biol. Chem. 279, 40545-40559. [DOI] [PubMed] [Google Scholar]
- 23.Levenson, J. M. & Sweatt, J. D. (2005) Nat. Rev. Neurosci. 6, 108-118. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell, C. P., Chen, Y., Kundakovich, M., Costa, E. & Grayson, D. R. (2005) J. Neurochem. 93, 483-492. [DOI] [PubMed] [Google Scholar]
- 25.Noh, J. S., Sharma, R. P., Veldic, M., Salvacion, A. A., Jia, X., Chen, Y., Costa, E., Guidotti, A. & Grayson, D. R. (2005) Proc. Natl. Acad. Sci. USA 102, 1749-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casey, D. E., Daniel, D. G., Wassef, A. A., Tracy, K. A., Wozniak, P. & Sommerville, K. W. (2003) Neuropsychopharmacology 28, 182-192. [DOI] [PubMed] [Google Scholar]
- 27.Wassef, A., Baker, J. & Kochan, L. D. (2003) J. Clin. Psychopharmacol. 23, 601-640. [DOI] [PubMed] [Google Scholar]
- 28.Dong, E., Agis-Balboa, R. C., Simonini, M. V., Grayson, D. R., Costa, E. & Guidotti, A. (2005) Proc. Natl. Acad. Sci. USA 102, 12578-12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito, A., Yamashita, T., Mariko, Y., Nosaka, Y., Tsuchiya, K., Ando, T., Suzuki, T., Tsuruo, T. & Nakanishi, O. (1999) Proc. Natl. Acad. Sci. USA 96, 4592-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu, E., Dul, E., Sung, C. M., Chen, Z., Kirkpatrick, R., Zhang, G. F., Johanson, K., Liu, R., Lago, A., Hoffman, G., et al. (2003) J. Pharmacol. Exp. Ther. 307, 720-728. [DOI] [PubMed] [Google Scholar]
- 31.Vannini, A., Volpari, C., Filocamo, G., Caroli Casavola, E., Brunetti, M., Renzoni, D., Chakravarty, P., Paolini, C., De Francesco, R., Galliani, P., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 15064-15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan, Q. C., Headlee, D., Acharya, M., Sparreboom, A., Trepel, J. B., Ye, J., Figg, W. D., Hwang, K., Chung, E. J., Murgo, A. et al. (2005) J. Clin. Oncol. 23, 3912-3922. [DOI] [PubMed] [Google Scholar]
- 33.Tremolizzo, L., Carboni, G., Ruzicka, W. B., Mitchell, C. P., Sugaya, I., Tueting, P., Sharma, R., Grayson, D. R., Costa, E. & Guidotti, A. (2002) Proc. Natl. Acad. Sci. USA 99, 17095-17100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waterborg, J. H. (2002) Biochem. Cell Biol. 80, 363-378. [DOI] [PubMed] [Google Scholar]
- 35.Marmorstein, R. & Roth, S. Y. (2001) Curr. Opin. Genet. Dev. 11, 155-161. [DOI] [PubMed] [Google Scholar]
- 36.de Ruijter, A. J., van Gennip, A. H., Caron, H. N., Kemp, S. & van Kuilenburg, A. B. (2003) Biochem. J. 15, 737-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khochbin, S. & Kao, H. Y. (2001) FEBS Lett. 494, 141-144. [DOI] [PubMed] [Google Scholar]
- 38.Johnstone, R. W. (2002) Nat. Rev. Drug Discov. 1, 287-299. [DOI] [PubMed] [Google Scholar]
- 39.Kornberg, R. D. & Lorch, L. (1999) Curr. Opin. Genet. Dev. 9, 148-151. [DOI] [PubMed] [Google Scholar]
- 40.Jenuwein, T. & Allis, C. D. (2001) Science 293, 1074-1080. [DOI] [PubMed] [Google Scholar]
- 41.Tremolizzo, L., Doueiri, M. S., Dong, E., Grayson, D. R., Davis, J. M., Pinna, G., Tueting, P., Rodriguez-Menendez, V., Costa, E. & Guidotti, A. (2005) Biol. Psychiatry 57, 500-509. [DOI] [PubMed] [Google Scholar]
- 42.Li, J., Guo, Y., Schroeder, F. A., Youngs, R. M., Schmidt, T. W., Ferris, C., Konradi, C. & Akbarian, S. (2004) J. Neurochem. 90, 1117-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]