Abstract
Estrogen is known to influence glucose homeostasis with dominant effects in the liver, but the role of estrogen receptors in muscle glucose metabolism is unknown. In the present study, we investigated the expression of the two estrogen receptors, ERα and ERβ, and their influence on regulation of the glucose transporter, GLUT4, and its associated structural protein, caveolin-1, in mouse gastrocnemius muscle. Immunohistochemical analysis revealed that ERα and ERβ are coexpressed in the nuclei of most muscle cells, and that their levels were not affected by absence of estradiol [in aromatase-knockout (ArKO) mice]. GLUT4 expression on the muscle cell membrane was not affected by loss of ERβ but was extremely reduced in ERα-/- mice and elevated in ArKO mice. RT-PCR confirmed a parallel reduction in GLUT4 mRNA levels in ERα-/- mice. Upon treatment of ArKO mice with the ERβ agonist 2,3-bis(4-hydroxyphenyl)propionitrile, GLUT4 expression was reduced. By immunofluorescence and Western blotting, caveolin-1 expression was higher in ArKO mice and lower in ERβ-/- and ERα-/- mice than in WT littermates. GLUT4 and caveolin-1 were colocalized in WT and ArKO mice but not in ERβ-/- and ERα-/- mice. These results reveal that ERα is a positive regulator of GLUT4 expression, whereas ERβ has a suppressive role. Both ERβ and ERα are necessary for optimal caveolin-1 expression. Taken together, these results indicate that colocalization of caveolin-1 and GLUT4 is not an absolute requirement for muscle glucose metabolism but that reduction in GLUT4 could be contributing to the insulin resistance observed in ERα-/- mice.
Keywords: caveolin-1, diabetes, aromatase
Estradiol (E2) is much more than a female reproductive hormone. It acts almost ubiquitously in the human body and is involved in physiological and pathological states in both males and females. E2 is known to modulate insulin sensitivity and, consequently, glucose homeostasis, but the mechanisms underlying this action are not clearly understood. Fluctuations in glucose homeostasis are observed during the menstrual cycle, and diabetes becomes more resistant to treatment during the luteal phase (1). When cycles are irregular or too long, the incidence of diabetes increases (2), and during the last trimester of gestation, when hormonal changes are pronounced, insulin action is decreased (3). Insulin gene expression is also influenced by E2 levels. At proestrus, when estrogen levels peak, insulin mRNA expression is decreased in the pancreas (4), and in the luteal phase of the cycle, the expression of some glucose transporters (GLUTs) in the uterus are also affected (5).
In diseases such as gestational diabetes mellitus and polycystic ovarian syndrome, which are characterized by disturbances in female gonadal hormones, there is insulin resistance accompanied by deranged carbohydrate metabolism and compromised glucose homeostasis. In both diseases, defects in glucose transporter expression have been observed (6, 7). Taken together, these changes clearly demonstrate that estrogen plays an important role in glucose homeostasis.
Insulin resistance is associated with decreased glucose uptake in insulin-sensitive tissues, i.e., skeletal muscle and white and brown adipose tissues (8). In these tissues, glucose uptake is maintained by one of the isoforms of the glucose transporter family, GLUT4 (9). When insulin interacts with its receptor on the cell membrane, an intracellular signaling cascade is activated, causing the phosphorylation of several substrates, including insulin receptor substrate, phosphatidylinositol 3-kinase, and protein kinase B (10). The final result of these events is the translocation of vesicles containing GLUT4 to the cell membrane, allowing glucose influx into the cell (11). The rate of glucose transport into muscle cells is limited by the concentration of GLUT4 at the cell surface
More than 50 years ago, plasma membrane invaginations called caveolae were first described, and since then many functions have been attributed to these structures. Caveolae play important physiological roles in cell metabolism through their involvement in vesicular transport, signal transduction, and protein anchorage (12). GLUT4 vesicles mainly anchor to caveolae in the plasma membrane of adipocytes, suggesting that insulin-stimulated glucose transport may occur in caveolae (13). No absolute requirement of GLUT4 in glucose transport into muscle cells has been demonstrated.
Because skeletal muscle is an important site for insulin resistance, we have investigated the expression of estrogen receptors α (ERα) and β (ERβ) and their colocalization in gastrocnemius muscle. Furthermore, to investigate whether GLUT4 and caveolin-1 in skeletal muscle are regulated by ERs, we examined the expression and colocalization of these two proteins in muscles from aromatase-knockout (ArKO), ERα-knockout (ERα-/-), and ERβ-knockout (ERβ-/-) mice and investigated the effects of ERα and ERβ agonists, 4,4′,4″-(4-propyl-1H-pyrazole-1,3,5-triyl)trisphenol (PPT) and 2,3-bis(4-hydroxyphenyl)propionitrile (DPN), in ArKO mice.
Results
Detection of ERα and ERβ in Skeletal Muscle by Immunohistochemistry. Gastrocnemius muscles of 8-month-old WT, ERα-/-, ERβ-/-, and ArKO male mice were examined for the presence of ERα and ERβ. In WT mice, immunohistochemistry revealed the presence of both ERα and ERβ in most nuclei of the muscle cells. In ERα-/- mouse gastrocnemius, the presence of ERα was not detected, but staining for ERβ was clearly observed. In ERβ-/- mice, ERα expression was normal. Both receptors were expressed in muscle from ArKO mice (data not shown).
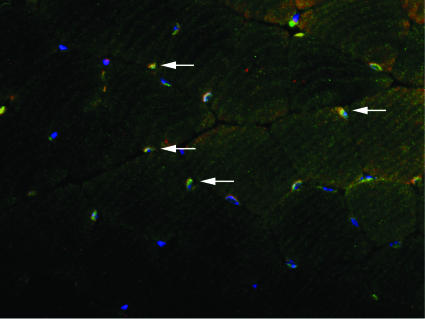
Colocalization of ERα and ERβ in Skeletal Muscle by Immunofluorescence. Gastrocnemius muscles from 8-month-old WT mice were examined by immunofluorescence for the colocalization of ERα and ERβ in the same nuclei of muscle cells. Double-staining with anti-ERα and anti-ERβ antibodies revealed the presence of ERα and ERβ in nuclei and colocalization of these receptors on the same nuclei of some cells (Fig. 1).
Fig. 1.
Colocalization of ERα and ERβ on some skeletal muscle nuclei of WT mice by immunofluorescence. Muscle was stained for ERα with Cy3 (red) and ERβ with FITC (green). Additionally, nuclei were stained with DAPI (blue). Arrows show the colocalization of the two receptors (yellow).
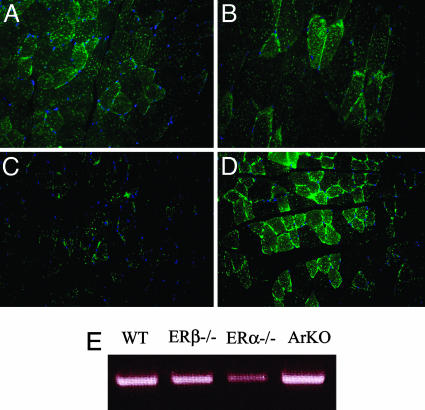
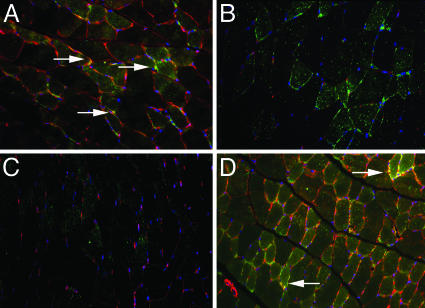
Expression and Distribution of GLUT4 in Skeletal Muscle of WT, ERα-/-, ERβ-/-, and ArKO Mice. Gastrocnemius muscles from 8-month-old WT, ERα-/-, ERβ-/-, and ArKO mice were evaluated for the expression and distribution of GLUT4. In WT (Fig. 2A) and ERβ-/- (Fig. 2B) mice, immunofluorescence showed the presence of GLUT4 on the cell membrane and vesicles localized in the cytoplasm. In ERα-/- (Fig. 2C) mice, the presence of GLUT4 on the cell membrane was markedly lower than that seen in WT and ERβ-/- mice. However, there were GLUT4-positive vesicles in the cytoplasm. In ArKO (Fig. 2D) mouse muscle, there was a substantial increase in GLUT4 expression on the cell membrane, but no changes were observed in GLUT4 in cytoplasmic vesicles. RT-PCR confirmed that GLUT4 mRNA levels were similar in WT, ERβ-/-, and ArKO mice but were decreased in ERα-/- mice (Fig. 2E).
Fig. 2.
Expression of GLUT4 on skeletal muscle of WT, ERβ-/-, ERα-/-, and ArKO mice by immunofluorescence. GLUT4 was stained with FITC revealing the presence of GLUT4 on the plasma membrane and cytosolic vesicles of WT (A) and ERβ-/- (B) mice. The presence of GLUT4 on the plasma membrane of ERα-/- mice was substantially reduced (C), whereas in ArKO mice it was significantly increased (D). Nuclei were also stained with DAPI (blue). RT-PCR confirmed that GLUT4 mRNA levels were similar in WT, ERβ-/-, and ArKO mice but were decreased in ERα-/- mice (E).
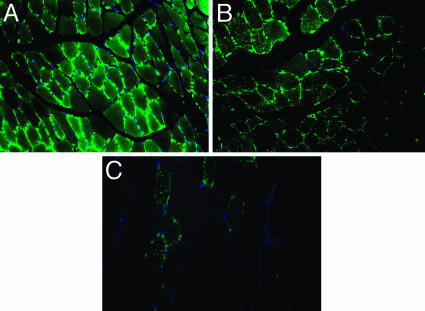
Expression and Distribution of GLUT4 in Skeletal Muscle of ArKO Mice Treated with ERα and ERβ Agonists, PPT and DPN. The very marked increase in GLUT4 expression in ArKO mice prompted an evaluation of the role of ERα and ERβ agonists on GLUT4 expression and distribution in the gastrocnemius muscle. In ArKO mice, GLUT4 expression was not affected by treatment of mice with the ERα agonist PPT (Fig. 3B). As shown in Fig. 3 A and B, the intensity of GLUT4 staining was similar in PPT- and vehicle-treated mice. However, treatment with the ERβ agonist DPN (Fig. 3C) resulted in marked decrease in GLUT4 expression, both on the cell membrane and in the cytosol.
Fig. 3.
Expression of GLUT4 on skeletal muscle of ArKO mice treated with vehicle or ERα and ERβ agonists, PPT and DPN, respectively. GLUT4 was stained with FITC, and immunofluorescence revealed the presence of GLUT4 on the plasma membrane and cytosol of ArKO mice treated with vehicle (A) and ERα agonist PPT (B). Treatment with ERβ agonist DPN, however, severely decreased GLUT4 expression on the plasma membrane and cytosol (C). Nuclei were also stained with DAPI (blue).
Expression of Caveolin-1 in Skeletal Muscle of WT, ERα-/-, ERβ-/-, and ArKO Mice. Gastrocnemius muscles from 8-month-old WT, ERα-/-, ERβ-/-, and ArKO mice were evaluated for the expression of caveolin-1. In WT (Fig. 4A) and ArKO (Fig. 4D), immunofluorescence showed the presence of caveolin-1 on the cell membrane. In ERβ-/- (Fig. 4B) and ERα-/- (Fig. 4C) mice, the presence of caveolin-1 on the cell membrane was lower than that seen in WT and ArKO mice. Western blotting with plasma membrane and microsomal fractions confirmed the decreased expression of caveolin-1 in ERβ-/- mice (Fig. 4E).
Fig. 4.
Expression of caveolin-1 on skeletal muscle of WT, ERβ-/-, ERα-/-, and ArKO mice by immunofluorescence. Staining with Cy3 (red) revealed the presence of caveolin-1 on the plasma membrane of WT (A) and ArKO (D) mice. However, caveolin-1 expression on the plasma membrane of ERβ-/- (B) and ERα-/- mice (C) was reduced. Nuclei were also stained with DAPI (blue). Western blotting with the plasma membrane and microsomal fractions confirmed the reduction of caveolin-1 in ERβ-/- mice (E).
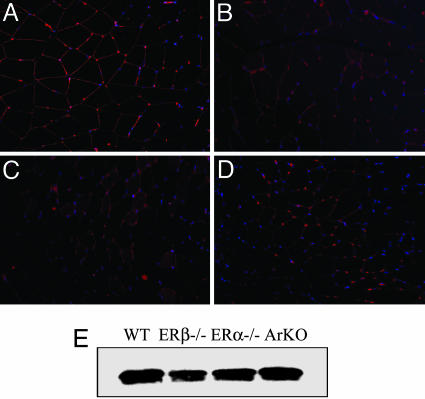
Colocalization of GLUT4 and Caveolin-1 in Skeletal Muscle of WT, ERα-/-, ERβ-/-, and ArKO Mice. To investigate whether GLUT4 is anchored on caveolae localized on the cell membrane, we evaluated the colocalization of GLUT4 and caveolin-1 proteins. Gastrocnemius muscle from 8-month-old WT, ERβ-/-, ERα-/-, and ArKO mice were examined by immunofluorescence. Double staining with both goat anti-GLUT4 and rabbit anti-caveolin-1 antibodies revealed the colocalization of these proteins in WT (Fig. 5A) and ArKO (Fig. 5D) mice on the plasma membrane. However, the colocalization was never observed in ERβ-/- (Fig. 5B) or ERα-/- (Fig. 5C) mice.
Fig. 5.
Colocalization of GLUT4 and caveolin-1 on skeletal muscle membranes of WT, ERβ-/-,ERα-/-, and ArKO mice by immunofluorescence. Muscle was stained for GLUT4 with FITC (green) and caveolin-1 with Cy3 (red). Additionally, nuclei were stained with DAPI (blue). Double staining revealed the colocalization of these proteins in WT (A) and ArKO (D) mice on the plasma membrane. However, the colocalization was never observed in ERβ-/- (B) or ERα-/- (C) mice. Arrows show the colocalization of the two receptors (yellow).
Discussion
There is much evidence that estrogen modulates insulin sensitivity and is involved in glucose homeostasis (14). This importance was confirmed in ERα-/- and ArKO mice, both of which develop adipocyte hyperplasia and hypertrophy, insulin resistance, and glucose intolerance in both sexes (15, 16). The role of ERβ in glucose metabolism has been less clear. Removal of E2/ERβ signaling in ERα-/- mice by ovariectomy decreases body and fat pad weights and adipocyte size and improves insulin and glucose metabolism (17, 18). These data suggest that ERβ opposes the effect of ERα on glucose tolerance, and that ERβ ligands may be diabetogenic. In the present study, administration of the selective ERβ ligand, DPN, to ArKO mice resulted in a substantial reduction in expression of GLUT4. GLUT4 plays a crucial role in glucose homeostasis, and it is a limiting step in the insulin-induced glucose uptake in skeletal muscle. Thus, the ERβ ligand, by reducing GLUT4, should result in a reduction in glucose tolerance. At present, no overall conclusion can be made about whether all ERβ ligands will be diabetogenic. The ERβ ligands developed by various pharmaceutical companies all appear to have tissue selectivity, and they may not all influence ERβ in skeletal muscle (19, 20). However, it might be a good precautionary measure to examine effects on GLUT4 early in the development of ERβ agonists that are being developed to target disease.
In adipocytes, GLUT4 must be anchored at caveolae on the plasma membrane for it to function in glucose transport. Caveolin-1 is an important structural component of caveolae. Our data show that, in the absence of ERβ or ERα, GLUT4 is not colocalized with caveolin-1 in the muscle. In ERα-/- mice, this apparent lack of colocalization is because GLUT4 levels are very reduced. In ERβ-/- mice, caveolin-1 on the plasma membrane is reduced. Because ERβ-/- mice are not diabetic, we have to conclude that colocalization of GLUT4 and caveolin-1 is not essential for glucose transport in skeletal muscle.
The colocalization of ERα and ERβ in the same nuclei has implications for the action of E2 on glucose tolerance in muscles. In cells where ERα and ERβ are expressed together and where they oppose each other's actions, E2 has very little effect, whereas ERα- and ERβ-selective ligands elicit distinct and opposing changes (21). In such tissues it is the ratio of ERα to ERβ that determines what type of action E2 will elicit. In the case of muscle and ER, manipulations that decrease ERβ [an action that androgens can elicit (22)] will improve glucose tolerance.
In human tissues, there is one additional factor that can influence the effect of E2 on glucose tolerance in muscle: the expression of ERβcx. This splice variant of ERβ is a repressor of ERα and its expression in muscle will favor ERβ signaling and lead to a diabetogenic state. Regulation of ERβcx in muscle during endocrine states such as late pregnancy might provide an explanation of why these states are associated with diabetes.
Materials and Methods
Animals. ERβ-/- mice were from our colony (23). ERα-/- mice were purchased from Taconic Europe (Ry, Denmark), and ArKO mice were provided by Evan Simpson (Prince Henry's Institute of Medical Research, Clayton, Australia). Mice were housed in the Karolinska University Hospital Animal Facility (Huddinge, Sweden) in a controlled environment on a 12-h light/12-h dark illumination schedule and fed a standard pellet diet with water provided ad libitum. Animals were asphyxiated by CO2, and gastrocnemius muscle was excised, frozen immediately for Western blotting, and processed for RT-PCR or fixed in 4% paraformaldehyde overnight and routinely embedded in paraffin wax for immunohistochemistry or immunofluorescence. We followed the guidelines for care and use of experimental animals that were issued by Stockholm's Södra Djurförsök-setiska Nämnd.
Chemicals and Antibodies. We purchased DPN and PPT from Tocris Cookson (Bristol, U.K.) and 4′,6-diamidino-2-phenylindole (DAPI) from Sigma-Aldrich. The following antibodies were used: rabbit polyclonal anti-ERα (MC-20) from Santa Cruz Biotechnology; biotinylated goat anti-rabbit and rabbit anti-chicken antibodies from Zymed Laboratories; FITC anti-chicken and Cy3 anti-rabbit from Jackson ImmunoResearch; rabbit affinity-purified anti-GLUT4 from FabGennix (Shreve-port, LA); FITC anti-rabbit from Jackson ImmunoResearch; rabbit anti-caveolin-1 was from BD Biosciences; and goat anti-GLUT4 was from Santa Cruz Biotechnology. The chicken polyclonal anti-ERβ 503 was produced in our laboratory (24).
Treatment of Mice with PPT or DPN. To observe the effect of DPN and PPT on GLUT4, 8-month-old male mice were used. Two mice were treated with DPN, and two were treated with PPT (30 μg per animal, 1 mg/kg of body weight). Mice were dosed every 24 h by s.c. injection for 3 days. Two control mice were treated with vehicle only.
Immunohistochemical Staining. Representative blocks of paraffin-embedded tissues were cut at 4-μm thickness, dewaxed, and rehydrated. For ER staining, antigens were retrieved by boiling in 10 mM citrate buffer (pH 7.0) for 20 min. The sections were incubated in 0.5% H2O2 in PBS for 30 min at room temperature to quench endogenous peroxidase and then incubated in 0.5% Triton X-100 in PBS for 30 min. To block the nonspecific binding, sections were incubated in 3% BSA for 1 h at 4°C. Sections were incubated with anti-ERα, anti-ERβ, or anti-GLUT4 antibodies at a dilution of 1:200, in 1% BSA and 0.1% Nonidet P-40 in PBS overnight at 4°C. For colocalization evaluation, sections were incubated with both anti-ERα and anti-ERβ antibodies. BSA replaced primary antibodies in negative controls. After washing, sections were incubated with the corresponding secondary antibodies (for ERα and ERβ, in 1:200 dilutions and for GLUT4, in 1:3000) for 1 h at room temperature. The Vectastain ABC kit (Vector Laboratories) was used for the avidin-biotin complex (ABC) method according to the manufacturer's instructions. Peroxidase activity was visualized with 3,3′-diaminobenzidine (DAKO). The sections were lightly counterstained with hematoxylin or DAPI, dehydrated through an ethanol series to xylene, and mounted. For immunofluorescence, slides were directly mounted in Vectashield antifading medium (Vector Laboratories). The sections were examined under a Zeiss fluorescence microscope with filters suitable for selectively detecting the fluorescence of FITC (green), Cy3 (red), or a light microscope. For colocalization, images from the same section but showing different antigen signals were overlaid. Cells where both FITC- and Cy3-conjugated secondary antibodies were present have an orange or yellow color.
Western Blotting. Plasma membrane and microsomal fractions were analyzed by Western blotting. Frozen tissues were homogenized for 1 min each with a Polytron in 25 ml of PBS containing protease inhibitor mixture according to the supplier's instructions (Roche Diagnostics). The protein content was measured by Bio-Rad protein assay with BSA as standard. Ten micrograms of protein was dissolved in SDS sample buffer and resolved on 4-20% gradient SDS/polyacrylamide gels (Invitrogen) in Tris·glycine buffer. Proteins were transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biosciences) by electroblotting in Tris·glycine buffer. Molecular weight markers were Precision Plus protein standards (Bio-Rad). The membrane was then incubated in blocking solution containing 10% fat-free milk and 0.1% Nonidet P-40 in PBS for 2 h at room temperature. Incubation with rabbit anti-caveolin-1 (1:5,000, BD Biosciences) was performed in blocking solution overnight at 4°C. For positive control, human endothelial cell lysate was used (BD Biosciences). After washing, secondary peroxidase-conjugated goat anti-rabbit antibody (1:3,000, Sigma) was applied in blocking solution for 1 h at room temperature. After washing, detection with an enhanced chemiluminescence kit (Amersham Pharmacia) was performed.
RNA Extraction and RT-PCR. RNA was extracted with TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Total RNA (2 μg) was reverse-transcribed by using random hexamers and reverse transcriptase (TaqMan reverse transcription reagents, Applied Biosystems) in a total volume of 40 μl. In total, 3 μl of synthesized cDNA was subjected to PCR amplification by using RED Taq Ready mix (Sigma). PCR primers and conditions for GLUT4 were as follows: forward, 5′-AAG ATG GCC ACG GAG AGA G-3′ and reverse, 5′-GTG GGT TGT GGC AGT GAG TC-3′ (58.1°C, 30 cycles). The housekeeping gene β-actin was used as a control with the following primers and conditions: forward, 5′-TGG CAC CAC ACC TTC TAC AA-3′ and reverse, 5′-TCA CGC ACG ATT TCC CTC TC-3′ (58.1°C, 30 cycles). The PCR products were separated on 1.8% agarose gels and visualized by ethidium bromide staining under UV illumination.
Acknowledgments
We thank Dr. Evan Simpson (Monash Medical Centre, Prince Henry's Institute of Medical Research, Clayton, Australia) for providing the ArKO mice. This study was supported by Brazil's Ministry of Education Grant CAPES/PDEE-BEX0145/05-0 and State of São Paulo Research Foundation Grant 02/13534-9. This work was also supported by grants from the European Union Network of Excellence Chemicals as Contaminants in the Food Chain: A network of excellence for research, risk assessment, and education (CASCADE), the Swedish Cancer Fund, and from KaroBio AB.
Author contributions: M.W. and J.-Å.G. designed research; R.P.A.B. performed research; R.P.A.B., U.F.M., M.W., and J.-Å.G. analyzed data; and R.P.A.B. and M.W. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: ArKO, aromatase-knockout; DPN, 2,3-bis(4-hydroxyphenyl)propionitrile; ER, estrogen receptor; E2, estradiol; GLUT, glucose transporter; PPT, 4,4′,4″-(4-propyl-1H-pyrazole-1,3,5-triyl)trisphenol.
References
- 1.Case, A. M. & Reid, R. L. (2001) Compr. Ther. 27, 65-71. [DOI] [PubMed] [Google Scholar]
- 2.Solomon, C. G., Hu, F. B., Dunaif, A., Rich-Edwards, J., Willett, W. C., Hunter, D. J., Colditz, G. A., Speizer, F. E. & Manson, J. E. (2001) J. Am. Med. Assoc. 286, 2421-2426. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan, T. A., Metzger, B. E., Freinkel, N. & Bergman, R. N. (1990) Am. J. Obstet. Gynecol. 162, 1008-1014. [DOI] [PubMed] [Google Scholar]
- 4.Morimoto, S., Cerbon, M. A., Alvarez-Alvarez, A., Romero-Navarro, G. & Diaz-Sanchez, V. (2001) Life Sci. 68, 2979-2985. [DOI] [PubMed] [Google Scholar]
- 5.Von Wolff, M., Ursel, S., Hahn, U., Steldinger, R. & Strowitzki, T. (2003) J. Clin. Endocrinol. Metab. 88, 3885-3892. [DOI] [PubMed] [Google Scholar]
- 6.Okuno, S., Akazawa, S., Yasuhi, I., Kawasaki, E., Matsumoto, K., Yamasaki, H., Matsuo, H., Yamaguchi, Y. & Nagataki, S. (1995) Horm. Metab. Res. 27, 231-234. [DOI] [PubMed] [Google Scholar]
- 7.Livingstone, C. & Collison, M. (2002) Clin. Sci. 102, 151-166. [DOI] [PubMed] [Google Scholar]
- 8.Bell, G. I. & Polonsky, K. S. (2001) Nature 414, 788-791. [DOI] [PubMed] [Google Scholar]
- 9.Ryder, J. W., Gilbert, M. & Zierath, J. R. (2001) Front. Biosci. 6, D154-D163. [DOI] [PubMed] [Google Scholar]
- 10.Tsakiridis, T., McDowell, H. E., Walker, T., Downes, C. P., Hundal, H. S., Vranic, M. & Klip, A. (1995) Endocrinology 136, 4315-4322. [DOI] [PubMed] [Google Scholar]
- 11.Zhou, L., Chen, H., Xu, P., Cong, L. N., Sciacchitano, S., Li, Y., Graham, D., Jacobs, A. R., Taylor, S. I. & Quon, M. J. (1999) Mol. Endocrinol. 13, 505-514. [DOI] [PubMed] [Google Scholar]
- 12.Cohen, A. W., Combs, T. P., Scherer, P. E. & Lisanti, M. P. (2003) Am. J. Physiol. 285, E1151-E1160. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson, M., Thorn, H., Parpal, S., Stralfors, P. & Gustavsson, J. (2002) FASEB J. 16, 249-251. [DOI] [PubMed] [Google Scholar]
- 14.Godsland, I. F. (2005) Diabetologia 48, 2213-2220. [DOI] [PubMed] [Google Scholar]
- 15.Takeda, K., Toda, K., Saibara, T., Nakagawa, M., Saika, K., Onishi, T., Sugiura, T. & Shizuta, Y. (2003) J. Endocrinol. 176, 237-246. [DOI] [PubMed] [Google Scholar]
- 16.Heine, P. A., Taylor, J. A., Iwamoto, G. A., Lubahn, D. B. & Cooke, P. S. (2000) Proc. Natl. Acad. Sci. USA 97, 12729-12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, M. E., Thorburn, A. W., Britt, K. L., Hewitt, K. N., Wreford, N. C., Proietto, J., Oz, O. K., Leury, B. J., Robertson, K. M., Yao, S. & Simpson, E. R. (2000) Proc. Natl. Acad. Sci. USA 97, 12735-12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naaz, A., Zakroczymski, M., Heine, P., Taylor, J., Saunders, P., Lubahn, D. & Cooke, P. S. (2002) Horm. Metab. Res. 34, 758-763. [DOI] [PubMed] [Google Scholar]
- 19.Follettie, M. T., Pinard, M., Keith, J. C., Jr., Wang, L., Chelsky, D., Hayward, C., Kearney, P., Thibault, P., Paramithiotis, E., Dorner, A. J. & Harris, H. A. (November 3, 2005) Endocrinology, 10.121/en.2005-0600. [DOI] [PubMed]
- 20.Komm, B. S., Kharode, Y. P., Bodine, P. V., Harris, H. A., Miller, C. P. & Lyttle, C. R. (2005) Endocrinology 146, 3999-4008. [DOI] [PubMed] [Google Scholar]
- 21.Helguero, L. A., Faulds, M. H., Gustafsson, J.-Å. & Haldosen, L. A. (2005) Oncogene 24, 6605-6616. [DOI] [PubMed] [Google Scholar]
- 22.Arnold, J. T., Le, H., McFann, K. K. & Blackman, M. R. (2005) Am. J. Physiol. 288, E573-E584. [DOI] [PubMed] [Google Scholar]
- 23.Krege, J. H., Hodgin, J. B., Couse, J. F., Enmark, E., Warner, M., Mahler, J. F., Sar, M., Korach, K. S., Gustafsson, J.-Å. & Smithies, O. (1998) Proc. Natl. Acad. Sci. USA 95, 15677-15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saji, S., Jensen, E. V., Nilsson, S., Rylander, T., Warner, M. & Gustafsson, J.-Å. (2000) Proc. Natl. Acad. Sci. USA 97, 337-342. [DOI] [PMC free article] [PubMed] [Google Scholar]