Abstract
G protein-coupled receptor ligand-dependent transactivation of growth factor receptors has been implicated in human cancer cell proliferation, migration, and cell survival. For example, prostaglandin E2 (PGE2)-induced transactivation of the EGF receptor (EGFR) in colorectal carcinoma cells is mediated by means of a c-Src-dependent mechanism and regulates cell proliferation and migration. Recent evidence indicates that β-arrestin 1 may act as an important mediator in G protein-coupled receptor-induced activation of c-Src. Whether β-arrestin 1 serves a functional role in these events is, however, unknown. We investigated the effects of PGE2 on colorectal cancer cells expressing WT and mutant β-arrestin 1. Here we report that PGE2 induces the association of a prostaglandin E receptor 4/β-arrestin 1/c-Src signaling complex resulting in the transactivation of the EGFR and downstream Akt (PKB) signaling. The interaction of β-arrestin 1 and c-Src is critical for the regulation of colorectal carcinoma cell migration in vitro as well as metastatic spread of disease to the liver in vivo. These results show that the prostaglandin E/β-arrestin 1/c-Src signaling complex is a crucial step in PGE2-mediated transactivation of the EGFR and may play a pivotal role in tumor metastasis. Furthermore, our data implicate a functional role for β-arrestin 1 as a mediator of cellular migration and metastasis.
Keywords: metastasis, prostaglandin E2, c-Src, EGF receptor, prostaglandin E receptor
Gprotein-coupled receptors (GPCRs) comprise the largest known family of plasma membrane receptors and consist of a seven-transmembrane-spanning region flanked by an extracellular N terminus and an intracellular C terminus. Upon ligand binding, these receptors couple to heterotrimeric G proteins (Gα- and Gβγ-subunits) and catalyze the exchange of GDP for GTP, thus initiating a multitude of signaling events into the cell. These include the classical activation of phosholipases (phosholipases A, C, and D), protein kinases (PKA and PKC), and lipid kinases (phosphatidylinositol 3-kinase) as well as increased intracellular calcium levels. The desensitization of GPCRs occurs through a multistep process. GPCR kinases are recruited to the receptor by liberated βγ-subunits and phosphorylate the receptor on the cytoplasmic tail and intracellular loops. This phosphorylation event triggers the association of arrestin, which then traffics the receptors to clathrin-coated pits for endocytosis (1).
Prostaglandins (PG) are important bioactive lipids which exert their effects through the activation of specific GPCRs as well as members of the peroxisome proliferator-activated receptor family. For example, PGE2 is the ligand for four prostaglandin E (EP) receptor isoforms termed EP1, EP2, EP3, and EP4. Stimulation of these receptors elicits different intracellular responses (2). The stimulation of the EP1 receptor induces an increase in intracellular calcium by means of the activation of phospholipase C. EP2 and EP4 receptors couple to Gαs proteins, which generate increased cAMP levels, whereas EP3 receptors mostly couple to Gαi proteins and reduce cAMP levels. Data derived from EP receptor knockout mice as well as EP receptor agonists and antagonists have indicated an important role for EP2 and EP4 receptors in the formation and growth of primary intestinal tumors (3).
Aside from the classical activation of downstream GPCR-linked signaling cascades, PGE2-induced stimulation of colorectal carcinoma cells can also transactivate growth factor receptors such as the EGF receptor (EGFR) (4, 5). The GPCR-induced activation of growth factor receptors can occur through many diverse mechanisms. GPCR activation of matrix metalloproteinases such as TNF-α-converting enzyme (ADAM 17) by means of PKC, Pyk2, or Src liberates peptide ligands, which results in the extracellular activation of growth factor receptors (6). GPCR-induced transactivation of the receptors can also occur through entirely intracellular mechanisms, including the direct activation of the receptor through Src-dependent signaling (4, 7). Interestingly, the recruitment of β-arrestin to stimulated GPCRs has been associated with the activation of Src (8).
Results and Discussion
Because the stimulation of colorectal carcinoma-derived cells with PGE2 induces the translocation of β-arrestin 1 (9) and PGE2 induces the activation of Src (4, 5), we examined whether β-arrestin 1 plays a role in this process. PGE2 stimulation of LS-174T and HCA-7 cells resulted in the association of β-arrestin 1 with the EP4 receptor and c-Src (Fig. 1A). The association of the EP4 receptor and β-arrestin 1 was observed at 2 min and began to decline by 10 min after treatment. However, the association of β-arrestin 1 and c-Src steadily increased during this time. Because the association of EP4 receptor/β-arrestin 1 and β-arrestin 1/c-Src could potentially be mutually exclusive, we also determined whether the EP4 receptor, β-arrestin 1, and c-Src formed a signaling complex (Fig. 1B). The EP4 receptor was detected in c-Src immunoprecipitates from both LS-174T and HCA-7 cells, indicating that PGE2 does induce the association of an EP4 receptor/β-arrestin 1/c-Src complex.
Fig. 1.
PGE2-induced association of the EP4 receptor/β-arrestin 1/c-Src signaling complex in LS-174T and HCA-7 cells. (A) Cells were incubated for the indicated times with 1 μM PGE2, and β-arrestin 1 was immunoprecipitated from isolated lysates. (Upper) Coprecipitated EP4 receptor and c-Src were detected by Western blot analysis. (Lower) Densitometry analysis. (B) Cells were incubated for the indicated times with 1 μM PGE2, and c-Src was immunoprecipitated from isolated lysates. (Upper) Coprecipitated EP4 receptor was detected by Western blot analysis. (Lower) Densitometry analysis. (C) Cells were incubated for the indicated times with 1 μM PGE2, and total cytosolic and membrane fractions were isolated. The translocation of β-arrestin 1 from the cytosol to the membrane was determined by Western blot analysis. (D) Cells were incubated for the indicated times with 1 μM PGE2, and whole-cell lysates were prepared. The phosphorylation status of β-arrestin 1 was determined by Western blot analysis with the use of phosphospecific (p-Ser-412) β-arrestin 1 antibodies. Equal protein is shown by total β-arrestin 1 Western blot analysis. Data shown are representative of three independent experiments.
In quiescent cells, arrestin is mainly localized diffusely in the cytosol. However, upon agonist stimulation of GPCRs and subsequent receptor phosphorylation, arrestin translocates from the cytosol to the membrane fraction, where it can then associate with GPCRs and direct the desensitization of the receptor (10). The treatment of LS-174T and HCA-7 cells with PGE2 induced the translocation of β-arrestin 1 from the cytosol to the membrane fraction (Fig. 1C). Together these observations indicate that PGE2 stimulation of the EP4 receptor induces the association of a membrane-associated signaling complex comprised of the EP4 receptor, β-arrestin 1, and c-Src.
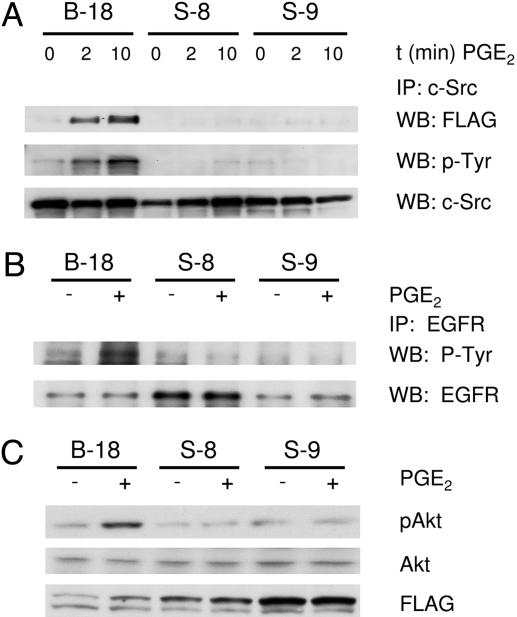
To determine whether the EP4/β-arrestin 1/c-Src complex was responsible for the PGE2-mediated activation of Src in LS-174T cells, LS-174T clones were generated that constitutively overexpress FLAG-tagged WT β-arrestin 1 (B-18) or FLAG-tagged mutant S412D-β-arrestin 1 (S-8 and S-9). The S412D-β-arrestin 1 mutant has a serine-to-aspartic acid point mutation at residue 412, which greatly reduces the association of β-arrestin 1 with Src (8). β-Arrestin 1 is normally phosphorylated on serine-412 in a quiescent state, and dephosphorylation at this site has been shown to be an important step in the regulation of β-arrestin 1 activity (11, 12). PGE2 also induced the loss of phosphorylation on serine-412 of β-arrestin 1 in LS-174T and HCA-7 cells (Fig. 1D). In cells expressing a WT β-arrestin 1-FLAG construct (B-18), PGE2 induced the association of β-arrestin 1 with c-Src (Fig. 2A). PGE2 also induced the activation of c-Src, as detected by tyrosine phosphorylation of c-Src in the B-18 cells (Fig. 2 A). PGE2 stimulation of two different LS-174T clones (S-8 and S-9) that overexpress the S412D-β-arrestin 1 mutant reveals that the β-arrestin 1/c-Src complex is critical for the activation of c-Src (Fig. 2 A). This finding was confirmed through the reduced association of β-arrestin 1 with c-Src and the inability of PGE2 to induce the phosphorylation (activation) of c-Src.
Fig. 2.
Effect of β-arrestin 1 S412D mutant on the association of c-Src and subsequent downstream signaling. (A) LS-174T cells expressing Flag epitope-tagged WT β-arrestin 1 (B-18) or mutant Flag epitope-tagged S412D-β-arrestin 1 (S-8 and S-9) were incubated for the indicated times with 1 μM PGE2, and c-Src was immunoprecipitated from whole-cell lysates. Coprecipitated Flag epitope-tagged β-arrestin was detected by Western blot analysis. The activation state of c-Src was determined on immunoprecipitates by Western blot analysis by using anti-phospho-tyrosine antibodies. Equal protein is shown by total c-Src Western blot analysis. (B) LS-174T cells expressing Flag epitope-tagged β-arrestin 1 were incubated in the absence or presence of 1 μM PGE2 for 15 min, and EGFR was immunoprecipitated from whole-cell lysates (see above). The activation state of the EGFR was determined on immunoprecipitates by Western blot analysis by using anti-phospho-tyrosine antibodies. Equal protein is shown by total EGFR Western blot analysis. (C) LS-174T cells expressing Flag epitope-tagged β-arrestin 1 (see above) were incubated in the absence or presence of 1 μM PGE2 for 15 min, and whole-cell lysates were prepared. The phosphorylation status of Akt was determined by Western blot analysis with the use of phosphospecific (p-Ser-473) Akt antibodies. Equal protein is shown by total Akt Western blot analysis. Data shown are representative of three independent experiments.
Because Src is involved in the PGE2-induced transactivation of the EGFR (4, 5), we sought to determine whether the association of β-arrestin 1 and c-Src is required for this transactivation. In cells expressing the WT β-arrestin 1-FLAG construct (B-18), PGE2 induced the transactivation of the EGFR (Fig. 2B). However, the stimulation of LS-174T clones which overexpress the S412D-β-arrestin 1 mutant (S-8 and S-9) with PGE2 indicated that the formation of the β-arrestin 1/c-Src complex is vital for the transactivation of the EGFR (Fig. 2B).
The activation of EGFR induces downstream signaling cascades such as phosphatidylinositol 3-kinase/Akt and Ras/Raf/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase. The induction of these signaling mediators results in a variety of cellular effects, including increased proliferation and migration. We have previously shown that PGE2, through the activation of Src and subsequent transactivation of the EGFR, increases Akt activity in colorectal carcinoma cells (4). We therefore sought to determine whether the EP4/β-arrestin 1/c-Src signaling complex plays a role in the PGE2-induced activation of Akt. In cells expressing WT β-arrestin 1-FLAG (B-18), PGE2 induced the activation of Akt as detected by phosphorylation of Akt on serine-473 (Fig. 2C). However, the stimulation of LS-174T clones that overexpress the S412D-β-arrestin 1 mutant (S-8 and S-9) with PGE2 did not induce the phosphorylation of Akt. These results indicate that the β-arrestin 1/c-Src complex is indeed necessary for the PGE2-induced activation of Akt (Fig. 2B).
Previous studies have shown that the S412D-β-arrestin 1 mutant can reduce desensitization and internalization of the β2 adrenergic receptor (8). Also, a β-arrestin 1 deletion mutant (319-418) can reduce the PGE2-induced internalization of the EP4 receptor (13). We therefore sought to determine whether the S412D-β-arrestin 1 mutant would affect the trafficking and internalization of the EP4 receptor in colorectal carcinoma cells. LS-174T, B-18, and S-9 cells were treated with 1 μM PGE2 or vehicle for 30 min. Detection of the EP4 receptor by immunofluorescence indicated that the EP4 receptor was internalized in the presence of PGE2 in the LS-174T and B-18 cells (Fig. 3). However, the internalization of the receptor was greatly inhibited in the S-9 clone, demonstrating that the S412D mutant does inhibit the internalization of the EP4 receptor.
Fig. 3.
Localization of the EP4 receptor upon PGE2 treatment. LS-174T, B-18, or S-9 cells were treated with 1 μM PGE2 or vehicle (DMSO) for 30 min. Cells were fixed, processed for detection of EP4 receptor (immunofluorescence), and visualized by using confocal microscopy.
The metastatic spread of tumor cells occurs in a multistep process (14). Tumor cells must detach from the primary tumor site and enter the circulatory system. These cells then travel to distant sites, extravasate from the vasculature, and reestablish growth. The expression of cyclooxygenase 2 has been associated with increased metastasis of breast, lung, and colon primary tumors (15-17), and cyclooxygenase-derived PGs such as PGE2 and thromboxane A2 have been shown to directly stimulate cell migration (18, 19). PGE2 can induce the in vitro migration of WT LS-174T cells (4, 18) as well as LS-174T cells overexpressing WT β-arrestin 1 (B-18) (Fig. 4A). Interestingly, in S412D-β-arrestin 1-expressing LS-174T cells (S-8 and S-9) we observed up to a 65% reduction of PGE2-induced migration in vitro (P < 0.0001). However, cells expressing WT or mutant β-arrestin 1 migrated at a similar rate in response to stimulation with EGF. We also observed a similar trend in a cell invasion assay (Fig. 4B). PGE2 induced the migration of B-18 cells through an extracellular matrix. However, as observed in Fig. 4A, the rate of cellular invasion was reduced in the S-8 and S-9 clones. Furthermore, all clones invaded similarly to EGF treatment. Taken together, these data indicate that the association of β-arrestin 1 with c-Src is an important step in the PGE2-mediated induction of migration and invasion. Furthermore, this interaction occurs upstream of EGFR activation.
Fig. 4.
Effect of β-arrestin 1/c-Src complex on cellular migration and invasion in vitro and metastasis in vivo. LS-174T cells expressing Flag epitope-tagged WT β-arrestin 1 (B-18) or mutant Flag epitope-tagged S412D-β-arrestin 1 (S-8 and S-9) were seeded onto the upper chamber of modified Boyden chambers in serum-free media (A) or serum-free media with a 1:10 dilution of Matrigel (B) (4). The assay was carried out for 24 h in the presence of 1 μM PGE2 or 100 ng/ml EGF as indicated. Migrated cells were counted and averaged from three wells from three independent experiments (average ± SD). (C) A total of 1 × 106 LS-174T cells expressing GFP, Flag epitope-tagged WT β-arrestin 1 (B-15, B-18, and B-22), mutant Flag epitope-tagged S412D-β-arrestin 1 (S-8, S-9, S-14, and S-22), or WT LS-174T were injected into the spleens of nude mice. After 4 weeks, livers were resected and weighed. Values represent the average ± SD of nine mice from three independent experiments (*, P < 0.001 compared with GFP control; **, P > 0.05 compared with GFP control). (D) Representative picture of gross liver specimen from C. (E) Tissue sections from metastatic-bearing livers from B-18- and S-9-injected mice. Hematoxylin and eosin staining and immunohistochemistry with pAkt and total Akt antibodies are shown. T indicates metastatic tumor.
To determine the role of the β-arrestin 1/c-Src complex on the metastasis of colorectal carcinoma cells in vivo, we used an orthotopic liver metastasis model in immunodeficient mice. Cells were injected into the spleens of nude mice. After 4 weeks, livers were resected and weighed to determine the burden of metastasis in the liver. In general, WT LS-174T cells did not metastasize to the liver (Fig. 4 C and D). Of all mice injected with WT LS-174T cells (n = 10), only one showed any sign of metastasis. LS-174T cells that constitutively express GFP showed a slight increase in metastases, indicating that either GFP or the selection process had an effect on the ability of these cells to metastasize in vivo. However, this increase was not significantly different from WT LS-174T cells (P > 0.05). Interestingly, three clonally derived LS-174T cell lines that overexpress β-arrestin 1 (B-15, B-18, and B-22) produced significant liver metastasis, with a >2-fold increase in liver weight when compared with GFP-expressing cells (Fig. 4 C and D; *, P < 0.001). However, clonal LS-174T cells that overexpress the S412D-β-arrestin 1 mutant (S-8, S-9, S-14, and S-22) inhibited this effect by nearly 50% (P < 0.005) and showed only a 1.3-fold increase over GFP-expressing cells (Fig. 4 C and D; **, P > 0.05).
Because in vitro data indicate the importance of β-arrestin 1 and downstream Akt activation (Figs. 1, 2, 3, 4 and ref. 4) as well as the role of Akt signaling (20-23) in cellular migration and invasion, we sought to determine the status of Akt signaling in our β-arrestin 1 liver metastases. Livers bearing metastatic disease from the B-18- and S-9-injected mice were sectioned, and immunohistochemistry of pAkt and total Akt was determined (Fig. 4E). We observed an increase in the phosphorylated form of Akt (pAkt) in the metastatic tumors derived from both the B-18 and S-9 clones when compared with normal adjacent liver. More importantly, we detected an increase in pAkt in the B-18 tumors when compared with the S-9-derived tumors. The level of Akt in these tumors was unchanged when compared with normal adjacent liver. These results indicate a causal link between in vitro signaling events and in vivo metastatic disease.
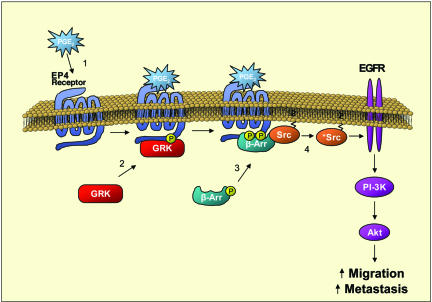
Cyclooxygenase 2 and the PGs resulting from its activity increase motility and invasion in many types of cancer (24, 25). Our data suggest that the EP4/β-arrestin 1/c-Src signaling complex is a critical link for PGE2-induced migration and invasion of colorectal carcinoma cells (Fig. 5). This finding is consistent with current reports indicating the association of the EP4 receptor with β-arrestin 1 through the use of colocalized GFP-tagged proteins (9, 13). PGE2-induced stimulation of the EP4 receptor triggers the GSK-dependent phosphorylation of intracellular domains, which induces the recruitment of β-arrestin 1 to the receptor as well as the dephosphorylation of β-arrestin 1 at serine-412 (Fig. 1). This event is critical in the signaling potential of β-arrestin 1. The dephosphorylation at serine-412 permits the association of β-arrestin 1 with c-Src, thus activating this tyrosine kinase (1, 8).
Fig. 5.
Model of PGE2-induced migration by means of the EP4 receptor/β-arrestin 1/c-Src signaling complex. Cyclooxygenase 2-derived PGE2 binds to and activates the EP4 receptor (part 1), which induces the phosphorylation of the receptor by GPCR kinase (part 2). The phosphorylation on intracellular loops of the EP4 receptor attracts β-arrestin 1. The translocation of β-arrestin 1 to the receptor triggers the dephosphorylation of β-arrestin 1 at serine-412, thus allowing its association with c-Src (part 3). β-Arrestin 1 activates c-Src (part 4), which initiates the transactivation of the EGFR and subsequent downstream signaling through Akt. The activation of this signaling cascade is involved in the migration and metastasis of colorectal carcinomas.
The PGE2-induced activation of c-Src can transactivate the EGFR by means of the liberation of extracellular ligand or a completely intracellular mechanism (4, 5). This stimulation of the EGFR can lead to the activation of Akt, which plays an important role in PGE2-mediated cell migration in vitro (4, 18). With the use of a serine-to-aspartic acid mutation at position 412 of β-arrestin 1 (which mimics the phosphorylated state of the protein), we now demonstrate that the PGE2-induced transactivation of the EGFR and subsequent downstream Akt signaling in LS-174T cells depend on the β-arrestin 1-mediated activation of c-Src (Fig. 2).
The functional consequence of a mutation at serine-412 of β-arrestin 1 was visualized by observing the rate of cell migration in vitro and metastatic spread in vivo (Fig. 4). PGE2 stimulates increased migration of colorectal carcinoma cells (4, 18). However, cells overexpressing the S412D mutation of β-arrestin 1 migrate with only 40% the efficiency of cells expressing WT β-arrestin 1. Interestingly, both cell lines migrated at similar rates in response to EGF, indicating that stimulation of this pathway downstream of β-arrestin 1 could rescue the inhibitory effect. More importantly, cells overexpressing WT β-arrestin 1 metastasized to the liver at a 2.5-fold higher rate than did cells expressing GFP. However, cells that express the S412D mutant β-arrestin 1 metastasized at a reduced rate when compared with WT β-arrestin 1. Because the PGE2-induced activation of Src, Akt, and EGFR is completely lost in cells expressing the S412D mutant, there is a differential effect on signaling and functional activity. Although the isolation of this signaling cascade gives rise to the observations in vitro, there are multiple factors that will have a net effect on metastasis in vivo. For example, there are >20 known proteins as well as multiple GPCR families that interact with β-arrestin 1 (26). The different effects of the β-arrestin 1 constructs on metastasis are most likely due to the activation of the Src family through serine-412, small guanine nucleotide exchange factors such as RhoA and Ral-GDS, and the mitogen-activated protein kinase cascade, including Raf and extracellular signal-regulated kinase 2.
Taken together, these results indicate an important role for the EP4 receptor/β-arrestin 1/c-Src complex in PGE2-mediated signaling and suggest that β-arrestin 1 may serve a pivotal role in colorectal carcinoma metastasis. Thus, novel approaches targeting the β-arrestin pathway may provide new therapeutic advances for treatment of patients with colorectal cancer. This disease accounts for the second highest number of cancer deaths in the United States, and novel approaches for more effective treatment would be dearly welcomed by both patients and physicians.
Materials and Methods
Materials. Antibodies to phospho-β-arrestin 1 serine-412, Akt, and phospho-Akt serine-473 were obtained from Cell Signaling Technology (Beverly, MA). Antibodies to EGFR and c-Src were obtained from Santa Cruz Biotechnology. Antibodies to phospho-tyrosine and EGF were purchased from Upstate Biotechnology. Anti-Flag and anti-β-actin antibodies were purchased from Sigma. PGE2 and EP4 receptor antibodies were purchased from Cayman Chemical (Ann Arbor, MI). The specificity of all antibodies was verified by using positive controls (data not shown).
Cell Culture and Stable Clone Selection. LS174T cells were purchased from the American Type Culture Collection, and HCA-7 cells were a generous gift from Susan Kirkland (Imperial College School of Medicine, London). Cells were maintained in McCoy's 5A medium containing 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin in a 5% CO2 atmosphere, washed in PBS, and incubated with serum-free media for 48 h before agonist treatment. LS-174T clones that stably express FLAG epitope-tagged β-arrrestin 1 constructs (8) were selected by using 600 μg/ml G418.
Whole-Cell Lysate Preparation. Quiescent cells were treated with 1 μM PGE2 at 37°C for various times as indicated in the experiments. The cells were rinsed twice in ice-cold PBS and scraped in 500 μl of lysis buffer (50 mM Hepes, pH 7.5/150 mM NaCl/1 mM EDTA/0.01% Triton X-100/10 μg/ml leupeptin/10 μg/ml antipain/1 mM phenylmethylsulfonyl fluoride/500 μM sodium orthovanadate/10 mM β-glycerophosphate). The cells were disrupted by brief sonication, and protein concentrations were determined with the Bio-Rad protein determination reagent.
Cytosol/Membrane Fractionation. Quiescent cells were treated with 1 μM PGE2 at 37°C for various times as indicated in the experiments. The cells were rinsed twice in ice-cold PBS and scraped in 500 μl of lysis buffer (50 mM Hepes, pH 7.5/150 mM NaCl/1 mM EDTA/10 μg/ml leupeptin/10 μg/ml antipain/1 mM phenylmethylsulfonyl fluoride/500 μM sodium orthovanadate/10 mM β-glycerophosphate). The cells were then disrupted by brief sonication and centrifuged at 100,000 × g for 60 min. The supernatant (cytosol) was removed, and the pellets (membrane) were resuspended in lysis buffer. Protein concentrations for the cytosol and membrane fractions were determined with the Bio-Rad protein determination reagent.
Immunoprecipitations. Quiescent cells were treated with 1 μM PGE2 at 37°C for various times as indicated in the experiments. The cells were rinsed twice in ice-cold PBS and scraped in 500 μl of immunoprecipitation buffer (25 mM Hepes, pH 7.5/150 mM NaCl/1 mM EDTA/1% Nonidet P-40/10% glycerol/0.25% sodium deoxycholate/10 μg/ml leupeptin/10 μg/ml antipain/1 mM sodium orthovanadate/10 mM β-glycerophosphate/1 mM phenylmethylsulfonyl fluoride). The cells were disrupted by brief sonication and centrifuged at 10,000 × g for 10 min. The supernatant was incubated for 1 h with gentle rocking at 4°C with antibody. Protein G-PLUS agarose beads from Santa Cruz Biotechnology were added, and samples were rocked at 4°C for an additional 3 h. The beads were collected by centrifugation and washed three times with immunoprecipitation buffer.
Western Blot Analysis. Equal amounts of samples were separated by SDS/PAGE and transferred to poly(vinylidene difluoride) membranes. The membranes were blocked in TTBS (TBS with 0.1% Tween 20) containing either 5% dry milk or BSA. Primary antibody incubations were performed in TTBS with either 5% dry milk or BSA overnight at 4°C. After washing, the membranes were incubated with the appropriate secondary peroxidase-conjugated antibody for 1 h in TTBS with either 5% dry milk or BSA. Immunoreactive proteins were visualized by using the enhanced chemiluminescence system from Amersham Pharmacia.
Cell Migration/Invasion. Cell migration and invasion was performed essentially as described in ref. 18. Briefly, 5 × 104 cells were suspended in 200 μl of serum-free McCoy's 5A media and placed in either a collagen-coated upper chamber or a 1:10 dilution of Matrigel (BD Bioscience) in an upper chamber. The lower chamber was filled with serum-free McCoy's 5A media containing 1 μM PGE2 or 100 ng/ml EGF as indicated. After overnight incubation, the cells were fixed in DiffQuick Fixative (Dade Diagnostics, Deerfield, IL) and stained in 0.1% crystal violet. Cells were removed from the upper surface of the filter with a cotton swab. Cells were counted on the under surface of the filter to determine migration. Values are expressed as the mean ± SD of three independent experiments.
Cell Metastasis. Nude mice were anesthetized by using inhaled isoflurane (2%) in accordance with veterinary standards at Vanderbilt University's animal care facility. The abdomen was prepped in a sterile fashion, and an incision was made in the midline, through which the spleen was extracted. A total of 1 × 106 cells were injected with a 27.5-gauge needle in 100 μl of PBS. The spleen was then resected by using electrocautery. Homeostasis was assured with electrocautery. The area was thoroughly irrigated with warm (37°C) sterile water. The abdominal cavity was closed in appropriate layers by using a 5-0 prolene suture. Mice were maintained for 4 weeks. Livers were resected, weighed, fixed overnight in 10% formalin, and stored in 70% ethanol.
Immunohistochemistry (IHC). IHC with antibodies to pAkt and total Akt as well as hematoxylin and eosin stains were performed in the Vanderbilt Institutional Immunohistochemistry Core Facility (Vanderbilt University Medical Center).
Immunofluorescence. Cells were seeded at 50,000 cells per well on Lab-Tek II chamber slides (Nalge Nunc). After 24 h, cells were treated with 1 μM PGE2 or vehicle (DMSO) for 30 min. Cells were rinsed in PBS, fixed for 5 min in 4% paraformaldehyde, and permeabilized for 5 min in 0.2% Triton X-100. Cells were blocked for 1 h in the presence of 1% BSA and then incubated for 16 h with EP4 antibodies (Santa Cruz Biotechnology). After washing in 1% BSA, cells were incubated with Alexa Fluor 488 antibodies (Molecular Probes) for 1 h. Cells were washed and mounted with PorLong Gold antifade reagent (Molecular Probes). Images were visualized by using a LSM 510 confocal microscope.
Acknowledgments
We thank Robert J. Lefkowitz (Howard Hughes Medical Institute, Duke University Medical Center, Durham, NC) for generously providing the β-arrestin 1 FLAG-tagged constructs and Kelly S. Parman at the Vanderbilt Institutional Immunohistochemistry Core Facility for helping obtain the immunohistochemistry results. This work was supported in part by U.S. Public Health Service Grants DK 47297, P30CA-68485, DK 62112, and PO1CA-77839 (to R.N.D.) and 1KO8DK70708-01 (to D.L.G.). R.N.D. is the B. F. Byrd Professor of Molecular Oncology and thanks the T. J. Martell Foundation and the National Colorectal Cancer Research Alliance for generous support.
Author contributions: F.G.B. and R.N.D. designed research; F.G.B., D.L.G., P.M., and Q.S. performed research; F.G.B. and L.M.M. analyzed data; and F.G.B. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: GPCR, G protein-coupled receptor; PG, prostaglandin; EP, prostaglandin E; EGFR, EGF receptor.
References
- 1.Perry, S. J. & Lefkowitz, R. J. (2002) Trends Cell Biol. 12, 130-138. [DOI] [PubMed] [Google Scholar]
- 2.Breyer, R. M., Bagdassarian, C. K., Myers, S. A. & Breyer, M. D. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 661-690. [DOI] [PubMed] [Google Scholar]
- 3.Regan, J. W. (2003) Life Sci. 74, 143-153. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan, F. G., Wang, D., Bargiacchi, F. & DuBois, R. N. (2003) J. Biol. Chem. 278, 35451-35457. [DOI] [PubMed] [Google Scholar]
- 5.Pai, R., Soreghan, B., Szabo, I. L., Pavelka, M., Baatar, D. & Tarnawski, A. S. (2002) Nat. Med. 8, 289-293. [DOI] [PubMed] [Google Scholar]
- 6.Gschwind, A., Zwick, E., Prenzel, N., Leserer, M. & Ullrich, A. (2001) Oncogene 20, 1594-1600. [DOI] [PubMed] [Google Scholar]
- 7.Pierce, K. L., Luttrell, L. M. & Lefkowitz, R. J. (2001) Oncogene 20, 1532-1539. [DOI] [PubMed] [Google Scholar]
- 8.Luttrell, L. M., Ferguson, S. S., Daaka, Y., Miller, W. E., Maudsley, S., Della Rocca, G. J., Lin, F., Kawakatsu, H., Owada, K., Luttrell, D. K., et al. (1999) Science 283, 655-661. [DOI] [PubMed] [Google Scholar]
- 9.Neuschafer-Rube, F., Hermosilla, R., Rehwald, M., Ronnstrand, L., Schulein, R., Wernstedt, C. & Puschel, G. P. (2004) Biochem. J. 379, 573-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurevich, V. V. & Gurevich, E. V. (2004) Trends Pharmacol. Sci. 25, 105-111. [DOI] [PubMed] [Google Scholar]
- 11.Lin, F. T., Miller, W. E., Luttrell, L. M. & Lefkowitz, R. J. (1999) J. Biol. Chem. 274, 15971-15974. [DOI] [PubMed] [Google Scholar]
- 12.Hupfeld, C. J., Resnik, J. L., Ugi, S. & Olefsky, J. M. (2005) J. Biol. Chem. 280, 1016-1023. [DOI] [PubMed] [Google Scholar]
- 13.Desai, S. & Ashby, B. (2001) FEBS Lett. 501, 156-160. [DOI] [PubMed] [Google Scholar]
- 14.Portera, C. A., Jr., Berman, R. S. & Ellis, L. M. (1998) Surg. Oncol. 7, 183-195. [DOI] [PubMed] [Google Scholar]
- 15.Kundu, N., Yang, Q., Dorsey, R. & Fulton, A. M. (2001) Int. J. Cancer 93, 681-686. [DOI] [PubMed] [Google Scholar]
- 16.Kozaki, K., Koshikawa, K., Tatematsu, Y., Miyaishi, O., Saito, H., Hida, T., Osada, H. & Takahashi, T. (2001) Oncogene 20, 4228-4234. [DOI] [PubMed] [Google Scholar]
- 17.Tsujii, M., Kawano, S. & DuBois, R. N. (1997) Proc. Natl. Acad. Sci. USA 94, 3336-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheng, H., Shao, J., Washington, M. K. & DuBois, R. N. (2001) J. Biol. Chem. 276, 18075-18081. [DOI] [PubMed] [Google Scholar]
- 19.Nie, D., Che, M., Zacharek, A., Qiao, Y., Li, L., Li, X., Lamberti, M., Tang, K., Cai, Y., Guo, Y., et al. (2004) Am. J. Pathol. 164, 429-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enomoto, A., Murakami, H., Asai, N., Morone, N., Watanabe, T., Kawai, K., Murakumo, Y., Usukura, J., Kaibuchi, K. & Takahashi, M. (2005) Dev. Cell 9, 389-402. [DOI] [PubMed] [Google Scholar]
- 21.Kim, D., Kim, S., Koh, H., Yoon, S. O., Chung, A. S., Cho, K. S. & Chung, J. (2001) FASEB J. 15, 1953-1962. [DOI] [PubMed] [Google Scholar]
- 22.Morales-Ruiz, M., Fulton, D., Sowa, G., Languino, L. R., Fujio, Y., Walsh, K. & Sessa, W. C. (2000) Circ. Res. 86, 892-896. [DOI] [PubMed] [Google Scholar]
- 23.Vivanco, I. & Sawyers, C. L. (2002) Nat. Rev. Cancer 2, 489-501. [DOI] [PubMed] [Google Scholar]
- 24.Kawai, N., Tsujii, M. & Tsuji, S. (2002) Prostaglandins Other Lipid Mediat. 68-69, 187-196. [DOI] [PubMed] [Google Scholar]
- 25.Singh, B., Berry, J. A., Shoher, A., Ramakrishnan, V. & Lucci, A. (2005) Int. J. Oncol. 26, 1393-1399. [PubMed] [Google Scholar]
- 26.Lefkowitz, R. J. & Shenoy, S. K. (2005) Science 308, 512-517. [DOI] [PubMed] [Google Scholar]