Abstract
WNK kinases are serine-threonine kinases with an atypical placement of the catalytic lysine. Intronic deletions with increased expression of a ubiquitous long WNK1 transcript cause pseudohypoaldosteronism type 2 (PHA II), characterized by hypertension and hyperkalemia. Here, we report that long WNK1 inhibited ROMK1 by stimulating its endocytosis. Inhibition of ROMK by long WNK1 was synergistic with, but not dependent on, WNK4. A smaller transcript of WNK1 lacking the N-terminal 1-437 amino acids is expressed highly in the kidney. Whether expression of the KS-WNK1 (kidney-specific, KS) is altered in PHA II is not known. We found that KS-WNK1 did not inhibit ROMK1 but reversed the inhibition of ROMK1 caused by long WNK1. Consistent with the lack of inhibition by KS-WNK1, we found that amino acids 1-491 of the long WNK1 were sufficient for inhibiting ROMK. Dietary K+ restriction decreases ROMK abundance in the renal cortical-collecting ducts by stimulating endocytosis, an adaptative response important for conservation of K+ during K+ deficiency. We found that K+ restriction in rats increased whole-kidney transcript of long WNK1 while decreasing that of KS-WNK1. Thus, KS-WNK1 is a physiological antagonist of long WNK1. Hyperkalemia in PHA II patients with PHA II mutations may be caused, at least partially, by increased expression of long WNK1 with or without decreased expression of KS-WNK1.
Keywords: dietary potassium intake, endocytosis, clathrin-coated vesicle, dynamin
WNK (with no lysine [K]) kinases are a new family of large serine-threonine protein kinases conserved in multicellular organisms with an atypical placement of the catalytic lysine. There are four mammalian WNK family members (1). WNK1, the first member identified, is >2,100 amino acids long (2). It contains an ≈270-aa kinase domain located near the amino terminus (e.g., amino acids 218-491 of the rat WNK1). WNK2, 3, and 4 are products of different genes and ≈1,200 to 1,600 amino acids in length (1, 2). The kinase domain of the four WNKs that share 85-90% sequence identity are unique in having the catalytic lysine located in the subdomain I instead of the conserved subdomain II of most protein kinases (1-3). Other conserved domains of WNK kinases include an autoinhibitory domain, 1-2 coiled-coil domains, and multiple PXXP prolinerich motifs for potential protein-protein interaction (1-4). Beyond the aforementioned conserved domains/motifs, sequence identity among the four WNKs is much lower and few homologous regions exist.
Pseudohypoaldosteronism type II (PHA II) is an autosomaldominant disease characterized by hypertension and hyperkalemia (5). Recently, Wilson et al. (6) reported that mutations of WNK1 and WNK4 cause PHA II. Mutations in the WNK1 gene are large deletions of the first intron leading to increased expression. Mutations in the WNK4 gene are missense mutations in the coding sequence outside the protein kinase domain. Several recent studies have examined the mechanisms for hypertension and hyperkalemia in PHA II patients. WNK4 inhibits the activity of the sodium chloride cotransporter. WNK4 mutants that cause disease fail to inhibit the sodium chloride cotransporter, suggesting that an increase in sodium chloride cotransporter activity in the distal convoluted tubule is a cause of hypertension (7, 8). Others have reported that WNK4 phosphorylates claudins 1-4, the tight-junction proteins involved in the regulation of paracellular ion permeability (9, 10). The paracellular chloride permeability is greater in cells expressing WNK4 mutants than in cells expressing wild-type proteins. Thus, hypertension in patients with WNK4 mutations may be caused by an increase in NaCl reabsorption through the Na-Cl cotransporter and the paracellular pathway. Wild-type WNK4 inhibits the ROMK1 channel and WNK4 mutants that cause disease exhibit increased inhibition of ROMK (11), suggesting that WNK4 mutations cause hyperkalemia by inhibiting ROMK.
Expression of WNK1 abolishes inhibition of the sodium chloride cotransporter caused by WNK4 in Xenopus oocytes (7), suggesting that WNK1 mutations cause hypertension by releasing WNK4-mediated inhibition of the cotransporter in the distal convoluted tubule. However, PHA II patients with WNK1 mutations are not particularly sensitive to thiazide diuretics (12). Moreover, patients with WNK1 mutations do not have hypercalciuria, whereas patients with WNK4 mutations have hypercalciuria that is ≈6-fold more sensitive to thiazide treatment than normal individuals (13, 14). A recent study by Xu et al. (15) shows that WNK1 activates SGK leading to activation of ENaC. Thus, hypertension in PHA II patents with WNK1 mutations may be caused by increased activity of Na-Cl cotransporter and ENaC. The mechanism for hyperkalemia in patients with WNK1 mutations is unknown.
Although WNK4 is expressed predominantly in kidney and several extrarenal epithelial tissues, WNK1 is widely expressed in multiple spliced forms (2, 16). A long WNK1 transcript (produced from 28 exons) encoding a polypeptide of >2,100 amino acids in length is expressed in all cell lines and tissues examined (2, 17-19). A shorter WNK1 transcript encoding a polypeptide (≈1,700 amino acids in length) lacking the amino terminal 1-437 amino acids of the long WNK1 is expressed highly in the kidney but not in other tissues (18, 19). The KS-WNK1 (KS, kidney-specific) is produced by replacing the first 4 exons with an alternative 5′ exon (exon 4A). The remaining exons 5-28 are the same as the long transcript. Quantitative analysis of WNK1 transcripts reveals that KS-WNK1 is expressed in kidney more abundantly than long WNK1 (≈85% vs. ≈15%) (18, 19). A large deletion of intron 1 causes increased expression of the long WNK1 isoform (6). Whether the expression of KS-WNK1 is altered in PHA II and the physiological role of KS-WNK1 are currently unknown.
K+ secretion by kidney is critical for controlling serum K+ levels and overall K+ homeostasis (20, 21). ROMK K+ channels present on the apical membrane of the distal renal tubules are important for baseline renal K+ secretion (20-23). Another type of K+ channels, maxi-K, are also present in the distal renal tubules and important for K+ secretion in response to increase in tubular fluid flow (23, 24). To maintain K+ homeostasis, the abundance of ROMK in the distal nephron decreases or increases during low or high dietary K+ intake, respectively (25, 26). Alteration of abundance of ROMK during changes of dietary K+ intake involves endocytosis and subsequent degradation of the channel protein (27, 28).
In the present study, we show that long WNK1 inhibits ROMK1, whereas KS-WNK1 reverses inhibition of ROMK1 caused by long WNK1. Dietary K+ restriction in rats increases the abundance of transcript for long WNK1 and decreases the abundance for KS-WNK1 in the kidney. These results suggest that the physiological role of KS-WNK1 as an antagonist of long WNK1. Furthermore, hyperkalemia in PHA II patients with WNK1 mutations may be caused, at least partially, by the inhibition of ROMK channels resulting from increased expression of long WNK1 with or without decreased expression of KS-WNK1.
Results
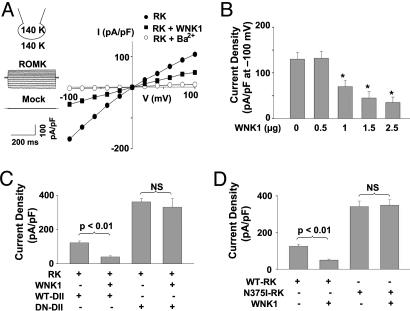
Long WNK1 Inhibits ROMK1 by Stimulating Endocytosis of the Channel. We examined regulation of ROMK1 by long WNK1 coexpressed in the human embryonic kidney (HEK) cells by using whole-cell patch-clamp recording. Cells transfected with ROMK1 exhibited the characteristic weak inward-rectifying Ba2+-sensitive K+ currents (Fig. 1A). K+ currents were not detected in mocktransfected cells (Fig. 1 A). Coexpression with long WNK1 inhibited K+ currents through ROMK1 (Fig. 1 A). Fig. 1B shows dose-dependent inhibition of ROMK1 current by long WNK1. The mean inward ROMK current density (at -100 mV) were 135 ± 14 pA/pF and 37 ± 13 pA/pF for without WNK1 and coexpression with 2.5-μg long WNK1 DNA, respectively (Fig. 1B). No further inhibition of ROMK1 was observed by coexpression with >2.5-μg WNK1 DNA (not shown). It has been reported that WNK4 regulates membrane trafficking of ion channels and transporters (7, 8, 11).
Fig. 1.
Effect of WNK1 on ROMK1 expressed in HEK cells. (A) Whole-cell recording, voltage-clamp protocol, and current-voltage (I-V) relationships of currents. (B) Dose-dependent inhibition of ROMK1 by WNK1. Cells were transfected with ROMK1 plus WNK1 (0-2.5 μg of plasmid DNA). In each experiment, the total amount of DNA for transfection was balanced by using empty vector. Ba2+-sensitive (after subtraction of residual currents in the presence of 10 mM Ba2+) inward current density is shown. *, P < 0.05 vs. ROMK1 alone. (C) Effect of wild-type (WT-DII) or dominant-negative (K44A) dynamin II (DN-DII) on WNK1 inhibition of ROMK1. (D) Effect of WNK1 on wild-type ROMK1 (WT-RK) vs. N375I ROMK1 mutant. Experiments above were repeated 3-5 times with similar results. NS, not significant.
ROMK1 undergoes clathrin-coated vesicle (CCV)-mediated endocytosis, a process believed to be an important mechanism for regulating K+ secretion in physiological and/or pathophysiological states (27, 28). To determine whether WNK1 inhibits ROMK1 by stimulating CCV-mediated endocytosis of the channel, we examined the effect of WNK1 on ROMK1 by coexpression with wild-type or a dominant-negative dynamin II. As reported previously, coexpression with dominant-negative dynamin II increased basal ROMK1 current density (Fig. 1C; 124 ± 10 pA/pF vs. 364 ± 20 pA/pF for coexpression with wild-type vs. dominant-negative dynamin II, respectively; P < 0.01), indicating that endocytosis of ROMK1 occurs in the basal state. Coexpression with dominant-negative dynamin II (lysine-44 to alanine; K44A) prevented the inhibition of ROMK1 by long WNK1. For comparison, coexpression with wild-type dynamin II had no effect on long WNK1-induced inhibition. Dynamin II may also be involved in internalization via caveolae (29). ROMK1 contains a canonical NPXY motif for internalization via CCV (30), and mutation of the critical asparagine-375 in the motif abolished endocytosis (28). We found that WNK1 failed to inhibit N375I mutant while inhibiting wild-type ROMK1 (Fig. 1D). Thus, WNK1 inhibits ROMK1 by stimulating endocytosis of the channel via CCV. The incomplete inhibition of ROMK by WNK1 (Fig. 1B) is probably due to limitation of endogenous endocytic machinery.
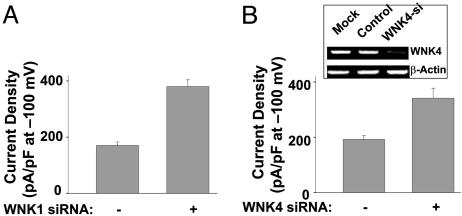
Endogenous WNK1 and WNK4 Are Important for Regulating ROMK1 Channels. WNK1 and WNK4 are widely expressed. In the next series of experiments, we examined whether endogenous WNK1 or WNK4 in HEK cells contributes to the basal endocytosis of ROMK1. We found that cells transfected by small interference RNA (siRNA) for WNK1 expressed ≈2.2 fold higher ROMK1 current density than cells transduced by control RNA oligonucleotide (current density: 380 ± 24 pA/pF vs. 170 ± 13 pA/pF for WNK1 siRNA vs. control RNA, respectively, P < 0.01; Fig. 2A). Knockdown of endogenous WNK1 by siRNA was confirmed by Western blot analysis of endogenous WNK1 in ref. 31. Similarly, cells transfected with WNK4 siRNA expressed higher ROMK1 currents (current density: 341 ± 36 pA/pF vs. 192 ± 15 pA/pF for WNK4 siRNA vs. control RNA, respectively, P < 0.01; Fig. 2B). Knockdown of endogenous WNK4 messenger RNA by siRNA was confirmed by reverse-transcription PCR analysis (Fig. 2B Inset). We could not consistently detect endogenous WNK4 protein by using the antibodies against WNK4 available to us.
Fig. 2.
Effect of WNK1 or WNK4 siRNA on ROMK1 expression in HEK cells. (A) Cells were cotransfected with ROMK1 (0.5 μg) plus WNK1 siRNA (200 nM in transfection mixture) or control oligonucleotide. (B) Cells were cotransfected with ROMK1 (0.5 μg) plus WNK4 siRNA (200 nM) or control oligonucleotide. (B Inset) mRNA expression of endogenous WNK4 analyzed by reversetranscription PCR. Cells were mock-transfected (Mock) or transfected with control oligos (Control) or siRNA for WNK4 (WNK4-si). Experiments above were repeated 2-3 times with similar results.
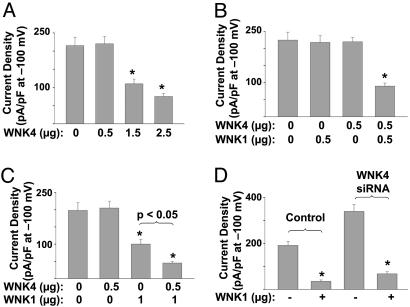
Regulation of ROMK1 by WNK1 Is Synergistic with but not Dependent on WNK4. It has been reported that WNK4 also inhibits ROMK by stimulating endocytosis (11). As shown in Fig. 3A, WNK4 inhibited ROMK1 dose-dependently. WNK4 did not inhibit N375I mutant (data not shown), confirming that endocytosis is involved. WNK1 interacts with WNK4 (7). We examined the relationships between WNK1 and WNK4 regulation of ROMK1. Although transfecting cells with 0.5 μg WNK1 plasmid DNA or WNK4 DNA alone did not cause inhibition of ROMK1, transfection with both WNK1 and 4 each at 0.5 μg DNA caused a significant inhibition of ROMK1 (Fig. 3B). Also, coexpression with 0.5 μg of WNK4 potentiated WNK1 inhibition of ROMK1 (Fig. 3C). These results indicate that WNK1 inhibits ROMK1 synergistically with WNK4. We further examined whether WNK1 inhibition of ROMK1 depends on WNK4 by knocking down endogenous WNK4. We found that WNK1 inhibited ROMK1 similarly in cells transfected with WNK4 siRNA and cells transfected with control oligonucleotide (Fig. 3D; 80% vs. 82% for siRNA vs. control, respectively), indicating that inhibition of ROMK1 by WNK1 does not depend on endogenous WNK4 in HEK cells.
Fig. 3.
Relationships between WNK1 and WNK4 regulation of ROMK1. (A) Dose-dependent inhibition of ROMK1 by WNK4. Cells were transfected with ROMK1 plus WNK4 (0-2.5 μg plasmid DNA). (B) Cells were cotransfected with ROMK1 plus 0.5 μg WNK1 and/or 0.5 μg WNK4. (C) Cells were cotransfected with ROMK1 plus 1 μg WNK1 and/or 0.5 μg WNK4. (D) Cells were cotransfected with ROMK1 (0.5 μg), WNK1 (2.5 μg), and WNK4 siRNA (200 nM) or control oligonucleotide. *, P < 0.05 vs. ROMK1 alone. Experiments above were repeated 3-5 times with similar results.
The Kinase Domain of WNK1 Is Required for Inhibition of ROMK1. We have recently shown that WNK1 activates serum- and glucocorticoid-inducible protein kinase SGK, leading to activation of the epithelial channel ENaC (15). The N-terminal 220 amino acids of WNK1 preceding the kinase domain are necessary and sufficient for activating SGK and ENaC. To determine whether the WNK1 kinase domain is necessary for regulating ROMK1, we compared the effects of amino acids 1-491 and 1-220 (Fig. 4A). We found that WNK1 (1-491) inhibited ROMK1 and the full-length WNK1, whereas WNK1 (1-220) had no effect (Fig. 4B), indicating that kinase domain is required for inhibition of ROMK1. Efficient expression of WNK1 (1-220) in HEK cells is shown in Fig. 4C. A construct containing kinase domain but no amino acids preceding it, WNK1 (218-491) did not cause inhibition of ROMK1 (data not shown). The level of expression of WNK1 (218-491) was comparable to that of WNK1 (1-491) (data not shown). Thus, both the WNK1 kinase domain and the preceding N terminus are required for regulation of ROMK1. Amino acids 486-555 of WNK1 contain an autoinhibitory domain to the kinase core (4) (Fig. 4A). Accordingly, WNK1 (1-555) construct does not exhibit kinase activity (4). Lysine-233 of WNK1 is the catalytic active lysine and WNK1 (1-491/K233M) is kinase-dead (2). We found that both WNK1 (1-555) and WNK1 (1-491/K233M) failed to inhibit ROMK1 (Fig. 4B). Efficient expression of each individual protein in cells was confirmed by Western blot analysis (Fig. 4C). Together, these results indicate that region of WNK1, including the kinase domain and preceding N terminus, contains amino acids sufficient for regulation of ROMK1.
Fig. 4.
Domain of WNK1 involved in regulation of ROMK1. (A) Domain structure of full-length long WNK1 and location of the catalytic lysine-233. Autoinhibitory domain (AID), kinase domain (KD), and N terminus preceding kinase domain (N-ter) are shown, but not drawn in scale. Fragments of WNK1 used are shown. (B) Cells were transfected with ROMK1 alone or cotransfected with indicated Myc-tagged WNK1 construct (each at 0.5 μg). Experiments above were repeated three times with similar results. *, P < 0.05 vs. ROMK1 alone. (C) Western blot analysis of each construct blotted by anti-Myc antibody. Arrowhead on the left indicates molecular mass in kilodaltons (kDa). Experiments above were repeated three times with similar results.
KS-WNK1 Does Not Inhibit ROMK1 but Reverses the Inhibition Caused by Long WNK1. The KS-WNK1 lacks the N-terminal 1-437 amino acids of the long WNK1, including most of the kinase domain (Fig. 5A) and is therefore kinase-defective. To examine whether the region of WNK1 beyond the kinase domain can inhibit ROMK1, we compared full-length WNK1, WNK1 (1-491), and KS-WNK1. We found that KS-WNK1 had no effect on ROMK1, whereas the full-length long WNK1 and WNK1 (1-491) both inhibited ROMK1 (Fig. 5B). These results support the idea that region of amino acids 1-491 of WNK1 is the only region necessary (and sufficient) for inhibition of ROMK1. The level of expression of KS-WNK1 in kidney is severalfold higher than that of long WNK1 (18, 19). We examined the hypothesis that KS-WNK1 may modulate long WNK1-induced inhibition of ROMK1 by coexpressing KS-WNK1 with ROMK1 plus fulllength WNK1 or with ROMK1 plus WNK1 (1-491). Interestingly, KS-WNK1 reversed inhibition of ROMK1 caused by full-length WNK1 (Fig. 5B), suggesting that it functions as an antagonist of long WNK1. Consistent with the finding that WNK1 (1-491) inhibits ROMK1 equally to the full-length WNK1, KS-WNK1 also reversed inhibition of ROMK1 caused by WNK1 (1-491) (Fig. 5B).
Fig. 5.
Role of KS-WNK1 in regulation of ROMK1. (A) Comparison of domain structure of long WNK1 (L-WNK1) with KS-WNK1. KS-WNK1 lacks the first 437 amino acids of L-WNK1 but contains unique 30 amino acids coded by an alternatively spliced exon 4A (the first exon of KS-WNK1). The vertical dotted line indicates position of amino acid in L-WNK1 equivalent to amino acid 31 of KS-WNK1. Amino acids of L-WNK1 and KS-WNK1 distal to the dotted line are identical. Amino acid 660 of L-WNK1 is equal to amino acid 253 of KS-WNK1. (B) Cells with transfected with ROMK alone or cotransfected with ROMK1 plus L-WNK1, WNK1 (1-491), and/or KS-WNK1 or KS-WNK1 (1-253) as indicated. (C) Lysates from mock-transfected cells or cells cotransfected with Myc-tagged WNK1 (1-491), Flag-tagged KS-WNK1 (1-253), and/or an unrelated Flag-tagged protein pod1 were immunoprecipitated by either anti-Myc or anti-Flag antibody and probed for Western blot analysis by the respective antibody as indicated. The molecular mass of Myc-WNK1 (1-491), Flag-KS-WNK1 (1-253), and Flag-pod1 are 60, 32, and 22 kDa, respectively (as indicated by arrowhead). Experiments above were repeated three times with similar results.
It has been reported that the region of amino acids 480-660 of long WNK1 interacts with the region of amino acids 1-491 (32). Amino acids 1-253 of KS-WNK1 contain the region of amino acids 480-660 of long WNK1 (Fig. 5A). We found that KS-WNK1 (1-253), like full-length KS-WNK1, was capable of reversing long WNK1-mediated inhibition of ROMK1 (Fig. 5B). KS-WNK1 (1-253) by itself had no effect on ROMK. We next examined the interaction between WNK1 (1-491) and KS-WNK1 (1-253) by coexpressing the respective Myc- and Flag-tagged proteins. As shown in Fig. 5C, anti-Myc antibody immunoprecipitated Myc-WNK1 (1-491) (indicated by a 60-kDa protein band), which coimmunoprecipitated Flag-KS-WNK1 (1-253) (indicated by a 32-kDa protein band). Pod1 is an unrelated transcription factor protein (33). As a control, Myc-WNK1 (1-491) did not coimmunoprecipitate Flag-pod1 [see lack of protein in 22 kDa position (predicted molecular mass of pod1); Fig. 5C]. Conversely, anti-Flag antibody immunoprecipitated Flag-KS-WNK1 (1-253), which coimmunoprecipitated Myc-WNK1 (1-491). Also, Flag-pod1 did not coimmunoprecipitate Myc-WNK1 (1-491) (Fig. 5C). These results suggest that KS-WNK1 antagonizes inhibition of ROMK1 by long WNK1 by binding to amino acids 1-491 of long WNK1.
Effects of Changes of Dietary K+ Intake on Expression of Long and KS-WNK1. ROMK channel in the apical membrane of the distal nephron is an exit pathway for baseline renal K+ secretion (20-22). To maintain K+ homeostasis, the abundance of ROMK in the apical membranes of distal nephron increases and decreases with high and low dietary K+ intake, respectively (25, 26). Dietary K+ restriction decreases the abundance of ROMK of cortical collecting ducts by stimulating endocytosis (27). To see whether the opposite regulation of ROMK1 by long and KS-WNK1 is physiologically important, we examined the expression of long and KS-WNK1 in rats fed K+-deficient (LK), control (CK) or high K+ (HK) diet. The abundance of transcript for K+-deficient and high K+ diet relative to the control K+ diet was determined by using quantitative real-time PCR. As shown in Fig. 6, the transcript for long WNK1 was apparently increased by feeding a K+-restriction diet (Fig. 6A; 115 ± 7% of the control, P = 0.05; mean ± SEM, n = 11 for each) but not significantly altered by a high K+ diet (88 ± 11% of the control, not significant). As reported in refs. 18 and 19, we found that KS-WNK1 is much more abundant than long WNK1 in rat kidney (91 ± 8% vs. 9 ± 4%). In contrast to the apparent increase for the long WNK1, the abundance of transcript for KS-WNK1 was decreased by K+-deficient diet (Fig. 6B,50 ± 6% of the control). Conversely, the transcript for KS-WNK1 was increased by high K+ diet (142 ± 10% of control). Besides the opposite direction of changes, the magnitude of changes of KS-WNK1 by variations of dietary K+ intake was much greater than that in long WNK1. The ratio (relative to control) of abundance of transcript for long over KS-WNK1 for K+-deficient and high K+ diet were calculated at 230% of control and 62% of control, respectively (Fig. 6C).
Fig. 6.
Effect of dietary K+ intake on long and KS-WNK1 expression in kidney. (A) Relative abundance of transcript (normalized to the control K+ diet) for long WNK1 in rat kidney fed low (LK), control (CK) or high K+ diet (HK). (B) Relative abundance for KS-WNK1. (C) Ratio of abundance of transcript for L-WNK1 vs. for KS-WNK1. Ratio relative to the control K+ diet is shown. *, P < 0.05 vs. CK.
Discussion
PHA II is an autosomal-dominant disease featured by hypertension and hyperkalemia (5, 6). A recent genetic study reported that mutations of WNK1 and WNK4 cause PHA II (6). Mutations in the WNK1 gene are large deletions of the first intron resulting in increased expression of the gene. How increased expression of WNK1 causes hyperkalemia is unknown. Schambelan et al. (34) found that infusion of sodium sulfate in substitution for NaCl ameliorates hyperkalemia in PHA II patients and proposed that hyperkalemia was due to reduced NaCl delivery to the cortical collecting ducts consequent to increased Cl- reabsorption in tubules proximal to the cortical collecting ducts. However, although all affected individuals develop hyperkalemia, only 50% of those develop hypertension (12-14). Also, hyperkalemia in PHA II of WNK1 and WNK4 mutations typically occurs ≈10 to 20 years before development of hypertension (12-14), suggesting that other mechanisms may also be involved. To support this observation, Kahle et al. (11) recently found that WNK4 inhibits ROMK. Here, we report that WNK1 inhibits ROMK1 by stimulating its endocytosis via CCV. These results suggest that inhibition of ROMK by WNK1 may contribute to hyperkalemia in PHA II with WNK1 mutations.
How does the finding of direct inhibition of K+ secretion through ROMK reconcile with the observation by Schambelan et al. (34)? Recent studies have found that maxi-K channels, although relatively quiescent at the basal state, are activated by an increase in the luminal fluid flow and predominantly responsible for flow-stimulated K+ secretion (23, 24). Thus, despite of inhibition of ROMK, increase in the distal Na+ and fluid delivery by infusion of nonreabsorbable sodium sulfate would stimulate K+ secretion through maxi-K channels to correct the hyperkalemia. Flow-stimulated K+ secretion through maxi-K can also explain the effectiveness of thiazide diuretics in ameliorating the hyperkalemia manifested by these patients (12, 13).
How does WNK1 inhibit ROMK1? Long WNK1 physically interacts with WNK4 (35), which also stimulates endocytosis of ROMK (11). Thus, it is interesting to know whether WNK1 regulates ROMK1 synergistically with WNK4. Our results indicate that WNK1 and WNK4 inhibit ROMK1 synergistically. However, knockdown of endogenous WNK4 by using siRNA does not affect inhibition of ROMK1 by WNK1, suggesting that regulation by WNK1 does not depend on WNK4. A presumed kinase-dead WNK4 mutant remains capable of inhibiting ROMK1, suggesting that kinase activity is not required (11). Our results indicate that inhibition of ROMK1 requires the WNK1 kinase domain. The WNK1 kinase domain, however, is not sufficient for regulating ROMK1; the N terminus before kinase domain is also required. The N terminus of WNK1 preceding the kinase domain shows little homology with WNK4. Thus, the mechanism by which WNKs stimulate endocytosis of ROMK1 cannot be deduced from sequence comparison alone. WNK1 and 4 may interact with different sets of proteins leading to stimulation of endocytosis of ROMK. Future experiments should focus on identification of these potential proteins.
WNK1 is expressed in multiple splice variants (2, 18, 19). At least two transcripts have been detected in kidney (18, 19). A longer transcript (≈12 kb in size) is ubiquitously expressed and encodes amino acids 1-491 necessary and sufficient for regulating ROMK1. A smaller kidney-specific transcript lacking the N-terminal 437 residues is highly expressed in kidney (18, 19). Leukocytes, in which increased WNK1 expression in PHA II were demonstrated (6), contain the ubiquitous long WNK1 but not the KS-WNK1 (18, 19). Limited by the availability of kidney tissue from patients with PHA II, the expression of KS-WNK1 has not been examined. Nevertheless, our results suggest that increased expression of the long WNK1 contributes to hyperkalemia in patients with WNK1 mutations. Expression of KS-WNK1 in PHA II, if altered, is likely reduced compared to the control. It has been shown that activation of ENaC by WNK1 requires the N-terminal amino acids 1-220 (15). Also, release of WNK4-mediated inhibition of Na-Cl cotransporter by WNK1 requires the kinase domain of WNK1 (35). Thus, it appears that hypertension in PHA II patients with WNK1 mutation is likely also caused by an increase in long WNK1, not KS-WNK1. Activation of ENaC and release of WNK4 inhibition of Na-Cl cotransporter likely contribute to pathogenesis of hypertension for patients with WNK1 mutations. These results of WNK1 on ENaC and Na-Cl cotransporter support our results on ROMK1. How deletion of intron 1 caused an increase in the expression of long WNK1 is not known. Interestingly, in contrast to the increased expression by large deletions of intron 1, insertion of a large piece of DNA (a ≈8-kb gene trap DNA) into intron 1 caused a decrease in the expression of long WNK1 in mice (36). Future experiments should examine the expression of KS-WNK1 in these mice.
The KS-WNK1 is expressed in the kidney much more abundantly than long WNK1 (18, 19). We found that KS-WNK1 does not inhibit ROMK1 but rather reverses the inhibition caused by long WNK1. Variations of dietary K+ intake in rats cause reciprocal changes of the long vs. KS-WNK1. These results suggest that KS-WNK1 functions as a physiological antagonist of the long WNK1. A recent report that KS-WNK1 inhibits long WNK1 regulation of Na-Cl cotransporter supports this idea (37). Dual control by both positive and negative regulators allows biological systems to respond to variations of external and/or internal milieu with much greater flexibility. Our findings that greater changes in the ratio of long vs. KS-WNK1 caused by variations of dietary K+ intake compared to changes in long or KS-WNK1 alone exemplifies this idea. Differential expression of WNK1 isoforms suggests that WNK1 will display widely variable tissue-specific regulatory properties depending on the forms that are present.
As a critical exit pathway for K+ secretion, ROMK channels are regulated by acute (such as arginine vasopressin) and long-term (such as dietary K+ intake) factors that affect K+ secretion (20-22). To maintain K+ homeostasis, the density of ROMK in cortical collecting ducts increases and decreases during high and low dietary K+ intake, respectively (25, 26). Low dietary K+ intake decreases ROMK, likely by stimulating endocytosis via CCVs followed by degradation via lysosomes (27, 28). Our results suggest that changes in the ratio of long vs. KS-WNK1 in kidney may, at least partly, mediate the regulation of ROMK by dietary K+ intake. Changes in the ratio of long vs. KS-WNK1 by dietary K+ intake occurred predominantly through changes of KS-WNK1. Thus, KS-WNK1 may be the primary mediator regulating renal K+ secretion in some settings.
Materials and Methods
DNA Constructs and siRNA for WNK1 and WNK4. GFP-ROMK1 was created by subcloning coding sequence of ROMK in-frame and downstream of GFP by using pEGFP-N3 vector. Full-length rat WNK1 and WNK4 (gifts of Melanie Cobb and Bing-e Xu, University of Texas Southwestern Medical Center) are in pCMV5-Myc vector (2, 15, 31, 32). WNK1 fragments were amplified by PCR with full-length rat WNK1 cDNA as the template and subcloned into pCMV5-Myc. KS-WNK1 (gift of Aniko Naray-Fejes-Toth, Dartmouth Medical School, Hanover, NH; ref. 38) was amplified by PCR and subcloned into pCMV5-myc vector. Fragments of KS-WNK1 were amplified by PCR and subcloned into a C-terminal Flag vector (pIRES-hrGFP-1a) (Stratagene). Point mutation was generated by site-directed mutagenesis (QuikChange kit, Stratagene) and confirmed by sequencing. Sense and antisense oligonucleotide for WNK1 siRNA were 5′-UGU CUA ACG AUG GCC GCU U dT dT and 5′-AAG CGG CCA UCG UUA GAC A dT dT, respectively. Sense and antisense oligonucleotide for WNK4 siRNA were CGG GCA CGC UCA AGA CGU AUU and 5′-P UAC GUC UUG AGC GUG CCC GUU, respectively.
Tissue Culture, Immunoprecipitation, and Western Blots. HEK293 cells were maintained in Dulbecco's modified Eagle's medium with 10% FBS/2 mM l-glutamine/100 units/ml penicillin/steptomycin and transfected as described in ref. 15. Transfected cells were harvested in isotonic lysis buffer containing 1% Triton X-100 and phosphatase and protease inhibitors as described in ref. 15. For coimmunoprecipitation experiments, cells were lysed with a Dounce homogenizer in buffer lacking Triton X-100. Proteins were immunoprecipitated from cell lysates by using monoclonal anti-Myc or anti-Flag (2, 15, 31, 32) antibodies at 1:100 dilution. For Western blots, total lysates or immunoprecipitates were resolved by SDS/PAGE and proteins were transferred onto nitrocellulose membranes. The membranes were incubated with the indicated anti-bodies and developed by using enhanced chemiluminescence.
Whole-Cell Patch-Clamp Recording of ROMK Channels. HEK cells were transfected with cDNAs (0.5 μg each) for GFP-ROMK. As indicated, cells were cotransfected with long WNK1, WNK4, KS-WNK1, wild-type, and/or dominant-negative rat dynamin II (gifts of Joseph Albanesi, University of Texas Southwestern Medical Center). In each experiment, the total amount of DNA for transfection was balanced by using empty vectors. Approximately 36-48 h after transfection, whole-cell currents were recorded by using an Axopatch 200B amplifier as described in refs. 15 and 39. Transfected cells were identified by using epifluorescent microscopy. The bath and pipette solution contained 145 mM KCl, 2 mM MgCl2,2 mM CaCl2, 10 mM Hepes (pH 7.4) and 145 mM KCl, 2 mM EDTA, 10 mM Hepes (pH 7.4), respectively. Capacitance and access resistance were monitored and 75% compensated. The voltage protocol consists of 0 mV holding potential and 400 ms steps from -100 to 100 mV in 20-mV increments. Statistical comparison was made by using the unpaired Student t test.
Experimental Animals, Diets, and Real-Time PCR. Male or female Sprague-Dawley rats (150-200 g) were used for the study. To study the effects of low dietary K+ intake, rats were pair-fed either a K+-deficient diet (no added K+; TD95006, Harlan TEKLAD, Madison, WI), a control K+ diet (K+ content 6.7 g/kg in KCl; TD88238) or a high K+ diet (K+ content 49.5 g/kg; TD94121) for 48-72 h as described in ref. 27. All animals were allowed to drink distilled water freely. Food intake and body weight were measured daily. Kidneys from control and K+-deficient rats were dissected immediately after killing. After homogenization, RNA was extracted from kidney by using RNAzol (Invitrogen) according to the manufacturer's instructions. Two hundred nanograms was used for RT with the TaqMan RT kit (Applied Biosystems). Real-time quantitative PCR was performed on ABI 7000 as described in Applied Biosystems User Bulletin no. 2 by using the TaqMan assay. Forward and reverse primers and TaqMan probe from the 5′ sequence of exon1 (WNK1 P1 forward, 5′-GGC ACT CCT GGC TTC CTT TC-3′; P1 reverse, 5′-ATC GGA GCT TGA GCC ATT CTT-3′; P1 TaqMan probe, 5′-CCT CCG GCT CCA GTC-3′) were used to amplify the full-length WNK1 isoform under P1 promoter control (18, 19). Primer from exon 4a (RP forward, 5′-GCT GCT GTT CTC AAA AGG ATT GTA T-3′) and from exon 5 (RP reverse, 5′-CAG GAA TTG CTA CTT TGT CAA AAC TG-3′) and TaqMan probe (5′-TGA GGG AGT GAA GCC A-3′) were used to amplify the kidney-specific isoform (18, 19). Relative WNK1 mRNA levels were calculated with 18s rRNA as the internal control.
Acknowledgments
We thank Drs. M. Cobb and B. Xu for WNK1 and WNK4 constructs and for discussion; Dr. M. Baum for reading of the manuscript; Dr. G. He (University of Texas Southwestern Medical Center) for the N375I point mutant of ROMK1; Drs. P. Igarashi and T. Hiesberger (University of Texas Southwestern Medical Center) for the Flag-pod1 construct; and members of the Huang laboratory for discussion. This work was supported, in part, by National Institute of Health Grants DK-54368, DK-59530, and DK-59530S1 (to C.-L.H.).
Author contributions: A.L., Z.L., and C.-L.H. designed research; A.L. and Z.L. performed research; A.L., Z.L., and C.-L.H. analyzed data; and C.-L.H. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: CCV, clathrin-coated vesicle; HEK, human embryonic kidney; KS, kidney-specific; PHA II, pseudohypoaldosteronism type II; siRNA, small interference RNA.
References
- 1.Verissimo, F. & Jordan, P. (2001) Oncogene 20, 5562-5569. [DOI] [PubMed] [Google Scholar]
- 2.Xu, B., English, J. M., Wilsbacher, J. L., Stippec, S., Goldsmith, E. J. & Cobb, M. H. (2000) J. Biol. Chem. 275, 16795-16801. [DOI] [PubMed] [Google Scholar]
- 3.Min, X., Lee, B. H., Cobb, M. H. & Goldsmith, E. J. (2004) Structure (London) 12, 1303-1311. [DOI] [PubMed] [Google Scholar]
- 4.Xu, B., Min, X., Stippec, S., Lee, B. H., Goldsmith, E. J. & Cobb, M. H. (2002) J. Biol. Chem. 277, 48456-48462. [DOI] [PubMed] [Google Scholar]
- 5.Gordon, R. D. (1986) Hypertension 8, 93-102. [DOI] [PubMed] [Google Scholar]
- 6.Wilson, F. H., Disse-Nicodeme, S., Choate, K. A., Ishikawa, K., Nelson-Williams, C., Desitter, I., Gunel, M., Milford, D. V., Lipkin, G. W., Achard, J. M., et al. (2001) Science 293, 1107-1112. [DOI] [PubMed] [Google Scholar]
- 7.Yang, C. L., Angell, J., Mitchell, R. & Ellison, D. H. (2003) J. Clin. Invest. 111, 1039-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson, F. H., Kahle, K. T., Sabath, E., Lalioti, M. D., Rapson, A. K., Hoover, R. S., Hebert, S. C., Gamba, G. & Lifton, R. P. (2003) Proc. Natl. Acad. Sci. USA 100, 680-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamauchi, K., Rai, T., Kobayashi, K., Sohara, E., Suzuki, T., Itoh, T., Suda, S., Hayama, A., Sasaki, S. & Uchida, S. (2004) Proc. Natl. Acad. Sci. USA 101, 4690-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahle, K.T., MacGregor, G. G., Wilson, F. H., Van Hoek, A. N., Brown, D., Ardito, T., Kashgarian, M., Giebisch, G., Hebert, S. C., Boulpaep, E. L. & Lifton, R. P. (2004) Proc. Natl. Acad. Sci. USA 101, 14877-14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahle, K. T., Wilson, F. H., Leng, Q., Lalioti, M. D., O'Connell, A. D., Dong, K., Rapson, A. K., MacGregor, G. G., Giebisch, G., Hebert, S. C., et al. (2003) Nat. Genet. 35: 372-376. [DOI] [PubMed] [Google Scholar]
- 12.Disse-Nicodeme, S., Achard, J. M., Desitter, I., Houot, A. M., Fournier, A., Corvol, P. & Jeunemaitre, X. (2000) Am. J. Hum. Genet. 67, 302-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayan, H., Vered, I., Mouallem, M., Tzadok-Witkon, M., Pauzner, R. & Farfel, Z. (2002) J. Clin. Endocrinol. Metab. 87, 3248-3254. [DOI] [PubMed] [Google Scholar]
- 14.Achard, J. M., Warnock, D. G., Disse-Nicodeme, S., Fiquet-Kempf, B., Corvol, P., Fournier, A. & Jeunemaitre, X. (2003) Am. J. Med. 114, 495-498. [DOI] [PubMed] [Google Scholar]
- 15.Xu, B.-e., Stippec, S., Chu, P.-Y., Lazrak, A., Li, X.-J., Lee, B.-H., English, J. M., Ortega, B., Huang, C.-L. & Cobb, M. H. (2005) Proc. Natl. Acad. Sci. USA 102, 10315-10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahle, K. T., Gimenez, I., Hassan, H., Wilson, F. H., Wong, R. D., Forbush, B., Aronson, P. S. & Lifton, R. P. (2004) Proc. Natl. Acad. Sci. USA 101, 2064-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu, Q., Modrek, B. & Lee, C. (2002) Nucleic Acids Res. 30, 3754-3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delaloy, C., Lu, J., Houot, A. M., Disse-Nicodeme, S., Gasc, J. M., Corvol, P. & Jeunemaitre, X. (2003) Mol. Cell. Biol. 23, 9208-9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Reilly, M., Marshall, E., Speirs, H. J. & Brown, R. W. (2003) J. Am. Soc. Nephrol. 14, 2447-2456. [DOI] [PubMed] [Google Scholar]
- 20.Giebisch. G. (1995) Kid. Int. 48, 1004-1009. [DOI] [PubMed] [Google Scholar]
- 21.Hebert, S. C. (1995) Kid. Int. 48, 1010-1016. [DOI] [PubMed] [Google Scholar]
- 22.Hebert, S. C., Desir, G., Giebisch, G. & Wang, W. (2005) Physiol. Rev. 85, 319-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woda, C. B., Bragin, A., Kleyman, T. R. & Satlin, L. M. (2001) Am. J. Physiol. 280, F786-F793. [DOI] [PubMed] [Google Scholar]
- 24.Pluznick, J. L., Wei, P., Grimm, P. R. & Sansom, S. C. (2005) Am. J. Physiol. 288, F846-F854. [DOI] [PubMed] [Google Scholar]
- 25.Wang, W. (2004) Annu. Rev. Physiol. 66, 547-569. [DOI] [PubMed] [Google Scholar]
- 26.Palmer, L. G., Antonian, L. & Frindt, G. (1994) J. Gen. Physiol. 104, 693-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu, P.-Y., Quigley, R., Babich, V. & Huang, C.-L. (2003) Am. J. Physiol. 285, F1179-F1187. [DOI] [PubMed] [Google Scholar]
- 28.Zeng, W.-Z., Babich, V., Ortega, B., Quigley, R., White, S. J., Welling, P. A. & Huang, C.-L. (2002) Am. J. Physiol. 283, F630-F639. [DOI] [PubMed] [Google Scholar]
- 29.Dessy, C., Kelly, R. A., Balligand, J. L. & Feron, O. (2000) EMBO J. 19, 4272-4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen, W. J., Goldstein J. L. & Brown, M. S. (1990) J. Biol. Chem. 265, 3116-3123. [PubMed] [Google Scholar]
- 31.Xu, B.-e., Stippec, S., Lazrak, A., Huang, C.-L. & Cobb, M. H. (2005) J. Biol. Chem. 280, 34218-34223. [DOI] [PubMed] [Google Scholar]
- 32.Lenertz, L. Y., Lee, B. H., Min, X., Xu, B. E., Wedin, K., Earnest, S., Goldsmith, E. J. & Cobb, M. H. (2005) J. Biol. Chem. 280, 26653-26658. [DOI] [PubMed] [Google Scholar]
- 33.Quaggin, S. E., Schwartz, L., Cui, S., Igarashi, P., Deimling, J., Post, M. & Rossant, J. (1999) Development (Cambridge, U.K.) 126, 5771-5783. [DOI] [PubMed] [Google Scholar]
- 34.Schambelan, M., Sebastian, A. & Rector, F. C., Jr. (1981) Kidney Int. 19, 716-727. [DOI] [PubMed] [Google Scholar]
- 35.Yang, C. L., Zhu, X., Wang, Z., Subramanya, A. R. & Ellison, D. H. (2005) J. Clin. Invest. 115, 1379-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zambrowicz, B. P., Abuin, A., Ramirez-Solis, R., Richter, L. J., Piggott, J., BeltrandelRio, H., Buxton, E. C., Edwards, J., Finch, R. A., Friddle, C. J., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 14109-14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subramanya, A.R., Yang, C. L., Zhu, X. & Ellison, D. H. (October 4, 2005) Am. J. Physiol., 10.1152/ajprenal.00280.2005. [DOI]
- 38.Naray-Fejes-Toth, A., Snyder, P. M. & Fejes-Toth, G. (2004) Proc. Natl. Acad. Sci. USA 101, 17434-17439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeh, B. I., Sun, T. J., Lee, J. Z., Chen, H. H. & Huang, C. L. (2003) J. Biol. Chem. 278, 51044-51052. [DOI] [PubMed] [Google Scholar]