Abstract
Trisomy 21 is the cause of Down's syndrome (DS) which is characterized by a number of phenotypes, including a brain which is small and hypocellular compared to that of euploid individuals. The cerebellum is disproportionately reduced. Ts65Dn mice are trisomic for orthologs of about half of the genes on human chromosome 21 and provide a genetic model for DS. These mice display a number of developmental anomalies analogous to those in DS, including a small cerebellum with a significantly decreased number of both granule and Purkinje cell neurons. Here we trace the origin of the granule cell deficit to precursors in early postnatal development, which show a substantially reduced mitogenic response to Hedgehog protein signaling. Purified cultures of trisomic granule cell precursors show a reduced but dose-dependent response to the Sonic hedgehog protein signal in vitro, demonstrating that this is a cell-autonomous deficit. Systemic treatment of newborn trisomic mice with a small molecule agonist of Hedgehog pathway activity increases mitosis and restores granule cell precursor populations in vivo. These results demonstrate a basis for and a potential therapeutic approach to a fundamental aspect of CNS pathology in DS.
Keywords: aneuploidy, neuronal deficit, sonic hedgehog, trisomy 21
Trisomy for human chromosome 21 (Chr21) is the most frequent genetic cause of mental retardation, and results in additional phenotypes that affect nearly every organ system in the body (1). Down's syndrome (DS) is associated with abnormal cognitive and language development, learning and memory impairments, and significant behavioral alterations (2). Total brain volume is reduced, and the cerebellum is reduced to an even greater degree (3, 4). The small, hypocellular brain exhibits a number of neuropathological changes including a paucity of granule cells in the cortex, abnormalities in dendritic spine morphology in the visual cortex, atypical synaptic density and length, and age-related neuronal degeneration (5–7).
Segmental trisomy for a segment of mouse Chr16 that is orthologous to human Chr21 and present in the T(1716)65Dn marker chromosome (Ts65Dn) (8), produces a number of features that directly parallel those in DS. These phenotypes include impairments in learning and memory (9–11), age-related degeneration of basal forebrain cholinergic neurons (12), abnormal synaptic structures (13, 14), fewer granule cell neurons and reduced cell proliferation in the dentate gyrus (H. Lorenzi and R.H.R., unpublished data, and refs. 15 and 16), and comparable anomalies in the craniofacial skeleton, mandible, and cranial vault (17). Additionally, Ts65Dn mice exhibit a significant reduction in size of the cerebellum measured as normalized volume or cross-sectional area at the midline (18, 19).
The cerebellum in Ts65Dn mice shows a significant reduction of both Purkinje cells and of the granule cell neurons (GC) whose cell bodies compose the internal granule layer (IGL). The resulting reduction in GC density in Ts65Dn mice suggested that a corresponding deficit might occur in humans with trisomy 21, which was confirmed (18), thus extending our understanding of DS. The parallel effect indicates that trisomy in Ts65Dn and in DS disturbs the same evolutionarily conserved genetic pathways in mouse and human with comparable results and validates the use of the mouse model to study this aspect of DS.
Results
During postnatal murine cerebellar development, granule cell precursors (GCP) near the surface of the cerebellum in the external germinal layer (EGL) proliferate, and their progeny migrate inward and differentiate to form the IGL (20). Purkinje cells mature and form a single cell layer at the interface of the IGL and the molecular layer. To characterize the etiology of the GCP deficit caused by trisomy, we surveyed overall growth of the Ts65Dn mouse and that of the brain and the cerebellum at seven time points beginning at the day of birth (postnatal day 0, P0) to determine the earliest point at which structural changes differentiate Ts65Dn and euploid cerebella.
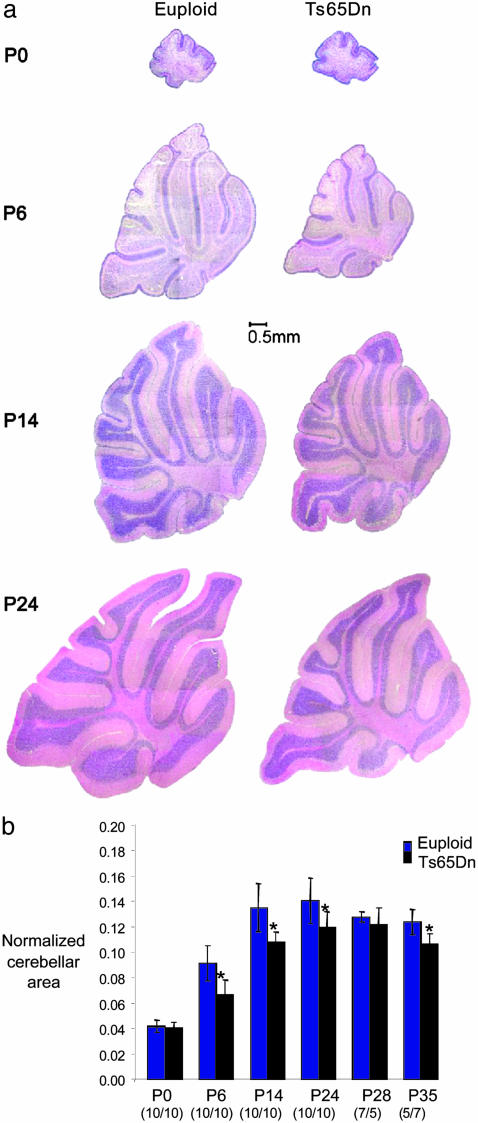
Delayed development of the trisomic cerebellum was revealed by comparison of several parameters in sagittal brain sections of 80 trisomic and 132 euploid mice at seven developmental time points from P0 to adult (Table 1). The trisomic cerebellum was the same size as euploid on the day of birth (P0), but significantly smaller than euploid by P6, and it remained smaller throughout development and into adulthood (Fig. 1). The EGL showed a similar pattern, with a similar thickness in euploid and trisomic mice at P0 but a reduced thickness in Ts65Dn by P6. The EGL persists similarly in trisomic and euploid mice, finally disappearing between P14 and P24 in both (Fig. 1a). The IGL was significantly reduced in thickness in midline sagittal sections from P14 to adulthood. In addition, GC density in the IGL was significantly reduced in trisomic mice from P6, the earliest point at which it could be measured, thus presaging the previously reported GC deficit in adult Ts65Dn mice and in DS (18).
Table 1. Reduced growth of Ts65Dn cerebellum during postnatal development.
| Age | Normalized cerebellar area, % | EGL thickness, % | IGL thickness, % | GC density, % |
|---|---|---|---|---|
| P0 | 96.7 | 93.9 | NA | NA |
| P6 | 73.1** | 76.9** | NA | 82* |
| P14 | 79.8** | NA | 85.2** | 87.4* |
| P24 | 85.2* | NA | 85.1** | 91** |
| P28 | 94.8 | NA | 85.0* | 92* |
| P35 | 86.0* | NA | 76.5* | 94 |
| Adult | 81.7* | NA | 86.8* | 76** |
Ts65Dn values are expressed as percentage of euploid. n = 10 for each genotype at each time point for P0, P6, P14, and Adult. At P24, Euploid, n = 6, Ts65Dn, n = 6. At P28 and P35, Euploid, n = 7, Ts65Dn, n = 7. NA, not applicable. *, Statistically significant by Student's test, P ≤ 0.05; **, statistically significant by Student's t test, P ≤ 0.001. Adult data are from Baxter et al. (18).
Fig. 1.
Ts65Dn cerebellum is the same size as euploid at birth, but shows specific growth deficits by P6. (a) Midline sagittal sections demonstrate grossly normal morphology but diminished growth in Ts65Dn cerebellum compared to euploid. The EGL is visible in posterior folia of both genotypes at P14 but has disappeared by P24. (b) Graphic representation of the midline cerebellar area as a fraction of total brain. An asterisk indicates a significant difference (P ≤ 0.05, Student's t test). The number of animals of each genotype is indicated.
These observations indicate that the reduced size and GC number in the adult Ts65Dn cerebellum originates shortly after birth when GCP are rapidly dividing in the EGL. Reduced cerebellar growth in trisomic mice could be due to increased apoptosis or decreased mitosis of GCP, or because of a small change in the number of precursors in the densely packed EGL at birth, which might not have been recognizable in the initial histological assessment. Analysis of midline sagittal sections at P6 by TUNEL assay (n = 5 Ts65Dn, 5 euploid) revealed very few or no apoptotic cells in the EGL in either trisomic or euploid mice (data not shown). However, the number of mitotic figures per unit length of the EGL was reduced in trisomic mice to 69% of euploid (P < 0.01, n = 9 Ts65Dn, 9 euploid).
To precisely quantify the number of GCP and mitotic GCP in the EGL at P0 and P6, we analyzed the entire cerebellum using unbiased stereology (21). At P0, the number of GCP was the same in euploid and Ts65Dn mice, but the number of mitotic GCP was significantly reduced in Ts65Dn, to 79% of mitotic GCP in euploid littermates (P = 0.05, Table 2). The reduced rate of mitosis at P0 resulted in a highly significant GCP reduction by P6, when euploid mice had 7.4 × 106 GCP vs. 5.3 × 106 cells in Ts65Dn (P = 0.001). Not surprisingly, the number of mitotic cells in trisomic mice at P6 was also reduced compared to euploid.
Table 2. Mitotic deficit in Ts65Dn granule cell precursors.
| Age | Strain (n) | GC precursors | Mitotic cells | Mitotic index, % |
|---|---|---|---|---|
| P0 | Euploid (6) | 951,995 (45,676) | 16,970* (1,790) | 1.8 |
| P0 | Ts65Dn (6) | 931,976 (62,961) | 13,354* (1,011) | 1.4 |
| P6 | Euploid (5) | 7,381,391† (144,660) | 135,359‡ (15,125) | 1.8 |
| P6 | Ts65Dn (5) | 5,269,919† (474,410) | 98,781‡ (13,184) | 1.9 |
Cell number is given as average and (s.e.). Significant differences between euploid and trisomic mice are indicated (†, P = 0.001; *, P = 0.05; and ‡, P = 0.04). At P0, n = 6 each for euploid and Ts65Dn; at P6, n = 5 for each.
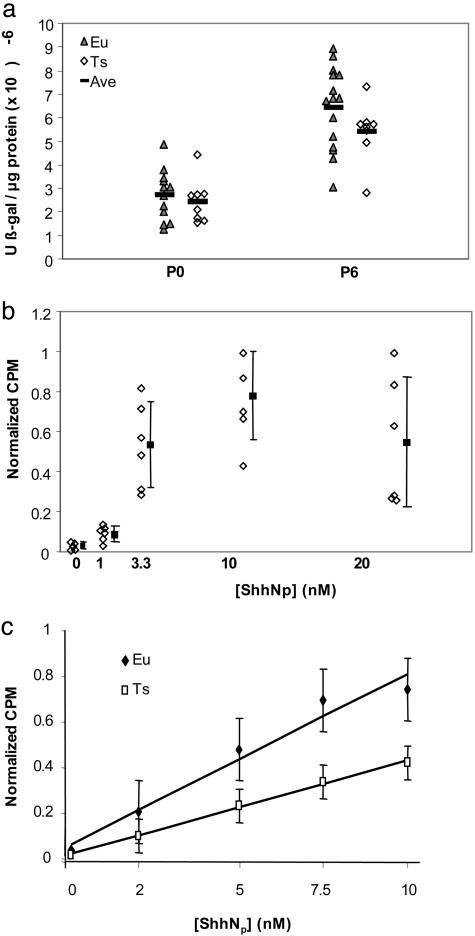
Sonic hedgehog (Shh) produced by Purkinje cells is a potent mitogen for GCP (22–24) and activates transcription of several genes, including Ptc. To determine whether the in vivo response of GCP to Shh is affected by trisomy, we crossed Ts65Dn to Ptc+/- transgenic mice in which lacZ coding sequences are inserted in frame with the Ptc ORF; β-galactosidase expression thus provides an indicator of Ptch transcription (25), which is up-regulated by Hedgehog (Hh) pathway activation. Although β-galactosidase activity normalized to total protein in cerebellum showed little difference at P0 (P < 0.28), the levels of β-galactosidase activity at P6 were reduced to 84% of euploid levels in trisomic mice (P = 0.08) (Fig. 2a). This reduced level of Ptc–lacZ expression, even in the presence of a reduced dosage of the pathway-suppressing Ptch protein, suggested that an impaired response to Shh in vivo may affect the growth of the trisomic Ts65Dn cerebellum.
Fig. 2.
Trisomic GCP are less responsive to Shh in vivo and in vitro. (a) Ptc is a target for increased transcription after Shh stimulation. β-galactosidase activity was measured as a function of total protein in euploid (Eu) or trisomic (Ts) cerebellum from Ptc-lacZ+/- mice. No difference was seen at P0 (P < 0.28, n = 8 Ts65Dn, 12 euploid). However, β-galactosidase activity in trisomic, Ptc-lacZ+/- mice was reduced to 84% of euploid levels at P6 (P = 0.08, n = 8 Ts65Dn, 15 euploid). (b) Mitogenic effect of dually lipidated ShhNp on euploid P6 GCP. [3H]dT-incorporation increased with ShhNp concentrations from 0 to 10 nM, but no further increase was seen at 20 nM. Error bars represent the standard deviation. (c) Average of six experiments showing a linear dose-response of mitogenesis to ShhNp in replicate euploid (filled diamonds) and trisomic (open boxes) GCP cultures. At each concentration of ShhNp, the trisomic response was significantly lower than euploid (P < 10-6). Total counts from multiple replicates at each concentration were normalized to the highest response at 10 nM ShhNp and averaged across six experiments (120 measurements each for euploid and trisomic cultures). Bars represent standard error.
Reduced Hh pathway activity in trisomic mice could result from impaired production or movement of the Shh signal from Purkinje cells or from a reduced response to Shh by GCP in the EGL. To test for reduced response, we isolated and cultured cerebellar GCP from euploid and trisomic mice at P6 and measured their ability to proliferate in response to Shh signal as indicated by incorporation of [3H]dT (22). We first tested the response of cultured GCP to ShhNp, the dually lipidated, naturally occurring form of the signal, which is significantly more potent than unmodified ShhN in cultured cell assays (26). After 48 h of culture, we saw almost no proliferation of cells in the absence of Shh, but noted significant proliferation in response to a range of Shh signal concentrations (Fig. 2b). A maximal response was noted at 10 nM ShhNp, a concentration substantially lower than that previously reported with unmodified Shh. Higher concentrations of ShhNp did not increase the mitogenic response, suggesting that the effect was saturated between 10 and 20 nM. Based on these results, we used concentrations of ShhNp ranging up to 10 nM for subsequent dose–response assays in GCP isolated from trisomic and euploid mice.
The ShhNp dose-response assay (Fig. 2b) was repeated six times, with pools of P6 GCP from at least two or more trisomic or euploid mice and with three to six replicates of euploid and trisomic GCP in each experiment (Fig. 2c). Extensive use of timed pregnancies was required to generate sufficient mice for these experiments, as Ts65Dn mice have small litters and a marked loss of trisomic offspring in the first 6 days after birth (27). Two important results were noted in these dose–response experiments. First, the mitogenic response of trisomic GCP was significantly lower than that of euploid cells at all concentrations of ShhNp, revealing a cell-autonomous deficit in the trisomic cells (Fig. 2c) (Student's t test P values <6 × 10-6 for 2, 5, 7.5, and 10 nM ShhNp). Second, although the mitogenic response of trisomic GCP was lower than that for euploid GCP, the trisomic cells did respond in a dose-dependent manner. Thus, in six independent experiments, the mitogenic response of Ts65Dn GCP at 10 nM ShhNp was comparable to the response of euploid cells at 4–5 nM ShhNp.
The ability of trisomic GCP to respond to Hh pathway stimulation, albeit in an attenuated manner, suggested that this response deficit might be overcome in vivo by increasing endogenous Hh pathway response. A series of related small molecule agonists has been described that trigger Hh pathway activity by binding to and activating the Smoothened component of the Hh response pathway (28, 29). These molecules cross the blood–brain barrier and produce, either alone or in synergy with Hh ligands, the effects of normal pathway stimulation in vitro and in vivo, including activation of reporter constructs (28, 29), induction of motor neuron differentiation (30), mitogenic stimulation of neural precursors in vivo (31), and even the substantial rescue of a deficit of Shh signal in utero (28).
We injected 20 μg/g of the Hh pathway agonist, SAG 1.1 (32), into newborn trisomic and euploid pups in a triolein vehicle. In contrast to the situation in untreated trisomic mice, in which the numbers of GCP and mitotic GCP were only ≈70% of those in euploid mice at P6 (Table 2), treatment with SAG 1.1 restored the numbers of both GCP and mitotic cells to euploid levels at P6 (Fig. 3 a and b). A trend toward increased GCP and mitotic GCP was also seen in euploid mice treated with SAG 1.1 (Fig. 3b).
Fig. 3.
Mitotic and GCP deficits of trisomic mice are reversed by injection of a Shh pathway agonist. Progeny of Ts65Dn mothers received a s.c. injection of 20 μg/g SAG 1.1 Shh agonist (32) on the day of birth. The animals were killed, genotyped, and assessed by stereology at P6. ANOVA with multiple comparisons was used to analyze results. (a) For GCP, the trisomic agonist, euploid vehicle, and euploid untreated groups were not different from each other, but were significantly increased relative to untreated trisomic mice (F = 5.6, P = 0.009, α = 0.05). (b) For mitotic cells, the trisomic agonist group was significantly different from trisomic untreated mice (F = 3.06, P = 0.06, α = 0.05), but not different from euploid or euploid vehicle groups.
Discussion and Conclusions
Our results indicate that failure to generate sufficient progeny from GCP is an important component of the GC deficit associated with reduced cerebellar size in adult Ts65Dn mice. On the day of birth, the number of progenitors is identical, but the number of mitotic GCP is significantly reduced in trisomic mice. By P6, the total number of precursor cells has been compromised such that normal levels of GCP production are not achieved in Ts65Dn mice. We show that an intrinsic deficit in the response of trisomic GCP to Shh underlies the reduced generation of GC in Ts65Dn mice. Introduction of a Shh pathway agonist early in development stimulated mitosis of GCP and corrected this deficit, such that the number of GCP and the rate of mitosis were normal 1 week after treatment.
The mouse cerebellum attains about a third of its adult size in the first week after birth, the period in which GCP populations and the rate of mitosis of those cells were restored in this study. Given the restoration to normal levels of both progenitors and the number of those cells that are mitotic at P6, it is likely that the effects of the single dose of SAG1.1 given at P0 would extend substantially farther into development. Cerebellar growth is mostly complete 5–6 weeks after birth in mouse, although remodeling continues. GC numbers decline slightly from 5 weeks as synaptogenesis is completed. The corresponding developmental processes do not coincide exactly in human beings, but occur roughly from late gestation through infancy.
Trisomy for human Chr21 is among the most complex genetic challenges compatible with substantial survival beyond term. In this study, a cerebellar phenotype of adult Ts65Dn mice that directly parallels an analogous deficit in DS was traced to a precise stage of development and to an intrinsic deficit in response to Hh signaling by GCP, a process critical for the generation of GC. This defect suggests the now testable hypothesis that one or more of the ≈130 clearly identified genes present within the trisomic segment of Chr16 in Ts65Dn mice (33) may function in the Hh signaling pathway.
The identification and reversal of a deficit in Hh pathway activity in trisomic mice was based on recognition of the disrupted cellular process through detailed analysis of the developmental timing and identification of affected cells. Similar information may illuminate the basis for other DS-associated cell deficits such as those of the hippocampus, which is especially affected in DS and undergoes a similar pattern of granule cell generation and migration to produce a laminated structure. If the trisomy-induced, cell-autonomous deficits in the response to Hh signaling identified here also occur in other developing cell populations, this could contribute to a range of anomalies seen in DS.
Methods
Animals. Founder B6EiC3H-a/A-Ts65Dn (Ts65Dn) and B6;129-Ptchtm1Mps (Ptc+/-) mice were obtained from The Jackson Laboratory. Both strains were maintained as an advanced intercross on a B6C3H genetic background. Ts65Dn mice were identified by FISH (35). Ptc+/- mice were identified by PCR using primers PTCP1-TGGGGTGGGATTAGATAAATGCC, PTCP2-TGTCTGTGTGTGCTCCTGAATCAC, PTCP3-CTGCGGCAAGTTTTTGGTTG, and P TCP4-AGGGCTTCTCGTTGGCTACAAG. Primers PTCP1 and -2 define the insert allele of 500 bp, whereas PTCP3 and -4 define the wild-type allele of ≈220 bp.
Image Analysis and Unbiased Stereology. After death, heads were fixed in 10% neutral buffered formalin or methacarn (60% methanol/30% chloroform/10% glacial acetic acid) for 12 h. Brains were removed and fixed an additional 24 h before processing, embedding, and sectioning.
Images of brain and cerebellum were analyzed by using the brainimage program (v.2.2.4, 1999) (35). Midline sagittal sections were stained with hematoxylin and eosin, and measurements of layer thickness and normalized cross-sectional area were determined by using brainimage (35). Determination of granule and Purkinje cell densities were performed as described (18) on two midline sagittal sections per mouse. The granule cell density in the IGL was measured by averaging the total number of cells within 12 independent, nonoverlapping 5,000-μm2 regions from folia IV, V, and VI for each mouse at P14–P35 and for 18 areas of 2,500 μm2 at P6. Statistical analyses on age-matched groups at each time point were performed by using Student's t test.
Unbiased stereology was performed according to established principles (21, 36). Brains from trisomic and euploid mice were sectioned completely, and alternate sections were stained with hematoxylin or cresyl violet stain to optimize visualization of nuclei and mitotic figures, respectively. Images were viewed by using stereo investigator 2000 (MicroBrightField, Colchester, VT) or stereologer (Systems Planning and Analysis, Alexandria, CA) software. Systematic random sampling using the optical “disector” methodology was used for enumeration of the cells. P0 GCP and mitotic cells were counted on every 12th section with a random starting point in the first 12 sections of the cerebellum, using a guard height of 5 μm. Granule cells were counted in dissectors with an area of 64 μm2 and a depth of 8 μm that were spaced every 100 μm. Mitotic cells were found in dissectors with an area of 900 μm2 and a depth of 10 μm with 70 μm spacing between dissectors. P6 GCP and mitotic cells were counted on every 18th section with a random starting point in the first 18 sections of the cerebellum. Guard height was 5 μm, and granule cells were counted in dissectors with an area of 64 μm2 and a depth of 8 μm spaced every 200 μm. Mitotic cells were found in dissectors with an area of 900 μm2 and a depth of 10 μm with 150 μm spacing between dissectors. Average CE ≤0.13 for all counts. Statistical differences were determined by using a Student's t test of ANOVA with post hoc least squares difference tests (α = 0.05) in SAS (Cary, NC).
β-Galactosidase Enzyme Assay. Cerebella from P0 or P6 mice from Ts65Dn × Ptc+/- matings were dissected and frozen on dry ice/ethanol, and stored at -80°C until analyzed. β-galactosidase assays were performed with the GalactoLight Plus kit (Tropix) using 2 μg (P0) or 10 μg (P6) of protein. Luminescence was measured with a Wallac 1450 MicroBeta (PerkinElmer) and compared to a standard curve of bacterial β-galactosidase. Each cerebellum was assayed three or more times from individually frozen aliquots of extract.
GCP Isolation and [3H]dT Incorporation. Pooled cerebella from two or more P6 mice of the same genotype were used for each of four experiments. Cerebellar cells were isolated essentially as described (22). After mechanical and enzymatic disruption, the tissue was triturated to obtain a single-cell suspension, collected by centrifugation and underlaid with a step gradient of 35% and 65% Percoll. GCP were collected from the 35/65% interface and resuspended in Neurobasal medium (Invitrogen). Trisomic and euploid cells from two or more cerebella in three or six replicate wells were pooled and plated at 105 cells per well in 96-well plates coated with poly(d-lysine) (Beckton Dickinson). Cells were cultured with indicated Shh concentrations and cultured at 37°C for 48 h and then pulsed with 1 μCi [3H]thymidine 6 h before harvesting (1 Ci = 37 GBq). Cells were harvested onto glass fiber filters, and incorporated radioactivity was quantified by liquid scintillation spectrophotometry.
Acknowledgments
Heidi St. John and Ann Lawler provided excellent assistance with production and typing of mice at early postnatal stages. Brendan Mullaney, Mollie Lange, and Mary Blue contributed technical expertise to histological preparations for stereology. We also thank K. Young for technical assistance and J. Chen for synthesis of SAG 1.1. P.A.B. is a Howard Hughes Medical Institute investigator. This work was supported by National Research Service Award HD43614 (to R.J.B.) and Public Health Service Award HD38384 (to R.H.R.).
Author contributions: R.J.R., L.L.B., P.A.B., and R.H.R. designed research; R.J.R., L.L.B., N.G.S., D.K.K., and R.H.R. performed research; R.J.R., L.L.B., N.G.S., D.K.K., P.A.B., and R.H.R. analyzed data; and R.J.R., L.L.B., N.G.S., D.K.K., P.A.B., and R.H.R. wrote the paper.
Conflict of interest statement: P.A.B. is a paid consultant for and P.A.B. and Johns Hopkins University hold stock in Curis, Inc.
Abbreviations: chr, chromosome; DS, Down's syndrome; IGL, internal granule layer; GCP, granule cell precursor; EGL, external germinal layer; Pn, postnatal day n; Shh, Sonic hedgehog; Hh, hedgehog.
References
- 1.Epstein, C. (1986) The Consequences of Chromosome Imbalance: Principles, Mechanisms, and Models (Cambridge Univ. Press, Cambridge, U.K.).
- 2.Pennington, B. F., Moon, J., Edgin, J., Stedron, J. & Nadel, L. (2003) Child Dev. 74, 75-93. [DOI] [PubMed] [Google Scholar]
- 3.Crome, R., Cowie, V. & Slater, E. (1966) J. Mental Defect. Res. 10, 69-72. [Google Scholar]
- 4.Aylward, E. H., Habbak, R., Warren, A. C., Pulsifer, M. B., Barta, P. E., Jerram, M. & Pearlson, G. D. (1997) Arch. Neurol. 54, 209-212. [DOI] [PubMed] [Google Scholar]
- 5.Wisniewski, K. E., Laure-Kamionowska, M. & Wisniewski, H. M. (1984) N. Engl. J. Med. 311, 1187-1188. [DOI] [PubMed] [Google Scholar]
- 6.Wisniewski, K. E. (1990) Am. J. Med. Genet. Suppl. 7, 274-281. [DOI] [PubMed] [Google Scholar]
- 7.Epstein, C. J. (2001) in The Metabolic & Molecular Bases of Inherited Disease, eds. Scriver, C. R., Beaudet, A. L., Sly, W. S. & Valle, D. (McGraw–Hill, New York), Vol. 1, pp. 1223-1256. [Google Scholar]
- 8.Davisson, M., Schmidt, C., Reeves, N., Irving, E., Akeson, E., Harris, B. & Bronson, R. (1993) Prog. Clin. Biol. Res. 384, 117-133. [PubMed] [Google Scholar]
- 9.Escorihuela, R. M., Fernandez-Teruel, A., Vallina, I. F., Baamonde, C., Lumbreras, M. A., Dierssen, M., Tobena, A. & Florez, J. (1995) Neurosci. Lett. 199, 143-146. [DOI] [PubMed] [Google Scholar]
- 10.Holtzman, D. M., Santucci, D., Kilbridge, J., Chua-Couzens, J., Fontana, D. J., Daniels, S. E., Johnson, R. M., Chen, K., Sun, Y., Carlson, E., et al. (1996) Proc. Natl. Acad. Sci. USA 93, 13333-13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reeves, R., Irving, N., Moran, T., Wohn, A., Kitt, C., Sisodia, S., Schmidt, C., Bronson, R. & Davisson, M. (1995) Nat. Genet. 11, 177-183. [DOI] [PubMed] [Google Scholar]
- 12.Cooper, J. D., Salehi, A., Delcroix, J. D., Howe, C. L., Belichenko, P. V., Chua-Couzens, J., Kilbridge, J. F., Carlson, E. J., Epstein, C. J. & Mobley, W. C. (2001) Proc. Natl. Acad. Sci. USA 98, 10439-10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurt, M. A., Davies, D. C., Kidd, M., Dierssen, M. & Florez, J. (2000) Brain Res. 858, 191-197. [DOI] [PubMed] [Google Scholar]
- 14.Belichenko, P. V., Masliah, E., Kleschevnikov, A. M., Villar, A. J., Epstein, C. J., Salehi, A. & Mobley, W. C. (2004) J. Comp. Neurol. 480, 281-298. [DOI] [PubMed] [Google Scholar]
- 15.Insausti, A. M., Megias, M., Crespo, D., Cruz-Orive, L. M., Dierssen, M., Vallina, I. F., Insausti, R., Florez, J. & Vallina, T. F. (1998) Neurosci. Lett. 253, 175-178. [DOI] [PubMed] [Google Scholar]
- 16.Rueda, N., Mostany, R., Pazos, A., Florez, J. & Martinez-Cue, C. (2005) Neurosci. Lett. 380, 197-201. [DOI] [PubMed] [Google Scholar]
- 17.Richtsmeier, J., Baxter, L. & Reeves, R. (2000) Dev. Dyn. 217, 137-145. [DOI] [PubMed] [Google Scholar]
- 18.Baxter, L. L., Moran, T. H., Richtsmeier, J. T., Troncoso, J. & Reeves, R. H. (2000) Hum. Mol. Genet. 9, 195-202. [DOI] [PubMed] [Google Scholar]
- 19.Olson, L. E., Roper, R. J., Baxter, L. L., Carlson, E. J., Epstein, C. J. & Reeves, R. H. (2004) Dev. Dyn. 230, 581-589. [DOI] [PubMed] [Google Scholar]
- 20.Hatten, M. E. & Heintz, N. (1995) Annu. Rev. Neurosci. 18, 385-408. [DOI] [PubMed] [Google Scholar]
- 21.Mouton, P. R. (2002) Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists (Johns Hopkins Univ. Press, Baltimore).
- 22.Wechsler-Reya, R. J. & Scott, M. P. (1999) Neuron 22, 103-114. [DOI] [PubMed] [Google Scholar]
- 23.Wallace, V. A. (1999) Curr. Biol. 9, 445-448. [DOI] [PubMed] [Google Scholar]
- 24.Dahmane, N. & Ruiz-i-Altaba, A. (1999) Development (Cambridge, U.K.) 126, 3089-3100. [DOI] [PubMed] [Google Scholar]
- 25.Goodrich, L. V., Milenkovic, L., Higgins, K. M. & Scott, M. P. (1997) Science 277, 1109-1113. [DOI] [PubMed] [Google Scholar]
- 26.Taipale, J., Chen, J. K., Cooper, M. K., Wang, B., Mann, R. K., Milenkovic, L., Scott, M. P. & Beachy, P. A. (2000) Nature 406, 1005-1009. [DOI] [PubMed] [Google Scholar]
- 27.Roper, R., St. John, H., Philip, J., Lawler, A. & Reeves, R. (2005) Genetics, Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 28.Frank-Kamenetsky, M., Zhang, X. M., Bottega, S., Guicherit, O., Wichterle, H., Dudek, H., Bumcrot, D., Wang, F. Y., Jones, S., Shulok, J., et al. (2002) J. Biol. 1, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen, J. K., Taipale, J., Young, K. E., Maiti, T. & Beachy, P. A. (2002) Proc. Natl. Acad. Sci. USA 99, 14071-14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wichterle, H., Lieberam, I., Porter, J. A. & Jessell, T. M. (2002) Cell 110, 385-397. [DOI] [PubMed] [Google Scholar]
- 31.Machold, R., Hayashi, S., Rutlin, M., Muzumdar, M. D., Nery, S., Corbin, J. G., Gritli-Linde, A., Dellovade, T., Porter, J. A., Rubin, L. L., et al. (2003) Neuron 39, 937-950. [DOI] [PubMed] [Google Scholar]
- 32.Chen, W., Ren, X. R., Nelson, C. D., Barak, L. S., Chen, J. K., Beachy, P. A., de Sauvage, F. & Lefkowitz, R. J. (2004) Science 306, 2257-2260. [DOI] [PubMed] [Google Scholar]
- 33.Antonarakis, S. E., Lyle, R., Dermitzakis, E. T., Reymond, A. & Deutsch, S. (2004) Nat. Rev. Genet. 5, 725-738. [DOI] [PubMed] [Google Scholar]
- 34.Moore, C. S., Lee, J. S., Birren, B., Stetten, G., Baxter, L. L. & Reeves, R. H. (1999) Genomics 59, 1-5. [DOI] [PubMed] [Google Scholar]
- 35.Ng, Y. R., Shiffman, S., Brosnan, T. J., Links, J. M., Beach, L. S., Judge, N. S., Xu, Y., Kelkar, U. V. & Reiss, A. L. (2001) J. Am. Med. Inform. Assoc. 8, 431-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long, J. M., Kalehua, A. N., Muth, N. J., Hengemihle, J. M., Jucker, M., Calhoun, M. E., Ingram, D. K. & Mouton, P. R. (1998) J. Neurosci. Methods 84, 101-108. [DOI] [PubMed] [Google Scholar]