Abstract
Herpes simplex virus (HSV) recombinants are being developed as vaccine vectors for the expression of heterologous antigens. There is concern, however, that preexisting HSV immunity may decrease their effectiveness. We have addressed this issue in an animal model. Immunized mice were inoculated with a replication-defective HSV-1 vector that expressed the Escherichia coli β-galactosidase protein as a model antigen. We assessed vector efficacy by analyzing the immunoglobulin G (IgG) antibody response and cellular proliferative response directed against β-galactosidase. We report that the ability of the vector to induce antibody or proliferative responses was not diminished by preexisting immunity to HSV. Of further note, the anti-HSV and anti-β-galactosidase IgG responses following vector administration were extremely durable in both immunized and naive mice. These results indicate that the ability of a replication-defective HSV-derived vaccine vector to elicit long-lived immune responses in mice is not impaired by prior HSV exposure.
Herpes simplex virus (HSV) recombinants and replication-defective HSV mutants are being evaluated as potential genital herpes vaccines (1, 10, 12, 17, 40, 43, 56, 66) and as novel vaccine vectors (50). Because HSV vectors are capable of infecting a wide range of tissues and host species, they are suitable for use in a variety of vaccination strategies. In particular, HSV has been shown to generate immune responses by various routes of inoculation, including intranasal administration. Finally, due to its large viral genome, multiple antigens may be expressed simultaneously from a single HSV vector to generate a combination vaccine.
HSV infection often results in a localized lesion within epithelial cells of the skin or a mucosal membrane. The innate immune response, consisting of macrophages, natural killer (NK) cells, cytokines, and complement proteins, may act to contain initial viral infection (27, 70). NK cell-mediated lysis (33) and numerous cytokines, including interleukin (IL)-12 (25, 68), IL-18 (21, 25), and gamma interferon and tumor necrosis factor alpha (26), have been reported to affect HSV pathogenesis in mouse models of disease.
The adaptive immune response to infection is comprised of CD8+ and CD4+ cells (42), and clearance of viral lesions may involve cytotoxic CD4+ T cells (38, 71). Replication-defective HSV strains are able to take advantage of the immunogenicity inherent to wild-type HSV but are much safer due to the incorporation of nonreverting mutations in essential viral genes (53). As a result, these HSV mutants are able to elicit robust and long-lived antiviral immunity (3, 41, 44, 46). In addition, analysis of cytokine expression and immunoglobulin G (IgG) antibody profiles following infection indicates that a Th1 type of cellular helper response is generated against wild-type HSV antigens and replication-defective HSV mutant-derived proteins (4-6, 52, 54).
The antibody response to HSV and HSV-encoded antigens is T cell dependent, and we have shown previously that it is also dependent on innate factors of the complement system (11). The presence of serum antibody alone does not protect the host from infection or primary disease but has been shown to reduce the spread of virus into the central nervous system and prevent the occurrence of viral encephalitis, which can result in death (69). Recently, it has been reported that antibody and helper T cells may act synergistically to enhance the rate of viral clearance following mucosal infection (47). Despite clearance of the virus from the periphery, HSV can establish a life-long latent infection within the sensory neurons that innervate the site of primary infection. In humans, periodic reactivation of this latent virus results in a recurring disease at or near the site of primary exposure.
Currently, many vector systems are being developed for use in vaccine design (24). In addition to HSV, other promising virus-derived vaccine systems include poxviruses (48, 54, 59), adenoviruses (31, 60, 61, 63), alphaviruses (7, 14, 58, 67), and poliovirus-derived vectors (37). One concern that affects all of these vaccine systems is the potential that prior host immunity may result in diminished efficacy of the vector or threaten the ability to use the same vector construct for repeated vaccinations. This has been reported to be the case for both poxvirus (16, 19) and adenovirus (55, 57, 64) vectors. In these instances, immune-mediated suppression of the vector was reversed in one of several ways: by altering the route of vaccination (2); by adding multiple booster vaccinations (16); or by changing the virus strain used for the vector (23, 39). Suppression of a viral vector as a result of prior infection, however, may not be a universal consequence, because both poliovirus- and alphavirus-derived vectors are reported to elicit similar antibody responses despite preexisting immunity (36, 37, 58). Cytotoxic T lymphocyte (CTL) responses in immune hosts have not been thoroughly investigated in any of these systems but may be diminished following poliovirus vector delivery (37).
Understanding the effects of preexisting immunity on HSV-derived vectors is of particular concern, considering the ubiquitous nature of HSV in the population. Recent estimates of HSV-1 infection in the adult population often range as high as 75% in the United States, while the rate of HSV-2 infection is currently 22% but continues to increase (9, 18). Several recent publications have described the use of HSV-derived gene therapy vectors in immunized mice, with various results. It has been reported that prior immunity had no effect on the efficacy of an HSV-mediated oncolytic therapy involving intratumoral injection (8). Nevertheless, another study has shown that prior immunity may decrease the ability of an HSV vector to complete in vivo gene transfer into brain tumors (28).
The consequence of preexisting immunity on the vaccine response generated by an HSV vector has not been reported previously. To address this issue, we used an animal model system in which immunized mice or mock-infected controls were inoculated with a replication-defective HSV recombinant virus expressing the Escherichia coli β-galactosidase (β-galactosidase) protein as a model antigen. By comparing the IgG antibody responses elicited by this vector between these two groups, we assayed vaccine efficacy in immune versus naive hosts. If prior HSV immunity were detrimental to vaccine efficacy, then we would have expected to see a quantitative decrease in the immune response directed towards β-galactosidase that was relative to the degree of immune-mediated vector suppression.
We report that the generation and durability of a β-galactosidase-specific IgG antibody response were equivalent in immune and naive animals. This result suggests that preexisting antiviral immunity does not impair the ability of an HSV-derived vaccine vector to elicit new antibody responses in immune mice and indicates that replication-defective HSV-derived vectors should be evaluated further as vaccine delivery vehicles.
MATERIALS AND METHODS
Cells and viruses.
Vero cells were purchased from the American Type Culture Collection (Manassas, Va.) and used for propagation of wild-type virus strains. The Vero-derived cell line V8-27 was used for propagation of replication-defective mutant viruses. V8-27 cells express the viral proteins ICP8 and ICP27 upon HSV infection (13). Cells were grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, Calif.) supplemented with 5% bovine calf serum, 5% fetal calf serum (HyClone, Logan, Utah), l-glutamine, penicillin G, and streptomycin (Glu-Pen-Strep; Irving Scientific, Santa Ana, Calif.) at 37°C with 5% CO2. Following viral infection, cells were maintained in DMEM containing 1% fetal bovine serum (DMEM-V) at 34°C until harvest.
The replication-defective HSV-1 recombinants and the parental wild-type KOS1.1 strain have been described previously (22). The d102 and d301 viruses contain large deletions within the UL29 gene. This gene encodes the ICP8 protein, which is essential for viral DNA replication. The HD-2 virus expresses the E. coli β-galactosidase protein fused in frame to the N terminus of ICP8 and has been used as an HSV-1 β-galactosidase vector.
Stocks of the viruses were generated as either infected-cell lysates, as described previously (32) except that cell pellets were resuspended in DMEM-V containing 20% glycerol, or cell-free virus stocks. For cell-free virus stocks, HD-2 virions were harvested from the supernatant of infected cells as follows. Infected cells and cellular debris were pelleted by low-speed centrifugation (1,000 × g) for 10 min at 25°C. Virus particles were collected from the clarified supernatant by high-speed centrifugation (30,000 × g) for 1 h at 4°C. The resulting virus pellet was resuspended in fresh DMEM-V containing 20% glycerol. All viruses were titered on the appropriate complementing cell line as described previously (32) using DMEM-V containing 0.1% human immune globulin (Massachusetts Public Health Biologic Laboratories, Boston, Mass.) as the overlay medium.
UV inactivation of HD-2 virus.
HD-2 virus was partially inactivated by exposure to 254-nm UV light for 10 min at a distance of 5 cm as described previously (45). Subsequent titering revealed that UV treatment reduced viral infectivity approximately 2,000-fold, from 2 × 109 PFU/ml to 1 × 106 PFU/ml.
Mice and inoculations.
Six-week-old female BALB/cJ mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and acclimated for 1 week prior to use. Groups of mice were immunized once by subcutaneous (s.c.) inoculation into the right flank with 2 × 106 PFU of KOS1.1, d301, or d102 virus, as indicated, or mock-infected by s.c. inoculation with uninfected Vero cell lysate. In some cases, a second identical inoculation was given 3 weeks later to boost host immunity. Four weeks after the final immunization, mice were inoculated s.c. in the left flank with 2 × 106 PFU of the HD-2 virus, followed by one or more booster inoculations with HD-2 at 3- or 4-week intervals. Inoculations consisted of virus stock diluted into a volume of 20 μl of sterile, endotoxin-free sodium chloride solution, 0.9% (Sigma, St. Louis, Mo.) per mouse. Mice were housed in accordance with National Institutes of Health (NIH) and Harvard University guidelines.
Serum collection, ELISA, and antibody neutralization.
Blood was collected from the tail vein of each mouse before immunization and at the times indicated during each experiment. Serum was prepared using Becton Dickinson Microtainer serum separators (VWR) and then stored at −20°C prior to analysis.
Enzyme-linked immunosorbent assays (ELISAs) to determine antigen-specific IgG titers were completed as described previously (11). Briefly, 96-well Nunc Maxisorp microtiter plates (VWR) were coated with HSV-1 antigen (Advanced Biotechnologies Inc., Columbia, Md.) at 50 ng per well or with β-galactosidase antigen (Sigma) at 250 ng per well in 50 μl of sodium bicarbonate buffer (pH 9.6) (Sigma) overnight at 4°C. Plates were blocked with phosphate-buffered saline (PBS, pH 7.4) containing 5% (wt/vol) nonfat milk for 1 h at 37°C and washed with PBS containing 0.05% Tween 20 (PBS/T) using a Skatron CellWasher 600 (Molecular Devices, Sunnyvale, Calif.). Serial twofold dilutions of mouse serum (from 1:100 to 1:12,800) in PBS/T were added and incubated for 2 h at 37°C. Following serum antibody binding, plates were washed with PBS/T and then incubated with a rabbit anti-mouse IgG secondary antibody conjugated to alkaline phosphatase (1:1,000 dilution; Sigma) for 1 h at 37°C. Plates were washed with PBS/T and developed by incubation with the alkaline phosphatase substrate p-nitrophenyl phosphate (Sigma) for 20 min at room temperature, and results were read at 405 nm on a VersaMax microplate reader (Molecular Devices, Sunnyvale, Calif.).
The IgG antibody titer indicated is the mean reciprocal log2 value of the last dilution resulting in an optical density (OD) reading 0.2 units above that of a control serum (background). In each case, negative OD readings at the 1:100 dilution were scored as positive at a 1:50 dilution (reciprocal dilution 50 = log2 5.65), and this value (5.65) was used as the limit of detection and subtracted from all results.
Neutralizing antibody titers were determined by plaque reduction in the presence of complement as reported previously (45) using twofold dilutions of pooled serum from at least five mice. Values reported are the reciprocal dilution at which at least 50% plaque reduction of HSV-1 strain KOS1.1 was observed.
β-Galactosidase enzyme assays.
β-Galactosidase enzymatic activity present in the virus stocks was determined using the β-galactosidase enzyme assay system (Promega, Madison, Wis.) according to the manufacturer's recommendations. Protein concentrations were calculated by comparison to a standard curve of freshly prepared β-galactosidase protein (Sigma) on the basis of OD410 values.
Cellular proliferation assay.
Cellular proliferation responses were measured as described previously (6). Single-cell suspensions were prepared from whole spleens in complete DMEM. Erythrocytes were lysed using the whole blood erythrocyte lysing kit (R&D Systems, Minneapolis, Minn.), and B cells were removed using Dynabeads Pan B (B220) magnetic beads (Dynal Inc., Lake Success, N.Y.). Lymphocytes were counted, and 2 × 105 cells were plated in quadruplicate wells of a flat-bottomed 96-well plate (Costar, Cambridge, Mass.) in the presence of soluble HSV-1 (Advanced Biotechnologies Inc.) or β-galactosidase (Sigma) (at 5 μg/well final concentration) or in DMEM alone to a final volume of 200 μl. After incubation for 4 days at 37°C, cells were labeled for 6 h with bromodeoxyuridine (BrdU). BrdU incorporation was measured using an ELISA-based assay (Cell Proliferation ELISA; Roche, Indianapolis, Ind.) using the accompanying protocol and reagents. Results are shown as a fold induction of cell proliferation, which was determined by dividing the signal obtained in the presence of HSV or β-galactosidase antigen by that observed with medium alone.
RESULTS
We used a mouse model of HSV infection with several recombinant viruses (Fig. 1) to test the efficacy of HSV-derived vaccine vectors in the presence of preexisting host immunity. In most experiments, groups of BALB/cJ mice were immunized by s.c. inoculation with 2 × 106 PFU of a replication-defective HSV-1, either d301 or d102. We chose this immunization protocol because a single s.c. inoculation with the replication-defective HSV-1 mutant d301 has been shown previously to protect mice from death following corneal challenge infection with a highly virulent HSV-1 strain, to significantly reduce shedding of challenge virus from the eye, to significantly enhance the rate of virus clearance from the eye following challenge, and to markedly diminish the establishment of latent infection by this challenge virus (44-46). By any of these measures, immunization with d301 was shown to be equivalent to immunization with its replication-competent parental virus KOS1.1 (44-46). In addition, immunization of mice with either d301 or KOS1.1 induced similar neutralizing antibody and T-cell proliferative responses (45, 46) and comparable primary and memory CTL responses (5).
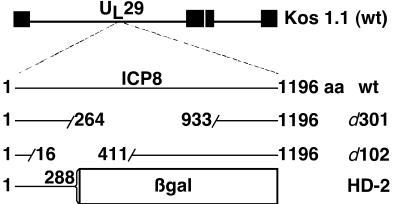
FIG. 1.
HSV-1 recombinants used in this study. HSV-1 viruses included the wild-type (wt) laboratory strain KOS1.1 and replication-defective KOS1.1-derived recombinants d301, d102, and HD-2. d301 and d102 each contain large deletions within the UL29 gene encoding the ICP8 protein. HD-2 encodes a truncated ICP8 protein fused to E. coli β-galactosidase (β-gal).
To directly compare immunity generated by a replication-competent virus, in one experiment a group of mice was immunized with the parental, replication-competent HSV-1 strain KOS1.1. Four weeks after immunization, mice were inoculated s.c. with 2 × 106 PFU of the replication-defective HSV-1 mutant HD-2 in the opposite flank. The HD-2 mutant expresses β-galactosidase fused to the N-terminal portion of the viral ICP8 protein and has been used as a model HSV-derived vaccine vector (β-galactosidase vector). B-cell responses were assayed by measuring the total IgG antibody responses directed towards HSV and β-galactosidase by ELISA at various times following immunization, primary β-galactosidase vector inoculation, and secondary β-galactosidase vector inoculation. In one experiment, cellular proliferative responses against HSV and β-galactosidase were determined to measure the induction of cellular immunity. By comparing the β-galactosidase-specific antibody responses between virus-inoculated and mock-infected animals, we determined the relative efficacy of the HSV vector in immune and naive mice.
IgG antibody responses in mice were proportional to the infectious dose of replication-defective virus vector and not total protein amount.
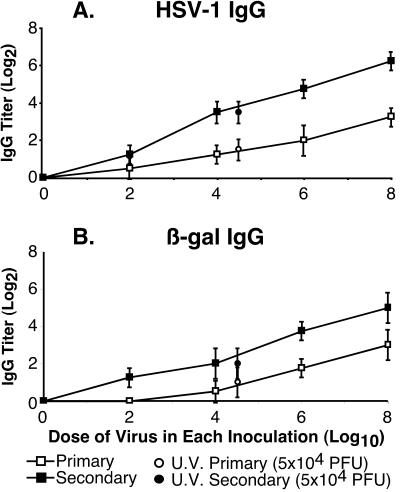
To establish that this system provided a quantitative measure of host immunity, we determined if the humoral immune response generated by the β-galactosidase vector was proportional to the dose of virus inoculated. Groups of six mice were inoculated s.c. in the flank with the indicated doses of the HSV-1 β-galactosidase-expressing recombinant HD-2, ranging from 0 (mock infection) to 108 PFU. Three weeks later, mice received an identical booster inoculation with HD-2. Serum samples were collected from each animal prior to infection, at 3 weeks following primary inoculation (primary), and at 3 weeks following secondary inoculation (secondary).
Anti-HSV and anti-β-galactosidase IgG titers were determined by ELISA (Fig. 2). Primary and secondary IgG titers to both HSV (Fig. 2A) and β-galactosidase (Fig. 2B) antigens were proportional to the dose of HD-2 virus. These results ensure that the vector inoculum had not surpassed a plateau for measuring increasing IgG antibody responses and that the IgG antibody response to the dose of β-galactosidase vector (2 × 106 PFU) used in subsequent experiments was within the linear range detected by ELISA.
FIG. 2.
Antibody responses as a function of viral dose. Groups of six mice were inoculated with various doses of HD-2 (0 to 108 PFU) or UV-treated HD-2 (108 PFU prior to treatment, 5 × 104 PFU following treatment), at weeks 0 and 3. Serum samples were collected at weeks 3 (primary) and 5 (secondary). HSV-1- (A) and β-galactosidase- (B) specific IgG titers were determined by ELISA, and results are shown as mean titer (log2) ± standard deviation.
In addition, one group of mice was inoculated with an equivalent volume of the highest-titer HD-2 virus that had been partially inactivated by treatment with UV light. This exposure resulted in a 2,000-fold reduction in the infectivity of the inoculum (from 1 × 108 PFU/mouse to 5 × 104 PFU/mouse). Following primary and secondary infection with UV-treated HD-2, we observed a reduction in the IgG antibody responses against both HSV-1 (Fig. 2A; UV primary and UV secondary) and β-galactosidase (Fig. 2B; UV primary and UV secondary) compared to the untreated HD-2 inoculum (108 PFU/mouse). Furthermore, the antibody response appeared to be proportional to the viral PFU and not to the amount of input protein, because the IgG antibody titers elicited by UV-treated HD-2 were similar to IgG titers resulting from untreated HD-2 inoculated at 104 PFU/mouse. These results indicated that the antibody response was mainly a result of viral infection and was not simply due to input viral or β-galactosidase antigen.
Prior HSV immunity did not diminish the induction of a durable β-galactosidase antibody response by an HSV vector.
To assess the effect of prior HSV-1 infection on the ability of an HSV-1-derived vector to generate IgG antibody responses, we immunized mice once with 2 × 106 PFU of a replication-defective HSV-1 mutant containing a deletion within the UL29 gene encoding the ICP8 protein, either d301 or d102, or mock-infected animals with uninfected Vero cell lysate, as indicated. To verify immunization following a single d301 inoculation, we measured anti-HSV neutralizing antibody in the serum of these mice, and we obtained a neutralization titer of 64. This value was similar to previous results with d301 in mice (45) and comparable to neutralizing titers observed in humans (range, 12 to 372) that have been reported previously (62).
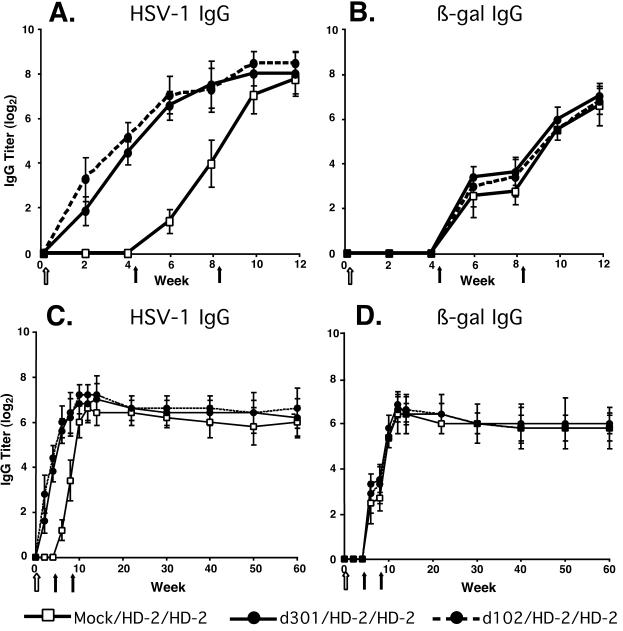
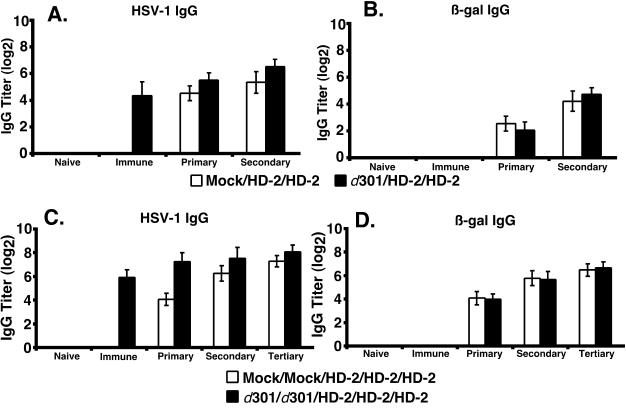
At 4 and 8 weeks after the immunization, all mice were inoculated with 2 × 106 PFU of the β-galactosidase vector HD-2. Serum samples were collected from the mice, and HSV-1-specific and β-galactosidase-specific IgG antibody titers were measured by ELISA (Fig. 3). Despite the induction of an antiviral antibody response following inoculation with d301 or d102 (Fig. 3A, week 4), indicative of preexisting immunity, the generation of β-galactosidase-specific antibody was equivalent between the immune groups and mock controls following HD-2 inoculation (Fig. 3B, weeks 4 to 12).
FIG. 3.
Induction and durability of IgG responses in mice. The generation of IgG antibody specific for HSV-1 (A) and β-galactosidase (B) and the durability of the IgG responses directed towards HSV-1 (C) and β-galactosidase (D) antigens are shown. Groups of six mice were immunized once with 2 × 106 PFU virus (solid circles) using either d301 (solid lines) or d102 (dashed lines) or mock infected (open squares) at week 0 (open arrow). At weeks 4 and 8, all mice were inoculated with 2 × 106 PFU of HD-2, expressing β-galactosidase (solid arrows). Serum samples were collected at the indicated times, and HSV-1- and β-galactosidase-specific IgG antibody titers were determined by ELISA. Results are shown as the mean reciprocal dilution (log2) ± standard deviation.
Previous reports have indicated that replication-competent and replication-defective HSV strains are capable of eliciting a stable antiviral neutralizing antibody response in experimentally infected mice (46) that persists for at least 7 months postinfection. To further address this issue, we evaluated the ability of HD-2 to elicit durable IgG antibody responses following inoculation in these immune mice. We continued to collect serum from the HD-2 inoculated mice up to one year following vector administration and boost, and we determined the HSV-1-specific and β-galactosidase-specific IgG antibody titers. We observed that inoculation with HD-2 generated a remarkably durable antibody response toward HSV-1 (Fig. 3C, weeks 15 to 60) and β-galactosidase (Fig. 3D, weeks 15 to 60) antigens and that prior HSV immunity did not alter the longevity of this antibody response. These results indicated that prior immunity to HSV-1 did not diminish the ability of an HSV-1-derived vector to generate durable, high-titer antibody responses.
HSV vectors were effective in the presence of boosted antiviral immunity.
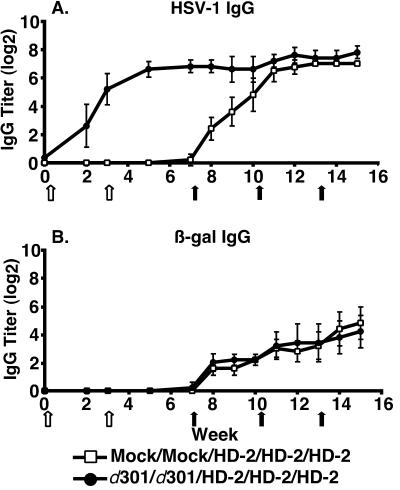
Because recurrent HSV infection may increase the host response directed against the virus, we enhanced the antiviral immunity to address vector efficacy in this situation. We immunized mice with 2 × 106 PFU of d301 and boosted the response with the same dose of d301 3 weeks later or mock-infected mice with uninfected Vero cell lysate at both times. The addition of a d301 boost resulted in an observed neutralizing antibody titer of 1,024 and an increase in the anti-HSV IgG titer (Fig. 4A, weeks 3 and 7). At 4, 7, and 10 weeks after the second immunization, mice were inoculated with 2 × 106 PFU of HD-2. Despite the increased anti-HSV response elicited with the immunization-boost regimen, equivalent β-galactosidase IgG titers were generated by HD-2 inoculation in mock-infected and immunized groups of animals (Fig. 4B, weeks 7 to 18). This result indicated that even a heightened level of HSV immunity was unable to reduce the capacity of an HSV vector to generate IgG antibody responses.
FIG. 4.
Induction of β-galactosidase IgG after two HSV-1 immunizations. Titers of IgG specific for HSV-1 (A) and β-galactosidase (B) are shown. Groups of six mice were immunized twice, at weeks 0 and 3 (open arrows), with 2 × 106 PFU of d301 virus (solid circles) or mock infected (open squares). At weeks 7, 10, and 13, all mice were inoculated with 2 × 106 PFU of HD-2 virus, expressing β-galactosidase (solid arrows). Serum samples were collected at the indicated times, and IgG was detected by ELISA. Results are shown as the mean reciprocal dilution (log2) ± standard deviation.
Antibody response was induced by a purified HSV vector in immune mice.
To confirm that the β-galactosidase-specific antibody responses that we observed were the result of vector-expressed antigen rather than protein present in the inoculum, we prepared cell-free stocks of the HD-2 virus from the supernatant of infected cells as described in Materials and Methods. β-Galactosidase enzyme activity was measured for virus stocks, and cell-free stocks were found to contain 65-fold less β-galactosidase activity than virus stocks prepared as infected-cell lysates.
We determined the effect of a single immunization with d301 on the efficacy of the cell-free HD-2 virus stock to elicit a β-galactosidase-specific IgG antibody response (Fig. 5A and B). Groups of mice were immunized once at week 0 by s.c. inoculation with 2 × 106 PFU of d301 or mock infected. At weeks 4 and 7, all mice were inoculated s.c. with 2 × 106 PFU of the cell-free HD-2 virus. Serum samples were collected at the beginning of the experiment (naïve), at week 3 (immune), at week 7 (primary), and at week 9 (secondary). Despite the generation of an HSV-specific immune response following a single d301 inoculation (Fig. 5A, immune), cell-free HD-2 virus elicited a β-galactosidase-specific IgG response (Fig. 5B, primary and secondary) that was similar in magnitude to previous results obtained with preparations of virus in infected-cell lysate (Fig. 3B).
FIG. 5.
Induction of β-galactosidase IgG by cell-free HD-2 virus after one or two immunizations. (A and B) Groups of six mice were immunized once with 2 × 106 PFU of d301 (solid bars) or mock infected (open bars) at week 0. At weeks 4 and 7, all mice were inoculated with 2 × 106 PFU of cell-free HD-2 virus, expressing β-galactosidase. IgG antibodies specific for HSV-1 (A) and β-galactosidase (B) were determined by ELISA using serum collected prior to week 0 (naïve), at week 3 (immune), at week 7 (primary), and at week 9 (secondary). (C and D) Groups of six mice were immunized twice with 2 × 106 PFU of d301 virus (solid bars) or mock infected (open bars) at weeks 0 and 3. All mice received 2 × 106 PFU of cell-free HD-2 at weeks 7, 10, and 13. Serum samples were collected prior to week 0 (naive), at week 6 (immune), at week 10 (primary), at week 12 (secondary), and at week 15 (tertiary). Titers of HSV-1- (C) and β-galactosidase- (D) specific IgG antibody were determined by ELISA. Results are shown as the mean reciprocal dilution (log2) ± standard deviation.
In a second experiment, the effects of two d301 immunizations on the cell-free HD-2 virus was assessed (Fig. 5C and 5D). Two groups of six mice were immunized by s.c. inoculation with 2 × 106 PFU of d301 or mock infected at weeks 0 and 3. At weeks 7, 10, and 13, all mice received 2 × 106 PFU of the cell-free HD-2 virus s.c. in the opposite flank. Naive serum was collected from each mouse prior to week 0, HSV immune serum was collected at week 6, and primary, secondary, and tertiary serum samples were collected at weeks 10, 12, and 15, respectively. Despite the increased level of anti-HSV immunity elicited by the second d301 inoculation (Fig. 5C, immune), the cell-free HD-2 virus generated β-galactosidase-specific IgG antibody responses that were equivalent between mock-treated and immune animals (Fig. 5D, primary, secondary, and tertiary).
Together, these experiments showed that the cell-free HD-2 recombinant virus elicited β-galactosidase-specific antibody responses in mice immunized either once or twice with d301 that were equivalent to those of mock-infected controls. These results also reiterate the point that the humoral responses generated by HD-2 are due to vector infection and not input antigen. We have shown that the IgG response to UV-treated virus is PFU dependent (Fig. 2, UV-treated viruses) and that the antibody response appears to be similar when either infected-cell lysate (Fig. 3 and 4) or partially purified virions (Fig. 5) are used for inoculation.
Immunity generated with replication-competent HSV-1 did not diminish antibody responses to an HSV vector.
In each of the previous experiments, replication-defective viruses were used to generate antiviral immunity. The experiments were designed in this way because we had previously shown that immunization with a replication-defective virus is as effective as immunization with a replication-competent virus for inducing protective immunity and because the immune phenotype of d301-immunized mice is comparable to that of KOS1.1-immunized animals (5, 45, 46). Because it may still be argued that the immunity generated by a replication-competent virus is qualitatively different from that elicited by a replication-defective virus in this model, we addressed whether prior infection with a replication-competent virus was able to inhibit an HSV vector.
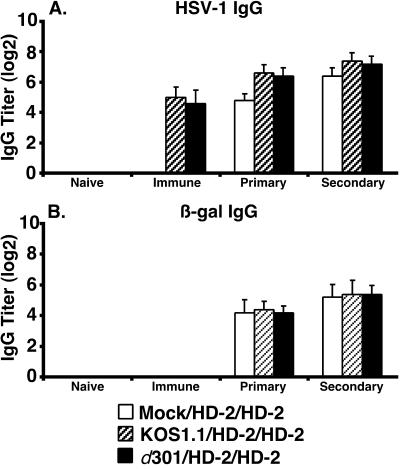
We compared the efficacy of the cell-free HD-2 vector in mice inoculated with either the replication-defective virus d301 or the replication-competent parental virus KOS1.1 (Fig. 6). Groups of mice were immunized by s.c. inoculation in the right flank with 2 × 106 PFU of either KOS1.1 or d301 or mock treated. A neutralizing antibody titer of 128 was measured in the KOS1.1-immunized mice, a value similar to that observed following a single d301 inoculation in mice and values previously reported for human serum (62). At weeks 4 and 7, all mice were inoculated s.c. with 2 × 106 PFU of cell-free HD-2 virus. Serum samples were collected before immunization (naive), at week 3 (immune), at week 7 (primary), and at week 10 (secondary). Despite phenotypic differences in their ability to replicate in vitro and in vivo, KOS1.1 and d301 viruses generated equivalent antiviral IgG responses (Fig. 6A, immune) compared to mock-infected animals. Despite preexisting antiviral immunity following KOS1.1 or d301 infection, HD-2 was able to elicit equivalent β-galactosidase-specific antibody responses in both groups of mice (Fig. 6B, primary and secondary). Thus, in this system, anti-HSV immunity induced by either replication-competent or replication-defective HSV strains did not diminish the vaccine efficacy of the HSV vector.
FIG. 6.
Induction of β-galactosidase IgG by cell-free HD-2 virus after immunization with replication-competent HSV-1. HSV-1- (A) and β-galactosidase- (B) specific IgG antibody responses are shown. Groups of six mice were immunized once with 2 × 106 PFU of either a replication-competent virus, KOS1.1 (hatched bars), or a replication-defective virus, d301 (solid bars), or mock infected (open bars) at week 0. All mice were inoculated with 2 × 106 PFU of cell-free HD-2 virus at weeks 4 and 7. Serum samples were collected prior to week 0 (naïve) and at week 3 (immune), week 7 (primary), and week 10 (secondary). IgG titers were determined by ELISA, and results are shown as the mean reciprocal dilution (log2) ± standard deviation.
Cellular proliferative responses to β-galactosidase were not affected by preexisting HSV immunity.
To confirm that β-galactosidase-specific cellular responses were being activated in these mice following vector inoculation, we analyzed cellular proliferation responses to HSV and β-galactosidase antigens. Mice were immunized with a single inoculation of 2 × 106 PFU of d301 or mock infected. At weeks 4 and 7, all mice were inoculated with 2 × 106 PFU of cell-free HD-2. Spleens were harvested at week 10 from two mice per group, and lymphocytes were isolated, pooled for analysis, and plated at 2 × 105 cells per well in a microtiter plate. Cells were cultured in the presence of soluble HSV or β-galactosidase (5 μg/well) for 4 days. Proliferation was measured by BrdU incorporation using an ELISA-based chemiluminescence assay.
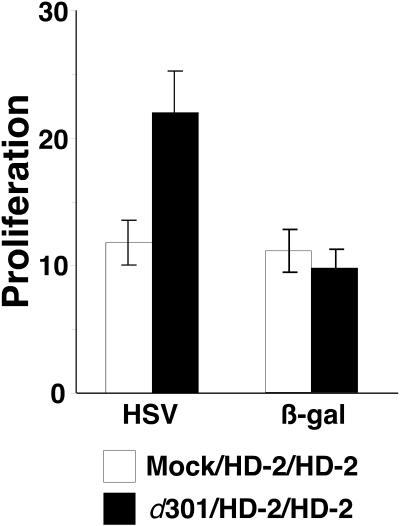
Following immunization and HD-2 inoculation, specific responses were elicited against both HSV and β-galactosidase antigens (Fig. 7). When cultured in the presence of HSV, a 12-fold induction was observed in cells from mock-immunized mice and a 22.5-fold induction was detected in cells from d301-immunized mice compared to cells incubated in medium alone. This difference in cellular activity can be attributed to the fact that the d301-immune mice had received one additional viral inoculation. When cells were cultured in the presence of β-galactosidase, an 11-fold induction was found for mock-immunized mice and a 9.5-fold induction was observed in immune animals. Because this difference was not statistically significant, the proliferative response to β-galactosidase did not appear to be diminished by prior d301 immunization. This result indicated that cellular responses to a vector-encoded antigen may also be unaffected in immune mice.
FIG. 7.
Cellular proliferative responses to β-galactosidase in immune mice. Proliferation was measured in the presence of HSV and β-galactosidase antigens. Mice were immunized once with 2 × 106 PFU of d301 or mock infected at week 0. At weeks 4 and 7, all mice were inoculated with 2 × 106 PFU of cell-free HD-2 virus. Spleens were harvested from two mock-immune (open bars) and two d301- immune (solid bars) mice and pooled for analysis. Single-cell suspensions were generated, and 2 × 105 cells were cultured in the presence of HSV or β-galactosidase antigen (5 μg per well) or medium alone for 4 days at 37°C. BrdU was added to each culture for the final 6 h, cells were fixed and lysed, and BrdU incorporation was measured in an ELISA-based assay. Results are shown as the mean fold proliferation (BrdU incorporation in the presence of antigen divided by incorporation in medium alone) of quadruplicate samples ± standard deviation.
DISCUSSION
HSV-1 and HSV-2 are highly prevalent in the human population. Thus, HSV-derived vaccine vectors are likely to encounter preexisting host immune responses upon in vivo delivery. As a result, the utility of these types of vectors will undoubtedly depend on their ability to elicit effective immune responses in the presence of prior antiviral immunity. In this study, using a mouse model, prior HSV infection did not diminish the magnitude or the durability of the IgG antibody response generated by a replication-defective HSV-1 vector expressing a model antigen, β-galactosidase. In addition, β-galactosidase-specific cellular proliferative responses were not reduced in immune mice, indicating that cellular immunity may also be unaffected by preexisting HSV immunity. Further studies are needed to determine if this property extends to immunization situations in human clinical trials.
These results are perhaps unexpected, because we have shown that the same inoculation protocols could immunize mice against wild-type HSV challenge (45, 46). However, immunization protects against disease, which involves multiple rounds of viral replication and spread (49). In contrast, replication-defective mutant viral vectors infect only one round of cells and do not spread. Thus, protective immunity may not block the primary infection of cells but may be mounted quickly enough to prevent spread of wild-type virus and induction of disease.
Possible role for viral immune evasion.
Prior immunity has been shown to decrease the efficacy of vaccine vector systems derived from certain other DNA viruses, such as poxviruses and adenoviruses (16, 19, 55, 57, 64). As a result, the use of these vaccine candidates may be limited by their inability to vaccinate immune individuals or to be readministered to a single patient.
It is unclear why HSV behaves differently than these other viruses, and a greater understanding of the subtle differences between HSV and these other viral systems will be necessary to determine the reasons for the observed variations in vaccine efficacy in vivo. We hypothesize that the ability of the HSV vectors to be efficacious in immune mice is due in part to the immune evasion properties of HSV. HSV encodes a number of immune-modulatory proteins that may aid in the protection of virions or virus-infected cells from immune-mediated clearance. For example, the interaction of glycoprotein C (gC) with the complement component C3 has been shown to protect virions from complement-dependent neutralization and to defend virus-infected cells from complement-mediated lysis (20, 35). Binding of HSV-1 gC to mouse C3 has been observed in vitro (29; unpublished result), consistent with a role for gC in enhancing primary infection or persistence of virus-infected cells in this system. In addition, a heterodimer of glycoproteins E (gE) and I (gI) forms an Fc gamma receptor that has been reported to protect virus-infected cells from antibody-dependent cellular cytotoxicity (15), though this Fc binding appears to be limited to human IgG and does not extend to mouse antibody (51).
There is some evidence that preexisting immunity can diminish the ability of an HSV gene therapy vector to transduce a tumor in mice following intravenous delivery (28) but not after direct intratumoral injection (8). These results suggest that the route of vector delivery is an important factor in the efficacy of HSV vectors and that serum factors may play a determining role in the effectiveness of these vectors. A recent report by Ikeda et al. (30) implicates the complement system in this antiviral serum response, because depletion of complement C3 using cobra venom factor in naïve mice resulted in increased vector efficacy, as measured by gene transduction into a brain tumor, following intravascular delivery of either an HSV-derived or adenovirus-derived vector. Therefore, HSV vectors appear to be labile when delivered directly into mouse serum. However, because HSV infection does not naturally occur intravenously, it is possible that the virus has evolved mechanisms that are better suited for infection at peripheral sites.
Potential mechanisms for immune durability.
A second surprising result is the durability of the antibody responses induced by the HSV vector. We propose that the ability of the vector to infect primary cells and the potential of virus-infected cells to persist in vivo may be important determinants of vaccine efficacy. Cellular cytopathic effects induced by the virus may significantly affect the ability of infected cells to maintain long-term antigen expression. The durability of the antibody response generated by HSV may also indicate that this virus is able to readily activate long-lived plasma cells (65). Alternatively, viral antigens may persist for extended periods on antigen-presenting cells, or the virus itself may persist at low levels and continue to express small amounts of antigen.
Clinical implications.
The results from this report may also influence the interpretation of experiments to test candidate genital herpes vaccines. We have shown previously, using the same virus (d301), dose (2 × 106 PFU), and route of inoculation (s.c.), that immunization with a replication-defective HSV protected mice from disease following challenge HSV infection, and always in the absence of sterilizing immunity (34, 43, 45, 46). The data presented here suggest that HSV will be able to infect a first round of cells regardless of preexisting host immunity against the virus. This may indicate that sterilizing immunity to HSV will be very difficult if not impossible to achieve by vaccination. In addition, our results show that antibody responses to novel viral antigens can be generated equally well in naive and HSV-immune hosts. Therefore, measuring seroconversion to an HSV-2-specific antigen, such as gG-2, following immunization may not be a true indication of a vaccine's ability to protect from disease. Rather, in vivo measures of viral replication and spread, such as clinical disease scores, reactivation profiles, and virus shedding, may continue to be the preferred endpoints for future HSV vaccine studies.
We have evaluated the efficacy of replication-defective HSV-derived vaccine vectors in immune animals by measuring the generation and durability of IgG antibody responses and have shown that the efficacy of an HSV vector is not diminished by prior HSV immunity, as judged by the ability of immune mice to generate β-galactosidase-specific IgG responses. This observation differs from the suppression that has been described for some vector systems in similar mouse models. This difference may be due to the ability of HSV to evade host immune responses in the periphery. In addition, we have shown that HSV vectors are able to elicit a durable immunity, characterized by HSV-specific and β-galactosidase-specific IgG antibody responses that are maintained for at least 1 year following vector administration. Notably, the durability of these responses is not diminished by prior immunization. Additional studies are needed to determine if this is due to induction of long-lived plasma cells, persistence of viral antigens, or maintenance of transcriptionally active viral genomes. These properties of HSV as a vector in the murine system would be very useful if they can be extended to the situation of human immunization.
Acknowledgments
We thank Darren Higgins for critical reviews of this work, Maurits Kleijnen for many helpful discussions, and Lisa Holik for assistance with preparing the manuscript for publication.
This work was supported by grants NS35138 and CA26345 from the NIH.
REFERENCES
- 1.Aurelian, L., H. Kokuba, and C. C. Smith. 1999. Vaccine potential of a herpes simplex virus type 2 mutant deleted in the PK domain of the large subunit of ribonucleotide reductase (ICP10). Vaccine 17:1951-1963. [DOI] [PubMed] [Google Scholar]
- 2.Belyakov, I. M., B. Moss, W. Strober, and J. Berzofsky. 1999. Mucosal vaccination overcomes the barriers to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proc. Natl. Acad. Sci. USA 96:4512-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boursnell, M. E. G., C. Entwisle, D. Blakeley, C. Roberts, I. A. Duncan, S. E. Chisholm, G. M. Martin, R. Jennings, D. Ni Challanain, I. Sobek, S. C. Inglis, and C. S. McLean. 1997. A genetically inactivated herpes simplex virus type 2 (HSV-2) vaccine provides effective protection against primary and recurrent HSV-2 disease. J. Infect. Dis. 175:16-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brehm, M., L. A. Samaniego, R. H. Bonneau, N. A. DeLuca, and S. S. Tevethia. 1999. Immunogenicity of herpes simplex virus type 1 mutants containing deletions in one or more alpha-genes: ICP4, ICP27, ICP22, and ICP0. J. Virol. 256:258-269. [DOI] [PubMed] [Google Scholar]
- 5.Brehm, M. A., R. H. Bonneau, D. M. Knipe, and S. S. Tevethia. 1997. Immunization with a replication deficient mutant of herpes simplex virus type 1 (HSV-1) induces a CD8+ cytotoxic T-lymphocyte (CTL) response and confers a level of protection comparable to wild-type HSV-1. J. Virol. 71:3534-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brubaker, J. O., C. M. Thompson, L. A. Morrison, D. M. Knipe, G. B. Siber, and R. W. Finberg. 1996. Th1-associated immune responses to beta-galactosidase expressed by replication-defective herpes simplex virus. J. Immunol. 157:1598-1604. [PubMed] [Google Scholar]
- 7.Caley, I. J., M. R. Betts, N. L. Davis, R. Swanstrom, J. A. Frelinger, and R. E. Johnston. 1999. Venezuelan equine encephalitis virus vectors expressing HIV-1 proteins: vector design strategies for improved vaccine efficacy. Vaccine 17:32124-33135. [DOI] [PubMed] [Google Scholar]
- 8.Chahlavi, A., S. D. Rabkin, T. Todo, P. Sundaresan, and R. Martuza. 1999. Effect of prior exposure to herpes simplex virus 1 on viral vector-mediated tumor therapy in immunocompetent mice. Gene Ther. 6:1751-1758. [DOI] [PubMed] [Google Scholar]
- 9.Corey, L., and H. H. Handsfield. 2000. Genital herpes and public health: Addressing a global problem. JAMA 283:791-794. [DOI] [PubMed] [Google Scholar]
- 10.Da Costa, X. J., N. Bourne, L. R. Stanberry, and D. M. Knipe. 1997. Construction and characterization of a replication-defective herpes simplex virus 2 ICP8 mutant strain and its use in immunization studies in a guinea pig model of genital herpes. Virology 232:1-12. [DOI] [PubMed] [Google Scholar]
- 11.Da Costa, X. J., M. A. Brockman, E. Alicot, M. Ma, M. B. Fischer, X. Zhou, D. M. Knipe, and M. C. Carroll. 1999. Humoral response to herpes simplex virus is complement-dependent. Proc. Natl. Acad. Sci. USA 96:12708-12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Da Costa, X. J., C. A. Jones, and D. M. Knipe. 1999. Immunization against genital herpes with a vaccine virus that has defects in productive and latent infection. Proc. Natl. Acad. Sci. USA 96:6994-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da Costa, X. J., L. A. Morrison, and D. M. Knipe. 2001. Comparison of different forms of herpes simplex replication-defective mutant viruses as vaccines in a mouse model of genital herpes. Virology 288:256-263. [DOI] [PubMed] [Google Scholar]
- 14.Davis, N. L., K. W. Brown, and R. E. Johnston. 1996. A viral vaccine vector that expresses foreign genes in lymph nodes and protects against mucosal challenge. J. Virol. 70:3781-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubin, G., E. Socolof, I. Frank, and H. M. Friedman. 1991. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J. Virol. 65:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etlinger, H. M., and W. Altenburger. 1991. Overcoming inhibition of antibody responses to a malaria recombinant vaccinia virus caused by prior exposure to wild-type virus. Vaccine 9:470-472. [DOI] [PubMed] [Google Scholar]
- 17.Farrell, H. E., C. S. McLean, C. Harley, S. Efstathiou, S. Inglis, and A. C. McLean. 1994. Vaccine potential of a herpes simplex virus type 1 mutant with an essential glycoprotein deleted. J. Virol. 68:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming, D. T., G. M. McQuillan, R. E. Johnson, A. J. Nahmias, S. O. Aral, F. K. Lee, and M. E. St. Louis. 1997. Herpes simplex virus type 2 in the United States, 1976 to 1994. N. Engl. J. Med. 337:1105-1111. [DOI] [PubMed] [Google Scholar]
- 19.Flexner, C., B. R. Murphy, J. F. Rooney, C. Wohlenberg, V. Yuferov, A. L. Notkins, and B. Moss. 1988. Successful vaccination with a polyvalent live vector despite existing immunity to an expressed antigen. Nature 335:259-262. [DOI] [PubMed] [Google Scholar]
- 20.Friedman, H. M., L. Wang, N. O. Fishman, J. D. Lambris, R. J. Eisenberg, G. H. Cohen, and J. Lubinski. 1996. Immune evasion properties of herpes simplex virus type 1 glycoprotein gC. J. Virol. 70:4253-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujioka, N., R. Akazawa, K. Ohashi, M. Fujii, M. Ikeda, and M. Kurimoto. 1999. Interleukin-18 protects mice against acute herpes simplex virus type 1 infection. J. Virol. 73:2401-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao, M., and D. M. Knipe. 1989. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J. Virol. 63:5258-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halbert, C. L., E. A. Rutledge, J. M. Allen, D. W. Russell, and A. D. Miller. 2000. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J. Virol. 74:1524-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hantman, M. J., E. L. Hohmann, C. G. Murphy, D. M. Knipe, and S. I. Miller. 1999. Antigen delivery systems: Development of recombinant live vaccines using viral or bacterial vectors, p. 779-791. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Stober, J. R. McGhee, and J. Bienenstock (ed.), Mucosal immunology. Academic Press, San Diego, Calif.
- 25.Harandi, A. M., B. Svennerholm, J. Holmgren, and K. Eriksson. 2001. Interleukin-12 (IL-12) and IL-18 are important in innate defense against genital herpes simplex virus type 2 infection in mice but are not required for the development of acquired gamma interferon-mediated protective immmunity. J. Virol. 75:6705-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heise, M. T., and H. W. Virgin, 4th. 1995. The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J. Virol. 69:904-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hendrzak, J. A., and P. S. Morahan. 1994. The role of macrophages and macrophage cytokines in host resistance to herpes simplex virus. Immunol. Ser. 60:601-617. [PubMed] [Google Scholar]
- 28.Herrlinger, U., C. M. Kramm, K. S. Aboody-Guterman, J. S. Silver, K. Ikeda, K. M. Johnston, P. A. Pechan, R. F. Barth, D. Finkelstein, E. A. Chiocca, D. N. Louis, and X. O. Breakefield. 1998. Pre-existing herpes simplex virus 1 (HSV-1) immunity decreases, but does not abolish, gene transfer to experimental brain tumors by a HSV-1 vector. Gene Ther. 5:809-819. [DOI] [PubMed] [Google Scholar]
- 29.Huemer, H. P., C. Larcher, S. van Drunen Littel-van den Hurk, and L. A. Babiuk. 1993. Species selective interaction of Alphaherpesvirinae with the “unspecific” immune system of the host. Arch. Virol. 130:353-364. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda, K., H. Wakimoto, T. Ichikawa, S. Jhung, F. H. Hochberg, D. N. Louis, and E. A. Chiocca. 2000. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J. Virol. 74:4765-4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juillard, V., P. Villefroy, D. Godfrin, A. Pavirani, A. Venet, and J. G. Guillet. 1995. Long-term humoral and cellular immunity induced by a single immunization with replication-defective adenovirus recombinant vector. Eur. J. Immunol. 25:3467-3473. [DOI] [PubMed] [Google Scholar]
- 32.Knipe, D. M., and A. E. Spang. 1982. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J. Virol. 43:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohl, S., N. C. Strynadka, R. S. Hodges, and L. Pereira. 1990. Analysis of the role of antibody-dependent cellular cytotoxic antibody activity in murine neonatal herpes simplex virus infection with antibodies to synthetic peptides of glycoprotein D and monoclonal antibodies to glycoprotein B. J. Clin. Investig. 86:273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuklin, N. A., M. Daheshia, P. C. Marconi, D. M. Krisky, R. J. Rouse, J. C. Glorioso, E. Manican, and B. T. Rouse. 1998. Modulation of mucosal and systemic immunity by enteric administration of nonreplicating herpes simplex virus expressing cytokines. Virology 240:245-253. [DOI] [PubMed] [Google Scholar]
- 35.Lubinski, J. M., L. Wang, A. M. Soulika, R. Burger, R. A. Wetsel, H. Colten, G. H. Cohen, R. J. Eisenberg, J. D. Lambris, and H. M. Friedman. 1998. Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo. J. Virol. 72:8257-8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandl, S., L. Hix, and R. Andino. 2001. Preexisting immunity to poliovirus does not impair the efficacy of recombinant poliovirus vaccine vectors. J. Virol. 75:622-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandl, S., L. J. Sigal, K. L. Rock, and R. Andino. 1998. Poliovirus vaccine vectors elicit antigen-specific cytotoxic T cells and protect mice against lethal challenge with malignant melanoma cells expressing a model antigen. Proc. Natl. Acad. Sci. USA 95:8216-8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manickan, E., R. J. Rouse, Z. Yu, W. S. Wire, and B. T. Rouse. 1995. Genetic immunization against herpes simplex virus. Protection is mediated by CD4+ T lymphocytes. J. Immunol. 155:259-265. [PubMed] [Google Scholar]
- 39.Mastrangeli, A., B. G. Harvey, J. Yao, G. Wolff, I. Kovesdi, R. G. Crystal, and E. Falck-Pedersen. 1996. “Sero-switch” adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum. Genet. Ther. 7:79-87. [DOI] [PubMed] [Google Scholar]
- 40.McLean, C. S., N. C. D., I. Duncan, M. E. Boursnell, R. Jennings, and S. C. Inglis. 1996. Induction of a protective immune response by mucosal vaccination with a DISC HSV-1 vaccine. Vaccine 14:987-992. [DOI] [PubMed] [Google Scholar]
- 41.McLean, C. S., M. Erturk, R. Jennings, D. Nichallanain, A. C. Minson, I. Duncan, M. E. G. Boursnell, and S. C. Inglis. 1994. Protective vaccination against primary and recurrent disease caused by herpes simplex virus (HSV) type 2 using a genetically disabled HSV-1. J. Infect. Dis. 170:1100-1109. [DOI] [PubMed] [Google Scholar]
- 42.Milligan, G. N., and D. Bernstein. 1995. Analysis of herpes simplex virus-specific T cells in the murine female gential tract following genital infection with herpes simplex type 2. Virology 212:481-489. [DOI] [PubMed] [Google Scholar]
- 43.Morrison, L. A., X. J. Da Costa, and D. M. Knipe. 1998. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology 243:178-187. [DOI] [PubMed] [Google Scholar]
- 44.Morrison, L. A., and D. M. Knipe. 1997. Contributions of antibody and T cell subsets to protection elicited by immunization with a replication-defective mutant of herpes simplex virus type 1. Virology 239:315-326. [DOI] [PubMed] [Google Scholar]
- 45.Morrison, L. A., and D. M. Knipe. 1994. Immunization with replication-defective mutants of herpes simplex virus type 1: Sites of immune intervention in pathogenesis of challenge virus infection. J. Virol. 68:689-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrison, L. A., and D. M. Knipe. 1996. Mechanisms of immunization with a replication-defective mutant of herpes simplex virus 1. Virology 220:402-413. [DOI] [PubMed] [Google Scholar]
- 47.Morrison, L. A., L. Zhu, and L. G. Thebeau. 2001. Vaccine-induced serum immunoglobin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T cells. J. Virol. 75:1195-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moss, B. 1996. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc. Natl. Acad. Sci. USA 93:11341-11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy, B. R., and R. M. Chanock. 2001. Immunization against viral diseases, p. 435-468. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 50.Murphy, C. G., W. T. Lucas, R. Means, S. Czajak, C. L. Hale, J. D. Lifson, A. Kauer, R. P. Johnson, D. M. Knipe, and R. C. Desrosiers. 2000. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J. Virol. 74:7745-7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagashunmugam, T., J. Lubinski, L. Wang, L. T. Goldstein, B. S. Weeks, P. Sundaresan, E. H. Kang, G. Dubin, and H. M. Friedman. 1998. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 72:5351-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen, L., D. M. Knipe, and R. W. Finberg. 1994. Mechanism of virus-induced Ig subclass shifts. J. Immunol. 152:478-484. [PubMed] [Google Scholar]
- 53.Nguyen, L. H., D. M. Knipe, and R. W. Finberg. 1992. Replication-defective mutants of herpes simplex virus (HSV) induce cellular immunity and protect against lethal HSV infection. J. Virol. 66:7067-7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paoletti, E. 1996. Applications of pox virus vectors to vaccination: an update. Proc. Natl. Acad. Sci. USA 93:11349-11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papp, Z., L. A. Babiuk, and M. E. Baca-Estrada. 1999. The effect of preexisting adenovirus-specific immunity on immune responses induced by recombinant adenovirus expressing glycoprotein D of bovine herpesvirus type 1. Vaccine 17:933-943. [DOI] [PubMed] [Google Scholar]
- 56.Parr, E. L., and M. B. Parr. 1999. Immune responses and protection against vaginal infection after nasal or vaginal immunization with attenuated herpes simplex virus type-2. Immunology 98:639-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parr, M. J., P. Y. Wen, M. Schaub, S. J. Khoury, M. H. Sayegh, and H. A. Fine. 1998. Immune parameters affecting adenoviral vector gene therapy in the brain. J. Neurovirol. 4:194-203. [DOI] [PubMed] [Google Scholar]
- 58.Pushko, P., M. Parker, G. V. Ludwig, N. L. Davis, R. E. Johnston, and J. F. Smith. 1997. Replication-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous pathogens in vivo. Virology 239:389-401. [DOI] [PubMed] [Google Scholar]
- 59.Ramirez, J. C., M. M. Gherardi, and M. Esteban. 2000. Biology of attenuated modified vaccinia virus Ankara recombinant vector in mice: virus fate and activation of B- and T-cell immune responses in comparison with the Western Reserve strain and advantages as a vaccine. J. Virol. 74:923-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramsay, A. J., S. J. Kent, R. A. Strugnell, A. Suhrbier, S. A. Thomson, and I. A. Ramshaw. 1999. Genetic vaccination strategies for enhanced cellular, humoral and mucosal immunity. Immunol. Rev. 171:27-44. [DOI] [PubMed] [Google Scholar]
- 61.Randrianarison-Jewtoukoff, V., and M. Perricaudet. 1995. Recombinant adenoviruses as vaccines. Biologicals 23:145-157. [DOI] [PubMed] [Google Scholar]
- 62.Rawls, W. E., K. Iwamoto, E. Adam, and J. L. Melnick. 1970. Measurement of antibodies to herpesvirus types 1 and 2 in human sera. J. Immunol. 104:599-606. [PubMed] [Google Scholar]
- 63.Schadeck, E. B., C. D. Partidos, A. R. Fooks, O. E. Obeid, G. W. Wilkinson, J. R. Stephenson, and M. W. Steward. 1999. CTL epitopes identified with a defective recombinant adenovirus expressing measles virus nucleoprotein and evaluation of their protective capacity in mice. Virus Res. 65:75-86. [DOI] [PubMed] [Google Scholar]
- 64.Schulick, A. H., G. Vassalli, P. F. Dunn, G. Dong, J. J. Rade, C. Zamarron, and D. A. Dichek. 1997. Established immunity precludes adenovirus-mediated gene transfer in rat carotid arteries. Potential for immunosuppression and vector engineering to overcome barriers of immunity. J. Clin. Investig. 99:209-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slifka, M. K., R. Antia, J. K. Whitmire, and R. Ahmed. 1998. Humoral immunity due to long-lived plasma cells. Immunity 8:363-372. [DOI] [PubMed] [Google Scholar]
- 66.Speck, P. G., S. Efstathiou, and A. C. Minson. 1996. In vivo complementation studies of a glycoprotein H-deleted herpes simplex virus-based vector. J. Gen. Virol. 77:2563-2568. [DOI] [PubMed] [Google Scholar]
- 67.Tubulekas, I., P. Berglund, M. Fleeton, and P. Liljestrom. 1997. Alphavirus expression vectors and their use as recombinant vaccines: a minireview. Gene 190:191-195. [DOI] [PubMed] [Google Scholar]
- 68.Vollstedt, S., M. Franchini, G. Alber, M. Ackermann, and M. Suter. 2001. Interleukin-12- and gamma interferon-dependent innate immunity are essential and sufficient for long-term survival of passively immunized mice infected with herpes simplex virus type 1. J. Virol. 75:9596-9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whitley, R. J. 1988. Herpes simplex virus infections of the central nervous system. A review. Am. J. Med. 85:61-67. [PubMed] [Google Scholar]
- 70.Wu, L., and P. S. Morahan. 1992. Macrophages and other nonspecific defenses: role in modulating resistance against herpes simplex virus. Curr. Top. Microbiol. Immunol. 179:89-110. [DOI] [PubMed] [Google Scholar]
- 71.Yasukawa, M., A. Inatsuki, and Y. Kobayashi. 1988. Helper activity in antigen-specific antibody production mediated by CD4+ human cytotoxic T cell clones directed against herpes simplex virus. J. Immunol. 140:3419-3425. [PubMed] [Google Scholar]