Abstract
Background
Consumption of chocolate has been often hypothesized to reduce the risk of cardiovascular disease (CVD) due to chocolate's high levels of stearic acid and antioxidant flavonoids. However, debate still lingers regarding the true long term beneficial cardiovascular effects of chocolate overall.
Methods
We reviewed English-language MEDLINE publications from 1966 through January 2005 for experimental, observational, and clinical studies of relations between cocoa, cacao, chocolate, stearic acid, flavonoids (including flavonols, flavanols, catechins, epicatechins, and procynadins) and the risk of cardiovascular disease (coronary heart disease (CHD), stroke). A total of 136 publications were selected based on relevance, and quality of design and methods. An updated meta-analysis of flavonoid intake and CHD mortality was also conducted.
Results
The body of short-term randomized feeding trials suggests cocoa and chocolate may exert beneficial effects on cardiovascular risk via effects on lowering blood pressure, anti-inflammation, anti-platelet function, higher HDL, decreased LDL oxidation. Additionally, a large body of trials of stearic acid suggests it is indeed cholesterol-neutral. However, epidemiologic studies of serum and dietary stearic acid are inconclusive due to many methodologic limitations. Meanwhile, the large body of prospective studies of flavonoids suggests the flavonoid content of chocolate may reduce risk of cardiovascular mortality. Our updated meta-analysis indicates that intake of flavonoids may lower risk of CHD mortality, RR = 0.81 (95% CI: 0.71–0.92) comparing highest and lowest tertiles.
Conclusion
Multiple lines of evidence from laboratory experiments and randomized trials suggest stearic acid may be neutral, while flavonoids are likely protective against CHD mortality. The highest priority now is to conduct larger randomized trials to definitively investigate the impact of chocolate consumption on long-term cardiovascular outcomes.
Introduction
Cardiovascular disease (CVD), as a group, is a leading cause of the death in the United States [1], and worldwide, causing over 16.7 million deaths globally in 2002 [2]. In 1990, greater than 85,000,000 disability-adjusted life-years were lost worldwide due to coronary heart disease (CHD) and stroke; this CVD disease burden is projected to rise to 143,000,000 disability-adjusted life-years by 2020 [2]. Studies suggest cardiovascular diseases may be preventable by lifestyle modifications, such as exercise and nutrition [3-7]. Additionally, the American Heart Association, American Diabetes Association, and the U.S. Preventive Services Task Force have each indicated the likely importance of diet for the prevention of CVD [8-10].
In the American diet, fruits, vegetables, tea, wine and chocolate are major sources of antioxidants, which have been shown to have protective effects against CVD [11,12]. One class of antioxidants, flavonoids, commonly found in such foods, have attracted great interest in potentially lowering risk of CVD. Since cocoa products contain greater antioxidant capacity and greater amounts of flavonoids per serving than all teas and red wines [12,13], it is important to explore chocolate's potential effects on CVD.
Since ancient times, chocolate has long been used as a medicinal remedy [14] and been proposed in medicine today for preventing various chronic diseases [15,16]. While chocolate has also sometimes been criticized for its saturated fat content, mostly in the form of long-chain stearic acid, chocolate has also been lauded for its antioxidant potential. However, to this date there are no long-term randomized feeding trials of chocolate to assess effects on actual cardiovascular events. Nevertheless, there have been many short-term trials of cocoa and chocolate examining effects on cardiovascular intermediates, and numerous epidemiology studies of stearic acid and flavonoids exploring associations with cardiovascular outcomes.
This systematic review serves to comprehensively evaluate the experimental and epidemiologic evidence of cocoa and chocolate products. Particularly, we focus on the controversial potential benefits of the chocolate components stearic acid and flavonoids; review their overall effects on CVD risk factor intermediates and CVD endpoints; and conduct a meta-analysis of total flavonoid intake and risk of CHD mortality.
Methods
We reviewed English-language MEDLINE publications from January 1965 through June 2005 for experimental, observational, and clinical studies of relations between the exposure search terms of chocolate, stearic acid, flavonoids (including flavonols, flavanols, catechins, epicatechins, and procynadins) and the outcome search terms of cardiovascular disease (coronary heart disease, ischemic heart disease, stroke), cholesterol, blood pressure, platelet, oxidation, and thrombosis. Approximately 400 papers were reviewed. Based on the relevance, strength, and quality of the design and methods, 136 publications were selected for inclusion.
We mainly focused on studies in humans, particularly randomized trials of either parallel or cross-over design, and prospective observational studies. Since no randomized trials have yet assessed chocolate in relation to definitive CVD outcomes, prospective observational studies evaluating chocolate sub-components and the risk of CVD outcomes were weighted equally in the overall evaluation. For overall objective evaluation, the strength of the evidence was evaluated by the design and quality of individual studies, the consistency of findings across studies, and the biologic plausibility of possible mechanisms. Finally, consistent with methods of the outdated prior analysis [17], an updated meta-analysis was conducted and relative risks estimates pooled using a random-effects model [18].
Review
Stearic acid in chocolate
Saturated fat has long been thought to contribute to atherosclerosis, and thus, adverse for CVD risk. However, stearic acid has been suggested to be a non-atherogenic type of dietary saturated fat. Stearic acid is a long-chain 18:0 saturated fatty acid found commonly in meats and dairy products. Cocoa butter, a fat derived from cocoa plants and predominantly found in dark chocolate [19], contains an average of 33% oleic acid (cis-18:1 monounsaturated), 25% palmitic acid (16:0 saturated), and 33% of stearic acid [20]. Thought it is generally considered that saturated fats overall adversely increase the total cholesterol and LDL levels [21-23], early studies have also suggested stearic acid may be non-cholesterolemic [21,22]. This has been confirmed in a series of studies and a meta-analysis of 60 controlled feeding trials which concludes stearic acid neither lowers HDL, nor increases LDL or total cholesterol [24-28]. The meta-analysis also estimates, that per 1% energy isocaloric replacement of stearic acid for carbohydrates, stearic acid intake is predicted to beneficially lower serum triglycerides by -17.0 nmol/L (p < 0.001) [26]. The most recent trial also shows the effects of stearic acid on lipids is even similar to oleic and linoleic acids [29].
Emerging studies have begun to explain how stearic acid in chocolate may be cholesterol-neutral. One suggested mechanism is stearic acid's lower absorption, which has been found in several animal and human studies [30-33], though only minimally in others [34,35]. These discrepancies may be attributed to the relative position of stearate on the triglyceride molecule which may affect its relative absorption rate [36,37]. This might also explain the suggestion that stearic acid from plants sources, such as cocoa, may be different from animal derived sources of stearic acid [38]. Furthermore, some feeding trials found lower absorption of cocoa buttered compared to corn oil [39], though not in others [40]. However, heterogeneity may be due to the dual-presence of calcium in chocolate, in which other trials found cocoa butter absorption further decreased 13% when supplemented with calcium (1% by weight) [41], as is done in chocolate manufacturing. Finally, another strongly supported protective mechanism relate to the relatively high percent desaturation of stearic acid to monosaturated oleic acid [35,42-45], a fat considered hypocholesterolemic [27,46-48] and protective against coronary heart disease [3,49].
Two other pathways suggested for potential benefit are stearic acid's potential anti-platelet and blood pressure reductions actions. Feeding trials have shown that stearic acid reduces mean platelet volume [50,51], an index of platelet activation. However, mixed findings have been observed regarding the relationship between stearic acids and factor VIIc coagulation factor, a predictor of fatal CHD [52-54]. Though an early study suggested that stearic acid may increase factor VIIc [55], no effect on levels of factor VIIc by stearic acid was observed in two other trials [56,57]. Moreover, additional trials have refuted the earlier small study and, in fact, shown that stearic acid lowered the levels of factor VIIc coagulation factor compared to palmitic [50,58] and other saturated fatty acids [58]. As for the relationship between stearic acid and blood pressure, two feeding trials found stearic acid did not adversely affect systolic blood pressure [28,59]. Furthermore, cross-sectional analysis within the Multiple Risk Factor Intervention Trial even found stearic acid levels may be inversely associated with diastolic blood pressure [60].
In summary, given the vast majority of studies showing stearic acid has beneficial or neutral effects on blood pressures and clotting parameters, it appears unlikely stearic acid intake would adversely affect CVD risk through these risk factors. Data indicates stearic acid does not adversely affect established traditional lipid risk factors, with even favorable lowering of serum triglycerides if isocalorically replaced for carbohydrates.
Stearic Acid Observational Studies
However, the observational studies of stearic acid's association with CVD are inconclusive. (Table 2) Among retrospective studies, a Japanese case-control study of serum levels reported no association for stenosis [61], a Norwegian study found lower odds of MI [62], while a Costa Rican study of dietary intake found higher risk of MI [63] with higher intake of stearic acid. However, the results from the Costa Rican study should not be given much weight since retrospective self-report of dietary intakes are notoriously inaccurate and susceptible to reporting bias [64]. Nevertheless, higher rates of CHD and CAD progression was found in several prospective studies [65-68], while stroke was not increased in another study [69].
Table 2.
Observational Studies of Stearic Acid and Cardiovascular Outcomes
| Author | Year | Study design | N, Population | Stearic acid assessment method | CHD/MI Outcomes | Other |
| Kromhout [141] | 1995 | Ecologic | 12,763 men, 16 cohorts of 7CS | Dietary intake | ↑ CHD mortality | |
| Simon [68, 69] | 1995 | Prospective | 96 cases, 96 controls, USA-MRFIT | Serum levels | ↑ CHD incidence | Null-stroke incidence |
| Watts [67] | 1996 | Prospective | 50 men, Australia | Dietary intake | ↑ CAD progression | |
| Hojo [61] | 1998 | Case-control | 71 cases, 60 controls, Japan | Serum levels | Null-stenosis | |
| Hu [65] | 1999 | Prospective | 80,082 women, USA-nurses | Dietary intake | ↑ CHD incidence | |
| Yli-Jama [62] | 2002 | Case-control | 103 cases, 104 controls, Norway | Serum levels | ↓ MI incidence | |
| Kabagambe [63] | 2003 | Case-control | 485 cases, 508 controls, Costa Rica | Dietary intake | ↑ MI incidence | |
| Wang [66] | 2003 | Prospective | 3591 whites, USA | Serum levels | ↑ CHD mortality |
Abbreviations: 7CS, 7 Countries Study; MRFIT, Multiple Risk Factor Intervention Trial;
* High stearic acid level among men from geographic areas of high IHD mortality
On the other hand, several limitations exist for observational studies of stearic acid. First, researchers have cautioned that analyses of dietary stearic acid are very difficult due to high correlations of stearic acid intake with other fatty acids (often r = 0.7 to 0.9), thus impeding optimal study of associations [65]. Additionally, the larger prospective study that found higher risk of CHD also noted chocolate was a very small contributor (5%) of total stearic acid intake, with red meats as primary sources of stearic acid. Finally, since there exists high interconversion of stearic acid to unsaturated fatty acids [35,42-45], studies involving serum levels of stearic acid do not answer the relevant causal question of dietary intake of stearic acid and risk of disease. The associations of long-term serum stearic acid levels represent the effects of post-conversion stearic acid levels after a large proportion of the original dietary stearic acid has already been converted away to monounsaturated fat, which is well-established to exert protective effects against CVD [3,27,46-49].
Thus, relatively little information can be inferred from observational studies of the association of stearic acid and CHD, and no epidemiologic study has, thus far, appropriately and optimally answered the causal question of the association of dietary stearic acid intake and risk of CVD. However, a sufficient body of strong evidence from short term randomized trials suggests stearic acid components in chocolate may be beneficial for cardiovascular health. However, further research in this area is warranted.
Flavonoids in chocolate
A 100 g bar of milk chocolate contains 170 mg of flavonoid antioxidants, procyanidins and flavanols [12]. It is estimated that chocolate is a leading source of procyanidin intake in Western nations (18–20%) [70,71]. Flavonoids belong to a class of antioxidants called polyphenols from plants [72]. The basic structure of flavonoids is a C6-C3-C6 backbone with two armomatic rings and varying degrees of hydroxylation differentiating one flavonoid type from another [73]. Flavonoids can be divided into various subclasses, important of which are flavones, flavonols, flavanones, catechins, anthocyanidins and isoflavones. Cocoa, is particularly rich in the flavonoids, epicatechin, catechin, and procyanidins (polymers of catechins and epicatechins) [74]. (Figure 1)
Figure 1.
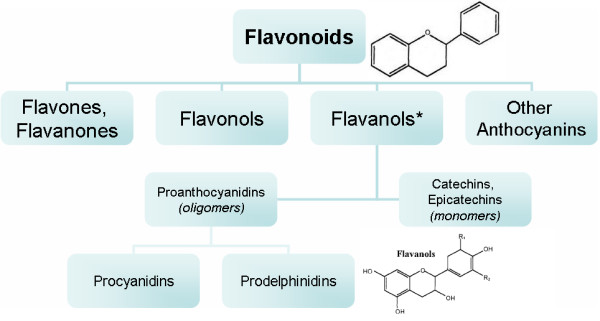
Structural skeleton of flavonoids and classification hierarchy of common flavonoids. *Flavanol is the predominate class of flavonoid found in cocoa and chocolate.
Various studies have compared the content of the flavanoids in cocoa with other food stuffs quantitatively. Figure 2 shows the comparative content of flavonoids in milk chocolate and dark chocolate versus other high-flavonoid foods. Cocoa has been shown to have the highest content of polyphenols (611 mg/serving) and flavanoids (564 mg/serving of epicatechin), greater than even tea and wine [13]. Per serving, dark chocolate contains substantially higher amounts of flavonoids than milk chocolate (951 mg of catechins per 40 g serving compared to 394 mg in white chocolate) [75], and levels of epicatechin in dark chocolate is comparable to red wine and tea [75]. Also of note, dark chocolate contains significantly greater amounts of total phenols as well as catechins than milk chocolate per serving (126+-7.4 μmol/g vs. 52.2+-20.2 μmol/g) [75]. In addition to dark chocolate having higher flavonoid content, the biologic effects of flavonoids may also be greater in dark chocolate because milk in milk chocolate may inhibit the intestinal absorption of flavanoids [76]. Finally, chocolate is also abundant in procyanidin flavonoids, comparable with levels in procyanidin-rich apples [77]. Thus, chocolate is a rich source of flavonoids, particularly catechins, epicatechins and procyanidins.
Figure 2.
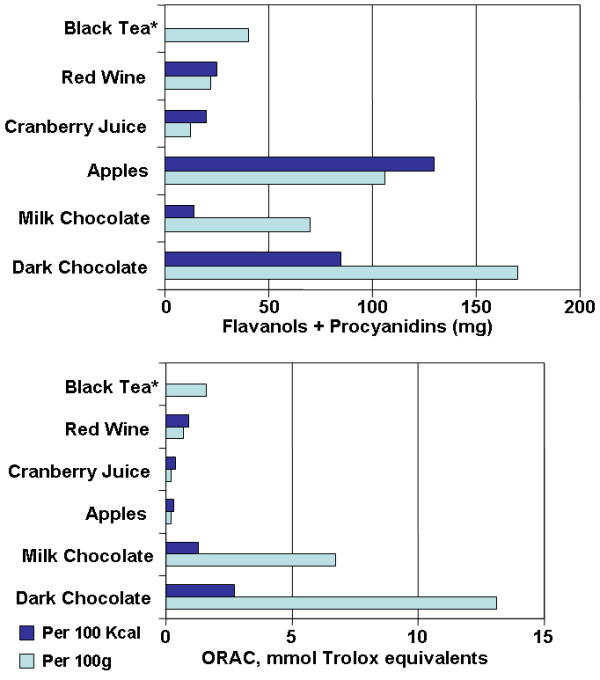
Flavonoid content and antioxidant capacity (ORAC) of milk chocolate and dark chocolate versus other high flavonoid foods. * Brewed, per 2 g bag/200 ml water. Antioxidant activity is reported as oxygen radical absorbance capacity (ORAC). Adapted from: Steinberg et al. J Am Diet Assoc 103: 215-23.
Mechanisms
Chocolate flavonoids have shown good dose-response bioavailability in humans [11,78,79]. There exists several mechanisms of how flavonoids may be protective against CVD; these include: antioxidant, anti-platelet, anti-inflammatory effects, as well as possibly increasing HDL, lowering blood pressure, and improving endothelial function. The body of trials involving chocolate flavonoids is summarized in Table 1.
Table 1.
Summary of Chocolate and Cocoa Feeding Trials
| Author | Year | No. Participants | Trial Design | Duration | Intervention | Outcome(s) |
| Kondo [83] | 1996 | 12 | Crossover | 1 meal, pre/post-meal measurement | Cocoa (35 g delipidated), vs. none | Decreased LDL oxidation |
| Rein [138] | 2000 | 30 | Parallel | 1 meal, 2 & 6 hrs | Cocoa beverage (300 ml, 19 g procyanidin), caffeinated beverage (17 mg caffeine), or water | Decreased platelet activation, decreased platelet function |
| Wang [79] | 2000 | 20 | Crossover | 1 meal, 1 week/phase | Procyanidin-rich chocolate (27, 53, 80 g), vs. none | Increased antioxidant capacity, decreased oxidative stress |
| Osakabe [88] | 2001 | 15 | Parallel | daily, 2 weeks | Cocoa powder (36 g/day), vs. sugar | Decreased LDL oxidation (increased lag time) |
| Wan [85] | 2001 | 23 | Crossover | daily, 4 weeks/phase | Cocoa powder (22 g/day) + dark chocolate (12 g/day), vs. average American diet | Decreased LDL oxidation (increased lag time), Increased HDL concentration |
| Schramm [101] | 2001 | 10 | Crossover | 1 meal, 2 & 6 hrs, 1 week/phase | Chocolate (35 g, high 4 mg/g vs. low 0.09 mg/g procyanidin) | Increased prostacyclin, decreased leukotriene (likely decreased platelet activation, anti-inflammatory) |
| Holt [95] | 2002 | 18 | Crossover | 1 meal, 2 hrs | Chocolate chips (25 g semi-sweet), vs. none | Decrease platelet function |
| Mathur [86] | 2002 | 25 | Crossover | daily, 6 weeks/phase | Dark chocolate (37 g/day), cocoa powder (31 g/day), vs. none | Decreased LDL oxidizability, marginal HDL increase |
| Pearson [92] | 2002 | 16 | Crossover | 1 meal, 1 day/phase | Cocoa beverage (300 ml, 19 g flavanol cocoa powder), cocoa beverage + aspirin, or aspirin | Decreased platelet activation, decreased platelet function, all additive of aspirin effects. |
| Heiss [99] | 2003 | 20 | Crossover | 1 meal, 1 day/phase | Cocoa beverages (100 ml, high or low flavan-3-ol) | Increased NO bioactivity, improved endothelial function |
| Innes [97] | 2003 | 30 | Parallel | 1 meal, 4 hrs | Dark (75% cocoa, highest flavonoid content), milk (20% cocoa), or white chocolate (no flavonoids) | Dark chocolate inhibited collagen-induced platelet aggregation |
| Murphy [94] | 2003 | 32 | Parallel | daily, 28 days | Cocoa flavonoid tablets (234 mg), vs. placebo | Decreased platelet function, no difference oxidation status |
| Serrafini [76] | 2003 | 12 | Crossover | 1 meal, 1 day/phase | Dark chocolate (100 g), dark chocolate (100 g) + milk (200 ml), or 200 g milk chocolate | Increase antioxidant capacity, in absence of milk |
| Taubert [118] | 2003 | 13 | Crossover | daily, 14 days/phase | Dark chocolate (100 g, 500 mg polyphenols), vs. white chocolate (90 g, 0 mg polyphenols) | Lower systolic and diastolic blood pressure with dark chocolate |
| Wiswedel [90] | 2004 | 20 | Crossover | 1 meal, 1 week washout | High flavanol (1.87 mg/ml) vs. low flavanol (0.14 mg/ml) cocoa beverage | Lower levels of lipid peroxidation indicators with high flavanol cocoa beverage |
| Engler [98] | 2004 | 21 | Parallel | daily, 2 weeks | Chocolate (high vs. low flavonoid) | Improved endothelial function, no difference oxidative stress, lipids with high flavonoid choc. |
| Mursu [115] | 2004 | 45 | Parallel | daily, 3 weeks | Dark chocolate, dark chocolate enriched with cocoa polyphenols, or white chocolate | Increased HDL concentration, no change LDL oxidizability |
| Grassi [116] | 2005 | 15 | Crossover | daily, 15 days/phase | Dark chocolate (100 g, 500 mg polyphenols), vs. white chocolate (90 g, 0 mg polyphenols) | Lower systolic blood pressure, improved insulin sensitivity, lower insulin resistance |
| Zhu [139] | 2005 | 8 | Parallel | 1 meal, 1–2–4–8 hrs | Cocoa beverage (high flavonoid); 0.25, 0.38, 0.50 g/kg body weight dose | Reduced susceptibility to free-radical induced hemolysis |
| Vlachopoulos [140] | 2005 | 17 | Crossover | 1 meal, 1 day/phase | Dark chocolate (100 g, 2.62 g procyanidin), vs. none | Improved endothelial function, vasodilation of brachial artery, no change in blood pressure |
| Fraga [119] | 2005 | 28 | Parallel | daily, 14 days | High flavanol milk chocolate (105 g, 168 mg flavanols) vs. low flavonoid chocolate (<5 mg flavanols) | Lower mean blood pressure, lower LDL cholesterol, lower oxidative stress markers in high flavanol chocolate group |
Central to the pathogenesis of atherosclerosis is the oxidation of low-density lipoprotein (LDL). The chemical structure of flavonoids gives the compound free radical scavenging ability, which means flavonoids may have antioxidant effects [80]. Various studies have confirmed the role of flavanoids as antioxidants in biological systems. Flavanoids in chocolate have been shown to exert potent antioxidant effects in vitro assays under artificial oxidative stress [13,81-84] as well increase antioxidant capacity as part of various chocolate feeding trials [79,85-89]. Additionally, because lipid soluble flavonoids may intercalate into the membranes of lipoprotein particles, studies have shown flavonoids to decrease lipid peroxidation of biological membranes [90]. Furthermore, a randomized trial also demonstrated that flavonoid-rich foods can protect human lymphocytes from oxidative damage in vivo [91].
Additionally, aggregation of platelets at the site of plaque rupture and endothelial dysfunction has been implicated in the pathogenesis of atherosclerosis. Current research has shown that a number of components of chocolate, particularly catechin and epicatechin, have significant antiplatelet effects, quantitatively similar to that of aspirin [92]. Randomized trials studying platelet activation markers, microparticle formation and primary platelet aggregation as end points have found that daily intake of cocoa beverages produces a significant reduction in all these endpoints among healthy volunteers [93-96]. There were also significant correlations between the reduction in these end points and the plasma concentrations of catechin and epicatechin [93-96]. Another study found a significant reduction in platelet activation in groups consuming 100 g of dark chocolate when compared to those consuming similar amounts of white chocolate and milk chocolate [97]. In addition, randomized trials have also shown that consumption of high-flavanoid dark chocolate is associated with a significant improvement of endothelial function, marked by increase in brachial artery flow mediated dilation [98-100], likely mediated by chocolate flavonoids increasing local production of nitric oxide [99,100].
Chocolate may also influence levels of leukotrienes and prostacyclins. Leukotrienes are potent vasocontrictors, proinflammatory agents and stimulate platelet aggregation, whereas prostacyclin is a vasodilator and inhibits platelet aggregation. Consumption of chocolate with high procyanidin content (147 mg) was shown in a feeding trial to significantly lower the levels of leukotrienes (29%) and increase the levels of prostacyclin (32%) when compared to a group consuming a low procyanidin (3.3 mg) chocolate [101]. In vitro studies have indeed demonstrated chocolate components to inhibit lipoxygenase pathways, which gives rise to proinflammatory leukotrienes [102,103]. Inflammation is now recognized as another independent mechanism in the pathogenesis of atherosclerosis, with various inflammatory markers having been shown to predict risk of future CVD events [104-108]. In addition to anti-inflammatory effects on the lipoxygenase pathway, cocoa polyphenols have also been shown to decrease inflammation via several mechanisms, namely: inhibition of mitogen induced activation of T cells, polyclonal activation of B cells, reduced expression of interleukin-2 (IL-2) messenger RNA, and reduced secretion of IL-2 by T cells[109] Other have also found chocolate procyanidins can modulate of a variety of other cytokines (e.g. IL-5, TNF-α, TGF-β), reducing their inflammatory effects [110-114].
Furthermore, multiple cocoa feeding trials have also found chocolate to increase HDL cholesterol [85,86,115], and decrease blood pressure [116-119]. Finally, there are also suggestive findings in a few trials that indicate high-flavonoid chocolate may also lower LDL cholesterol [119], and improve insulin sensitivity [116].
Thus, the large body of evidence from laboratory findings and randomized trials suggest that high-flavonoid chocolate may protect against LDL oxidation, inhibit platelet aggregation, improve endothelial function, increase HDL, lower blood pressure, and reduce inflammation – thereby protective against risk of CVD.
Flavonoid Observational Studies
Mechanistic studies involving stearic acid and flavonoids have only assessed effects on intermediate cardiovascular endpoints. However, one cannot always assume effects from short term trials effects will necessarily translate into long term effects on CVD outcomes. Therefore, one needs to examine observational studies followed to CVD events. While one small study found moderate consumption of candy and chocolate was associated with lower all-cause mortality [120], this analysis neither isolates chocolate nor CVD events. Thus, in absence of specific studies of chocolate flavonoids and risk of CVD, studies of all flavonoids are the best available evidence to infer risk.
The prospective studies of flavonoids and risk of CVD are summarized in Table 3. The earliest international ecologic study suggested flavonoid intake may be associated with lower rates of CHD mortality [121]. While some studies report flavonoid intake is not associated with CHD incidence [122-124], two other prospective studies suggested flavonoids may lower risk of MI [125,126]. For stroke, the evidence is fairly consistent. Other than one small early study which found a significantly lower risk of stroke with higher total flavonoid intake [127], most studies indicated no association for risk of stroke [124,128-130]. However, most of these studies had insufficient power to adequately study stroke, nor enough power to stratify on various subtypes of stroke with different etiologies.
Table 3.
Prospective Studies of Flavonoids and Cardiovascular Outcomes
| Author | Year | Study type | N, Population | Follow-up Years | Flavonoid Type | CHD/MI Incidence | CHD/MI Mortality | Stroke Mortality | Comments: |
| Hertog [125, 142]*, Keli [127] | 1993, 1996 | Prospective | 552 to 806 Men, Dutch | 5, then 10* | Total Flavonoids | ↓ | ↓ | ↓ | *Update 1997 analysis finds even stronger CHD association [142] |
| Knekt [131] | 1996 | Prospective | 5133 M+W, Finland | 26 | Total Flavonoids | ↓ | |||
| Rimm [123] | 1996 | Prospective | 34789 Men, USA | 6 | Total Flavonoids | Null | ↓* | *marginal significance, if past history of CVD | |
| Hertog [133] | 1997 | Prospective | 1900 Men, UK | 14 | Total Flavonoids | Null | ↑* | *marginal significance, *milk consumed w/tea | |
| Yochum [130] | 1999 | Prospective | 34492 PostM women, Iowa | 10 | Total Flavonoids | ↓ | Null | ||
| Hirvonen [126, 129] | 2000, 2001 | Prospective | 23596 Men, Finland | 6.1 | Total Flavonoids | ↓ MI | ↓* | Null | *suggestive, but non-significant |
| Arts [143] | 2001 | Prospective | 806 men, Dutch | 10 | Catechins (Flavonoid) | ↓ | Null | ||
| Arts [128] | 2001 | Prospective | 34492 PostM women, Iowa | 13 | Catechins (Flavonoid) | ↓ | |||
| Geleinjse [122] | 2002 | Prospective | 4807 M+W, Dutch | 5.6 | Total Tea Flavonoids | Null | ↓ | ||
| Knekt [132] | 2002 | Prospective | 10054 M+W Finland | 28 | Specific flavonoids | ↓ | ↓ | also ↓ type 2 diabetes | |
| Sesso [124] | 2003 | Prospective | 38445 women, USA | 6.9 | Total Flavonoids | Null | Null | Null | |
| META-ANALYSIS (updated)** | Total Flavonoids → CHD Mortality | RR = 0.81 (95% CI: 0.71–0.92)* | (extreme tertiles) | ||||||
**Updated meta-analysis includes: all studies of "total flavonoids" and CHD mortality; comparison of top vs. bottom tertile.
However, the most extensively consistent finding is the association between flavonoid intake and CHD mortality. A total of eight cohort studies found risk of lower CHD mortality with total or specific flavonoid intake [71,121,123,125,126,128,130-132], with one study finding marginally protective association among men with prior CVD conditions [123]. Only one study reported absolutely no association between flavonoid intake and CHD mortality [133]. However, as noted by the authors of one of the studies, a high background consumption of milk with tea intake may have led to the null finding [133], since milk intake has been shown to prevent the intestinal absorption of flavonoids [76].
A meta-analysis of the 7 prospective studies prior to September 2001 found that, overall, flavonoids may be protective against CHD mortality [17]. However, this meta-analysis did not include a large subsequent cohort study of 38,445 women [124], which found a non-significant inverse association between flavonoid intake and CHD mortality. However, results from our updated meta-analysis still indicate a significant protective association exists between flavonoid intake and risk of CHD mortality, RR = 0.81 (95% CI: 0.71–0.92), comparing highest vs. lowest tertiles.
However, a limitation of inference exists in that flavonoids consists of a wide variety of polyphenol compounds, the variety of which may differ between studies due to varying sources of dietary flavonoids. Nonetheless, dark chocolate does contain substantially more flavanols than tea, apple, onions, and red wine [12]. Additionally, chocolate has all the flavonoids of tea [134], has 4 times the catechins of tea [134], has many flavonoids not found in tea [135], and substantially contributes to the total flavonoid intake in the diet of many countries [136]. However, inference from observational studies on the protective effect of flavonoids in chocolate on CVD risk is somewhat indirect and may need to be examined by further studies.
Overall, these epidemiologic findings, combined with the large body of evidence from short term randomized chocolate feeding trials, suggests flavonoid intake from chocolate is likely protective against CVD, particularly CHD mortality. Additionally, given that dark chocolate has substantially higher levels of flavonoids than milk chocolate, and that milk may inhibit absorption of flavonoids – it would be more prudent to consume high flavonoid dark chocolate rather than milk chocolate.
Conclusion
According to the International Cocoa Organization, production has risen from 1.2 million tons per year in 1960 to 3.2 million tons per year in 2004 [137]. Given the rapidly increasing world consumption of chocolate and rising global rates of CVD, it is important to establish chocolate's association with CVD risk. The projected increase in global consumption could have profound effects if chocolate consumption does have implications for CVD.
Based upon our systematic review, multiple lines of evidence from laboratory experiments and randomized trials suggest stearic acid may be neutral, while flavonoids are likely protective against CVD, the latter of which is well supported by prospective observational studies that suggest flavonoids may lower the risk of CHD mortality. Though it has been approximated that eating 50 g of dark chocolate per day may reduce one's risk of CVD by 10.5% (95% CI: 7.0%–13.5%) [16], such crude estimates were based on results from studies of short duration, extrapolated to long term CVD outcomes. Therefore, the highest priority now is to conduct long-term randomized feeding trials, beyond short term studies of CVD risk factor intermediates, in order to definitively investigate the impact of chocolate consumption on cardiovascular outcomes.
Abbreviations
CHD, Coronary heart disease
CVD, Cardiovascular disease
CI, Confidence interval
HDL, High-density lipoprotein
IL, Interleukin
LDL, Low-density lipoprotein
NO, Nitric oxide
MI, Myocardial infarction
RR, Relative risk
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
All authors contributed to systematically reviewing articles. E.L.D. led the drafting of the manuscript, insights into nutritional metabolism, and S.G. provided further insights into clinical disease etiology.
Acknowledgments
Acknowledgements
We'd like to thank Dr. Eric Rimm for his encouragement and support.
Contributor Information
Eric L Ding, Email: eding@jhu.edu.
Susan M Hutfless, Email: shutfles@hsph.harvard.edu.
Xin Ding, Email: xinding@hsph.harvard.edu.
Saket Girotra, Email: sgirotra@post.harvard.edu.
References
- American Heart Association: Heart Disease and Stroke Statistics: 2004 Update. Dallas, TX , American Heart Association; 2003. [Google Scholar]
- Mackay J, Mensah G. The Atlas of Heart Disease and Stroke. The World Health Organization. http://www.who.int/cardiovascular_diseases/resources/atlas/en/
- Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288:2569–2578. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288:1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- Weisburger JH. Eat to live, not live to eat. Nutrition. 2000;16:767–773. doi: 10.1016/S0899-9007(00)00400-7. [DOI] [PubMed] [Google Scholar]
- Ding EL, Mozaffarian D. Optimal Dietary Habits for the Prevention of Stroke. Semin Neurol. 2006;26 doi: 10.1055/s-2006-933305. In press. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- Eyre H, Kahn R, Robertson RM, Clark NG, Doyle C, Hong Y, Gansler T, Glynn T, Smith RA, Taubert K, Thun MJ. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Stroke. 2004;35:1999–2010. doi: 10.1161/01.CIR.0000133321.00456.00. [DOI] [PubMed] [Google Scholar]
- Goldstein LB, Adams R, Becker K, Furberg CD, Gorelick PB, Hademenos G, Hill M, Howard G, Howard VJ, Jacobs B, Levine SR, Mosca L, Sacco RL, Sherman DG, Wolf PA, del Zoppo GJ. Primary prevention of ischemic stroke: A statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 2001;32:280–299. doi: 10.1161/01.str.32.1.280. [DOI] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. Guide to Clinical Preventive Services: Report of the U.S. Preventive Services Task Force. xcii. Baltimore, MD , Williams & Wilkins; 1996. p. 953. [Google Scholar]
- Kris-Etherton PM, Keen CL. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr Opin Lipidol. 2002;13:41–49. doi: 10.1097/00041433-200202000-00007. [DOI] [PubMed] [Google Scholar]
- Steinberg FM, Bearden MM, Keen CL. Cocoa and chocolate flavonoids: implications for cardiovascular health. J Am Diet Assoc. 2003;103:215–223. doi: 10.1053/jada.2003.50028. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kim YJ, Lee HJ, Lee CY. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J Agric Food Chem. 2003;51:7292–7295. doi: 10.1021/jf0344385. [DOI] [PubMed] [Google Scholar]
- Dillinger TL, Barriga P, Escarcega S, Jimenez M, Salazar Lowe D, Grivetti LE. Food of the gods: cure for humanity? A cultural history of the medicinal and ritual use of chocolate. J Nutr. 2000;130:2057S–72S. doi: 10.1093/jn/130.8.2057S. [DOI] [PubMed] [Google Scholar]
- Weisburger JH. Chemopreventive effects of cocoa polyphenols on chronic diseases. Exp Biol Med (Maywood) 2001;226:891–897. doi: 10.1177/153537020122601003. [DOI] [PubMed] [Google Scholar]
- Franco OH, Bonneux L, de Laet C, Peeters A, Steyerberg EW, Mackenbach JP. The Polymeal: a more natural, safer, and probably tastier (than the Polypill) strategy to reduce cardiovascular disease by more than 75% Bmj. 2004;329:1447–1450. doi: 10.1136/bmj.329.7480.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley RR, Neil HA. The relation between dietary flavonol intake and coronary heart disease mortality: a meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2003;57:904–908. doi: 10.1038/sj.ejcn.1601624. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Mustad V, Derr J. Effects of dietary stearic acid on plasma lipids and thrombosis. Nutrition Today. 1993.
- USDA National Nutrient Database http://www.nal.usda.gov/fnic/foodcomp/search/
- Keys A, Anderson JT, Grande F. Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet. 1957;273:959–966. doi: 10.1016/S0140-6736(57)91998-0. [DOI] [PubMed] [Google Scholar]
- Hegsted DM, McGandy RB, Myers ML, Stare FJ. Quantitative effects of dietary fat on serum cholesterol in man. Am J Clin Nutr. 1965;17:281–295. doi: 10.1093/ajcn/17.5.281. [DOI] [PubMed] [Google Scholar]
- Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr. 2001;20:5–19. doi: 10.1080/07315724.2001.10719008. [DOI] [PubMed] [Google Scholar]
- Bonanome A, Grundy SM. Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels. N Engl J Med. 1988;318:1244–1248. doi: 10.1056/NEJM198805123181905. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Mustad VA. Chocolate feeding studies: a novel approach for evaluating the plasma lipid effects of stearic acid. Am J Clin Nutr. 1994;60:1029S–1036S. doi: 10.1093/ajcn/60.6.1029S. [DOI] [PubMed] [Google Scholar]
- Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Yu S. Individual fatty acid effects on plasma lipids and lipoproteins: human studies. Am J Clin Nutr. 1997;65:1628S–1644S. doi: 10.1093/ajcn/65.5.1628S. [DOI] [PubMed] [Google Scholar]
- Storm H, Thomsen C, Pedersen E, Rasmussen O, Christiansen C, Hermansen K. Comparison of a carbohydrate-rich diet and diets rich in stearic or palmitic acid in NIDDM patients. Effects on lipids, glycemic control, and diurnal blood pressure. Diabetes Care. 1997;20:1807–1813. doi: 10.2337/diacare.20.12.1807. [DOI] [PubMed] [Google Scholar]
- Thijssen MA, Mensink RP. Small differences in the effects of stearic acid, oleic acid, and linoleic acid on the serum lipoprotein profile of humans. Am J Clin Nutr. 2005;82:510–516. doi: 10.1093/ajcn.82.3.510. [DOI] [PubMed] [Google Scholar]
- Dougherty RM, Allman MA, Iacono JM. Effects of diets containing high or low amounts of stearic acid on plasma lipoprotein fractions and fecal fatty acid excretion of men. Am J Clin Nutr. 1995;61:1120–1128. doi: 10.1093/ajcn/61.4.1120. [DOI] [PubMed] [Google Scholar]
- Jones AE, Stolinski M, Smith RD, Murphy JL, Wootton SA. Effect of fatty acid chain length and saturation on the gastrointestinal handling and metabolic disposal of dietary fatty acids in women. Br J Nutr. 1999;81:37–43. [PubMed] [Google Scholar]
- Baer DJ, Judd JT, Kris-Etherton PM, Zhao G, Emken EA. Stearic acid absorption and its metabolizable energy value are minimally lower than those of other fatty acids in healthy men fed mixed diets. J Nutr. 2003;133:4129–4134. doi: 10.1093/jn/133.12.4129. [DOI] [PubMed] [Google Scholar]
- Denke MA, Grundy SM. Effects of fats high in stearic acid on lipid and lipoprotein concentrations in men. Am J Clin Nutr. 1991;54:1036–1040. doi: 10.1093/ajcn/54.6.1036. [DOI] [PubMed] [Google Scholar]
- Bonanome A, Grundy SM. Intestinal absorption of stearic acid after consumption of high fat meals in humans. J Nutr. 1989;119:1556–1560. doi: 10.1093/jn/119.11.1556. [DOI] [PubMed] [Google Scholar]
- Emken EA, Adlof RO, Rohwedder WK, Gulley RM. Influence of linoleic acid on desaturation and uptake of deuterium-labeled palmitic and stearic acids in humans. Biochim Biophys Acta. 1993;1170:173–181. doi: 10.1016/0005-2760(93)90068-k. [DOI] [PubMed] [Google Scholar]
- Nestel PJ, Pomeroy S, Kay S, Sasahara T, Yamashita T. Effect of a stearic acid-rich, structured triacylglycerol on plasma lipid concentrations. Am J Clin Nutr. 1998;68:1196–1201. doi: 10.1093/ajcn/68.6.1196. [DOI] [PubMed] [Google Scholar]
- Brink EJ, Haddeman E, de Fouw NJ, Weststrate JA. Positional distribution of stearic acid and oleic acid in a triacylglycerol and dietary calcium concentration determines the apparent absorption of these fatty acids in rats. J Nutr. 1995;125:2379–2387. doi: 10.1093/jn/125.9.2379. [DOI] [PubMed] [Google Scholar]
- Li D. Relationship between the concentrations of plasma phospholipid stearic acid and plasma lipoprotein lipids in healthy men. Clin Sci (Lond) 2001;100:25–32. [PubMed] [Google Scholar]
- Mitchell DC, McMahon KE, Shively CA, Apgar JL, Kris-Etherton PM. Digestibility of cocoa butter and corn oil in human subjects: a preliminary study. Am J Clin Nutr. 1989;50:983–986. doi: 10.1093/ajcn/50.5.983. [DOI] [PubMed] [Google Scholar]
- Shahkhalili Y, Duruz E, Acheson K. Digestibility of cocoa butter from chocolate in humans: a comparison with corn-oil. Eur J Clin Nutr. 2000;54:120–125. doi: 10.1038/sj.ejcn.1600905. [DOI] [PubMed] [Google Scholar]
- Shahkhalili Y, Murset C, Meirim I, Duruz E, Guinchard S, Cavadini C, Acheson K. Calcium supplementation of chocolate: effect on cocoa butter digestibility and blood lipids in humans. Am J Clin Nutr. 2001;73:246–252. doi: 10.1093/ajcn/73.2.246. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Influence of stearic acid on cholesterol metabolism relative to other long-chain fatty acids. Am J Clin Nutr. 1994;60:986S–990S. doi: 10.1093/ajcn/60.6.986S. [DOI] [PubMed] [Google Scholar]
- Bonanome A, Bennett M, Grundy SM. Metabolic effects of dietary stearic acid in mice: changes in the fatty acid composition of triglycerides and phospholipids in various tissues. Atherosclerosis. 1992;94:119–127. doi: 10.1016/0021-9150(92)90236-A. [DOI] [PubMed] [Google Scholar]
- Elovson J. Conversions of palmitic and stearic acid in the intact rat. Biochim Biophys Acta. 1965;106:291–303. doi: 10.1016/0005-2760(65)90037-8. [DOI] [PubMed] [Google Scholar]
- Rhee SK, Kayani AJ, Ciszek A, Brenna JT. Desaturation and interconversion of dietary stearic and palmitic acids in human plasma and lipoproteins. Am J Clin Nutr. 1997;65:451–458. doi: 10.1093/ajcn/65.2.451. [DOI] [PubMed] [Google Scholar]
- Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J Lipid Res. 1985;26:194–202. [PubMed] [Google Scholar]
- Kris-Etherton PM, Pearson TA, Wan Y, Hargrove RL, Moriarty K, Fishell V, Etherton TD. High-monounsaturated fatty acid diets lower both plasma cholesterol and triacylglycerol concentrations. Am J Clin Nutr. 1999;70:1009–1015. doi: 10.1093/ajcn/70.6.1009. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Binkoski AE, Zhao G, Coval SM, Clemmer KF, Hecker KD, Jacques H, Etherton TD. Dietary fat: assessing the evidence in support of a moderate-fat diet; the benchmark based on lipoprotein metabolism. Proc Nutr Soc. 2002;61:287–298. doi: 10.1079/PNS2002157. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton P, Daniels SR, Eckel RH, Engler M, Howard BV, Krauss RM, Lichtenstein AH, Sacks F, St Jeor S, Stampfer M, Eckel RH, Grundy SM, Appel LJ, Byers T, Campos H, Cooney G, Denke MA, Howard BV, Kennedy E, Krauss RM, Kris-Etherton P, Lichtenstein AH, Marckmann P, Pearson TA, Riccardi G, Rudel LL, Rudrum M, Sacks F, Stein DT, Tracy RP, Ursin V, Vogel RA, Zock PL, Bazzarre TL, Clark J. Summary of the scientific conference on dietary fatty acids and cardiovascular health: conference summary from the nutrition committee of the American Heart Association. Circulation. 2001;103:1034–1039. doi: 10.1161/01.cir.103.7.1034. [DOI] [PubMed] [Google Scholar]
- Kelly FD, Sinclair AJ, Mann NJ, Turner AH, Abedin L, Li D. A stearic acid-rich diet improves thrombogenic and atherogenic risk factor profiles in healthy males. Eur J Clin Nutr. 2001;55:88–96. doi: 10.1038/sj.ejcn.1601122. [DOI] [PubMed] [Google Scholar]
- Kelly FD, Sinclair AJ, Mann NJ, Turner AH, Raffin FL, Blandford MV, Pike MJ. Short-term diets enriched in stearic or palmitic acids do not alter plasma lipids, platelet aggregation or platelet activation status. Eur J Clin Nutr. 2002;56:490–499. doi: 10.1038/sj.ejcn.1601332. [DOI] [PubMed] [Google Scholar]
- Meade TW, Mellows S, Brozovic M, Miller GJ, Chakrabarti RR, North WR, Haines AP, Stirling Y, Imeson JD, Thompson SG. Haemostatic function and ischaemic heart disease: principal results of the Northwick Park Heart Study. Lancet. 1986;2:533–537. doi: 10.1016/S0140-6736(86)90111-X. [DOI] [PubMed] [Google Scholar]
- Meade TW, Ruddock V, Stirling Y, Chakrabarti R, Miller GJ. Fibrinolytic activity, clotting factors, and long-term incidence of ischaemic heart disease in the Northwick Park Heart Study. Lancet. 1993;342:1076–1079. doi: 10.1016/0140-6736(93)92062-X. [DOI] [PubMed] [Google Scholar]
- Assmann G, Cullen P, Heinrich J, Schulte H. Hemostatic variables in the prediction of coronary risk: results of the 8 year follow-up of healthy men in the Munster Heart Study (PROCAM). Prospective Cardiovascular Munster Study. Isr J Med Sci. 1996;32:364–370. [PubMed] [Google Scholar]
- Mitropoulos KA, Miller GJ, Martin JC, Reeves BE, Cooper J. Dietary fat induces changes in factor VII coagulant activity through effects on plasma free stearic acid concentration. Arterioscler Thromb. 1994;14:214–222. doi: 10.1161/01.atv.14.2.214. [DOI] [PubMed] [Google Scholar]
- Mutanen M, Aro A. Coagulation and fibrinolysis factors in healthy subjects consuming high stearic or trans fatty acid diets. Thromb Haemost. 1997;77:99–104. [PubMed] [Google Scholar]
- Tholstrup T, Marckmann P, Vessby B, Sandstrom B. Effect of fats high in individual saturated fatty acids on plasma lipoprotein[a] levels in young healthy men. J Lipid Res. 1995;36:1447–1452. [PubMed] [Google Scholar]
- Tholstrup T, Marckmann P, Jespersen J, Sandstrom B. Fat high in stearic acid favorably affects blood lipids and factor VII coagulant activity in comparison with fats high in palmitic acid or high in myristic and lauric acids. Am J Clin Nutr. 1994;59:371–377. doi: 10.1093/ajcn/59.2.371. [DOI] [PubMed] [Google Scholar]
- Zock PL, Blijlevens RA, de Vries JH, Katan MB. Effects of stearic acid and trans fatty acids versus linoleic acid on blood pressure in normotensive women and men. Eur J Clin Nutr. 1993;47:437–444. [PubMed] [Google Scholar]
- Simon JA, Fong J, Bernert JTJ. Serum fatty acids and blood pressure. Hypertension. 1996;27:303–307. doi: 10.1161/01.hyp.27.2.303. [DOI] [PubMed] [Google Scholar]
- Hojo N, Fukushima T, Isobe A, Gao T, Shiwaku K, Ishida K, Ohta N, Yamane Y. Effect of serum fatty acid composition on coronary atherosclerosis in Japan. Int J Cardiol. 1998;66:31–38. doi: 10.1016/S0167-5273(98)00199-5. [DOI] [PubMed] [Google Scholar]
- Yli-Jama P, Meyer HE, Ringstad J, Pedersen JI. Serum free fatty acid pattern and risk of myocardial infarction: a case-control study. J Intern Med. 2002;251:19–28. doi: 10.1046/j.1365-2796.2002.00922.x. [DOI] [PubMed] [Google Scholar]
- Kabagambe EK, Baylin A, Siles X, Campos H. Individual saturated fatty acids and nonfatal acute myocardial infarction in Costa Rica. Eur J Clin Nutr. 2003;57:1447–1457. doi: 10.1038/sj.ejcn.1601709. [DOI] [PubMed] [Google Scholar]
- Willett WC. Nutritional Epidemiology. 2nd Edition. Oxford , Oxford University Press; 1998. [Google Scholar]
- Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr. 1999;70:1001–1008. doi: 10.1093/ajcn/70.6.1001. [DOI] [PubMed] [Google Scholar]
- Wang L, Folsom AR, Eckfeldt JH. Plasma fatty acid composition and incidence of coronary heart disease in middle aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Nutr Metab Cardiovasc Dis. 2003;13:256–266. doi: 10.1016/S0939-4753(03)80029-7. [DOI] [PubMed] [Google Scholar]
- Watts GF, Jackson P, Burke V, Lewis B. Dietary fatty acids and progression of coronary artery disease in men. Am J Clin Nutr. 1996;64:202–209. doi: 10.1093/ajcn/64.2.202. [DOI] [PubMed] [Google Scholar]
- Simon JA, Hodgkins ML, Browner WS, Neuhaus JM, Bernert JTJ, Hulley SB. Serum fatty acids and the risk of coronary heart disease. Am J Epidemiol. 1995;142:469–476. doi: 10.1093/oxfordjournals.aje.a117662. [DOI] [PubMed] [Google Scholar]
- Simon JA, Fong J, Bernert JTJ, Browner WS. Serum fatty acids and the risk of stroke. Stroke. 1995;26:778–782. doi: 10.1161/01.str.26.5.778. [DOI] [PubMed] [Google Scholar]
- Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, Gebhardt S, Prior RL. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J Nutr. 2004;134:613–617. doi: 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- Arts IC, Hollman PC, Feskens EJ, Bueno de Mesquita HB, Kromhout D. Catechin intake and associated dietary and lifestyle factors in a representative sample of Dutch men and women. Eur J Clin Nutr. 2001;55:76–81. doi: 10.1038/sj.ejcn.1601115. [DOI] [PubMed] [Google Scholar]
- Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- Tapiero H, Tew KD, Ba GN, Mathe G. Polyphenols: do they play a role in the prevention of human pathologies? Biomed Pharmacother. 2002;56:200–207. doi: 10.1016/S0753-3322(02)00178-6. [DOI] [PubMed] [Google Scholar]
- Natsume M, Osakabe N, Yamagishi M, Takizawa T, Nakamura T, Miyatake H, Hatano T, Yoshida T. Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC. Biosci Biotechnol Biochem. 2000;64:2581–2587. doi: 10.1271/bbb.64.2581. [DOI] [PubMed] [Google Scholar]
- Vinson JA, Proch J, Zubik L. Phenol antioxidant quantity and quality in foods: cocoa, dark chocolate, and milk chocolate. J Agric Food Chem. 1999;47:4821–4824. doi: 10.1021/jf990312p. [DOI] [PubMed] [Google Scholar]
- Serafini M, Bugianesi R, Maiani G, Valtuena S, De Santis S, Crozier A. Plasma antioxidants from chocolate. Nature. 2003;424:1013. doi: 10.1038/4241013a. [DOI] [PubMed] [Google Scholar]
- Hammerstone JF, Lazarus SA, Schmitz HH. Procyanidin content and variation in some commonly consumed foods. J Nutr. 2000;130:2086S–92S. doi: 10.1093/jn/130.8.2086S. [DOI] [PubMed] [Google Scholar]
- Richelle M, Tavazzi I, Enslen M, Offord EA. Plasma kinetics in man of epicatechin from black chocolate. Eur J Clin Nutr. 1999;53:22–26. doi: 10.1038/sj.ejcn.1600673. [DOI] [PubMed] [Google Scholar]
- Wang JF, Schramm DD, Holt RR, Ensunsa JL, Fraga CG, Schmitz HH, Keen CL. A dose-response effect from chocolate consumption on plasma epicatechin and oxidative damage. J Nutr. 2000;130:2115S–9S. doi: 10.1093/jn/130.8.2115S. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Osakabe N, Natsume M, Adachi T, Yamagishi M, Hirano R, Takizawa T, Itakura H, Kondo K. Effects of cacao liquor polyphenols on the susceptibility of low-density lipoprotein to oxidation in hypercholesterolemic rabbits. J Atheroscler Thromb. 2000;7:164–168. doi: 10.5551/jat1994.7.164. [DOI] [PubMed] [Google Scholar]
- Osakabe N, Yasuda A, Natsume M, Takizawa T, Terao J, Kondo K. Catechins and their oligomers linked by C4 --> C8 bonds are major cacao polyphenols and protect low-density lipoprotein from oxidation in vitro. Exp Biol Med (Maywood) 2002;227:51–56. doi: 10.1177/153537020222700109. [DOI] [PubMed] [Google Scholar]
- Kondo K, Hirano R, Matsumoto A, Igarashi O, Itakura H. Inhibition of LDL oxidation by cocoa. Lancet. 1996;348:1514. doi: 10.1016/S0140-6736(05)65927-2. [DOI] [PubMed] [Google Scholar]
- Waterhouse AL, Shirley JR, Donovan JL. Antioxidants in chocolate. Lancet. 1996;348:834. doi: 10.1016/S0140-6736(05)65262-2. [DOI] [PubMed] [Google Scholar]
- Wan Y, Vinson JA, Etherton TD, Proch J, Lazarus SA, Kris-Etherton PM. Effects of cocoa powder and dark chocolate on LDL oxidative susceptibility and prostaglandin concentrations in humans. Am J Clin Nutr. 2001;74:596–602. doi: 10.1093/ajcn/74.5.596. [DOI] [PubMed] [Google Scholar]
- Mathur S, Devaraj S, Grundy SM, Jialal I. Cocoa products decrease low density lipoprotein oxidative susceptibility but do not affect biomarkers of inflammation in humans. J Nutr. 2002;132:3663–3667. doi: 10.1093/jn/132.12.3663. [DOI] [PubMed] [Google Scholar]
- Rein D, Lotito S, Holt RR, Keen CL, Schmitz HH, Fraga CG. Epicatechin in human plasma: in vivo determination and effect of chocolate consumption on plasma oxidation status. J Nutr. 2000;130:2109S–14S. doi: 10.1093/jn/130.8.2109S. [DOI] [PubMed] [Google Scholar]
- Osakabe N, Baba S, Yasuda A, Iwamoto T, Kamiyama M, Takizawa T, Itakura H, Kondo K. Daily cocoa intake reduces the susceptibility of low-density lipoprotein to oxidation as demonstrated in healthy human volunteers. Free Radic Res. 2001;34:93–99. doi: 10.1080/10715760100300091. [DOI] [PubMed] [Google Scholar]
- Adamson GE, Lazarus SA, Mitchell AE, Prior RL, Cao G, Jacobs PH, Kremers BG, Hammerstone JF, Rucker RB, Ritter KA, Schmitz HH. HPLC method for the quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacity. J Agric Food Chem. 1999;47:4184–4188. doi: 10.1021/jf990317m. [DOI] [PubMed] [Google Scholar]
- Wiswedel I, Hirsch D, Kropf S, Gruening M, Pfister E, Schewe T, Sies H. Flavanol-rich cocoa drink lowers plasma F(2)-isoprostane concentrations in humans. Free Radic Biol Med. 2004;37:411–421. doi: 10.1016/j.freeradbiomed.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Lean ME, Noroozi M, Kelly I, Burns J, Talwar D, Sattar N, Crozier A. Dietary flavonols protect diabetic human lymphocytes against oxidative damage to DNA. Diabetes. 1999;48:176–181. doi: 10.2337/diabetes.48.1.176. [DOI] [PubMed] [Google Scholar]
- Pearson DA, Paglieroni TG, Rein D, Wun T, Schramm DD, Wang JF, Holt RR, Gosselin R, Schmitz HH, Keen CL. The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb Res. 2002;106:191–197. doi: 10.1016/S0049-3848(02)00128-7. [DOI] [PubMed] [Google Scholar]
- Rein D, Paglieroni TG, Pearson DA, Wun T, Schmitz HH, Gosselin R, Keen CL. Cocoa and wine polyphenols modulate platelet activation and function. J Nutr. 2000;130:2120S–6S. doi: 10.1093/jn/130.8.2120S. [DOI] [PubMed] [Google Scholar]
- Murphy KJ, Chronopoulos AK, Singh I, Francis MA, Moriarty H, Pike MJ, Turner AH, Mann NJ, Sinclair AJ. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am J Clin Nutr. 2003;77:1466–1473. doi: 10.1093/ajcn/77.6.1466. [DOI] [PubMed] [Google Scholar]
- Holt RR, Schramm DD, Keen CL, Lazarus SA, Schmitz HH. Chocolate consumption and platelet function. Jama. 2002;287:2212–2213. doi: 10.1001/jama.287.17.2212. [DOI] [PubMed] [Google Scholar]
- Rein D, Paglieroni TG, Wun T, Pearson DA, Schmitz HH, Gosselin R, Keen CL. Cocoa inhibits platelet activation and function. Am J Clin Nutr. 2000;72:30–35. doi: 10.1093/ajcn/72.1.30. [DOI] [PubMed] [Google Scholar]
- Innes AJ, Kennedy G, McLaren M, Bancroft AJ, Belch JJ. Dark chocolate inhibits platelet aggregation in healthy volunteers. Platelets. 2003;14:325–327. doi: 10.1080/0953710031000123681. [DOI] [PubMed] [Google Scholar]
- Engler MB, Engler MM, Chen CY, Malloy MJ, Browne A, Chiu EY, Kwak HK, Milbury P, Paul SM, Blumberg J, Mietus-Snyder ML. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr. 2004;23:197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- Heiss C, Dejam A, Kleinbongard P, Schewe T, Sies H, Kelm M. Vascular effects of cocoa rich in flavan-3-ols. JAMA. 2003;290:1030–1031. doi: 10.1001/jama.290.8.1030. [DOI] [PubMed] [Google Scholar]
- Karim M, McCormick K, Kappagoda CT. Effects of cocoa extracts on endothelium-dependent relaxation. J Nutr. 2000;130:2105S–8S. doi: 10.1093/jn/130.8.2105S. [DOI] [PubMed] [Google Scholar]
- Schramm DD, Wang JF, Holt RR, Ensunsa JL, Gonsalves JL, Lazarus SA, Schmitz HH, German JB, Keen CL. Chocolate procyanidins decrease the leukotriene-prostacyclin ratio in humans and human aortic endothelial cells. Am J Clin Nutr. 2001;73:36–40. doi: 10.1093/ajcn/73.1.36. [DOI] [PubMed] [Google Scholar]
- Schewe T, Sadik C, Klotz LO, Yoshimoto T, Kuhn H, Sies H. Polyphenols of cocoa: inhibition of mammalian 15-lipoxygenase. Biol Chem. 2001;382:1687–1696. doi: 10.1515/BC.2001.204. [DOI] [PubMed] [Google Scholar]
- Schewe T, Kuhn H, Sies H. Flavonoids of cocoa inhibit recombinant human 5-lipoxygenase. J Nutr. 2002;132:1825–1829. doi: 10.1093/jn/132.7.1825. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- Margolis KL, Manson JE, Greenland P, Rodabough RJ, Bray PF, Safford M, Grimm RHJ, Howard BV, Assaf AR, Prentice R. Leukocyte count as a predictor of cardiovascular events and mortality in postmenopausal women: the Women's Health Initiative Observational Study. Arch Intern Med. 2005;165:500–508. doi: 10.1001/archinte.165.5.500. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- Sanbongi C, Suzuki N, Sakane T. Polyphenols in chocolate, which have antioxidant activity, modulate immune functions in humans in vitro. Cell Immunol. 1997;177:129–136. doi: 10.1006/cimm.1997.1109. [DOI] [PubMed] [Google Scholar]
- Mao TK, Powell J, Van de Water J, Keen CL, Schmitz HH, Hammerstone JF, Gershwin ME. The effect of cocoa procyanidins on the transcription and secretion of interleukin 1 beta in peripheral blood mononuclear cells. Life Sci. 2000;66:1377–1386. doi: 10.1016/S0024-3205(00)00449-5. [DOI] [PubMed] [Google Scholar]
- Mao T, Van De Water J, Keen CL, Schmitz HH, Gershwin ME. Cocoa procyanidins and human cytokine transcription and secretion. J Nutr. 2000;130:2093S–9S. doi: 10.1093/jn/130.8.2093S. [DOI] [PubMed] [Google Scholar]
- Mao TK, Van de Water J, Keen CL, Schmitz HH, Gershwin ME. Effect of cocoa flavanols and their related oligomers on the secretion of interleukin-5 in peripheral blood mononuclear cells. J Med Food. 2002;5:17–22. doi: 10.1089/109662002753723188. [DOI] [PubMed] [Google Scholar]
- Mao TK, van de Water J, Keen CL, Schmitz HH, Gershwin ME. Modulation of TNF-alpha secretion in peripheral blood mononuclear cells by cocoa flavanols and procyanidins. Dev Immunol. 2002;9:135–141. doi: 10.1080/1044667031000137601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao TK, Van De Water J, Keen CL, Schmitz HH, Gershwin ME. Cocoa flavonols and procyanidins promote transforming growth factor-beta1 homeostasis in peripheral blood mononuclear cells. Exp Biol Med (Maywood) 2003;228:93–99. doi: 10.1177/153537020322800113. [DOI] [PubMed] [Google Scholar]
- Mursu J, Voutilainen S, Nurmi T, Rissanen TH, Virtanen JK, Kaikkonen J, Nyyssonen K, Salonen JT. Dark Chocolate Consumption Increases HDL Cholesterol Concentration and Chocolate Fatty Acids May Inhibit Lipid Peroxidation in Healthy Humans. Free Radic Biol Med. 2004;37:1351–1359. doi: 10.1016/j.freeradbiomed.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, Desideri G, Blumberg JB, Ferri C. Cocoa Reduces Blood Pressure and Insulin Resistance and Improves Endothelium-Dependent Vasodilation in Hypertensives. Hypertension. 2005. p. 01.HYP.0000174990.46027.70. [DOI] [PubMed]
- Taubert D, Berkels R, Roesen R, Klaus W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA. 2003;290:1029–1030. doi: 10.1001/jama.290.8.1029. [DOI] [PubMed] [Google Scholar]
- Fraga CG, Actis-Goretta L, Ottaviani JI, Carrasquedo F, Lotito SB, Lazarus S, Schmitz HH, Keen CL. Regular consumption of a flavanol-rich chocolate can improve oxidant stress in young soccer players. Clin Dev Immunol. 2005;12:11–17. doi: 10.1080/10446670410001722159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IM, Paffenbarger RSJ. Life is sweet: candy consumption and longevity. BMJ. 1998;317:1683–1684. doi: 10.1136/bmj.317.7174.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, et Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995;155:381–386. doi: 10.1001/archinte.155.4.381. [DOI] [PubMed] [Google Scholar]
- Geleijnse JM, Launer LJ, Van der Kuip DA, Hofman A, Witteman JC. Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam Study. Am J Clin Nutr. 2002;75:880–886. doi: 10.1093/ajcn/75.5.880. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Relation between Intake of Flavonoids and Risk for Coronary Heart Disease in Male Health Professionals. Ann Intern Med. 1996;125:384–389. doi: 10.7326/0003-4819-125-5-199609010-00005. [DOI] [PubMed] [Google Scholar]
- Sesso HD, Gaziano JM, Liu S, Buring JE. Flavonoid intake and the risk of cardiovascular disease in women. Am J Clin Nutr. 2003;77:1400–1408. doi: 10.1093/ajcn/77.6.1400. [DOI] [PubMed] [Google Scholar]
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- Hirvonen T, Pietinen P, Virtanen M, Ovaskainen ML, Hakkinen S, Albanes D, Virtamo J. Intake of flavonols and flavones and risk of coronary heart disease in male smokers. Epidemiology. 2001;12:62–67. doi: 10.1097/00001648-200101000-00011. [DOI] [PubMed] [Google Scholar]
- Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996;156:637–642. doi: 10.1001/archinte.156.6.637. [DOI] [PubMed] [Google Scholar]
- Arts IC, Jacobs DRJ, Harnack LJ, Gross M, Folsom AR. Dietary catechins in relation to coronary heart disease death among postmenopausal women. Epidemiology. 2001;12:668–675. doi: 10.1097/00001648-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Intake of flavonoids, carotenoids, vitamins C and E, and risk of stroke in male smokers. Stroke. 2000;31:2301–2306. doi: 10.1161/01.str.31.10.2301. [DOI] [PubMed] [Google Scholar]
- Yochum L, Kushi LH, Meyer K, Folsom AR. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am J Epidemiol. 1999;149:943–949. doi: 10.1093/oxfordjournals.aje.a009738. [DOI] [PubMed] [Google Scholar]
- Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996;312:478–481. doi: 10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- Hertog MG, Sweetnam PM, Fehily AM, Elwood PC, Kromhout D. Antioxidant flavonols and ischemic heart disease in a Welsh population of men: the Caerphilly Study. Am J Clin Nutr. 1997;65:1489–1494. doi: 10.1093/ajcn/65.5.1489. [DOI] [PubMed] [Google Scholar]
- Arts IC, Hollman PC, Kromhout D. Chocolate as a source of tea flavonoids. Lancet. 1999;354:488. doi: 10.1016/S0140-6736(99)02267-9. [DOI] [PubMed] [Google Scholar]
- Lazarus SA, Hammerstone JF, Schmitz HH. Chocolate contains additional flavonoids not found in tea. Lancet. 1999;354:1825. doi: 10.1016/S0140-6736(05)70599-7. [DOI] [PubMed] [Google Scholar]
- Dreosti IE. Antioxidant polyphenols in tea, cocoa, and wine. Nutrition. 2000;16:692–694. doi: 10.1016/S0899-9007(00)00304-X. [DOI] [PubMed] [Google Scholar]
- International Cocoa Association . Annual Report 2003-2004. London, UK , http://www.icco.org/anrep/anrep0304english.pdf Accessed Sept. 18 2005; 2005. [Google Scholar]
- Zhu QY, Schramm DD, Gross HB, Holt RR, Kim SH, Yamaguchi T, Kwik-Uribe CL, Keen CL. Influence of cocoa flavanols and procyanidins on free radical-induced human erythrocyte hemolysis. Clin Dev Immunol. 2005;12:27–34. doi: 10.1080/17402520512331329514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, Alexopoulos N, Economou E, Andreadou I, Stefanadis C. Effect of Dark Chocolate on Arterial Function in Healthy Individuals. American Journal of Hypertension. 2005;18:785. doi: 10.1016/j.amjhyper.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Kromhout D, Menotti A, Bloemberg B, Aravanis C, Blackburn H, Buzina R, Dontas AS, Fidanza F, Giampaoli S, Jansen A. Dietary saturated and trans fatty acids and cholesterol and 25-year mortality from coronary heart disease: the Seven Countries Study. Prev Med. 1995;24:308–315. doi: 10.1006/pmed.1995.1049. [DOI] [PubMed] [Google Scholar]
- Hertog MG, Feskens EJ, Kromhout D. Antioxidant flavonols and coronary heart disease risk. Lancet. 1997;349:699. doi: 10.1016/S0140-6736(05)60135-3. [DOI] [PubMed] [Google Scholar]
- Arts IC, Hollman PC, Feskens EJ, Bueno de Mesquita HB, Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am J Clin Nutr. 2001;74:227–232. doi: 10.1093/ajcn/74.2.227. [DOI] [PubMed] [Google Scholar]


