Abstract
The purpose of this investigation was to assess the ability of the hippocampus to withstand a metabolic challenge following chronic stress. An N-methyl-d-aspartate receptor excitotoxin (ibotenic acid, IBO) was infused into the CA3 region of the hippocampus following a period of restraint for 6 h/day/21 days. Following the end of restraint when CA3 dendritic retraction persists (3 to 4 days), rats were infused with IBO (or vehicle) into the CA3 region of the hippocampus. Stressed male rats showed significantly more CA3 damage after IBO infusion relative to controls and the saline-infused side. Moreover, IBO-exacerbation of damage in males was not observed in the CA3 region 3 to 4 days after acute stress (6 h restraint), nor in the CA1 region after chronic stress. Females were also examined and chronic stress did not exacerbate IBO damage in the CA3 region. Overall, these results demonstrate that chronic stress compromises the ability of the hippocampus to withstand a metabolic challenge days after the chronic stress regimen has subsided in male rats. Whether the conditions surrounding CA3 dendritic retraction in females represents vulnerability is less clear and warrants further investigation.
Keywords: glucocorticoids, CA3, excitotoxicity, NMDA, sex difference
The hippocampus contributes to the regulation of the hypothalamic–pituitary–adrenal (HPA) axis, an important component of the stress response. In many conditions associated with an overactive HPA axis, the hippocampus is compromised, including Alzheimer's disease (de Leon et al., 1993), chronic depressive disorder (Sheline et al., 1996), acquired immunodeficiency syndrome (Oberfield et al., 1994), obesity (Raber, 1998), aging (Lupien et al., 1998), and Cushing's disease (Starkman et al., 1992). Glucocorticoids (GC) secreted from chronic HPA activation or administered exogenously may be a causal factor in hippocampal damage (Sapolsky et al., 1985; de Leon et al., 1988; Uno et al., 1989). Removal of GC appears to attenuate damage (Landfield et al., 1981): when Cushing's patients are treated to reduce GC hypersecretion, decreases in hippocampal volume are partially reversed (Starkman et al., 1999)
Sapolsky et al. (1986) conceptualized the destructive relationship between stress and hippocampal damage in the glucocorticoid cascade hypothesis. This hypothesis states that chronic stress can desensitize the hippocampus to stress steroids (Sapolsky et al., 1984), which allows GC to remain elevated, thereby making the hippocampus vulnerable to aging and/or metabolic challenges. For the effect of GC on aging, prolonged GC alone fail to damage the hippocampus in rats (Sousa et al., 1998), tree shrews (Vollmann-Honsdorf et al., 1997), macaques (Leverenz et al., 1999), or humans (Müller et al., 2001), which raises doubt about GC's direct influence on aging (for review, see Landfield and Eldridge, 1994). In contrast, hippocampal vulnerability after GC exposure is supported by many studies using rats. For instance, hippocampal damage caused by ischemia is potentiated by acute and simultaneous elevations of GC (Sapolsky and Pulsinelli, 1985; Morse and Davis, 1990). Moreover, hippocampal injury produced by neurotoxins is enhanced by acute elevations of GC or stress (Sapolsky, 1985a; Sapolsky et al., 1988; Stein-Behrens et al., 1994) and attenuated by reducing GC (Stein and Sapolsky, 1988; Krugers et al., 1998). However, the majority of studies that support this aspect of the glucocorticoid cascade hypothesis have used an acute stressor or a single GC treatment to investigate hippocampal vulnerability. In a few studies, the hippocampus was challenged during a prolonged period of GC elevation (Sapolsky, 1985b, 1986), confounding the effects of acute and chronic GC actions on hippocampal vulnerability. Thus, whether chronic stress makes the hippocampus vulnerable to metabolic insults after stress has subsided remains unknown.
The purpose of this investigation was to assess the influence of chronic stress on the vulnerability of the hippocampus to a metabolic challenge. Rats were chronically stressed by 6 h of restraint each day for 21 days. This chronic stressor was chosen because it induces CA3 hippocampal dendritic retraction (Watanabe et al., 1992a,b,c; Magariños et al., 1996, 1999) and hippocampal-dependent memory deficits (Luine et al., 1994; Conrad et al., 1996; Wright et al., 2003) without neuron loss (Fuchs et al., 2001). In this paradigm, chronic stress-induced morphological (Conrad et al., 1999) and behavioral changes (Luine et al., 1994; Conrad et al., 1996) endure for at least 4 days after stress ends. However, hippocampal dendritic retraction recovers 10 days following chronic stress (Conrad et al., 1999), necessitating that the metabolic challenge occur within 4 days following stress termination when hippocampal dendritic retraction persists. In addition, the CA3 region was investigated first because it develops stress-induced dendritic retraction (Magariños and McEwen, 1995a) before other hippocampal areas (Sousa et al., 2000). If chronic stress made the CA3 region vulnerable to metabolic challenge, then control studies were performed to answer the following questions: (1) Does the influence of chronic stress and metabolic challenge generalize to other hippocampal regions such as the CA1 area under similar circumstances? (2) Can acute stress compromise the CA3 region under these conditions of metabolic challenge? Female rats were included because they are less affected by chronic stress on hippocampal morphology (Galea et al., 1997) and function (Baran et al., 2002; Conrad et al., 2003) and therefore, may show reduced susceptibility to metabolic challenge.
EXPERIMENTAL PROCEDURES
Subjects
Arizona State University's Institutional Animal Care and Use Committee approved all procedures, in accordance with the applicable portions of the Animal Welfare Act and “Guide for the Care and Use of Laboratory Animals” by Department of Health and Human Resources. All efforts were made to minimize the number of animals used and their suffering. Male (n=64) and female (n=36) Sprague–Dawley rats (Charles River, Hartford, CT, USA) were same sex, pair-housed in temperature and light-controlled chambers. Upon arrival, female and male rat littermates weighed approximately 175 and 225 g, respectively. Lights were on a 12-h schedule (lights on at 6:00 AM) and rats were provided with food and water ad libitum. Rats acclimated to the facilities for 1 week and then were randomly assigned to the experimental groups, with control and stressed rats housed in separate chambers.
Restraint procedure
Half of the experimental male and female rats were stressed by being placed in wire mesh restrainers. Two different sizes of restrainers were used, with rats being placed in the larger restrainer as they outgrew the smaller one (7 inch circumference×9 1/2 inches long, 9 inch circumference×11 inches long). During restraint, rats were placed back in their home cages. Restraint started at 8:00 AM and ended at 2:00 PM, and was performed once (acute stress) or repeated for 21 days (chronic stress). Controls remained undisturbed during the period of restraint.
Ibotenic acid (IBO) infusion procedure
Three or 4 days from the last restraint session, IBO (Sigma, St. Louis, MO, USA) was dissolved in phosphate-buffered saline (PBS; 0.1 M, pH=7.4) and stereotaxically infused into the hippocampus. IBO was infused within the time frame that CA3 dendritic retraction is known to persist (Conrad et al., 1999). Moreover, no differences were observed between animals receiving the infusion 3 or 4 days after the restraint stress. Using aseptic surgical procedures, rats were given atropine (i.p.; 0.1 ml of 0.54 mg/ml) and then were deeply anesthetized with sodium pentobarbital (45 mg/kg, i.p.) 10 min later. A stereotaxic frame (Kopf Instruments, Tujunga, CA, USA) held the head securely and the top of the skull was exposed to reveal the midsaggital suture. Bregma and lambda were leveled (interaural line 0) and a 5 μl Hamilton syringe was lowered to the coordinates targeting midway between the CA3 and CA1 region (anterior/posterior −3.6, medial/lateral±3.5, dorsal/ventral −3.6). IBO (1 μl of 1 μg/μl) was infused unilaterally for 2 min and the needle left in place for an additional 2 min to allow the neurotoxin to diffuse. PBS (1 μl) was infused on the contralateral side, which was counter-balanced among rats. The dose of IBO was chosen to avoid a ceiling effect in non-treated control males, which was defined as 100% damage throughout most of the hippocampal CA3 region. After infusion, the skin was sutured and rats recovered in clean cages without bedding, then were placed on top of heating pads set on the lowest temperature. Once the rats awakened from anesthesia, they were placed in clean cages with fresh bedding and returned to their chambers.
Perfusion and histology
Observing IBO-induced cell death and microglia proliferation requires at least a 7-day wait after infusion (DeGiorgio et al., 2002). Longer delays are not necessary because histological verification of damage 120–370 days from IBO lesions in monkeys were comparable to magnetic resonance images taken in the same animals 6–11 days from infusion (Málková et al., 2001). Thus, rats were transcardially perfused exactly 7 days after IBO infusion after an overdose of sodium pentobarbital (100 mg/kg, i.p.). Approximately 100 ml of 0.1 M PBS (pH=7.4) were used to clear the blood, followed by 100 ml of 4% PBS-buffered formalin. The brains were removed and stored in 4% buffered formalin until tissue sectioning. Two days prior to sectioning, brains were placed in 30% sucrose for cryoprotection. Brains were cut (30 μm) in the frontal plane using a Micron cryostat (San Marcos, CA, USA) at −22 °C and mounted on gelatin-chromium-subbed slides until the dorsal hippocampus was exhaustively sectioned. Lost sections were noted for accurate reconstruction. For tissue staining, sections were defatted using a xylene substitute (CitriSolv; Fisher, Pittsburg, PA, USA; 15 min), followed by a series of ethanol dilutions (95%×2; 70%, distilled water×3 for 5 min each) to dehydrate the sections. Cell bodies were stained in 0.1% Cresyl Violet (5–10 min), then re-hydrated (distilled water, 80%; 95%×2; 100% ethanol×2 for 2 min each). Sections were placed in CitriSolv until cover slipped with Permount (VWR).
Histological quantification
Sections were coded and the code was sealed until the analysis was complete. The section containing the needle tip was identified at a magnification of 100× using an Olympus microscope (model BX50) and referred to as the infusion site (Fig. 1A). Damage to the CA3 region was determined by identifying the absence of pyramidal cells in the CA3 region. The extent of this damage was then quantified using a calibrated grid (0.0125 inch divisions, total length=5/8 inch) in the ocular lens of the microscope. The distance representing damage was divided by the distance of the total CA3 region and then converted to a percentage (Fig. 1A–D). The CA3 region measured was consistent with the area expressing CA3 dendritic retraction after exposure to chronic stress (Magariños and McEwen, 1995a,b). Sections in the anterior and posterior planes of the infusion site were averaged to determine percentage of CA3 damage at each 120 μm increment from the infusion site. Therefore, four 30 μm sections were averaged in the posterior and anterior directions from the infusion site to provide one value at each 120 μm increment. In the few cases that sections were lost, the distance of the lost section was skipped and the average damage of the remaining sections was calculated. Lesion accuracy was determined by two factors: 1) verifying that the needle tip was within the medial–lateral borders of the CA3 region and 2) ensuring that at least 20% damage occurred to the CA3 region.
Fig. 1.
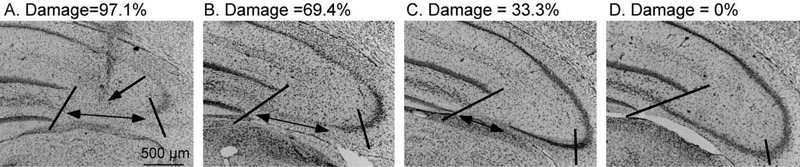
Example of quantification of IBO-induced CA3 damage. The section containing the needle tip was identified (A). The single-headed arrow points to the region of the needle tip, indicated by pronounced gliosis. This example represents an ideal needle placement and corresponds to the first control rat in Fig. 2. The percent CA3 damage was determined by quantifying the length of CA3 that lacked pyramidal cells, indicated by the double-headed arrow. This value was divided by the total length of the CA3 region that previously expressed stress-induced dendritic retraction, which is indicated by the two slashed lines. In panel A, this region represents 97.1% damage. As the distance from the needle tip increases, the percent of CA3 damage decreases (B=69.4%, C=33.3%) until no damage is detected, D=0%. Magnification=80×.
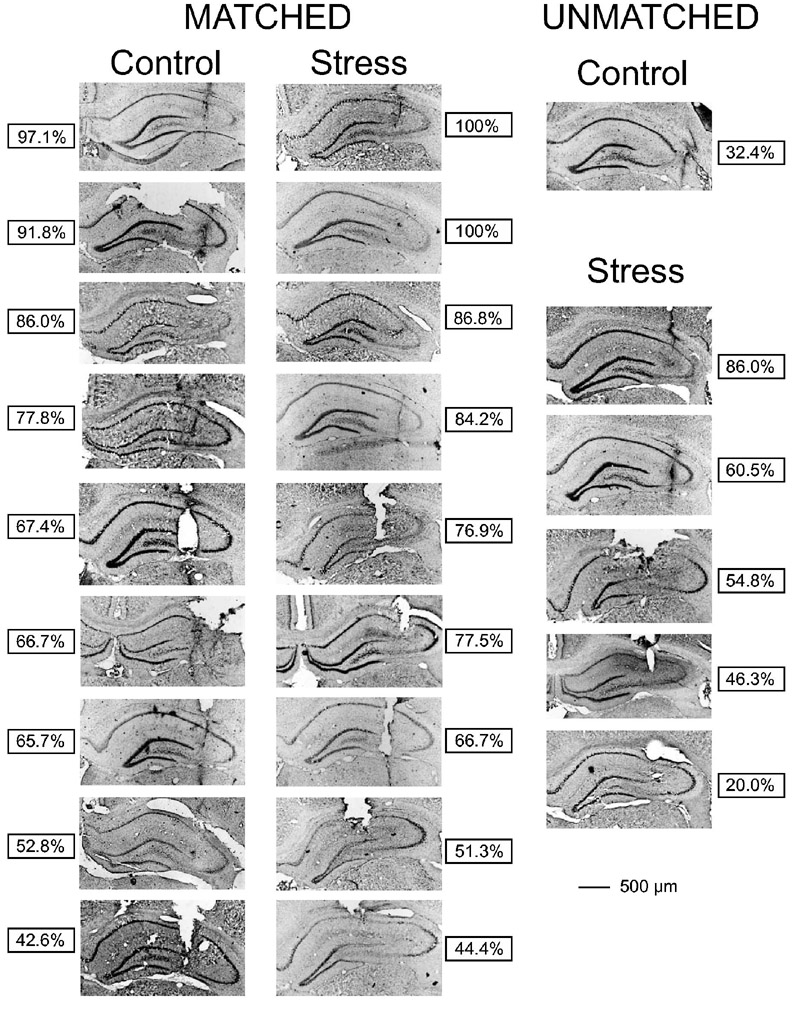
To analyze the histological data, control and stressed rats were paired according to placement of the infusion needle because IBO-induced damage varies depending upon needle tip location. Brains were matched to ensure similarity between rats in both the position of the needle tip within the hippocampus and the percentage of damage to the CA3 region. Needle tip location was matched using its position from all three planes: anterior–posterior, medial–lateral, and dorsal–ventral. Matching needle placements and percentage of damage at the needle tip proved difficult among four treatment groups (male, female, control, stress) because the females generally had more damage from IBO than the males. Consequently, matching was performed between treatments (control, stress) within each sex.
Statistics
The independent variable for this experiment was treatment (control, stress). The dependent measure for histology was percentage of damage. The histological data were transformed using the equation for proportions, 2 arcsin (square root of Y), which allows an analysis of variance (ANOVA) to be performed (Neter et al., 1985; Cohen et al., 2003). Data were analyzed using a two-way ANOVA with distance as the repeated measure. Chronically stressed rats were weighed on days 1, 7, 14, and 21 of restraint and weight was analyzed using a two-way ANOVA with day as the repeated measure. Data are represented by means±S.E.M. Reliability for determining damage averaged 96.4%±1.4% (inter-rater reliability) and 97.0%±1.7% (intra-rater reliability).
RESULTS
Based upon the section containing the needle tip, nine pairings were made between control and stressed rats with similar needle placements and percentage of CA3 damage (Fig. 2). One control and five stressed rats were not paired because the location of the needle tip and/or the amount of CA3 damage at the needle tip differed by more than 10%. Rats with less than 20% CA3 damage at the needle tip had poor needle placements (lateral, medial, etc.) and were removed from the analysis. Of the paired rats, males exposed to chronic stress exhibited more IBO-induced damage to the CA3 region compared to controls (Fig. 3A). This result was confirmed by a main effect of treatment, F(1,16)=4.814, P<0.05, following a two-way ANOVA with distance as the repeated measure. In addition, a main effect of distance, F(7,112)=43.801, P<0.0001 indicated that as the distance from the needle tip increased, the IBO-induced damage decreased for both control and stressed rats. There was no significant interaction between treatment and distance, F(7,112)=0.778, P=0.61. A one-way ANOVA was performed on the sections containing the needle tip, showing no statistical difference between control and stressed rats, F(1,16)=0.534, P=0.476. Mechanical disruption was rare and was illustrated on the IBO-infused side in two rats (Fig. 2E). These two were paired because mechanical disruption was comparable. In addition, these sections had similar needle tip location and percentage of CA3 damage at the needle tip. Determining CA3 damage in adjacent sections without mechanical damage and confirming that the CA3 damage was similar verified that the CA3 damage estimates for these two were accurate. Moreover, damage caused by mechanical disruption in two hippocampi infused with PBS (damage in the section containing the needle tip=2.3% and 4.7%) was no longer detectible within three sections (90 μm) of the infusion point. None of the other hippocampi infused with PBS showed any signs of CA3 damage. For the rats that were not matched and expressed greater than 20% CA3 damage, five were in the stress group (average damage=53.5%±10.7%) and one was in the control group (damage=32.4%).
Fig. 2.
A histological summary of IBO-induced CA3 damage in male rats. After determining the section containing the needle tip, the amount of damage in the CA3 region was determined without knowledge of treatment group. Rats were matched based upon similar needle-tip locations and CA3 damage. CA3 damage had to be greater than 20% and be within a 10% range of the other treatment to be matched. Nine pairings were made in ascending order of percent CA3 damage with the greatest CA3 damage illustrated by the first pair and the least CA3 damage illustrated by the ninth pair. Brains that were not matched are represented in the far right column. The boxes indicate the percent of damage in the section containing the needle tip.
Fig. 3.
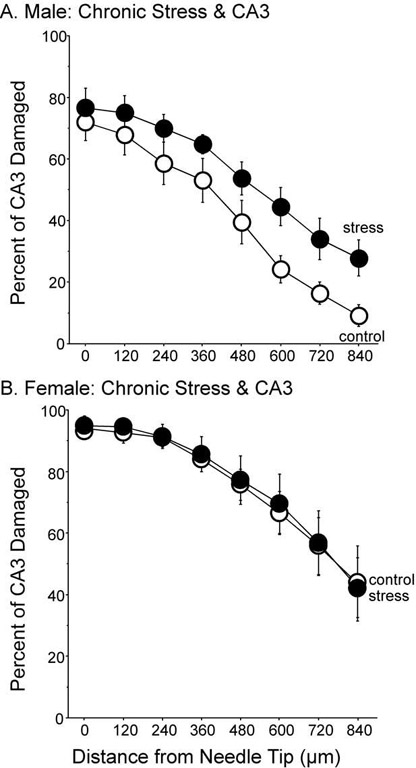
Percent of CA3 damage after IBO infusion and chronic stress. (A) Males with a history of chronic stress expressed more IBO-induced CA3 damage compared to controls, F(1,16)=5.308, P<0.05. Damage in the section containing the needle tip did not differ among groups. Nine subjects per group. (B) In females, a history of chronic stress did not alter IBO-induced damage in the CA3 region, F(1,14)=0.017, P=0.899. Eight subjects per group. Data represent means±S.E.M.
Sixteen female rats were paired based upon the needle tip location in the CA3 region and percent of damage at the infusion point. Stress had no effect on the amount of IBO-induced damage in females (Fig. 3B). A two-way ANOVA revealed no significant effect of treatment F(1,14)=0.0547, P=0.819, nor interaction between treatment and distance, F(7,98)=0.310, P=0.948. A main effect of distance, F(7,98)=69.71, P<0.0001, and post hoc tests indicated that IBO-induced CA3 damage was greater at the infusion point and less at distances farther away from the infusion point. Of the rats that were excluded from the analysis, 11 had at least 20% CA3 damage but were not matched (control, n=3, CA3 damage=62.7%±13.7%; stress, n=8, CA3 damage=48.3%±5.8%) and the remaining nine had missed placements or poor histology.
For damage to the CA1 region in males, seven pairings were made between control and stressed rats with similar needle tip placements and percentage of CA1 damage. Exposure to chronic stress did not alter the CA1 region damaged by IBO infusion (Fig. 4A), as indicated by no significant main effect of treatment, F(1,12)=0.423, P=0.52, nor a significant interaction between treatment and distance, F(7,84)=0.547, P=0.79. As observed with the CA3 region, the amount of IBO-induced damage decreased as the distance from the needle tip increased for both stress and control males, F(7, 84)=26.31, P<0.001. Many rats were not paired because of the variability of needle tip locations. Of the sections with more than 20% CA1 damage, five were controls (average damage=37.0%±5.0%) and five were stressed (average damage=50.0%±13.9%).
Fig. 4.
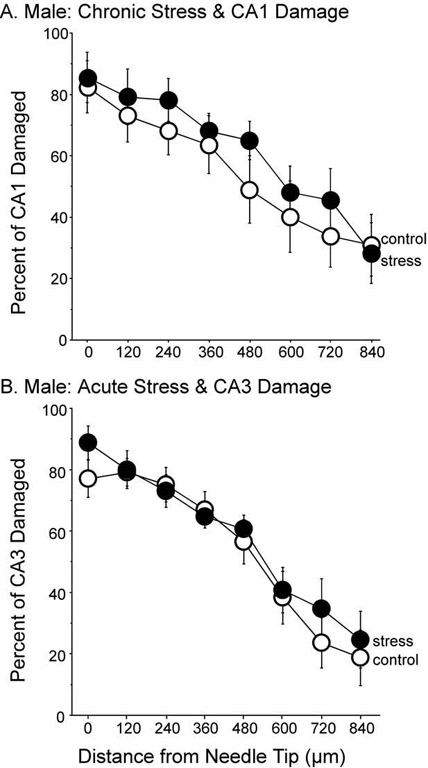
(A) Percent of CA1 damage after IBO infusion in male rats exposed to chronic stress. A history of chronic stress had no influence on the amount of damage inflicted in the CA1 region after IBO infusion, F(1,12)=0.434, P=0.522. Seven subjects per group. (B) Percent of CA3 damage after IBO infusion in male rats exposed to acute stress. A single session of restraint did not alter IBO-induced CA3 damage, F(1,10)=0.139, P=0.717. Six subjects per group. Data represent means±S.E.M.
Measures of body weights were taken to verify that the restraint procedure was stressful (Magariños and McEwen, 1995b). Body weights were similar prior to the start of restraint, but stressed rats gained weight more slowly after the restraint procedure began (Table 1). The slow weight gain of the chronically stressed rats was confirmed by a significant interaction between treatment and week, F(2,84)=25.7, P<0.001, and least significant difference (LSD) post hoc tests.
Table 1.
Body weights
| Group | Subjects | Day 1 | Day 7 | Day 14 | Day 21 |
|---|---|---|---|---|---|
| For males only: Treat×Day interaction F(3,111)=8.632, P<0.0001 | |||||
| Male-Con | 19 | 287.6±14.3 | 353.6±12.9 | 381.2±12.5 | 398.4±11.5 |
| Male-Str | 20 | 277.7±11.7 | 317.8±7.6 | 348.3±7.6 | 356.4±4.6 |
| For females only: Treat×Day interaction F(3,114)=6.325, P<0.001 | |||||
| Female-Con | 20 | 214.1±2.8 | 234.7±3.2 | 240.3±3.6 | 243.2±3.5 |
| Female-Str | 20 | 215.1±2.8 | 226.0±3.6 | 232.1±3.9 | 239.5±3.7 |
Con, control; Str, stress.
Ischemia and neurotoxins such as kainic acid can be more damaging to the hippocampus when they occur within 24 hours of GC elevations (Sapolsky, 1985a,b, 1986). An acute stress procedure was performed on another set of 20 male rats to determine whether the IBO-induced CA3 damage in the male rats was caused by elevations of GC arising from the last day of restraint or the cumulative actions of stress on the CA3 region. The delay between the restraint, infusion and killing was identical to the chronic stress paradigm. Twelve rats with similar needle tip location and percentages of CA3 damage were paired. One restraint session 3 or 4 days before IBO infusion had no effect on IBO-induced damage of the CA3 region (Fig. 4B). A main effect of treatment was not significant, F(1,10)=0.114, P=0.74, and neither was the interaction between treatment and distance, F(7,70)=0.681, P=0.69. As expected, the amount of CA3 damage decreased with increasing distance from the needle tip, F(7,70)=31.90, P<0.0001. Of the eight rats that were excluded in the analysis, seven had needle tip placements that were inaccurate. The one unmatched rat with accurate needle tip placement was a control with 53.8% CA3 damage.
DISCUSSION
These results support the view that chronic restraint selectively compromises the hippocampal CA3 region in young adult males. IBO damaged more neurons in the CA3 region of chronically stressed males compared to the contralateral, vehicle-infused side and to IBO-infused controls. To determine whether these effects of chronic restraint were widespread within the hippocampus, CA1 damage after IBO infusion was investigated and found to be similar between chronically stressed males and controls. Moreover, acute stress a few days earlier did not exacerbate damage in the CA3 region after IBO infusion, suggesting that circumstances surrounding chronic stress were necessary for CA3 vulnerability to IBO. Unlike males, females did not exhibit differences in IBO neurotoxicity within the CA3 region after chronic stress. Hence, CA3 and CA1 susceptibility to the effects of acute and chronic restraint, respectively, were not investigated further in females. These results indicate that chronic stress compromises the hippocampus in a region- and gender-specific manner, making only the CA3 region in males vulnerable to IBO neurotoxicity.
Three critical factors in interpreting the results of IBO treatment are 1) amount, 2) location, and 3) timing. First, the amount and volume of IBO infused was chosen to kill cells without unwanted spread of neurotoxin (Jarrard, 1989). Therefore, damage caused by IBO in the current study represents selective actions of IBO on N-methyl-d-aspartate (NMDA) receptors (Keilhoff et al., 1990) within the hippocampus and implicates the NMDA receptor as a mechanism by which chronic stress damages the CA3 region. Although IBO may act on many different cell types (Jarrard, 2002), it is not possible to speculate which cell type, if any, is most influenced by IBO. Second, infusion amount was measured indirectly by quantifying damage at the infusion site where IBO levels would be greatest. Matching for IBO amount and location was paramount to compare rats with similar levels of neuronal damage. Damage at the infusion site was statistically similar between stress and control groups, indicating that our matching process was not biased. Moreover, data were represented by percentage of damage to control for unequal tissue shrinkage, and the IBO-directed damage was not caused by the mechanical disruption of the needle because the side infused with PBS had minimal injury. Third, the 3 to 4 day delay between the final restraint and timing of IBO infusion was chosen to avoid acute GC elevations in the last restraint session from influencing IBO damage, as previous work showed that hippocampal damage caused by ischemia or neurotoxin was exacerbated within 24 h of acute GC elevations (Sapolsky, 1985a,b, 1986; Sapolsky and Pulsinelli, 1985). Indeed, acute restraint was not sufficient to exacerbate IBO-induced damage in the CA3 region. Therefore, IBO infusions were performed at an interval that was not influenced by elevated GC from the final restraint. These data support the interpretation that chronic stress is necessary for IBO to exacerbate damage in the CA3 region.
In this study, body weights were measured instead of corticosterone levels, adrenal, thymus or testis weights because reduced body weight can be measured non-invasively and significantly corresponds to chronic restraint exposure (Watanabe et al., 1992b; Magariños and McEwen, 1995a,b; Magariños et al., 1999; Conrad et al., 2001, 2003). A caveat is that restraint may reduce access to food, suggesting that inaccessibility to food and not stress perception altered weight gain. However, ongoing work from our lab contradicts this interpretation because food restriction to maintain body weight at 85% from baseline shows that chronically restrained rats require significantly more food (16±1 g) than controls (13±1 g). Moreover, providing stress levels of corticosterone in the drinking water over 3 weeks attenuates body weight gain (Conrad et al., 2004). These results illustrate that rats with unlimited access to food will still exhibit reduced body weight following prolonged exposure to corticosterone. Thus, reduced weight gain from chronic stress does not appear to be caused by decreased food intake.
Unlike males, chronically stressed females did not exhibit enhanced IBO-directed damage; however, CA3 cell damage in females was generally greater than in the males (95% relative to 75% at the needle tip). Direct comparison between the sexes was problematic because females had smaller brains and better needle placements than males. Moreover, estrogen increases glutamate binding to hippocampal NMDA receptors (Woolley et al., 1997), which may lead to more IBO-induced damage in females. However, these factors do not explain why stress failed to exacerbate IBO-induced hippocampal damage in females. Perhaps the damage observed in females reached a ceiling effect. This seems unlikely as a ceiling effect was not observed at distances farthest from the infusion site where stress also failed to influence IBO-induced damage in females. Another possibility is that females are less affected by chronic stress than males, in terms of hippocampal morphology (Galea et al., 1997) and function (Conrad et al., 2003). Although the stage of the estrous cycle was not determined in these females, the possibility that even small amounts of ovarian hormones protected the CA3 region of females cannot be discounted. Ovarian hormones protect the brain from IBO infusion after chronic stress because hippocampal damage caused by ischemia or neurotoxicity is reduced by estrogen (Goodman et al., 1996; Green et al., 2001; Jover et al., 2002) and estrogen+progesterone (Vongher and Frye, 1999).
Another interpretation for why females demonstrated less IBO-induced hippocampal damage may be the perception of the restraint procedure. Although both males and females exhibited less weight gain when chronically stressed, males showed nearly 11% weight difference by the end of stress compared to their same-sex controls, whereas stressed produced a difference of just 4% for the females. These data may suggest that the females perceived the restraint procedure as less stressful than the males. In contrast to this interpretation, female rats show greater corticosterone levels in response to restraint compared to males (Galea et al., 1997). Moreover, corticosterone levels stay elevated throughout the 21 day restraint paradigm in females, but attenuate by the end of restraint in males (Galea et al., 1997). A more parsimonious position is to defer interpretation until corticosterone levels and estrous cycle can be determined in future studies.
These data share some parallels with humans, suggesting that males may be more susceptible than females to the detrimental effects of stress. Psychosocial stressors elevate salivary free-cortisol in men compared to women in their follicular phase (low estrogen and progesterone), while basal HPA functioning remains similar (Kirschbaum et al., 1999). Moreover, cortisol levels secreted after stress negatively correlate with cognitive ability in men, but not women (Wolf et al., 2001). The animal and human literature provides compelling evidence that future studies include females when investigating the influence of stress because females exhibit a unique response to stress that warrants further research.
Interpretation and significance
Changes in hippocampal neurochemistry may underlie CA3 susceptibility to IBO neurotoxicity after chronic stress. Perhaps exposure to chronic stress increases hippocampal glutaminergic receptor density or affinity, thereby making CA3 neurons more vulnerable to IBO. However, rats exposed to the same paradigm of chronic restraint do not show increases in hippocampal NMDA or non-NMDA receptor binding (Watanabe et al., 1995). In Alzheimer's disease, where HPA activity is elevated, hippocampal NMDA receptor number does not generally decrease (Geddes et al., 1986). One report even suggests that 8 days of GC treatment down-regulates HPA activity and protects against hypoxia/ischemia (Krugers et al., 1995), implying that chronic stress would protect hippocampal neurons from IBO. A recent study observed that chronic exposure to stress levels of GC alters the pharmacological properties of hippocampal GABAA receptors (Orchinik et al., 2001), suggesting that the reduced inhibitory environment of the hippocampus may make CA3 neurons more susceptible to neurotoxicity. However, the CA1 and CA3 regions show comparable changes to GABAA sensitivity (Orchinik et al., 2001), implying that both the CA1 and CA3 regions should respond similarly after chronic stress and IBO infusion in the current study.
Although they were not quantified, alterations in spine density may also explain some of the findings. Chronic stress increases post-synaptic spine density on CA3 neurons (Sunanda et al., 1995) and increased spine number is associated with enhanced synaptic activity (Sánchez-Toscano et al., 1991). This stress-induced NMDA receptor supersensitivity may make CA3 neurons more vulnerable to IBO. Estrogen can also enhance CA1, but not CA3 hippocampal spine density (Woolley et al., 1990a; Woolley and McEwen, 1994; Woolley, 1998) by enhancing NMDA receptor sensitivity on these neurons (Woolley et al., 1997). Additional studies comparing the effects of IBO infusion on CA1 and CA3 damage in females during estrus and proestrus would clarify the relationship between ovarian hormones and hippocampal damage.
Another interpretation is that dendritic retraction may be critical in the susceptibility of CA3 neurons to neurotoxicity. For instance, 3 weeks of stress or GC treatment causes dendritic retraction in the CA3 area, but not in the CA1, CA2 or dentate gyrus regions (Woolley et al., 1990b; Magariños and McEwen, 1995a). Concurrent with these findings, the CA3 area was more susceptible to IBO compared to the CA1 region. CA1 dendritic retraction has been reported (Sousa et al., 2000) when the restraint procedure continued for 4 weeks instead of 3 (Watanabe et al., 1992c; Magariños and McEwen, 1995a), showing that a difference of 1 week can be critical. Consequently, 3 weeks of restraint may have been sufficient to induce CA3, but not CA1 dendritic retraction. Moreover, one session of restraint is not enough to cause CA3 dendritic retraction (Luine et al., 1996) and IBO infusions in the present study did not exacerbate CA3 damage in acutely stressed rats.
In summary, seminal work showed that hippocampal damage caused by metabolic challenges was exacerbated by acute stress or GC (Sapolsky and Pulsinelli, 1985). The results of the current study are the first to demonstrate that morphological and/or chemical changes caused by chronic stress may make the hippocampus vulnerable to further insult in the days following the end of chronic stress.
Acknowledgements
This work was funded by MH64727 (Conrad), a research incentive award from ASU College of Liberal Arts and Sciences (Conrad), and the Howard Hughes Medical Institute through the Undergraduate Biology Enrichment Program (Jackson and Wise). Preliminary data were presented at the 32nd Annual Meeting of the Society for Neuroscience in November 2002. The contributions of following individuals are gratefully acknowledged: Sarah Baran, Rudy Bellani, Angelique Ferayorni, George G. Gifford, Katherine A. Grote, Rebecca J. Hobbs, Jonathan Kleen, Donald D. MacMillan, Jacques P. McKissick, Jamie Plishka, Jaroslaw S. Strzelec, Sergey Tsekhanov, and Ryan Wright.
Abbreviations
- ANOVA
analysis of variance
- CA
cornus ammonis
- GC
glucocorticoids
- HPA
hypothalamic–pituitary–adrenal
- IBO
ibotenic acid
- NMDA
N-methyl-d-aspartate
- PBS
phosphate-buffered saline
REFERENCES
- Baran SE, Wright RL, Jackson JL, Kleen JK, Tsekhanov S, Wise L, Zachow KA, Conrad CD. Ovariectomized female rats demonstrate enhanced spatial memory on the Y-maze following chronic stress while acute estrogen treatment may attenuate performance. Soc Neurosci Abst. 2002;28:370.12. [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd Lawrence Erlbaum Associates, Inc; Mahwah, New Jersey: 2003. [Google Scholar]
- Conrad CD, Galea LAM, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y-Maze and this effect is blocked by tianeptine pre-treatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Magariños AM, LeDoux JE, McEwen BS. Repeated restraint stress increases fear conditioning, independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Mauldin-Jourdain ML, Hobbs RJ. Metryapone reveals that previous chronic stress differentially impairs hippocampal-dependent memory. Stress. 2001;4:305–318. doi: 10.3109/10253890109014754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol Learn Mem. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Conrad CD, MacMillan DD, II, Tsekhanov S, Wright RL, Baran SE, Fuchs RA. Influence of chronic corticosterone and glucocorticoid receptor antagonism in the amygdala on fear conditioning. Neurobiol Learn Mem. 2004 doi: 10.1016/j.nlm.2004.01.002. in press. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, McRae T, Tsai JR, George AE, Marcus DL, Freedman M, Wolf AP, McEwen B. Abnormal cortisol response in Alzheimer's disease linked to hippocampal atrophy. Lancet. 1988;2:391–392. doi: 10.1016/s0140-6736(88)92855-3. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Golomb J, George AE, Convit A, Tarshish CY, McRae T, De Santi S, Smith G, Ferris SH, Noz M, Rusinek H. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. Am J Neuroradiol. 1993;14:897–906. [PMC free article] [PubMed] [Google Scholar]
- DeGiorgio LA, Attardi B, Shimizu Y, Ogata M, Volpe BT. 17β-Estradiol treatment retards excitotoxic delayed degeneration in substantia nigra reticulata neurons. Brain Res. 2002;936:15–20. doi: 10.1016/s0006-8993(02)02482-4. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Flügge G, Ohl F, Lucassen P, Vollmann-Honsdorf GK, Michaelis T. Psychosocial stress, glucocorticoids, and structural alterations in the tree shrew hippocampus. Physiol Behav. 2001;73:285–291. doi: 10.1016/s0031-9384(01)00497-8. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Geddes JW, Chang-Chui H, Cooper SM, Lott IT, Cotman CW. Density and distribution of NMDA receptors in the human hippocampus in Alzheimer's disease. Brain Res. 1986;399:156–161. doi: 10.1016/0006-8993(86)90611-6. [DOI] [PubMed] [Google Scholar]
- Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid β-peptide toxicity in hippocampal neurons. J Neurochem. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- Green PS, Yang S-H, Nilsson KR, Kumar AS, Covey DF, Simpkins JW. The nonfeminizing enantiomer of 17β-estradiol exerts protective effects in neuronal cultures and a rat model of cerebral ischemia. Endocrinology. 2001;142:400–406. doi: 10.1210/endo.142.1.7888. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. On the use of ibotenic acid to lesion selectively different components of the hippocampal formation. J Neurosci Methods. 1989;29:251–259. doi: 10.1016/0165-0270(89)90149-0. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. Use of excitotoxins to lesion the hippocampus: update. Hippocampus. 2002;12:405–414. doi: 10.1002/hipo.10054. [DOI] [PubMed] [Google Scholar]
- Jover T, Tanaka H, Calderone A, Oguro K, Bennett MVL, Etgen AM, Zukin RS. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22:2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilhoff G, Wolf G, Stastny F, Schmidt W. Quinolinate neurotoxicity and glutamatergic structures. Neuroscience. 1990;34:235–242. doi: 10.1016/0306-4522(90)90317-w. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomat Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Knollema S, Kemper RHA, Ter Horst GJ, Korf J. Down-regulation of the hypothalamo-pituitary-adrenal axis reduces brain damage and number of seizures following hypoxia/ischaemia in rats. Brain Res. 1995;690:41–47. doi: 10.1016/0006-8993(95)00585-e. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Kemper RHA, Korf J, Ter Horst GJ, Knollema S. Metyrapone reduces rat brain damage and seizures after hypoxiaischemia: an effect independent of modulation of plasma corticosterone levels? J Cereb Blood Flow Metab. 1998;18:386–390. doi: 10.1097/00004647-199804000-00006. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Baskin RK, Pitler TA. Brain aging correlates: retardation by hormonal-pharmacological treatments. Science. 1981;214:581–584. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Eldridge JC. Evolving aspects of the glucocorticoid hypothesis of brain aging: hormonal modulation of neuronal calcium homeostasis. Neurobiol Aging. 1994;15:579–588. doi: 10.1016/0197-4580(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Leverenz JB, Wilkinson CW, Wamble M, Corbin S, Grabber JE, Raskind MA, Peskind ER. Effect of chronic high-dose exogenous cortisol on hippocampal neuronal number in aged nonhuman primates. J Neurosci. 1999;19:2356–2361. doi: 10.1523/JNEUROSCI.19-06-02356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Luine V, Martinez C, Villegas M, Magariños AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiol Behav. 1996;59:27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NPV, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995a;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995b;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazapine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- Málková L, Lex CK, Mishkin M, Saunders RC. MRI-based evaluation of locus and extent of neurotoxic lesions in monkeys. Hippocampus. 2001;11:361–370. doi: 10.1002/hipo.1050. [DOI] [PubMed] [Google Scholar]
- Morse JK, Davis JN. Regulation of ischemic hippocampal damage in the gerbil: adrenalectomy alters the rate of CA1 cell disappearance. Exp Neurol. 1990;110:86–92. doi: 10.1016/0014-4886(90)90053-u. [DOI] [PubMed] [Google Scholar]
- Müller MB, Lucassen PJ, Yassouridis A, Hoogendijk WJG, Holsboer F, Swaab DF. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci. 2001;14:1603–1612. doi: 10.1046/j.0953-816x.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- Neter J, Wasserman W, Kutner MH. Applied linear statistical models. 2nd Irwin; Homewood, IL: 1985. [Google Scholar]
- Oberfield SE, Cowan L, Levine LS, George A, David R, Litt A, Rojas V, Kairam R. Altered cortisol response and hippocampal atrophy in pediatric HIV disease. J Acquir Immune Defic Syndr. 1994;7:57–62. [PubMed] [Google Scholar]
- Orchinik M, Carroll SS, Li Y-H, McEwen BS, Weiland NG. Heterogeneity of hippocampal GABAA receptors: regulation by corticosterone. J Neurosci. 2001;21:330–339. doi: 10.1523/JNEUROSCI.21-01-00330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J. Detrimental effects of chronic hypothalamic-pituitary-adrenal axis activation. Mol Neurobiol. 1998;18:1–22. doi: 10.1007/BF02741457. [DOI] [PubMed] [Google Scholar]
- Sánchez-Toscano F, Sánchez M-d-M, Garzon J. Changes in the number of dendritic spines in the medial preoptic area during a premature long-term social isolation in rats. Neurosci Lett. 1991;122:1–3. doi: 10.1016/0304-3940(91)90178-v. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology. 1984;114:287–292. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoid toxicity in the hippocampus: temporal aspects of neuronal vulnerability. Brain Res. 1985a;359:300–305. doi: 10.1016/0006-8993(85)91440-4. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. A mechanism for glucocorticoid toxicity in the hippocampus: increased neuronal vulnerability to metabolic insults. J Neurosci. 1985b;5:1228–1232. doi: 10.1523/JNEUROSCI.05-05-01228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Pulsinelli WA. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229:1397–1399. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoid toxicity in the hippocampus: temporal aspects of synergy with kainic acid. Neuroendocrinology. 1986;43:440–444. doi: 10.1159/000124561. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Packan DR, Vale WW. Glucocorticoid toxicity in the hippocampus: in vitro demonstration. Brain Res. 1988;453:367–371. doi: 10.1016/0006-8993(88)90180-1. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JC, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Almeida OF, Holsboer F, Paula-Barbosa MM, Madeira MD. Maintenance of hippocampal cell numbers in young and aged rats submitted to chronic unpredictable stress: comparison with the effects of corticosterone treatment. Stress. 1998;2:237–249. doi: 10.3109/10253899809167288. [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OFX, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing's syndrome. Biol Psychol. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing's disease. Biol Psychiatry. 1999;46:1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- Stein BA, Sapolsky RM. Chemical adrenalectomy reduces hippocampal damage induced by kainic acid. Brain Res. 1988;473:175–180. doi: 10.1016/0006-8993(88)90332-0. [DOI] [PubMed] [Google Scholar]
- Stein-Behrens B, Mattson MP, Chang I, Yeh M, Sapolsky R. Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. J Neurosci. 1994;14:5373–5380. doi: 10.1523/JNEUROSCI.14-09-05373.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunanda Rao MS, Raju TR. Effect of chronic restraint stress on dendritic spines and excrescences of hippocampal CA3 pyramidal neurons: a quantitative study. Brain Res. 1995;694:312–317. doi: 10.1016/0006-8993(95)00822-8. [DOI] [PubMed] [Google Scholar]
- Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmann-Honsdorf GK, Flügge G, Fuchs E. Chronic psychosocial stress does not affect the number of pyramidal neurons in tree shrew hippocampus. Neurosci Lett. 1997;233:121–124. doi: 10.1016/s0304-3940(97)00647-2. [DOI] [PubMed] [Google Scholar]
- Vongher JM, Frye CA. Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacol Biochem Behav. 1999;64:777–785. doi: 10.1016/s0091-3057(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992a;2:431–436. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharmacol. 1992b;222:157–162. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992c;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Weiland NG, McEwen BS. Effects of adrenal steroid manipulations and repeated restraint stress on dynorphin mRNA levels and excitatory amino acid receptor binding in hippocampus. Brain Res. 1995;680:217–225. doi: 10.1016/0006-8993(95)00235-i. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990a;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990b;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-d-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic output: correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- Wright RL, Harman JS, Bellani R, Barab SE, Jackson JL, Kleen JK, McLaughlin KJ, Tsekhanov S, Zachow K, Conrad CD. Chronic stress and hippocampal lesion impair Y-maze performance when salient internal cues are absent but not when salient internal cues are present. Soc Neurosci Abstr. 2003;29:507.4. [Google Scholar]