Abstract
In the present study, the regulation of the sphingosine-recycling pathway in A549 human lung adenocarcinoma cells by oxidative stress was investigated. The generation of endogenous long-chain ceramide in response to exogenous C6-cer (C6-ceramide), which is FB1 (fumonisin B1)-sensitive, was employed to probe the sphingosine-recycling pathway. The data showed that ceramide formation via this pathway was significantly blocked by GSH and NAC (N-acetylcysteine) whereas it was enhanced by H2O2, as detected by both palmitate labelling and HPLC/MS. Similar data were also obtained using a novel approach that measures the incorporation of 17Sph (sphingosine containing 17 carbons) of 17C6-cer (C6-cer containing a 17Sph backbone) into long-chain 17C16-cer in cells by HPLC/MS, which was significantly decreased and increased in response to GSH and H2O2 respectively. TNF (tumour necrosis factor)-α, which decreases the levels of endogenous GSH, increased the generation of C16-cer in response to C6-cer, and this was blocked by exogenous GSH or NAC, or by the overexpression of TPx I (thioredoxin peroxidase I), an enzyme that reduces the generation of intracellular ROS (reactive oxygen species). Additional data showed that ROS regulated both the deacylation and reacylation steps of C6-cer. At a functional level, C6-cer inhibited the DNA-binding function of the c-Myc/Max oncogene. Inhibition of the generation of longchain ceramide in response to C6-cer by FB1 or NAC significantly blocked the modulation of the c-Myc/Max function. These data demonstrate that the sphingosine-recycling pathway for the generation of endogenous long-chain ceramide in response to exogenous C6-cer is regulated by ROS, and plays an important biological role in controlling c-Myc function.
Keywords: ceramide, fumonisin B1, c-Myc, oxidative stress, sphingosine, thioredoxin peroxidase
Abbreviations: AP-1, activator protein-1; BFA, brefeldin A; CDase, ceramidase; Cn-cer, ceramide with a usual sphingosine backbone of 18 carbons and a fatty acid chain containing n carbons; 17Cn-cer, ceramide with a sphingosine backbone of 17 carbons and a fatty acid chain containing n carbons; DCFH-DA, 2′,7′-dichlorofluorescein diacetate; e, erythro; EMSA, electrophoretic mobility-shift assay; FB1, fumonisin B1; MYR, myriocin; NAC, N-acetylcysteine; ROS, reactive oxygen species; RT, reverse transcription; siRNA, small interfering RNA; 17Sph, sphingosine containing 17 carbons; SPT, serine palmitoyltransferase; TNF, tumour necrosis factor; TPx I, thioredoxin peroxidase I
INTRODUCTION
The sphingolipid ceramide plays a role as an effector molecule that is involved in intracellular signalling, and regulates anti-proliferative responses such as apoptosis, growth arrest, differentiation and senescence [1]. Intracellular levels of ceramides are critical in regulating these processes, and these levels can be influenced by external inducers including cytokines [TNF (tumour necrosis factor), Fas, nerve growth factor], ‘environmental’ stresses (hyperthermia/heat, UV radiation, hypoxia/reperfusion), chemotherapeutic drugs (doxorubicin, etoposide and gemcitabine) and other agents, such as dexamethasone, lipopolysaccharide and sitosterol [2]. The generation of endogenous ceramide can be achieved mainly via the de novo pathway, or the activation of sphingomyelinases, which then release ceramide from sphingomyelin [3].
In addition, it has been shown previously that exogenous short-chain ceramides can also induce the generation of endogenous long-chain ceramides. For example, treatment of U937 human myeloid leukaemia cells with 25 μM cell-permeant C6-cer (C6-ceramide) triggered sustained endogenous ceramide generation at 24 h, which was inhibited by FB1 (fumonisin B1), an inhibitor of (dihydro)ceramide synthase, a key enzyme in the de novo ceramide synthesis pathway [4]. Previously, we demonstrated the generation of long-chain ceramide from short-chain ceramide in a process that involved deacylation of the exogenous ceramide, probably by a CDase (ceramidase) (or a deacylase), and reacylation of the generated sphingosine by ceramide synthase to form long-chain ceramide [5]. This process was found to be stereospecific whereby only the D-erythro isomers of C2- and C6-cer served as possible substrates for the sphingosine-recycling pathway [5]. Treatment of A549 cells with L-erythro-C2- (L-e-C2-) and C6-cer, or D-e-dihydro-C6-cer, did not result in the incorporation of labelled palmitate into long-chain ceramides after short-term treatment (2–6 h). These findings indicated that the stereochemistry, and the 4,5-trans double bond are critical in selecting the appropriate substrates for this pathway [5].
Importantly, a biological role for the generation of long-chain ceramides in response to exogenous short-chain ceramides was demonstrated previously by the inhibition of telomerase activity in A549 cells [5]. On the other hand, treatment of HL-60 cells with D-e-C6- or L-e-C6-cer equally induced apoptosis, indicating that the induction of apoptosis in these cells by exogenous ceramide may not involve the recycling pathway [5]. These results therefore show the ability of exogenous ceramide to undergo sphingosine recycling to generate endogenous long-chain ceramide, which in turn induces various biological responses, which might be cell-line-specific, and may depend on the availability of specific enzymes that act in this pathway. Nevertheless, these data suggest that some of the important biological roles of endogenous ceramides generated by the sphingosine-recycling pathway can be probed by employing exogenous short-chain ceramides, and thus any insight into the regulation of this pathway would be of great significance and interest.
Among the regulators of intracellular levels of ceramide, ROS (reactive oxygen species) play a crucial role. ROS regulate critical steps of the signal transduction cascades and many important cellular events, including protein phosphorylation, gene expression, transcription factor activation, DNA synthesis and cellular proliferation. On the other hand, antioxidants such as GSH and NAC (N-acetylcysteine), a GSH precursor, exert opposing effects [6]. Both ROS and antioxidants appear to interact with ceramide-mediated processes. For example, in A549 cells, 10 mM GSH significantly decreased apoptosis mediated by 25 μM C6-cer at 12 and 24 h [7]. In another study, neutral sphingomyelinase was reversibly inhibited by GSH in MCF7 human breast carcinoma cells. Treatment of MCF7 cells with TNF induced a marked decrease in the level of cellular GSH, which was accompanied by hydrolysis of sphingomyelin and generation of ceramide. Pre-treatment of cells with GSH, GSH-methyl ester, or NAC inhibited the TNF-induced sphingomyelin hydrolysis, ceramide generation, and cell death [8]. In neuronal and vascular cells, ROS play critical roles in mediating downstream effects of ceramide, including mitochondrial iron uptake, cytochrome c release, caspase 3 activation and apoptosis [9].
Although multiple previous studies have demonstrated the operation of a sphingolipid-recycling/salvage pathway for ceramide generation [4,5], mechanisms that regulate this pathway are still unknown. Therefore mechanisms that control the generation of long-chain ceramide in response to short-chain ceramide via the sphingosine-recycling pathway in A549 cells were examined in the present study. Results show for the first time that the sphingosine-recycling pathway is highly regulated by ROS at both the deacylation and reacylation steps. The role of ROS in response to TNF-α in the regulation of the sphingosine-recycling pathway is also demonstrated. In addition, a novel 17Sph (sphingosine containing 17 carbons instead of the usual 18)/MS approach using 17C6-cer (C6-cer containing a 17Sph backbone), was employed, and the role of ROS in the incorporation of 17Sph into 17C16-cer in these cells was confirmed further by HPLC/MS. More importantly, data presented show a novel biological role for the long-chain ceramide generated via the sphingosine-recycling pathway in response to C6-cer in the inhibition of the DNA-binding function of the c-Myc oncogene.
EXPERIMENTAL
Cell lines and culture conditions
A549 human lung adenocarcinoma cells were obtained from Dr Alice Boylan (Medical University of South Carolina). The MCF-7 human breast cancer cell line was obtained from the A.T.C.C. Cells were maintained in growth medium containing 10% (v/v) foetal calf serum and 100 ng/ml each of penicillin and streptomycin (Invitrogen) at 37 °C in 5% CO2. Sphingosine, short- and long-chain ceramides, and 17C6-cer were obtained from the Synthetic Lipidomics Core at the Department of Biochemistry and Molecular Biology (Medical University of South Carolina). 17Sph was purchased from Avanti Polar Lipids. FB1 was obtained from Alexis. MYR (myriocin), DCFH-DA (2′,7′-dichlorofluorescin diacetate), GSH, H2O2, NAC and TNF-α were purchased from Sigma. Exogenous short-chain ceramides were dissolved in ethanol at a concentration of 50–100 mM, and were then added directly to the medium containing 10% (v/v) foetal calf serum to obtain a final concentration of 1–20 μM. The proportion of ethanol was 0.02%, which had no effect on cell growth and/or survival. Fibroblasts from a patient with Farber's disease were obtained from Dr R. M. Boustany (Duke University Medical Center, Durham, NC, U.S.A.).
[3H]Palmitate labelling
Cells (1×105), grown in six-well plates, were treated with 1 μCi/ml of [3H]palmitate (Amersham Biosciences) with or without 5 μM sphingosine or 20 μM short-chain ceramides in the presence or absence of antioxidants (GSH or NAC), H2O2, TNF-α, FB1, MYR or BFA (brefeldin A) at various time points. The lipids were extracted using the method of Bligh and Dyer [9a] and separated by TLC using a solvent system containing chloroform, methanol and 2 M NH4OH (40:10:1), as described in [5].
SPT (serine palmitoyltransferase) enzyme activity assay
Inhibition of SPT by MYR was measured after treatment of A549 cells with various concentrations of MYR (10–100 nM) for 1 h as described previously [10]. In short, SPT activity in 100 μg of microsomal fractions was determined in 100 mM Hepes, pH 8.3, 5 mM dithiothreitol, 2.5 mM EDTA and 50 μM pyridoxal 5′-phosphate. The reaction is initiated with 200 μM palmitoyl-CoA and 2 μCi of L-[3H]serine, with a final serine concentration of 1 mM. The reaction mixtures were incubated at 37 °C for 30 min before the termination of the reaction with 200 μl of 0.5 M NH4OH. The organic soluble counts were extracted and quantified by liquid-scintillation counting as described in [10]. A reaction without microsomes was used as a blank, and measurements were made in triplicate.
Analysis of endogenous ceramides by HPLC/MS
The cellular levels of endogenous ceramides generated in response to C6-cer were measured using HPLC/MS as described previously [11]. Ceramide levels were normalized to total protein levels. The incorporation of 17Sph into endogenous 17C16-cer in response to treatment with exogenous 17C6-cer was detected by HPLC/MS (J. Bielawski, Z. M. Szulc and A. Bielawska, unpublished work).
Measurement of the levels of intracellular ROS
The ROS levels in cells were measured using DCFH-DA as described by the manufacturer. DCFH-DA is rapidly taken up by cells, and the diacetate residues are cleaved by esterases, liberating H2-DCF which accumulates intracellularly because of its low membrane permeability. When H2-DCF reacts with ROS, a structural change of the compound is responsible for intense fluorescence emission. In short, to measure the intracellular levels of ROS, cells were washed with PBS, DCFH-DA was added at a concentration of 20 μM for 20 min at 37 °C, and fluorescence was measured using a Wallac Victor3 1420 Multilabel Counter with excitation and emission wavelengths of 485 and 535 nm respectively.
TPx I (thioredoxin peroxidase I) transfections and RT (reverse transcription)–PCR
A549 cells (3×106/ml) were transfected with PCRTM 3.1-Uni vector (Invitrogen) with and without full-length TPx I cDNA (10 μg) [12] using the Effectene transfection agent (Qiagen). A heterogeneous population of positive clones were selected with G418 (Life Technologies) and were maintained in medium containing 10% (v/v) foetal bovine serum and 0.5 mg/ml G418. To determine the expression of TPx I, the mRNA levels of TPx I were analysed by RT–PCR. Total RNA (1 μg), isolated using an RNA-isolation kit (Qiagen), was used in RT reactions as described by the manufacturer. The resulting total cDNA was then used in PCR to measure the mRNA levels of TPx I using primers 5′-CGATTAAGCCCAACGTGTGGAT-3′ and 3′-GGAGGGCGTCACTATTCAGC-5′.
EMSA (electrophoretic mobility-shift assay)
Nuclear extracts (5–8 μg of protein) were isolated as described previously [13] from cells grown in the presence or absence of C6-cer, and were then pre-incubated in 15 μl of binding buffer containing 1.5 μg of poly(dI-dC)·(dI-dC) (Amersham Biosciences) with or without oligonucleotides containing unlabelled E-box and AP-1 (activator protein-1) sequences used as specific and non-specific competitors respectively at 25 °C for 15 min. Then, 3–5 ng of 5′-end-labelled DNA probes (50000 c.p.m./ng) were added to the reaction mixture and incubated at 25 °C for 15 min. End-labelling of DNA fragments with [γ-32P]ATP (Amersham Biosciences) was performed using T4 DNA kinase (Promega). The reaction mixtures were separated on native 5% (w/v) polyacrylamide gels and visualized by autoradiography [13,14]. The double-stranded oligomers were purchased from Santa Cruz Biotechnology.
Statistical analysis
Statistical analysis of the data was performed using Student's t test, and P<0.05 was considered to be significant.
RESULTS
Time-dependence and substrate specificity of the generation of endogenous long-chain ceramide via the sphingosine-recycling pathway
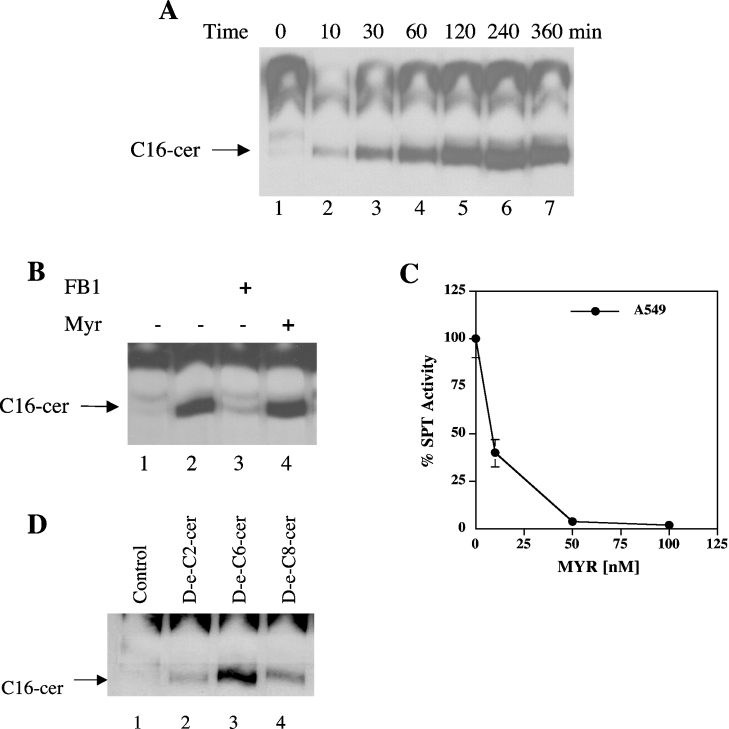
The generation of endogenous C16-cer in response to treatment with exogenous C6-cer (20 μM) at various time points (10 min–6 h) in A549 cells was studied in the presence of [3H]palmitate (1 μCi/ml). The data showed that the minimum time requirement for the recycling, as analysed by the incorporation of [3H]palmitate into C16-cer, was within 10 min of C6-cer treatment, and reached higher levels of long-chain ceramide generation at 30 min (Figure 1A, lanes 1–4) with an optimum conversion at 2 h, which was saturated between 4 and 6 h (Figure 1A, lanes 5–7). Consistent with our previous data [5], treatment of A549 cells with C6-cer for 2 h caused a significant increase (approx. 6-fold) in the generation of palmitate-labelled C16-cer when compared with the control, which did not receive any C6-cer (Figure 1B, lanes 2 and 1 respectively). This process was almost completely inhibited by pre-treatment (for 1 h) with 50 μM FB1, an inhibitor of (dihydro)ceramide synthase, but not by 50 nM MYR, an inhibitor of SPT (Figure 1B, lanes 3 and 4 respectively), demonstrating the functional recycling of ceramide generation in A549 cells. As shown in Figure 1(C), the effectiveness of 50 nM MYR in the inhibition of SPT (>95%) in A549 cells was also confirmed by measuring the enzyme activity of SPT using microsomes from cells treated with increasing concentrations of MYR for 1 h.
Figure 1. Time-dependence and substrate specificity of the recycling pathway for the generation of ceramide.
(A) Cells were treated with 20 μM C6-cer for various lengths of time (10–360 min; lanes 2–7), and the generation of long-chain ceramide was measured and compared with controls (without exogenous ceramide; lane 1) using [3H]palmitate labelling. (B) Cells were treated with (lane 2) or without (lane 1) 20 μM C6-cer in the presence of 1 μCi/ml [3H]palmitate for 2 h. The effects of pre-treatment with FB1 (50 μM; lane 3) and MYR (50 nM; lane 4) on the generation of endogenous ceramide via recycling were measured as described in the Experimental section. (C) The inhibition of SPT activity by MYR in A549 cells was examined after treatment with increasing concentrations of MYR (10–500 nM) for 1 h, and the enzyme activity was measured as described in the Experimental section. Results are means±S.D. (when not visible, error bars are smaller than the diameter of the points on the graph). (D) Cells were treated with 20 μM unlabelled D-e-C2, C6- and C8-cer for 2 h (lanes 2–4 respectively), and their effects on the generation of long-chain ceramide was measured and compared with controls (lane 1) in the presence of [3H]palmitate. The results shown are representative of at least two independent experiments performed in duplicate.
To evaluate the substrate specificity of long-chain ceramide generation via the sphingosine-recycling pathway, A549 cells were treated with various exogenous ceramides, which contained different length fatty acid chains (at 20 μM for 2 h). As shown in Figure 1(D), treatment of cells with D-e-C2-, C6- and C8-cer (lanes 2–4) resulted in the generation of C16-cer. Previous data showed also the stereospecificity of this pathway, which preferred D-e-C6-cer, but not L-e- or D-e-dihydro-C6-cer [5]. Taken together, these data show that the generation of endogenous C16-cer in response to exogenous ceramide is highly specific to the D-e-configurations of C2-, C6- and C8-cer, and D-e-C6-cer appeared to be the optimal substrate for this process (see Figure 1D, lane 3).
Role of ROS in the regulation of the generation of long-chain ceramide via the sphingosine-recycling pathway
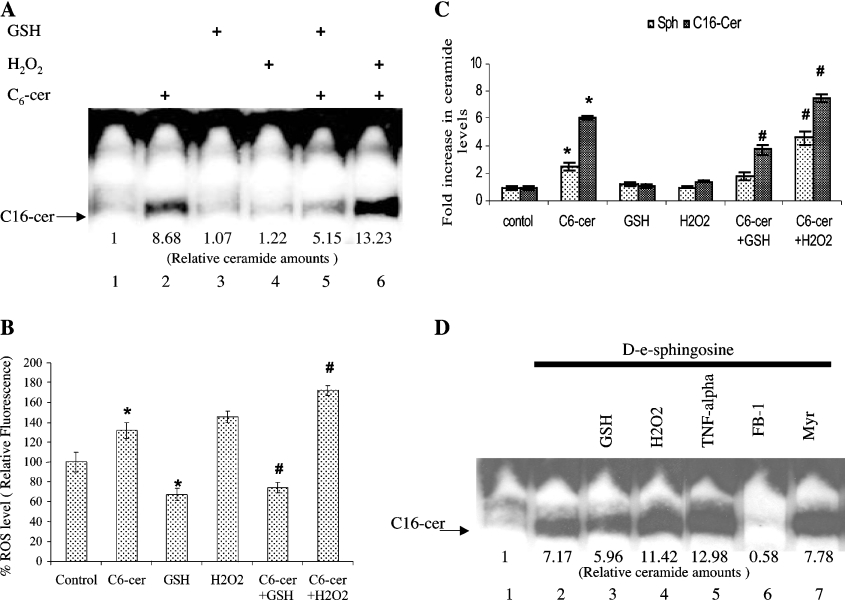
Next, it became important to gain some insight into the mechanisms that regulate the sphingosine-recycling pathway. Since ROS are known to regulate ceramide levels, the role of ROS in the sphingosine-recycling pathway was examined. A549 cells were pre-treated with 15 mM GSH or 100 μM H2O2 for 2 h. This pre-treatment was followed by the addition of [3H]palmitate (1 μCi/ml) with or without unlabelled D-e-C6-cer (20 μM) for an additional 2 h. The results in Figure 2(A) show that GSH significantly decreased (by approx. 40%) the incorporation of [3H]palmitate into C16-cer (lane 5), whereas the presence of H2O2 increased this process approx. 1.5-fold (lane 6) as compared with cells that were treated with C6-cer alone (lane 2). The presence of GSH or H2O2 alone, in the absence of C6-cer, did not have any significant effect in the incorporation of [3H]palmitate into C16-cer (Figure 2A, lanes 3 and 4). Then, the intracellular levels of ROS in response to C6-cer in the presence or absence of GSH and H2O2 were measured using DCFH-DA and fluorimetry as described in the Experimental section. Treatment of cells with C6-cer resulted in the elevation of intracellular ROS levels by approx. 31%, which was blocked in the presence of 15 mM GSH. However, when cells were pre-treated with 100 μM H2O2, ROS levels were increased by approx. 46 and 72% in the absence or presence of C6-cer respectively (Figure 2B). These data show that treatment of cells with C6-cer results in the generation of ROS, which in turn regulate the generation of long-chain ceramide via the recycling pathway.
Figure 2. Regulation of the recycling pathway for the generation of ceramide by ROS.
(A) Cells were pre-treated with 15 mM GSH (lane 5) or 100 μM H2O2 (lane 6) for 2 h, which was followed by treatment with 20 μM C6-cer for an additional 2 h (lane 2), and their effects on the generation of long-chain ceramide formation was measured by palmitate labelling, compared with controls (lane 1). Lanes 3 and 4 contain samples treated with GSH and H2O2 alone. The quantification of ceramide bands are shown beneath the lanes. (B) Cells were treated with GSH and H2O2 as in (A) in the presence or absence of C6-cer, and endogenous ROS levels were measured using DCFH-DA as indicated in the Experimental section. (C) The levels of endogenous ceramides were determined using HPLC/MS after treatment of cells with C6-cer (2 h) following 15 mM GSH or 100 μM H2O2 for 2 h. The results shown are representative of three independent experiments performed in duplicate. (D) Cells were treated with or without 5 μM D-e-Sph in the presence of 1 μCi/ml of [3H]palmitate for 2 h, without (lane 2), or with (lane 3) 15 mM GSH, 100 μM H2O2 (lane 4), 2 nM TNF-α (lane 5), 50 μM FB1 (lane 6) or 50 nM MYR (lane 7). Lane 1 contains untreated control. Results are means±S.D. * and #, P<0.05 compared with controls or ceramide alone respectively.
To evaluate further the regulation of the sphingosine-recycling pathway by ROS in these cells, total endogenous ceramide levels were also measured by HPLC/MS in response to C6-cer treatment in the presence or absence of GSH or H2O2 (Figure 2C). As expected, treatment of cells with C6-cer alone resulted in a 6-fold increase in the generation of endogenous C16-cer, going from 140 pmol/0.1 mg of protein for untreated cells to 854 pmol/0.1 mg of protein in cells treated with C6-cer (Figure 2C). The presence of GSH decreased the levels of C16-cer generation by approx. 50% in response to exogenous C6-cer at 2 h (Figure 2C). On the other hand, treatment of cells with C6-cer in the presence of H2O2 caused an approx. 7.2-fold increase in the levels of total endogenous C16-cer, which is approx. 20% higher than the effects of C6-cer alone (Figure 2C). Interestingly, the data in Figure 2(C) also show that C6-cer induced an increase in the levels of free sphingosine, most likely generated by the deacylation of C6-cer. Importantly, treatment with GSH or H2O2 in the presence of C6-cer resulted in decreased and increased levels of sphingosine respectively (Figure 2C). These results suggest that the regulation of sphingosine recycling by ROS might involve, at least, the deacylation step.
In order to investigate whether ROS also regulate the reacylation step, cells were treated with free exogenous sphingosine and labelled palmitate, in the presence of GSH and H2O2, in order to bypass the deacylation step. The results showed that GSH decreased the generation of endogenous long-chain ceramides (approx. 40%), whereas H2O2 increased those levels to 160% when compared with the effects of exogenous sphingosine alone (Figure 2D, lanes 3, 4 and 2 respectively). In addition, TNF-α, which is known to decrease intracellular GSH levels, also enhanced the generation of long-chain ceramide in response to exogenous sphingosine compared with sphingosine alone (Figure 2D, lanes 5 and 2 respectively). Thus ROS also regulate the recycling pathway at the reacylation step. Pre-treatment with MYR had no significant effect on the acylation of exogenous sphingosine, whereas FB1 blocked this step completely (Figure 2D, lanes 7 and 6 respectively).
Taken together, these data demonstrate that the regulation of the recycling pathway for the generation of endogenous ceramide by ROS appears to be controlled at both the deacylation and reacylation steps.
The use of a novel 17C-sphingolipid/MS approach to study the regulation of the sphingosine-recycling pathway by ROS
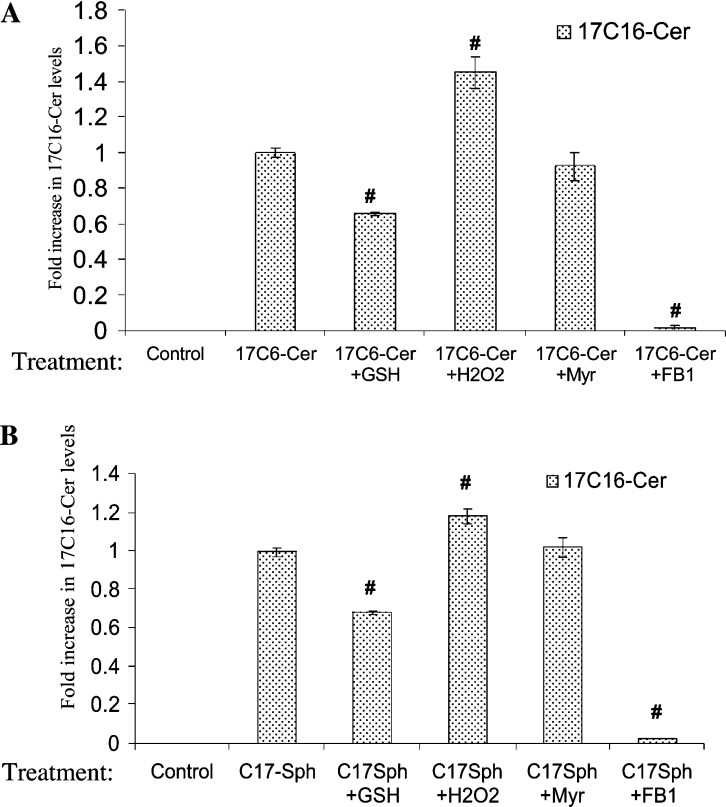
To better understand the regulation of the sphingosine-recycling pathway by ROS, we employed a novel 17C-sphingolipid/MS approach in which cells were treated with 17C6-cer, and the effects of GSH, H2O2, FB1 and MYR on the incorporation of 17Sph into endogenous 17C16-cer were examined by HPLC/MS. As shown in Figure 3(A), 17Sph was incorporated into 17C16-cer significantly upon treatment of cells with 17C6-cer, and it was blocked almost completely by FB1, and not by MYR, demonstrating the incorporation of 17Sph into 17C16-cer through the action of a FB1-sensitive ceramide synthase. Consistent with the previous data obtained by palmitate labelling and HPLC/MS, treatment of cells with GSH inhibited the incorporation of 17Sph into 17C16-cer by approx. 35%, whereas H2O2 caused an approx. 42% increase in this process, when compared with 17C6-cer treatment alone (Figure 3A). The absolute values of 17C16-cer in response to exogenous ceramide in the absence or presence of GSH and H2O2 were 10, 6.5 and 14.2 pmol/nmol of Pi respectively.
Figure 3. The detection of the recycling of 17Sph into long-chain endogenous ceramide by HPLC/MS.
The effects of GSH, H2O2, FB1 and MYR on the recycling pathway were examined in extracts obtained from cells treated with 2 μM 17C6-cer (A) or 17Sph (B). The incorporation of 17Sph into 17C16-cer was detected by HPLC/MS as described in the Experimental section. The results shown are means±S.D. and are representative of two independent experiments performed in duplicate. #, P<0.05 compared with ceramide or sphingosine alone.
The role of ROS in the reacylation step of the recycling pathway was also examined and confirmed by this approach, in which the incorporation of exogenously added 17Sph into 17C16-cer was significantly decreased by GSH (approx. 30%), and slightly increased (approx. 20%) in response to H2O2 when compared with 17Sph-treatment (Figure 3B). The metabolism of exogenous 17Sph into 17C16-cer was also responsive to FB1, and not to MYR (Figure 3B), as expected. Therefore these data demonstrate further the role of ROS in the regulation of the sphingosine-recycling pathway in A549 cells. However, the role of ROS in the reacylation step appears to be less detectable, based on the effects of GSH and H2O2 on the generation of long-chain ceramide in response to 17Sph compared with 17C16-cer (compare Figures 3B with Figure 3A). The absolute values of 17C16-cer in response to exogenous 17Sph in the absence or presence of GSH and H2O2 were 24, 16.8 and 30 pmol/nmol of Pi respectively.
Regulation of the sphingosine-recycling pathway by TNF-α via ROS production
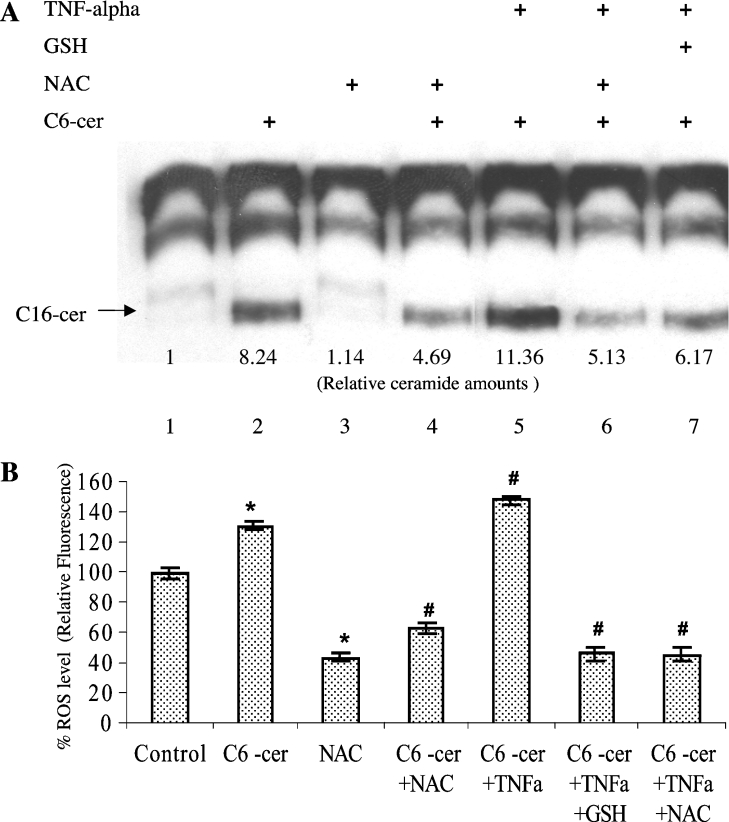
The role of ROS in the generation of ceramide via the sphingosine-recycling pathway was also examined in response to TNF-α, which is known to cause a decrease in the endogenous levels of GSH [15]. Consistent with its effects on the reacylation step (see Figure 2D, lanes 2 and 5), pre-treatment of cells with 1 nM TNF-α for 1 h caused an increase in the incorporation of [3H]palmitate into C16-cer by approx. 40% (Figure 4A, lane 5), when compared with cells treated with C6-cer alone (Figure 4A, lane 2). The effect of 1 nM TNF on the generation of C16-cer in response to C6-cer was significantly blocked in the presence of either 15 mM GSH or 10 mM NAC (Figure 4A, lanes 6 and 7). Similar results were also obtained using the MCF-7 human breast cancer cells, in which TNF-α increased the generation of long-chain ceramide in response to C6-cer, and those effects were blocked almost completely by GSH or NAC (results not shown). Intracellular levels of ROS were also measured using DCFH-DA (Figure 4B), and the data showed that treatment with TNF-α in combination with C6-cer induced the generation of ROS by approx. 48%, whereas C6-cer alone caused an approx. 32% increase in ROS production as compared with untreated controls. Importantly, GSH and NAC pre-treatment significantly decreased the levels of intracellular ROS even in the presence of TNF and C6-cer in combination (Figure 4B), indicating a role for TNF in the regulation of the recycling pathway via the control of ROS production and/or cellular redox status. TNF-α (1 nM) alone did not have significant effects on the levels of ROS or generation of long-chain ceramide (results not shown).
Figure 4. Role of TNF-α in the regulation of the recycling pathway.
(A) The role of TNF-α, which is known to regulate intracellular GSH levels, was examined using palmitate labelling experiments in cells treated with 1 nM TNF-α and D-e-C6-cer in combination for 2 h in the absence (lanes 5) or presence (lane 6) of NAC, or presence of GSH (lane 7). Lanes 2–4 contain samples treated with ceramide, GSH or NAC alone respectively. Lane 1 contains untreated controls. (B) The effects of TNF-α in the presence of exogenous ceramide on the generation of ROS were measured using DCFH-DA. Results are means±S.D. * and #, P<0.05 compared with untreated or ceramide (alone)-treated controls respectively.
Down-regulation of the sphingosine-recycling pathway by TPx I via decreasing intracellular ROS levels
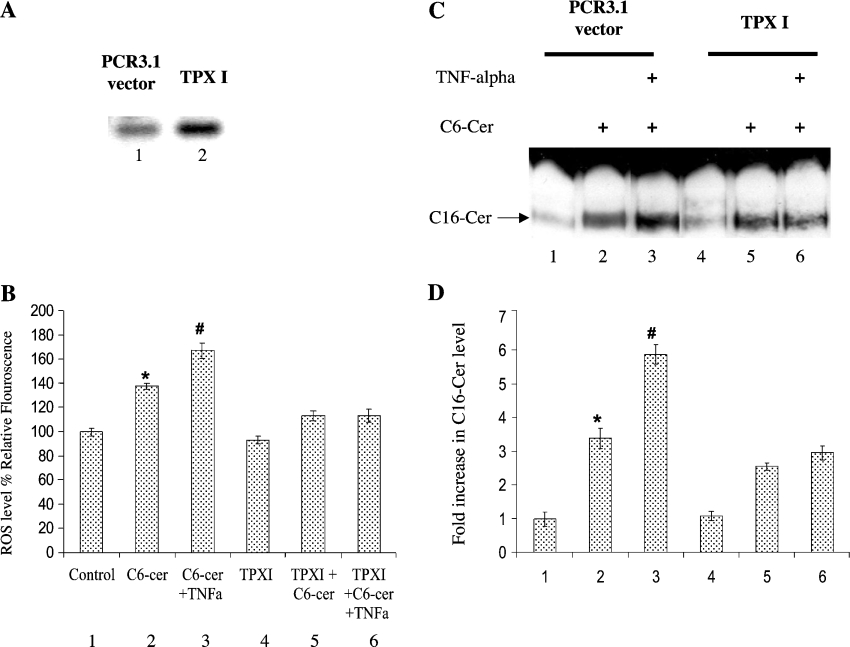
To examine further the role of ROS-mediated regulation of ceramide generation in response to TNF, we transfected A549 cells with a cDNA encoding the full-length mammalian TPx I protein, which is a known regulator of cytoplasmic ROS. After transfection, the overexpression of TPx I was demonstrated using RT–PCR (Figure 5A). Interestingly, TPx I attenuated the intracellular levels of ROS in response to C6-cer in the absence or presence of TNF (Figure 5B). We next evaluated endogenous long-chain ceramide generation in response to C6-cer using [3H]palmitate (Figures 5C and 5D). The data showed that overexpression of TPx I decreased sphingosine recycling by approx. 40% when compared with vector controls in response to exogenous ceramide (Figure 5C, lanes 5 and 2 respectively). More importantly, TPx I overexpression almost completely blocked the effects of TNF-α on the incorporation of labelled palmitate into C16-cer, when compared with vector controls in the presence of C6-cer (Figure 5C, lanes 6 and 3 respectively). The radioactivity in these bands was quantified, and reported in Figure 5D, which confirms the down-regulation of the sphingosine-recycling pathway by TPx I in response to TNF and C6-cer.
Figure 5. Regulation of ROS and recycling pathway by TPx I.
A549 cells were transfected with vector (PCR3.1) or TPXI. (A) The mRNA levels of TPx I were measured by RT–PCR, showing the overexpression of TPXI in transfected A549 cells (lane 2) compared with vector-transfected controls (lane 1). (B) Control vector- and TPx I-transfected cells were grown with or without 20 μM C6-cer in the presence and absence of 1 nM TNF-α, and the levels of ROS were measured using DCFH-DA. (C) The same treatments as in (B) were applied in the presence of 1 μCi/ml [3H]palmitate for 2 h, and long-chain ceramide formation was measured by TLC. (D) Radioactivity was measured in ceramide bands from the TLC plate shown in (C). The results shown are means±S.D. and are representative of three independent experiments performed in duplicate. * and #, P<0.05 compared with untreated or ceramide (alone)-treated controls respectively.
The role of the Golgi complex in the regulation of the sphingosine-recycling pathway mainly at the deacylation step
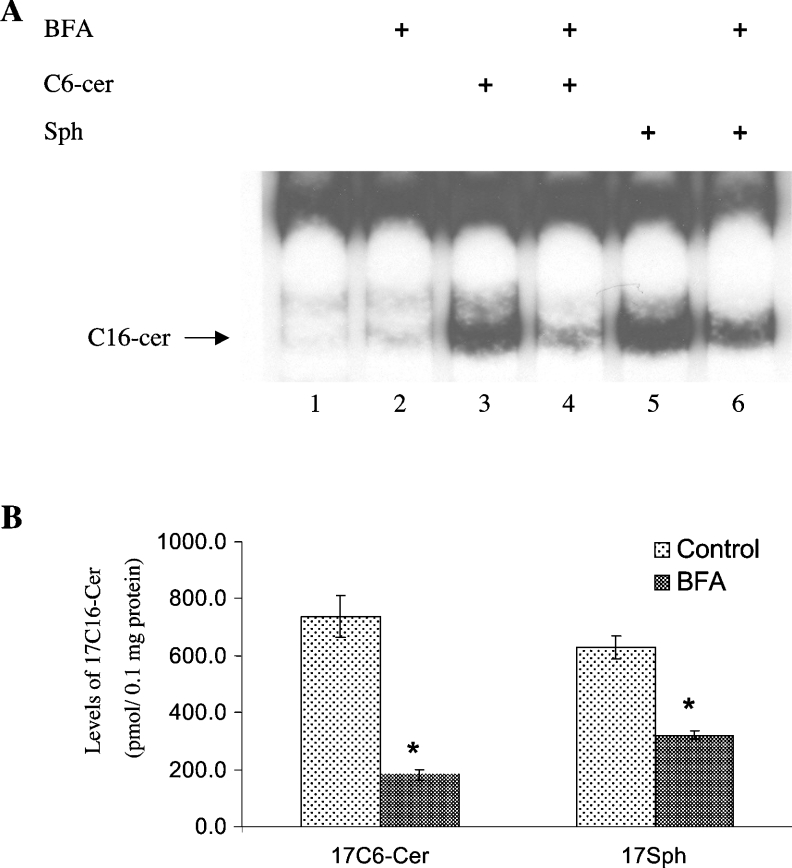
The importance of the deacylation and reacylation steps in the regulation of the sphingosine-recycling pathway was also determined after treatment of cells with BFA, a mycotoxin that causes disassembly of the Golgi apparatus. BFA inhibited the generation of C16-cer in response to C6-cer significantly, by approx. 80% (Figure 6A, lane 4), as shown by palmitate labelling experiments. However, when cells were treated with exogenous D-e-Sph to bypass the deacylation step, BFA had a smaller effect on the generation of C16-cer, blocking its generation by approx. 44% (Figure 6A, lane 6). These data were confirmed further by treatment of cells with 17C6-cer in the absence or presence of BFA. The results showed that BFA was very efficient in inhibiting the recycling of 17C6-cer (75%), whereas its effect on the incorporation of exogenous 17Sph into 17C16-cer was less significant (49%) (Figure 6B). These data demonstrate that BFA regulates the recycling pathway for the generation of long-chain ceramide mainly at the deacylation step, which might require an intact Golgi complex.
Figure 6. Golgi complex disassembly inhibits sphingosine acylation and recycling pathway.
(A) Cells were pre-treated with 10 μg/ml BFA for 1 h (lanes 2, 4 and 6) before treatment with 20 μM D-e-C6-cer (lanes 3 and 4) or 5 μM D-e-Sph (lanes 5 and 6) in the presence of [3H]palmitate for 2 h, and the formation of long-chain ceramide was examined by TLC. (B) Cells were treated with 17C6-cer and 17Sph in the presence or absence of BFA, and the generation of 17C16-cer was measured by HPLC/MS. The results are means±S.D. and are representative of two independent experiments performed in duplicate. *P<0.05 compared with ceramide- or sphingosine-treated controls.
The role of the endogenous C16-cer generated via the recycling pathway in response to exogenous C6-cer in the regulation of c-Myc inactivation
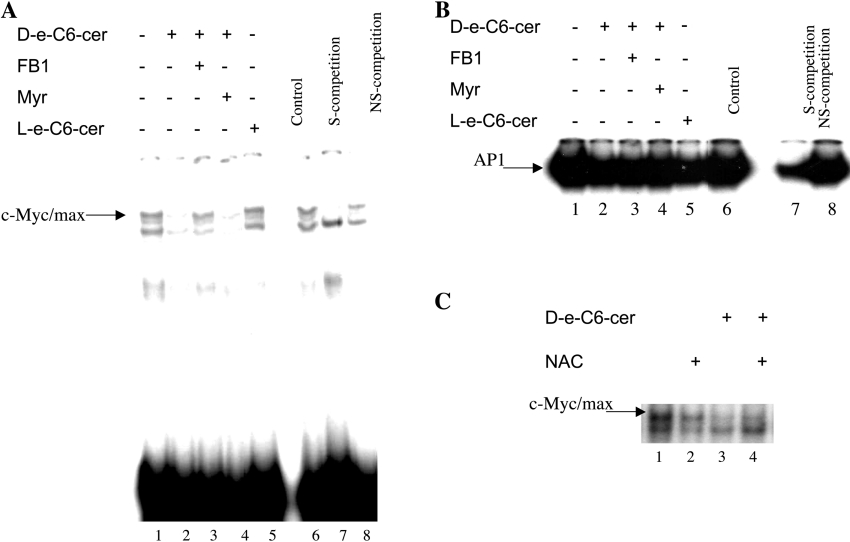
A role for endogenous C16-cer generated via the sphingosine-recycling pathway (in response to C6-cer treatment) in the inhibition of telomerase activity in A549 cells after long-term treatment (24 h) was shown previously [5]. Previous studies also defined a mechanism by which C6-cer inhibited the DNA-binding activity of the c-Myc transcription factor to the E-box sequence in the promoter of the hTERT (human telomerase reverse transcriptase) [16]. Our results suggested that endogenous ceramide formed from sphingosine recycling may mediate the inhibition of c-Myc [5,16]. Therefore, in the present study, cells were treated with 20 μM C6-cer for 2 h, with or without pre-treatment with FB1 and MYR (24 h), and then the DNA-binding function of the c-Myc/Max transcription factor was analysed by EMSAs as described above. The results showed that treatment of cells with 20 μM C6-cer for 2 h resulted in the inhibition of c-Myc/Max function, which was blocked completely by FB1, and not by MYR, suggesting an important role for the sphingosine-recycling pathway in C6-cer-mediated inhibition of c-Myc (Figure 7A, lanes 2–4). Also, treatment of cells with L-e-C6-cer (20 μM for 2 h), which is not metabolized for the generation of long-chain ceramides in these cells [5], did not have any inhibitory effect on the DNA-binding function of c-Myc (Figure 7A, lane 5). The specificity of the c-Myc-binding in EMSAs was confirmed by using unlabelled oligonucleotides containing E-box or Sp1 sequences at a 100-fold molar ratio as specific and non-specific competitors (Figure 7A, lanes 7 and 8). In contrast, treatment of cells with C6-cer (20 μM for 2 h) did not have any significant effects on the DNA-binding function of the AP-1 complex compared with untreated controls, demonstrating that the inhibition of c-Myc function by ceramide is not due to a general lipid effect (Figure 7B). The equal amounts of protein in these extracts were also confirmed by the detection of equal levels of Sp3 protein by Western blot analysis (results not shown). Also, pre-treatment with NAC, which blocked the recycling pathway significantly, partially blocked the inhibition of c-Myc/Max DNA binding in response to C6-cer (Figure 7C, lanes 4 and 3 respectively). These data demonstrate a specific role for the endogenous long-chain ceramide generated via the sphingosine-recycling pathway in the inhibition of c-Myc oncogene in response to exogenous C6-cer.
Figure 7. Endogenous long-chain ceramide generated via recycling is involved in the regulation of c-Myc/Max DNA-binding activity.
(A) The role of ceramide generated via the recycling pathway in the DNA-binding function of c-Myc/Max was determined by EMSAs using 32P-labelled double-stranded E-box oligomer, and nuclear extracts of cells pre-treated with 50 μM FB1 (lane 3) or 50 nM MYR (lane 4) in the presence of C6-cer for an additional 2 h. Lane 5 contains extracts obtained from cells treated with L-e-C6-cer, which cannot be recycled. The specificity of c-Myc/Max binding was confirmed using a 100-fold excess amount of unlabelled oligomers containing c-Myc (E-box) (lane 7) and Sp1-recognition (lane 8) sequences used as specific (S-competition) and non-specific (NS-competition) competitors respectively. The results shown are representative of two independent experiments. (B) The effects of C6-cer on the DNA-binding function of AP-1 were examined by EMSA using a 32P-labelled double-stranded oligomer containing the AP-1-recognition sequence, and nuclear extracts obtained from cells which were treated in the presence (lane 2) or absence (lane 1) of 20 μM D-e-C6-cer for 2 h. The effects of FB1, MYR and L-e-C6-cer on AP-1 function in the presence of ceramide was also examined (lanes 3–5). (C) The effects of NAC alone (lane 2) or in response to exogenous ceramide (lane 4) were examined by EMSA, and compared with the effects of ceramide alone (lane 3).
DISCUSSION
Previous data have established the biochemical mechanisms of the generation of endogenous long-chain ceramide in response to exogenous short-chain ceramide via the sphingosine-recycling pathway [5]; however, the mechanisms that regulate this pathway have not been described. The data obtained by using three alternative approaches to measure the generation of endogenous long-chain ceramides (by palmitate labelling in the presence of C6-cer, and treatment of cells with C6-cer or 17C6-cer coupled with HPLC/MS) demonstrate, for the first time, that the sphingosine-recycling pathway is regulated by intracellular ROS. Antioxidants, such as GSH and NAC decreased long-chain ceramide generated in response to exogenous ceramide, and H2O2 was found to have the opposite effect, inducing the sphingosine-recycling pathway. Interestingly, TNF-α-mediated induction of this pathway was also dependent on ROS. In addition, the data presented showed a novel function for the endogenous ceramide generated via the sphingosine recycling in the inhibition of the DNA-binding function of the c-Myc/Max transcription factor. Taken together, these data show for the first time that the generation of ceramide via sphingosine recycling is highly regulated by ROS, and is involved in the regulation of important biological events, such as the DNA-binding function of c-Myc, which is known to regulate the expression of telomerase [16].
In the experiments reported in the present paper, the effects of GSH or H2O2 on sphingosine recycling were accompanied by significant changes in sphingosine levels generated in response to exogenous ceramide, such that GSH decreased and H2O2 increased the amount of sphingosine generated. This indicates that regulation of this pathway by ROS is mediated, at least partially, by controlling the first step in the process, which is the deacylation of C6-cer.
The effects of exogenous sphingosine on the recycling pathway were also studied, and the data showed that treatment of cells with exogenous sphingosine was able to generate endogenous ceramide in a similar fashion to the treatment with short-chain ceramides. Incorporation of sphingosine into long-chain ceramide was modulated by ROS, and sphingosine recycling was inhibited by FB1, which is believed to compete with the sphingoid-base-binding site of (dihydro)ceramide synthase and interfere with utilization of the co-substrate fatty acyl-CoA [17]. These data, in addition to the results obtained with C6-cer, demonstrate that ROS play a role in the regulation of sphingosine recycling at both the deacylation and reacylation steps. Interestingly, consistent with previous studies [18,19], treatment with exogenous ceramide caused an approx. 30% increase in ROS production in A549 cells, and TNF-α enhanced further the effects of C6-cer approx. 16% more in the generation of ROS, which was effectively blocked by the overexpression of TPx I. Since TPx I is a cytoplasmic enzyme, these data suggest that ceramide-mediated ROS generation in the cytoplasm might be involved in the regulation of the sphingosine-recycling pathway. The precise mechanisms by which ceramide increases the generation of ROS are not known, however, and need to be determined.
Interestingly, BFA, a mycotoxin that causes rapid disassembly of the Golgi and redistribution of its components to the cell membrane and endoplasmic reticulum [20], was shown to inhibit sphingosine recycling [5], but did not significantly inhibit endogenous ceramide generation in response to sphingosine where the deacylation step was bypassed. These data suggest that the sphingosine-recycling pathway might require an intact Golgi complex, and that the enzyme responsible for deacylation of exogenous short-chain ceramide might reside in the Golgi.
CDases are enzymes that catalyse the cleavage of the N-acyl linkage of ceramide to give rise to sphingosine and non-esterified (‘free’) fatty acid. Acid CDase is a lysosomal enzyme [21–23], alkaline CDase is found in the endoplasmic reticulum and Golgi complex [24,25], whereas neutral CDase is a mitochondrial enzyme [26]. Interestingly, it was shown that acid and alkaline CDases have reversible activity via CoA-independent ceramide synthesis [27]. To identify which CDase is responsible for the deacylation of C6-cer for sphingosine recycling, the expression of all major CDases (acid, neutral and alkaline CDases) was partially inhibited using siRNAs (small interfering RNAs), and their effects on sphingosine recycling were assessed (results not shown). Unfortunately, none of the known CDases appeared to play a role in the sphingosine-recycling pathway. However, it should be noted that, because of the residual activity, which remains after partial inhibition of CDases using siRNA treatments, the identification of the CDase might be difficult. Nevertheless, studies using Farber's disease fibroblasts, which are deficient in acid CDase, showed no altered levels of sphingosine recycling, negating further a role for acid CDase in this process (results not shown). This is not so surprising, as these CDases do not use short-chain ceramides as their optimal substrates in vitro. Interestingly, it was shown previously that chloroquine, a weak base that raises the pH of lysosomes and inhibits acid CDase [28,29], was able to inhibit the shunting pathway, where native or partially hydrolysed glycosphingolipids are recycled from lysosomes to the Golgi [30]. Although the enzyme responsible for the deacylation of C6-cer still remains unknown, this study provided some insights into biochemical properties of this enzyme; it requires the C4,5 double bond, exhibits a limited chain length preference (D-e-C2-<C6->C8-cer), and is stereoisomer specific.
In parallel with these findings, one newly discovered carrier protein is CERT (ceramide transfer protein), which transports ceramide from the endoplasmic reticulum to the Golgi for the synthesis of sphingomyelin [31]. In addition, there are reports which suggest that cellular cytoskeleton proteins may provide important channels for the trafficking of lipids between intracellular compartments. For example, SW13 cells deficient in vimentin intermediate filaments had decreased ability to glycosylate ceramide, a process that takes place in Golgi [30]. These data show also that intracellular trafficking might be an essential part of sphingosine recycling, and this needs to be evaluated.
Moreover, to better study recycling and its regulation by ROS, we treated cells with novel analogues of 17Sph and 17C6-cer, and followed their metabolism to 17C16-cer in the presence or absence of antioxidants by HPLC/MS. As expected, the role of ROS in the regulation of sphingosine recycling was clearly demonstrated using this approach. Curiously, the effects of H2O2 on sphingosine recycling were much lower when measured using 17Sph (causing only an approx. 20% increase, as shown in Figure 3B) by HPLC/MS than measured by palmitate labelling (causing approx. 40% increase, as shown in Figure 2D). The exact reasons for this discrepancy are not known, and need to be examined further; however, in both techniques, GSH decreased, whereas H2O2 increased the effects of exogenous sphingosine on the generation of long-chain ceramide, demonstrating a role for ROS in the reacylation step.
Importantly, it was shown in the present study that sphingosine recycling is essential for some of the downstream effects of exogenous ceramides. We showed that after 2 h of short-chain ceramide treatment and sphingosine recycling, the binding of c-Myc/Max to DNA was decreased significantly. Inhibition of sphingosine recycling by FB1, and not MYR, blocked the modulation of c-Myc/Max function in response to C6-cer. On the other hand, L-e-C6-cer, which is not recycled [5], did not effect the DNA-binding of c-Myc/max. Therefore these data suggest that the generation of long-chain ceramides, and not the presence of exogenous short-chain ceramides, might be important for the inhibition of c-Myc function. However, the precise mechanism by which long-chain endogenous ceramide generated via the sphingosine-recycling pathway inhibits c-Myc is still unknown and needs to be determined. The role of ubiquitination and/or dephosphorylation in the stability of c-Myc protein has been demonstrated recently [32], and in the light of these data, it will be of great interest to determine the immediate role of the sphingosine-recycling pathway in these post-translational modifications of c-Myc.
In a recent study, the role of endogenous ceramide generated via the sphingosine-recycling/salvage pathway in response to PMA in the inhibition of juxtanuclear translocation of protein kinase C βII has been demonstrated [33]. These studies suggest that the recycling/salvage pathway can be operational in response not only to exogenous short-chain ceramides, but also to other agonists, such as PMA.
In conclusion, the results of the present study show for the first time using multiple experimental approaches and molecular tools that the sphingosine-recycling pathway, which results in the generation of long-chain ceramide in response to exogenous short-chain ceramide, is highly regulated by ROS, and that endogenous ceramide generated via this pathway plays important biological roles, such as inhibition of the DNA-binding function of c-Myc/Max (the present study) and telomerase function [5].
Acknowledgments
This work is funded by research grants from the National Institutes of Health (CA-88932) and the National Science Foundation/EPSCoR (EPS-0132573) to B. O. We thank Dr Lina M. Obeid and Dr S. G. Rhee for providing us with the human TPx I cDNA. We also thank the members of the Ogretmen laboratory for their technical help and discussions, and Dr D. K. Perry for helping us with the SPT enzyme analysis.
References
- 1.Hannun Y. A. Functions of ceramide in coordinating cellular response to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 2.Ogretmen B., Hannun Y. A. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Rev. Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 3.Merrill A. H., Jr, Schmelz E. M., Dillehay D. L., Spiegel S., Shayman J. A., Schroeder J. J., Riley R. T., Voss K. A., Wang E. Sphingolipids–the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol. Appl. Pharmacol. 1997;142:208–225. doi: 10.1006/taap.1996.8029. [DOI] [PubMed] [Google Scholar]
- 4.Jaffrezou J. P., Maestre N., de Mas-Mansat V., Bezombes C., Levade T., Laurent G. Positive feedback control of neutral sphingomyelinase activity by ceramide. FASEB J. 1998;12:999–106. doi: 10.1096/fasebj.12.11.999. [DOI] [PubMed] [Google Scholar]
- 5.Ogretmen B., Pettus B. J., Rossi M. J., Wood R., Usta J., Szulc Z., Bielawska A., Obeid L. M., Hannun Y. A. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line: role for endogenous ceramide in mediating the action of exogenous ceramide. J. Biol. Chem. 2002;277:12960–12969. doi: 10.1074/jbc.M110699200. [DOI] [PubMed] [Google Scholar]
- 6.Hoidal J. R. Reactive oxygen species and cell signaling. Am. J. Respir. Cell Mol. Biol. 2001;25:661–663. doi: 10.1165/ajrcmb.25.6.f213. [DOI] [PubMed] [Google Scholar]
- 7.Lavrentiadou S. N., Chan C., Kawcak T., Ravid T., Tsaba A., van der Vliet A., Rasooly R., Goldkorn T. Ceramide-mediated apoptosis in lung epithelial cells is regulated by glutathione. Am. J. Respir. Cell Mol. Biol. 2001;25:676–684. doi: 10.1165/ajrcmb.25.6.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B., Andrieu-Abadie N., Levade T., Zhang P., Obeid L. M., Hannun Y. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-α-induced cell death. J. Biol. Chem. 1998;273:11313–11320. doi: 10.1074/jbc.273.18.11313. [DOI] [PubMed] [Google Scholar]
- 9.Matsunaga T., Kotamraju S., Kalivendi S. V., Dhanasekaran A., Joseph J., Kalyanaraman B. Ceramide-induced intracellular oxidant formation, iron signaling, and apoptosis in endothelial cells: protective role of endogenous nitric oxide. J. Biol. Chem. 2004;279:28614–28624. doi: 10.1074/jbc.M400977200. [DOI] [PubMed] [Google Scholar]
- 9a.Bligh E. G., Dyer W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 10.Williams R. D., Wang E., Merrill A. H., Jr Enzymology of long-chain base synthesis by liver: characterization of serine palmitoyltransferase in rat liver microsomes. Arch. Biochem. Biophys. 1984;228:282–291. doi: 10.1016/0003-9861(84)90069-9. [DOI] [PubMed] [Google Scholar]
- 11.Koybasi S., Senkal C. E., Sundararaj K., Spassieva S., Bielawski J., Osta W., Day T. A., Jiang J. C., Jazwinski S. M., Hannun Y. A., et al. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J. Biol. Chem. 2004;279:44311–44319. doi: 10.1074/jbc.M406920200. [DOI] [PubMed] [Google Scholar]
- 12.Chae H. Z., Robison K., Poole L. B., Church G., Storz G., Rhee S. G. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flamigni F., Faenza I., Marmiroli S., Stanic I., Giaccari A., Muscari C., Stefanelli C., Rossoni C. Inhibition of the expression of ornithine decarboxylase and c-Myc by cell-permeant ceramide in difluoromethylornithine-resistant leukaemia cells. Biochem. J. 1997;324:783–789. doi: 10.1042/bj3240783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogretmen B., Safa A. R. Identification and characterization of the MDR1 promoter-enhancing factor 1 (MEF1) in the multidrug resistant HL60/VCR human acute myeloid leukemia cell line. Biochemistry. 2000;39:194–204. doi: 10.1021/bi991943f. [DOI] [PubMed] [Google Scholar]
- 15.Hayter H. L., Pettus B. J., Ito F., Obeid L. M., Hannun Y. A. TNFα-induced glutathione depletion lies downstream of cPLA2 in L929 cells. FEBS Lett. 2001;507:151–156. doi: 10.1016/s0014-5793(01)02967-2. [DOI] [PubMed] [Google Scholar]
- 16.Ogretmen B., Kraveka J. M., Schady D., Usta J., Hannun Y. A., Obeid L. M. Molecular mechanisms of ceramide-mediated telomerase inhibition in the A549 human lung adenocarcinoma cell line. J. Biol. Chem. 2001;276:32506–32514. doi: 10.1074/jbc.M101350200. [DOI] [PubMed] [Google Scholar]
- 17.Humpf H. U., Schmelz E. M., Meredith F. I., Vesper H., Vales T. R., Wang E., Menaldino D. S., Liotta D. C., Merrill A. H., Jr Acylation of naturally occurring and synthetic 1-deoxysphinganines by ceramide synthase: formation of N-palmitoyl-aminopentol produces a toxic metabolite of hydrolyzed fumonisin, AP1, and a new category of ceramide synthase inhibitor. J. Biol. Chem. 1998;273:19060–19064. doi: 10.1074/jbc.273.30.19060. [DOI] [PubMed] [Google Scholar]
- 18.Phillips D. C., Allen K., Griffiths H. R. Synthetic ceramides induce growth arrest or apoptosis by altering cellular redox status. Arch. Biochem. Biophys. 2002;407:15–24. doi: 10.1016/s0003-9861(02)00496-4. [DOI] [PubMed] [Google Scholar]
- 19.Lavrentiadou S. N., Chan C., Kawcak T., Ravid T., Tsaba A., van der Vliet A., Rasooly R., Goldkorn T. Ceramide-mediated apoptosis in lung epithelial cells is regulated by glutathione. Am. J. Respir. Cell Mol. Biol. 2001;25:676–684. doi: 10.1165/ajrcmb.25.6.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mironov A., Colanzi A., Silletta M. G., Fiucci G., Flati S., Fusella A., Polishchuk R., Mironov A., Jr, Di Tullio G., Weigert R., et al. Role of NAD+ and ADP-ribosylation in the maintenance of the Golgi structure. J. Cell Biol. 1997;139:1109–1118. doi: 10.1083/jcb.139.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernardo K., Hurwitz R., Zenk T., Desnick R. J., Ferlinz K., Schuchman E. H., Sandhoff K. Purification, characterization, and biosynthesis of human acid ceramidase. J. Biol. Chem. 1995;270:11098–11102. doi: 10.1074/jbc.270.19.11098. [DOI] [PubMed] [Google Scholar]
- 22.Ferlinz K., Kopal G., Bernardo K., Linke T., Bar J., Breiden B., Neumann U., Lang F., Schuchman E. H., Sandhoff K. Human acid ceramidase: processing, glycosylation, and lysosomal targeting. J. Biol. Chem. 2001;276:35352–35360. doi: 10.1074/jbc.M103066200. [DOI] [PubMed] [Google Scholar]
- 23.He X., Okino N., Dhami R., Dagan A., Gatt S., Schulze H., Sandhoff K., Schuchman E. H. Purification and characterization of recombinant, human acid ceramidase: catalytic reactions and interactions with acid sphingomyelinase. J. Biol. Chem. 2003;278:32978–32986. doi: 10.1074/jbc.M301936200. [DOI] [PubMed] [Google Scholar]
- 24.El Bawab S., Roddy P., Qian T., Bielawska A., Lemasters J. J., Hannun Y. A. Molecular cloning and characterization of a human mitochondrial ceramidase. J. Biol. Chem. 2000;275:21508–21512. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- 25.Mao C., Xu R., Szulc Z. M., Bielawski J., Becker K. P., Bielawska A., Galadari S. H., Hu W., Obeid L. M. Cloning and characterization of a mouse endoplasmic reticulum alkaline ceramidase: an enzyme that preferentially regulates metabolism of very long chain ceramides. J. Biol. Chem. 2003;278:31184–31191. doi: 10.1074/jbc.M303875200. [DOI] [PubMed] [Google Scholar]
- 26.Mao C., Xu R., Szulc Z. M., Bielawska A., Galadari S. H., Obeid L. M. Cloning and characterization of a novel human alkaline ceramidase: a mammalian enzyme that hydrolyzes phytoceramide. J. Biol. Chem. 2001;276:26577–26588. doi: 10.1074/jbc.M102818200. [DOI] [PubMed] [Google Scholar]
- 27.Okino N., He X., Gatt S., Sandhoff K., Ito M., Schuchman E. H. The reverse activity of human acid ceramidase. J. Biol. Chem. 2003;278:29948–29953. doi: 10.1074/jbc.M303310200. [DOI] [PubMed] [Google Scholar]
- 28.Maxfield F. R. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J. Cell Biol. 1982;95:676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dean R. T., Jessup W., Roberts C. R. Effects of exogenous amines on mammalian cells, with particular reference to membrane flow. Biochem. J. 1984;217:27–40. doi: 10.1042/bj2170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillard B. K., Clement R., Colucci-Guyon E., Babinet C., Schwarzmann G., Taki T., Kasama T., Marcus D. M. Decreased synthesis of glycosphingolipids in cells lacking vimentin intermediate filaments. Exp. Cell Res. 1998;242:561–572. doi: 10.1006/excr.1998.4126. [DOI] [PubMed] [Google Scholar]
- 31.Hanada K., Kumagai K., Yasuda S., Miura Y., Kawano M., Fukasawa M., Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature (London) 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- 32.Yeh E., Cunningham M., Arnold H., Chasse D., Monteith T., Ivaldi G., Hahn W. C., Stukenberg P. T., Shenolikar S., Uchida T., et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat. Cell Biol. 2004;6:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- 33.Becker K. P., Kitatani K., Idkowiak-Baldys J., Bielawski J., Hannun Y. A. Selective inhibition of juxtanuclear translocation of protein kinase C βII by a negative feedback mechanism involving ceramide formed from the salvage pathway. J. Biol. Chem. 2004;280:2606–2612. doi: 10.1074/jbc.M409066200. [DOI] [PubMed] [Google Scholar]