Abstract
We previously found that scavenger receptor cysteine-rich gp-340 (glycoprotein-340), isolated from lung or saliva, directly inhibits human IAVs (influenza A viruses). We now show that salivary gp-340 has broad antiviral activity against human, equine and porcine IAV strains. Although lung and salivary gp-340 are identical in protein sequence, salivary gp-340 from one donor had significantly greater antiviral activity against avian-like IAV strains which preferentially bind sialic acids in α(2,3) linkage. A greater density of α(2,3)-linked sialic acids was present on the salivary gp-340 from this donor as compared with salivary gp-340 from another donor or several preparations of lung gp-340. Hence, the specificity of sialic acid linkages on gp-340 is an important determinant of anti-IAV activity. Gp-340 binds to SP-D (surfactant protein D), and we previously showed that lung gp-340 has co-operative interactions with SP-D in viral neutralization and aggregation assays. We now report that salivary gp-340 can, in some cases, strongly antagonize certain antiviral activities of SP-D. This effect was associated with greater binding of salivary gp-340 to the carbohydrate recognition domain of SP-D as compared with the binding of lung gp-340. These findings may relate to inter-individual variations in innate defence against highly pathogenic IAV and to effects of aspiration of oral contents on SP-D-mediated lung functions.
Keywords: collectin, glycoprotein-340, influenza A virus, innate immunity, saliva, surfactant protein D
Abbreviations: Aichi, A2/Aichi68/H3N2; BALF, bronchoalveolar lavage fluids; Braz79, Phil82/BS, A/Brazil79/H1N1; classic swine, A/Swine/Iowa90; CRD, carbohydrate recognition domain; Equine, A/equine2/Miami63/H3N8; gp-340, glycoprotein-340; HA, haemagglutination; IAV, influenza A virus; MAA, Maackia amurensis agglutinin; Mem71, A/Mem71H-BelN/H3N1; NK, natural killer; Phil82, A/Philippines/82/H3N2; PR-8, A/Puerto Rico/34/H1N1; pSP-D or RhSP-D, porcine or recombinant human surfactant protein D respectively; RF-SP-DnCRD, trimeric neck and CRD construct of RhSP-D; SNA, Sambucus nigra agglutinin; SP, surfactant protein; TMB, tetramethylbenzidine
INTRODUCTION
IAV (influenza A virus) is a major cause of morbidity and mortality, even in developed countries in non-pandemic years; annual mortality from influenza in the U.S.A. is approx. 40000 [1]. Mortality in non-pandemic years is concentrated in the elderly, but IAV also causes work loss in healthy adults [2] and is a major contributor to hospitalization in children [3,4]. The 1918 pandemic strain that caused approx. 30 million deaths worldwide was recently shown to be of avian origin [5–7]. In recent years, there have been numerous instances of transmission of avian strains to humans [8,9], and a pandemic is considered likely in the near future by the World Health Organization [9]. There is also concern regarding the use of highly pathogenic IAV strains in bioterrorism. Currently available vaccines and antivirals can have a large impact on IAV. However, there is need for improved treatments and additional knowledge regarding the role of innate and adaptive immune responses in mediating susceptibility of individual subjects.
Recent studies have partly elucidated the complex interplay of innate and adaptive immune responses during IAV infection. It is now clear that innate immune mediators play critical roles during the early phase of IAV infection. These innate mediators include soluble components [e.g. the collectins, SPs (surfactant proteins) -A and -D, tumour necrosis factor α, Type I interferons, scavenger-receptor cysteine-rich gp-340 (glycoprotein-340) and interfering RNA] [10–17] and cellular components [e.g. NK (natural killer) cells, macrophages and neutrophils] [18–20]. The ability of IAV strains to modify their antigenic properties through small incremental mutations (drift) and major changes resulting from exchange of genome segments with those of animal strains (reassortment) results in continued emergence of strains for which prior adaptive immune responses are ineffective.
We have demonstrated that SP-D and, to a lesser extent, SP-A, inhibit infectivity of IAV and contribute strongly to the antiviral activity of BALF (bronchoalveolar lavage fluids) [10,11]. Collectins neutralize IAV by binding to viral envelope proteins, aggregate IAV particles, and promote IAV uptake by neutrophils [21]. SP-D is not only present in the lung but in a variety of mucosal or other epithelial surfaces and in blood [22,23], where it can act as an initial defence against IAV or other pathogens. In vivo studies have recently confirmed initial in vitro findings regarding SP-D and SP-A [15,16,24–26].
We have shown that the saliva of healthy volunteer donors has significant anti-influenza activity. Innate immune factors were shown to contribute strongly to the antiviral activity of saliva, because removal of IgA only slightly reduced the activity [11]. The antiviral activity of saliva differed qualitatively from that of BALF. In the case of BALF, calcium-dependent lectin activity, contributed largely by SP-D, was predominant [10,11]. In contrast, such calcium-dependent lectin activity makes a much smaller contribution to the antiviral activity of saliva. One of the innate immune factors contributing to its IAV neutralizing activity was shown to be salivary agglutinin (or salivary gp-340). The mechanism of action of gp-340 involves non-calcium-dependent binding of IAV to sialic acid moieties on gp-340 [11].
Lung gp-340 also contributes to the antiviral activity of BALF. However, the contribution of lung gp-340 to the overall anti-influenza activity of BALF was less than the contribution of salivary gp-340 to saliva. Lung and salivary gp-340 share the same protein sequence and reactivity with anti-gp340 antibodies [27,28]. One difference between salivary and lung gp-340 is in glycosylation; salivary gp-340 reacts with antibodies against Sialyl-Lewisx carbohydrate epitope, whereas lung gp-340 does not [28]. Initial studies of salivary gp-340 isolated from a single donor showed increased activity against the PR-8 strain of IAV compared with lung gp-340 [11]. This finding was of interest, since this mouse adapted human strain shares with avian and other animal strains an increased affinity for α(2,3)-linked sialic acids.
The goals of the current study were to characterize the viral specificity of salivary gp-340, to determine if there are donor-dependent differences in activity of salivary gp-340, and to study interactions of salivary gp-340 with SP-D in antiviral assays.
MATERIALS AND METHODS
Buffers and reagents
Dulbecco's phosphate-buffered saline with (PBS++) and without (PBS) calcium and magnesium were purchased from GIBCO BRL. Endotoxin and other chemical reagents were obtained from Sigma–Aldrich.
Virus preparation
IAV was grown in the chorioallantoic fluid of 10-day-old chicken eggs and purified on a discontinuous sucrose gradient as previously described [29]. The virus was dialysed against PBS to remove sucrose, divided into aliquots and stored at −80 °C until needed. The A/Philippines/82/H3N2 (Phil82), Phil82/BS, A/Brazil79/H1N1 (Braz79) and A/Mem71H-BelN/H3N1 (Mem71) strains were kindly provided by Dr E. Margot Anders (Department of Microbiology and Immunology, University of Melbourne, Melbourne, Australia). The A/Puerto Rico/34/H1N1 (PR-8) strain was kindly provided by Dr Jon Abramson (Department of Pediatrics, Wake Forest University, NC, U.S.A.). The A2/Aichi68/H3N2 (Aichi), A/equine2/Miami63/H3N8 (Equine) and Sendai strains were provided by American Type Culture Collection. The inactivated A/Swine/Iowa90 (Classic Swine) strain was kindly given by Dr R. Webster and Mr S. Krauss (Department of Virology and Molecular Biology, St. Jude Children's Research Hospital, Memphis, TN, U.S.A.). The HA (haemagglutination) titres of virus preparations were determined by titration of virus samples in PBS with thoroughly washed human type O, Rh(−) red blood cells as described [30]. After thawing, the viral stocks contained approx. 5×108 plaque-forming units/ml.
Source of normal donor saliva and BALF
Normal donor saliva was obtained by simple expectoration into 50 ml tubes, followed by centrifugation at 10000 g to remove mucinous precipitate and addition of 1% penicillin and streptomycin to inhibit bacterial growth. BALF were obtained from healthy volunteer donors. Between 150 and 200 ml of normal saline was instilled for the lavage. Fluids obtained by this procedure were subjected to an initial centrifugation (150 g) to remove cells and large particulate matter. BALF and saliva were obtained after informed consent as approved by the Boston University School of Medicine Institutional Review Board for Human Research.
Lung gp-340, SP-D and SP-A preparations
RhSP-D (recombinant human SP-D) was produced in CHO-K1 cells as previously described [31]. Using gel filtration, RhSP-D was fractionated into preparations predominantly composed of trimers, dodecamers and multimers as described [32]. For the present studies the dodecameric fraction of RhSP-D was used unless otherwise specified. SP-A was purified from human alveolarproteinosis fluid as described in [16]. Natural human SP-D dodecamers were isolated from amniotic fluid as described [33]. pSP-D (porcine SP-D) was purified from BALF as described previously [34]. pSP-D was composed of a mixture of dodecameric and more highly multimerized forms. RfSP-DnCRD is a truncated trimeric construct made up of the neck and CRD (carbohydrate recognition domain) only of RhSP-D. This was produced in Escherichia coli as described [35]. The collectin preparations used in the present study were tested for degree of contamination with endotoxin using a quantitative endotoxin assay (Limulus Amebocyte Lysate; Bio-Whittaker). The final concentrations of endotoxin in samples containing the highest concentrations of collectins were approx. 20–100 pg/ml (or 6–12 Endotoxin Units/ml using internal assay standard).
Lung gp-340 was isolated from human BALF as described using Mono-Q fast flow columns (Pharmacia) and gel-permeation chromatography [11]. The purity of the resulting gp-340 was analysed by chromatography and SDS/PAGE (polyacrylamide gradient 4–15% gels). The gp-340 preparation was free of SP-D and SP-A or other major contaminants. The salivary gp-340 preparation was prepared as described in [27]. Parotid saliva was precipitated at 4 °C, centrifuged at 5000 g for 15 min at 4 °C, resuspended in Tris with 10 mM EDTA and 0.05% CHAPS. Samples were applied to an Uno Q-6 column (Bio-Rad) and eluted with a linear gradient of 0–0.5 M NaCl. The salivary and lung gp-340 preparations had similar migration on SDS/PAGE as previously reported [27].
Sialic acid linkage analysis of gp-340
Presence and linkage patterns of terminally linked sialic acids on gp-340 preparations were analysed by ELISA, using digoxigenin-labelled lectins MAA (Maackia amurensis agglutinin) [for sialic acids in α(2,3) linkage to galactose] and SNA (Sambucus nigra agglutinin) [for sialic acids in α(2,6) linkage to galactose or N-acetylgalactosamine]. Lectin binding was assessed according to the instructions supplied by the manufacturer (DIG Glycan Differentiation Kit; Roche Diagnostics GmbH). Controls for α(2,3)- and α(2,6)-linked sialic acids were fetuin and transferrin, respectively. Approximately equal binding to these controls was found. ELISA plates were coated overnight with gp-340 (2 μg/ml) or control proteins in the presence of coating buffer. Following washes in TBS [20 mM Tris/0.9% (w/v) NaCl (pH 7.4)], lectins were added for 1 h. Detection of the bound lectins was accomplished with peroxidase-labelled anti-digoxigenin Fab fragments (1:5000 dilution for 30 min). Final detection was made with TMB (tetramethylbenzidine) peroxidase substrate solution (Bio-Rad) and the reaction was stopped with H2SO4. A450 values were read and ratios of MAA/SNA binding were obtained.
Measurement of aggregation of IAV or bacteria
Aggregation of IAV particles was assessed following addition of various concentrations of collectins by monitoring changes in light transmission on a highly sensitive SLM/Aminco 8000C (SLM Instruments) spectrofluorimeter as described [36]. The aggregation of viral particles or liposomes is demonstrated by a decline in light transmission (i.e. increased turbidity). Salmonella typhimurium (TV119 strain) was graciously provided Dr H. Nikaido (Department of Molecular and Cell Biology, University of California, Berkeley, CA, U.S.A.). Aggregation of the bacteria results in increased light transmission as previously described [37,38].
Assessment of binding of SP-D to gp-340
Binding was determined using solid-phase ELISA. Plates were coated with lung or salivary gp-340 or PBS containing fatty-acid-free BSA (Sigma–Aldrich) overnight [39]. The plates were then washed and incubated with PBS containing 25% fatty-acid-free BSA for blocking. After further washes, RhSP-D or pSP-D was added for 30 min at 37 °C. After further washing, the bound collectins were detected with appropriate antibodies. RhSP-D was detected with a rabbit polyclonal antibody. pSP-D was detected with rabbit polyclonal antibody raised specifically against pSP-D. The bound antibodies were detected with goat anti-rabbit or donkey anti-mouse F(ab′)2 fragments coupled to horseradish peroxidase (Jackson Immunochemicals), and bound secondary antibodies were detected with TMB peroxidase substrates (Bio-Rad). The reaction was stopped using 0.5 M H2SO4. The absorbance was measured on an ELISA plate reader at 450 nm. Experiments were performed in duplicate.
Fluorescent focus assay of IAV infectivity
The fluorescent focus assay was performed as described in [11], 6 h after viral inoculation of Madin–Darby canine kidney cells. Detection of infected cells was achieved by fluorescent microscopy using a monoclonal antibody directed against the influenza A viral nucleoprotein (provided by Dr Nancy Cox, Center for Disease Control, Atlanta, GA, U.S.A.).
Statistics
Statistical comparisons were made using Student's paired, two-tailed t test or ANOVA with post hoc test (Tukey's).
RESULTS
Characterization of anti-influenza activity of salivary gp-340
Salivary gp-340 has broad antiviral activity against various IAV strains: spectrum of activity is partially dependent on saliva donor
To better characterize the antiviral activity of salivary gp-340 we prepared a panel of human IAV and related viral strains and compared HA inhibitory activity of salivary gp-340 from two donors with that of lung gp-340 against these strains. We chose strains based on the preference of their HA for binding to sialic acids in either in an α(2,3) or an α(2,6) linkage (indicated in Table 1) or for their biological relevance (e.g. commonly circulating human strains of the H3N2 and H1N1 type or representative animal strains). As shown in Table 1, salivary gp-340 from donor 1 had greater activity than lung gp-340 or salivary gp-340 from donor 2 against the PR-8 and Sendai (parainfluenza) viral strains which are highly selective for α(2,3)-linked sialic acids [40,41]. The activity of salivary gp-340 from donor 1 was also greater than that of lung gp-340 against Phil82/BS. Phil82/BS is a strain developed by Anders et al. [42] and is resistant to inhibition by collectins due to loss of a high-mannose oligosaccharide attachment adjacent to the sialic acid-binding pocket of the HA. This alteration results in a relative increase in affinity for α(2,3)-linked sialic acids [40]. HA inhibition results obtained with lung gp-340 were consistent for three different preparations of the protein (results not shown).
Table 1. HA inhibitory activity of gp-340 preparations.
The results are expressed as the means±S.E.M. of four or more experiments testing the ability of the gp-340 preparations to inhibit 40 HA units of the indicated viral strains. The concentrations shown are minimal amounts of the proteins (in ng/ml) needed to achieve complete inhibition of the virus. *P<0.05 compared with either lung gp-340 or salivary gp-340 of donor 2. ND, not done.
| Amount required for inhibition (ng/ml) | ||||
|---|---|---|---|---|
| Salivary gp-340 | ||||
| Viral strain | Sialic acid binding preference | Lung gp-340 | Donor 1 | Donor 2 |
| Phil82 | α(2,6)-Linkage | 312±43 | 291±69 | 268±38 |
| Braz79 | α(2,6)-Linkage | 341±77 | 347±75 | ND |
| PR-8 | α(2,3)-Linkage | 275±25 | 74±3* | 204±51 |
| Sendai | α(2,3)-Linkage | ND | 126±8* | 245±10 |
| Phil82/BS | α(2,6) and α(2,3)-Linkage | 210±37 | 84±12* | ND |
Salivary gp-340 of donor 1 also had strong inhibitory activity against the equine strain of IAV, which is highly selective for α(2,3)-linked sialic acids (e.g. 112±68 ng/ml required to inhibit 40 HA units; n=4), as compared with its activity against the α(2,6)-specific H3N2 Aichi human IAV strain (249±65; n=4; P<0.009 compared with equine strain; results not shown).
Given the relevance of porcine IAV to interspecies transmission, we also tested activity of salivary gp-340 against a representative porcine IAV strain. Salivary gp-340 from donors 1 and 2 had HA inhibitory activity against the classic swine strain (A/Swine/Iowa/3421/90) at concentrations present in saliva (e.g. 343±114 and 191±38 ng/ml of salivary gp-340 of donor 1 and 2, respectively, inhibited HA activity of this strain; n=3). RhSP-D had no activity against this strain in parallel experiments (results not shown).
The varied activity of different gp-340 preparations against specific viral strains depends on the preponderance of specific sialic acid linkages on the proteins
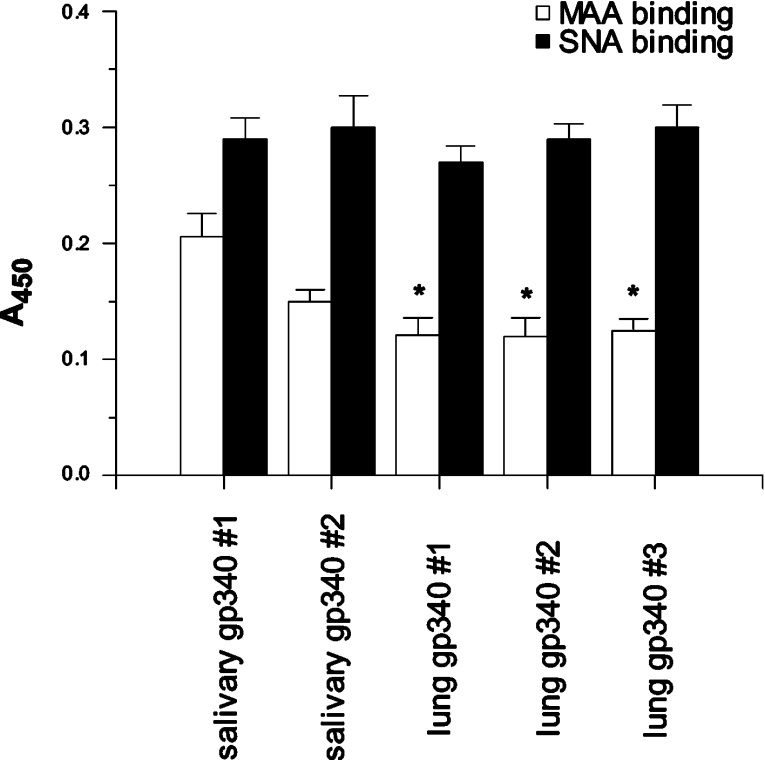
We developed an ELISA to make quantitative comparisons of the amounts of α(2,3)- and α(2,6)-linked sialic acids occurring on the different gp-340 preparations used in these studies. The amounts of α(2,3)- to α(2,6)-linked sialic acids were determined using MAA and SNA lectins respectively. As shown in Figure 1, salivary gp-340 from donor 1 had significantly greater density of α(2,3)-linked sialic acids compared with three different lung gp-340 preparations. Similar findings were obtained with salivary gp-340 of donor 1 isolated from three separate saliva donations (results not shown). Hence the increased abundance of α(2,3)-linked sialic acids was a consistent finding for this donor. The density of α(2,6)-linked sialic acids was similar for all the gp-340 preparations tested. Although the amount of α(2,3)-linked sialic acids on salivary gp-340 of donor 1 was greater than that of donor 2 in all experiments, the difference was not significant (P<0.1). However, the MAA/SNA binding ratio for salivary gp-340 of donor 1 (72±7%) was significantly greater than for donor 2 (47±2%; P<0.02). MAA/SNA binding ratios for the three lung gp-340 preparations (i.e. 43±4, 42±6, and 42±5%) were very similar and all significantly lower than the ratio for salivary gp-340 of donor 1 (P<0.04 for all). Overall the results indicate a greater density of α(2,3)-linked sialic acids on salivary gp-340 of donor 1 compared with the other preparations. The lung gp-340 preparations were obtained from BALF from different donors than those who provided saliva, so that we cannot yet comment on whether salivary and lung gp-340 from the same donor could vary in type of sialic acid linkages.
Figure 1. Comparison of density of α(2,3)- and α(2,6)-linked sialic acids on salivary and lung gp-340 preparations.
The amounts of specific sialic acids were measured by ELISA as described, using the MAA and SNA lectins for detection of α(2,3)- and α(2,6)-linked sialic acids respectively. The binding of lectins was obtained from four separate experiments and the results are expressed as the means±S.E.M. (using A450 values). MAA binding was significantly greater for salivary gp-340 of donor 1 compared with that of three different lung gp-340 preparations (P<0.04 for all), indicating a greater density of α(2,3)-linked sialic acids on salivary gp-340 of donor 1.
Because these results gave further confirmation that the IAV-inhibiting activity of gp-340 depends on its sialylation, we tested whether the neuraminidase inhibitor oseltamivir increased its inhibitory activity by preventing hydrolysis of the sialic acids by the viral neuraminidase. Prior studies demonstrated that SP-A and mucin (which have a similar mechanism of inhibition) have increased HA inhibiting activity in presence of oselatamivir [43]; however, no enhancement of the activity of salivary gp-340 was found (results not shown). This result suggests that the sialic acids present on salivary gp-340 are not readily hydrolysed by the viral neuraminidase (in contrast to those present on mucin).
Salivary gp-340 can compete against anti-microbial activities of SP-D
Competitive effects of salivary gp-340 of donor 1 when combined with SP-D
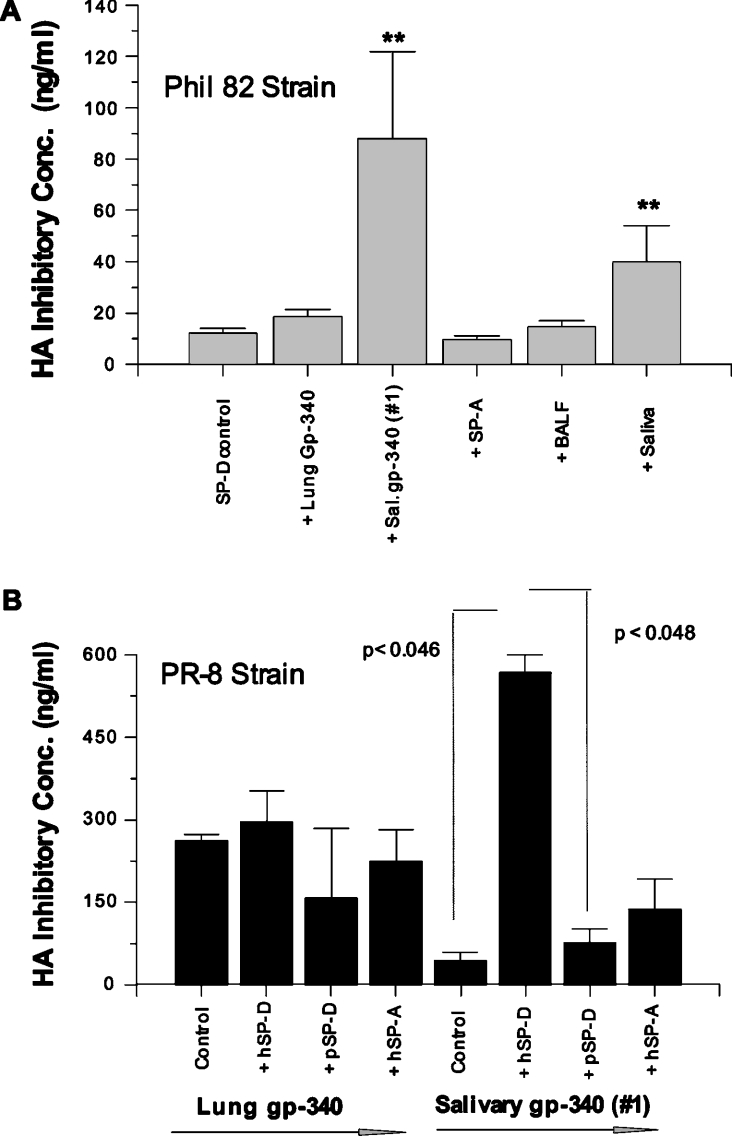
We have reported that lung gp-340 has co-operative activities on viral neutralizing and aggregating activities when combined with RhSP-D [43]. We compared interactions of salivary gp-340 preparations with SP-D on various antiviral assays and unexpectedly found that salivary gp-340 of donor 1 had strong competitive activity. In the HA inhibition assay, lung gp-340 did not significantly alter the activity of RhSP-D. In contrast, salivary gp-340 obtained from donor 1 strongly interfered with the activity of RhSP-D (Figure 2A). Normal donor saliva also interfered with the activity of RhSP-D, whereas SP-A and normal donor BALF did not. The PR-8 strain is resistant to inhibition by RhSP-D, but is inhibited by gp-340 [10,11]. Hence this strain provides a means of testing whether there is reciprocal inhibition by RhSP-D of the activity of gp-340 against PR-8. RhSP-D markedly interfered with the HA inhibitory activity of salivary gp-340 from donor 1 against the PR-8 strain (Figure 2B). A similar effect was not observed when pSP-D or pSP-A were combined with this preparation of salivary gp-340. RhSP-D did not inhibit the activity of lung gp-340 against PR-8.
Figure 2. Salivary gp-340 of donor 1 interferes with the HA inhibitory activity of RhSP-D and vice-versa.
HA inhibition was measured using Phil82 (SP-D sensitive) and PR-8 (SP-D resistant) strains of IAV. (A) HA inhibitory activity of RhSP-D alone or in combination with lung gp-340, salivary gp-340 (donor 1), SP-A, normal donor BALF or saliva. Since fixed concentrations of IAV were used, a loss of activity is indicated when greater concentrations of RhSP-D or gp-340 were required for inhibition. As indicated by **, salivary gp-340 and saliva significantly reduced HA inhibitory activity of RhSP-D (P< 0.03; n=4). In (B), the HA inhibitory activity of lung or salivary gp-340 against the PR-8 strain was tested in the absence of other proteins or combined with human SP-A or SP-D or pSP-D as indicated. RhSP-D significantly interfered with the HA inhibitory activity of salivary gp-340 for the PR-8 strain as shown.
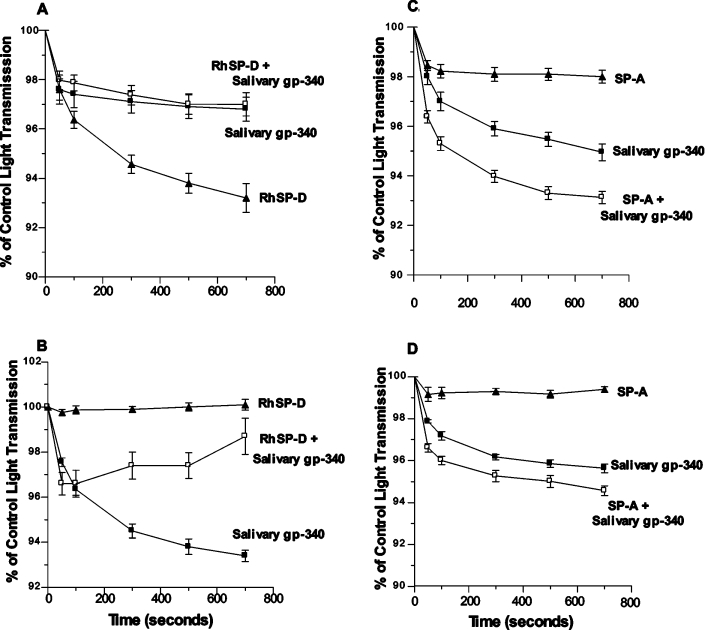
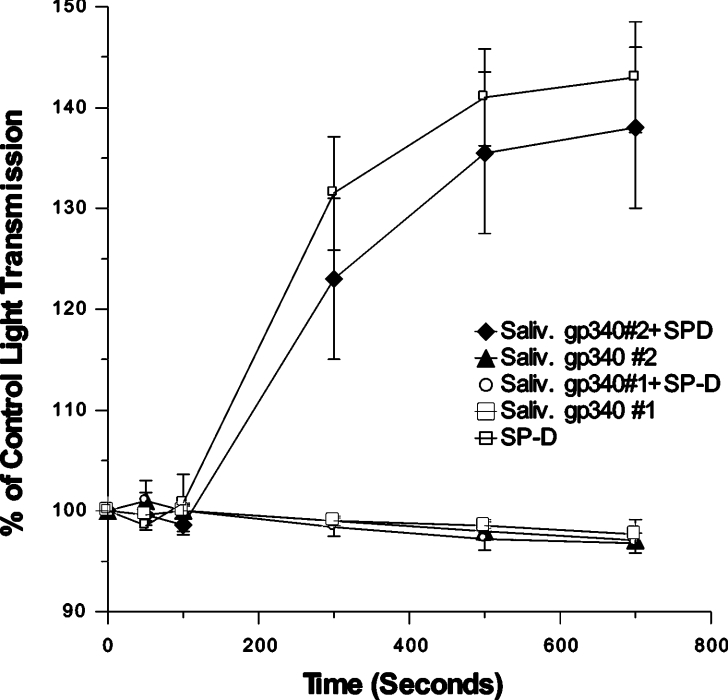
Similar antagonism was found when salivary gp-340 of donor 1 was combined with SP-D in viral neutralization assays. RhSP-D alone (20 ng/ml) reduced infectious foci formed by the Phil82 strain of IAV to 14±1% of control. However, pre-incubation of RhSP-D with 20 ng/ml of salivary gp-340 of donor 1 resulted in a much smaller reduction of infectious foci (e.g. to 60±11% of control; n=4; P<0.01). Finally, treatment of IAV with RhSP-D or SP-A causes viral aggregation. In marked contrast with the strong co-operative effect of adding lung gp-340 to SP-D in this assay [43], salivary gp-340 greatly reduced the aggregating activity of SP-D (Figure 3). Again a reciprocal effect was observed in which RhSP-D inhibited the aggregating activity of salivary gp-340 of donor 1 against the SP-D-resistant PR-8 strain (Figure 3). In contrast, salivary gp-340 of donor 1 had additive effects when combined with SP-A in these assays.
Figure 3. Salivary gp-340 (donor 1) interferes with viral aggregation induced by RhSP-D but not by SP-A.
Aggregation of stirred suspensions of the Phil82 or PR-8 strain of IAV in response to salivary gp-340 (donor 1), RhSP-D, SP-A, or combinations of these proteins, was assessed by measuring decreases in light transmission as described. The results are expressed as the means±S.E.M. for four or more experiments. In (A), both salivary gp-340 and RhSP-D independently induced significant viral aggregation of Phil82 strain; however, addition of salivary gp-340 to RhSP-D significantly reduced the aggregating activity of SP-D (P<0.05). In (B), the PR-8 strain did not aggregate in response to RhSP-D, but did aggregate in response to salivary gp-340. Pre-incubation of salivary gp-340 with RhSP-D markedly reduced its aggregating activity (P<0.05). Aggregation of the Phil82 strain (C) or PR-8 strain (D) induced by salivary gp-340 was increased by addition of SP-A (P<0.05 in either case).
The competitive effect of the salivary gp-340 of donor 1 was not observed with that of donor 2
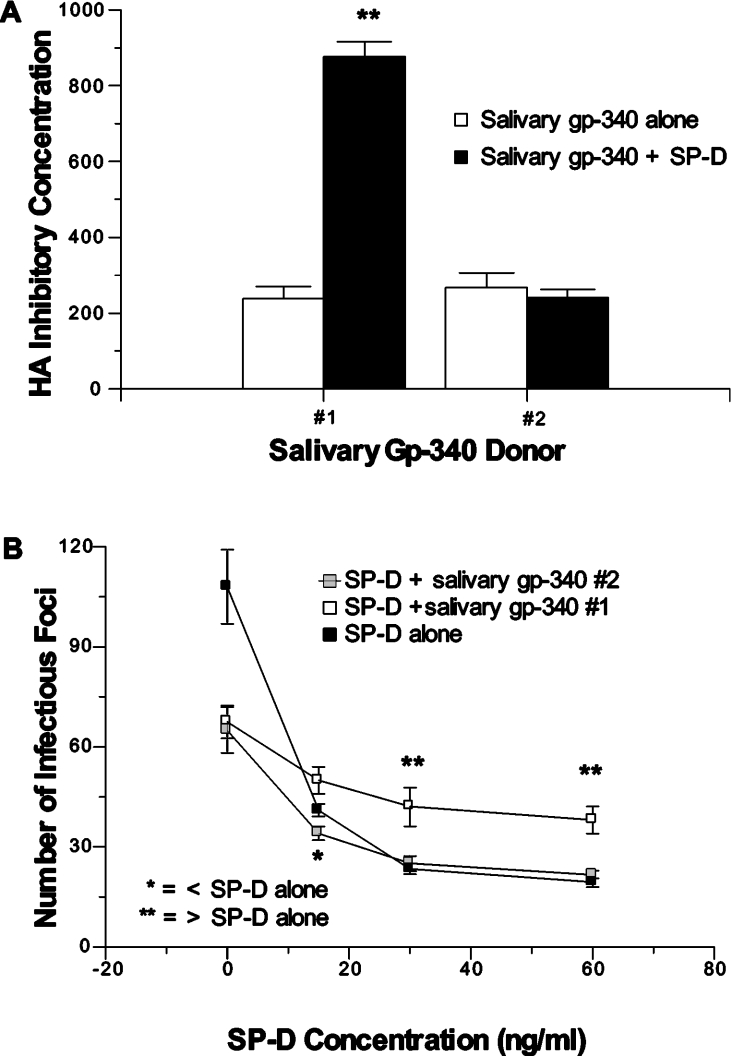
These various competitive effects were donor dependent, because salivary gp-340 from donor 2 did not significantly alter HA inhibitory activity (Figure 4A), neutralizing activity (Figure 4B) or viral aggregating activity (results not shown) of RhSP-D. To determine if the competitive effects of salivary gp-340 of donor 1 apply to interactions of SP-D with bacteria as well, we tested aggregation of the Salmonella typhimurium strain TV119 induced by SP-D alone or SP-D combined with the two salivary gp-340 preparations (Figure 5). This bacterial strain was strongly aggregated by RhSP-D and the effect was negated by pre-incubation of RhSP-D with the salivary gp-340 of donor 1 (but not that of donor 2).
Figure 4. Competitive effects of salivary gp-340 and RhSP-D in antiviral assays are dependent on donor of salivary gp-340.
The effect of adding salivary gp-340 to RhSP-D on HA inhibiting (A) and neutralizing activity (B) against the Phil82 viral strain was tested using preparations of salivary gp-340 derived from two different donors in parallel. Salivary gp-340 of donor 1 again interfered with these antiviral activities of RhSP-D, but that of donor 2 did not. ** Indicates instances where activity of RhSP-D combined with salivary gp-340 of donor 1 was significantly reduced (P<0.05) compared with RhSP-D alone. * Indicates an instance where neutralizing activity of RhSP-D was significantly greater after addition of salivary gp-340 of donor 2 (P<0.05).
Figure 5. Salivary gp-340 of donor 1 but not donor 2 competes with bacterial aggregating activity of RhSP-D.
RhSP-D strongly agglutinated S. typhimurium (TV119 strain). In this case agglutination is reflected in increased light transmission through the suspension. Addition of salivary gp-340 of donor 1 completely inhibited this aggregating activity of RhSP-D, but that of donor 2 did not. The results are expressed as the means±S.E.M. (n=4).
Association of binding of SP-D to gp-340 and competitive activity
The above results strongly suggested that RhSP-D binds to salivary gp-340 of donor 1 in such a way that neither protein can interact as effectively with viral or bacterial ligands. As shown in Table 2, salivary gp-340 of donor 1 bound more strongly than lung gp-340 (or salivary gp-340 of donor 2) to RhSP-D. The salivary gp-340 of donor 1 also bound more strongly than lung gp-340 to natural human SP-D dodecamers obtained from amniotic fluid (i.e. mean binding of 160 ng/ml of natural human SP-D dodecamers, expressed as A450 values: 0.21±0.017 for salivary gp-340 of donor 1, compared with 0.10±0.01 for lung gp-340; n=6; P<0.05; results not shown).
Table 2. Comparison of binding of RhSP-D and pSP-D to lung and salivary gp-340 of donor 1.
The results are expressed as the means±S.E.M. (n=4–6) A450 from ELISA testing binding of 125 ng/ml of the indicated SP-D preparations to either lung or salivary gp-340. RhSP-D bound significantly more to salivary gp-340 of donor 1 than to either lung gp-340 or salivary gp-340 of donor 2 (**P<0.005). pSP-D also bound significantly more to salivary gp-340 of donor 1 than to lung gp-340 (*P<0.005). Binding of pSP-D to lung gp-340 or salivary gp-340 of donor 1 was significantly reduced compared with binding of RhSP-D (P<0.003 for each comparison). However, it should be noted that different antibodies were used to measure binding of RhSP-D and pSP-D. ND, not done.
| A450 | |||
|---|---|---|---|
| Salivary gp-340 | |||
| Lung gp-340 | Donor 1 | Donor 2 | |
| RhSP-D | 0.75±0.1 | 2.14±0.17** | 0.92±0.21 |
| pSP-D | 0.143±0.02 | 0.62±0.1* | ND |
We also tested the binding of pSP-D to lung gp-340 and salivary gp-340 of donor 1. pSP-D also bound significantly more to the latter preparation of gp-340 (Table 2). Of interest, however, there was a marked reduction in apparent binding of pSP-D to either gp-340 preparation compared with binding of RhSP-D (P<0.003 for either comparison). It is hard to draw firm conclusions from these results because of the need to use different antibodies to detect hSP-D and pSP-D. However, the reduced binding of pSP-D to gp-340 is consistent with the finding that pSP-D and salivary gp-340 (donor 1) did not inhibit each other's antiviral activity (Figure 2).
One possible explanation for the greater binding of RhSP-D to the salivary gp-340 of donor 1 could be increased endotoxin bound to the gp-340 preparation. The salivary gp-340 preparations were isolated directly from parotid saliva. However, endotoxin contamination is likely, given the presence of bacteria in the oral cavity. Indeed, both salivary gp-340 preparations contained significant amounts of endotoxin (i.e. 10 and 11.5 ng/μg of protein for salivary gp-340 of donors 1 and 2 respectively), whereas three different lung gp-340 preparations did not have measurable endotoxin (i.e. <12.5 pg/μg of protein). We pre-incubated lung gp-340 or the salivary gp-340 of donor 2 with two different endotoxin preparations (0.5–8 μg of endotoxin added) and tested the effect of the added endotoxin on binding to SP-D. No increase in binding of SP-D was noted (e.g. 0.32±0.04 compared with 0.29±0.03 for lung gp-340 control and lung gp-340 pre-incubated with 2 μg/ml endotoxin respectively; n=3; A450 means±S.E.M.; 50 ng/ml RhSP-D added). Note also that the level of endotoxin present in both salivary gp-340 preparations was comparable despite differences in binding to SP-D. Hence, endotoxin contamination is unlikely to account for differences in binding to SP-D.
Gp-340 is known to bind to the CRD of SP-D. To confirm that the CRD of SP-D is responsible for competitive effects of combining SP-D with salivary gp-340 of donor 1 in antiviral assays, we used the recombinant trimeric fragment, RfSP-DnCRD, which is composed only of the neck and CRDs of human SP-D. This construct has no measurable viral neutralizing activity or HA inhibiting activity on its own [35]. As shown in Figure 6, RfSP-DnCRD significantly interfered with the HA inhibiting activity of salivary gp-340 of donor 1 but not of donor 2 against the Sendai viral strain. This indicates that the neck and CRDs of human SP-D are sufficient to interfere with the antiviral activity of the salivary gp-340 from donor 1.
Figure 6. RfSP-DnCRD, a trimeric neck and CRD fragment of human SP-D, interferes with HA inhibiting activity of salivary gp-340 of donor 1 but not donor 2.
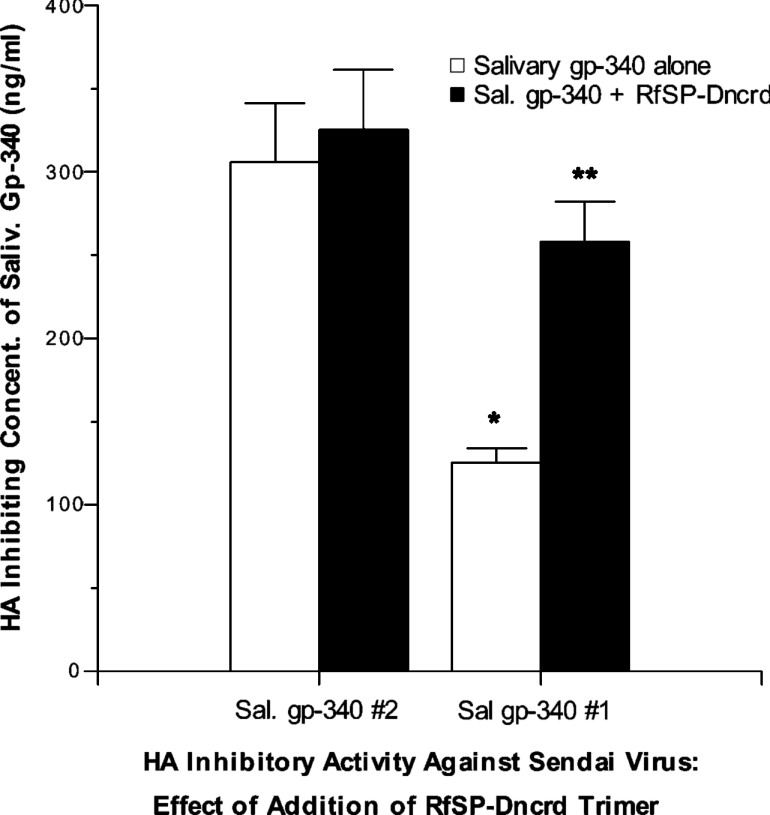
HA inhibitory activity of salivary gp-340 from donors 1 and 2 was measured as in Figure 3 using Sendai virus. As in prior experiments, the salivary gp-340 of donor 1 inhibited HA activity of this virus at significantly lower concentrations than that of donor 2 (*P< 0.05). RfSP-DnCRD had no HA inhibiting activity on its own against Sendai virus (results not shown). However, RfSP-DnCRD (black bars) significantly interfered with the HA inhibitory activity of salivary gp-340 of donor 1 (**P<0.03) without affecting that of donor 2. The results are expressed as the means±S.E.M. (n=4).
DISCUSSION
Our goal in these studies was to better characterize the anti-IAV activities of salivary gp-340 and its functional interactions with SP-D. The studies support two novel, significant conclusions: (i) the antiviral activity of salivary gp-340 against specific IAV strains varies among donors and depends on the pattern of sialylation on gp-340, and (ii) interactions with SP-D also vary among donors and (in contrast with lung gp-340) can be strongly antagonistic in some cases.
IAV inhibitory activities of gp-340 depend on specific patterns of sialylation of the protein
The antibacterial activity of salivary and lung gp-340 resides in a repeating peptide sequence present in the scavenger-receptor-like domains of the proteins [44]. The HIV neutralizing activity of gp-340 involves calcium-dependent binding to the HIV envelope protein at a site distinct from the binding site of CD4, but possibly overlapping with the chemokine receptor binding site [45,46]. Both of these interactions differ mechanistically from the interaction of salivary gp-340 with IAV. Gp-340 acts as a γ-inhibitor of IAV (i.e. it provides a decoy sialic acid ligand for the viral HA, thereby preventing the HA from binding to cellular receptors). This mechanism is not calcium-dependent. There are several other important inhibitors of IAV that act in this manner, including α2-macroglobulin [47], SP-A [48] and mucins [47]. Binding of IAV to activating receptors of NK cells [19] and neutrophils [49–51] is mediated by similar mechanisms. SP-D and other secreted collectins [e.g. MBL (mannose binding lectin), conglutinin and CL43 (collectin-43)] have a distinct mechanism of inhibition (called β-inhibition) that involves calcium-dependent binding of the collectin to mannosylated carbohydrates on the envelope proteins of IAV. pSP-D differs from all other forms of SP-D studied in that it has a highly sialylated N-linked carbohydrate attachment on its CRD [34]. As a result, pSP-D inhibits IAV via both γ- and β-inhibition [40,52].
We now demonstrate that the inhibitory activity of salivary and lung gp-340 varies depending on the abundance of specific sialic acid linkages on the protein. Using a panel of IAV strains, we show that salivary gp-340 obtained from one donor has an increased concentration of α(2,3)-linked sialic acids and that this conferred increased activity against several IAV strains that have higher affinity for this linkage pattern. Salivary gp-340 obtained from another donor (and several preparations of lung gp-340) had lower densities of α(2,3)-linked sialic acids and these preparations had, as a result, less activity against viral strains preferring the α(2,3)-linkage. Because avian strains have a high affinity for α(2,3)-linked sialic acids, and saliva is present at a major portal of entry of the virus, this could indicate that some humans differ in their resistance to these strains. The finding that both preparations of salivary gp-340 inhibited a prototypical swine IAV strain that is not inhibited by SP-D [52] suggests that salivary gp-340 could play a role in inhibiting transmission of porcine strains to humans. Avian and swine (porcine) strains of IAV are widely viewed as potential sources of pandemic IAV.
It is noteworthy that several different preparations of lung gp-340 had similar relative densities of α(2,3)- and α(2,6)-linked sialic acids [i.e. having a greater concentration of α(2,6)-linked sialic acids]. Although the two proteins are encoded by the same gene, salivary gp-340 was previously found to have increased reactivity with antibodies against Sialyl-Lewisx antigen which contains an α(2,3)-linked sialic acid [28]. It will require more studies to establish whether glycosylation differences between lung and salivary gp-340 occur frequently or within the same individual.
Salivary gp-340 can inhibit antiviral activities of SP-D and vice versa
We previously found that lung gp-340 has co-operative interactions with human SP-D in viral neutralization and aggregation assays. However, we concluded that these co-operative effects did not depend on the binding of lung gp-340 to RhSP-D and that binding of lung gp-340 to RhSP-D interfered with co-operative antiviral activity. This conclusion was based on the finding that pSP-D showed low levels of binding to lung gp-340, but increased co-operative activity when combined with lung gp-340 [43]. The lower binding of pSP-D resulted from the N-linked sugar on its CRD, possibly as a consequence of charge repulsion between this negatively charged, highly sialylated sugar and similar negatively charged sugars on gp-340.
Salivary gp-340 from donor 1 bound more strongly than lung gp-340 to RhSP-D, and this appears to be related to the finding that these preparations reciprocally inhibited each other's antiviral activities. Again, pSP-D bound less than RhSP-D to salivary gp-340 and did not have competitive interactions when combined with salivary gp-340. Gp-340 binds to the CRD of SP-D; however, the binding site is different from the carbohydrate-binding site, as demonstrated using monoclonal antibodies directed against each site [43]. Nonetheless, the sites of carbohydrate and gp-340 binding appear to be close enough to result in some steric hindrance between binding to lung gp-340 and binding to IAV. Binding of salivary agglutinin from donor 1 to SP-D strongly inhibited the ability of either protein to interact with IAV. The trimeric lectin domains of human SP-D were shown to mediate the observed inhibition of salivary gp-340 activity of donor 1 (Figure 6). Hence, dodecameric structure and the N-terminal domains of SP-D were not required.
The fact that salivary gp-340 of donor 1 had additive effects in viral aggregation assays when combined with SP-A supports the concept that the competitive effect observed when this preparation of salivary gp-340 was combined with RhSP-D results from specific binding interactions with the CRD of SP-D. Since the competitive effect also occurred in bacterial aggregation, it is likely that the effect depends on binding of salivary gp-340 of donor 1 to RhSP-D, and not to specific interactions of either protein with IAV. The most likely interpretation is that salivary gp-340 of donor 1 forms a complex with SP-D due to strong binding between the proteins and this inhibits interaction of either protein with other ligands.
The finding, that salivary gp-340 from donor 1 antagonized actions of RhSP-D, was reproducible with different preparations of the protein from this donor and was distinct from interactions of salivary gp-340 from another donor. We excluded differences in endotoxin contamination as a cause for the increased binding. We were unable to show any significant binding of SP-D to BSA derivitized with the Sialyl-Lewisx antigen, and addition of Sialyl-Lewisx–BSA did not alter HA inhibition activity of SP-D (results not shown). Hence, it seems unlikely that increased expression of Sialyl-Lewisx on salivary gp-340 relates directly to the increased binding to SP-D to salivary gp-340 of donor 1. Further studies will be needed to determine the mechanism of increased binding. It will also be necessary to study lung and salivary gp-340 isolated from a larger panel of donors to determine the frequency of variations in sialylation, binding to SP-D, and competitive interactions with SP-D. Finally it will be important to determine whether lung and salivary gp-340 obtained from the same donor have the same or different patterns of sialylation.
Conclusions
In summary, salivary gp-340 has broad-spectrum activity against IAV strains, which varies among donors depending on the relative abundance of specific sialic acid linkages on gp-340. These results may be relevant to the innate host defence of different individuals against IAV and in particular to resistance against avian type IAV strains. The finding that salivary gp-340 and whole saliva can in some cases antagonize anti-microbial activities of SP-D could relate to pulmonary complications following aspiration of oral contents. Confirmation of these hypotheses will, however, require large-scale clinical studies.
Acknowledgments
This work was supported by National Institutes of Health grants HL69031 (K.L.H.) and HL29594 and 44015 (E.C.). U.H. was supported by grants from the Danish Medical Research Council (no. 9902278), the Novo Nordic Foundation, the Fifth (European Community) Framework Program (contract no. QLK2000-00325), and the Benzon Foundation. We gratefully acknowledge the kind gift of human SP-A from Dr Jeffrey Whitsett (Children's Hospital Medical Center, Cincinnati, OH, U.S.A.).
References
- 1.Morens D. Influenza-related mortality: considerations for practice and public health. JAMA, J. Am. Med. Assoc. 2003;289:227–229. doi: 10.1001/jama.289.2.227. [DOI] [PubMed] [Google Scholar]
- 2.Nichol K., Lind A., Margolis K., Murdoch M., McFadden R., Hauge M., Magnan S., Drake M. The effectiveness of vaccination against influenza in healthy working adults. N. Engl. J. Med. 1995;333:889–893. doi: 10.1056/NEJM199510053331401. [DOI] [PubMed] [Google Scholar]
- 3.Izurieta H. S., Thompson W. W., Kramarz P., Shay D. K., Davis R. L., DeStefano F., Black S., Shinefield H., Fukuda K. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N. Engl. J. Med. 2000;342:232–239. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 4.Chiu S., Lau Y. L., Chan K., Wong W., Peiris J. M. Influenza-related hospitalizations among children in Hong Kong. N. Engl. J. Med. 2002;347:2097–2103. doi: 10.1056/NEJMoa020546. [DOI] [PubMed] [Google Scholar]
- 5.Tumpey T., Garcia-Sastre A., Taubenberger J., Palese P., Swayne D. Pathogenicity and immunogenicity of influenza viruses with genes from the 1918 pandemic virus. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3166–3171. doi: 10.1073/pnas.0308391100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamblin S., Haire L., Russell R., Stevens D., Xiao B., Ha Y., Vasisht N., Steinhauer D., Daniels R., Elliot A., et al. The structure and receptor-binding properties of the 1918 influenza hemagglutinin. Science (Washington, D.C.) 2004;303:1838–1842. doi: 10.1126/science.1093155. [DOI] [PubMed] [Google Scholar]
- 7.Stevens J., Corper A., Basler C., Taubenberger J., Palese P., Wilson I. Structure of the uncleaved human H1 hemagglutinin from the extinct 1918 influenza virus. Science (Washington, D.C.) 2004;303:1866–1870. doi: 10.1126/science.1093373. [DOI] [PubMed] [Google Scholar]
- 8.Trampuz A., Prabhu R. M., Smith T. F., Baddour L. M. Avian Influenza: A new pandemic threat? Mayo Clin. Proc. 2004;79:523–530. doi: 10.4065/79.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webby R. J., Webster R. G. Are we ready for pandemic influenza? Science (Washington, D.C.) 2003;302:1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
- 10.Hartshorn K. L., Crouch E. C., White M. R., Eggleton P., Tauber A. I., Chang D., Sastry K. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J. Clin. Invest. 1994;94:311–319. doi: 10.1172/JCI117323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartshorn K. L., White M. R., Mogues T., Ligtenberg T., Crouch E., Holmskov U. Lung and salivary scavenger receptor glycoprotein-340 contribute to the host defence against influenza A viruses. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L1066–L1076. doi: 10.1152/ajplung.00057.2003. [DOI] [PubMed] [Google Scholar]
- 12.Seo S., Webster R. Tumour necrosis factor α exerts powerful anti-influenza virus effects in lung epithelial cells. J. Virol. 2002;76:1071–1076. doi: 10.1128/JVI.76.3.1071-1076.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X., Li M., Zheng H., Muster T., Palese P., Beg A. A., Garcia-Sastre A. Influenza A virus NS1 protein prevents activation of NFκB and induction of α/β interferon. J. Virol. 2000;74:11566–11573. doi: 10.1128/jvi.74.24.11566-11573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanshoot P., Cupac D., Berkhout B. Inhibition of virus replication by RNA interference. J. Biomed. Sci. 2003;10:607–616. doi: 10.1159/000073526. [DOI] [PubMed] [Google Scholar]
- 15.LeVine A., Whitsett J., Hartshorn K., Korfhagen T. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J. Immunol. 2001;167:5868–5873. doi: 10.4049/jimmunol.167.10.5868. [DOI] [PubMed] [Google Scholar]
- 16.LeVine A., Hartshorn K., Elliot J., Whitsett J., Korfhagen T. Surfactant protein A modulates both innate and adaptive defence responses to pulmonary influenza A virus infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;282:L563–L572. doi: 10.1152/ajplung.00280.2001. [DOI] [PubMed] [Google Scholar]
- 17.Hawgood S., Brown C., Edmondson J., Stumbaugh A., Allen L., Goerke J., Clark H., Poulain F. Pulmonary collectins modulate strain-specific influenza A virus infection and host responses. J. Virol. 2004;78:8565–8572. doi: 10.1128/JVI.78.16.8565-8572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong J. H., Sprenger H., Hinder F., Bender A., Schmidt A., Horch S., Nain M., Gemsa D. Influenza A infection of macrophages. Enhanced tumour necrosis factor α (TNF-α) gene expression and lipopolysaccharide-triggered TNF-α release. J. Immunol. 1991;147:3507–3513. [PubMed] [Google Scholar]
- 19.Mandelboim O., Lieberman N., Lev M., Paul L., Arnon T., Bushkin Y., Davis D., Strominger J., Yewdell J., Porgador A. Recognition of haemagglutinins of virus-infected cells by NKp46 activates lysis by NK cells. Nature (London) 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 20.Hartshorn K. L., Daigneault D. E., Tauber A. I. Phagocyte responses to viral infection. In: Gallin J. I., Goldstein I. M., Snyderman R., editors. Inflammation: Basic Principles and Clinical Correlates. New York: Raven Press; 1992. pp. 1017–1031. [Google Scholar]
- 21.Crouch E., Hartshorn K., Ofek I. Collectins and pulmonary innate immunity. Immunol. Rev. 2000;173:52–65. doi: 10.1034/j.1600-065x.2000.917311.x. [DOI] [PubMed] [Google Scholar]
- 22.Madsen J., Kliem A., Tornoe I., Skjolt K., Koch C., Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J. Immunol. 2000;164:5866–5870. doi: 10.4049/jimmunol.164.11.5866. [DOI] [PubMed] [Google Scholar]
- 23.Husby S., Herskind A., Jensenius J., Holmkov U. Heritability estimates for the constitutional levels of the collectins, mannan-binding lectin and lung surfactant protein D. Immunology. 2002;106:389–394. doi: 10.1046/j.1365-2567.2002.01436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reading P., Allison J., Crouch E., Anders E. Increased susceptibility of diabetic mice to influenza virus infection: compromise of collectin-mediated host defence of the lung by glucose. J. Virol. 1998;72:6884–6887. doi: 10.1128/jvi.72.8.6884-6887.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reading P., Morey L., Crouch E., Anders E. Collectin-mediated antiviral host defence of the lung: evidence from influenza virus infection of mice. J. Virol. 1997;71:8204–8212. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown-Augsburger P., Hartshorn K., Chang D., Rust K., Fliszar C., Welgus H., Crouch E. Site directed mutagenesis of Cys15 and Cys20 of pulmonary surfactant protein D: expression of a trimeric protein with altered anti-viral properties. J. Biol. Chem. 1996;271:13724–13730. doi: 10.1074/jbc.271.23.13724. [DOI] [PubMed] [Google Scholar]
- 27.Ligtenberg T., Bikker F., Groenink J., Tornoe I., Leth-Larsen R., Veerman E., Amerongen A. N., Holmskov U. Human salivary agglutinin binds to lung surfactant protein D and is identical with scavenger receptor protein gp-340. Biochem. J. 2001;359:243–248. doi: 10.1042/0264-6021:3590243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prakobphol A., Xu F., Hoang V., Larsson T., Bergstrom J., Johansson I., Frangsmyr L., Holmskov U., Leffler H., Nilsson C., et al. Salivary agglutinin, which binds Streptococcus mutans and Helicobacter pylori, is the lung scavenger receptor cysteine-rich protein gp340. J. Biol. Chem. 2000;275:39860–39866. doi: 10.1074/jbc.M006928200. [DOI] [PubMed] [Google Scholar]
- 29.Hartshorn K. L., Collamer M., Auerbach M., Myers J. B., Pavlotsky N., Tauber A. I. Effects of influenza A virus on human neutrophil calcium metabolism. J. Immunol. 1988;141:1295–1301. [PubMed] [Google Scholar]
- 30.Hartshorn K. L., Tauber A. I. The influenza virus-infected phagocyte: a model of deactivation. Hematol. Oncol. Clin. North Am. 1988;2:301–315. [PubMed] [Google Scholar]
- 31.Hartshorn K., Chang D., Rust K., White M., Heuser J., Crouch E. Interactions of recombinant human pulmonary surfactant protein D and SPD multimers with influenza A. Am. J. Physiol. 1996;271:L753–L762. doi: 10.1152/ajplung.1996.271.5.L753. [DOI] [PubMed] [Google Scholar]
- 32.White M., Crouch E., Chang D., Sastry K., Guo N., Engelich G., Takahashi K., Ezekowitz R., Hartshorn K. Enhanced antiviral and opsonic activity of a human mannose binding lectin and surfactant protein D fusion protein. J. Immunol. 2000;165:2108–2155. doi: 10.4049/jimmunol.165.4.2108. [DOI] [PubMed] [Google Scholar]
- 33.Leth-Larsen R., Garred P., Jensenius H., Meschi J., Hartshorn K., Madsen J., Tornoe I., Madsen H. O., Sorensen G., Crouch E., Holmskov U. A common polymorphism in the SFTPD gene influences assembly, function, and concentration of surfactant protein D. J. Immunol. 2005;174:1532–1538. doi: 10.4049/jimmunol.174.3.1532. [DOI] [PubMed] [Google Scholar]
- 34.van Eijk M., van de Lest C. H., Batenburg J. J., Vaandrager A. B., Meschi J., Hartshorn K. L., van Golde L. M., Haagsman H. P. Porcine surfactant protein D is N-glycosylated in its carbohydrate recognition domain and is assembled into differently charged oligomers. Am. J. Respir. Cell Mol. Biol. 2002;26:739–747. doi: 10.1165/ajrcmb.26.6.4520. [DOI] [PubMed] [Google Scholar]
- 35.Crouch E., Tu Y., Briner D., McDonald B., Smith K., Holmskov U., Hartshorn K. Ligand binding of human surfactant protein D: expression of a mutant trimeric collectin that shows enhanced interactions with influenza A virus. J. Biol. Chem. 2005;280:17046–17056. doi: 10.1074/jbc.M413932200. [DOI] [PubMed] [Google Scholar]
- 36.Hartshorn K. L., Sastry K., Brown D., White M. R., Okarma T. B., Lee Y., Tauber A. I. Conglutinin acts as an opsonin for influenza A viruses. J. Immunol. 1993;151:1–9. [PubMed] [Google Scholar]
- 37.Hartshorn K., Crouch E., White M., Colamussi M., Kakkanatt A., Tauber B., Shepherd V., Sastry K. Pulmonary surfactant proteins A and D enhance neutrophil uptake of bacteria. Am. J. Physiol. 1998;274:L958–L969. doi: 10.1152/ajplung.1998.274.6.L958. [DOI] [PubMed] [Google Scholar]
- 38.Hartshorn K., White M., Crouch E. Contributions of the N- and C-terminal domains of surfactant protein D to the binding, aggregation and phagocyte uptake of bacteria. Infect. Immun. 2002;70:6129–6139. doi: 10.1128/IAI.70.11.6129-6139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeSilva N. S., Ofek I., Crouch E. C. Interactions of surfactant protein D with fatty acids. Am. J. Respir. Cell Mol. Biol. 2003;29:757–770. doi: 10.1165/rcmb.2003-0186OC. [DOI] [PubMed] [Google Scholar]
- 40.van Eijk M., White M. R., Batenburg J. J., Vaandrager A. B., van Golde L. M., Haagsman H. P., Hartshorn K. L. Interactions of influenza A virus with sialic acids present on porcine surfactant protein D. Am. J. Respir. Cell Mol. Biol. 2004;30:871–879. doi: 10.1165/rcmb.2003-0355OC. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki T., Portner A., Scroggs R. A., Uchikawa M., Koyama N., Matsuo K., Suzuki Y., Takimoto T. Receptor specificities of human respiroviruses. J. Virol. 2001;75:4604–4613. doi: 10.1128/JVI.75.10.4604-4613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartley C. A., Jackson D. C., Anders M. E. Two distinct serum mannose-binding lectins functions as B inhibitors of influenza virus: identification of bovine serum B inhibitor as conglutinin. J. Virol. 1992;66:4358–4363. doi: 10.1128/jvi.66.7.4358-4363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White M. R., Crouch E., van Eijk M., Hartshorn M., Pemberton L., Tornoe I., Holmskov U., Hartshorn K. L. Co-operative anti-influenza activities of respiratory innate immune proteins and neuraminidase inhibitor. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;288:L831–L840. doi: 10.1152/ajplung.00365.2004. [DOI] [PubMed] [Google Scholar]
- 44.Bikker F., Ligtenberg A., Nazmi K., Veerman E., van't Hof W., Bolscher J., Poustka A., Amerongen A. N., Mollenhauer J. Identification of the bacteria-binding peptide domain on salivary agglutinin (gp-340/DMBT1), a member of the scavenger receptor cysteine-rich superfamily. J. Biol. Chem. 2002;277:32109–32115. doi: 10.1074/jbc.M203788200. [DOI] [PubMed] [Google Scholar]
- 45.Wu Z., Ryk D. V., Davis C., Abrams W., Chaiken I., Magnani J., Malamud D. Salivary agglutinin inhibits HIV type 1 infectivity through interaction with viral glycoprotein 120. AIDS Res. Hum. Retroviruses. 2003;19:201–209. doi: 10.1089/088922203763315704. [DOI] [PubMed] [Google Scholar]
- 46.Wu Z., Golub E., Abrams W. R., Malamud D. gp340 (SAG) binds to the V3 sequence of gp120 important for chemokine receptor interaction. AIDS Res. Hum. Retroviruses. 2004;20:600–607. doi: 10.1089/0889222041217400. [DOI] [PubMed] [Google Scholar]
- 47.Ryan-Poirier K. A., Kawaoka Y. α2-Macroglobulin is the major neutralizing inhibitor of influenza A virus in pig serum. Virology. 1993;193:974–976. doi: 10.1006/viro.1993.1208. [DOI] [PubMed] [Google Scholar]
- 48.Benne C. A., Kraaijeveld C. A., van-Strijp J. A. G., Brouwer E., Harmsen M., Verhoef J., van-Golde L. M. G., van-Iwaarden J. F. Interactions of surfactant protein A with influenza A viruses: binding and neutralization. J. Infect. Dis. 1995;171:335–341. doi: 10.1093/infdis/171.2.335. [DOI] [PubMed] [Google Scholar]
- 49.Daigneault D. E., Hartshorn K. L., Liou L. S., Abbruzi G. M., White M. R., Oh S., Tauber A. I. Influenza A virus binding to human neutrophils and cross-linking requirements for activation. Blood. 1992;80:3227–3234. [PubMed] [Google Scholar]
- 50.Hartshorn K. L., Daigneault D. E., White M. R., Tauber A. I. Anomalous features of human neutrophil activation by influenza A virus are shared by related viruses and sialic acid-binding lectins. J. Leukocyte Biol. 1992;51:230–236. doi: 10.1002/jlb.51.3.230. [DOI] [PubMed] [Google Scholar]
- 51.Hartshorn K. L., Liou L. S., White M. R., Kazhdan M. M., Tauber J. L. Neutrophil deactivation by influenza A virus: role of hemagglutinin binding to specific sialic acid-bearing cellular proteins. J. Immunol. 1995;154:3952–3960. [PubMed] [Google Scholar]
- 52.van Eijk M., White M. R., Crouch E. C., Batenburg J. J., Vaandrager A. B., Van Golde L. M., Haagsman H. P., Hartshorn K. L. Porcine pulmonary collectins show distinct interactions with influenza A viruses: role of the N-linked oligosaccharides in the carbohydrate recognition domain. J. Immunol. 2003;171:1431–1440. doi: 10.4049/jimmunol.171.3.1431. [DOI] [PubMed] [Google Scholar]